Abstract
Age-related macular disease (AMD) is a multifactorial degenerative condition affecting the central area of the retina. Patients with AMD report that eye care practitioners are not giving consistent advice regarding nutrition and reported confusion as to what advice, if any, to follow. The aim of this study was to design and conduct a preliminary evaluation of a flowchart to support eye care practitioners in providing accurate, evidence-based nutritional advice to their patients. A flowchart was designed to take practitioners through a decision-making process that would determine whether a patient matched the Age-Related Eye Disease Study (AREDS) 2 eligibility criteria for supplementation. The flowchart was evaluated using a qualified and student optometrist cohort, with both cohorts completing confidence scales and students completing clinical scenarios. Qualified participants showed a significant increase in confidence scores from the initial survey (M = 69.7%, standard deviation [SD] = 16.2%) to the second survey after use of the flowchart for 2 weeks (M = 82.1%, SD = 11.6%; t(45) = 7.33, p < .001; rs = .61, p < .001). The student participants also increased confidence scored after receiving the flowchart (M of first survey = 41.7, SD = 14.6; M of second survey = 69.1, SD = 1.7; t(25) = 7.92, d = .81, p < .001) and increased the number of correct answers on five clinical scenarios. Overall, the flowchart has proved to be useful in boosting the self-efficacy of both qualified practitioners and student practitioners, as well as improving clinical decisions made by student practitioners.
Keywords: Age-related macular degeneration, clinical decision-making aid, flowchart, nutrition
Introduction
Age-related macular disease (AMD) is a multifactorial degenerative condition affecting the central area of the retina. It is the leading cause of visual impairment and blindness registration in the developed world (Royal National Institute of Blind People [RNIB], 2010). A rapidly ageing population has raised the priority of reducing the risk for age-related eye diseases that impair sight and quality of life. As there are currently 9.7 million people aged 65 and older in the United Kingdom and by 2020 one in five UK citizens will be aged 65 or older (National Institute for Health and Care Excellence, 2008), it is imperative that more is learnt about AMD and done to help those with the condition.
The role of oxidation in the development of AMD has prompted interest in the use of antioxidant supplementation for reducing the risk of progression of the condition. The Age-Related Eye Disease Study (AREDS; 2001) investigators reported that taking a supplement containing vitamins E and C, beta-carotene, and zinc reduced risk of progression of the disease by 25%. Since then, the carotenoids lutein (L) and zeaxanthin (Z) have been identified as nutrients that can provide a protective role against progression of AMD due to their antioxidant and photo protective properties (Beatty, Nolan, Kavanagh, & O’Donovan, 2004). Recently, the Age-Related Eye Disease Study 2 (AREDS 2) (Chew et al., 2013) found that people who took a supplement containing L and Z had their risk of progression reduced by 18%.
Despite results from AREDS studies, there remains confusion among patients and practitioners about what supplements to take and what foods should be consumed (Kent, 2007). Many patients turn to organisations such as the Macular Society – the UK charity that supports people with AMD and provides information via its helpline, website, and journals. Following the results of AREDS 2, the Macular Society have advocated the use of AREDS 2 formulation, where appropriate, and eating vegetables that are L&Z rich such as eggs, spinach, and kale (Hosseini, Mosallaei, & Kalameh, 2009). However, recent surveys of its members found that many were not taking a clinically proven nutritional supplement (Stevens, Bartlett, Cooke, & Walsh, 2014) or consuming adequate amounts of L&Z (Stevens, Bartlett, & Cooke, 2015).
Aggressive marketing of particular nutritional formulations makes it difficult for patients and practitioners to make research-based choices. Research has shown that given more choices, patients can become overwhelmed (Kent, 2007). Supplements are not regulated in the same manner as medication in the United Kingdom (EU Directive, 2002), and it is very difficult to identify which supplements are likely to be of any benefit.
In a similar way, the information available in newspapers and magazines and on the Internet can be conflicting as to which are the best dietary sources of lutein and zeaxanthin. Other barriers that prevent patients from taking preventative measures include poor communication with practitioners, misinformation in the marketplace, and age-related compliance problems (Kent, 2007).
