Abstract
The skin is the first line of defense against dehydration and external environmental aggressions. It constantly renews itself throughout adult life mainly due to the activity of tissue-specific stem cells. In this review, we discuss fundamental characteristics of different stem cell populations within the skin and how they are able to contribute to normal skin homeostasis. We also examine the most recent results regarding the cell-intrinsic and -extrinsic components of the stem cell niche within the adult skin epithelium. Finally, we address the recent efforts to understand how abnormal regulation of stem cell activity contributes to the initiation and progression of skin-associated cancers.
1. INTRODUCTION
The skin serves as a highly dynamic and adaptable outer coating for the bodies of many animal species, protecting against the external environment and providing tactile function for touch sensation. These roles are engendered by multiple types of differentiated cells within the interfollicular epidermis (IFE) and by the formation of epidermal appendages such as hair follicles (HFs), sebaceous glands (SGs), and sweat glands. In order for organisms to navigate a continuously changing external environment, specialized cell types in the skin continually regenerate through the action of several distinct epithelial stem cell (SC) populations that self-renew and generate cells with unipotent and multipotent differentiation potential (Lee & Tumbar, 2012; Sennett & Rendl, 2012).
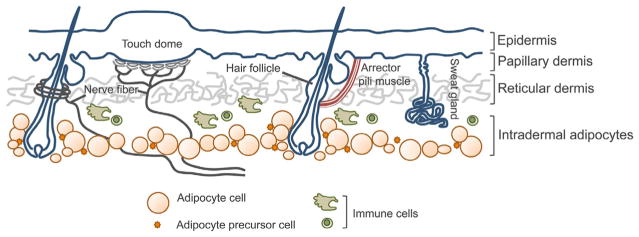
The IFE and its appendages interact with the dermis, which is rich with connective tissue and a multitude of cells that confer structure and function to the epithelial cells (Fig. 4.1). Beneath the basement membrane, three main cell layers exist to support the epithelium. The uppermost papillary dermis contains fine matrix fibers, while a second layer of the reticular dermis is composed of large fibers of matrix molecules (Dick, 1947). A thick layer of dermal adipocytes resides below the reticular dermis (Chase, Montagna, & Malone, 1953). These layers are permeated with additional cell types including inflammatory cells, neurons, blood vessels, and muscle cells. The function of the dermal cell types in controlling epithelial SCs in the skin is just starting to emerge.
Figure 4.1.
Schematic cross-section representation of mammalian skin. The skin is composed of a multitude of cell types and skin appendages that need to interact efficiently and accurately to ensure normal tissue homeostasis.
This review will describe the organization and cellular hierarchy of epithelial SCs in the skin. We will highlight the cellular and molecular mechanisms that regulate epithelial SC populations within the outermost IFE and its appendages, HFs, SGs, and sweat glands with an emphasis on recent work in the area. Finally, we will also highlight recent work that sheds light into mechanisms of SC deregulation and their contribution to epidermal cancer formation and progression.
2. STEM CELLS IN THE INTERFOLLICULAR EPIDERMIS
The outermost layer of mammalian skin is comprised of a multilayered or stratified epidermis of the IFE that is anchored to the underlying papillary dermis via integrin-mediated adhesion to a basement membrane (reviewed in Blanpain & Fuchs, 2006). The epidermal cells that adhere to the basement membrane are proliferative keratinocytes of the basal layer. Epidermal keratinocytes are formed during embryonic development from the surface ectoderm and generate differentiated suprabasal cells through asymmetric cell divisions (Lechler & Fuchs, 2005). Cells in the outermost epidermal layer (stratum corneum) tightly adhere to one other and form a protein–lipid matrix that ultimately creates the skin’s essential barrier (reviewed in Sandilands, Sutherland, Irvine, & McLean, 2009). The cells of the stratum corneum are constantly shed and thus, proliferative basal cells fuel the continual reformation of these dedicated cells of the IFE.
Classic experiments analyzing IFE homeostasis via morphology and proliferation proposed the existence of an epidermal proliferative unit (EPU) in which a central slow-cycling basal cell generates a defined number of rapidly dividing progenitor cells that differentiate into a restricted number of “units” (Loeffler, Potten, & Wichmann, 1987; Mackenzie, 1969, 1970; Potten, 1981; Potten, Wichmann, Loeffler, Dobek, & Major, 1982). More recently, extensive and quantitative analyses of basal cell progeny using genetic lineage tracing was performed in several mouse models (Clayton et al., 2007; Doupé, Klein, Simons, & Jones, 2010; Mascré et al., 2012). The ground-breaking initial studies used mouse models expressing tamoxifen-regulated cre recombinase driven by an inducible CYP1A1 promoter (AhcreERT), crossed to a YFP reporter strain (Clayton et al., 2007; Doupé et al., 2010). Low-dose tamoxifen administration allowed single-cell labeling within the tail and ear IFE and the ability to follow clone generation long term. Interestingly, the average size of persisting clones increased linearly with time, which is contrary to the previously proposed restricted size of the EPU. Furthermore, mathematic analysis of the clone generation in these studies suggested that basal cells could generate proliferative or differentiated progeny stochastically. However, whether these experiments labeled the most primitive SC within the IFE was unclear.
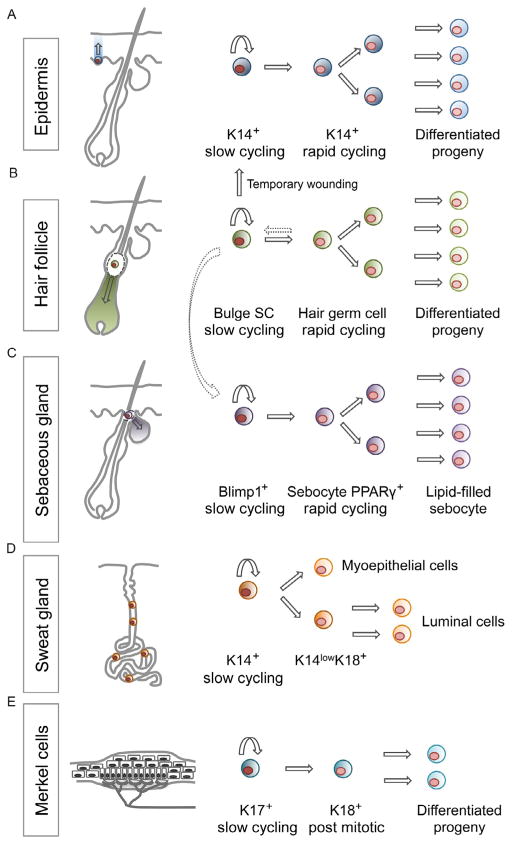
More recently, comparing lineage tracing in the IFE of mouse models expressing either an inducible CreER driven by the keratin 14 (K14) promoter or a fragment of the Involucrin (Inv) promoter reveal a hieracherical and heterogeneous nature of progenitor cells in the IFE (Mascré et al., 2012). In the InvCreER mouse model, persistent labeled clones followed the same cell-fate dynamics and linear growth patterns as the clones generated in the AhCre model (Clayton et al., 2007; Doupé et al., 2010). By contrast, the persistent clones generated in the K14CreER mouse model displayed stochastic fate decisions but were restricted in their growth potential, consistent with an EPU-type model and supporting the existence of a slow-cycling SC within the IFE. Molecular characterization of basal cells labeled in the K14CreER and InvCreER mouse models further supported the labeling of two distinct cell types within the IFE. Together, these studies reveal a hierarchy of cells within the IFE: a slow-cycling SC (marked by K14CreER) that gives rise to more rapidly cycling committed progenitors (marked by AhCreER or InvCreER) that subsequently undergo terminal differentiation (Fig. 4.2). These studies provide significant knowledge regarding the molecular characteristics of the slow-cycling IFE progenitors that will allow the future identification of novel markers as well as the further analysis of the molecular regulation of these cells during skin homeostasis.
