Abstract
Brain tumor incidence shows an upward trend in recent years; brain tumors account for 5% of adult tumors, while in children, this figure has increased to 70%. Moreover, 20%–30% of malignant tumors will eventually metastasize into the brain. Both benign and malignant tumors can cause an increase in intracranial pressure and brain tissue compression, leading to central nervous system (CNS) damage which endangers the patients' lives. Despite the many approaches to treating brain tumors and the progress that has been made, only modest gains in survival time of brain tumor patients have been achieved. At present, chemotherapy is the treatment of choice for many cancers, but the special structure of the blood–brain barrier (BBB) limits most chemotherapeutic agents from passing through the BBB and penetrating into tumors in the brain. The BBB microenvironment contains numerous cell types, including endothelial cells, astrocytes, peripheral cells and microglia, and extracellular matrix (ECM). Many chemical components of natural products are reported to regulate the BBB microenvironment near brain tumors and assist in their treatment. This review focuses on the composition and function of the BBB microenvironment under both physiological and pathological conditions, and the current research progress in regulating the BBB microenvironment by natural products to promote the treatment of brain tumors.
KEY WORDS: Blood--brain barrier, Microenvironment, Natural products, Brain tumors, Glioma, Extracellular matrix
Graphical abstract
The blood–brain barrier (BBB) microenvironment is closely related to the occurrence and development of glioma. Natural products are of great importance for remodeling the BBB microenvironment and can be effectively used in assisting the treatment of brain tumors.
1. Introduction
Brain and central nervous system (CNS) tumors are the most common neoplasm among those 0–19 years old, with an average annual age-adjusted incidence rate of 5.42 per 100,0001. The annual incidence of adult glioblastoma is 7.2 per 100,000, making it the most common adult primary intrinsic brain tumor2. The BBB excludes drugs from entering the brain, due to the special structure of the microvasculature. Despite many methods of surgical resection, radiotherapy, and chemotherapy for treatment, outcomes have remained dismal3. Recently, remodeling the tumor microenvironment has become a promising way of enhancing tumor therapy for various advanced cancers; however, research on the effects of BBB microenvironment on brain tumor therapy is still in its infancy. The BBB microenvironment includes endothelial cells, astrocytes, peripheral cells, microglia, and extracellular matrix (ECM). These cells also play a key role in controlling the formation and morphology of brain tumors. Regulation of the BBB microenvironment by natural products shows increasing potential for assisting in the treatment of brain tumors. Natural products affect a number of factors secreted by some tumor-associated cells and influence tumor biology. In this review, we describe the composition and function of the BBB microenvironment and highlight the effects of natural products on regulating the brain tumor BBB microenvironment to treat brain tumors. The signaling pathways, factors, interactions described in this review provide a perspective on regulating the BBB microenvironment for brain tumor therapy.
2. The composition and function of the BBB microenvironment
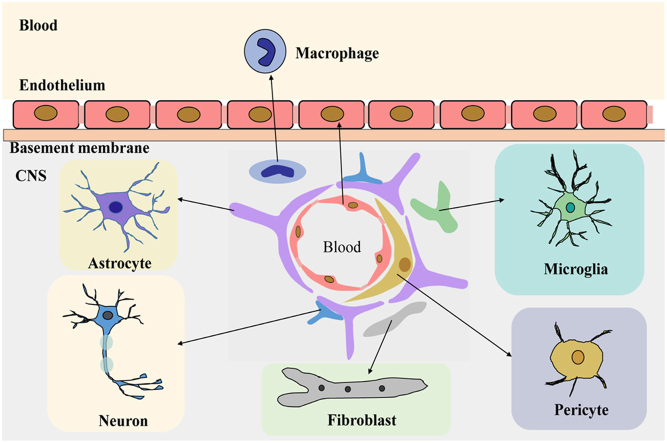
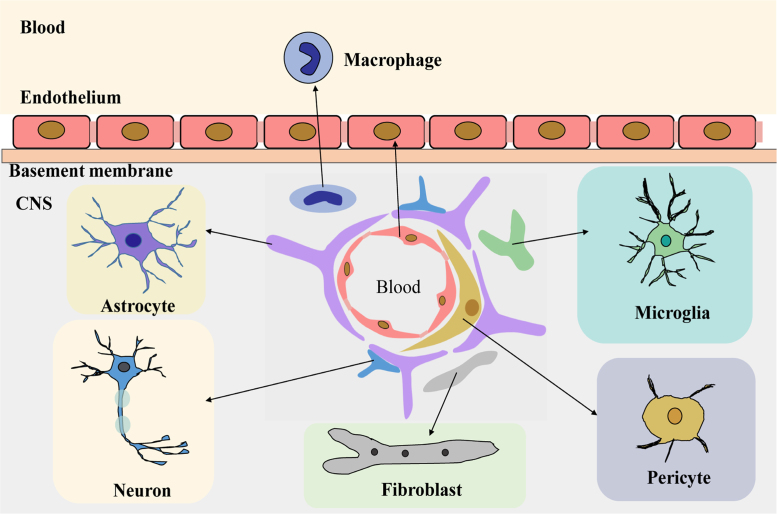
The BBB is an important structure which maintains the balance of the CNS microenvironment and maintains the normal functioning of the brain4. The BBB microenvironment (Fig. 1) is constituted of endothelial cells, astrocytes, pericytes, macrophage, fibroblasts, neuronal cells, basal membranes, microglia, and other cell types5. In addition, there are many transporters on the BBB, including P-glycoprotein (P-gp) and other multidrug resistance-related proteins6., 7., which can reduce the apparent distribution of drugs in the brain.
Figure 1.
The composition of the blood–brain barrier microenvironment.
Astrocytes are involved in nerve signal transmission, nutrient transport, maintaining the balance of brain microenvironment and ECM ion balance buffering. Astrocytes also participate in the inflammatory response of the CNS8., 9. and maintain the integrity of the BBB10.
Peripheral cells are multifunctional cells, with immune function in the CNS neurovascular unit11. Peripheral cells surround the endothelial cells and play an important role in the BBB microenvironment and in maintaining the BBB function by secreting growth factors and ECM.
Microglia are a kind of long-standing immune cell in the human brain. They can stimulate the opening of BBB, leukocyte extravasation, and angiogenesis12. Fibroblasts, when co-cultured with glioblastoma cells, can induce production and activation of matrix metalloproteinase MMP2, and its activators membrane type 1 metalloprotease (MT1-MMP) and MT2-MMP13, which affect the growth progression of gliomas14.
Other cells like endothelial cells in the BBB microenvironment maintain the normal function and integrity of the BBB by forming tight junctions that limit transcytosis4. CNS neurons bind chemicals and convey electrical signals. They can regulate the ionic microenvironment of the synaptic and axonal regions of the nerve cell, which are essential to the nerve signal transduction15. The basement membrane is attached as a support tissue to the neurovascular unit cells, forming a substrate for cellular differentiation and gene expression9.
3. The formation, morphology and classification of brain tumors
Benign tumors are nodular, lobulated or cystic with a clear border and often with an envelope. Brain tumors are usually solitary. Under electron microscopy, the orthotopic brain gliomas have mitochondria with dense matrix cohesive structures, indicating an active state. The mitochondria extend and tightly connect to the endoplasmic reticulum16. Fibrous astrocytomas show vascular abnormalities, including fibrosis, hyaline degeneration and angiogenesis under optical microscopy. There are fenestra and vesicles among capillary endothelial cells17.
The formation of brain metastases can be divided into several consecutive processes, including epithelial-mesenchymal transition, escape from the primary tumor, entry into the vascular circulation system, survival in the blood, interaction with cerebral vascular endothelial cells through the BBB extravasation, the formation of metastatic tumor sites, and colonization in the brain18. An important condition for colonization is the formation of a metastatic niche, which is facilitated by angiogenesis stimulated by the interaction between tumor cells and endothelial cells. Tumor cells and surrounding inflammatory cells infiltrating into tumor tissue are able to produce a class of angiogenic factors, such as vascular endothelial growth factor (VEGF). These factors can stimulate endothelial cell division, proliferation, and migration, promote vascular basement membrane degradation, induction of host capillary germination and increase capillary permeability.
The most common brain tumor is glioma, which is derived from glial cells, including glioblastoma multiforme (GBM), astrocytoma, oligodendrocyte glioma and ependymona. GBM is a kind of highly invasive primary brain tumor. Its malignant cells usually infiltrate into surrounding tissue and destroy cerebral blood flow through a variety of biomechanical and biochemical mechanisms19. It is of poor prognosis with high recurrence rate, and survival time general less than two years20., 21.. It is a highly aggressive tumor with a large number of neovessels, necrosis and intense resistance22. In addition, microglias and macrophages are present in polymorphic glioblastomas, promoting tumor growth23. Astrocytoma is the most common glioma, accounting for 70% to 80% of gliomas. It can grow anywhere in the brain or spinal cord.
4. The glioma microenvironment
The tumor microenvironment is a dynamic network, including tumor cells, surrounding cells, and ECM and interstitial tissue, and it is a key factor affecting tumor metastasis24. The glioma microenvironment includes glioma-associated microglia and macrophages which are the most abundant immune cells in the tumor. The glioma microenvironment is a potential therapeutic target20., 25.. In addition, primary tumors have a strong host response at the beginning and contain a large number of immune cells, such as microglia and tumor-infiltrating macrophages. The process of tumor pathology not only is controlled by the gene composition, but also depends on the interaction with immune cells in the tumor microenvironment. It is well known that malignant tumors are immunosuppressive diseases, in which malignant tumor cells can control immune cells to support their growth and glioma-associated immune cells can promote cancer development by supporting angiogenesis and promoting tumor cell invasion, proliferation and survival26. The composition of brain tumors' BBB microenvironment is the same as the normal BBB microenvironment. While tumor blood vessels are highly irregular, the distance between the blood vessels is much greater than in the normal brain27.
4.1. Cell components
4.1.1. Microglia promote tumor growth in the progression of gliomas
Microglia, accounting for up to 30% of the weight of a brain tumor, are an important component of the microenvironment28. Microglia infiltrate most of the gliomas. They release cytokines, leading to degradation of the ECM and stimulation of the signaling pathways that promote glioma cell invasion29. The complex microenvironment of malignant gliomas is dynamic, and plays an important role in promoting tumor growth in the progression of gliomas. Kees et al.30 demonstrated that small glial cells isolated from tumor cells indeed supported the growth, migration, and invasion of tumor cells. The impact of microglia on glioma migration might relate to the production of MT1-MMP that is produced by microglia in response to soluble factors released from glioma cells. Microglia cells produce abundant pro-MMP-2, which can be quickly converted into matrix metalloprotease 2 (MMP-2) by MT1-MMP. Glioma cells also release MMP-2 that is promoted by MT1-MMP released from microglia31. Besides, microglia can promote the proliferation of glioma cells by increasing the activity of MMP-2 before invasion. In the glioma microenvironment, the morphology of microglia changes from a branch-like form into an amoebiform. The motility of microglia around tumor blood vessels is high, indicating an interaction between microglia and tumor blood vessels32.