Patients reported that eye care practitioners were not giving consistent advice regarding nutrition and reported confusion as to what advice, if any, to follow. However, there are currently no guidelines for patients for practitioners to follow when advising patients about nutrition. Practitioners are often unsure, or lack confidence, when giving advice outside of their area of expertise, even when this advice is consistent with general health advice (Turner, Nicholson, & Sanders, 2011).
The aim of this study was to design and conduct a preliminary evaluation of a clinical decision-making aid (CDA) to support eye care practitioners in providing accurate, evidence-based nutritional advice to their patients. As treatment for advanced AMD accounts for 1% of the total National Health Service (NHS) drugs budget (Owen et al., 2012; Vision 2020 UK, 2012), educating AMD patients about nutrition could have significant impact on NHS resources and patient quality of life.
Method
Design of the decision-making aid
In medicine, flowcharts have been used to aid diagnosis, treatment options and advice given to patients (Bailey et al., 2016). Because they use symbols or diagrams, they are able to be used in settings where time is limited, since users can absorb information very quickly. Flowcharts are often space efficient too and can be placed on clinic walls or pin boards so practitioners can have easy access. As such, a flowchart makes an ideal CDA for all practitioners to use and an ideal way to implement an intervention for consistent nutritional advice for patients with AMD.
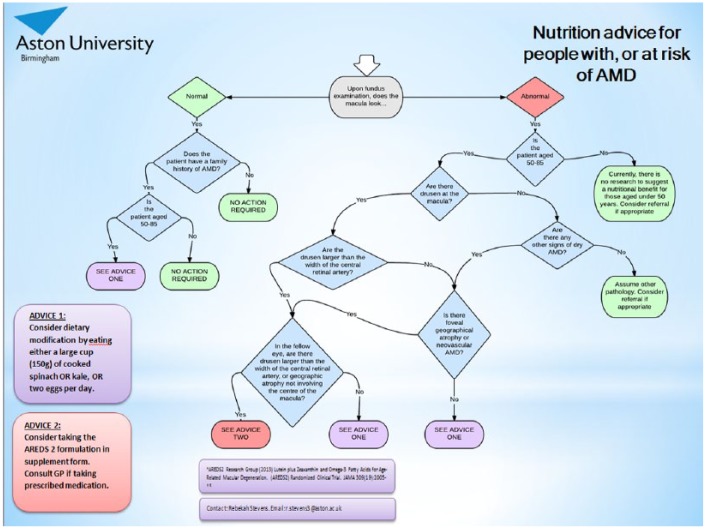
A flowchart was designed to take practitioners through a decision-making process that would determine whether a patient matched the AREDS 2 eligibility criteria for supplementation. The question to be answered by the flowchart was: ‘what nutritional advice should be given to patients with, or at risk of, AMD’? The most recent large-scale clinical research available to answer this question is the AREDS 2 (Chew et al., 2013); the study’s inclusion and exclusion criteria can be used to decide when it is appropriate to advise the AREDS 2 nutritional formulation.
The flowchart (Figure 1) was initially drafted in Microsoft Word using the shapes function. The top of the flowchart started with consideration of the retinal examination. If a patient had a normal macula, but had a family history of AMD, a branch of decisions was created to determine whether they would benefit from dietary modification. If the patient did have a non-normal macula, the branches following determined whether the patient fitted into the AREDS 2 inclusion criteria or whether they would benefit from dietary modification only. If the findings were not related to AMD, referral for an ophthalmological opinion was advised. The final outcomes were split into either dietary modification (advice one) or supplementation (advice two).
Figure 1.
Flowchart given to eye care practitioners to assist in nutrition advice. Practitioners to start with the ‘better’ eye.
Once the design had been decided upon, the chart was then created in flowchart software Lucidchart (Lucid Software Inc., Draper, UT, USA), with ‘yes’ and ‘no’ decision lines.
Evaluation of the flowchart
Pilot testing of the flowchart was conducted by sending the flowchart to 25 optometrists connected to Aston University who worked in hospital, independent, multiple and university practices by email, asking for their input into the usability of such an aid. The optometrists were asked to comment on any aspect of the flowchart. All 25 provided positive feedback, with some also providing useful comments about how to amend the aid to make it more user-friendly – for instance by defining terms like ‘geographic atrophy’ with photos. These suggestions were incorporated into the final version.
