Abstract
Potassium 2-(1-hydroxypentyl)-benzoate (d,l-PHPB), a new drug candidate for ischemic stroke at the phase II clinic trial, has been shown to protect neurons by inhibiting oxidative injury and reducing neuron apoptosis in previous studies. But the mechanisms of d,l-PHPB remain to be studied. In this study, a neuron–astrocytes co-culture system was used to elucidate the roles of astrocytes in neuroprotection of d,l-PHPB under oxygen-glucose deprivation/reoxygenation (OGD/R) condition. Our data showed that d,l-PHPB reduced neuronal apoptosis in mono-culture system and this effect was enhanced in neuron–astrocyte co-culture system under the OGD/R condition. Meanwhile, d,l-PHPB obviously increased the levels of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), which were mainly secreted from astrocytes, in the co-culture system after OGD/R. The PI3K/AKT and ERK signaling pathways as well as the p-TRKA/B receptors were involved in the process. In addition, the levels of TNF-α and IL-1β secreted from astrocytes after OGD/R were markedly reduced after d,l-PHPB treatment, which was mainly due to the suppression of phosphorylated p38. In conclusion, the present study demonstrates that the neuroprotective effects of d,l-PHPB were improved by astrocytes, mainly mediated by increasing the release of BDNF/NGF and attenuating inflammatory cytokines.
KEY WORDS: d,l-PHPB; Oxygen-glucose deprivation; Neuron apoptosis; Astrocytes; Neuro--astrocyte co-culture
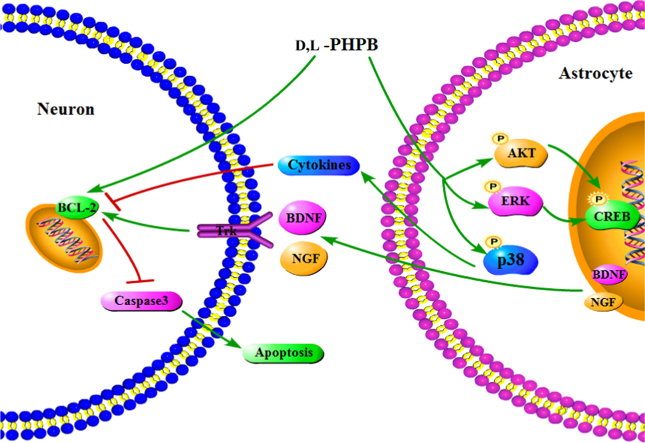
Graphical abstract
The present study demonstrated that the neuroprotective effects of d,l-PHPB were improved by astrocytes, mainly mediated by increasing the levels of BDNF/NGF and reducing inflammatory cytokines.
1. Introduction
Brain ischemic stroke is a leading cause of death and disability. Most of researches have focused on neurons injury after brain ischemia, because neurons are far more susceptible to ischemic injury than their neighboring astrocytes1. Astrocytes, as the most abundant cell type in brain, distribute in all regions of the central nervous system (CNS). Aastrocytes had been regarded as the passive partners of neurons in the CNS. However, this view was challenged by more and more evidences that astrocytes may play the important roles in the CNS2, 3. Astrocytes may provide the metabolic and trophic support to neurons, participate in the synaptic function and neuroplasticity, and maintain the extracellular balance of ions, fluid, and transmitters. In addition, astrocytes respond to a variety of injury of CNS, such as cerebral ischemia4, 5, neurodegenerative disease6 and infection.
Potassium 2-(1-hydroxypentyl)-benzoate (d,l-PHPB) is a novel drug candidate for the treatment of cerebral ischemia. Previous study7 showed that d,l-PHPB improved the neurobehavioral deficits and reduced infarct volume in the cerebral ischemic animal model. It might protect neurons against H2O2-induced apoptosis by modulating the protein kinase C (PKC) signaling pathway8. Recently, d,l-PHPB was shown to improve learning and memory deficits, reduce oxidative stress and glia activation in the cerebral area of hypo-perfused rats9 and attenuate amyloid and τ pathologies in a mouse model of Alzheimer׳s disease10. These results suggested that the neuroprotective roles of d,l-PHPB in cerebral ischemia might be related to astrocytic functions, but the details are still unclear.
Most studies of neuroprotective agents were carried out separately on neurons or astrocytes. However, in brain both cells are interacted closely at physiological and pathological condition. Therefore, to understand the function of astrocytes under the cerebral ischemia and the effects of astrocytes on neuroprotective agent are very important. In the present study, we investigated the protective effects of d,l-PHPB on neuronal injury in vitro after oxygen-glucose deprivation/reoxygenation (OGD/R) using a neuron–astrocyte co-culture system. It was found that the neuronal protective effects of d,l-PHPB were different in presence and absence of astrocytes. The relevant mechanisms were studied.
2. Materials and methods
2.1. Animals
Neonatal pups of Wistar rats (within 24 h after birth) were obtained from Vital River Laboratories, Beijing, China. All experimental protocols in this study were approved by the Laboratories Institutional Animal Care and Use Committee of the Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
2.2. Materials
d,l-PHPB (purity > 98% by HPLC) was synthesized by the Department of Medical Synthetic Chemistry, Institute of Materia Medica (Beijing, China) and dissolved in phosphate buffered saline (PBS) with the current use. DMEM/F12, DMEM and Neurobasal-A media were purchased from Invitrogen Co. (Carlsbad, CA, USA). Hoechst 33342 dye was obtained from Sigma–Aldrich Co. (St. Louis, MO, USA). Brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF) ELISA kits, as well as tumor necrosis factor-α (TNF-α), interleukelin-1β (IL-1β) and interleukelin-6 (IL-6) ELISA kits were purchased from BOSTER Co. (Wuhan, China). Antibodies used in Western blot analysis, including phosphorylated tyrosine kinase receptorA/B (TRKA/B), protein kinase B (AKT), extracellular regulated protein kinases (ERK), cAMP-response element binding protein (CREB) and p38 antibodies, as well as their respective total protein and β-actin antibodies, were obtained from Cell Signaling Technology Co. (Danvers, MA, USA). Other antibodies, including cleaved-caspase 3, B-cell lymphoma-2 (BCL-2), BCL-2 assaciated X protein (BAX), phosphorylated c-Jun N-terminal kinase (JNK) and total JNK, phosphatidylinositol 3-kinase (PI3K) and glial fibrillary acidic protein (GFAP) antibodies were obtained from Santa Cruz Biotechnology Co. (San Diego, CA, USA). Anti-mouse and anti-rabbit IgG were purchased from Zhongshan Golden Bridge Biotechnology Co. (Beijing, China). All other reagents were analytical grade.
