Abstract
The important role of epidermal appendages especially the sebaceous gland has only recently been recognised. In particular, it has been convincingly shown that normal development and maintenance of the sebaceous gland are required for skin homeostasis since atrophic sebaceous glands and disturbances in sebaceous lipid composition result in major defects of the physiological barrier and maintenance of the skin. Consequently, it is important to unravel the signalling network controlling proper sebaceous lineage differentiation in mammalian skin and to understand the underlying mechanisms leading to severe skin diseases, including abnormal proliferation and differentiation of the gland, defects of the lipid metabolism and barrier, as well as sebaceous tumour formation. Over the last years, results from transgenic and knock out mouse models manipulating distinct signalling pathways in the skin as well as the detailed analysis of human sebaceous gland-derived cell lines provided new insights into crucial mediators balancing proliferation and differentiation of the sebaceous gland. Here, we discuss our current knowledge of in vivo mechanisms of sebaceous gland development, maintenance and disorders and highlight recent contributions to the field of sebaceous gland biology.
Keywords: sebocytes, sebaceous gland, stem cell, hair follicle, epidermis, skin
1. Introduction
Our bodies are encased by an intricate organ, the skin, which serves as a protective barrier against external environmental insults and loss of internal bodily fluids [1]. These functions of the skin exist through the formation of a multilayered epithelium of the interfollicular epidermis (IFE) in which the keratinocytes form tight intracellular adhesions. The epidermal cells can also form a major appendage of the epidermis, the pilosebaceous unit, which consists of the hair follicle (HF) and sebaceous gland (SG) (Figure 1). In most cases, the SG remains intimately associated with the follicular structure, residing above the HF bulge stem cell compartment. Of the three lineages of the skin epithelium, the IFE, the HF and the SG, the SG remains the least understood. In this review, we will discuss the current knowledge and latest developments regarding the regulation of the formation and homeostasis of the SG.
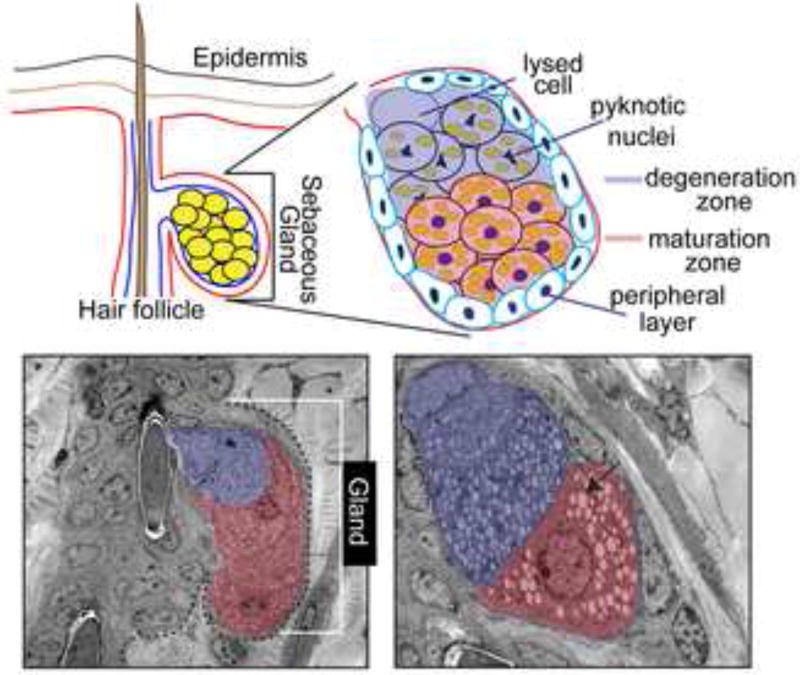
Figure 1.
Schematic illustrations (top) and electron microscopic (EM) images of cross-sections of the sebaceous gland presenting an overview (left) and more detailed view (right) of different stages of sebocyte differentiation in mouse skin. Arrow indicates lipid droplets accumulating in differentiating sebocytes. EM images are provided by E.Fuchs and A.Passoli (Rockefeller University, New York).
2. Organization and Functions of the Sebaceous Gland
In its mature form, SGs are acinar holocrine-secreting appendages of the epidermis (Figure 1). As crucial component of the pilosebaceous unit, the SG is connected to the junctional zone (JZ) of the HF, an area that is localized to the upper permanent part of the HF. This region lies below the follicular infundibulum and above the bulge region and isthmus of the HF. The basal layer of the gland is composed of proliferative cells that are contiguous with the outermost layer of the HF. Lipid-filled sebocytes, specialized keratinocytes of the SG, exist in the inner compartment of the gland. Lysis of these cells results in release of distinct lipids or sebum through a specialized canal onto the skin’s surface (Figure 1). SGs can form independently from the HF and in some cases, form specialized glands in distinct regions of the skin, e.g. Meibomian glands of the eyelid or Fordyce’s spots of the oral epithelium.
The continuous sebum production and regeneration of SGs requires replenishment of sebocytes by undifferentiated cells of the proliferative basal layer. In many ways, the physiological processes of the SG are similar to those of the continual renewal of the IFE. Both epithelial lineages are constantly renewed throughout life and are sustained by cell division of basal keratinocytes. These basal cells are surrounded by a basement membrane that separates the epithelium from the neighboring dermal tissue. Although sebocyte maturation of the gland is quite different from squamous differentiation of the IFE, both tissues produce specialized lipids and undergo a well-defined program of cellular differentiation.
As the sebocytes of the gland prepare for holocrine secretion of sebum, the cells undergo stages of maturation at distinct locations in the gland (Figure 1). Next to the single layer of basal keratinocytes of the peripheral zone, follows the maturation zone. This compartment of the gland contains enlarged sebocytes with lipid droplets [2]. As the sebocytes become more mature and progressively accumulate lipids, they are pushed towards the center of the glandular structure into the necrosis zone. Here, fully mature sebocytes have pyknotic nuclei, indicative that they are about to degenerate. This process of degeneration also occurs in the outermost layers of the IFE, yet this specialized cell death is poorly understood. Eventually, the lysis of maturated sebocytes results in the release of the lipid-containing sebum through a specialized excretory duct of the gland into the follicular canal to finally reach the skin surface (Figure 1).
It is well established that SGs contribute significantly to the barrier of mammalian skin. Although the complex physiological functions are not completely understood to date, all SGs produce and release sebum, an oily and waxy material. This complex mixture of lipids acts to reduce water loss from the surface of the skin and executes a thermoregulatory function. In addition, sebum has anti-microbial activity and is a source of antioxidants, including vitamin E [3]. Generally, sebum contains triglycerides, diglycerides, free fatty acids, wax esters, squalene and some cholesterol. Particularly, wax esters and squalene are specific to SGs and are not produced by other tissues. However, the relative amount of each of the lipid components varies between different types of SGs (HF-associated or free SGs with specific functions) and among mammalian species.
Mature sebocytes are commonly detected through lipophilic dyes, e.g. Oil Red O or Nile Red. Furthermore, sebocytes can be identified by immunostaining with antibodies against keratin 7 [4], milk fat globulins like EMA (epithelial membrane antigen) [5], proteins associated with lipid droplets, e.g. adipophilin and enzymes of the lipid metabolism, including fatty acid synthase [6] and stearoyl coenzyme A desaturase 1 (SCD1) which are implemented in important aspects of SG biology and will be discussed later in more detail.