Figure 4.2.
Different stem cell populations regulate normal epidermal homeostasis. Schematic representation of the different stem cell population found within different skin compartments. (A) Different lineage tracing experiment have shown that the IFE is maintained by the presence of K14+ progenitor basal cells that are able to generate the differentiated lineages that comprise the squamous epithelium of the skin. (B and C) Within the pilosebaceous unit, a slow cycling SC residing in the bulge is able to give rise to all the different HF lineages and regenerate a new hair follicle during anagen. They can also contribute to the IFE after wounding and to the SG population that is usually maintained by a resident pool of Blimp1+ slow-cycling residing progenitors. (D) In the sweat gland, a K14+ progenitor was shown to be able to regenerate a full functioning gland capable of sebum production. Finally, within the touch dome structure (E), K17+ slow-cycling cells have been shown to give rise to K18+ rapid-cycling progenitor that can further generate differentiated progeny.
Heterogeneity also exists within the slow-cycling SCs of the IFE. Within the majority of the murine epidermis, orthokeratotic differentiation generates cells that are spinous, granular, and have lost nuclei in the stratum corneum (Didierjean, Wrench, & Saurat, 1983; Schweizer & Marks, 1977). In the tail, however, IFE regions between the organized arrays of HFs postnatally develop a parakeratotic program postnatally, resulting in IFE regions lacking the granular layer and retention of nuclei within the stratum corneum (Didierjean et al., 1983; Schweizer & Marks, 1977). Careful genetic lineage analysis of the clonogenic behavior of K14CreER marked cells in these different regions revealed that IFE SCs are restricted to a particular compartment and display distinct proliferative behavior, suggesting that heterogeneous, unipotent SCs exist within the IFE (Gomez, Chua, Miremadi, Quist, & Headon, 2013).
Within the adult IFE, a separate epithelial SC niche exists for Merkel cells, specialized sensory cells that allow mammals to respond to mechanical stimuli during touch sensations (Fig. 4.2). Mature Merkel cells reside in touch domes, which are clusters of cells that are innervated by afferent somatosensory nerve fibers at the dermal–epidermal border in specialized skin regions (Merkel, 1875). These unique cells express both cytokeratins and neuroendocrine proteins and thus their developmental origin was unclear until several recent studies demonstrated that these cells derive from K14 expressing cells of the developing epidermis (Morrison, Miesegaes, Lumpkin, & Maricich, 2009; Van Keymeulen et al., 2009). Once mature touch domes are formed in the epidermis, K17+ keratinocytes within the epidermal touch dome maintain mature Merkel cells during homeostasis, turning over every 2 months in adult skin (Doucet, Woo, Ruiz, & Owens, 2013). Ablation of K17+ cells results in Merkel cell loss and the inability of sensory afferents to innervate the skin, indicating a functional role for Merkel cell progenitor cells in maintaining the neuronal niche in the skin (Doucet et al., 2013). How the surrounding keratinocytes and interacting neurons control Merkel cell progenitors will be an interesting area for future investigation.
3. STEM CELLS IN THE PILOSEBACEOUS UNIT
During epidermal development, basal progenitor cells are specified to appendage cell fates such as the pilosebaceous unit containing the HF and the SG (Millar, 2002). The SG is a continually regenerative gland that produces sebum, specialized lipids that are released into the hair canal onto the skin’s surface through lysis of differentiated sebocytes (Niemann & Horsley, 2012). The HF is maintained through a three-stage regenerative process of the hair cycle, which begins with HF growth (anagen), where the lower portion of the HF grows into the dermis and produces differentiated lineages that allow hair production (Lee & Tumbar, 2012; Sennett & Rendl, 2012). Eventually, hair growth ends and a destructive phase (catagen) starts, where the lower portion of the HF dies and regresses, leaving a permanent region, called the bulge. The final stage involves a resting phase (telogen) before the next regenerative hair cycle starts when bulge SCs are activated to start growth of a new follicle.
The initial identification of the SC properties of bulge cells took advantage of their slow-cycling nature, which allowed retention of nucleotide analogs such as BrdU and tritiated thymidine during pulse-chase experiments (Bickenbach, 1981; Cotsarelis, Sun, & Lavker, 1990). Innovative genetic mouse models were also developed that allowed fluorescent labeling of histone H2B in epithelial cells and after a lengthy chase period, bulge cells were the primary cell-type labeled within the skin (Tumbar et al., 2004). These experiments demonstrated that the cell cycle of bulge cells was slower than the other epithelial cells of the skin.
Genetic lineage tracing experiments using multiple mouse models with cell-type-specific expression of cre recombinase have demonstrated that several populations of cells within the pilosebaceous unit have the capacity to generate lineages of the HF and SG (Fig. 4.2) (Jaks et al., 2008; Jensen et al., 2009; Morris et al., 2004; Petersson et al., 2011; Snippert et al., 2010), as well as the epidermis after wounding (Ito et al., 2005; Levy, Lindon, Zheng, Harfe, & Morgan, 2007; Snippert et al., 2010). The isolation and transplantation of putative follicular SCs based on microdissection or based on fluorescence-activated cell sorting for bulge markers such CD34 and α6-integrin also demonstrated the multipotency of SCs within the HF bulge (Blanpain, Lowry, Geoghegan, Polak, & Fuchs, 2004; Claudinot, Nicolas, Oshima, Rochat, & Barrandon, 2005). In sum, these studies have demonstrated that bulge cells are multipotent, contributing temporarily to the epidermis during wounding and primarily to the homeostasis of HF and SG.
The activation of HF SCs during hair cycling involves several cellular processes including proliferation and migration of bulge cells and hair germ cells (Greco, Chen, Rendl, Schober, & Pasolli, 2009; Rompolas et al., 2012; Zhang, Cheong, Ciapurin, McDermitt, & Tumbar, 2009). The activated SCs generate proliferative progeny and differentiate to form the inner root sheath and the HF shaft (Ito et al., 2005; Oshima, Rochat, Kedzia, Kobayashi, & Barrandon, 2001; Tumbar et al., 2004).