4.1.2. Astrocytes activate the glioma microenvironment
Astrocytes, which are the main stromal cells in the brain, can activate the glioma microenvironment, leading to the generation of a layer of activated astrocytes around the glioma. Activated astrocytes are an essential part of the glioma microenvironment33. It has been demonstrated that glioma cells activate astrocytes with upregulation of WNT/β-catenin signaling that can increase migration and invasion capabilities of glioma cells. In addition, glioma cells can stimulate adjacent astrocytes to degrade ECM and to promote tumor invasion34. Non-neoplastic astrocytes can be transformed in an active glioma microenvironment, and then secrete factors that affect the biological characteristics of the tumor28. Tumor cells located in the microenvironment often interact with astrocytes to promote tumor growth by secreting stromal cell-derived factor35, and astrocyte elevated gene36, both of which are overexpressed in human brain tumors37., 38..
Astrocytes also play an important role in the process of tumor metastasis. By in vitro experiments it has been confirmed that astrocyte-derived molecules have a tumorigenic effect39. An immunohistochemical study of human glioma biopsies revealed that astrocytes were located around gliomas40. Neurological disorders are often accompanied with astrocyte hypertrophy and aggregation, and an increase in the number of astrocytes41., 42.. The glioma can restore the proliferation properties of astrocytes. For example, under pathological conditions activated astrocytes can proliferate43 and astrocytes around the tumor, instead of removing glutamate with neurotoxicity, will respond to glioma cell signaling and release glutamate44. Gliomas can use astrocytes to secrete MMP and to enhance glioma cell invasion through soluble cascades45., 46., 47.. Astrocytes can also produce and release pro-MMP-2, which can be activated by cellular MT1-MMP.
4.1.3. Macrophages promote tumor growth with poor prognostics
Macrophages are also an important part of the glioma microenvironment. Tumor-associated macrophages have been proven to promote tumor growth with poor prognostics48. According to the homing characteristics of macrophages, they can pass through the endothelial barrier to the brain tumor site by secreting cytokines in the diseased tissue49. Macrophages can be a potential target for brain tumor therapy. A study of intracranial injection of GL261 cells into Fas-induced macrophage-depleted transgenic mice showed that the mice had a lower tumor mitotic index, lower microvessel density, and slower growth of the tumor50. Macrophages are predominantly classified as type M1, classically activated macrophage, and M2, alternatively activated macrophage. M1 macrophages play a role in immune surveillance by secreting pro-inflammatory cytokines and chemokines, and full-time presentation of antigens to participate in positive immune responses. M2 type macrophages have only weak antigen presenting ability and act in the immune regulation by the secretion of inhibitory cytokines interleukin-10 and/or tumor growth factor β (TGF-β).
4.1.4. Endothelial cells enhance glioblastoma stem cell proliferation and migration
Endothelial cells can secrete interleukin-8 to enhance glioblastoma stem cell proliferation and migration51. Endothelial cells can emit physical signals by directly contacting glioma cells, and communication by paracrine signals52. Normal endothelial cells maintain the integrity and normal function of the BBB. The endothelial cell is the most important structural component of the BBB, and changes in the phosphorylation state of the tight junction protein (ZO-1 or occluding) are critical to the control of BBB vascular permeability53. In areas of bulky tumors, the connection of endothelial cells is very loose and almost lacks integrity. In the invasive peripheral regions of a tumor, the close connection of endothelial cells is slightly leaky, while in the sparsely invaded regions distant from the tumor bulk, the connection of endothelial cells is very tight54.
Other cell components like peripheral cells are essential for the formation of microenvironment and survival of tumor-associated epithelial cells, promoting the growth of malignant tumors55. Brain tumors can cause detachment of peripheral cells and astrocytes, and dynamic sensory pressure of pericardial cell11. Neurons surrounding gliomas in rats have a shrunken dendritic arbor and severe functional changes such as increased spontaneous activity and decreased visual response.56 As the glioma worsens, neurons are slowly depleted57.
4.2. ECM
ECM includes fibronectin, tumor growth factor (TGF) chemokines, laminin and hyaluronic acid, among which fibronectin, laminin and hyaluronic acid can promote the adhesion and migration of glioma cells58., 59.. Chemokines in glioma cells, such as chemoattractant protein families, interleukin families, EGF, TGF and tenascin, can stimulate signal transduction of malignant tumors through their receptors60. Fibronectin regulates cell adhesion, growth, metastasis, proliferation and wound healing61. Interleukin and EGF are found overexpressed in gliomas62., 63., 64..
4.2.1. Fibronectin
Fibronectin is an ECM protein that has important physiological and pathologic function in development and adulthood. Fibronectins subtypes, extra domain-A (EDA) and extra domain-B (EDB), are important markers of angiogenesis and play a vital role in the development of tumor cells. EDA is 100% homologous between human and mouse, while EDB is identical between human and mouse65. EDA plays an important role in a variety of processes, including cell adhesion66, myofibroblast differentiation67, cell cycle, and mitotic signal transduction68. EDB knockout will lead to cell growth delays69. EDB can regulate the expression of VEGF, endothelial cell proliferation, and tubule formation70. According to solubility and tissue distribution, fibronectin has two main forms: one is circulating blood plasma fibronectin, and the other is cell fibronectin. Both can be aggregated into the ECM of connective tissues71.
4.2.2. Lactic acid
Healthy cells rely on mitochondrial oxidation of carbohydrate molecules to release ATP. The fast growth of tumor tissue leads to insufficient oxygen supply, transforming the energy source of tumor cells from an aerobic oxidation pathway to an anaerobic one. Anaerobic oxidation produces a large amount of lactic acid released into the extracellular matrix, which is responsible for the formation of an extracellular acidic microenvironment72.
4.2.3. Fatty acids
Tumor cells can convert nutrients to acetyl-CoA under the action of tumor genetic signals. Acetyl-CoA forms fatty acids through biochemical reactions under the action of the acetyl-CoA carboxylase and fatty acid synthetase, and fatty acids can directly or indirectly promote the proliferation and survival of tumor cells73.
4.2.4. Tenascin
Tenascin is an ECM protein that is ubiquitously expressed in gliomas, particularly in glioblastomas63. It participates in CNS development74 and contributes to tumor cell adhesion, invasion, migration and proliferation75., 76..
4.2.5. Vasoactive peptides
Vasoactive substances in brain tumors are more likely to be activated than in normal brain77. Vasoactive peptides can increase the penetration of the drug to the brain tumor site, and they include leukotriene and bradykinin78.
4.2.6. Others
Another ECM, EGF, is the earliest discovered growth factor, and plays an important role in regulating cell growth, proliferation and differentiation. It can stimulate thymus regeneration, accelerate lymphoid T cell-, B cell-, and phagocytic cell-formation, improve immune function and phagocytosis of cancer cells, and thus treat cancer and tumors. The human EGF receptor 3 is a target for anti-tumor therapy. Interleukin receptors are overexpressed in malignant gliomas. Targeting interleukin receptors can promote tumor cell death via elongation factor 2 inhibition64. TGF-β is a protein that regulates various aspects in tumor growth and survival64. It can inhibit epithelial and endothelial cell growth, promote ECM expression, such as collagen and fibronectin, and inhibit ECM degradation.
4.3. The correlation between glioma and BBB in the occurrence and development of glioma
The BBB microenvironment is composed of endothelial cells, astrocytes, microglia, pericytes, as well as the ECM, and various chemokines that are secreted in the brain. In the occurrence and development of glioma, functionality changes of the BBB and supporting cells lead to BBB dysfunction. The compromised BBB allows an influx of inflammatory cytokines, including TNF-α, TGF-β and interleukins, to enter the brain. Chemokines can recruit immune cells (astrocytes, macrophages, etc.) from the blood or from within the brain to secrete MMP-2 and MMP-9 that alter BBB permeability. The tight junction protein occludin is vulnerable to attack by MMPs, which seems to have implications in glioma. In addition, in the occurrence of glioma, the brain tumor capillaries form a blood–brain tumor barrier (BBTB) that is variably distinct from the BBB and includes existing and newly formed blood vessels that contribute to the delivery of oxygen and nutrients to the tumor and facilitate glioma cell migration to other parts of the brain54. In low-grade gliomas, the normal vascularization and the function of the BBTB remains mostly intact and resembles the BBB as under normal conditions79. However, in high-grade gliomas, BBTB is characterized by major alterations of normal vascular function resulting in a disrupted, ‘leaky’ BBTB, which may allow more inflammatory cytokines to enter the brain80. The brain endothelial cell is highly reactive because it serves as both a source of, and a target for, reactive oxygen species (ROS) and inflammatory proteins. Astrocyte end-feet cover over 99% of cerebral capillaries81, leading to critical cell–cell interactions that directly regulate BBB characteristics and are fundamental for homeostasis, defense, and regeneration of the CNS82. The physical dislocation of astrocytes from the blood vessels and subsequent encasing of vessels by gliomas disrupts astrocyte–vascular coupling and will result in loss of astrocytes function. In addition, aberrant expression of chemokines, like IL-1, by glioma cells can activate astrocytes83.
Microglia, the primary immune cells of the brain, are distributed in the CNS and are activated in response to systemic inflammation and several CNS pathophysiologies84. An activated microglia-associated tumor microenvironment will lead to glioma progression. Activated microglia are associated with not only altered tight junctions protein expression and increased BBB permeability, but also produce high levels of neurotoxic and proinflammatory mediators, such as nitric oxide (NO) and TNF-α, all of which result in cell injury and neuronal death85., 86.. Neuroinflammation and oxidative stress lead to BBB breakdown, which are implicated in the pathogenesis of CNS disease87.
Pericyte activation mainly results from the widespread parenchymal diffusion of factors produced locally by the glioma, from glioma-derived factors such as exosomes delivered by the systemic circulation88, or instead, from the action of elevated intracranial pressure resulting from hypoxia. Svensson et al.89, showed that pericytes become activated in most areas of the brain in response to GL261 mouse gliomas. Non-tumor-derived pericytes infiltrate the glioma extensively and integrate with the vasculature. Montagne et al.83 showed that pericyte injury and possibly early degeneration correlate with increased BBB permeability within the hippocampus, a region known to be affected by pericyte loss and BBB breakdown on post-mortem tissue analysis.
