Abstract
Morphotype switch is a cellular response to external and internal cues. The Cryptococcus neoformans species complex can undergo morphological transitions between the yeast and the hypha form, and such morphological changes profoundly affect cryptococcal interaction with various hosts. Filamentation in Cryptococcus was historically considered a mating response towards pheromone. Recent studies indicate the existence of pheromone-independent signaling pathways but their identity or the effectors remain unknown. Here, we demonstrated that glucosamine stimulated the C. neoformans species complex to undergo self-filamentation. Glucosamine-stimulated filamentation was independent of the key components of the pheromone pathway, which is distinct from pheromone-elicited filamentation. Glucosamine stimulated self-filamentation in H99, a highly virulent serotype A clinical isolate and a widely used reference strain. Through a genetic screen of the deletion sets made in the H99 background, we found that Crz1, a transcription factor downstream of calcineurin, was essential for glucosamine-stimulated filamentation despite its dispensability for pheromone-mediated filamentation. Glucosamine promoted Crz1 translocation from the cytoplasm to the nucleus. Interestingly, multiple components of the high osmolality glycerol response (HOG) pathway, consisting of the phosphorelay system and some of the Hog1 MAPK module, acted as repressors of glucosamine-elicited filamentation through their calcineurin-opposing effect on Crz1’s nuclear translocation. Surprisingly, glucosamine-stimulated filamentation did not require Hog1 itself and was distinct from the conventional general stress response. The results demonstrate that Cryptococcus can resort to multiple genetic pathways for morphological transition in response to different stimuli. Given that the filamentous form attenuates cryptococcal virulence and is immune-stimulatory in mammalian models, the findings suggest that morphogenesis is a fertile ground for future investigation into novel means to compromise cryptococcal pathogenesis.
Author summary
Cryptococcal meningitis claims half a million lives each year. There is no clinically available vaccine and the current antifungal therapies have serious limitations. Thus identifying cryptococcal specific programs that can be targeted for antifungal or vaccine development is of great value. We have shown previously that switching from the yeast to the hypha form drastically attenuates/abolishes cryptococcal virulence. Cryptococcal cells in the filamentous form also trigger host immune responses that can protect the host from a subsequent lethal challenge. However, self-filamentation is rarely observed in serotype A isolates that are responsible for the vast majority of cryptococcosis cases. In this study, we found that glucosamine stimulated self-filamentation in genetically distinct strains of the Cryptococcus species complex, including the most commonly used serotype A reference strain H99. We demonstrated that filamentation elicited by glucosamine did not depend on the pheromone pathway, but it requires the calcineurin transcription factor Crz1. Glucosamine promotes nuclear translocation of Crz1, which is positively controlled by the phosphatase calcineurin and is suppressed by the HOG pathway. These findings raise the possibility of manipulating genetic pathways controlling fungal morphogenesis against diseases caused by the Cryptococcus species complex.
Introduction
The opportunistic environmental fungal pathogen, Cryptococcus neoformans, is a leading killer of HIV-infected patients worldwide [1, 2]. It causes 1 million infections and more than half a million deaths each year worldwide [1]. C. neoformans is a species complex containing several serotypes (A, D, and AD hybrids) [3]. Among these serotypes, serotype A (C. neoformans var. grubii) causes approximately 95% of all cryptococcosis cases [4, 5], whereas serotype D (C. neoformans var. neoformans) is responsible for about 5% of the cases. The current anti-cryptococcal treatments rely primarily on azole antifungals with or without the induction therapy with amphotericin B [6]. The mortality rates of cryptococcosis are unacceptably high (~10–75%) [1, 7–9]. To further compound the problem, the emergence of resistance to azole drugs has been observed in multiple regions around the world [10–14] and relapse frequently occurs following treatment largely due to failure to clear the original infection [12, 15]. Thus, it is of great value to identify cryptococcal specific programs that can be used for new antifungal or vaccine development.
Morphotype switch between yeast and hypha is a cellular adaptation tightly linked to the virulence of dimorphic fungal pathogens [16–18]. In Candida albicans, proteins associated with hyphal growth are shown to impact its pathogenicity and some of them provide the bases for vaccine development [19–23]. Our previous studies in C. neoformans demonstrated that morphotypes (yeast or filament) are tightly linked to pathogenicity of this fungus as well [24–26]. Znf2, the master regulator of filamentation, is a potent anti-virulent factor. Deletion of this zinc finger transcription factor locks cells in yeast form and enhances fungal virulence in a murine model of cryptococcosis. Conversely, overexpression of ZNF2 drives cells to the hyphal form and attenuates/abolishes the ability of C. neoformans to cause fatal infections [24–26]. Cryptococcus cells with ZNF2 overexpression stimulate protective immune responses in the host and provide protection to the animal against a subsequent challenge by the highly virulent serotype A clinical isolate H99 [26]. These findings indicate that activation of the filamentation program could drastically compromise cryptococcal virulence. Thus, the yeast-to-hypha morphological transition provides an important avenue to explore alternative measures for the prevention and/or treatment of cryptococcal infections.