Similar to the heterogeneity in IFE SCs (Gomez et al., 2013), the permanent portion of the HF contains heterogeneous group of cells with differing capacities for contributing to homeostasis of epithelial lineages of the skin (Goldstein & Horsley, 2012). At the junction between the IFE and HF, cells expressing SC antigen-1 and/or leucine-rich repeats and immunoglobulin-like domain 1 (Lrig1) exist and are able to generate IFE and SG cells but are limited in their contribution to the HF lineage (Jensen et al., 2009, 2008). Below the junctional zone and at the base of the SG, several slow-cycling cells exist such as MTS24 expressing cells (Nijhof et al., 2006) and also leucine-rich repeat containing G protein-coupled receptor 6 or B-lymphocyte-induced maturation protein 1 (Blimp1) positive cells that can generate SG lineages (Horsley et al., 2006; Snippert et al., 2010). The cells that primarily generate HF lineages are located at the base of the isthmus and throughout the CD34+, K15+ region of the bulge (Blanpain et al., 2004; Brownell, Guevara, Bai, Loomis, & Joyner, 2011; Morris et al., 2004; Snippert et al., 2010; Tumbar et al., 2004). At the base of this compartment and within the hair germ, leucine-rich repeat containing G protein-coupled receptor 5 (Lgr5) positive cells can give rise to different HF lineages (Jaks et al., 2008). These populations are not restricted but dynamically interact: K15+ bulge cells can migrate to the SG where they locally self-renew and continue to contribute to the normal gland homeostasis (Petersson et al., 2011). Additionally, Lgr5+descendants of bulge cells can repopulate the bulge at the end of hair HF regression (Hsu, Pasolli, & Fuchs, 2011).
4. STEM CELLS IN THE SWEAT GLAND
Eccrine sweat glands are an additional epidermal appendage that generate sweat for mammalian thermoregulation. Sweat fluids are generated by specialized epithelial cells in a coiled structure and are secreted into luminal structures into a duct that opens directly onto the skin surface (Lobitz & Dobson, 1961). Similar to the mammary gland, the secretory coil of the sweat gland contains an outer basal layer of myoepithelial cells that express K5, K14, and smooth muscle actin. An inner suprabasal layer of luminal cells is positive for K8, K18, and K19 (Langbein et al., 2005; Moll & Moll, 1992; Schön, Benwood, O’Connell-Willstaedt, & Rheinwald, 1999). In contrast to the dramatic cellular changes during puberty and pregnancy in the mammary gland (Richert, Schwertfeger, Ryder, & Anderson, 2000), the sweat gland presents little sign of continual renewal (Lu et al., 2012).
Initial experiments to address the existence of sweat gland progenitors identified cell divisions in basal cells of the gland’s duct region (Lobitz, Holyoke, & Montagna, 1954). Other studies analyzed the regenerative potential of sweat glands in vitro by showing proliferation of dissociated gland cells in culture and in vivo by demonstrating the contribution of sweat gland for epidermal reconstitution after superficial skin injuries (Biedermann et al., 2010; Miller, Burke, Rader, Coulombe, & Lavker, 1998). Recent work has identified K14+ progenitors that can differentiate into myoepithelial cells and can also stratify to form the suprabasal layer of K14low/K18+ cell that give rise to luminal cells (Lu et al., 2012) (Fig. 4.2). Furthermore, lineage tracing and EdU pulse-chase experiments were able to show that both sweat gland progenitor cell types participate in repair of the epidermis after wounding but the sweat gland itself remains relatively quiescent throughout the wound repair process (Lu et al., 2012). The plasticity of sweat gland progenitor cells was illustrated by transplantation studies in which progenitor cells were injected into cleared mammary fat pads and were able to reconstitute an entire sweat gland. By contrast, luminal cells were unable to contribute to de novo formation of sweat glands (Lu et al., 2012). Further studies will be necessary to define the molecular mechanisms that regulate sweat glands homeostasis and their contribution to epidermal healing.
5. COMPONENTS OF ADULT STEM CELL NICHES IN THE SKIN
The identification, isolation, and characterization of different populations of SCs within the adult skin epithelium identified layers of molecular mechanisms that act intrinsically to regulate SC behavior. Recent reviews have discussed several studies that identify molecular mechanisms that regulate SC behavior in the skin (Arwert, Hoste, & Watt, 2012; Blanpain & Fuchs, 2006; Hsu et al., 2011; Lee & Dai, 2013; Lee & Tumbar, 2012). Several signaling pathways are involved in regulating SC behavior in the skin including Wnt, BMP, FGF, and PDGF (Festa et al., 2011; Greco et al., 2009; Kandyba et al., 2013; Kobielak, Stokes, de la Cruz, Polak, & Fuchset, 2007; Myung, Takeo, Ito, & Atit, 2013; Oshimori & Fuchs, 2012; Plikus et al., 2008). In addition, the involvement of dermal cell types has recently come to light (Goldstein & Horsley, 2012). Here, we will highlight recent developments regarding the intrinsic and extrinsic regulation of epithelial SCs.
5.1. Intrinsic regulation of stem cell function
Cellular metabolism and environmental assaults can result in DNA damage, especially in the skin, which directly receives UV irradiation from the sun and environmental mutagens that can induce genomic instability. Interestingly, HFSCs are more resistant to radiation-induced damage compared to other epithelial skin cells (Sotiropoulou et al., 2010). To attain this resistance, HFSCs express high levels of the antiapoptotic protein B-cell lymphoma 2 (Bcl2) and transiently express p53 to promote survival. In addition, breast cancer 1 (Brca1) is essential for DNA damage repair (Gudmundsdottir & Ashworth, 2006; Moynahan & Jasin, 2010) and epidermal deletion of Brca1 leads to defects in HF formation as well as induction of caspase-dependent apoptosis that leads to hyperproliferation and subsequent exhaustion of adult SCs (Sotiropoulou et al., 2013). The differential regulation of DNA damage in SCs also exists within other tissues (Mandal, Blanpain, & Rossi, 2011) and may be similar to the mechanisms that act in SCs in the IFE and other epidermal appendages.
Several transcriptional regulators of SC function in the HF have been identified and are shared among SCs of other tissues including transcription factor 3 and 4 (TCF3/4), nuclear factor of activated T-cells 1 (NFATc1) and sex determining region Y-box 9 (Sox9) (Blanpain & Fuchs, 2006; Nguyen et al., 2009; Nguyen, Rendl, & Fuchs, 2006; Nowak, Polak, Pasolli, & Fuchs, 2008). In addition, Lgr5 (Barker et al., 2007) and the atypical HOP homeobox protein Hopx are expressed by an intestinal SC epithelial pool at the base of the crypt (Takeda, Jain, LeBoeuf, Wang, & Lu, 2011). In the HF, Hopx is expressed within bulge cells and can contribute to all HF lineages upon HF growth as well as to IFE cells upon wounding (Takeda, Jain, LeBoeuf, & Padmanabhan, 2013). Lower bulge cells expressing the SC marker Lgr5 also express Hopx, are able to escape apoptosis during the HF death phase and contribute long-term to bulge cell maintenance (Takeda et al., 2013).
The transcription factor LIM homeobox protein 2 (Lhx2) is another homeobox protein that has been implicated in regulating morphogenesis and patterning of ectodermal derivatives and in SC maintenance and quiescence within the HF SC niche (Mardaryev et al., 2011; Rhee, Polak, & Fuchs, 2006; Törnqvist, Sandberg, Hägglund, & Carlsson, 2010). Lhx2 is expressed in the bulge and secondary hair germ where it co-localizes with the SC markers Sox9, Tcf4, and Lgr5. In response to skin injury, Lhx2+cells within the bulge and secondary hair germ proliferate and contribute to skin re-epithelialization via positive regulation of Sox9 and Tcf4 while inhibiting HF cycling through negatively regulating Lgr5 (Mardaryev et al., 2011). These and many other studies have provided novel insights of how Wnt and BMP signaling pathways and transcriptional regulation networks modulate activity of epithelial SCs during normal homeostasis and in response to injury (Blanpain & Fuchs, 2006; Lee & Tumbar, 2012; Sennett & Rendl, 2012).