A key outcome of using the flowchart is to increase the confidence of practitioners in giving advice on dietary intake. Self-efficacy is a measure of the confidence that a person has in their ability to perform a behaviour, such as give dietary advice to AMD patients (Lyons, Dunson-Strane, & Sherman, 2014). Self-efficacy was measured to assess the impact of using the flowchart on practitioner’s confidence. Evaluating self-efficacy using scales gives researchers an idea of whether a subject is likely to accomplish a task in the future. Self-efficacy scales have been used in many surveys of medical practitioners’ confidence in performing certain procedures or giving advice to patients (Chapin, Coleman, & Varner, 2011; Lyons et al., 2014; Turner et al., 2011). Moreover, a survey of clinicians perceptions of computerised protocols demonstrated that the biggest predictor of intention to use a computerised protocol was beliefs about self-efficacy (AREDS 2, 2013), highlighting how important self-efficacy is in following advice.
A survey was created that asked participants to rate their confidence and self-efficacy out of 100 (in 10 increment steps) when performing certain tasks such as giving nutritional advice to patients or classifying the type of AMD seen in a patient. This survey was created using Bristol Online Survey software (University of Bristol, Bristol, UK).The seven-item statements included in the survey are shown in Table 1.
Table 1.
Seven-item scale assessing self-efficacy.
| A. I am confident that I could classify the type of AMD a patient has based on retinal signs |
| B. I am confident that I can advise a patient with AMD on the relationship between AMD and nutrition |
| C. I am confident that I can advise a patient with AMD on what foods to eat that might be beneficial for their condition |
| D. I am confident that I can advise a patient with AMD on the quantities of foods that might be beneficial for their eye health |
| E. I am confident that I can advise a patient with AMD on when nutritional supplementation may be beneficial |
| F. I am confident that I can advise a patient with AMD on what supplements to take and what dosage to recommend |
| G. I am confident with talking about nutrition to those at risk of AMD |
AMD: age-related macular disease.
These items were chosen because they cover all decisions that need to be contemplated when determining when and what nutritional advice should be given to a patient based on the AREDS 2 criteria, that is, determining that a patient had drusen and geographic atrophy, advising a patient that AMD and nutrition are linked, determining what foods are beneficial and how much, knowing which patients require supplementation, which supplements to take (and how much), and advising those that have a family history of AMD or are at risk.
Demographic information was also elicited by questions on age, gender, number of years practising (where appropriate), the country practising in (as nutritional advice has been shown to vary in other countries [Smick, 2014]), and the number of AMD patients seen each week.
A self-efficacy survey was repeated by participants after using the flowchart. This survey was the same as the initial survey and used the same items to rate the participant’s confidence and self-efficacy out of 0 to 100 in 10 increment steps.
Ethical approval
The procedures for both qualified practitioners and student practitioners followed were in accordance with the ethical standards of the Aston University Ethics Committee on human experimentation that conform to the Declaration of Helsinki 1975 (DoH, 2009), revised Hong Kong 1989; application number 717.
Recruitment and delivery protocol
The project was two-pronged as it was conducted on two groups of participants: qualified practitioners and student practitioners.
Qualified practitioner recruitment
Two professional optometry journals agreed to run a 200-word feature of the study. Readers were advised to email their interest in the study to R.S. and they would receive a document with the study information. The inclusion criteria for the qualified practitioner study were that the participant was a qualified optometrist or ophthalmologist that had completed their pre-registration period.
Delivery protocol
Participants were sent a URL to the initial survey on Bristol Online Surveys where they could enter their demographic details and complete the self-efficacy questionnaire prior to receiving the flowchart. At the end of the survey, a further link sent them to an Aston University website which provided them study information, with the flowchart and a list of frequently asked questions. Participants entered an email address so that we could send them the follow-up survey 2 weeks later. Participants were sent a URL to the final survey, where they completed the self-efficacy questionnaire for the second time. If they had any other questions, they could email the researcher (three participants wanted extra clarification regarding supplements).