C. neoformans species is not considered a conventional dimorphic fungus due to the historical association of the yeast-to-hypha transition with mating. The mating response is controlled by the pheromone pathway composed of the pheromone, the pheromone receptor, the Cpk1 mitogen-activated protein kinase (MAPK) module, and the ultimate HMG domain transcription factor Mat2 [25, 27–32]. The pheromone pathway promotes self-filamentation during unisexual development or dikaryotic filamentation during a-α bisexual development. As expected, the pheromone pathway is activated under mating-inducing conditions (e.g. dehydration, nutrition limitation, V8 juice, and darkness). However, the host environment is not favorable for mating and the pheromone pathway exerts no or minimal impact on virulence [25, 29, 33, 34]. Recent studies with C. neoformans serotype D isolates indicate that the pheromone pathway is essential for filamentation during a-α bisexual mating [24, 25, 35–37], but it is not necessary for self-filamentation in a unisexual population under certain conditions [38–41]. Given the largely unisexual population of C. neoformans (α >99%, a <1%), it is important to identify pheromone-independent pathways that can control self-filamentation.
Although all serotypes of the C. neoformans species complex are expected to possess the ability to undergo self-filamentation, self-filamentation is often observed in serotype D isolates and rarely in serotype A isolates [42–45], including the highly virulent clinical isolate and the most widely used serotype A reference strain H99 [46–48]. This hinders the investigation of morphological transition in C. neoformans as many resources are generated for the H99 background, including a congenic pair, gene deletion sets, a well-annotated genome, and vast literatures about cryptococcal biology and pathology [49–52]. The rarity of self-filamentation in serotype A isolates challenge the possibility of mitigating the diseases caused by the C. neoformans species complex through activating the filamentation program.
Here, we found that glucosamine stimulated self-filamentation in both serotype D and serotype A strains, including H99. Although we found that both N-acetyl-glucosamine (GlcNAc) and glucosamine could stimulate filamentation in another fungal pathogen C. albicans, GlcNAc showed no effect on filamentation in C. neoformans. We demonstrated that filamentation in C. neoformans evoked by glucosamine was independent of the pheromone pathway. By genetic screens, we discovered that Crz1, a transcription factor downstream of calcineurin, was required for this process. The requirement of Crz1 for filamentation is specific to the response elicited by glucosamine, as Crz1 is not critical for filamentation elicited by pheromone [38]. We demonstrated that glucosamine promoted the translocation of Crz1 from the cytoplasm to the nucleus where it could exert its function as a transcription factor. Not surprisingly, we found that the catalytic and regulatory subunits of the phosphatase calcineurin, Cna1 and Cnb1, were essential for the nuclear translocation of Crz1 and for filamentation. Interestingly, multiple components in the HOG pathway, except Hog1 itself, acted as repressors of glucosamine-elicited filamentation through their calcineurin-opposing effect on Crz1’s nuclear translocation. Deletion of these kinases increased the basal level of nucleus-localized Crz1. These findings indicate that C. neoformans can resort to different genetic pathways for morphological transition in response to different stimuli, paving the way for future investigation to identify signals and targets that can be used to manipulate morphogenesis of this fungal pathogen in vivo.
Results
Glucosamine stimulates H99 and other strains to undergo self-filamentation
Wild-type H99 does not undergo self-filamentation under all mating-inducing conditions. Here, we decided to test the effect of different carbon sources based on previous studies in other dimorphic fungal pathogens such as Candida albicans [53–56], Histoplasma capsulatum, and Blastomyces dermatitidis [57] where N-acetyl-glucosamine (GlcNAc) activates hyphal growth. Here, we used YP medium (1% yeast extract and 2% peptone) as the base medium and supplemented it with different carbon sources at the final concentrations of 2%. We included 6-carbon sugars (glucose, galactose, and inositol), amino sugars (N-methyl-glucosamine, N-acetyl glucosamine, and glucosamine), and other carbon sources (glycerol, ethanol, and sodium acetate). None of the carbon sources tested stimulated filamentation in H99, with the exception of glucosamine (Fig 1A). Filamentation induced by glucosamine was unlikely to be an effect of carbon repression, as the non-metabolizable glucose analog 2-deoxyl glucose did not trigger filamentation in H99 (Fig 1A). The filamentation stimulated by glucosamine was also unlikely to be a general effect due to the activation of the hexamine metabolism pathway, as N-methyl-glucosamine and N-acetyl glucosamine both failed to stimulate filamentation in H99 (Fig 1A).