5. Natural products regulating the BBB microenvironment
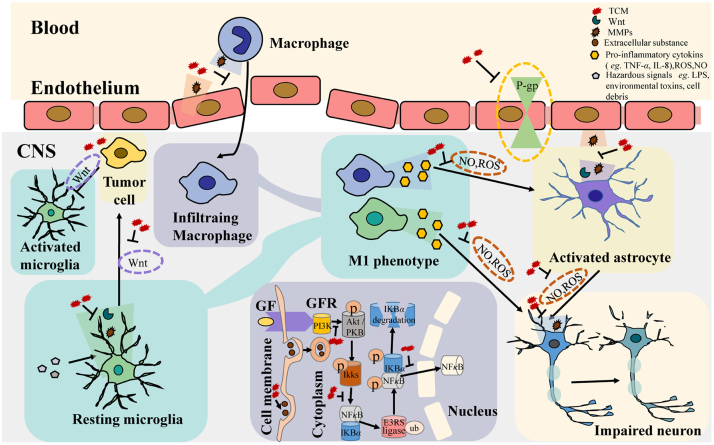
Surgical treatment, chemotherapy, and radiation therapy are common treatments for brain tumors. Surgical treatment is generally used for benign tumors with high survival rates. Chemotherapy has made great progress, but due to the barrier function of BBB, brain tumor chemotherapy is still subject to many restrictions. Chemotherapeutics can enter into tumor cells only after passing the vascular endothelium and is dependent upon their lipophilicity. This process reduces the speed and efficiency of drug action. Regulation of the BBB microenvironment by natural products shows increasing prospect for assisting in the treatment of brain tumors. Natural products can regulate the BBB microenvironment by acting on P-gp, endocytosis, and MMPs, etc. (Fig. 2). In the BBB microenvironment, MMPs can be secreted by macrophages, endothelium, astrocytes, microglias, and neurons. Endocytosis is a common process for transporting extracellular material into the cells, which happens in endothelial cells90, astrocytes91, macrophage92, pericytes93, fibroblasts94, neuronal cells95 and microglia96. P-gp is expressed on endothelial cells and functions as a transporter in the BBB. Generation of NO and ROS is a feature of genuine immune system cells as well as other cells involved in immune reactions. The WNT/β-catenin signaling pathway not only is involved in many biological processes of other components of BBB microenvironment but also contains oncogenes and tumor suppressor genes, which are closely related to the occurrence of tumors.
Figure 2.
Natural products regulate the blood–brain barrier microenvironment by (1) downregulating MMPs; (2) inhibiting P-gp; (3) reducing NO and ROS production; (4) promoting endocytosis; (5) downregulating the WNT pathway; and (6) regulating the PI3K/Akt/BDNF/TRKB-ERK/NF-κB signaling pathway.
5.1. Modulating MMP-2 and MMP-9
MMP-2 and MMP-9 belong to a family of MMPs which are secreted by endothelial cells, astrocytes, pericytes, fibroblasts, neuronal cells, and microglia and function to dynamically balance the degradation and remodeling of ECM. In addition, MMP-9 binds to CD44 to release stored TGF-β1, and may be involved in angiogenesis by releasing VEGF.
5.1.1. Shikonin
Shikonin, extracted from lithospermum, is an active derivative of anthraquinone. Zhang et al.97 showed that shikonin inhibited the migration and invasion of glioma cells by inhibiting the expression of MMP-2 and MMP-9, which are secreted by microglia and can promote the proliferation and invasion of glioma98. Glial-to-mesenchymal transition proteins play a prominent role in tumor invasion, while shikonin can reduce the expression of these proteins and is a naphthoquinone with anti-tumor properties. Matias et al.99 showed that a combination of shikonin and temozolomide can decrease glioblastomas-derived cells in their proliferation and migration capacities. These decreases were followed by the suppression of glial-to-mesenchymal transition through a reduction in expression of MMP-2 and MMP-9 via inactivation of PI3K/AKT signaling. Wang et al.100 showed that shikonin can inhibit the over-expression of MMP-9 and effectively protected brain by regulating inflammatory responses and maintaining BBB permeability. Shikonin can inhibit the MMPs generated by both BBB microenvironment components and tumor-derived cells.
5.1.2. Osthole
Osthole is an active substance extracted from the common cnidium fruit which is an umbelliferous plant. Osthole can inhibit proliferation and promote the apoptosis of U87 cells by regulating the expression of miR16 and inhibiting the expression of MMP-9101. Duan et al.102 showed that osthole can inhibit inflammatory reactions, reduce MMP-2 activity and activate mitogen-activated protein kinase (MAPK) cascades. In addition, several studies showed that osthole can regulate MMP-2 and MMP-9 in the A549 human lung cancer cells and breast cancer cells103., 104., 105.. It can also regulate MMPs secreted by myocardial cells, vascular endothelial cells, and smooth muscle cells106.
5.1.3. Resveratrol
Resveratrol is a polyphenolic antioxidant found in peanuts, grapes, and red wines. Despite the low bioavailability of resveratrol, its antitumor effect has been demonstrated both in cell experiments in vitro and murine models107., 108., 109.. The invasion and metastasis of glioblasts in early glioblastoma are highly related to glioblastoma progression and recurrence. Resveratrol can reduce glioblast invasion by inhibiting PI3K/Akt/NF-κB signaling and decreasing MMP-2 expression109. Hao et al.110 showed that resveratrol can inhibit proliferation and migration of human retinal pigment epithelial cells in a concentration-dependent manner with downregulation of MMP-9. Jiao et al.111 proved that resveratrol can inhibit the invasion of glioblastoma-initiating cells via the inhibition of PI3K/Akt/NF-κB signal transduction and the suppression of MMP-2 expression. Wei et al.112 showed that resveratrol can attenuate the BBB dysfunction via regulation of MMP-9 to maintain the integrity of BBB. Resveratrol can not only regulate MMP, but also inhibit PI3K/Akt/NF-κB signal.
5.1.4. (+)-Aeroplysinin-1
Aeroplysinin-1 is extracted from sponge Aplisina aerophoba and inhibits differentiation and proliferation of epithelial cells. Aeroplysinin-1 can also decrease gene expression of MMP-2, and inhibit the migration and invasion of glioma cells113. In previously published work describing aeroplysinin-1 as a potent anti-angiogenic compound, two molecular targets for its effects, namely, MMP-2 and urokinase were identified114. Martínez-Poveda et al.115 confirmed MMP-2 as a molecular target of aeroplysinin-1, which can inhibit the expression of MMP-2.
5.1.5. Oligomeric procyanidins
Oligomer procyanidins, natural components from grape seeds, have been shown to have an inhibitory effect on VEGF and MMP-2. It can also reduce tumor invasion through an HIF-1α-dependent way116.
5.2. Regulating PI3K/AKT/BDNF/TRKB-ERK/NF-κB signaling pathway
Brain-derived neurotropic factor (BDNF), a member of the neurotropic family of growth factors, plays an important role in the development, differentiation and maintenance of neurons. BDNF can promote neuronal cell survival in a concentration-dependent manner. Tropomyosin-related kinase B (TRKB) is a receptor protein involved in the development and maturation of the CNS117. BDNF has a high affinity for TRKB and was demonstrated to promote the protein expression of TRKB118. Members of the TRKB downstream signaling cascade, including PI3K/PKB and ERK/MAPK, have been reported to be responsive to BDNF119., 120.. Several studies have assumed that BDNF largely activates the ERK/MAPK pathway121., 122., 123.. In comparison, other studies have reported that activation of the ERK/MAPK pathway leads to cell death and PI3K/PKB is the main pathway involved in the protection of neurons induced by BDNF124. Both pathways are critical for neuroprotection induced by BDNF. Nuclear factor-κB (NF-κB), a transcription factor usually activated by inflammatory stimuli and cellular stresses, has an important role in regulating the survival and growth of the cell. NF-E2-related factor-2 (NRF2), a transcription factor, mediates cytoprotective antioxidant responses and thus prevents cells from the damage induced by ROS and other harmful chemicals in various types of cancers125. The serine/threonine kinase AKT is a downstream effector of PI3K and is involved in cell survival and anti-apoptotic signaling126. Recently, a study demonstrated that cell signaling is mediated by PI3K/Akt via induction of the NF-κB, which is associated with cell migration, proliferation and angiogenesis127. NF-κB provides a mechanistic link between cancer and inflammation and might also regulate tumor angiogenesis and invasiveness128.
5.2.1. Tenuifoliside A
Tenuifoliside A is an active ingredient extracted from Polygala tenuifolia. Brain glioma-derived factors can improve the migration and proliferation of microglia by promoting overexpression of focal adhesion kinase and activating the PI3K/Akt pathway, thus promoting microglia accumulation in the region of the tumors. The signal from the brain glioma cells can activate the ERK/p38MAPK pathway, inhibit STAT1 and NF-κB signaling, and cause microglia immunodeficiency129. Dong et al.130 found that tenuifoliside A can increase the phosphorylation of ERK and AKT by blocking the signaling pathway of ERK and PI3K and increase the release of BDNF. The results showed that tenuifoliside A provided a neuroprotective effect on C6 glioma cells by regulating the BDNF/TRKB-ERK/PI3K-CREB signaling pathway.
5.2.2. Betulinic acid
Betulinic acid is a pentacyclic triterpenoid extracted from white birch. It can directly target mitochondria and reduce the mitochondrial membrane potential131. In addition, betulinic acid can also inhibit the NF-κB signaling pathway to suppress inflammation and modulate the immune response in glioma132. Clinical studies demonstrated overexpression of NF-κB as well as downregulation of the P53 network in metastatic tumors. Shankar et al.133 showed that it induces apoptosis by stabilizing P53 and downregulating NF-κB pathway in human prostate cancer cells. Su et al.134 showed that betulinic acid can suppress the expression of c-Myc, cyclin-D1, BCL-xL and the downstream gene targets of NF-κB and STAT3.
5.2.3. Withania somnifera
Withania somnifera is also known as Indian ginseng. Withania somnifera extract can inhibit microglia invasion by decreasing the production of inflammatory cytokines such as NF-κB and activator protein 1, and reduces TNF-α/ROS production, downregulating MMPs135. Sun et al.136 showed that Withania somnifera played a role in immunomodulatory effects. It suppressed oxidative and inflammatory responses in microglial cells by downregulating the NF-κB and upregulating the NRF2 pathways at the same time. Kataria et al.137 proved that Withania somnifera reduced the intracranial tumor volumes in vivo and suppressed the tumor-promoting proteins NF-κB, p-AKT, VEGF, heat shock protein 70, polysialylated form of neural cell adhesion molecule (PSA-NCAM), and cyclin D1 in the rat model of orthotopic glioma allograft.
5.3. Inhibiting P-gp or pinocytosis
P-gp, an ATP-dependent drug efflux pump for xenobiotic compounds with broad substrate specificity, is expressed on endothelial cells. It is responsible for decreasing drug accumulation in multidrug-resistant cells and often mediates the development of resistance to anticancer drugs. P-gp also functions as a transporter in the blood–brain barrier. Pinocytosis, known as fluid endocytosis and bulk-phase pinocytosis, is a mode of endocytosis in which small particles are brought into the cell, forming an invagination, and then suspended within small vesicles. Inhibition of P-gp function or facilitating pinocytosis may enhance chemotherapeutics uptake and help in glioma therapy.
5.3.1. Procyanidine
Procyanidine is isolated from the bark of Pinusmassoniana. Procyanidine is extensively studied for the effective inhibition of P-gp on the BBB, which can increase the accumulation of some drugs in the brain138. It significantly increases the accumulation of rhodamine 123 in cells by inhibiting its efflux in a dose-dependent manner139. Zhao et al.140 showed that procyanidine reverses P-gp-associated multi-drug resistance by inhibiting the function and expression of P-gp through downregulation of NF-κB activity.