The delivery protocol can be summarised as follows:
Stage 1 – Complete baseline self-efficacy survey
↓
Stage 2 – Receive flowchart and use for 2 weeks
↓
Stage 3 – Complete final self-efficacy survey
Student practitioner recruitment
For student optometrists, it was important that only students who had reached a sufficient level of knowledge about AMD were included in the study. Final year students have reached an appropriate level in their retinal knowledge to make clinic management decisions, based on the curriculum they will have covered. Aston University currently has one of the largest optometry schools in the United Kingdom, with an intake of approximately 140 students each year. Final year students at Aston University were informed about the study via email and announcements in lectures. If they wished to participate, they needed to come to the data collection session. The data were all collected in one tutorial session.
Delivery protocol
If a student wished to participate, they attended a one-off tutorial session. Participants were allocated to group ‘1’ or ‘2’ by numbering them alternately. Students from both groups were asked to view five pairs of retinal photos depicting hypothetical clinical scenarios of patients in various stages of AMD. This type of task is similar to questions that the students will have encountered in university assessments – please see Figure 2 for the scenarios. Information about the patient’s age, the type of AMD they had, and if there was any family history of AMD was given underneath the photographs.
Figure 2.
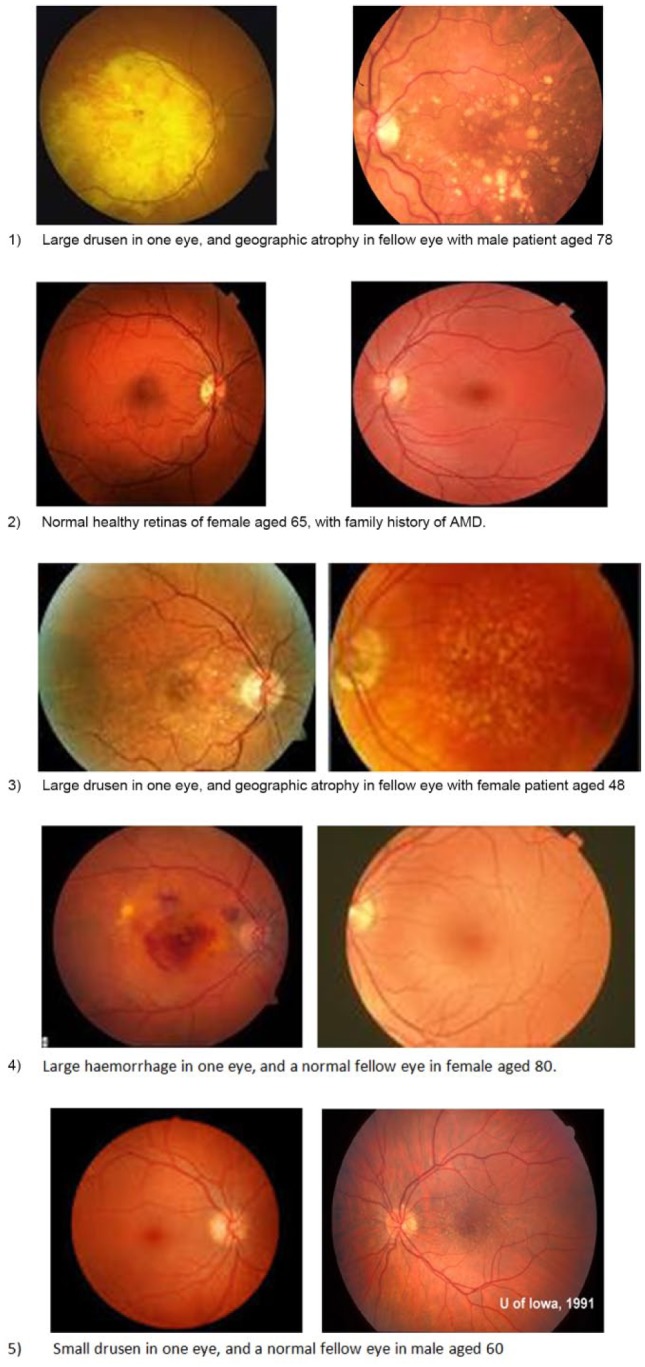
Student clinical scenarios.