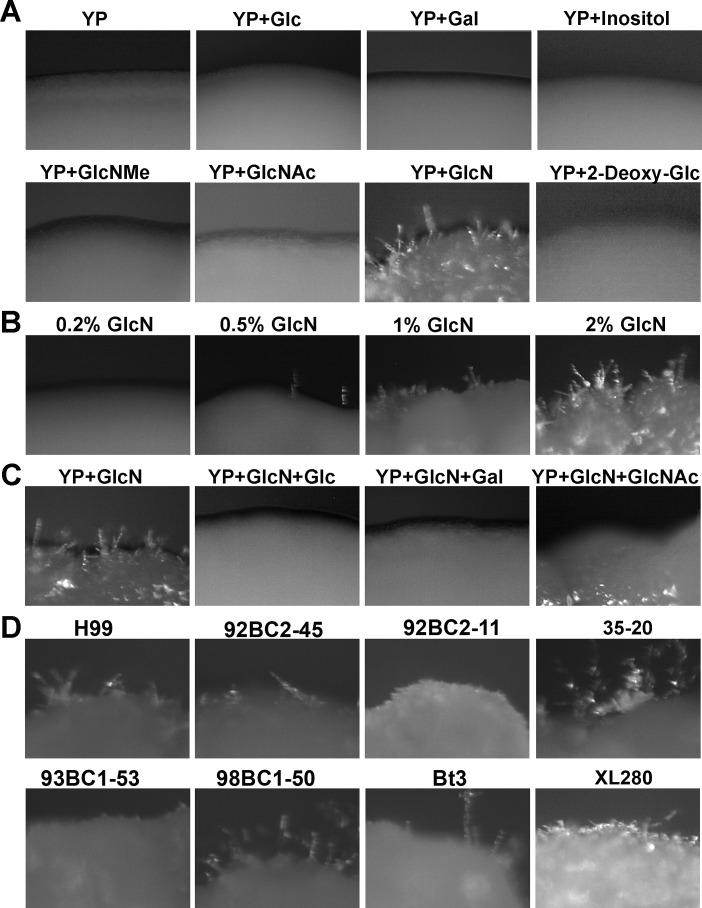
Fig 1. Glucosamine stimulates self-filamentation in H99 and other cryptococcal isolates.
(A) The effect of the addition of different six-carbon sugars and hexamines at 2% to YP base medium. H99 was cultured on YP, YP+Glc (glucose), YP+Gal (galactose), YP+Inositol, YP+GlcNMe (N-Methyl-glucosamine), YP+GlcNAc (N-Acetyl-glucosamine), YP+GlcN (glucosamine), and YP+2-Dexoyl-Glc (2-Deoxyl-glucose) for 7 days. (B) The dose-dependent effect of glucosamine on self-filamentation in H99. H99 was cultured on YP+GlcN at final concentration of 0, 0.2%, 0.5%, 1%, and 2% for 7 days. (C) The inhibitory effect of other carbon sources on GlcN-induced filamentation. H99 was cultured on the indicated media for 7 days. H99 cultured on YP+GlcN (2%) was used as control. Other carbon sources, such as glucose, galactose, or N-Acetyl-glucosamine (2%) were added to the YP+GlcN medium. (D) The effect of glucosamine on filamentation is not specific to H99. 92BC2-45 (serotype A), 92BC2-11 (serotype A), 93BC1-53 (serotype D), 35–20 VNI (serotype A), 98BC1-50 (serotype D), Bt3 strain (serotype A), and XL280α (serotype D) were cultured on YP+GlcN medium for 7 days.
The effect of glucosamine on filamentation was dose-dependent, as glucosamine at lower concentrations (<0.5%) did not evoke obvious hyphal growth in H99 (Fig 1B). Robust filamentation was observed when glucosamine was present at concentrations higher than 1% (Fig 1B). The addition of other carbon sources (e.g. glucose, galactose, or GlcNAc) inhibited filamentation in H99 (Fig 1C). This suggests potential competitive inhibition of glucosamine by other carbon sources. Although not all strains could produce hyphae on the glucosamine medium, glucosamine-stimulated filamentation was not limited to H99. Some other isolates of either serotype A or serotype D (e.g. XL280) self-filamented on glucosamine medium (Fig 1D). Interestingly, glucosamine-stimulated filamentation not only in C. neoformans, but also in some C. albicans strains (S1 Fig). This suggests that glucosamine could be a general signal for fungal morphogenesis.