3. Development of the sebaceous gland
The complex structure of the skin epithelium is generated during development as the surface ectoderm becomes restricted to the epidermal lineages. A single layer of epidermal cells starts to proliferate and differentiate to stratify and form the IFE. Specialized mesenchymal cells also develop underneath the epidermis and signal to direct the commitment of epidermal cells towards the HF lineage (Figure 2) [7, 8].
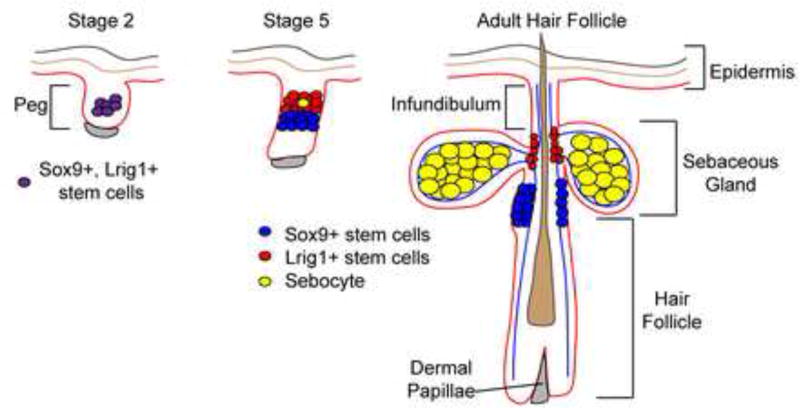
Figure 2.
Model depicting stem cell compartments contributing to hair follicle and sebaceous gland morphogenesis. An early common progenitor cell generates stem cells localized at distinct hair follicle compartments in adult skin.
The development of the pilosebaceous unit starts with the generation of the follicular compartment when progenitor cells begin to grow down into the mesenchyme to generate all cell lineages of the HF [7, 9]. On the basis of several morphological criteria, the process of formation of the pilosebaceous unit has been classified into eight distinct stages [7, 10]. The SG is the last lineage to develop at stage 5 (bulbous peg stage) of HF morphogenesis and remains permanently associated with the upper part of the HF. In mouse skin, the first sebocytes become evident and can be detected by expression of marker molecules shortly after birth [9, 11]. Certain types of HFs are associated with two prominent SGs that are originating in one small cluster of sebocytes. Once this small population of sebocytes reaches a critical size it splits to generate two sebaceous cell cluster at stage 7 of HF morphogenesis and subsequently, these develop into prominent and fully differentiated glands [11].
In humans, sebaceous glands develop in the 13th–14th week of fetal life [12], where they contribute to the production of the vernix caseosa, the protective wax-like substance that coats embryonic skin. Postnatally, SGs are diminished [13] and produce little sebum. During puberty, elevated hormonal levels induce SGs to enlarge and produce sebum.
Recent work has begun answering a major outstanding question regarding SG development: When do SG progenitors form and which cells act as progenitors for sebocytes? During HF morphogenesis, distinct stem cell compartments are established as determined by the expression of specific mRNAs and proteins [11, 14]. In the early HF placodes (stage 2), cells express Sox9 and Lrig1. Genetic lineage tracing experiments examining the fates of Sox9 expressing cells in the early HF placode (stage 2) have demonstrated that these infant cells can generate the HF and sebaceous gland cells [14]. Furthermore, ablation of Sox9 results in the absence of SGs, supporting the requirement of these cells for the sebocyte fate [14].
As HF morphogenesis precedes, this early Sox9+, Lrig1+ progenitor cell generates distinct stem cell pools just before the first sebocytes emerge [11]. The Sox9 expressing cells separate from the Lrig1+ stem cell pool and are confined to the bulge region of the HF. In contrast, Lrig1 positive cells remain at the upper part of the HF where mature sebocytes are about to emerge (Figure 2) [11, 15].
Careful analysis of the proliferation of cells during sebocyte formation suggests that Lrig1+ cells generate sebocytes. The first postmitotic mature sebocytes of the developing gland form in the Lrig1+ cell compartment of the follicle [11]. In addition, the majority of Lrig1+ cells exhibit a mitotic spindle that is oriented toward mature sebocytes, suggesting that these cells divide asymmetrically to generate sebocytes. Given the ability of Sox9+ bulge cells to maintain Lrig1+ cells of the mature HF [16], the Sox9+ cells may also be linked to sebocytes during homeostasis of the gland as well. Genetic lineage tracing of Lrig1+ and Sox9+ cells during distinct stages of SG development will ultimately define the lineage relationship between the stem cells of the follicle and the SG lineage.
4. Regulation of homeostasis of the sebaceous gland
How are the SG regenerated and maintained on a cellular level? Collective experimental data provided over recent years support two different models: 1. unipotent progenitor cells located at the periphery of the SG are committed to the SG lineage and drive SG renewal [15, 17, 18]. 2. Alternatively, HF stem cells are activated and mobilized to regenerate the SG [16]. While these models are not mutually exclusive, both scenarios require a tight control of activation and mobilization of either multipotent HF stem cells or unipotent SG progenitor cells and crucial signaling cues steering the sebaceous lineage fate. However, the molecular mechanisms underlying these processes are not well understood.
Interestingly, recent elegant lineage tracing studies of HF stem cells using promoters for different genes imply the existence of a hierarchical organization of diverse stem and progenitor cell compartments. In particular, lineage tracing the progeny of keratin 15 positive bulge stem cells as well as Lgr6 expressing isthmus stem cells has demonstrated their capacity to replenish the sebocyte lineage [16, 19]. Furthermore, progeny of bulge stem cells give rise to stem and progenitor cells of the SG indicating that different stem cell pools of the pilosebaceous unit do not operate autonomously in the process of HF and SG regeneration but are intimately linked in their function [16]. This would explain the interdependence of HFs and SGs often seen in mutant mouse models where the destruction of one tissue leads to the collapse of another [20, 21].
Together, the data suggest that the process of continuous SG regeneration is regulated by signals of two main cell fate determination centers. Within the HF bulge, stem cells respond at least to three different sets of signals to either replenish the bulge stem cell pool or to renew the HF or the SG. Currently, it is not known how this is controlled on a molecular level. One explanation would be that the HF bulge constitutes a heterogeneous population of stem cells that are characterized by different cell intrinsic properties and therefore respond differently towards environmental cues. Alternatively, bulge stem cells could exist in different states of activation and mobilization and consequently are primed for a particular cellular decision [22]. Another cell fate determination center controls the final decision of stem and progenitor cells to ultimately commit to the SG lineage and is localized at the junctional zone of the HF and probably at the region where the SG attaches to the HF [15–17]. Here, individual cells are instructed to migrate towards the periphery of the SG and to initiate a program of sebocyte differentiation.