5.3.2. Scillarenin
Scillarenin is an active ingredient extracted from the scilla. Scillarenin can inhibit P-gp activity in the BBB endothelial cells, improving the efficacy of tumor therapy141.
5.3.3. Kainic acid
Kainic acid can change the microenvironment around the blood vessels by increasing the pinocytosis of endothelial cells and opening the BBB142.
5.4. Inhibiting WNT/β-catenin
The WNT/β-catenin signaling pathway itself contains an oncogene and a tumor suppressor gene, which are closely related to the occurrence of tumor. Activating WNT/β-catenin signaling pathway will accelerate tumor growth. Lin et al.143 proved that silencing of β-catenin in mesangial cells in vitro using siRNA decreased the mRNA expression levels of the pro-apoptotic proteins (cleaved caspase-3 and BAX) and the fibrosis-associated proteins (fibronectin, and collagens I/III/IV). In addition, β-catenin silencing attenuated TNF-α-induced apoptosis in mesangial cells. Many investigations suggested that WNT/β-catenin signaling is importantly involved in pathogenesis of fibrotic diseases144. For instance, the expression of β-catenin is significantly increased in keloid tissues, and the stimulation of fibroblasts by TGF-β results in upregulation of WNT/β-catenin signaling145.
5.4.1. Rhodiola crenulata
Rhodiola crenulata, a well-known medicinal Tibetan herb, is mainly grown in high-altitude regions of Tibet, Sichuan and Yunnan in China. Recently, increasing numbers of studies have been published on the potential pharmacological activities of Rhodiola crenulate146. Rhodiola crenulata extracts can effectively suppress proliferation, stimulate differentiation, and eliminate tumor sphere formation of GBM cells in vitro. The mechanism may be related to the inhibition of WNT/β-catenin signaling pathway147. Bassa et al.148 showed that Rhodiola crenulata can reduce the transcriptional activity of β-catenin and positive responses of estrogen receptor, leading to reduced proliferation and tumor sphere formation.
5.4.2. Trichosanthin
Trichosanthin is an active ingredient extracted from the roots of melon. Trichosanthin is also a plant toxin belonging to the family of ribosome-inactivating proteins. It has various biological and pharmacological activities, including anti-tumor and immunoregulatory effects149. It has dose-dependent inhibitory effect on the invasion, migration and proliferation of human glioma cells and can induce tumor cell apoptosis by the inhibition of WNT/β-catenin signal pathways150.
On one hand, activated WNT/β-catenin signaling pathways directly facilitate the invasion, migration and proliferation of human glioma cells, and induce tumor cell apoptosis. On the other hand, the pathways decrease the levels of fibrosis-associated proteins (fibronectin and collagens I/III/IV) by the inhibition of WNT/β-catenin signal pathways. These will facilitate the treatment of glioma.
5.5. Adjusting the NO level
As is known, NO is an endothelium-derived relaxing factor and contributes to vessel homeostasis by inhibiting contraction and growth of vascular smooth muscle, platelet aggregation, and leukocyte adhesion to the endothelium. In addition, NO has been recognized as one of the most versatile players in the immune system. It is involved in the pathogenesis and control of tumors, autoimmune processes and chronic degenerative diseases. The role of NO in the immune system is simply defined. NO is a product of macrophages activated by microbial compounds, cytokines, or both. It is derived from the amino acid l-arginine by the enzymatic activity of inducible nitric oxide synthase (iNOS or NOS2) and functions as a tumoricidal molecule in vitro and in vivo151. Generation of NO is a feature of genuine immune system cells (dendritic cells, NK cells, mast cells and phagocytic cells including monocytes, macrophages, microglia, Kupffer cells, eosinophils, and neutrophils) as well as other cells involved in immune reactions (such as endothelial cells, epithelial cells, vascular smooth muscle cells and fibroblasts)152.
5.5.1. Salicin
Salicin is an active substance isolated from the twigs of Salix glandulosa Seemen. It was shown that salicin can inhibit the production of NO due to the carboxyl structure of C-7 and the acetyl structure of C-3. It can also increase the production of nerve growth factor in C6 glioma cells153., 154.. Kong et al.155 showed that salicin can reduce ROS production and activation of the extracellular signal-regulated kinase pathway.
5.5.2. Lignan glycosides
Lignan glycosides are isolated from the roots of Wasabia japonica. They can induce neuroprotective effects by stimulating the nerve growth factor in C6 glioma cells. It can stimulate lipopolysaccharide level in BV2 cells to affect NO levels156., 157..
5.6. Effects on oxidative stress
Oxidative stress is a state of BBB microenvironment under interaction with glioma, and is defined by an imbalance between increased levels of ROS and a low activity of antioxidant mechanisms. An increased oxidative stress state can induce damage to the cellular structure and potentially destroy tissues. Oxidative stress can activate a variety of transcription factors, including NF-κB, P53, AP-1, β-catenin/WNT, and NRF2. The activation of these transcription factors leads to the expression of over 500 different genes, including those for growth factors, chemokines, inflammatory cytokines, cell cycle regulatory molecules, and anti-inflammatory molecules158. Recently, considerable evidence has demonstrated that ROS are involved in the relationship between cancer and chronic inflammation159., 160.. Indeed, an important characteristic of tumor promoters is their ability to recruit inflammatory cells and to stimulate them to generate ROS161. Reuter et al.158 clearly demonstrated the role of ROS state in different phases of tumorigenesis, such as cellular transformation, proliferation, invasion, angiogenesis, promotion, survival and metastasis.
5.6.1. β-Caryophyllene
β-Caryophyllene can reduce neuronal damage by reducing oxidative stress injury and inflammatory cytokine-induced BBB damage162, maintaining BBB integrity. Park et al.163 found that β-caryophyllene oxide not only inhibited the activation of the PI3K/AKT/mTOR/S6K1 signaling cascade, but also caused the activation of ERK, JNK, and P38 MAPK in tumor cells. β-Caryophyllene oxide induces increased ROS generation from mitochondria, which is associated with the induction of apoptosis.
5.6.2. Curcumin
Curcumin is the main active ingredient isolated from food spices. Under a hypoxic state it can increase the permeability of BBB by upregulating the expression of heme oxygenase-1 (HO-1) in microvascular endothelial cells164. In addition, Xiao et al.165 showed that curcumin can protect neurons from TNF-α-triggered excessive ROS production and cellular apoptosis. Correspondingly, it promoted mRNA expression of anti-oxidative enzymes like HO-1, catalase and superoxide dismutase-2. Santos-Parker et al.166 showed that curcumin improved resistance artery endothelial function by increasing vascular NO bioavailability and reducing oxidative stress, as well as conduit artery endothelial function.
5.6.3. Shizukahenriol
Shizukahenriol is a natural product isolated from the Chloranthus henryi Hemsl. It activates NRF2, which inhibits oxidative stress. In addition, it can induce the expression of NRF2-related antioxidant HO-1, glutamate-cysteine ligase, which consists of both the modifier and catalytic subunits in BV-2 microglial cells. This natural product can also inhibit the production of inflammatory molecules, such as NO and TNF-α, and inhibit translocation of NF-κB and P65 to the nucleus167.
6. Outlook
Despite the progress that has been made by chemotherapy, we are still far from desired therapeutic effects due to the special structure of the BBB. Regulation of BBB microenvironment will play an important role in assisting the treatment of brain tumors. Many studies have used natural products to regulate the BBB microenvironment, most of which focused on regulating oxidative stress, MMPs, WNT/β-catenin and PI3K/AKT/BDNF/TRKB-ERK/NF-κB pathways (Table 1). In fact, there are many other factors that can be affected by natural products in the BBB microenvironment. Further encapsulation of natural products into nanoparticles has been proven to be effective for in vivo studies168. Microglia and macrophages in the tumor microenvironment are potential therapeutic targets. Natural products can be used to target microglia and macrophages to remodel the tumor microenvironment for better treatment of brain tumors. However, the mechanisms of BBB regulation through natural products has not been fully explored. Further study on the mechanism by which these natural substances regulate BBB permeability are still needed.
Table 1.
Relationships between BBB constituents and different cytokines during the occurrence and development of glioma.