Glucosamine-stimulated filamentation requires Znf2 but not the pheromone sensing pathway controlled by Mat2
C. neoformans typically undergoes yeast-to-hypha transition during a-α bisexual mating or during unisexual development. Two transcription factors, Mat2 and Znf2, were demonstrated to be critical for hyphal growth during sexual development [24, 25, 37]. Mat2 controls the pheromone pathway and plays a central role in cell fusion [25]. Under mating-inducing conditions (e.g. on V8 medium), Mat2 activates Znf2, the master regulator of filamentation [24, 25]. However, under mating-suppressing conditions (e.g. on YPD medium), overexpression of MAT2 fails to activate Znf2 despite high levels of pheromone and C. neoformans remains in the yeast form [24].
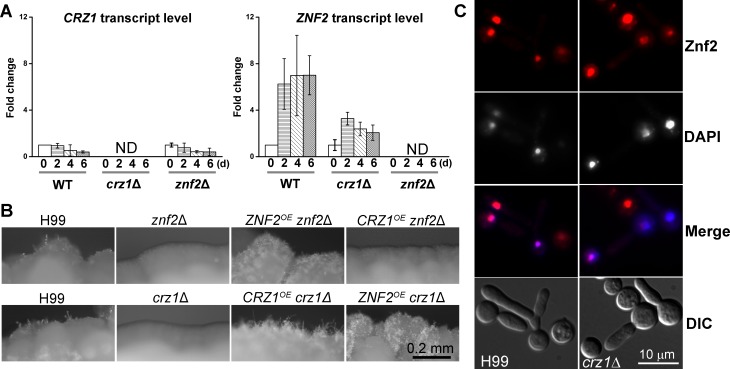
We first examined the effect of glucosamine on the activity of Mat2 and Znf2 in wild-type H99. We measured the transcript levels of their target genes, which reflected the activities of these transcription factors. The pheromone gene MFα is the most upregulated gene controlled by Mat2 during both bisexual and unisexual development [25, 37]. The filamentation marker gene CFL1 is one of the highly expressed genes upregulated by Znf2 [24, 58, 59]. We found that the transcript levels of MFα and CFL1 increased more than 100 and 300 folds respectively when H99 was cultured on glucosamine medium compared to that of the base medium at 96 hours (Fig 2A), indicating the activation of both Mat2 and Znf2.
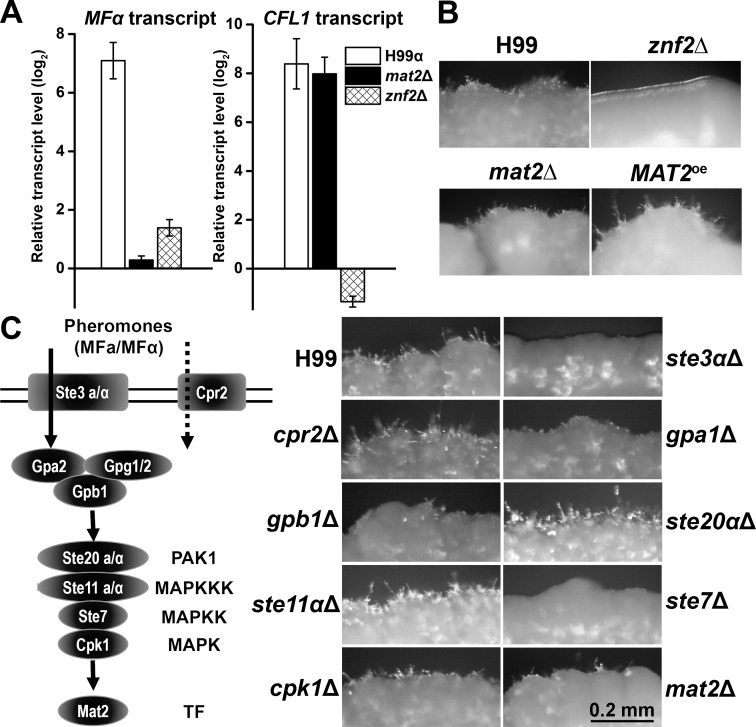
Fig 2. Glucosamine-stimulated filamentation is independent of the Mat2-controlled pheromone pathway but requires the morphogenesis regulator Znf2.