2.3. Neuron–astrocyte co-culture
Astrocytes were cultured and purified as described previously11. Neonatal pups of Wistar rats were decapitated and the cerebral hemispheres were immediately transferred to the cold DMEM/F12 media, then the meninges were carefully removed under a dissecting microscope. The cerebral tissue was treated with a 0.25% trypsin solution for 20 min at 37 °C. An equal volume of DMEM/F12 medium containing 10% fetal bovine serum (FBS) was added to stop the trypsin and the mixture was centrifuged at 800 × g for 3–5 min. The pellet was resuspended with the medium. The cultures were incubated at 37 °C in a humid 5% CO2/95% air environment. The complete medium was changed after 24 h and half medium was changed every three days. After about 12 days, the astrocytes were treated with 0.25% trypsin solution for 3–5 min at 37 °C and were harvested. The destiny of the astrocytes was adjusted to 2×105 cells/mL. And they were planted on the upper chamber of transwell (0.4 μm, Corning, NY, USA).
Rat hippocampal neurons were isolated and cultured from Wistar rats postnatal within 24 h according to protocols modified from Kaech and Banke12. Hippocampus were isolated and incubated in trypsin (0.25%) for 20 min at 37 °C to obtain neurons. Then, neurons were planted on the lower chamber of transwell at the destiny of 4 × 105 cells/mL. Neurons were firstly cultured in DMEM with 10% FBS and 10% horse serum on poly-d-lysine (Sigma–Aldrich) coated glass coverslips. After 4 h the medium was removed and changed to Neurobasal-A medium with 2% B27 supplement (Invitrogen). Then culture medium was half-changed every 3 days. A final concentration of 10 μmol/L cytosine arabinoside (AraC, Sigma–Aldrich) was added on day in vitro (DIV) 2, and removed on DIV 3. At the same time, the upper chamber containing astrocytes was inserted above the lower chamber containing neurons to establish the neuron–astrocyte co-culture system. And the medium was change to Neurobasal-A with 2% B27 supplement (2%). Until DIV 7, the neuron–astrocytes were used to OGD/R treatment.
2.4. OGD/R model
OGD/R experiments were performed based on previous procedures13 by replacing the culture medium (Neurobasal-A) with deoxygenated glucose-free Earl׳s balanced salt solution (BSS).The composition of BSS solution was (in mmol/L): NaCl 140, KCl 5, CaCl2 2, HEPES 20, glycine 0.03, and adjusted to pH 7.4. Prior to use, BSS was equilibrated with the anaerobic gas mixture (5% CO2, 1% O2 and 94% N2) by bubbling for 15 min, adjusted to pH 7.4 if necessary, and heated to 37 °C. Cultures were then subjected to a hypoxic environment of 5% CO2, 1% O2 and 94% N2 at 37 °C for 2 h. OGD was terminated by washing two times, replacing the culture medium, and returning the cultures to a normoxic incubator maintained at 37 °C and 5% CO2 to keep culturing for 24 h.
2.5. Assay of apoptosis by Hoechst 33342 staining
Morphological changes of apoptosis in neurons were studied by Hoechst33342 staining14. The neuron–astrocyte co-culture system on DIV 7 were treated with OGD (2 h)/R (24 h) while being administrated with d,l-PHPB (1 or 10 μmol/L). Then, Hoechst33342 dissolved in PBS (10 mg/L) was added to neurons and incubated for 30 min at 37 °C in the dark. The cells were then fixed with 4% paraformaldehyde for 10 min at room temperature, after which they were examined by the fluroescence microscopy (UV excitation and emission = 360 and 450 nm, respectively). The percentage of the apoptotic cells was calculated by the ratio of apoptotic cells to the total cells counted. At least 500 cells were counted from more than 3 random microscopic fields.
2.6. ELISA analysis
The neurons cultured alone, astrocytes cultured alone, and neuron–astrocyte co-culture system on DIV 7 were treated with OGD (2 h)/R (24 h) while being administrated with d,l-PHPB (1 or 10 μmol/L), respectively. The supernatant of them were harvested respectively for determined the levels of secreted BDNF, NGF, TNF-α, IL-1β and IL-6. Additionally, the neurons and astrocytes were harvested in the ice-cold PBS, and centrifuged at 4 °C for 5 min at 3000 × g. Cytoplasm contents in neurons or astrocytes were obtained by using lysis buffer containing 150 mmol/L NaCl, 10 mmol/L Tris, 1% NP-40, 1 mmol/L EGTA, 1 mmol/L Na3VO4, 10 mmol/L NaF, 10% glycerol and complete protease inhibitor.
The levels of BDNF, NGF, TNF-α, IL-1β and IL-6 in the supernatant and cytoplasm of neurons or astrocytes were measured by ELISA according to the manufacturer׳s instructions.
2.7. Western blot analysis
After OGD (2 h)/R (24 h), neurons and astrocytes were rinsed with PBS for 2 times, and the total protein was extracted from the cells by using lysis buffer containing 150 mmol/L NaCl, 10 mmol/L Tris, 1% NP-40, 1 mmol/L EGTA, 1 mmol/L Na3VO4, 10 mmol/L NaF, 10% glycerol and complete protease inhibitor. Protein concentration was determined by the Bradford method. Equal amounts of proteins (40 μg) were run on a 10% polyacrylamide gel, transferred on to a polyvinylidene fluoride (PVDF) membrane, blocked with 5% fat-free milk in Tris-buffered saline with Tween-20 (TBST) for 2 h, and subsequently incubated with primary antibodies overnight. After washing with TBST for 5 times, the membranes were incubated with secondary antibodies at room temperature for 1 h. Membranes were developed with chemiluminescence reagents and band intensities were quantified using the Quantity-One software (BioRad, Hercules, CA, USA). The values were normalized to β-actin intensity levels.
2.8. Statistical analysis
All data were expressed as mean ± S.E.M. of at least 3 independent experiments. One-way analysis of variance (ANOVA) and student׳s t-test using SPSS version 10.0 software were used for statistical analysis. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Astrocytes attenuated OGD/R-induced neuron apoptosis in neuron–astrocyte co-culture system
In the central nervous system, astrocytes always accompanies with the entire development process of neurons, thus the interaction between neurons and astrocytes is very important for illustrating the mechanism of nervous system diseases, especially for ischemic damage. Therefore, we established neuron–astrocyte co-culture system by using transwell chamber, where neurons were cultured in the lower chamber and astrocytes were cultured in the upper one. Meanwhile, we established OGD/R model in vitro to investigate the neuronal injury and observe the effects of neuroprotective agent.