Molecular mechanisms governing sebaceous gland physiology
Signaling cascades
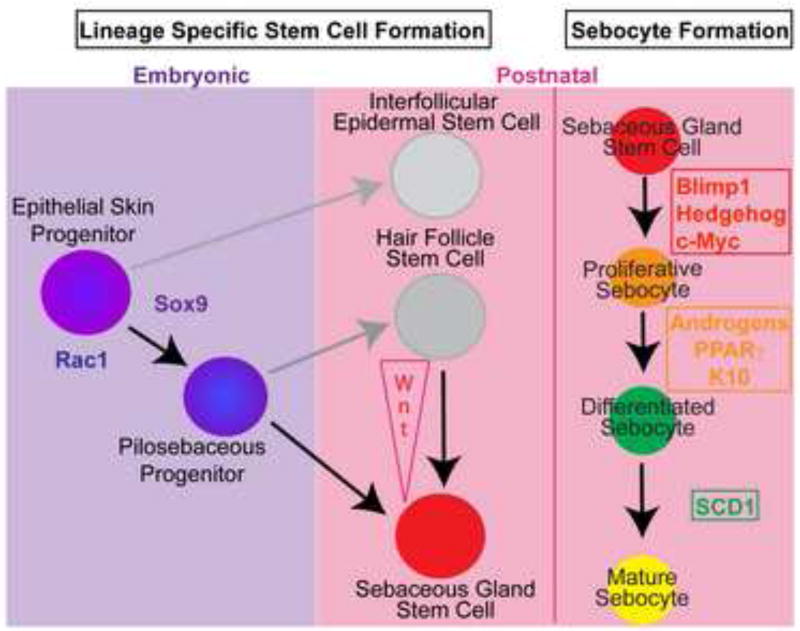
One pathway that is crucial in directing cell fate decisions in HF stem cells is canonical Wnt/β-catenin signaling. In particular, it has been well documented that the transcription factors TCF3 and Lef1, which are important down-stream mediators of Wnt/β-catenin activity, regulate bulge stem cell activation for HF renewal and HF differentiation [23–25]. In contrast, blocking TCF/Lef1 signaling activity is an important requisite to allow stem and progenitor cells to commit to SG lineage differentiation [24, 26]. In mice expressing a dominant-negative Lef1 (ΔNLef1) under control of the keratin 14 promoter, SGs develop at the expenses of HFs [24, 26]. Interestingly, blocking functional TCF/Lef1 signaling in bulge stem cells results in the formation of additional cell fate determination centers for SG specification and an increase in sebocyte differentiation [16]. In contrast, expression of the Wnt/β-catenin signaling mediator TCF3 in mouse epidermis induces the suppression of sebocyte transcriptional regulators and the lack of SG formation in vivo [27]. Furthermore, overexpression of Smad7 in basal keratinocytes shifts the balance of lineage choice towards SG differentiation thereby leading to SG hyperplasia. In this mouse model, Smad7 reduces canonical Wnt activity by directly binding to β-catenin and recruiting Smurf2, a ubiquitin E3 ligase that leads to degradation of cytoplasmic β-catenin [28]. Taken together, these data demonstrate that sebocyte cell specification and proper formation of SG require the suppression of canonical Wnt/β-catenin signaling in stem and progenitor cells of mammalian skin (Figure 3).
Figure 3.
Schematic overview of stem cell contribution to different epidermal lineages and regulatory pathways controlling the establishment and subsequent differentiation of sebaceous gland stem cells. The process of sebocyte maturation is characterized by defined steps of cellular differentiation each regulated by signaling activities of specific molecules.
Another well-characterized signaling pathway involved in sebocyte proliferation is hedgehog signaling. Hedgehog ligands initiate signaling by binding and inactivating the transmembrane receptor patched-1, which relieves its inhibition of the co-receptor smoothened. This de-repression of smoothened induces a cascade of downstream reactions in primary cilia that culminate in the activation of the Gli transcription factors and result in transcription of hedgehog target genes [29]. Expression of an activated smoothened receptor in mouse epidermis results in formation of ectopic SGs. Conversely, inhibition of hedgehog signaling by overexpression of a dominant-negative Gli mutant can suppress sebocyte differentiation [30]. Interestingly, overexpression of a dominant-negative Lef1 in a human sebocyte cell line induces hedgehog expression and this is accompanied with an increase in proliferation and differentiation in vitro [31]. Thus, these data suggest an intimate crosstalk of TCF/Lef1 and hedgehog signaling in the process of SG cell proliferation and maturation.
In addition to hedgehog and canonical Wnt activity, stimulation of Ras signaling also can promote SG cell fate. Particularly, expression of an oncogenic constitutively active mutant of KRas (KRas G12D) at a physiological level in bulge stem cells generated hyperproliferative and enlarged SGs indicating that small GTPases could play an important function in SG homeostasis [32].
Transcriptional regulation
One of the most well established regulators of SG development are the androgens, nuclear receptors that respond to hormones such as testosterone and estrogen. Hormonal regulation of SG function is evident based on the increased production of sebum in adolescents during puberty. Additionally, castrated men, who are unable to generate testosterone, exhibit diminished sebum production [33]. Androgens act directly on androgen receptors expressed by sebocytes [34, 35]. Testosterone can induce sebocyte proliferation and sebum production, which can be blocked by estrogens and antiandrogenic steroids [36–38].
In addition to the direct action of androgens on sebocyte proliferation and function, sebocytes can also metabolize and synthesize androgen hormones [39]. Sebocytes express the enzymes involved in androgen metabolism and can convert testosterone to the more effective 5α-dihydrotestosterone (5α-DHT) [39]. Moreover, sebocytes can inactivate testosterone [40], allowing sebaceous glands to alter androgen levels within the skin. Whether these actions act in a cell nonautonomous manner on sebocytes themselves is not known.
Two transcription factors, Blimp1 and c-Myc, that regulate proliferation and lineage commitment in other tissues can influence sebaceous gland formation (Figure 3). Blimp1 (PRDM-1) is a transcriptional repressor that was first identified as a master regulator of differentiation of plasma cells from B cells [41]. Blimp1 is expressed in a small population of cells that reside adjacent to the sebaceous gland in the upper hair follicle and through genetic lineage tracing experiments were shown to produce the proliferative and differentiated cells of the SG, demonstrating that these cells are progenitors for the gland [17]. The conditional loss of Blimp1 in the epidermis results in enlarged SGs due to increased sebocyte proliferation, leading to an increase in the number of cells in the gland. As a result of this defect, the Blimp1 conditional null (cKO) mice produce an excess of sebum and have oily skin, evident externally around 1 month of age.
Blimp1 cKO mice also display elevated c-Myc expression, suggesting that Blimp1 represses c-Myc to control SG homeostasis. The phenotype of the epithelial specific Blimp1 cKO mice is similar to transgenic mice that overexpress c-Myc in the epithelium. Activation of c-Myc in the skin epithelium results in an enlargement of the SG and remarkably, leads to the ectopic formation of sebocytes in the HF and the IFE [42–44]. These data indicate that Blimp1 and c-Myc can act as a gatekeeper for the progenitor cell population for the SG.