Epithelial SCs are also regulated post-transcriptionally and translationally in part by microRNAs (miRNAs), which are small noncoding RNAs that alter RNA translation or stability to control gene expression. Complete ablation of miRNA production by deletion of the upstream processing enzyme Dicer in mice results in perinatal lethality and severe HF defects (Andl et al., 2006; Yi et al., 2006). Among these defects are undeveloped and misaligned HFs, increased apoptosis and lack of K15+ and CD34+ cells within the bulge compartment suggesting that miRNAs, in general, are important for HF SC maintenance (Andl et al., 2006).
Several miRNAs are spatiotemporally regulated within the IFE and the HFSCs. MiR203 was shown to be preferentially enriched in the IFE versus the HF (Andl et al., 2006; Yi, Poy, Stoffel, & Fuchs, 2008) and is sufficient to promote IFE differentiation and suppress self-renewal in the IFE by controlling the expression of p63 (Andl et al., 2006; Yi et al., 2008). Additionally, miR203 is transcriptionally activated during asymmetric cell division in the developing epidermis, localizing to the differentiated daughter cell, where it promotes cell cycle exit and abolishes self-renewal in a process involving co-suppression of p63, S-phase kinase-associated protein 2 (Skp2), and musashi RNA-binding protein 2 (Msi2) (Jackson et al., 2013).
An additional miRNA, miR125b is sufficient to alter IFE homeostasis and abrogate hair specification (Zhang, Stokes, Polak, & Fuchs, 2011). MiR31 can also alter HFSC activity by targeting fibroblast growth factor 10 (Fgf10), distal-less homeobox 3 (Dlx3), several keratin genes and also components of the Wnt and BMP signaling pathways (Mardaryev et al., 2010). The differential regulation of several miRNAs in the epithelium of the skin suggests that roles for additional miRNAs will be defined as this burgeoning field continues to expand.
Another level of regulation of skin SCs occurs through modification of histones and DNA to epigenetically regulate transcription (Calo & Wysocka, 2013). Several epigenetic factors play a role in epidermal differentiation (Mulder et al., 2012). Histone acetylation and methylation through histone deacetylase and methyltransferase activity, respectively, regulate IFE development (Driskell et al., 2012; LeBoeuf et al., 2010) and homeostasis (Driskell et al., 2012). Maintenance of repressive histone modifications via the polycomb repressor complex, enhancer of zeste homolog 1 (Ezh1) and Ezh2 are essential for IFE differentiation and for HF morphogenesis and maintenance (Bardot et al., 2013; Ezhkova et al., 2011). Merkel cells also require Ezh2 proteins for their maintenance through the regulation of the transcription factor Sox2 (Bardot et al., 2013). Histone methylation controlled by the demethylase Jumonji domain containing 3 (JmjD3) is essential for IFE differentiation (Sen, Webster, Barragan, Chang, & Khavari, 2008), while the demethylase Jumonji/jmjc domain-containing protein 2 (Jarid2) is required to maintain IFE basal progenitors (Mejetta et al., 2011). In addition, the DNA methyltransferase 1 (DNMT1) and the ubiquitin like, containing PHD and RING finger domain-1 (UHRF1) are expressed in basal cells and are downregulated once cells enter the differentiation program suggesting that they are also involved in regulating stemness. Deletion of DNMT1 in human skin regeneration assays induced premature differentiation of progenitors and progressive tissue loss further demonstrating its importance for self-renewal (Sen, Reuter, Webster, Zhu, & Khavari, 2010).
Additional control of SC function occurs through the regulation of gene expression by altering nucleosome positioning through the action of chromatin remodeling complexes such as the SWI/SNF complex (Kidder, Palmer, & Knott, 2009). By rearranging nucleosome positions within the chromatin, these complexes regulate RNA polymerase II occupancy and thus transcriptional initiation in an ATP-dependent manner (Liu, Balliano, & Hayes, 2011). At the crux of these complexes, brahma-related gene 1 (Brg1) acts as a catalytic subunit and regulates SC proliferation and differentiation. In the HF, it was recently shown that Brg1 is dynamically activated after SC activation in the skin. Deletion of Brg1 with the bulge-specific NFATc1-Cre induced precocious HF regression, loss of HFSCs, and progressive hair loss (Xiong et al., 2013). Molecularly, Brg1 and Shh act in a molecular loop, where Brg1 regulates Shh expression and Shh activates Brg1 expression within the follicle (Xiong et al., 2013). Whether Brg1 regulates additional genes to control HFSC function will be an interesting area of future investigation.
5.2. Cell extrinsic regulation of SC function
The intrinsic regulation of epithelial SCs in the skin is influenced by multiple cell types, including follicle-associated melanocytes and other cells within the dermis (Goldstein & Horsley, 2012). Of primary importance, the dermal papillae (DP) is a mesenchymal cell population that abuts the HF, induces its morphogenesis and remains associated with the follicle throughout its life cycle (Driskell, Clavel, Rendl, & Watt, 2011). The association of the DP with the follicle is essential for the activation of HF growth (Chi, Wu, & Morgan, 2013; Rompolas et al., 2012) and the size of the DP can define the size and shape of the hair follicle (Chi et al., 2013). Several signaling ligands are expressed by the DP (Rendl, Lewis, & Fuchs, 2005) and the identification of DP specific Cre lines (Enshell-Seijffers, Lindon, Kashiwagi, & Morgan, 2010; Grisanti et al., 2013) will allow the identification of the molecular mechanisms by which these cells control HFSC activity.
Below the DP, a depot of dermal adipocytes displays dynamic changes in size during the hair cycle. Both adipocyte hypertrophy and adipogenesis contribute to the growth of the adipocyte depot in the skin. The production of immature adipocyte precursor cells during adipogenesis is both necessary and sufficient to drive hair cycling (Festa et al., 2011). Adipogenesis also occurs after acute wounding and inhibition of adipogenesis can alter fibroblast function in the skin, leading to wound closure failure (Schmidt & Horsley, 2013). The molecular mechanisms by which adipocyte lineage cells function in the skin will reveal novel components of the skin SC niche.
Permeating through the dermal cell layers, somatosensory nerve fibers innervate the touch dome in the IFE and surround the HF in a piloneural collar (Lumpkin, Marshall, & Nelson, 2010). The HF cells of each follicle type may provide unknown cues for the distinct neural innervation. Interestingly, each hair follicle type is innervated by distinct mechanoreceptors which converge within the dorsal horn of the spinal column to process touch sensations (Li et al., 2011). Afferents from the dorsal root ganglion produce glutamate that is essential for the proper development of the piloneural mechanoreceptors. The neural innervation also provides signals for the HF, such as Shh and can promote the contribution of HF cells to wound healing and SG homeostasis (Brownell et al., 2011).