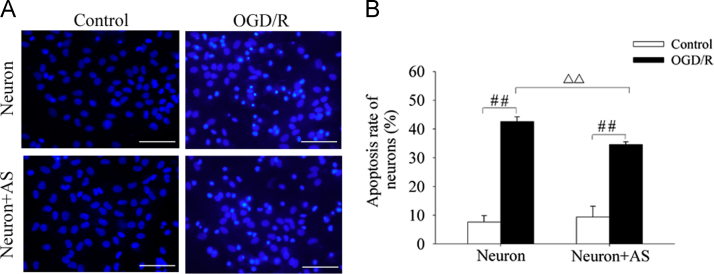
The neuron apoptosis after exposure to OGD (2 h)/R (24 h) was determined by staining with Hoechst 33342 dye. In this experiment, the apoptotic cells were characterized by the condensed nuclear chromatin and nuclear fragmentation. The results showed that OGD (2 h)/R (24 h) induced obvious neuron apoptosis with fluorescent nuclear dye, and the number of apoptotic neurons in the neuron–astrocyte co-culture system was significantly less than that in neurons alone culture system (Fig. 1A and B). These results indicated that astrocytes might protect neurons and attenuate cell apoptosis under OGD/R conditions.
Figure 1.
Effect of OGD/R on neuron apoptosis in neurons mono-culture (Neuron) and neuron–astrocyte co-culture (Neuron+AS) system. (A) Representative images of Hoechst 33342 staining of neurons. (B) Quantitative determination of apoptotic rate of neurons. Values were expressed as mean ± S.E.M. (n = 4), ####P < 0.01 versus control group; △△P < 0.01 versus OGD/R group of Neuron. Scale bars, 100 μm.
We also used flow cytometry to detect neuron apoptosis, and the results were consistent with above Hoechst 33342 staining study (Appendix A, Appendix A).
3.2. The neuronal protection of d,l-PHPB was improved by astrocytes in the co-culture system
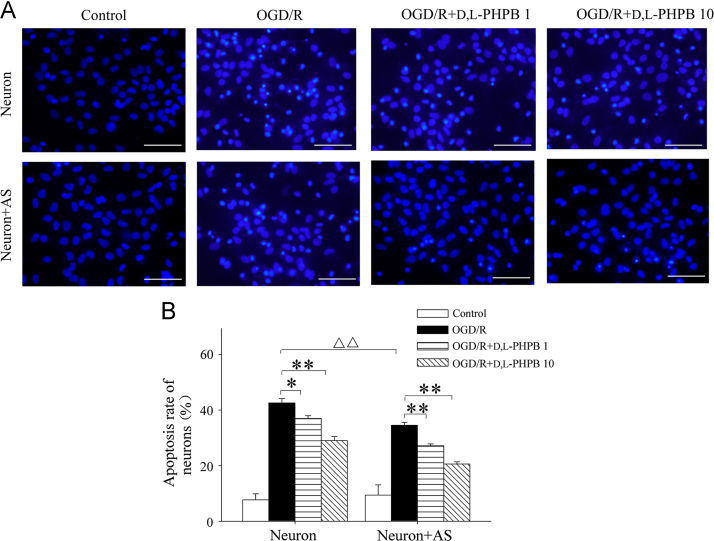
In order to explore the effects of d,l-PHPB on OGD/R-induced neuron apoptosis in the presence of astrocytes, d,l-PHPB (1 or 10 μmol/L) was given to the neuron–astrocyte co-culture system at the same time of OGD/R stimulation. In the Hoechst 33342 staining experiment, the fluorescent imaging showed that the cell nuclear condensation and nuclear fragmentation were significantly reduced in d,l-PHPB treated groups (Fig. 2A). By using quantitative analysis, the neurons apoptotic rate of the model group was 34.6%, while in d,l-PHPB treated (10 μmol/L) group the rate reduced to 20.6% (Fig. 2B). Whereas, when absent of astrocytes, d,l-PHPB reduced neuron apoptosis rate from 42.6% to 29.0%. Compared with the experiment without astrocytes, we found that the neuroprotective effects of d,l-PHPB were enhanced by astrocytes (Fig. 2B). In this study we also used flow cytometry to detect neuron apoptosis, and the results were consistent with above Hoechst 33342 staining results again (Appendix A, Appendix A).
Figure 2.
d,l-PHPB (1 or 10 μmol/L) inhibited OGD/R-induced neuron apoptosis in Neuron and Neuron+AS system. (A) Representative images of Hoechst 33342 staining of neurons. (B) Quantitative determination of apoptotic rate of neurons. Values were expressed as mean ± S.E.M. (n = 4), *P < 0.05, **P < 0.01 versus model group; ####P < 0.01 versus OGD/R group; △△P < 0.01 versus OGD/R group of Neuron. Scale bars, 100 μm.
3.3. d,l-PHPB reduced cleaved-caspase 3 expression and increased BCL-2 expression in neurons after OGD/R in neuron–astrocyte co-culture system
To reveal the mechanism of the protective effects of d,l-PHPB against OGD/R-induced neuron apoptosis, we used Western blot to measure the expression levels of apoptosis related protein.