(A) The CFL1 and MFα transcript levels in wild-type H99, the znf2Δ mutant, and the mat2Δ mutant on glucosamine medium for 4 days relative to those on the control YP medium. The house keeping gene TEF1 is used as the internal control. (B) The wild-type H99 strain, the znf2Δ mutant, the mat2Δ mutant, and the MAT2oe strain were cultured on YP+GlcN medium for 6 days. (C) The left panel depicts some of the key components of the pheromone pathway. The right panel shows images of colonies of wild-type H99, the ste3Δ mutant, the cpr2Δ mutant, the gpa1Δ mutant, the gpb1Δ mutant, the ste20αΔ mutant, the ste11αΔ mutant, the ste7Δ mutant, the cpk1Δ mutant, and the mat2Δ mutant cultured on medium for 7 days.
To examine if self-filamentation in H99 evoked by glucosamine relies on Mat2 or/and Znf2, we tested the znf2Δ mutant and the mat2Δ mutant on glucosamine medium. As expected, the MFα transcript level was no longer induced by glucosamine in the mat2Δ mutant (Fig 2A). By contrast, a strong induction of the filamentation marker CFL1 at a level comparable to that in wild-type H99 was observed in the mat2Δ mutant (Fig 2A). Consistent with the expression of the filamentation marker CFL1, the mat2Δ mutant self-filamented on glucosamine medium (Fig 2B). A strain overexpressing MAT2 (MAT2oe) also self-filamented on glucosamine medium. The result indicates that Mat2 is not essential for glucosamine-stimulated filamentation.
In contrast to the mat2Δ mutant, there was no increase but rather a modest reduction in the CFL1 transcript level in the znf2Δ mutant on glucosamine medium compared to that of the base medium (Fig 2A). The MFα transcript level increased slightly (~4 fold) in this mutant (Fig 2A). The low level of CFL1 in the znf2Δ mutant was consistent with its non-filamentous phenotype on glucosamine medium (Fig 2B). Collectively, these observations indicate that Znf2, but not Mat2, is required for glucosamine-stimulated filamentation in H99. The self-filamentation observed in H99 and the mat2Δ mutant was a response to glucosamine. The wild-type H99, the mat2Δ mutant, or the znf2Δ mutant is incapable of self-filamentation on V8 medium (S2 Fig).
To determine if the dispensability of Mat2 and the essentiality of Znf2 in glucosamine-stimulated filamentation are conserved in C. neoformans, we further tested the mat2Δ mutant and the znf2Δ mutant made in the serotype D XL280 background. No hyphal growth was observed in the znf2Δ mutant while the mat2Δ mutant filamented similarly as the wild-type control on glucosamine medium (S2 Fig). This is again different from filamentation observed on mating-inducing V8 medium where Mat2 is required (S2 Fig)[25, 38]. This result corroborates the conclusion that filamentation elicited by glucosamine requires the morphogenesis regulator Znf2, but not the pheromone pathway regulator Mat2.
To further verify that the pheromone pathway is not critical for glucosamine-stimulated filamentation, we tested additional mutants in the H99 background with disruption in the following key components of this pathway (Fig 2C), namely the pheromone receptor Ste3 [60, 61], the pheromone receptor like protein Cpr2 [62], Gβ subunit Gpb1 [64], a PAK kinase Ste20α [65], the MAPK kinase kinase Ste11α [33], the MAPK kinase Ste7 [32], and the MAPK Cpk1 [32]. We also included Gα subunit Gpa1 [63] that regulates mating through the cAMP/PKA pathway in our test. Except for the ste3αΔ and the ste7Δ mutants, all other gene deletion mutants tested filamented on glucosamine medium (Fig 2C). The ste3αΔ could eventually produce some filaments after prolonged incubation. To verify that the blocked filamentation observed in the ste7Δ mutant is not an artifact, we tested multiple ste7Δ isolates generated in both mating type a and α backgrounds. All the ste7Δ mutants tested showed only yeast growth on glucosamine medium, indicating the unique role of Ste7 in filamentation compared to other components of the pheromone pathway. Collectively, the results indicate that the pheromone pathway overall is dispensable for filamentation induced by glucosamine.
Identify the possible pathways involved in glucosamine-stimulated self-filamentation through genetic screens
To identify genes that are involved in filamentation triggered by glucosamine, we screened approximately 2500 gene deletion mutants made in the H99 background for altered filamentation on glucosamine medium. The strains screened included the partial genome deletion set generated by Dr. Hiten Madhani’s group in 2015, and the transcription factor and the kinase deletion sets generated by Dr. Yong-Sun Bahn’s group [66, 67]. Among the deletion mutants tested, two genes encoding the glucosamine-6-phosphate deaminase Gnd1 (gene locus # CNAG_06098) and the glucosamine 6-phosphate N-acetyltransferase Gnat1 (gene locus # CNAG_05695) are involved in the hexamine metabolism pathway (S3A Fig). The gnd1Δ mutant was unable to grow in the presence of glucosamine (even at concentrations lower than 0.1%) (S3B Fig), suggesting that the Gnd1 is essential for the growth of C. neoformans under such conditions. GNAT1 was not essential for growth on glucosamine medium. However, the gnat1Δ mutant filamented as well as, if not better than, the wild-type H99 on glucosamine medium (S3C Fig). The result suggests that the hexamine metabolism is unlikely to be responsible for the filamentous growth elicited by glucosamine.