Genome-wide association studies have implicated inflammatory cells in the regulation of hair loss (Petukhova et al., 2010), suggesting that these cells may be instrumental in SC function in the skin. Supporting a role for immune cells in hair cycling, mice lacking γδT cells through deletion of TCRδ have defects in hair cycling (Kloepper, Kawai, Bertolini, Kanekura, & Paus, 2013). Inflammatory cells also play many roles in wound healing (Eming, Krieg, & Davidson, 2007), and γδT cells can induce HF neogenesis from healed epithelial cells after wounding via the production of Fgf9 (Gay et al., 2013). Functional roles of other immune cells may reveal their functions in the control of additional epithelial SCs in the skin.
6. STEM CELLS IN EPITHELIAL SKIN CANCERS
Several types of epithelial cancers form in the skin. Papillomas, basal cell carcinomas (BCCs), and squamous cell carcinomas (SCCs) are found in the IFE, while pilomatriomas, trichofolliculomas, and SG carcinomas are associated with the pilosebaceous unit. Resident epidermal SCs have been proposed to initiate epithelial tumorigenesis. Furthermore, maintenance of tumors following initiation is thought to be driven by tumor cells with SC characteristics such as self-renewal, and slow-cycling, properties (Al-Hajj & Clarke, 2004).
BCCs comprise 80% of epithelial skin cancers and are often associated with activating mutations in the Shh pathway via inactivation of the repressive receptor Patched (Ptch), expression/activation of the transcriptional mediators GLI family zinc finger (Gli) proteins, or activation of the Shh signal transducer, smoothened (Smo) (Athar, Tang, Lee, Kopelovich, & Kim, 2006; Rogers et al., 2010). The morphology of BCCs is variable, forming various subtypes including superficial and nodular tumors, suggesting that different cellular origins may exist.
Based on the similarities of keratin expression between BCCs and HF progenitor cells (Asada, 1993) and the inability of BCCs to develop in irradiated mice with only one allele of Ptch1 during HF SC quiescence (Mancuso et al., 2006), it was initially hypothesized that HF progenitor cells were the cells of origin for BCCs. However, genetic lineage-tracing experiments in mice expressing an active Gli2 demonstrated that BCCs can arise from several epithelial cell types including the bulge, SGs, and IFE (Grachtchouk et al., 2011). Similarly, Lgr5+ bulge progeny required wounding to generate BCC-like lesions upon genetic Gli1 activation (Kasper et al., 2011). Expression of a constitutively active form of Smo in a cell-type specific manner was shown to generate BCCs preferentially from cells in the IFE and infundibulum rather than from bulge cells (Youssef et al., 2010). Taken together, these data suggest that BCC may have multiple origins and that wounding promotes BCC expansion.
Abnormal Wnt signaling has been associated with the development of epidermal tumors. In fact expression of a constitutively active form of β-catenin promotes the development of pilomatricomas and trichofolliculomas and mutations of lymphoid enhancer-binding factor 1 (lef1) that normally inactivates β-catenin is associated with human SG tumors (Chan, Gat, McNiff, & Fuchs, 1999; Gat, DasGupta, Degenstein, & Fuchs, 1998; Takeda et al., 2006). Inducible activation of β-catenin under the K14 promoter lead to the formation of lesions similar to pilomatricomas that regress when β-catenin is no longer activated (Lo Celso, Prowse, & Watt, 2004). Furthermore, expression of ΔNLef1 in epidermis induced SG tumors (Niemann, Owens, Hülsken, Birchmeier, & Watt, 2002; Niemann, Owens, Schettina, & Watt, 2007) and ablation of β-catenin also under the K14 promoter caused a regression of chemically induced papillomas (Malanchi et al., 2008). Different SC populations react differently to Wnt signaling-associated tumor formation stimuli. For example, sustained β-catenin activity under the K15 promoter leads to an increase in proliferation, expression of Wnt target genes within the bulge compartment, and inability to form pilomatricomas even when a wounding stimulus is enforced, while sustained β-catenin activity under a truncated K5 promoter (expressed in SG and HF bulb) causes conversion of SG into HF structures that develop and resemble benign tumors (Baker, Verstuyf, Jensen, & Watt, 2010).
SCCs encompass 20% of epithelia-derived tumors in the skin and have metastatic potential following papilloma formation (Alam & Ratner, 2001). Induction of SCCs in mice can occur via UV irradiation, chemical carcinogenesis protocols using 7,12-dimethylbenz(a)anthracene (DMBA) to induce Ras mutations followed by promotion with 12-O-tetradecanoylphorbol-13-acetate or genetic expression of oncogenes within the epithelium. Clone analysis of human skin with p53 mutations suggested that SCCs derived from the dermal–epidermal junction and from HFs (Jonason et al., 1996). Similar results were obtained when a mutated activated form of Ras was expressed in the proliferative cells of the IFE and HF (Brown, Strathdee, Bryson, Lambie, & Balmain, 1998).
To further elucidate the cellular origin of Ras induced SCCs, cell-type-specific expression of mutant Kras was induced in mouse models controlled by bulge-specific promoters, K15 or K19 (Lapouge et al., 2011; White et al., 2011). Activation of Kras in the bulge led to papilloma formation, whereas tumorigenesis was not induced when Kras mutations were targeted to the transiently amplifying cells of the HF. Interestingly, activation of Kras in InvCre-expressing cells also generated papillomas (Lapouge et al., 2011). Since this promoter can drive expression in IFE progenitor cells (Mascré et al., 2012), IFE cells may also contribute to SCC formation. The contribution of bulge cells to papilloma formation was further confirmed by the presence of progeny from K15+ bulge cells in murine papillomas after induction of Ras mutations with DMBA (Kangsamaksin, Park, Trempus, & Morris, 2007).
To determine if cells with SC potential exist within SCCs, transplantation assays of purified cells into immunocompromised mice have revealed that CD34 or α6β1 integrin expression can enrich for tumor-initiating cells in this assay (Malanchi et al., 2008; Schober & Fuchs, 2011). Importantly, ablation of TGFβ signaling enhanced the proliferation of integrinhi, CD34+ cells, and their tumorigenic potential (Schober & Fuchs, 2011). By applying quantitative clonal analysis to SCC tumor growth in vivo, Blanpain and colleagues found a minority of cells within papillomas with dramatic growth potential (Driessens, Beck, Caauwe, Simons, & Blanpain, 2012). By contrast, metastatic SCCs displayed clones of cells with increased replicative and abrogated differentiation potential. Interestingly, a hierarchical organization of tumor cells also exists in benign intestinal adenomas and metastatic brain tumors such as glioblastomas (Chen et al., 2012; Schepers et al., 2012). In glioblastomas, when the highly proliferative progeny of the cells are ablated with chemotherapeutic drugs, the other cells with SC properties can repopulate the tumors. When these cells with more primitive potential were selectively ablated with genetic tools in the presence of chemotherapeutic drugs, tumor growth was significantly hampered. Together, these studies provide strong evidence for the ability of cancer cells to acquire SC properties to fuel tumorigenesis and suggest that targeting both primitive and more developed cells within tumors will be important for effective cancer therapies.
7. CONCLUDING REMARKS
Several advances have been made toward understanding the cellular and molecular mechanisms that control epidermal SC quiescence and differentiation in multiple lineages in the skin. Important progress achieved in recent years to develop mouse models to target and disrupt distinct SC pools within the epidermis have provided precious information that begins to unravel not only the complexity of the different epidermal SC niches but also the interactions between these niches. Future in-depth studies looking at the different SC niche signals and how they are affected during disease will definitely contribute to the better understanding of epidermal SC biology and the consequent application toward treatment of SC-related pathologies such as cancer.