Given the ability of sebocytes to accumulate lipids, several developmental pathways control the formation of both lipid-laden adipocytes and sebum-rich sebocytes. One of these similarities is the importance of peroxisome proliferator-activated receptor γ (PPARγ) in SG development. PPARs act by forming heterodimers with retinoid × receptors and serve as transcriptional regulators of a variety of genes including those involved in lipid metabolism in adipose tissue, liver and skin [45]. PPARγ is expressed predominantly in adipose tissue and is key to promoting adipocyte differentiation [46]. PPARs are also expressed in an immortalized human sebocyte cell line (SZ95) and treatment of these cells with linoleic acid, which can activate PPARα and PPARγ, also increases the intracellular content of lipids within these cells [47].
In the SG, PPARγ is expressed in differentiating sebocytes [48]. Although PPARγ null mice are not viable, PPARγ null ES cells have been found to contribute poorly to the SGs of chimeric mice, lending some supporting functional evidence to its role in vivo [46]. Additional evidence comes from the use of PPAR ligands in vitro and in vivo. Co-administration of androgens and ligands for PPARs can augment sebum accumulation in rat preputial glands, which also contain sebocytes [48]. Furthermore, treatment with PPARγ ligands can induce sebocyte differentiation in vitro and increase sebum production in humans [49]. Surprisingly, no defects in SGs are reported in mice lacking PPARγ in the skin epithelium [50]. Deletion of PPARγ in the bulge stem cells with the keratin 15 promoter results in scarring alopecia after 3 months (discussed later), suggesting that sebocytes derived from bulge cells require PPARγ for proper homeostasis of the gland [51].
Lipid metabolism
The regulation of lipid metabolism is another shared feature of sebocytes and adipocytes as they mature into lipid-laden cells. Several enzymes that regulate lipid metabolism are essential for important aspects of SG physiology. Mice lacking fatty acid transport protein (FATP)-4 display reduced pilosebaceous formation including SGs [52], highlighting the importance of lipid transport for pilosebaceous formation. Once fatty acids are internalized, several metabolic steps are involved in the conversion of these lipids to sebum. SCD1 is a key enzyme involved in mammalian lipid metabolism that converts saturated fatty acids into mono-unsaturated fatty acids, allowing them to be used for further metabolic steps in sebum production. Targeted deletion of SCD1 (stearoyl coenzymeA desaturase 1) or mutations within the SCD1 gene in the asebia mouse lead to atrophy of sebocyte containing Meibomian glands of the eyelid and skin SGs [20, 53–55]. Additionally, diaglyceride acyltransferase (DGAT) enzymes are also essential for SG homeostasis. DGATs convert triglycerides into products for downstream metabolic steps and mice lacking DGAT1 display atrophic SGs [56], indicating the importance of triglyceride synthesis in sebocyte homeostasis. Finally, modulation of lipid homeostasis in transgenic mice overexpressing the lipid binding protein, apolipoprotein C-I, in the skin results in atrophic SGs lacking sebum [57]. Together, these mouse models pinpoint the importance of lipid metabolism in the generation of sebum and the homeostasis of the SG. Since most of these mouse models also display defects in the HF and IFE as well, a link between lipid metabolism and other epithelial lineages could occur through SG function or an independent role of lipids in these lineage cells.
5. Pathophysiological conditions of the sebaceous gland
Acne
Acne vulgaris is a common condition affecting 80% of people 11–30 years of age [58]. The development of acne pathogenesis is multifactorial, including hormonal, microbiological, and immunological mechanisms that ultimately result from aberrant SG biology [40]. Clinical features of acne are seborrhea (excessive grease), blocked pores (comedones), inflammatory lesions and scarring. The exact sequence of events that results in acne is not clear but involve androgen induced increases in sebum production, keratinization, colonization by Propionibacterium acnes and inflammation. Follicles containing comedones due to increased keratinization provides an anaerobic and lipid-rich environment for the growth of P. acnes. This infection leads to inflammation and can lead to extensive scarring in infected individuals.
Currently, the most effective treatments for acne are isotretinoin (13-cis retinoic acid (13-cis RA)) or hormones. The mechanism of action for these treatments, while complex, leads to reduced sebum production. While the exact mechanism by which isotretinoin blocks acne formation is not known, it normalizes keratinization, reduces SG size and sebum production, and decreases inflammation. While isotretinoin is effective in reducing sebum levels [59], given that 13-cis RA is associated with severe side effects including teratogenicity, novel therapeutics are needed for acne treatment. Hormonal therapies are designed to inhibit the effect of androgens on SG enlargement. Estrogens, androgen receptor antagonists, or drugs that block androgen production from the ovary or adrenal gland, such as oral contraceptives are commonly used for acne treatment and can reduce sebum production [59].
Androgenic and Scarring Alopecia
Scarring alopecia (SA) describes a number of disorders that lead to the destruction of the pilosebaceous unit [60]. The primary causative event resulting in the loss of hair in these disorders is not known but could be inflammatory in nature, leading to a fibrotic tissue replacing the HFs. SG dysfunction has been suggested to lead to SA and their loss and inflammation may precede HF destruction [60, 61]. Several mouse models support the involvement of SGs in SA pathogenesis. Asebia mice, which display SA, harbor a mutation in the stearoyl codestaturase-1 (SCD-1) gene which leads to hypoplastic SGs and minimal to no sebum production [62]. Another spontaneous mouse model, defolliculated, also displays alopecia and lacks sebum production [63]. Furthermore, conditional deletion of PPARγ in hair follicle stem cells using an inducible K15-CrePR mouse model results in a scarring alopecia type phenotype [51]. Since these mice do not display all of the clinical features of human SA including epidermal atrophy, a precise role for SG dysfunction in SA has not been identified.
Another form of alopecia, androgenic alopecia (AGA), is characterized by hypertrophy of SGs [64]. Clinical phenotypes of AGA, can be recapitulated by transgenic mice overexpressing prostaglandin G/H synthase (Ptgs2) in the skin via the keratin 14 (K14) promoter, suggesting that prostaglandin levels may influence the pathogenesis of AGA. Consistent with this model, prostaglandin D2 synthase (PTGDS) and its product PGD2 are elevated in bald scalp of men with AGA [65].
Sebaceous tumors
Several types of tumors can arise from sebocytes in the skin. Sebaceous adenoma and sebaceoma represent benign epithelial tumors with sebaceous cell differentiation [66]. Sebaceous carcinomas are rare and constitute malignant and aggressive tumors that undergo SG differentiation [67]. Sebaceous neoplasms are often associated with the epidermal nevus syndrome and Muir-Torre syndrome (MTS) [68]. MTS is an autosomal dominant tumor syndrome and affected patients with defects in DNA mismatch repair genes including MLH-1 and MSH-2 develop internal malignancy, most commonly colorectal carcinoma [69].
A high proportion of human sebaceous adenomas have amino acid substitutions in the N-terminus of Lef1, which impair the binding of Lef1 to β-catenin resulting in the inhibition of β-catenin-dependent transcription of Wnt target genes [70]. Accordingly, transgenic mice expressing the N-terminal mutant Lef1 in basal keratinocytes of the skin (K14ΔNLef1 mice) develop sebaceous adenomas spontaneously [24, 26]. Upon treatment with a carcinogen, K14ΔNLef1 transgenics form sebaceous adenoma and papilloma with sebocyte differentiation in high frequency [71]. Experiments investigating the underlying mechanisms for sebaceous tumor development revealed that K14ΔNLef1 epidermis failed to upregulate p53 and p21 proteins during tumorigenesis and this correlated with impaired induction of the tumor suppressor ARF [71]. These data demonstrate that Lef1 mutations play a dual role in skin cancer, promoting tumor growth by preventing p53 activity and specifying the sebaceous tumor type by inhibiting Wnt/β-catenin signaling. Conversely, activation of canonical Wnt signaling drives HF morphogenesis and the formation of skin tumors with hair differentiation [72–74]. Together, these data illustrate that important signaling mechanisms directing the cell fate commitment of tissue stem cells are reapplied in the process of tumor type specification.