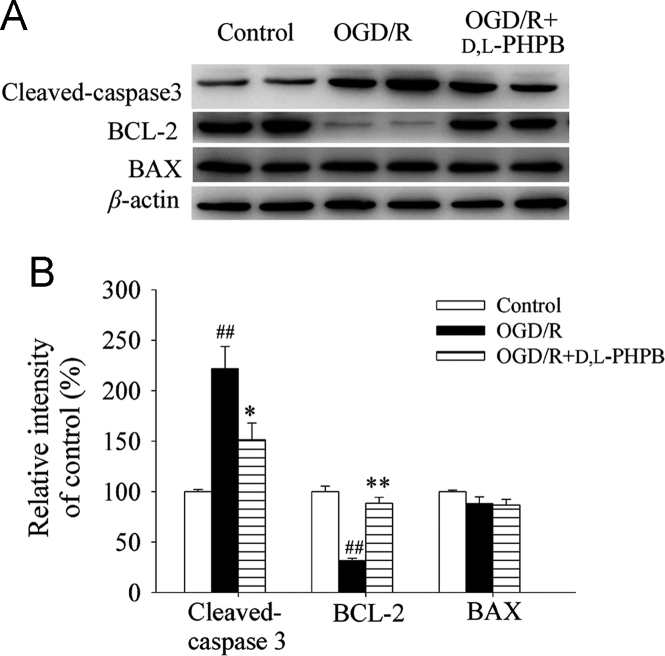
Caspase 3 is an activated death protease and crucial mediators of apoptosis. It is activated by the proteolytic cleavage of its inactive zymogen into the cleaved active subunit (17 kDa) fragment. In the present study, we measured levels of cleaved-caspase 3 using the Western blot analysis. As shown in Fig. 3, the expression of cleaved-caspase 3 after OGD (2 h)/R (24 h) was increased by 121.6% compared with the control group (P < 0.01). However, the elevation of cleaved-caspase 3 was significantly attenuated by d,l-PHPB treatment. At the concentration of 10 μmol/L, d,l-PHPB reduced expression level of the cleaved-caspase 3 by 70.3% compared with the OGD/R group (P < 0.05).
Figure 3.
d,l-PHPB (10 μmol/L) reduced cleaved-caspase 3 expression and increased BCL-2 expression in neurons after OGD/R in Neuron+AS system. Quantified results were normalized to β-actin expression. Values were expressed as percentages compared with the control group (set to 100%), and represented as mean ± S.E.M. (n = 8). ####P < 0.01 versus control group; and *P < 0.05, **P < 0.01 versus OGD/R group.
Furthermore, we evaluated the effect of d,l-PHPB on the expressions of anti-apoptotic protein BCL-2 and pro-apoptotic protein BAX. Compared with the control group, OGD (2 h)/R (24 h) treatment could significantly reduce the expression level of BCL-2 (P < 0.01, Fig. 3). At the concentration of 10 μmol/L, d,l-PHPB robustly increased BCL-2 level by 56.7% compared with OGD/R group (P < 0.01, Fig. 3), whereas we did not see any significant change in expression of BAX (Fig. 3). The results indicated that the anti-apoptosis effect of d,l-PHPB might be attributed to regulation of the upstream apoptosis-related genes.
3.4. d,l-PHPB promoted BDNF and NGF synthesis and secretion from astrocytes after OGD/R in neuron–astrocyte co-culture system
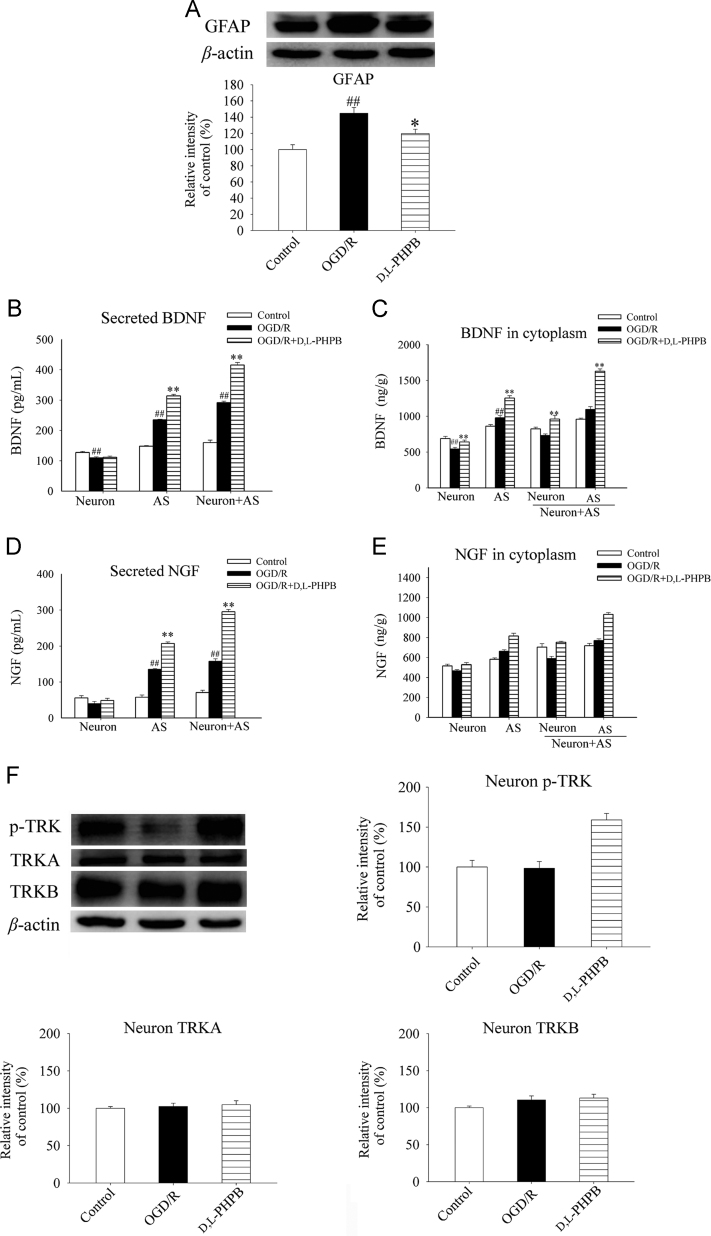
Though above results indicated that d,l-PHPB had protective effects against neuron apoptosis in the presence of astrocytes. However, whether this effect of d,l-PHPB was related to astrocytes remains to be studied. Generally, astrocytes were markedly activated and it might be accompanied with the increased expression of the special marker GFAP in the cerebral ischemia progress. In the present study, we used Western blot analysis to measure the levels of GFAP expression of astrocytes in neuron–astrocyte co-culture system after OGD (2 h)/R (24 h). The results showed that the activation of astrocytes was significantly suppressed by d,l-PHPB treatment (P < 0.05, Fig. 4A).
Figure 4.
d,l-PHPB (10 μmol/L) promoted BDNF and NGF synthesis and secretion from astrocytes after OGD/R in Neuron+AS system. (A) and (F) Representative Western blot of GFAP, p-TRKA/B and total TRKA/B, and their quantified results. Quantified results were normalized to β-actin expression. Values were expressed as percentages compared with the control group (set to 100%), and represented as mean ± S.E.M. (n = 8). ####P < 0.01 versus control group; and *P < 0.05, **P < 0.01 versus OGD/R group. (B)–(E) Levels of BDNF and NGF assayed by ELISA analysis. Values were expressed as mean ± S.E.M. (n = 6). ####P < 0.01 versus control group; and *P < 0.05, **P < 0.01 versus OGD/R group.