Acknowledgments
We thank the Horsley lab members for critical reading of the chapter and valuable discussions. A. M. B. T. was a Fundação para a Ciência e Tecnologia postdoctoral fellow. V. H. is a Pew Scholar in Biomedical Research and is funded by the NIH (AR060295) and the state of CT (12-SCB-YALE-01 and 12-SCA-YALE-09).
References
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. The New England Journal of Medicine. 2001;344:975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- Andl T, Murchison EP, Liu F, Zhang Y, Yunta-Gonzalez M, Tobias JW, et al. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Current Biology. 2006;16:1041–1049. doi: 10.1016/j.cub.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nature Reviews Cancer. 2012;12:170–180. doi: 10.1038/nrc3217. [DOI] [PubMed] [Google Scholar]
- Asada M, Schaart FM, de Almeida HL, Jr, Korge B, Kurokawa I, Asada Y, et al. Solid basal cell epithelioma (BCE) possibly originates from the outer root sheath of the hair follicle. Acta Dermato Venereologica. 1993;73(4):286–292. [PubMed] [Google Scholar]
- Athar M, Tang X, Lee JL, Kopelovich L, Kim AL. Hedgehog signalling in skin development and cancer. Experimental Dermatology. 2006;15:667–677. doi: 10.1111/j.1600-0625.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- Baker CM, Verstuyf A, Jensen KB, Watt FM. Differential sensitivity of epidermal cell subpopulations to beta-catenin-induced ectopic hair follicle formation. Developmental Biology. 2010;343:40–50. doi: 10.1016/j.ydbio.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardot ES, Valdes VJ, Zhang J, Perdigoto CN, Nicolis S, Hearn SA, et al. Polycomb subunits Ezh1 and Ezh2 regulate the Merkel cell differentiation program in skin stem cells. EMBO Journal. 2013 doi: 10.1038/emboj.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Bickenbach JR. Identification and behavior of label-retaining cells in oral mucosa and skin. Journal of Dental Research. 1981;60(Spec No C):1611–1620. doi: 10.1177/002203458106000311011. [DOI] [PubMed] [Google Scholar]
- Biedermann T, Pontiggia L, Böttcher-Haberzeth S, Tharakan S, Braziulis E, Schiestl C, et al. Human eccrine sweat gland cells can reconstitute a stratified epidermis. The Journal of Investigative Dermatology. 2010;130:1996–2009. doi: 10.1038/jid.2010.83. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annual Review of Cell and Developmental Biology. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Brown K, Strathdee D, Bryson S, Lambie W, Balmain A. The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Current Biology. 1998;8:516–524. doi: 10.1016/s0960-9822(98)70203-9. [DOI] [PubMed] [Google Scholar]
- Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo E, Wysocka J. Modification of enhancer chromatin: What, how, and why? Mol Cell. 2013;49:825–837. doi: 10.1016/j.molcel.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nature Genetics. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- Chase HB, Montagna W, Malone JD. Changes in the skin in relation to the hair growth cycle. The Anatomical Record. 1953;116:75–81. doi: 10.1002/ar.1091160107. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W, Wu E, Morgan BA. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development. 2013;140:1676–1683. doi: 10.1242/dev.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14677–14682. doi: 10.1073/pnas.0507250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E, Doupé DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Dick JC. Observations on the elastic tissue of the skin with a note on the reticular layer at the junction of the dermis and epidermis. Journal of Anatomy. 1947;81:201–211. [PMC free article] [PubMed] [Google Scholar]
- Didierjean L, Wrench R, Saurat JH. Expression of cytoplasmic antigens linked to orthokeratosis during the development of parakeratosis in newborn mouse tail epidermis. Differentiation. 1983;23:250–255. doi: 10.1111/j.1432-0436.1982.tb01290.x. [DOI] [PubMed] [Google Scholar]
- Doucet YS, Woo SH, Ruiz ME, Owens DM. The touch dome defines an epidermal niche specialized for mechanosensory signaling. Cell Reports. 2013;3:1759–1765. doi: 10.1016/j.celrep.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupé DP, Klein AM, Simons BD, Jones PH. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Developmental Cell. 2010;18:317–323. doi: 10.1016/j.devcel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–530. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. Journal of Cell Science. 2011;124:1179–1182. doi: 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell I, Oda H, Blanco S, Nascimento E, Humphreys P, Frye M. The histone methyltransferase Setd8 acts in concert with c-Myc and is required to maintain skin. The EMBO Journal. 2012;31:616–629. doi: 10.1038/emboj.2011.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: Molecular and cellular mechanisms. The Journal of Investigative Dermatology. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Developmental Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhkova E, Lien WH, Stokes N, Pasolli HA, Silva JM, Fuchs E. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes and Development. 2011;25:485–498. doi: 10.1101/gad.2019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler PD, et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nature Medicine. 2013;19:916–923. doi: 10.1038/nm.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J, Horsley V. Home sweet home: Skin stem cell niches. Cellular and Molecular Life Sciences. 2012;69:2573–2582. doi: 10.1007/s00018-012-0943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez C, Chua W, Miremadi A, Quist S, Headon DJ. The interfollicular epidermis of adult mouse tail comprises two distinct cell lineages that are differentially regulated by Wnt, Edaradd, and Lrig1. Stem Cell Reports. 2013;1:19–27. doi: 10.1016/j.stemcr.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grachtchouk M, Pero J, Yang SH, Ermilov AN, Michael LE, Wang A, et al. Basal cell carcinomas in mice arise from hair follicle stem cells and multiple epithelial progenitor populations. The Journal of Clinical Investigation. 2011;121:1768–1781. doi: 10.1172/JCI46307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti L, Clavel C, Cai X, Rezza A, Tsai SY, Sennett R, et al. Tbx18 targets dermal condensates for labeling, isolation, and gene ablation during embryonic hair follicle formation. The Journal of Investigative Dermatology. 2013;133:344–353. doi: 10.1038/jid.2012.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsdottir K, Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene. 2006;25:5864–5874. doi: 10.1038/sj.onc.1209874. [DOI] [PubMed] [Google Scholar]
- Horsley V, O’Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature Medicine. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Jackson SJ, Zhang Z, Feng D, Flagg M, O’Loughlin E, Wang D, et al. Rapid and widespread suppression of self-renewal by microRNA-203 during epidermal differentiation. Development. 2013;140:1882–1891. doi: 10.1242/dev.089649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nature Genetics. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen UB, Yan X, Triel C, Woo SH, Christensen R, Owens DM. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. Journal of Cell Science. 2008;121:609–617. doi: 10.1242/jcs.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonason AS, Kunala S, Price GJ, Restifo RJ, Spinelli HM, Persing JA, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14025–14029. doi: 10.1073/pnas.93.24.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandyba E, Leung Y, Chen YB, Widelitz R, Chuong CM, Kobielak K. Competitive balance of intrabulge BMP/Wnt signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1351–1356. doi: 10.1073/pnas.1121312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangsamaksin T, Park HJ, Trempus CS, Morris RJ. A perspective on murine keratinocyte stem cells as targets of chemically induced skin cancer. Molecular Carcinogenesis. 2007;46:579–584. doi: 10.1002/mc.20355. [DOI] [PubMed] [Google Scholar]