| BBB element | Function | Related cytokine |
|---|---|---|
| Endothelial cells | Maintaining the BBB function | MMP |
| Astrocytes | The inflammatory response of the CNS8., 9. and maintaining the integrity of the BBB10 | MMP, TGF-β, AEG-143, IL-1β, IL-17, TNF, CXCL1294., 95., 96. |
| Pericytes | Secreting growth factors and ECM and absorbing soluble molecules into the cerebrospinal fluid9 | MMP, platelet-derived growth factor-β, (PDGFβ), IL-1β, IL-6, TNF-α, ROS, NO97., 98., 99., 100., 101., 102. |
| Microglia | Stimulating the opening of BBB, leukocyte extravasation and angiogenesis12 | MMP, TNF-α, IL-1β, CCL2, CXCL1, CXCL10103 |
| Macrophages | Promoting tumor growth with poor prognostics52 | MMP, IL-10, TGF-β, NO, TNF-α104., 105. |
| Fibroblasts | Inducing production and activation of MMP13 | MMP, IL-6, IL-8106., 107. |
| Neurons | Increasing spontaneous activity and decreasing visual response60 | MMP |
Acknowledgments
This work was supported by the Shanghai Rising-Star Program of China (13QA1403400), Shanghai Talent Development Funds (201665), Natural Science Foundation of China (81773909), Excellent Youth Program of Shanghai Municipal Commission of Health and Family Planning (2017YQ060), Medical Profession Scholarship of Shanghai University of Traditional Chinese Medicine, and The Open Project Program of Key Lab of Smart Drug Delivery, Fudan University (SDD2017-01).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Gittleman H., Ostrom Q.T., Farah P.D., Ondracek A., Chen Y., Wolinsky Y. Descriptive epidemiology of pituitary tumors in the United States, 2004–2009. J Neurosurg. 2014;121:527–535. doi: 10.3171/2014.5.JNS131819. [DOI] [PubMed] [Google Scholar]
- 2.Williams M.J., Singleton W.G., Lowis S.P., Malik K., Kurian K.M. Therapeutic targeting of histone modifications in adult and pediatric high-grade glioma. Front Oncol. 2017;7:45. doi: 10.3389/fonc.2017.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fangusaro J. Pediatric high grade glioma: a review and update on tumor clinical characteristics and biology. Front Oncol. 2012;2:105. doi: 10.3389/fonc.2012.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow B.W., Gu C. The molecular constituents of the blood–brain barrier. Trends Neurosci. 2015;38:598–608. doi: 10.1016/j.tins.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood--brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schinkel A.H. P-Glycoprotein, a gatekeeper in the blood–brain barrier. Adv Drug Deliv Rev. 1999;36:179–194. doi: 10.1016/s0169-409x(98)00085-4. [DOI] [PubMed] [Google Scholar]
- 7.Tamai I., Tsuji A. Transporter-mediated permeation of drugs across the blood–brain barrier. J Pharm Sci. 2000;89:1371–1388. doi: 10.1002/1520-6017(200011)89:11<1371::aid-jps1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Fidler I.J. The role of the organ microenvironment in brain metastasis. Semin Cancer Biol. 2011;21:107–112. doi: 10.1016/j.semcancer.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez J.I., Cayrol R., Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta Mol Basis Dis. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Lécuyer M.A., Kebir H., Prat A. Glial influences on BBB functions and molecular players in immune cell trafficking. Biochim Biophys Acta Mol Basis Dis. 2016;1862:472–482. doi: 10.1016/j.bbadis.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 11.ElAli A., Thériault P., Rivest S. The role of pericytes in neurovascular unit remodeling in brain disorders. Int J Mol Sci. 2014;15:6453–6474. doi: 10.3390/ijms15046453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stankovic D.N., Teodorczyk M., Ploen R., Zipp F., Schmidt M.H. Microglia–blood vessel interactions: a double-edged sword in brain pathologies. Acta Neuropathol. 2016;131:347–363. doi: 10.1007/s00401-015-1524-y. [DOI] [PubMed] [Google Scholar]
- 13.Beliën A.T., Paganetti P.A., Schwab M.E. Membrane-type 1 matrix metalloprotease (MT1-MMP) enables invasive migration of glioma cells in central nervous system white matter. J Cell Biol. 1999;144:373–384. doi: 10.1083/jcb.144.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sameshima T., Nabeshima K., Toole B.P., Yokogami K., Okada Y., Goya T. Glioma cell extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147) stimulates production of membrane-type matrix metalloproteinases and activated gelatinase A in co-cultures with brain-derived fibroblasts. Cancer Lett. 2000;157:177–184. doi: 10.1016/s0304-3835(00)00485-7. [DOI] [PubMed] [Google Scholar]
- 15.Abbott N.J., Patabendige A.A., Dolman D.E., Yusof S.R., Begley D.J. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Arismendi-Morillo G., Hoa N.T., Ge L., Jadus M.R. Mitochondrial network in glioma's invadopodia displays an activated state both in situ and in vitro: potential functional implications. Ultrastruct Pathol. 2012;36:409–414. doi: 10.3109/01913123.2012.694582. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi H., Kubota T., Sato K., Arishima H. Ultrastructure of capillary endothelium in pilocytic astrocytomas. Brain Tumor Pathol. 2004;21:23–26. doi: 10.1007/BF02482173. [DOI] [PubMed] [Google Scholar]
- 18.Klumpp L., Sezgin E.C., Eckert F., Huber S.M. Ion channels in brain metastasis. Int J Mol Sci. 2016;17:1513. doi: 10.3390/ijms17091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chow D.S., Horenstein C.I., Canoll P., Lignelli A., Hillman E.M., Filippi C.G. Glioblastoma induces vascular dysregulation in nonenhancing peritumoral regions in humans. Am J Roentgenol. 2016;206:1073–1081. doi: 10.2214/AJR.15.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs V.L., Landry R.P., Liu Y., Romero-Sandoval E.A., De Leo J.A. Propentofylline decreases tumor growth in a rodent model of glioblastoma multiforme by a direct mechanism on microglia. Neuro Oncol. 2012;14:119–131. doi: 10.1093/neuonc/nor194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu F., Li G., Gao J., Sun Y., Liu P., Gao H. SPOCK1 is upregulated in recurrent glioblastoma and contributes to metastasis and temozolomide resistance. Cell Prolif. 2016;49:195–206. doi: 10.1111/cpr.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Hindy N., Keyvani K., Pagenstecher A., Hindy P., Sandalcioglu I.E., Sure U. Implications of Dll4-Notch signaling activation in primary glioblastoma multiforme. Neuro Oncol. 2013;15:1366–1378. doi: 10.1093/neuonc/not071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pham K., Luo D., Liu C., Harrison J.K. CCL5, CCR1 and CCR5 in murine glioblastoma: immune cell infiltration and survival rates are not dependent on individual expression of either CCR1 or CCR5. J Neuroimmunol. 2012;246:10–17. doi: 10.1016/j.jneuroim.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spano D., Zollo M. Tumor microenvironment: a main actor in the metastasis process. Clin Exp Metastas- 2012;29:381–395. doi: 10.1007/s10585-012-9457-5. [DOI] [PubMed] [Google Scholar]
- 25.De I., Steffen M.D., Clark P.A., Patros C.J., Sokn E., Bishop S.M. CSF1 overexpression promotes high-grade glioma formation without impacting the polarization status of glioma-associated microglia and macrophages. Cancer Res. 2016;76:2552–2560. doi: 10.1158/0008-5472.CAN-15-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glass R., Synowitz M. CNS macrophages and peripheral myeloid cells in brain tumours. Acta Neuropathol. 2014;128:347–362. doi: 10.1007/s00401-014-1274-2. [DOI] [PubMed] [Google Scholar]
- 27.Schlageter K.E., Molnar P., Lapin G.D., Groothuis D.R. Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvasc Res. 1999;58:312–328. doi: 10.1006/mvre.1999.2188. [DOI] [PubMed] [Google Scholar]
- 28.Charles N.A., Holland E.C., Gilbertson R., Glass R., Kettenmann H. The brain tumor microenvironment. Glia. 2012;60:502–514. doi: 10.1002/glia.21264. [DOI] [PubMed] [Google Scholar]
- 29.Rolón-Reyes K., Kucheryavykh Y.V., Cubano L.A., Inyushin M., Skatchkov S.N., Eaton M.J. Microglia activate migration of glioma cells through a Pyk2 intracellular pathway. PLoS One. 2015;10:e0131059. doi: 10.1371/journal.pone.0131059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kees T., Lohr J., Noack J., Mora R., Gdynia G., Tödt G. Microglia isolated from patients with glioma gain antitumor activities on poly (I:c) stimulation. Neuro Oncol. 2012;14:64–78. doi: 10.1093/neuonc/nor182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada T., Yoshiyama Y., Sato H., Seiki M., Shinagawa A., Takahashi M. White matter microglia produce membrane-type matrix metalloprotease, an activator of gelatinase A, in human brain tissues. Acta Neuropathol. 1995;90:421–424. doi: 10.1007/BF00294800. [DOI] [PubMed] [Google Scholar]
- 32.Bayerl S.H., Niesner R., Cseresnyes Z., Radbruch H., Pohlan J., Brandenburg S. Time lapse in vivo microscopy reveals distinct dynamics of microglia-tumor environment interactions—a new role for the tumor perivascular space as highway for trafficking microglia. Glia. 2016;64:1210–1226. doi: 10.1002/glia.22994. [DOI] [PubMed] [Google Scholar]
- 33.Sin W.C., Aftab Q., Bechberger J.F., Leung J.H., Chen H., Naus C.C. Astrocytes promote glioma invasion via the gap junction protein connexin43. Oncogene. 2016;35:1504–1516. doi: 10.1038/onc.2015.210. [DOI] [PubMed] [Google Scholar]
- 34.Lu P., Wang Y., Liu X., Wang H., Zhang X., Wang K. Malignant gliomas induce and exploit astrocytic mesenchymal-like transition by activating canonical Wnt/β-catenin signaling. Med Oncol. 2016;33:66. doi: 10.1007/s12032-016-0778-0. [DOI] [PubMed] [Google Scholar]
- 35.Barbero S., Bajetto A., Bonavia R., Porcile C., Piccioli P., Pirani P. Expression of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1 in human brain tumors and their involvement in glial proliferation in vitro. Ann New Y Acad Sci. 2002;973:60–69. doi: 10.1111/j.1749-6632.2002.tb04607.x. [DOI] [PubMed] [Google Scholar]
- 36.Hoelzinger D.B., Demuth T., Berens M.E. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst. 2007;99:1583–1593. doi: 10.1093/jnci/djm187. [DOI] [PubMed] [Google Scholar]
- 37.Emdad L., Sarkar D., Su Z.Z., Lee D.C., Kang D.C., Bruce J.N. Astrocyte elevated gene-1: recent insights into a novel gene involved in tumor progression, metastasis and neurodegeneration. Pharmacol Ther. 2007;114:155–170. doi: 10.1016/j.pharmthera.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rempel S.A., Dudas S., Ge S., Gutiérrez J.A. Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clin Cancer Res. 2000;6:102–111. [PubMed] [Google Scholar]