Astrocytes provide many supportive functions essential for neuronal survival. One critical function of astrocytes is to provide neurotropic support by synthesis and secretion of BDNF and NGF. Our studies found that BDNF and NGF were mainly synthetized and secreted from astrocytes after OGD (2 h)/R (24 h), and d,l-PHPB treatment further promoted this progress, but the levels of BDNF and NGF in neurons were not changed at the same condition (Fig. 4B–E). In addition, d,l-PHPB significantly increased the expression of phosphorylated TRKA/B on the neurons (P < 0.01), but had no effect on the expression of total TRKA or TRKB (Fig. 4F). These data suggested that the neuroprotective effect of d,l-PHPB might be mediated by BDNF/TRKB and NGF/TRKA signaling pathway. Thus, the improvement of neurotropic effect of astrocytes may be one of the mechanisms of d,l-PHPB against OGD/R-induced neuron apoptosis.
3.5. d,l-PHPB up-regulated PI3K/AKT and ERK signaling pathway in astrocytes after OGD/R in the co-culture system
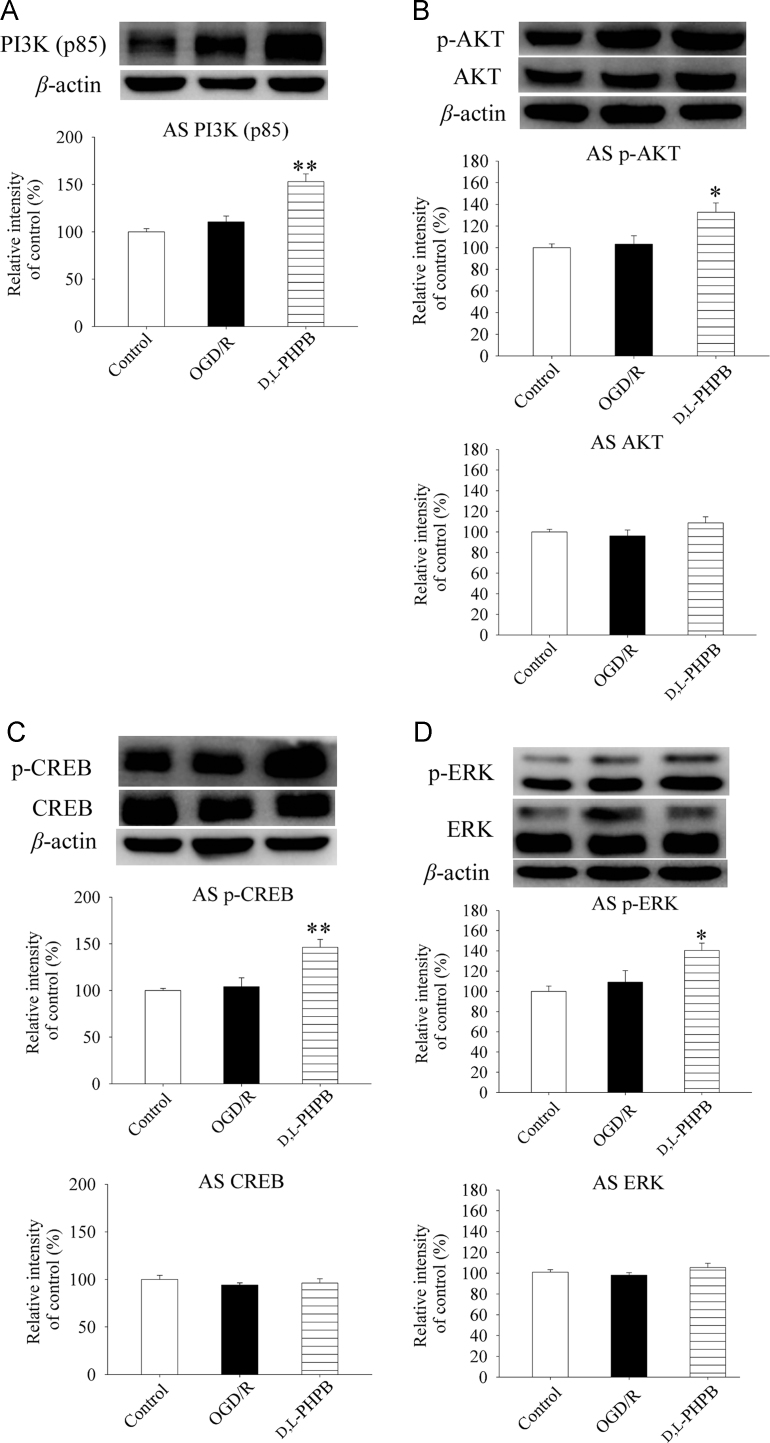
The gene transcription process of BDNF and NGF is regulated by active CREB, which is associated with phosphorylation of AKT and ERK. We further investigated the effects of d,l-PHPB on PI3K/AKT and ERK signaling pathways in astrocytes by Western blot analysis to clarify the mechanism of BDNF and NGF synthesis in astrocytes. The results showed that d,l-PHPB increased the levels of PI3K (p85), p-AKT (Ser473), p-ERK and p-CREB in astrocytes by 41.9%, 29.6%, 31.3% and 42.1%, respectively (Fig. 5A–D). Whereas, we did not found significant differences in the total AKT, ERK and CREB levels among the groups. The above results suggested that d,l-PHPB promoted BDNF and NGF synthesis in astrocytes by up-regulating PI3K/AKT and ERK signaling pathway in astrocytes.
Figure 5.
d,l-PHPB (10 μmol/L) up-regulated PI3K/AKT and ERK signaling pathway after OGD/R in Neuron+AS system. (A)–(D) Representative Western blot of PI3K(p85), p-AKT (Ser437), p-CREB and p-ERK, as well as total AKT, CREB and ERK, and their quantified results. Quantified results were normalized to β-actin expression. Values were expressed as percentages compared with the control group (set to 100%), and represented as mean ± S.E.M. (n = 8). ####P < 0.01 versus control group; and *P < 0.05, **P < 0.01 versus OGD/R group.