The students had to then pick the most appropriate nutritional advice to give to each patient from a list of 10 possibilities:
No action required.
Consider dietary modification by eating either a large cup (150 g) of cooked spinach OR kale, OR two eggs every day.
Consider dietary modification by eating either a large cup (150 g) of uncooked spinach OR kale, OR two eggs every day.
Consider dietary modification by eating either a small cup (75 g) of cooked spinach OR kale every day.
Consider dietary modification by eating two bananas or two mangoes every day.
Consider taking supplementation of vitamin C 500 mg, vitamin E 400 IU, lutein 10 mg, xeaxanthin 2 mg, zinc 25 mg, and copper 2 mg every day. Consult general practitioner (GP) if taking prescribed medication.
Consider taking vitamin C 250 mg, vitamin E 800 IU, lutein 1 mg, xeaxanthin 5 mg, zinc 250 mg, and copper 20 mg every day. Consult GP if taking prescribed medication.
Consider taking supplementation of lutein 10 mg and zeaxanthin 2 mg every day.
Consider taking supplementation of Ginkgo biloba and cod liver oil every day.
Refer immediately for wet AMD treatment.
For each scenario, there was only one correct answer. After this exercise, the participants were asked to complete the self-efficacy survey.
After the students had picked and marked their answers on the given sheet (which were then taken away), the groups were separated into opposite sides of the lecture hall. Group 1 were given a continuing education article about the AREDS 2 from Optician journal (AREDS 2, 2013). Group 2 were given the flowchart and the same frequently asked questions that the qualified practitioners received. The participants were then asked to look at the same five clinical scenarios and again pick the most appropriate advice for the patient. Afterwards, the students were asked to complete the self-efficacy survey for a second time. After the exercise, students from both groups were given the correct answers and both packs of information for their own studies.
The delivery protocol can be summarised as follows:
Students split into groups
← →
Flowchart group Article group
↓
Stage 1 – complete clinical scenarios
↓
Stage 2 – complete baseline self-efficacy survey
↓
Stage 3 – receive flowchart or article
↓
Stage 4 – repeat clinical scenarios
↓
Stage 5 – complete final self-efficacy survey
Participants
Table 2 shows the number of qualified practitioner participants completing the surveys.
Table 2.
Number of qualified practitioner participants completing the surveys, and average age and years practising.
| Males | Females | Total | Average age in years | Years practising | |
|---|---|---|---|---|---|
| First survey participants | 38 | 33 | 71 | 48.1 ± 12 | 23.6 ± 12.7 |
| Second survey participants | 25 | 21 | 46 | 45.2 ± 11.5 | 22 ± 11.7 |
A Shapiro–Wilk test for normality (where p > .05), and a visual inspection of the data’s histograms, normal Q-Q, and box plots showed that the qualified practitioner male survey data were normally distributed, the female data, however, were not. The skewness and kurtosis values for females were also outside of the +1.96 to −1.96 normal range.
A total of 51 final year optometry students participated in the study, 8 males and 43 females with a mean age of 21.7 ± 2.9 years. Of the 51 participants, 25 were allocated to the AREDS 2 article group (group 1, ‘article’) and 26 participants were allocated to the flowchart information group (group 2, ‘flowchart’). Ethnicity was an optional and open question and participants were able to write freely what they felt their ethnicity was in their own words.
The ethnicity of the participants (in percentage) was White 17.6, Indian 15.7, Pakistani 7.8, Sri Lankan 1.9, Bangladeshi 1.9, Arab 1.9, Palestine 1.9, and Asian 11.7%. NB, not all participants answered this as it was optional.
A Shapiro–Wilk test for normality (where p > .05), and a visual inspection of the data’s histograms, normal Q-Q, and box plots showed that the student survey data were normally distributed.
Results
Statistical analysis
Data from Excel were used in statistical software IBM SPSS to first find the reliability of the survey using Cronbach’s alpha and Pearson’s correlation. Comparisons between self-efficacy levels were next performed using paired t-tests for practitioners. Two-factor analysis of variance (ANOVA) with group (control vs flowchart) and time (baseline, follow-up) as factors was used to analyse differences in self-efficacy and scenario answers in the sample of student practitioners.