- 39.O'Brien E.R., Howarth C., Sibson N.R. The role of astrocytes in CNS tumors: pre-clinical models and novel imaging approaches. Front Cell Neurosci. 2013;7:40. doi: 10.3389/fncel.2013.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagashima G., Suzuki R., Asai J., Fujimoto T. Immunohistochemical analysis of reactive astrocytes around glioblastoma: an immunohistochemical study of postmortem glioblastoma cases. Clin Neurol Neurosurg. 2002;104:125–131. doi: 10.1016/s0303-8467(01)00197-4. [DOI] [PubMed] [Google Scholar]
- 41.Gimsa U., Mitchison N.A., Brunner-Weinzierl M.C. Immune privilege as an intrinsic CNS property: astrocytes protect the CNS against T-cell-mediated neuroinflammation. Mediat Inflamm. 2013;2013:320519. doi: 10.1155/2013/320519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rousselet E., Traver S., Monnet Y., Perrin A., Mandjee N., Hild A. Tumor necrosis factor-like weak inducer of apoptosis induces astrocyte proliferation through the activation of transforming-growth factor-α/epidermal growth factor receptor signaling pathway. Mol Pharmacol. 2012;82:948–957. doi: 10.1124/mol.112.079608. [DOI] [PubMed] [Google Scholar]
- 43.Guizzetti M., Kavanagh T.J., Costa L.G. Measurements of astrocyte proliferation. In: Costa L.G., Giordano G., Guizzetti M., editors. In vitro neurotoxicology. Humana Press; New York: 2011. [Google Scholar]
- 44.Buckingham S.C., Robel S. Glutamate and tumor-associated epilepsy: glial cell dysfunction in the peritumoral environment. Neurochem Int. 2013;63:696–701. doi: 10.1016/j.neuint.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Couldwell W.T., Yong V.W., Dore-Duffy P., Freedman M.S., Antel J.P. Production of soluble autocrine inhibitory factors by human glioma cell lines. J Neurol Sci. 1992;110:178–185. doi: 10.1016/0022-510x(92)90026-h. [DOI] [PubMed] [Google Scholar]
- 46.Lal P.G., Ghirnikar R.S., Eng L.F. Astrocyte-astrocytoma cell line interactions in culture. J Neurosci Res. 1996;44:216–222. doi: 10.1002/(SICI)1097-4547(19960501)44:3<216::AID-JNR2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 47.Le D.M., Besson A., Fogg D.K., Choi K.S., Waisman D.M., Goodyer C.G. Exploitation of astrocytes by glioma cells to facilitate invasiveness: a mechanism involving matrix metalloproteinase-2 and the urokinase-type plasminogen activator-plasmin cascade. J Neurosci. 2003;23:4034–4043. doi: 10.1523/JNEUROSCI.23-10-04034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lapa C., Linsenmann T., Lückerath K., Samnick S., Herrmann K., Stoffer C. Tumor-associated macrophages in glioblastoma multiforme—a suitable target for somatostatin receptor-based imaging and therapy?. PLoS One. 2015;10:e0122269. doi: 10.1371/journal.pone.0122269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pang L., Qin J., Han L., Zhao W., Liang J., Xie Z. Exploiting macrophages as targeted carrier to guide nanoparticles into glioma. Oncotarget. 2016;7:37081–37091. doi: 10.18632/oncotarget.9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabrusiewicz K., Hossain M.B., Cortes-Santiago N., Fan X., Kaminska B., Marini F.C. Macrophage ablation reduces M2-Like populations and jeopardizes tumor growth in a MAFIA-based glioma model. Neoplasia. 2015;17:374–384. doi: 10.1016/j.neo.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Infanger D.W., Cho Y., Lopez B.S., Mohanan S., Liu S.C., Gursel D. Glioblastoma stem cells are regulated by interleukin-8 signaling in a tumoral perivascular niche. Cancer Res. 2013;73:7079–7089. doi: 10.1158/0008-5472.CAN-13-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rape A., Ananthanarayanan B., Kumar S. Engineering strategies to mimic the glioblastoma microenvironment. Adv Drug Deliv Rev. 2014;79–80:172–183. doi: 10.1016/j.addr.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nico B., Ribatti D. Morphofunctional aspects of the blood–brain barrier. Curr Drug Metab. 2012;13:50–60. doi: 10.2174/138920012798356970. [DOI] [PubMed] [Google Scholar]
- 54.Van Tellingen O., Yetkin-Arik B., De Gooijer M.C., Wesseling P., Wurdinger T., de Vries H.E. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Song S., Ewald A.J., Stallcup W., Werb Z., Bergers G. PDGFRβ+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vannini E., Olimpico F., Middei S., Ammassari-Teule M., de Graaf E.L., McDonnell L. Electrophysiology of glioma: a Rho GTPase-activating protein reduces tumor growth and spares neuron structure and function. Neuro Oncol. 2016;18:1634–1643. doi: 10.1093/neuonc/now114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dilmanian F.A., Button T.M., Le Duc G., Zhong N., Peña L.A., Smith J.A. Response of rat intracranial 9L gliosarcoma to microbeam radiation therapy. Neuro Oncol. 2002;4:26–38. doi: 10.1215/15228517-4-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giese A., Loo M.A., Rief M.D., Tran N., Berens M.E. Substrates for astrocytoma invasion. Neurosurgery. 1995;37:294–302. doi: 10.1227/00006123-199508000-00015. [DOI] [PubMed] [Google Scholar]
- 59.Koochekpour S., Pilkington G.J., Merzak A. Hyaluronic acid/CD44H interaction induces cell detachment and stimulates migration and invasion of human glioma cells in vitro. Int J Cancer. 1995;63:450–454. doi: 10.1002/ijc.2910630325. [DOI] [PubMed] [Google Scholar]
- 60.Zhou Y., Larsen P.H., Hao C., Yong V.W. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem. 2002;277:49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- 61.Schwarzbauer J.E. Alternative splicing of fibronectin: three variants, three functions. Bioessays. 1991;13:527–533. doi: 10.1002/bies.950131006. [DOI] [PubMed] [Google Scholar]
- 62.Ding D., Kanaly C.W., Bigner D.D., Cummings T.J., Herndon J.E., Pastan I. Convection-enhanced delivery of free gadolinium with the recombinant immunotoxin MR1-1. J Neuro-Oncol. 2010;98:1–7. doi: 10.1007/s11060-009-0046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bourdon M.A., Wikstrand C.J., Furthmayr H., Matthews T.J., Bigner D.D. Human glioma-mesenchymal extracellular matrix antigen defined by monoclonal antibody. Cancer Res. 1983;43:2796–2805. [PubMed] [Google Scholar]
- 64.Serwer L.P., James C.D. Challenges in drug delivery to tumors of the central nervous system: an overview of pharmacological and surgical considerations. Adv Drug Deliv Rev. 2012;64:590–597. doi: 10.1016/j.addr.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 65.White E.S., Muro A.F. Fibronectin splice variants: understanding their multiple roles in health and disease using engineered mouse models. IUBMB Life. 2011;63:538–546. doi: 10.1002/iub.493. [DOI] [PubMed] [Google Scholar]
- 66.Xia P., Culp L.A. Adhesion activity in fibronectin's alternatively spliced domain EDa (EIIIA): complementarity to plasma fibronectin functions. Exp Cell Res. 1995;217:517–527. doi: 10.1006/excr.1995.1117. [DOI] [PubMed] [Google Scholar]
- 67.Serini G., Bochaton-Piallat M.L., Ropraz P., Geinoz A., Borsi L., Zardi L. Vol. 142. 1998. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-β1; pp. 873–881. (J Cell Biol). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manabe R., Oh-e N., Sekiguchi K. Alternatively spliced EDA segment regulates fibronectin-dependent cell cycle progression and mitogenic signal transduction. J Biol Chem. 1999;274:5919–5924. doi: 10.1074/jbc.274.9.5919. [DOI] [PubMed] [Google Scholar]
- 69.Fukuda T., Yoshida N., Kataoka Y., Manabe R., Mizuno-Horikawa Y., Sato M. Mice lacking the EDB segment of fibronectin develop normally but exhibit reduced cell growth and fibronectin matrix assembly in vitro. Cancer Res. 2002;62:5603–5610. [PubMed] [Google Scholar]
- 70.Khan Z.A., Chan B.M., Uniyal S., Barbin Y.P., Farhangkhoee H., Chen S. EDB fibronectin and angiogenesis—a novel mechanistic pathway. Angiogenesis. 2005;8:183–196. doi: 10.1007/s10456-005-9017-6. [DOI] [PubMed] [Google Scholar]
- 71.Kuusela P., Ruoslahti E., Vaheri A. Polypeptides of a glycoprotein antigen (SF) present in serum and surface of normal but not of transformed chicken fibroblasts. Biochim Biophys Acta Prot Struct. 1975;379:295–303. doi: 10.1016/0005-2795(75)90032-x. [DOI] [PubMed] [Google Scholar]
- 72.Vander H.M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim J., DeBerardinis R.J. Blocking fatty acid synthesis reduces lung tumor growth in mice. Nat Med. 2016;22:1077–1078. doi: 10.1038/nm.4195. [DOI] [PubMed] [Google Scholar]
- 74.Apostolova I., Irintchev A., Schachner M. Tenascin-R restricts posttraumatic remodeling of motoneuron innervation and functional recovery after spinal cord injury in adult mice. J Neurosci. 2006;26:7849–7859. doi: 10.1523/JNEUROSCI.1526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xia S., Lal B., Tung B., Wang S., Goodwin C.R., Laterra J. Tumor microenvironment tenascin-C promotes glioblastoma invasion and negatively regulates tumor proliferation. Neuro Oncol. 2016;18:507–517. doi: 10.1093/neuonc/nov171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rolle K., Nowak S., Wyszko E., Nowak M., Zukiel R., Piestrzeniewicz R. Promising human brain tumors therapy with interference RNA intervention (iRNAi) Cancer Biol Ther. 2010;9:397–407. doi: 10.4161/cbt.9.5.10958. [DOI] [PubMed] [Google Scholar]
- 77.Black K.L., King W.A., Ikezaki K. Selective opening of the blood–tumour barrier by intracarotid infusion of leukotriene C4. In: Reulen H.J., Baethmann A., Fenstermacher J., Marmarou A., Spatz M., editors. Brain edema VIII. Acta neurochirurgica. Springer; Vienna: 1990. pp. 140–141. [DOI] [PubMed] [Google Scholar]
- 78.Nakano S., Matsukado K., Black K.L. Increased brain tumor microvessel permeability after intracarotid bradykinin infusion is mediated by nitric oxide. Cancer Res. 1996;56:4027–4031. [PubMed] [Google Scholar]
- 79.Machein M.R., Kullmer J., Fiebich B.L., Plate K.H., Warnke P.C. Vascular endothelial growth factor expression, vascular volume, and capillary permeability in human brain tumors. Neurosurgery. 1999;44:732–741. doi: 10.1097/00006123-199904000-00022. [DOI] [PubMed] [Google Scholar]
- 80.Demeule M., Régina A., Jodoin J., Laplante A., Dagenais C., Berthelet F. Drug transport to the brain: key roles for the efflux pump P-glycoprotein in the blood–brain barrier. Vasc Pharmacol. 2002;38:339–348. doi: 10.1016/s1537-1891(02)00201-x. [DOI] [PubMed] [Google Scholar]
- 81.Kacem K., Lacombe P., Seylaz J., Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23:1–10. [PubMed] [Google Scholar]