Importantly, a similar regulation of stem cells and tumor cells has also been shown for c-Myc. Transgenic mice overexpressing c-Myc under control of the keratin 14 promoter exhibit an increase in size and number of the SG and are predisposed to develop sebaceous adenomas in response to carcinogens [75, 76].
Another example demonstrating that genetic alterations can alter the fate of tumor cells came from experiments manipulating signaling through AP-1. In particular, blocking AP-1 alters the tumor phenotype induced in response to chemical carcinogenesis from squamous skin lesions towards sebaceous adenomas. In contrast, induction of AP-1 in sebaceous skin tumors resulted in formation of squamous tumors [77]. Interestingly, the changes of differentiation induced by AP-1 function are dependent on alterations of Wnt/β-catenin signaling. Thus, block of AP-1 signaling leads to inhibition of β-catenin/TCF/Lef1 function and subsequently promotes sebocyte differentiation within the tumors [77].
Aging
Throughout adult life, the number of SGs remains approximately the same. However, the size of the glands tends to increase with age [78]. Furthermore, skin aging is associated with hormonal changes of the SG and altered lipid composition of the sebum is found [78, 79]. Compared to young adults, the turnover of the SG is slowed down in aged skin. This suggests age-related alterations in cellular and molecular mechanism of SG regeneration. It has been demonstrated that skin stem cells are maintained throughout adult life [80]. However, signaling activities of the surrounding tissue is altered in aged skin, including decreased expression of Igfbp3 (insulin-like growth factor binding protein 3) [81]. Impaired Igf/Igfbp signaling has tremendous implications for the differentiation of pilosebaceous cells [82, 83]. These data indicate that stromaderived signals affected during aging could control important aspects of SG cellular turn over. In addition to SG enlargement, HF shortening is indicative for ageing of the pilosebaceous unit. Therefore, one can speculate that age-associated changes in signaling could lead to differences in activation of bulge stem cells and local progenitor cells of the SG. For instance, stem cells could be mobilized for SG regeneration whereas HF renewal is decreased in aged skin.
6. Summary and perspective
Recent discoveries by many laboratories are unraveling numerous facets of the molecular, cellular and metabolic functions of the SG. Despite the tremendous progress that has been made in our understanding how SGs develop and regenerate, many important questions are still remaining. Until now, the molecular cues governing the establishment of the distinct stem cell compartments during morphogenesis of the epidermal appendages are not known. In particular, what are the signals that generate Lrig1+ve stem cells and do these cells contribute solely to the sebocyte lineage during development? Although many different molecules contributing to the sebocyte differentiation program have been identified, it is less clear, how these factors, including signaling pathways, hormones, nuclear receptors, transcription factors and components of the lipid metabolism are synchronized and work together. In the future, it will be important to dissect the instructive signals of the surrounding environment that clearly plays an important role in controlling normal SG function. Finally, which cell gives rise to sebaceous skin tumors and what are the underlying detailed mechanisms of propagation and differentiation of tumor cells? Undoubtedly, future experiments will lead to a better understanding of the cellular and molecular cues that direct normal SG function and be beneficial for the development of improved treatments for SG disorders.
Table 1.
Genetic mouse models with SG phenotypes
| Genetic deletion mouse model | Gene | SG phenotype | Reference |
| Asebia | SCD-1 | SG atropy | [53] |
| Bareskin, Rex (denuded), Reduced Coat 2 | Gsdm3 | SG loss | [84] |
| Blimp1fl/fl; K14-Cre | Prdm1 | SG hyperplasia | [17] |
| C/EBPα/βfl/fl;K14CreER | C/EBPα/β | Block in SG differentiation | [80] |
| Depilated | Zdhhc21 | SG hyperplasia | [85] |
| DGAT1−/− | Dgat1 | SG atrophy | [55] |
| FATP4−/− | FATP4 | Loss of SG | [51] |
| FGFR2bfl/fl; K5-Cre | Fgfr2b | SG atrophy | [86] |
| K10−/− | K10 | SG hyperplasia | [87] |
| Lmna−/− | Lmna | Hypoplastic SG | [88] |
| Rac1fl/fl; K14-Cre | Rac1 | Loss of SGs | [89] |
| Rhino | Hr | SG atropy | [90] |
| Rough coat | Mpzl3 | SG hyperplasia | [91] |
| Sox9fl/fl;K14-Cre | Sox9 | No SG development | [14] |
| Traf6−/− | Traf6 | Impaired SG formation | [92] |
| Transgenic mouse model | Gene | SG phenotype | Reference |
| CMV/βactin-Epgn | EPGN | SG hyperplasia | [93] |
| K14-ΔNLef1/K15-ΔNLef1 | Dominant negative Lef1 | SG tumor formation | [24] |
| K5-Cox2 | Cox2 | SG hyperplasia | [94] |
| K5-Gli2ΔC4 | Dominant negative Gli2 | Lack of SG development | [30] |
| K5-Smad7 | Smad7 | Precocious SG development | [28] |
| K6-Odc1 | Odc1 | SG hyperplasia | [95] |
| MMTV-TGFA | TGFA | SG hyperplasia | [96] |
| NSE-noggin | Noggin | Ectopic sebocyte differentiation | [97] |
| K14c-MycER K14.Myc2 | c-Myc | Ectopic sebocytes; SG hyperplasia | [41–43] |
Highlights.