3.6. d,l-PHPB inhibited inflammatory cytokines secretion from astrocytes after OGD/R treatment
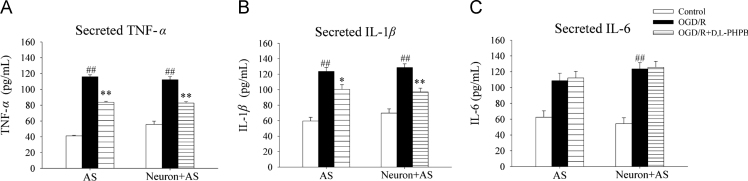
Neuroinflammation is a critical characteristic of astrocytes by the synthesis and secretion of various inflammatory cytokines. We measured the effect of d,l-PHPB on the levels of inflammatory cytokines, e.g. TNF-α, IL-1β and IL-6 in the present study. The results showed that the levels of TNF-α, IL-1β and IL-6 in the supernatant of neuron–astrocyte co-culture system after OGD (2 h)/R (24 h) were increased significantly. And the increases came mainly from astrocytes. d,l-PHPB decreased the levels of TNF-α and IL-1β, but not IL-6 (Fig. 6).
Figure 6.
d,l-PHPB (10 μmol/L) inhibited inflammatory cytokines secretion from astrocytes after OGD/R in Neuron+AS system. Levels of TNF-α, IL-1β and IL-6 assayed by ELISA analysis. Values were expressed as mean ± S.E.M. (n = 6). ####P < 0.01 versus control group; and *P < 0.05, **P < 0.01 versus OGD/R group.
3.7. d,l-PHPB down-regulated p38 but not JNK signaling pathway in astrocytes after OGD/R
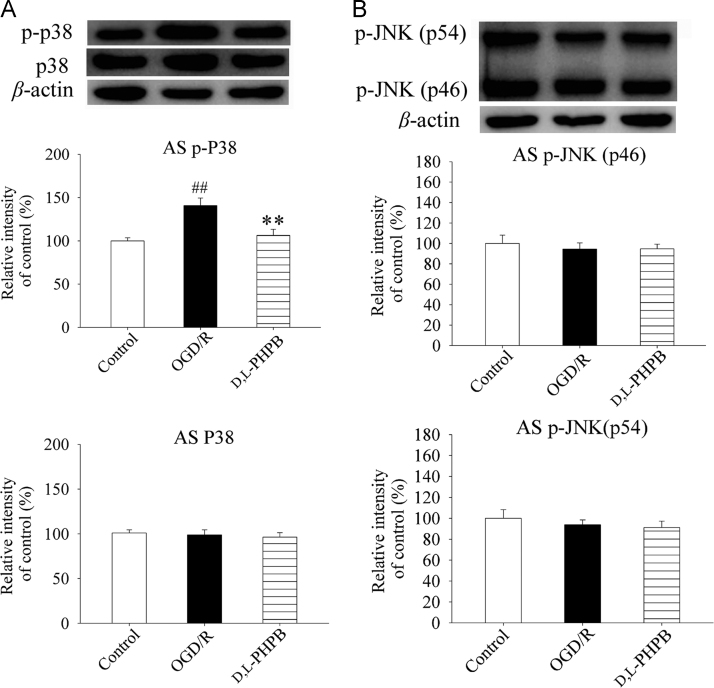
It has been shown that p38 and JNK play an important role in inflammatory cytokines production after cerebral ischemia. Thus, we further investigated the effects of d,l-PHPB on p38 and JNK signaling pathway in astrocytes by Western blot to illustrate the mechanism of d,l-PHPB on inflammatory cytokines secretion from astrocytes after OGD/R. The results showed that d,l-PHPB decreased the levels of p-p38 by 34.4% in astrocytes compared with the model group (Fig. 7A), but had no effect on the levels of p-JNK in astrocytes (Fig. 7B). The above results suggested that d,l-PHPB inhibited inflammatory cytokines production from astrocytes by down-regulating p38 signaling pathway in the co-culture system.
Figure 7.
d,l-PHPB (10 μmol/L) down-regulated p38 but not JNK signaling pathway after OGD/R in Neuron+AS system. (A) and (B) Representative Western blot of p-p38 and p-JNK, as well as total p38, and their quantified results. Quantified results were normalized to β-actin expression. Values were expressed as percentages compared with the control group (set to 100%), and represented as mean ± S.E.M. (n = 8). ####P < 0.01 versus control group; and **P < 0.01 versus OGD/R group.
4. Discussion
As a promising candidate for the treatment of cerebral ischemia, although d,l-PHPB has been shown to reduce infarct volume and improve the neurobehavioral deficits in cerebral ischemic animal model, the mechanisms of the drug candidate remain to be studied.
In order to study further the interaction between neurons and astrocytes, we established neuron–astrocyte co-culture system by using transwell chamber, where neurons were cultured in the lower chamber and astrocytes were cultured in the upper one. In this system, neurons and astrocytes shared the same medium and various ions or cytokines secreted by neurons or astrocytes could interact between both cells15. Thus, the neuron–astrocyte co-culture system was particularly suitable for the aim of the present study.
Meanwhile, we established OGD/R model in vitro to simulate ischemic and reperfusion conditions. By using Hoechst 33342 staining, we found that OGD (2 h)/R (24 h) induced obvious neuronal apoptosis; however, in the neuron–astrocyte co-culture system the number of apoptotic neurons was less than that in neuronal mono-culture system. This indicated that astrocytes protected neurons under OGD/R conditions.
Furthermore, in order to explore whether d,l-PHPB affected OGD/R-induced neuron apoptosis in the presence of astrocytes, d,l-PHPB (1 or 10 μmol/L) was given to the neuron–astrocyte co-culture system at the same time with OGD/R stimulation. It was showed that d,l-PHPB (1 or 10 μmol/L) significantly reduced the neuronal apoptotic rate induced by OGD (2 h)/R (24 h) to 20.6% in the neuron–astrocyte co-culture system. When absent of astrocytes, d,l-PHPB only reduced neuron apoptosis rate to 29.0%. The results demonstrated that the protective effects of d,l-PHPB on neurons were related with astrocytes.