Qualified practitioner study: results
Reliability of the survey
Reliability of the scale items in the surveys was confirmed using Cronbach’s alpha coefficient (a measure of internal consistency, i.e., how closely related a set of items are as a group). Alpha values were high on each occasion = 0.87 for the first survey, alpha = 0.90 for the second survey. Test–retest correlations between scores at both time points confirmed the reliability of the scale (r = .70, p < .001).
Sample characteristics
For average confidence scores, there were no differences found between the age of participants, gender, or the number of years practising as an optometrist. Hence, these variables did not appear to influence confidence levels.
There were no demographic differences between the participants who did not complete second survey compared with the participants who did complete the second survey. Chi-square tests showed no significant differences between those who completed the two surveys and gender, ethnicity, age, and number of years practising. Confidence for first survey (on a scale of 0–100) = 69.0 ± 18.8, 70.6 ± 12.8, 69.8 ± 15.8. Confidence for second survey (0–100) = 80 ± 14, 84.6 ± 7.3, 82.3 ± 10.7.
Differences between surveys
A paired-samples t-test showed that there was a significant increase in confidence (self-efficacy) scores from the initial survey (M = 69.7, standard deviation [SD] = 16.2) to the second survey after use of the flowchart for 2 weeks (M = 82.1, SD = 11.6; t(45) = 7.33, p < .001). The effect size was found to be large according to Cohen’s categorisation scheme (Cohen’s d = .88).
Student practitioner study: results
Reliability of the survey
Cronbach’s alpha coefficient was again used to assess the internal consistency of the self-efficacy scale items in the surveys. Alpha values were high at both time points (alpha = 0.84 for the first survey, alpha = 0.92 for the second survey). There was a large correlation between scored at the two time points (r = .50, p = .01); this provides evidence of test–retest reliability.
Confidence scores
A paired t-test showed that there was a significant increase in confidence scores from the initial survey to the second survey after receiving educational materials (see Table 3): group ‘article’ (M of first survey = 42.5, SD = 15.7; M of second survey = 64.8, SD = 12.3; t(24) = 7.84, d = .67, p = .01), group ‘flowchart’ (M of first survey = 41.7, SD = 14.6; M of second survey = 69.1, SD = 1.7; t(25) = 7.92, d = .81, p < .001).
Table 3.
Mean confidence scores for student participants.
| Group | Survey 1 | SD | Survey 2 | SD |
|---|---|---|---|---|
| Article | 42.5 | 15.7 | 64.8 | 12.3 |
| Flowchart | 41.7 | 14.6 | 69.1 | 1.7 |
SD: standard deviation.
There was not a statistically significant difference in second survey’s confidence scores between the two groups, although the group ‘flowchart’ scores were higher than group ‘article’. A two-factor ANOVA, with group as an independent groups factor and time as a repeated measures factor, showed that the main effect of time was significant F(1,49) = 277.70, p < .001. This means that scores were higher at the second time point. In contrast, there was no significant main effect of group which means there was not a statistically significant difference in confidence scores between the two groups. Finally, there was no statistically significant interaction between the confidence scores in the flowchart and article groups and time F ratio F(1,49) = 1.24, p = .270 – although the group ‘flowchart’ scores were higher than group ‘article’.
Scenario questions
Table 4 depicts the mean correct answers given to the five clinical scenarios in the baseline (initial) survey and then in the second survey according to each group. For statistical analysis, a correct answer was given a value of ‘1’ and incorrect answers were given a value of ‘0’.
Table 4.
Mean score of correct answers to clinical scenarios (to 2 d.p.).
| Group | Mean initial score | Initial SD | Mean second score | Second SD |
|---|---|---|---|---|
| Article | 0.28 | 0.40 | 0.54 | 0.45 |
| Flowchart | 0.35 | 0.42 | 0.68 | 0.34 |
SD: standard deviation.
A mixed-measures two-factor ANOVA was run to compare the main effects of time and group and the interaction between the factors. The main effect of time yielded was significant F(1,49) = 85.43, p < .001 indicating the number of correct answers was higher at the second time point. However, there was no significant interaction effect with the two groups (group ‘article’ and group ‘flowchart’) F(1,49) = 1.39, p = .245, but there was a significant difference between groups F(1,49) = 4.12, p = .48. An independent t-test also showed that there was a significant difference between the two group’s answers in the second survey: group ‘flowchart’ answered significantly more correctly than group ‘article’ t(24) = 2.21, p = .03.