Sebaceous gland development
Sebaceous gland homeostasis
Clinical manifestations of sebaceous gland biology
Acknowledgments
C.N. is grateful for financial support by the German Research Foundation (SFB 572 and SFB 829) and the German Cancer Foundation. V.H. is a Pew Scholar Biomedical Research and is funded by the NIH (R01AR060295) and CT Innovations (12-SCB-YALE-01). We also thank Elaine Fuchs and Amalia Pasolli (Rockefeller University) for providing EM images.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–73. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thody AJ, Shuster S. Control and function of sebaceous glands. Physiol Rev. 1989;69:383–416. doi: 10.1152/physrev.1989.69.2.383. [DOI] [PubMed] [Google Scholar]
- 3.Smith KR, Thiboutot DM. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J Lipid Res. 2008;49:271–81. doi: 10.1194/jlr.R700015-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Zouboulis CC, Xia LQ, Detmar M, Bogdanoff B, Giannakopoulos G, Gollnick H, et al. Culture of human sebocytes and markers of sebocytic differentiation in vitro. Skin Pharmacol. 1991;4:74–83. doi: 10.1159/000210927. [DOI] [PubMed] [Google Scholar]
- 5.Latham JA, Redfern CP, Thody AJ, De Kretser TA. Immunohistochemical markers of human sebaceous gland differentiation. J Histochem Cytochem. 1989;37:729–34. doi: 10.1177/37.5.2467930. [DOI] [PubMed] [Google Scholar]
- 6.Kusakabe T, Maeda M, Hoshi N, Sugino T, Watanabe K, Fukuda T, et al. Fatty acid synthase is expressed mainly in adult hormone-sensitive cells or cells with high lipid metabolism and in proliferating fetal cells. J Histochem Cytochem. 2000;48:613–22. doi: 10.1177/002215540004800505. [DOI] [PubMed] [Google Scholar]
- 7.Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–25. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–61. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- 9.Paus R, Müller-Röver S, Van Der Veen C, Maurer M, Eichmüller S, Ling G, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–32. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- 10.Müller-Röver S, Handjiski B, van der Veen C, Eichmüller S, Foitzik K, McKay I, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 11.Frances D, Niemann C. Stem cell dynamics in sebaceous gland morphogenesis in mouse skin. Dev Biol. 2012;363:138–46. doi: 10.1016/j.ydbio.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 12.Serri F, Montagna W, Huber WM. Studies of skin of fetus and the child. The distribution of alkaline phosphatase in the skin of the fetus. Arch Dermatol. 1963;87:234–45. doi: 10.1001/archderm.1963.01590140096016. [DOI] [PubMed] [Google Scholar]
- 13.Strauss JS, Pochi PE. The human sebaceous gland: its regulation by steroidal hormones and its use as an end organ for assaying androgenicity in vivo. Recent Prog Horm Res. 1963;19:385–444. [PubMed] [Google Scholar]
- 14.Nowak J, Polak L, Pasolli H, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4:427–39. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersson M, Brylka H, Kraus A, John S, Rappl G, Schettina P, et al. TCF/Lef1 activity controls establishment of diverse stem and progenitor cell compartments in mouse epidermis. EMBO J. 2011;30:3004–18. doi: 10.1038/emboj.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horsley V, O'Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghazizadeh S, Taichman LB. Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. EMBO J. 2001;20:1215–22. doi: 10.1093/emboj/20.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–9. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 20.Binczek E, Jenke B, Holz B, Günter RH, Thevis M, Stoffel W. Obesity resistance of the stearoyl-CoA desaturase-deficient (scd1−/−) mouse results from disruption of the epidermal lipid barrier and adaptive thermoregulation. Biol Chem. 2007;388:405–18. doi: 10.1515/BC.2007.046. [DOI] [PubMed] [Google Scholar]
- 21.Selleri S, Seltmann H, Gariboldi S, Shirai YF, Balsari A, Zouboulis CC, et al. Doxorubicin-induced alopecia is associated with sebaceous gland degeneration. J Invest Dermatol. 2006;126:711–20. doi: 10.1038/sj.jid.5700175. [DOI] [PubMed] [Google Scholar]
- 22.Janich P, Pascual G, Merlos-Suárez A, Batlle E, Ripperger J, Albrecht U, et al. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480:209–14. doi: 10.1038/nature10649. [DOI] [PubMed] [Google Scholar]
- 23.Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niemann C, Owens DM, Hülsken J, Birchmeier W, Watt FM. Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development. 2002;129:95–109. doi: 10.1242/dev.129.1.95. [DOI] [PubMed] [Google Scholar]
- 25.Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174:715–21. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen H, Rendl M, Fuchs E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 2006;127:171–83. doi: 10.1016/j.cell.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 28.Han G, Li AG, Liang YY, Owens P, He W, Lu S, et al. Smad7-induced beta-catenin degradation alters epidermal appendage development. Dev Cell. 2006;11:301–12. doi: 10.1016/j.devcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303–12. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Allen M, Grachtchouk M, Sheng H, Grachtchouk V, Wang A, Wei L, et al. Hedgehog signaling regulates sebaceous gland development. Am J Pathol. 2003;163:2173–8. doi: 10.1016/S0002-9440(10)63574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niemann C, Unden AB, Lyle S, Zouboulis CC, Toftgård R, Watt FM. Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11873–80. doi: 10.1073/pnas.1834202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lapouge G, Youssef KK, Vokaer B, Achouri Y, Michaux C, Sotiropoulou PA, et al. Identifying the cellular origin of squamous skin tumors. Proc Natl Acad Sci U S A. 2011;108:7431–6. doi: 10.1073/pnas.1012720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pochi PE, Strauss JS, Mescon H. Sebum secretion and urinary fractional 17-ketosteroid and total 17-hydroxycorticoid excretion in male castrates. J Invest Dermatol. 1962;39:475–83. doi: 10.1038/jid.1962.146. [DOI] [PubMed] [Google Scholar]
- 34.Choudhry R, Hodgins MB, Van der Kwast TH, Brinkmann AO, Boersma WJ. Localization of androgen receptors in human skin by immunohistochemistry: implications for the hormonal regulation of hair growth, sebaceous glands and sweat glands. J Endocrinol. 1992;133:467–75. doi: 10.1677/joe.0.1330467. [DOI] [PubMed] [Google Scholar]
- 35.Zouboulis CC, Akamatsu H, Stephanek K, Orfanos CE. Androgens affect the activity of human sebocytes in culture in a manner dependent on the localization of the sebaceous glands and their effect is antagonized by spironolactone. Skin Pharmacol. 1994;7:33–40. doi: 10.1159/000211271. [DOI] [PubMed] [Google Scholar]
- 36.Ebling FJ. The action of an anti-androgenic steroid, 17-alpha-methyl-B-nortestosterone, on sebum secretion in rats treated with testosterone. J Endocrinol. 1967;38:181–5. doi: 10.1677/joe.0.0380181. [DOI] [PubMed] [Google Scholar]