The neuron apoptosis can be controlled by some apoptosis-related genes. BCL-2 family proteins play a key role in the apoptosis by regulating the permeability of the outer mitochondrial membrane. This family comprises of both pro- (e.g. BAX, BAD, BID and BIK) and anti-apoptotic members (e.g. BCL-2, BCL-w, BCL-xl). It is apparent that members of BCL-2 family interact with each other to form a dynamic equilibrium maintaining the normal cell survival16. In addition, the caspases are crucial for the cell death. It was known that caspases are synthesized as inactive pro-enzymes and are activated by the proteolytic cleavage. There are several members for the family. The caspase 3 has been identified as a key mediator of apoptosis in the neuronal cells. In this experiment, we measured the expression levels of apoptosis related protein and found that the expression of cleaved-caspase 3 after OGD (2 h)/R (24 h) was increased by 121.6% compared with the control group (P < 0.01). However, the elevation of cleaved-caspase 3 was significantly attenuated by d,l-PHPB treatment. In addition, we evaluated the effect of d,l-PHPB on the expressions of anti-apoptotic protein BCL-2 and pro-apoptotic protein BAX. The treatment of d,l-PHPB at the concentration of 10 μmol/L robustly increased BCL-2 level compared with OGD/R group. But we did not find any significant changes in protein expression of BAX. These results indicated that the anti-apoptosis effect of d,l-PHPB might be attributed to its regulation to upstream apoptosis-related genes.
In addition, the expression level of GFAP, the special marker of astrocytes17, in neuron–astrocyte co-culture system after OGD (2 h)/R (24 h) was signicantly suppressed by d,l-PHPB treatment, but its neurotrophy effect of astrocytes was improved. Concretely, BDNF and NGF were mainly synthetized and secreted from astrocytes after OGD (2 h)/R (24 h), and d,l-PHPB treatment further promoted this progress. Thus, improving neurotrophic effect of astrocytes may be an important mechanisms of d,l-PHPB against OGD/R-induced neuron apoptosis.
It is well known that the gene transcription process of BDNF and NGF is regulated by active CREB, associated with phosphorylation of AKT and ERK18, 19. Moreover, it has been reported that the activity of PI3K/AKT pathway was significantly increased during ischemia20, 21, and the activation of ERK signaling pathway was critical for delayed neuroprotection after brain ischemia22. We found that d,l-PHPB activated PI3K/AKT and ERK signaling pathway in astrocytes after OGD/R in the co-culture system. Our results suggested that d,l-PHPB promoted BDNF and NGF synthesis in astrocytes by up-regulating PI3K/AKT and ERK signaling pathway in astrocytes.
In addition, after BDNF and NGF are secreted from astrocytes, they usually affect neuronal survival by being bound to TRKB and TRKA receptors on neurons, respectively. We found in the present study that d,l-PHPB significantly increased the expression of phosphorylated TRKA/B on the neurons, but had no effect on the expression of total TRKA or TRKB. These data suggested that the neuroprotection effect of d,l-PHPB might be mediated by BDNF/TRKB and NGF/TRKA signaling pathway.
Neuroinflammation effect is another critical characteristic of astrocytes by the synthesis and secretion of various inflammatory cytokines23. Thus, we measured the effect of d,l-PHPB on the levels of inflammatory cytokines and found that the levels of TNF-α, IL-1β and IL-6 in neuron–astrocyte co-culture system after OGD (2 h)/R (24 h) increased significantly. d,l-PHPB decreased the levels of TNF-α and IL-1β, but not IL-6.
It has been shown that MAPK signaling pathways positively regulate transcription of inflammatory genes, such as those coding for TNF-α, IL-1β, and COX2. Inhibition of MAPK signaling pathways, especially p38 and JNKs, could lead to a reduction in pro-inflammatory molecule production by inflammatory cells, particularly microglia/astrocytes in which MAPK cascades are highly activated after an ischemic injury24, 25, 26, 27. We found that d,l-PHPB suppressed the expression of p-p38 in astrocytes, but had no effect on the levels of p-JNK in astrocytes. These results illustrated that d,l-PHPB inhibited inflammatory cytokines production from astrocytes, which may be related to p38 signaling pathway in astrocytes.
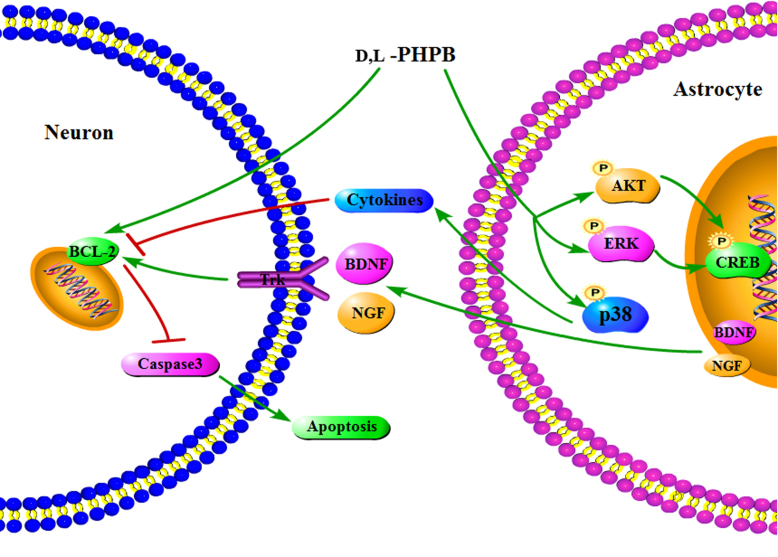
In conclusion, the present study demonstrates that d,l-PHPB plays the neuroprotective role on OGD/R-induced neuronal apoptosis, which is partially by improving the supporting function of astrocytes. The neurotrophy and neuro-inflammatory signaling pathways in astrocytes and the roles of d,l-PHPB are shown in Fig. 8.
Figure 8.