- 82.Abbott N.J., Rönnbäck L., Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 83.Montagne A., Barnes S.R., Sweeney M.D., Halliday M.R., Sagare A.P., Zhao Z. Blood–brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sanchez-Covarrubias L., Slosky L.M., Thompson B.J., Davis T.P., Ronaldson P.T. Transporters at CNS barrier sites: obstacles or opportunities for drug delivery. Curr Pharm Des. 2014;20:1422–1449. doi: 10.2174/13816128113199990463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ronaldson P.T., Davis T.P. Blood–brain barrier integrity and glial support: mechanisms that can be targeted for novel therapeutic approaches in stroke. Curr Pharm Des. 2012;18:3624–3644. doi: 10.2174/138161212802002625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huber J.D., Campos C.R., Mark K.S., Davis T.P. Alterations in blood–brain barrier ICAM-1 expression and brain microglial activation after lambda-carrageenan-induced inflammatory pain. Am J Physiol Heart Circ Physiol. 2006;290:H732–H740. doi: 10.1152/ajpheart.00747.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Erdő F., Denes L., De Lange E. Age-associated physiological and pathological changes at the blood--brain barrier: a review. J Cereb Blood Flow Metab. 2017;37:4–24. doi: 10.1177/0271678X16679420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kucharzewska P., Christianson H.C., Welch J.E., Svensson K.J., Fredlund E., Ringnér M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci USA. 2013;110:7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Svensson A., Özen I., Genové G., Paul G., Bengzon J. Endogenous brain pericytes are widely activated and contribute to mouse glioma microvasculature. PLoS One. 2015;10:e0123553. doi: 10.1371/journal.pone.0123553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tae N., Lee S., Kim O., Park J., Na S., Lee J.H. Syntenin promotes VEGF-induced VEGFR2 endocytosis and angiogenesis by increasing ephrin-B2 function in endothelial cells. Oncotarget. 2017;8:38886–38901. doi: 10.18632/oncotarget.16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie H., Cui Y., Hou S., Wang J., Miao J., Deng F. Evaluation of connexin 43 redistribution and endocytosis in astrocytes subjected to ischemia/reperfusion or oxygen-glucose deprivation and reoxygenation. Biomed Res Int. 2017;2017:5064683. doi: 10.1155/2017/5064683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cui X., Wan B., Yang Y., Ren X., Guo L.H. Length effects on the dynamic process of cellular uptake and exocytosis of single-walled carbon nanotubes in murine macrophage cells. Sci Rep. 2017;7:1518. doi: 10.1038/s41598-017-01746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Candela P., Saint-Pol J., Kuntz M., Boucau M.C., Lamartiniere Y., Gosselet F. In vitro discrimination of the role of LRP1 at the BBB cellular level: focus on brain capillary endothelial cells and brain pericytes. Brain Res. 2015;1594:15–26. doi: 10.1016/j.brainres.2014.10.047. [DOI] [PubMed] [Google Scholar]
- 94.Hilgemann D.W., Fine M., Linder M.E., Jennings B.C., Lin M.J. Massive endocytosis triggered by surface membrane palmitoylation under mitochondrial control in BHK fibroblasts. Elife. 2013;2:e01293. doi: 10.7554/eLife.01293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu X.S., Zhang Z., Zhao W.D., Wang D., Luo F., Wu L.G. Calcineurin is universally involved in vesicle endocytosis at neuronal and nonneuronal secretory cells. Cell Rep. 2014;7:982–988. doi: 10.1016/j.celrep.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kawabe K, Takano K, Moriyama M, Nakamura Y. Microglia endocytose amyloid β through the binding of transglutaminase 2 and milk fat globule EGF factor 8 protein. Neurochem Res 2017. Available form: 10.1007/s11064-017-2284-y. [DOI] [PubMed]
- 97.Zhang F.Y., Hu Y., Que Z.Y., Wang P., Liu Y.H., Wang Z.H. Shikonin inhibits the migration and invasion of human glioblastoma cells by targeting phosphorylated β-catenin and phosphorylated PI3K/Akt: a potential mechanism for the anti-glioma efficacy of a traditional Chinese herbal medicine. Int J Mol Sci. 2015;16:23823–23848. doi: 10.3390/ijms161023823. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Choi Y.W., Lee K.P., Kim J.M., Kang S., Park S.J., Lee J.M. Petatewalide B, a novel compound from Petasitesjaponicus with anti-allergic activity. J Ethnopharmacol. 2016;178:17–24. doi: 10.1016/j.jep.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 99.Matias D., Balça-Silva J., Dubois L.G., Pontes B., Ferrer V.P., Rosário L. Dual treatment with shikonin and temozolomide reduces glioblastoma tumor growth, migration and glial-to-mesenchymal transition. Cell Oncol. 2017;40:247–261. doi: 10.1007/s13402-017-0320-1. [DOI] [PubMed] [Google Scholar]
- 100.Wang L., Li Z., Zhang X., Wang S., Zhu C., Miao J. Protective effect of shikonin in experimental ischemic stroke: attenuated TLR4, p-p38MAPK, NF-κB, TNF-α and MMP-9 expression, up-regulated claudin-5 expression, ameliorated BBB permeability. Neurochem Res. 2014;39:97–106. doi: 10.1007/s11064-013-1194-x. [DOI] [PubMed] [Google Scholar]
- 101.Lin K., Gao Z., Shang B., Sui S., Fu Q. Osthole suppresses the proliferation and accelerates the apoptosis of human glioma cells via the upregulation of microRNA-16 and downregulation of MMP-9. Mol Med Rep. 2015;12:4592–4597. doi: 10.3892/mmr.2015.3929. [DOI] [PubMed] [Google Scholar]
- 102.Duan J., Yang Y., Liu H., Dou P.C., Tan S.Y. Osthole ameliorates acute myocardial infarction in rats by decreasing the expression of inflammatory-related cytokines, diminishing MMP-2 expression and activating p-ERK. Int J Mol Med. 2016;37:207–216. doi: 10.3892/ijmm.2015.2402. [DOI] [PubMed] [Google Scholar]
- 103.Chen R., Xue J., Xie M. Osthole regulates TGF-β1 and MMP-2/9 expressions via activation of PPARα/γ in cultured mouse cardiac fibroblasts stimulated with angiotensin II. J Pharm Pharm Sci. 2013;16:732–741. doi: 10.18433/j3hk5c. [DOI] [PubMed] [Google Scholar]
- 104.Xu X.M., Zhang Y., Qu D., Feng X.W., Chen Y., Zhao L. Osthole suppresses migration and invasion of A549 human lung cancer cells through inhibition of matrix metalloproteinase-2 and matrix metallopeptidase-9 in vitro. Mol Med Rep. 2012;6:1018–1022. doi: 10.3892/mmr.2012.1044. [DOI] [PubMed] [Google Scholar]
- 105.Kao S.J., Su J.L., Chen C.K., Yu M.C., Bai K.J., Chang J.H. Osthole inhibits the invasive ability of human lung adenocarcinoma cells via suppression of NF-κB-mediated matrix metalloproteinase-9 expression. Toxicol Appl Pharmacol. 2012;261:105–115. doi: 10.1016/j.taap.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 106.Yang D., Gu T., Wang T., Tang Q., Ma C. Effects of osthole on migration and invasion in breast cancer cells. Biosci Biotechnol Biochem. 2010;74:1430–1434. doi: 10.1271/bbb.100110. [DOI] [PubMed] [Google Scholar]
- 107.Jang M., Cai L., Udeani G.O., Slowing K.V., Thomas C.F., Beecher C.W.W. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 108.Oi N., Jeong C.H., Nadas J., Cho Y.Y., Pugliese A., Pugliese A.M. Resveratrol, a red wine polyphenol, suppresses pancreatic cancer by inhibiting leukotriene A4 hydrolase. Cancer Res. 2010;70:9755–9764. doi: 10.1158/0008-5472.CAN-10-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Puissant A., Robert G., Fenouille N., Luciano F., Cassuto J.P., Raynaud S. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 2010;70:1042–1052. doi: 10.1158/0008-5472.CAN-09-3537. [DOI] [PubMed] [Google Scholar]
- 110.Hao X.N., Wang W.J., Chen J., Zhou Q., Qu Y.X., Liu X.Y. Effects of resveratrol on ARPE-19 cell proliferation and migration via regulating the expression of proliferating cell nuclear antigen, P21, P27 and p38MAPK/MMP-9. Int J Ophthalmol. 2016;9:1725–1731. doi: 10.18240/ijo.2016.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jiao Y., Li H., Liu Y., Guo A., Xu X., Qu X. Resveratrol inhibits the invasion of glioblastoma-initiating cells via down-regulation of the PI3K/Akt/NF-κB signaling pathway. Nutrients. 2015;7:4383–4402. doi: 10.3390/nu7064383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wei H., Wang S., Zhen L., Yang Q., Wu Z., Lei X. Resveratrol attenuates the blood–brain barrier dysfunction by regulation of the MMP-9/TIMP-1 balance after cerebral ischemia reperfusion in rats. J Mol Neurosci. 2015;55:872–879. doi: 10.1007/s12031-014-0441-1. [DOI] [PubMed] [Google Scholar]
- 113.Gentile E., Liuzzi G.M. Marine pharmacology: therapeutic targeting of matrix metalloproteinases in neuroinflammation. Drug Discov Today. 2017;22:299–313. doi: 10.1016/j.drudis.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 114.Rodríguez-Nieto S., González-Iriarte M., Carmona R., Muñoz-Chápuli R., Medina M.A., Quesada A.R. Antiangiogenic activity of aeroplysinin-1, a brominated compound isolated from a marine sponge. FASEB J. 2002;16:261–263. doi: 10.1096/fj.01-0427fje. [DOI] [PubMed] [Google Scholar]
- 115.Martínez-Poveda B., García-Vilas J.A., Cárdenas C., Melgarejo E., Quesada A.R., Medina M.A. The brominated compound aeroplysinin-1 inhibits proliferation and the expression of key pro-inflammatory molecules in human endothelial and monocyte cells. PLoS One. 2013;8:e55203. doi: 10.1371/journal.pone.0055203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zheng H.L., Yang J., Hou Y., Sun B., Zhang Q., Mou Y. Oligomer procyanidins (F2) isolated from grape seeds inhibits tumor angiogenesis and cell invasion by targeting HIF-1αin vitro. Int J Oncol. 2015;46:708–720. doi: 10.3892/ijo.2014.2744. [DOI] [PubMed] [Google Scholar]
- 117.Liu Y., Tao L., Fu X., Zhao Y., Xu X. BDNF protects retinal neurons from hyperglycemia through the TrkB/ERK/MAPK pathway. Mol Med Rep. 2013;7:1773–1778. doi: 10.3892/mmr.2013.1433. [DOI] [PubMed] [Google Scholar]
- 118.Yoshii A., Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70:304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun X., Zhou H., Luo X., Li S., Yu D., Hua J. Neuroprotection of brain-derived neurotrophic factor against hypoxic injury in vitro requires activation of extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. Int J Dev Neurosci. 2008;26:363–370. doi: 10.1016/j.ijdevneu.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 120.Almeida R.D., Manadas B.J., Melo C.V., Gomes J.R., Mendes C.S., Grãos M.M. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- 121.Bonni A., Brunet A., West A.E., Datta S.R., Takasu M.A., Greenberg M.E. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 122.Satoh T., Nakatsuka D., Watanabe Y., Nagata I., Kikuchi H., Namura S. Neuroprotection by MAPK/ERK kinase inhibition with U0126 against oxidative stress in a mouse neuronal cell line and rat primary cultured cortical neurons. Neurosci Lett. 2000;288:163–166. doi: 10.1016/s0304-3940(00)01229-5. [DOI] [PubMed] [Google Scholar]
- 123.Jin K., Mao X.O., Zhu Y., Greenberg D.A. MEK and ERK protect hypoxic cortical neurons via phosphorylation of Bad. J Neurochem. 2002;80:119–125. doi: 10.1046/j.0022-3042.2001.00678.x. [DOI] [PubMed] [Google Scholar]