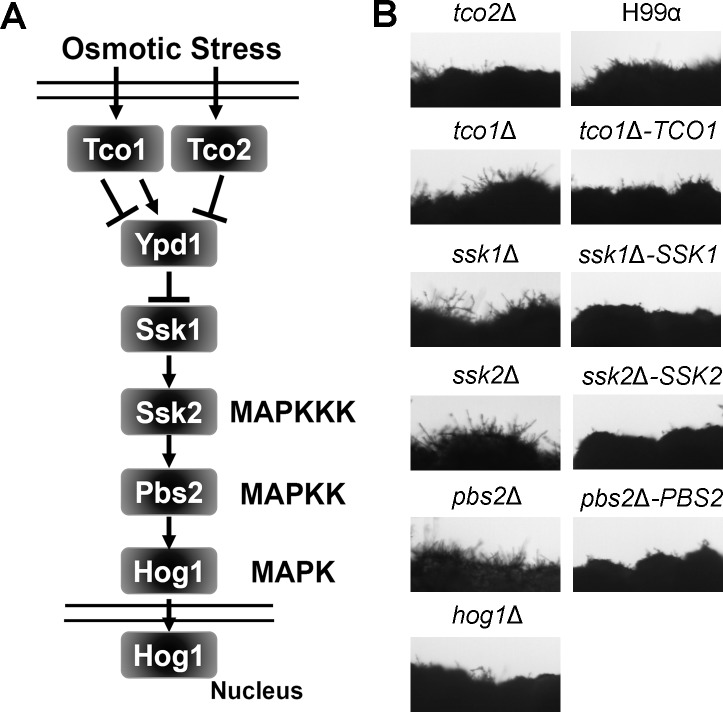
We classified the mutants screened with altered filamentation into four groups: non-filamentous group, decreased filamentation, increased filamentation, and hyper-filamentation (S4 Fig and S1 Table). The fact that mutants with reduced/abolished filamentation and mutants with enhanced filamentation were recovered from the screen indicates that there are both repressors and activators of filamentation in response to glucosamine. Among mutants with enhanced filamentation, several were in the HOG pathway [68, 69] (Fig 3A), including the tco1Δ, ssk1Δ, ssk2Δ, and pbs2Δ mutants (Fig 3B). However, disruption of Hog1 itself, the downstream MAPK of this pathway, did not impact filamentation (Fig 3B). This observation suggests that glucosamine may not trigger the same response as osmotic stress. Consistent with this idea, the crz1Δ mutant is as resistant to osmotic stress caused by NaCl as the wild type (more details later. See S8 Fig).
Fig 3. Multiple components of the HOG pathway suppress filamentation on glucosamine medium.
(A) A diagram depicting the major components of the osmotic sensing HOG pathway in Cryptococcus. (B) The wild-type H99, the tco1Δ mutant, the tco1Δ-TCO1 complemented strain, the tco2Δ mutant, the ssk1Δ mutant, the ssk1Δ-SSK1 complemented strain, the ssk2Δ mutant, the ssk2Δ-SSK2 complemented strain, the pbs2Δ mutant, the pbs2Δ-PBS2 complemented strain, and the hog1Δ mutant were cultured on glucosamine medium (1.6% glucosamine) for 7 days.