Discussion
A novel flowchart outlining advice on diet increased the self-efficacy to give dietary advice to patients with AMD among samples of qualified and student eye care practitioners. In addition, student practitioners given the flowchart made more correct decisions for hypothetical clinical scenarios than student practitioners given AREDS information.
Qualified eye care practitioners
The qualified eye care practitioners felt more confident giving nutrition advice to AMD patients after using the flowchart for 2 weeks. This is regardless of the gender or age of the participant, or number of years practising. Qualified practitioners gave positive feedback about the flowchart and felt that it had enhanced their clinical practise skills. The results from this study replicate those of past studies showing that using a clinical decision aid can boost health professional’s self-efficacy in making clinical decisions (Phansalkara, Weir, Morrisa, & Warner, 2008). In a study investigating GP’s attitudes towards using guidelines, participants responded best to guidelines in a flowchart format, no more than two sides of A4 paper (Watkins, Harvey, Langley, Grey, & Faulkner, 1999). This result has been found to occur in patient decision aids (PDAs) also – improvements were seen in knowledge and decisional self-efficacy (Bailey et al., 2016). Overall, the flowchart has been shown to be a useful tool in a qualified eye care practitioner population.
Student practitioners
Student optometric practitioners feel more confident giving nutrition advice after having received either generic clinical information or after using the flowchart. While those using the flowchart reported higher self-efficacy scores than those who received the continuing education article, this difference was not statistically significant. This may reflect a lack of power in this analysis, due to the sample size, or that receiving information after the clinical scenario task, which was challenging, makes student practitioners feel more confident. Nevertheless, receiving the flowchart led to participants making more correct decisions receiving the article. Impressively, flowchart participants appeared to improve the most at the hardest scenarios. It was apparent that the clinical scenario tasks appeared to vary in difficulty – scenario three had much fewer correct answers in the first survey overall (2% with group flowchart, 0% with group article) when compared to scenario four (88.2% both groups flowchart and article). Once the participants had received their educational materials, Group ‘article’ increased the average correct responses in scenario three to 8%, but group ‘flowchart’ increased the average correct responses to 57.7% – this is a 25-fold increase. Group ‘flowchart’ appeared to have performed best at the tasks which were the hardest.
Study strengths and weaknesses
This study has a number of strengths. First, by showing that the flowchart boosted self-efficacy in two groups, we are confident that this decision aid has the potential to increase self-efficacy in different groups. By collecting data from qualified practitioner study, we were able to evaluate the impact of the flowchart in the real world. Finally, the clinical scenarios were a good method for determining increased knowledge at a specific time – scenarios are used to teach students about clinical practice; improving their performance is a good marker for success in clinical decision making – and were useful as it was only possible to get the participants together once. This shows that the effects of the flowchart are generalisable. We acknowledge that as well as these strengths this study also has a number of weaknesses. Some qualified practitioners dropped out from the study, despite having three email reminders. In the course of busy clinical practice, it may be that a greater incentive was required to encourage completion of the study. In addition, this was an opportunistic sample – we did not know how many participants would respond to the two advertisements, so we set restrictions on the time period that a potential participant could respond with interest. The sample size is relatively small, as it was an opportunistic sample – it was logistically difficult for all students in the year group to attend due to timetable variations.