The scheme of neuroprotective mechanism of d,l-PHPB after OGD/R.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.apsb.2017.06.006.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Rossi D.J., Brady J.D., Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gouix E., Buisson A., Nieoullon A., Kerkerian-Le Goff L., Tauskela J.S., Blondeau N. Oxygen glucose deprivation-induced astrocyte dysfunction provokes neuronal death through oxidative stress. Pharmacol Res. 2014;87:8–17. doi: 10.1016/j.phrs.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Nedergaard M., Ransom B., Goldman S.A. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Iadecola C., Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci. 2011;14:1363–1368. doi: 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panickar K.S., Norenberg M.D. Astrocytes in cerebral ischemic injury: morphological and general considerations. Glia. 2005;50:287–298. doi: 10.1002/glia.20181. [DOI] [PubMed] [Google Scholar]
- 6.Rossi D., Volterra A. Astrocytic dysfunction: insights on the role in neurodegeneration. Brain Res Bull. 2009;80:224–232. doi: 10.1016/j.brainresbull.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Wang L, Li J, Wang XL. 2-(1-Hydroxypentyl)-benzoate increases cerebral blood flow and reduces infarct volume in rats model of transient focal cerebral ischemia. J Pharmacol Exp Ther 200;317:973–9. [DOI] [PubMed]
- 8.Hu Y., Peng Y., Long Y., Xu S., Feng N., Wang L. Potassium 2-(1-hydroxypentyl)-benzoate attenuated hydrogen peroxide-induced apoptosis in neuroblastoma SK-N-SH cells. Eur J Pharmacol. 2012;680:49–54. doi: 10.1016/j.ejphar.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Zhao W., Xu S., Peng Y., Ji X., Cao D., Li J. Potassium 2-(1-hydroxypentyl)-benzoate improves learning and memory deficits in chronic cerebral hypoperfused rats. Neurosci Lett. 2013;541:155–160. doi: 10.1016/j.neulet.2013.01.053. [DOI] [PubMed] [Google Scholar]
- 10.Peng Y., Hu Y., Xu S., Rong X., Li J., Li P. Potassium 2-(1-hydroxypentyl)-benzoate improves memory deficits and attenuates amyloid and τ pathologies in a mouse model of Alzheimer׳s disease. J Pharmacol Exp Ther. 2014;350:361–374. doi: 10.1124/jpet.114.213140. [DOI] [PubMed] [Google Scholar]
- 11.Jana M., Jana A., Pal U., Pahan K. A simplified method for isolating highly purified neurons, oligodendrocytes, astrocytes, and microglia from the same human fetal brain tissue. Neurochem Res. 2007;32:2015–2022. doi: 10.1007/s11064-007-9340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaech S., Banker G. Culturing hippocampal neurons. Nat Protoc. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- 13.Gouix E., Buisson A., Nieoullon A., Kerkerian-Le Goff L., Tauskela J.S., Blondeau N. Oxygen glucose deprivation-induced astrocyte dysfunction provokes neuronal death through oxidative stress. Pharmacol Res. 2014;87:8–17. doi: 10.1016/j.phrs.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Yu Y., Du J.R., Wang C.Y., Qian Z.M. Protection against hydrogen peroxide-induced injury by Z-ligustilide in PC12 cells. Exp Brain Res. 2008;184:307–312. doi: 10.1007/s00221-007-1100-3. [DOI] [PubMed] [Google Scholar]
- 15.Saeed Y., Rehman A., Xie B., Xu J., Hong M., Hong Q. Astroglial U87 cells protect neuronal SH-SY5Y cells from indirect effect of radiation by reducing DNA damage and inhibiting Fas mediated apoptotic pathway in coculture system. Neurochem Res. 2015;40:1644–1654. doi: 10.1007/s11064-015-1642-x. [DOI] [PubMed] [Google Scholar]
- 16.Cory S. Adams JM.The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 17.Marek R., Caruso M., Rostami A., Grinspan J.B., Das Sarma J. Magnetic cell sorting: a fast and effective method of concurrent isolation of high purity viable astrocytes and microglia from neonatal mouse brain tissue. J Neurosci Methods. 2008;175:108–118. doi: 10.1016/j.jneumeth.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Kim J., Lee S., Choi B.R., Yang H., Hwang Y., Park J.H. Sulforaphane epigenetically enhances neuronal BDNF expression and TrkB signaling pathways. Mol Nutr Food Res. 2017;61:1–13. doi: 10.1002/mnfr.201600194. [DOI] [PubMed] [Google Scholar]
- 19.Pius-Sadowska E., Kawa M.P., Kłos P., Rogińska D., Rudnicki M., Boehlke M. Alteration of selected neurotrophic factors and their receptor expression in mouse brain response to whole-brain irradiation. Radiat Res. 2016;186:489–507. doi: 10.1667/RR14457.1. [DOI] [PubMed] [Google Scholar]
- 20.Zhou J., Du T., Li B., Rong Y., Verkhratsky A., Peng L. Crosstalk between MAPK/ERK and PI3K/AKT signal pathways during brain ischemia/reperfusion. ASN Neuro. 2015;7:1–16. doi: 10.1177/1759091415602463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon D.S., Kwon C.H., Kim J.H., Woo J.S., Jung J.S., Kim Y.K. Signal transduction of MEK/ERK and PI3K/Akt activation by hypoxia/reoxygenation in renal epithelial cells. Eur J Cell Biol. 2006;85:1189–1199. doi: 10.1016/j.ejcb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Irving E.A., Barone F.C., Reith A.D., Hadingham S.J., Parsons A.A. Differential activation of MAPK/ERK and p38/SAPK in neurons and glia following focal cerebral ischaemia in the rat. Brain Res Mol Brain Res. 2000;77:65–75. doi: 10.1016/s0169-328x(00)00043-7. [DOI] [PubMed] [Google Scholar]
- 23.Ben Haim L., Carrillo-de Sauvage M.A., Ceyzériat K., Escartin C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci. 2015;9:278. doi: 10.3389/fncel.2015.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo R.B., Wang G.F., Zhao A.P., Gu J., Sun X.L., Hu G. Paeoniflorin protects against ischemia-induced brain damages in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses. PLoS One. 2012;7:e49701. doi: 10.1371/journal.pone.0049701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X., Zhu C., Qiu L., Hagberg H., Sandberg M., Blomgren K. Activation of ERK1/2 after neonatal rat cerebral hypoxia-ischaemia. J Neurochem. 2003;86:351–362. doi: 10.1046/j.1471-4159.2003.01838.x. [DOI] [PubMed] [Google Scholar]
- 26.Barone F.C., Irving E.A., Ray A.M., Lee J.C., Kassis S., Kumar S. Inhibition of p38 mitogen-activated protein kinase provides neuroprotection in cerebral focal ischemia. Med Res Rev. 2001;21:129–145. doi: 10.1002/1098-1128(200103)21:2<129::aid-med1003>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 27.Piao C.S., Kim J.B., Han P.L., Lee J.K. Administration of the p38 MAPK inhibitor SB203580 affords brain protection with a wide therapeutic window against focal ischemic insult. J Neurosci Res. 2003;73:537–544. doi: 10.1002/jnr.10671. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material