- Kasper M, Jaks V, Are A, Bergström Å, Schwäger A, Svärd J, et al. Wounding enhances epidermal tumorigenesis by recruiting hair follicle keratinocytes. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4099–4104. doi: 10.1073/pnas.1014489108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidder BL, Palmer S, Knott JG. SWI/SNF-Brg1 regulates self-renewal and occupies core pluripotency-related genes in embryonic stem cells. Stem Cells. 2009;27:317–328. doi: 10.1634/stemcells.2008-0710. [DOI] [PubMed] [Google Scholar]
- Kloepper JE, Kawai K, Bertolini M, Kanekura T, Paus R. Loss of γδ T cells results in hair cycling defects. The Journal of Investigative Dermatology. 2013;133:1666–1669. doi: 10.1038/jid.2013.17. [DOI] [PubMed] [Google Scholar]
- Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbein L, Rogers MA, Praetzel S, Cribier B, Peltre B, Gassler N, et al. Characterization of a novel human type II epithelial keratin K1b, specifically expressed in eccrine sweat glands. The Journal of Investigative Dermatology. 2005;125:428–444. doi: 10.1111/j.0022-202X.2005.23860.x. [DOI] [PubMed] [Google Scholar]
- Lapouge G, Youssef KK, Vokaer B, Achouri Y, Michaux C, Sotiropoulou PA, et al. Identifying the cellular origin of squamous skin tumors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7431–7436. doi: 10.1073/pnas.1012720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBoeuf M, Terrell A, Trivedi S, Sinha S, Epstein JA, Olson EN, et al. Hdac1 and Hdac2 act redundantly to control p63 and p53 functions in epidermal progenitor cells. Developmental Cell. 2010;19:807–818. doi: 10.1016/j.devcel.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Dai X. Transcriptional control of epidermal stem cells. Advances in Experimental Medicine and Biology. 2013;786:157–173. doi: 10.1007/978-94-007-6621-1_9. [DOI] [PubMed] [Google Scholar]
- Lee J, Tumbar T. Hairy tale of signaling in hair follicle development and cycling. Seminars in Cell and Developmental Biology. 2012;23:906–916. doi: 10.1016/j.semcdb.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. The FASEB Journal. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Balliano A, Hayes JJ. Mechanism(s) of SWI/SNF-induced nucleosome mobilization. Chembiochem. 2011;12:196–204. doi: 10.1002/cbic.201000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Celso C, Prowse C, Watt FM. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131:1787–1799. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- Lobitz WC, Dobson RL. Dermatology: The eccrine sweat glands. Annual Review of Medicine. 1961;12:289–298. doi: 10.1146/annurev.me.12.020161.001445. [DOI] [PubMed] [Google Scholar]
- Lobitz WC, Holyoke JB, Montagna W. Responses of the human eccrine sweat duct to controlled injury: Growth center of the epidermal sweat duct unit. The Journal of Investigative Dermatology. 1954;23:329–344. doi: 10.1038/jid.1954.116. [DOI] [PubMed] [Google Scholar]
- Loeffler M, Potten CS, Wichmann HE. Epidermal cell proliferation. II. A comprehensive mathematical model of cell proliferation and migration in the basal layer predicts some unusual properties of epidermal stem cells. Virchows Archiv B Cell Pathology. 1987;53:286–300. [PubMed] [Google Scholar]
- Lu CP, Polak L, Rocha AS, Pasolli HA, Chen SC, Sharma N, et al. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Marshall KL, Nelson AM. The cell biology of touch. The Journal of Cell Biology. 2010;191:237–248. doi: 10.1083/jcb.201006074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie JC. Ordered structure of the stratum corneum of mammalian skin. Nature. 1969;222:881–882. doi: 10.1038/222881a0. [DOI] [PubMed] [Google Scholar]
- Mackenzie IC. Relationship between mitosis and the ordered structure of the stratum corneum in mouse epidermis. Nature. 1970;226:653–655. doi: 10.1038/226653a0. [DOI] [PubMed] [Google Scholar]
- Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- Mandal PK, Blanpain C, Rossi DJ. DNA damage response in adult stem cells: Pathways and consequences. Nature Reviews Molecular Cell Biology. 2011;12:198–202. doi: 10.1038/nrm3060. [DOI] [PubMed] [Google Scholar]
- Mardaryev AN, Ahmed MI, Vlahov NV, Fessing MY, Gill JH, Sharov AA, et al. Micro-RNA-31 controls hair cycle-associated changes in gene expression programs of the skin and hair follicle. The FASEB Journal. 2010;24:3869–3881. doi: 10.1096/fj.10-160663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardaryev AN, Meier N, Poterlowicz K, Sharov AA, Sharova TY, Ahmed MI, et al. Lhx2 differentially regulates Sox9, Tcf4 and Lgr5 in hair follicle stem cells to promote epidermal regeneration after injury. Development. 2011;138:4843–4852. doi: 10.1242/dev.070284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso M, Leonardi S, Tanori M, Pasquali E, Pierdomenico M, Rebessi S, et al. Hair Cycle–Dependent Basal Cell Carcinoma Tumorigenesis in Ptc1neo67/+ Mice Exposed to Radiation. Cancer Research. 2006;66(13):6606–6614. doi: 10.1158/0008-5472.CAN-05-3690. [DOI] [PubMed] [Google Scholar]
- Mascré G, Dekoninck S, Drogat B, Youssef KK, Broheé S, Sotiropoulou PA, et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- Mejetta S, Morey L, Pascual G, Kuebler B, Mysliwiec MR, Lee Y, et al. Jarid2 regulates mouse epidermal stem cell activation and differentiation. The EMBO Journal. 2011;30:3635–3646. doi: 10.1038/emboj.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel F. Tastzellen und Tastkörperchen bei den Haustieren und beim Menschen. Archiv für mikroskopische Anatomie und Entwicklungsmechanik. 1875;11:636–652. [Google Scholar]
- Millar SE. Molecular mechanisms regulating hair follicle development. The Journal of Investigative Dermatology. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- Miller SJ, Burke EM, Rader MD, Coulombe PA, Lavker RM. Re-epithelialization of porcine skin by the sweat apparatus. The Journal of Investigative Dermatology. 1998;110:13–19. doi: 10.1046/j.1523-1747.1998.00087.x. [DOI] [PubMed] [Google Scholar]
- Moll I, Moll R. Changes of expression of intermediate filament proteins during ontogenesis of eccrine sweat glands. The Journal of Investigative Dermatology. 1992;98:777–785. doi: 10.1111/1523-1747.ep12499950. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, et al. Capturing and profiling adult hair follicle stem cells. Nature Biotechnology. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Morrison KM, Miesegaes GR, Lumpkin EA, Maricich SM. Mammalian Merkel cells are descended from the epidermal lineage. Developmental Biology. 2009;336:76–83. doi: 10.1016/j.ydbio.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynahan ME, Jasin M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nature Reviews Molecular Cell Biology. 2010;11:196–207. doi: 10.1038/nrm2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder KW, Wang X, Escriu C, Ito Y, Schwarz RF, Gillis J, et al. Diverse epigenetic strategies interact to control epidermal differentiation. Nature Cell Biology. 2012;14:753–763. doi: 10.1038/ncb2520. [DOI] [PubMed] [Google Scholar]
- Myung PS, Takeo M, Ito M, Atit RP. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. The Journal of Investigative Dermatology. 2013;133:31–41. doi: 10.1038/jid.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Merrill BJ, Polak L, Nikolova M, Rendl M, Shaver TM, et al. Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nature Genetics. 2009;41:1068–1075. doi: 10.1038/ng.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Rendl M, Fuchs E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 2006;127:171–183. doi: 10.1016/j.cell.2006.07.036. [DOI] [PubMed] [Google Scholar]