- 37.Ebling FJ. Hormonal factors influencing the response of the sebaceous gland to androgens. Proc R Soc Med. 1969;62:890–1. doi: 10.1177/003591576906200910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fritsch M, Orfanos CE, Zouboulis CC. Sebocytes are the key regulators of androgen homeostasis in human skin. J Invest Dermatol. 2001;116:793–800. doi: 10.1046/j.1523-1747.2001.01312.x. [DOI] [PubMed] [Google Scholar]
- 39.Makrantonaki E, Zouboulis CC. Testosterone metabolism to 5alpha-dihydrotestosterone and synthesis of sebaceous lipids is regulated by the peroxisome proliferator-activated receptor ligand linoleic acid in human sebocytes. Br J Dermatol. 2007;156:428–32. doi: 10.1111/j.1365-2133.2006.07671.x. [DOI] [PubMed] [Google Scholar]
- 40.Seiffert K, Seltmann H, Fritsch M, Zouboulis CC. Inhibition of 5alpha-reductase activity in SZ95 sebocytes and HaCaT keratinocytes in vitro. Horm Metab Res. 2007;39:141–8. doi: 10.1055/s-2007-961814. [DOI] [PubMed] [Google Scholar]
- 41.Calame K. Blimp-1's maiden flight. J Immunol. 2010;185:3–4. doi: 10.4049/jimmunol.1090044. [DOI] [PubMed] [Google Scholar]
- 42.Bull JJ, Pelengaris S, Hendrix S, Chronnell CM, Khan M, Philpott MP. Ectopic expression of c-Myc in the skin affects the hair growth cycle and causes an enlargement of the sebaceous gland. Br J Dermatol. 2005;152:1125–33. doi: 10.1111/j.1365-2133.2005.06458.x. [DOI] [PubMed] [Google Scholar]
- 43.Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol. 2001;11:558–68. doi: 10.1016/s0960-9822(01)00154-3. [DOI] [PubMed] [Google Scholar]
- 44.Braun K, Niemann C, Jensen U, Sundberg J, Silva-Vargas V, Watt F. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130:5241–55. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 45.Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–71. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 46.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–7. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 47.Chen W, Yang CC, Sheu HM, Seltmann H, Zouboulis CC. Expression of peroxisome proliferator-activated receptor and CCAAT/enhancer binding protein transcription factors in cultured human sebocytes. J Invest Dermatol. 2003;121:441–7. doi: 10.1046/j.1523-1747.2003.12411.x. [DOI] [PubMed] [Google Scholar]
- 48.Rosenfield RL, Deplewski D, Kentsis A, Ciletti N. Mechanisms of androgen induction of sebocyte differentiation. Dermatology. 1998;196:43–6. doi: 10.1159/000017864. [DOI] [PubMed] [Google Scholar]
- 49.Trivedi NR, Cong Z, Nelson AM, Albert AJ, Rosamilia LL, Sivarajah S, et al. Peroxisome proliferator-activated receptors increase human sebum production. J Invest Dermatol. 2006;126:2002–9. doi: 10.1038/sj.jid.5700336. [DOI] [PubMed] [Google Scholar]
- 50.Mao-Qiang M, Fowler AJ, Schmuth M, Lau P, Chang S, Brown BE, et al. Peroxisome-proliferator-activated receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. J Invest Dermatol. 2004;123:305–12. doi: 10.1111/j.0022-202X.2004.23235.x. [DOI] [PubMed] [Google Scholar]
- 51.Karnik P, Tekeste Z, McCormick TS, Gilliam AC, Price VH, Cooper KD, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243–57. doi: 10.1038/jid.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herrmann T, van der Hoeven F, Grone HJ, Stewart AF, Langbein L, Kaiser I, et al. Mice with targeted disruption of the fatty acid transport protein 4 (Fatp 4, Slc27a4) gene show features of lethal restrictive dermopathy. J Cell Biol. 2003;161:1105–15. doi: 10.1083/jcb.200207080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng Y, Eilertsen KJ, Ge L, Zhang L, Sundberg JP, Prouty SM, et al. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268–70. doi: 10.1038/15446. [DOI] [PubMed] [Google Scholar]
- 54.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem. 2000;275:30132–8. doi: 10.1074/jbc.M005488200. [DOI] [PubMed] [Google Scholar]
- 55.Sundberg JP, Boggess D, Sundberg BA, Eilertsen K, Parimoo S, Filippi M, et al. Asebia-2J (Scd1(ab2J)): a new allele and a model for scarring alopecia. Am J Pathol. 2000;156:2067–75. doi: 10.1016/S0002-9440(10)65078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen H, Smith S, Tow B, Elias P, Farese RJ. Leptin modulates the effects of acyl CoA:diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J Clin Invest. 2002;109:175–81. doi: 10.1172/JCI13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jong MC, Gijbels MJ, Dahlmans VE, Gorp PJ, Koopman SJ, Ponec M, et al. Hyperlipidemia and cutaneous abnormalities in transgenic mice overexpressing human apolipoprotein C1. J Clin Invest. 1998;101:145–52. doi: 10.1172/JCI791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halvorsen JA, Vleugels RA, Bjertness E, Lien L. A population-based study of acne and body mass index in adolescents. Arch Dermatol. 2012;148:131–2. doi: 10.1001/archderm.148.1.131. [DOI] [PubMed] [Google Scholar]
- 59.Gollnick H, Cunliffe W, Berson D, Dreno B, Finlay A, Leyden JJ, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49:S1–37. doi: 10.1067/mjd.2003.618. [DOI] [PubMed] [Google Scholar]
- 60.Al-Zaid T, Vanderweil S, Zembowicz A, Lyle S. Sebaceous gland loss and inflammation in scarring alopecia: a potential role in pathogenesis. J Am Acad Dermatol. 2011;65:597–603. doi: 10.1016/j.jaad.2010.09.774. [DOI] [PubMed] [Google Scholar]
- 61.Stenn KS, Sundberg JP, Sperling LC. Hair follicle biology, the sebaceous gland, and scarring alopecias. Arch Dermatol. 1999;135:973–4. doi: 10.1001/archderm.135.8.973. [DOI] [PubMed] [Google Scholar]
- 62.Stenn KS. Insights from the asebia mouse: a molecular sebaceous gland defect leading to cicatricial alopecia. J Cutan Pathol. 2001;28:445–7. doi: 10.1034/j.1600-0560.2001.028009445.x. [DOI] [PubMed] [Google Scholar]
- 63.Porter RM, Jahoda CA, Lunny DP, Henderson G, Ross J, McLean WH, et al. Defolliculated (dfl): a dominant mouse mutation leading to poor sebaceous gland differentiation and total elimination of pelage follicles. J Invest Dermatol. 2002;119:32–7. doi: 10.1046/j.1523-1747.2002.01806.x. [DOI] [PubMed] [Google Scholar]
- 64.Lattanand A, Johnson WC. Male pattern alopecia a histopathologic and histochemical study. J Cutan Pathol. 1975;2:58–70. doi: 10.1111/j.1600-0560.1975.tb00209.x. [DOI] [PubMed] [Google Scholar]
- 65.Garza LA, Liu Y, Yang Z, Alagesan B, Lawson JA, Norberg SM, et al. Prostaglandin d2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci Transl Med. 2012;4:126ra34. doi: 10.1126/scitranslmed.3003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Troy JL, Ackerman AB. Sebaceoma. A distinctive benign neoplasm of adnexal epithelium differentiating toward sebaceous cells. Am J Dermatopathol. 1984;6:7–13. [PubMed] [Google Scholar]
- 67.Nelson BR, Hamlet KR, Gillard M, Railan D, Johnson TM. Sebaceous carcinoma. J Am Acad Dermatol. 1995;33:1–15. doi: 10.1016/0190-9622(95)90001-2. quiz 6–8. [DOI] [PubMed] [Google Scholar]