- 124.Klöcker N., Kermer P., Weishaupt J.H., Labes M., Ankerhold R., Bähr M. Brain-derived neurotrophic factor-mediated neuroprotection of adult rat retinal ganglion cells in vivo does not exclusively depend on phosphatidyl-inositol-3ʹ-kinase/protein kinase B signaling. J Neurosci. 2000;20:6962–6967. doi: 10.1523/JNEUROSCI.20-18-06962.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baird L., Dinkova-Kostova A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 126.Li W., Ma J., Ma Q., Li B., Han L., Liu J. Resveratrol inhibits the epithelial-mesenchymal transition of pancreatic cancer cells via suppression of the PI-3K/Akt/NF-κB pathway. Curr Med Chem. 2013;20:4185–4194. doi: 10.2174/09298673113209990251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gilmore T.D. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 128.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 129.Zhou T., Yang Y., Zhang H., Che Y., Wang W., Lv H. Serenoa Repens induces growth arrest, apoptosis and inactivation of STAT3 signaling in human glioma cells. Technol Cancer Res Treat. 2015;14:729–736. doi: 10.7785/tcrt.2012.500417. [DOI] [PubMed] [Google Scholar]
- 130.Dong X.Z., Huang C.L., Yu B.Y., Hu Y., Mu L.H., Liu P. Effect of tenuifoliside A isolated from Polygala tenuifolia on the ERK and PI3K pathways in C6 glioma cells. Phytomedicine. 2014;21:1178–1188. doi: 10.1016/j.phymed.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 131.Fulda S. Betulinic acid for cancer treatment and prevention. Int J Mol Sci. 2008;9:1096–1107. doi: 10.3390/ijms9061096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ehrhardt H., Fulda S., Führer M., Debatin K.M., Jeremias I. Betulinic acid-induced apoptosis in leukemia cells. Leukemia. 2004;18:1406–1412. doi: 10.1038/sj.leu.2403406. [DOI] [PubMed] [Google Scholar]
- 133.Shankar E., Zhang A., Franco D., Gupta S. Betulinic acid-mediated apoptosis in human prostate cancer cells involves p53 and nuclear factor-kappa B (NF-κB) pathways. Molecules. 2017;22 doi: 10.3390/molecules22020264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Su D., Gao Y.Q., Dai W.B., Hu Y., Wu Y.F., Mei Q.X. Helicteric acid, oleanic acid, and betulinic acid, three triterpenes from Helicteres angustifolia L., inhibit proliferation and induce apoptosis in HT-29 colorectal cancer cells via suppressing NF-κB and STAT3 Signaling. Evid Based Complement Altern Med. 2017;2017:5180707. doi: 10.1155/2017/5180707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gupta M., Kaur G. Aqueous extract from the Withania somnifera leaves as a potential anti-neuroinflammatory agent: a mechanistic study. J Neuroinflamm. 2016;13:193. doi: 10.1186/s12974-016-0650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun G.Y., Li R., Cui J., Hannink M., Gu Z., Fritsche K.L. Withania somnifera and its withanolides attenuate oxidative and inflammatory responses and up-regulate antioxidant responses in BV-2 microglial cells. Neuromolecular Med. 2016;18:241–252. doi: 10.1007/s12017-016-8411-0. [DOI] [PubMed] [Google Scholar]
- 137.Kataria H., Kumar S., Chaudhary H., Kaur G. Withania somnifera suppresses tumor growth of intracranial allograft of glioma cells. Mol Neurobiol. 2016;53:4143–4158. doi: 10.1007/s12035-015-9320-1. [DOI] [PubMed] [Google Scholar]
- 138.Abdallah H.M., Al-Abd A.M., El-Dine R.S., El-Halawany A.M. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: a review. J Adv Res. 2015;6:45–62. doi: 10.1016/j.jare.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.He L., Zhao C., Yan M., Zhang L.Y., Xia Y.Z. Inhibition of P-glycoprotein function by procyanidine on blood–brain barrier. Phytother Res. 2009;23:933–937. doi: 10.1002/ptr.2781. [DOI] [PubMed] [Google Scholar]
- 140.Zhao B.X., Sun Y.B., Wang S.Q., Duan L., Huo Q.L., Ren F. Grape seed procyanidin reversal of p-glycoprotein associated multi-drug resistance via down-regulation of NF-κB and MAPK/ERK mediated YB-1 activity in A2780/T cells. PLoS One. 2013;8:e71071. doi: 10.1371/journal.pone.0071071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mahringer A., Karamustafa S., Klotz D., Kahl S., Konkimalla V.B., Wang Y. Inhibition of P-glycoprotein at the blood–brain barrier by phytochemicals derived from traditional Chinese medicine. Cancer Genom Proteom. 2010;7:191–205. [PubMed] [Google Scholar]
- 142.Nitsch C., Hubauer H. Distant blood–brain barrier opening in subfields of the rat hippocampus after intrastriatal injections of kainic acid but not ibotenic acid. Neurosci Lett. 1986;64:53–58. doi: 10.1016/0304-3940(86)90662-2. [DOI] [PubMed] [Google Scholar]
- 143.Lin X., Zha Y., Zeng X.Z., Dong R., Wang Q.H., Wang D.T. Role of the Wnt/β-catenin signaling pathway in inducing apoptosis and renal fibrosis in 5/6-nephrectomized rats. Mol Med Rep. 2017;15:3575–3582. doi: 10.3892/mmr.2017.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim K.I., Jeong D.S., Yoon T.J., Jung E.C., Lee J.H., Kim C.D. Inhibition of collagen production by ICG-001, a small molecule inhibitor for Wnt/β-catenin signaling, in skin fibroblasts. J Dermatol Sci. 2017;86:76–78. doi: 10.1016/j.jdermsci.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 145.Sato M. Upregulation of the Wnt/β-catenin pathway induced by transforming growth factor-β in hypertrophic scars and keloids. Acta Derm Venereol. 2006;86:300–307. doi: 10.2340/00015555-0101. [DOI] [PubMed] [Google Scholar]
- 146.Fu Y., Li L., Hao S., Guan R., Fan G., Shi C. Draft genome sequence of the Tibetan medicinal herb, Rhodiola crenulata. Gigascience. 2017;6:1–5. doi: 10.1093/gigascience/gix033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mora M.C., Bassa L.M., Wong K.E., Tirabassi M.V., Arenas R.B., Schneider S.S. Rhodiola crenulata inhibits Wnt/β-catenin signaling in glioblastoma. J Surg Res. 2015;197:247–255. doi: 10.1016/j.jss.2015.02.074. [DOI] [PubMed] [Google Scholar]
- 148.Bassa L.M., Jacobs C., Gregory K., Henchey E., Ser-Dolansky J., Schneider S.S. Rhodiola crenulata induces an early estrogenic response and reduces proliferation and tumorsphere formation over time in MCF7 breast cancer cells. Phytomedicine. 2016;23:87–94. doi: 10.1016/j.phymed.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 149.Li C., Zeng M., Chi H., Shen J., Ng T.B., Jin G. Trichosanthin increases granzyme B penetration into tumor cells by upregulation of CI-MPR on the cell surface. Oncotarget. 2017;8:26460–26470. doi: 10.18632/oncotarget.15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Miao J., Jiang Y., Wang D., Zhou J., Fan C., Jiao F. Trichosanthin suppresses the proliferation of glioma cells by inhibiting LGR5 expression and the Wnt/β-catenin signaling pathway. Oncol Rep. 2015;34:2845–2852. doi: 10.3892/or.2015.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 152.Valenti S., Cuttica C.M., Giusti M., Giordano G. Nitric oxide modulates Leydig cell function in vitro: is this a way of communication between the immune and endocrine system in the testis? Ann N Y Acad Sci. 1999;876:298–300. doi: 10.1111/j.1749-6632.1999.tb07652.x. [DOI] [PubMed] [Google Scholar]
- 153.Kim C.S., Subedi L., Park K.J., Kim S.Y., Choi S.U., Kim K.H. Salicin derivatives from Salix glandulosa and their biological activities. Fitoterapia. 2015;106:147–152. doi: 10.1016/j.fitote.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 154.Ramachandran C., Portalatin G., Quirin K.W., Escalon E., Khatib Z., Melnick S.J. Inhibition of AKT signaling by supercritical CO2 extract of mango ginger (Curcumaamada Roxb.) in human glioblastoma cells. J Complement Integr Med. 2015;12:307–315. doi: 10.1515/jcim-2015-0005. [DOI] [PubMed] [Google Scholar]
- 155.Kong C.S., Kim K.H., Choi J.S., Kim J.E., Park C., Jeong J.W. Salicin, an extract from white willow bark, inhibits angiogenesis by blocking the ROS-ERK pathways. Phytother Res. 2014;28:1246–1251. doi: 10.1002/ptr.5126. [DOI] [PubMed] [Google Scholar]
- 156.Kim C.S., Subedi L., Kwon O.W., Park H.B., Kim S.Y., Choi S.U. Wasabisides A-E, lignan glycosides from the roots of Wasabia Japonica. J Nat Prod. 2016;79:2652–2657. doi: 10.1021/acs.jnatprod.6b00582. [DOI] [PubMed] [Google Scholar]
- 157.Kim C.S., Subedi L., Kim S.Y., Choi S.U., Kim K.H., Lee K.R. Diterpenes from the trunk of Abies holophylla and their potential neuroprotective and anti-inflammatory activities. J Nat Prod. 2016;79:387–394. doi: 10.1021/acs.jnatprod.5b01053. [DOI] [PubMed] [Google Scholar]
- 158.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked?. Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weitzman S.A., Gordon L.I. Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood. 1990;76:655–663. [PubMed] [Google Scholar]
- 160.Rosin M.P., Saad D.Z., Ward A.J., Anwar W.A. Involvement of inflammatory reactions and elevated cell proliferation in the development of bladder cancer in schistosomiasis patients. Mutat Res. 1994;305:283–292. doi: 10.1016/0027-5107(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 161.Frenkel K. Carcinogen-mediated oxidant formation and oxidative DNA damage. Pharmacol Ther. 1992;53:127–166. doi: 10.1016/0163-7258(92)90047-4. [DOI] [PubMed] [Google Scholar]
- 162.Tian X., Peng J., Zhong J., Yang M., Pang J., Lou J. β-Caryophyllene protects in vitro neurovascular unit against oxygen-glucose deprivation and re-oxygenation-induced injury. J Neurochem. 2016;139:757–768. doi: 10.1111/jnc.13833. [DOI] [PubMed] [Google Scholar]
- 163.Park K.R., Nam D., Yun H.M., Lee S.G., Jang H.J., Sethi G. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011;312:178–188. doi: 10.1016/j.canlet.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 164.Wang Y.F., Gu Y.T., Qin G.H., Zhong L., Meng Y.N. Curcumin ameliorates the permeability of the blood–brain barrier during hypoxia by upregulating heme oxygenase-1 expression in brain microvascular endothelial cells. J Mol Neurosci. 2013;51:344–351. doi: 10.1007/s12031-013-9989-4. [DOI] [PubMed] [Google Scholar]
- 165.Xiao L., Ding M., Fernandez A., Zhao P., Jin L., Li X. Curcumin alleviates lumbar radiculopathy by reducing neuroinflammation, oxidative stress and nociceptive factors. Eur Cell Mater. 2017;33:279–293. doi: 10.22203/eCM.v033a21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Santos-Parker J.R., Strahler T.R., Bassett C.J., Bispham N.Z., Chonchol M.B., Seals D.R. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging. 2017;9:187–208. doi: 10.18632/aging.101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Park J.H., Choi J.W., Ju E.J., Pae A.N., Park K.D. Antioxidant and anti-inflammatory activities of a natural compound, shizukahenriol, through Nrf2 activation. Molecules. 2015;20:15989–16003. doi: 10.3390/molecules200915989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dietrich J.B. Alteration of blood–brain barrier function by methamphetamine and cocaine. Cell Tissue Res. 2009;336:385–392. doi: 10.1007/s00441-009-0777-y. [DOI] [PubMed] [Google Scholar]