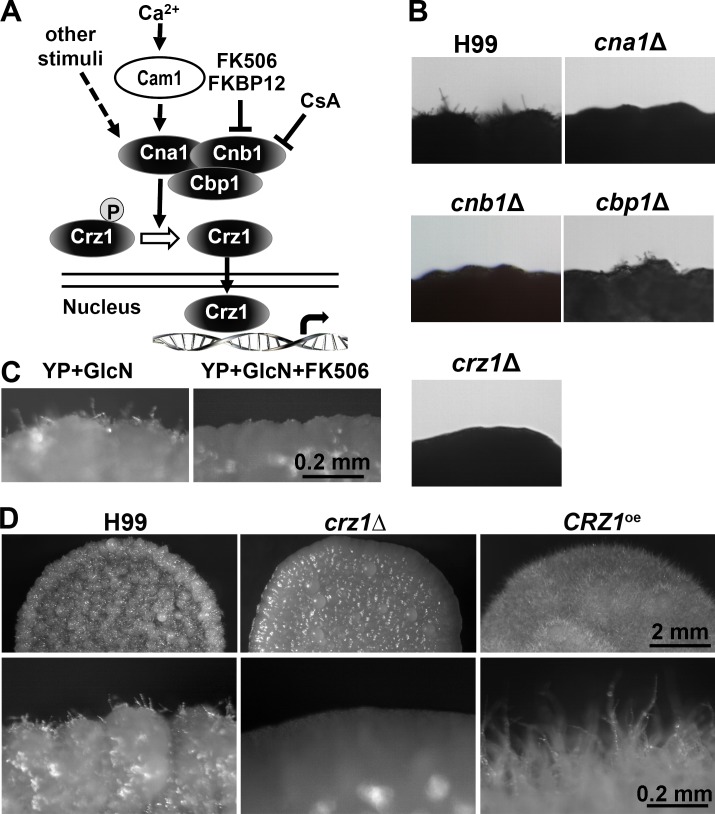
Among the gene deletion mutants that showed blocked filamentation on glucosamine medium were the calcineurin mutants. These include the mutants with disruption in genes encoding the calcineurin catalytic subunit Cna1 [70, 71], the calcineurin regulatory subunit Cnb1 [70, 72], and the calcineurin downstream zinc finger transcription factor Crz1 (aka Sp1) [73–75] (Fig 4A and 4B, S1 Table). The mutant defective in the calcineurin binding protein Cbp1 [76, 77] showed reduced filamentation (Fig 4B). Treatment with the calcineurin inhibitor FK506 blocked the wild-type H99 from undergoing filamentation on glucosamine medium (Fig 4C), a phenotype similar to the cna1Δ, cnb1Δ, and crz11Δ mutants (Fig 4B).
Fig 4. The calcineurin pathway is required for glucosamine-induced self-filamentation.
(A) A diagram depicting the calcineurin pathway. (B) The wild-type H99, the cna1Δ mutant, the cnb1Δ mutant, the cbp1Δ mutant, and two independent crz1Δ mutants were cultured on glucosamine medium for 7 days. (C) The wild-type H99 was cultured on glucosamine medium with or without the calcineurin inhibitor FK506. (D) The wild-type H99, the crz1Δ mutant, and the CRZ1oe strain (PGPD1-CRZ1) were cultured on glucosamine medium for 7 days.
Thus, the two pathways appear to exert opposing effects on glucosamine-stimulated self-filamentation in H99: the phosphorelay system and Ssk2-Pbs2 upstream of the Hog1 MAPK pathway suppress filamentation while the calcineurin pathway is required for filamentation.
Crz1 controls glucosamine-stimulated filamentation and functions upstream of Znf2
Calcineurin transduces signals (e.g. elevated level of calcium) by dephosphorylating the downstream targets (Fig 4A). The transcription factor Crz1 is one of the targets of calcineurin, and not all responses controlled by calcineurin depend on Crz1 [78, 79]. For instance, the cna1Δ and cnb1Δ mutants displayed severe growth defect at 37°C and these mutants were hyper-sensitive to cell wall stress induced by Calcofluor White or Congo red [73–75, 78] (S5 Fig). By contrast, the crz1Δ mutant showed only slightly increased sensitivity to cell wall stress and heat stress (S5 Fig). Nonetheless, the crz1Δ mutant, like the calcineurin mutants (cna1Δ and cnb1Δ), was abolished in filamentation induced by glucosamine (Fig 4B). This suggests that Crz1 is a major effector of the calcineurin pathway in regulating filamentation in response to glucosamine.
We then examined if overexpression of CRZ1 could promote filamentation on glucosamine medium. To this end, we placed the CRZ1 gene under the control of the constitutively active GPD1 promoter [24, 80]. We introduced these constructs into the wild-type H99 or the crz1Δ mutant. We found that overexpression of CRZ1 enhanced filamentation (Fig 4D and Fig 5B). The enhancement in filamentation by CRZ1 overexpression was specific to the induction by glucosamine, as CRZ1 overexpression did not confer self-filamentation to either wild-type H99 or the corresponding crz1Δ mutant when cells were cultured alone on V8 medium (S6A Fig). Furthermore, the deletion of CRZ1 or the overexpression of CRZ1 did not affect the ability of the strain to cross with a wild-type partner of the opposite mating type based on the observation that there was no notable difference between the crosses crz1Δ α x a, CRZ1oe α x a, and α x a (S6B Fig). These findings suggest that alteration of the expression level of CRZ1 does not impact mating efficiency controlled by the pheromone pathway, consistent with the recent finding in a serotype D strain [38].
Fig 5. Crz1 acts upstream of Znf2 in regulating filamentation induced by glucosamine.