- 68.Eisen DB, Michael DJ. Sebaceous lesions and their associated syndromes: part II. J Am Acad Dermatol. 2009;61:563–78. doi: 10.1016/j.jaad.2009.04.059. quiz 79–80. [DOI] [PubMed] [Google Scholar]
- 69.Mathiak M, Rütten A, Mangold E, Fischer HP, Ruzicka T, Friedl W, et al. Loss of DNA mismatch repair proteins in skin tumors from patients with Muir-Torre syndrome and MSH2 or MLH1 germline mutations: establishment of immunohistochemical analysis as a screening test. Am J Surg Pathol. 2002;26:338–43. doi: 10.1097/00000478-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 70.Takeda H, Lyle S, Lazar AJ, Zouboulis CC, Smyth I, Watt FM. Human sebaceous tumors harbor inactivating mutations in LEF1. Nat Med. 2006;12:395–7. doi: 10.1038/nm1386. [DOI] [PubMed] [Google Scholar]
- 71.Niemann C, Owens DM, Schettina P, Watt FM. Dual role of inactivating Lef1 mutations in epidermis: tumor promotion and specification of tumor type. Cancer Res. 2007;67:2916–21. doi: 10.1158/0008-5472.CAN-06-3427. [DOI] [PubMed] [Google Scholar]
- 72.Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–14. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 73.Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 1999;21:410–3. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- 74.Lazar AJ, Calonje E, Grayson W, Dei Tos AP, Mihm MC, Redston M, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J Cutan Pathol. 2005;32:148–57. doi: 10.1111/j.0303-6987.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 75.Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet. 2001;28:165–8. doi: 10.1038/88889. [DOI] [PubMed] [Google Scholar]
- 76.Honeycutt KA, Waikel RL, Koster MI, Wang XJ, Roop DR. The effect of c-myc on stem cell fate influences skin tumor phenotype. Mol Carcinog. 2010;49:315–9. doi: 10.1002/mc.20617. [DOI] [PubMed] [Google Scholar]
- 77.Gerdes MJ, Myakishev M, Frost NA, Rishi V, Moitra J, Acharya A, et al. Activator protein-1 activity regulates epithelial tumor cell identity. Cancer Res. 2006;66:7578–88. doi: 10.1158/0008-5472.CAN-06-1247. [DOI] [PubMed] [Google Scholar]
- 78.Zouboulis CC, Boschnakow A. Chronological ageing and photoageing of the human sebaceous gland. Clin Exp Dermatol. 2001;26:600–7. doi: 10.1046/j.1365-2230.2001.00894.x. [DOI] [PubMed] [Google Scholar]
- 79.Makrantonaki E, Adjaye J, Herwig R, Brink TC, Groth D, Hultschig C, et al. Age-specific hormonal decline is accompanied by transcriptional changes in human sebocytes in vitro. Aging Cell. 2006;5:331–44. doi: 10.1111/j.1474-9726.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- 80.Stern MM, Bickenbach JR. Epidermal stem cells are resistant to cellular aging. Aging Cell. 2007;6:439–52. doi: 10.1111/j.1474-9726.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- 81.Giangreco A, Qin M, Pintar JE, Watt FM. Epidermal stem cells are retained in vivo throughout skin aging. Aging Cell. 2008;7:250–9. doi: 10.1111/j.1474-9726.2008.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deplewski D, Rosenfield RL. Growth hormone and insulin-like growth factors have different effects on sebaceous cell growth and differentiation. Endocrinology. 1999;140:4089–94. doi: 10.1210/endo.140.9.6957. [DOI] [PubMed] [Google Scholar]
- 83.Weger N, Schlake T. Igf-I signalling controls the hair growth cycle and the differentiation of hair shafts. J Invest Dermatol. 2005;125:873–82. doi: 10.1111/j.0022-202X.2005.23946.x. [DOI] [PubMed] [Google Scholar]
- 84.Runkel F, Marquardt A, Stoeger C, Kochmann E, Simon D, Kohnke B, et al. The dominant alopecia phenotypes Bareskin, Rex-denuded, and Reduced Coat 2 are caused by mutations in gasdermin 3. Genomics. 2004;84:824–35. doi: 10.1016/j.ygeno.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 85.Mill P, Lee AW, Fukata Y, Tsutsumi R, Fukata M, Keighren M, et al. Palmitoylation regulates epidermal homeostasis and hair follicle differentiation. PLoS Genet. 2009;5:e1000748. doi: 10.1371/journal.pgen.1000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grose R, Fantl V, Werner S, Chioni AM, Jarosz M, Rudling R, et al. The role of fibroblast growth factor receptor 2b in skin homeostasis and cancer development. EMBO J. 2007;26:1268–78. doi: 10.1038/sj.emboj.7601583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reichelt J, Breiden B, Sandhoff K, Magin TM. Loss of keratin 10 is accompanied by increased sebocyte proliferation and differentiation. Eur J Cell Biol. 2004;83:747–59. doi: 10.1078/0171-9335-00429. [DOI] [PubMed] [Google Scholar]
- 88.Odgren PR, Pratt CH, Mackay CA, Mason-Savas A, Curtain M, Shopland L, et al. Disheveled hair and ear (Dhe), a spontaneous mouse Lmna mutation modeling human laminopathies. PLoS One. 2010;5:e9959. doi: 10.1371/journal.pone.0009959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benitah SA, Frye M, Glogauer M, Watt FM. Stem cell depletion through epidermal deletion of Rac1. Science. 2005;309:933–5. doi: 10.1126/science.1113579. [DOI] [PubMed] [Google Scholar]
- 90.Mann SJ. Hair loss and cyst formation in hairless and rhino mutant mice. Anat Rec. 1971;170:485–99. doi: 10.1002/ar.1091700409. [DOI] [PubMed] [Google Scholar]
- 91.Cao T, Racz P, Szauter KM, Groma G, Nakamatsu GY, Fogelgren B, et al. Mutation in Mpzl3, a novel [corrected] gene encoding a predicted [corrected] adhesion protein, in the rough coat (rc) mice with severe skin and hair abnormalities. J Invest Dermatol. 2007;127:1375–86. doi: 10.1038/sj.jid.5700706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Naito A, Yoshida H, Nishioka E, Satoh M, Azuma S, Yamamoto T, et al. TRAF6-deficient mice display hypohidrotic ectodermal dysplasia. Proc Natl Acad Sci U S A. 2002;99:8766–71. doi: 10.1073/pnas.132636999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dahlhoff M, Müller AK, Wolf E, Werner S, Schneider MR. Epigen transgenic mice develop enlarged sebaceous glands. J Invest Dermatol. 2010;130:623–6. doi: 10.1038/jid.2009.251. [DOI] [PubMed] [Google Scholar]
- 94.Neufang G, Furstenberger G, Heidt M, Marks F, Müller-Decker K. Abnormal differentiation of epidermis in transgenic mice constitutively expressing cyclooxygenase-2 in skin. Proc Natl Acad Sci U S A. 2001;98:7629–34. doi: 10.1073/pnas.121574098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Megosh L, Gilmour SK, Rosson D, Soler AP, Blessing M, Sawicki JA, et al. Increased frequency of spontaneous skin tumors in transgenic mice which overexpress ornithine decarboxylase. Cancer Res. 1995;55:4205–9. [PubMed] [Google Scholar]
- 96.Matsui Y, Halter SA, Holt JT, Hogan BL, Coffey RJ. Development of mammary hyperplasia and neoplasia in MMTV-TGF alpha transgenic mice. Cell. 1990;61:1147–55. doi: 10.1016/0092-8674(90)90077-r. [DOI] [PubMed] [Google Scholar]
- 97.Guha U, Mecklenburg L, Cowin P, Kan L, O'Guin WM, D'Vizio D, et al. Bone morphogenetic protein signaling regulates postnatal hair follicle differentiation and cycling. Am J Pathol. 2004;165:729–40. doi: 10.1016/S0002-9440(10)63336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]