- Niemann C, Horsley V. Development and homeostasis of the sebaceous gland. Seminars in Cell and Developmental Biology. 2012;23:928–936. doi: 10.1016/j.semcdb.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann C, Owens DM, Hülsken J, Birchmeier W, Watt FM. Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development. 2002;129:95–109. doi: 10.1242/dev.129.1.95. [DOI] [PubMed] [Google Scholar]
- Niemann C, Owens DM, Schettina P, Watt FM. Dual role of inactivating Lef1 mutations in epidermis: Tumor promotion and specification of tumor type. Cancer Research. 2007;67:2916–2921. doi: 10.1158/0008-5472.CAN-06-3427. [DOI] [PubMed] [Google Scholar]
- Nijhof JGW, Braun KM, Giangreco A, van Pelt C, Kawamoto H, Boyd RL, et al. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development. 2006;133:3027–3037. doi: 10.1242/dev.02443. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Oshimori N, Fuchs E. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell. 2012;10:63–75. doi: 10.1016/j.stem.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson M, Brylka H, Kraus A, John S, Rappl G, Schettina P, et al. TCF/Lef1 activity controls establishment of diverse stem and progenitor cell compartments in mouse epidermis. The EMBO Journal. 2011;30:3004–3018. doi: 10.1038/emboj.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113–117. doi: 10.1038/nature09114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, la Cruz de D, Baker RE, Maini PK, Maxson R, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS. Cell replacement in epidermis (keratopoiesis) via discrete units of proliferation. International Review of Cytology. 1981;69:271–318. doi: 10.1016/s0074-7696(08)62326-8. [DOI] [PubMed] [Google Scholar]
- Potten CS, Wichmann HE, Loeffler M, Dobek K, Major D. Evidence for discrete cell kinetic subpopulations in mouse epidermis based on mathematical analysis. Cell and Tissue Kinetics. 1982;15:305–329. doi: 10.1111/j.1365-2184.1982.tb01050.x. [DOI] [PubMed] [Google Scholar]
- Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biology. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee H, Polak L, Fuchs E. Lhx2 maintains stem cell character in hair follicles. Science. 2006;312:1946–1949. doi: 10.1126/science.1128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert MM, Schwertfeger KL, Ryder JW, Anderson SM. An atlas of mouse mammary gland development. Journal of Mammary Gland Biology and Neoplasia. 2000;5:227–241. doi: 10.1023/a:1026499523505. [DOI] [PubMed] [Google Scholar]
- Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Archives of Dermatology. 2010;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- Rompolas P, Deschene ER, Zito G, Gonzalez DG, Saotome I, Haberman AM, et al. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature. 2012;487:496–499. doi: 10.1038/nature11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandilands A, Sutherland C, Irvine AD, McLean WHI. Filaggrin in the frontline: Role in skin barrier function and disease. Journal of Cell Science. 2009;122:1285–1294. doi: 10.1242/jcs.033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development. 2013;140:1517–1527. doi: 10.1242/dev.087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober M, Fuchs E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-β and integrin/focal adhesion kinase (FAK) signaling. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10544–10549. doi: 10.1073/pnas.1107807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön M, Benwood J, O’Connell-Willstaedt T, Rheinwald JG. Human sweat gland myoepithelial cells express a unique set of cytokeratins and reveal the potential for alternative epithelial and mesenchymal differentiation states in culture. Journal of Cell Science. 1999;112(Pt 12):1925–1936. doi: 10.1242/jcs.112.12.1925. [DOI] [PubMed] [Google Scholar]
- Schweizer J, Marks F. A developmental study of the distribution and frequency of Langerhans cells in relation to formation of patterning in mouse tail epidermis. The Journal of Investigative Dermatology. 1977;69:198–204. doi: 10.1111/1523-1747.ep12506298. [DOI] [PubMed] [Google Scholar]
- Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature. 2010;463:563–567. doi: 10.1038/nature08683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen GL, Webster DE, Barragan DI, Chang HY, Khavari PA. Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. Genes and Development. 2008;22:1865–1870. doi: 10.1101/gad.1673508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennett R, Rendl M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Seminars in Cell and Developmental Biology. 2012;23:917–927. doi: 10.1016/j.semcdb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou PA, Candi A, Mascré G, De Clercq S, Youssef KK, Lapouge G, et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nature Cell Biology. 2010;12:572–582. doi: 10.1038/ncb2059. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou PA, Karambelas AE, Debaugnies M, Candi A, Bouwman P, Moers V, et al. BRCA1 deficiency in skin epidermis leads to selective loss of hair follicle stem cells and their progeny. Genes and Development. 2013;27:39–51. doi: 10.1101/gad.206573.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, Jain R, LeBoeuf MR, Padmanabhan A. Hopx expression defines a subset of multipotent hair follicle stem cells and a progenitor population primed to give rise to K6+ niche cells. Development. 2013;140:1655–1664. doi: 10.1242/dev.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Lyle S, Lazar AJF, Zouboulis CC, Smyth I, Watt FM. Human sebaceous tumors harbor inactivating mutations in LEF1. Nature Medicine. 2006;12:395–397. doi: 10.1038/nm1386. [DOI] [PubMed] [Google Scholar]
- Törnqvist G, Sandberg A, Hägglund AC, Carlsson L. Cyclic expression of lhx2 regulates hair formation. PLoS Genetics. 2010;6(4):e1000904. doi: 10.1371/journal.pgen.1000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keymeulen A, Mascré G, Youseff KK, Harel I, Michaux C, De Geest N, et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. The Journal of Cell Biology. 2009;187:91–100. doi: 10.1083/jcb.200907080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AC, Tran K, Khuu J, Dang C, Cui Y, Binder SW, et al. Defining the origins of Ras/p53-mediated squamous cell carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7425–7430. doi: 10.1073/pnas.1012670108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Li W, Shang C, Chen RM, Han P, Yang J, et al. Brg1 governs a positive feedback circuit in the hair follicle for tissue regeneration and repair. Developmental Cell. 2013;25:169–181. doi: 10.1016/j.devcel.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Yi R, O’Carroll D, Pasolli HA, Zhang Z, Dietrich FS, Tarakhovsky A, et al. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nature Genetics. 2006;38:356–362. doi: 10.1038/ng1744. [DOI] [PubMed] [Google Scholar]
- Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature. 2008;452:225–229. doi: 10.1038/nature06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef KK, Van Keymeulen A, Lapouge G, Beck B, Michaux C, Achouri Y, et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nature Cell Biology. 2010;12:299–305. doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell. 2009;5:267–278. doi: 10.1016/j.stem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Stokes N, Polak L, Fuchs E. Specific microRNAs are preferentially expressed by skin stem cells to balance self-renewal and early lineage commitment. Cell Stem Cell. 2011;8:294–308. doi: 10.1016/j.stem.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]