Conclusion
Overall, the flowchart has proved to be useful in boosting the self-efficacy of both qualified practitioners and student practitioners, as well as improving clinical decisions made by student practitioners. The current results show that a brief, low-cost, clinical decision aid can boost self-efficacy and improve clinical decision making.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Age-Related Eye Disease Study Research Group. (2001). A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Archives of Ophthalmology, 119, 1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study 2. (2013, May 19). What does it mean in practice? Optician, p. 20. [Google Scholar]
- Bailey R. A., Pfiefer M., Shillington A. C., Harshaw Q., Funnell M. M., VanWingen J., Col N. (2016). Effect of a patient decision aid (PDA) for type 2 diabetes on knowledge, decisional self-efficacy, and decisional conflict. BMC Health Services Research, 16, Article 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty S., Nolan J., Kavanagh H., O’Donovan O. (2004). Macular pigment optical density and its relationship with serum and dietary levels of lutein and zeaxanthin. Archives of Biochemistry and Biophysics, 430, 70–76. [DOI] [PubMed] [Google Scholar]
- Chapin J. R., Coleman G., Varner E. (2011). Yes we can! Improving medical screening for intimate partner violence through self-efficacy. Journal of Injury & Violence Research, 3, 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew E. Y., Clemon T. E., SanGiovanni J. P., Danis R., Ferris F. L., Elman M., . . . Speduto R. (2013). Lutein plus zeaxanthin and omega-3 fatty acids for age-related macular degeneration the age-related eye disease study 2 (AREDS2) randomized clinical trial. Journal of the American Medical Association, 309, 2005–2015. [DOI] [PubMed] [Google Scholar]
- Declaration of Helsinki. (2009). Ethical principles for medical research involving human subjects. Journal of the Indian Medical Association, 107, 403–405. [PubMed] [Google Scholar]
- EU Directive. (2002). Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. Official Journal of the European Communities: Legislation, 45, 51–57. [Google Scholar]
- Hosseini H. R. J., Mosallaei M., Kalameh K. A. (2009). The effect of nutrition and supplements on ocular health. Iranian Red Crescent Medical Journal, 11(1), 10–17. [Google Scholar]
- Kent C. (2007). AMD and nutrition: The missing message. Review of Ophthalmology, 14(8), 31–37. [Google Scholar]
- Lyons B., Dunson-Strane T., Sherman F. (2014). The joys of caring for older adults: Training practitioners to empower older adults. Journal of Community Health, 39, 464–470. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (NICE). (2008). Mental wellbeing and older people. Public health guidline (PH16). London: NICE [Google Scholar]
- Owen C., Jarrar Z., Wormald R., Cook D. G., Fletcher A. E., Rudnicka A. R. (2012). The estimated prevalence and incidence of late stage age related macular degeneration in the UK. British Journal of Ophthalmology, 96, 752–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phansalkara S., Weir C. R., Morrisa A. H., Warner H. R. (2008). Clinicians’ perceptions about use of computerized protocols: A multicenter study. International Journal of Medical Informatics, 77, 184–193. [DOI] [PubMed] [Google Scholar]
- Royal National Institute of Blind People. (2013). AMD information. Retrieved from http://www.rnib.org.uk/eyehealth/eyeconditions/conditionsac/pages/amd.aspx
- Smick K. (2014). U.S. Optometrists begin global initiative of eye disease prevention. Points de Vue. Available at: http://www.pointsdevue.com/article/us-optometrists-begin-global-initiative-eye-disease-prevention
- Stevens R., Bartlett H. E., Cooke R. (2015). Dietary analysis and nutritional behaviour in people with and without age-related macular disease. Clinical Nutrition ESPEN, 10, e112–e117. [DOI] [PubMed] [Google Scholar]
- Stevens R., Bartlett H. E., Cooke R., Walsh R. (2014). Age-related macular degeneration patients’ awareness of nutritional factors. British Journal of Visual Impairment, 32, 77–93. [Google Scholar]
- Turner K. M. T., Nicholson J. M., Sanders M. R. (2011). The role of practitioner self-efficacy, training, program and workplace factors on the implementation of an evidence-based parenting intervention in primary care. Journal of Primary Prevention, 32, 95–112. [DOI] [PubMed] [Google Scholar]
- Vision 2020 UK. (2012). The Macular Disease Society press release: NHS seriously underestimating cost of elderly eye disease. Retrieved from http://www.vision2020uk.org.uk/macular-disease-society-press-release-nhs-seriously-underestimating-cost-of-elderly-eye-disease
- Watkins C., Harvey I., Langley C., Grey S., Faulkner A. (1999). General practitioners’ use of guidelines in the consultation and their attitudes to them. British Journal of General Practice, 49, 11–15. [PMC free article] [PubMed] [Google Scholar]