(A) Transcript levels of CRZ1 and ZNF2 in the wild-type H99, the crz1Δ mutant, and the znf2Δ mutant cultured on glucosamine medium for 2 days, 4 days, and 6 days compared to the control of 0 days. (B) The wild-type H99, the znf2Δ mutant, the ZNF2oe znf2Δ strain, the CRZ1oe znf2Δ strain, the crz1Δ mutant, the ZNF2oe crz1Δ strain, and the CRZ1oe crz1Δ strain were cultured on glucosamine medium for 7 days. The overexpression of both CRZ1 and ZNF2 was driven by the constitutively active GPD1 promoter and the inducible CTR4 promoter respectively. (C) The subcellular localization of mCherry-Znf2 in the crz1Δ mutant and in the wild-type strain H99 on glucosamine medium. DAPI was used to indicate nuclear localization.
To examine the genetic relationship between Crz1 and Znf2 in the regulation of filamentation in response to glucosamine, we first measured the transcript levels of ZNF2 and CRZ1 in the znf2Δ mutant and the crz1Δ mutant. The CRZ1 transcript level on glucosamine medium was comparable to that of the base medium in wild type and its transcript level was also comparable between the wild type and the znf2Δ mutant (Fig 5A). This result indicates that neither Znf2 nor glucosamine has much impact on CRZ1 at the transcript level. On the other hand, the ZNF2 transcript level in wild-type H99 increased more than 6 fold on glucosamine medium and the degree of induction was much reduced in the crz1Δ mutant (2–3 fold) (Fig 5A). This suggests that deletion of CRZ1 attenuated the induction of ZNF2 elicited by glucosamine. Furthermore, overexpression of CRZ1 failed to confer filamentation to the znf2Δ mutant while overexpression of ZNF2 restored filamentation in the crz1Δ mutant on glucosamine medium (Fig 5B). Collectively, these epistatic results indicate that Crz1 functions upstream of Znf2 in response to glucosamine.
We then examined if disruption of Crz1 affects the subcellular localization of Znf2 after the Znf2 protein is made. For this purpose, we introduced the PCTR4-mCherry-ZNF2 construct into the crz1Δ mutant and the wild-type H99 background. The mCherry-Znf2 signal was localized to the nucleus in both the crz1Δ mutant background and the wild-type background (Fig 5C). Collectively, the results suggest that Crz1 regulates ZNF2 at the transcript level and it functions upstream of Znf2, and Crz1 does not affect the subcellular localization of the Znf2 protein.
Crz1 translocates from the cytosol to the nucleus in response to glucosamine
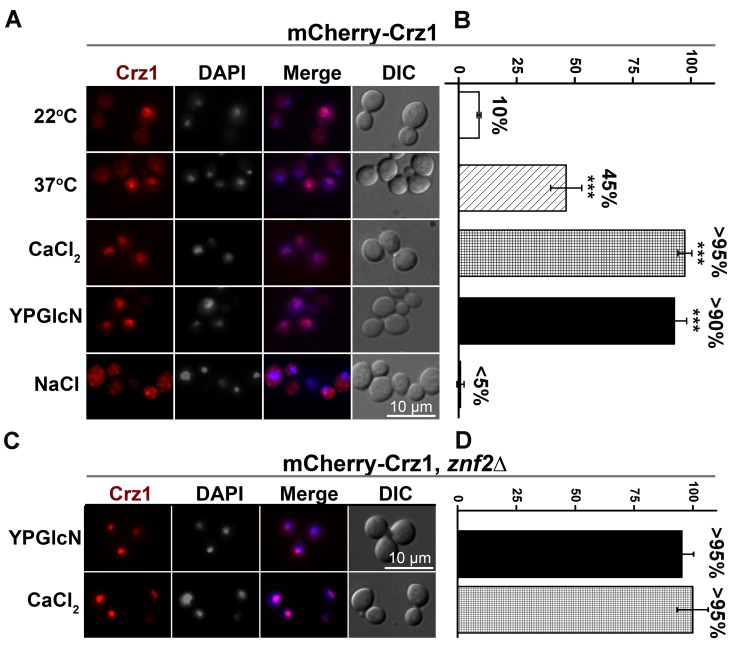
Calcineurin is known to dephosphorylate Crz1 in response to certain stimuli like calcium or heat shock. Dephosphorylation causes the translocation of Crz1 from the cytosol to the nucleus for it to function as a transcription factor in C. neoformans [73–75] (Fig 4A). To examine if glucosamine affects the subcellular translocation of Crz1, we placed mCherry tagged Crz1 under the control of the constitutively active GPD1 promoter. The exposure to either calcium or high temperature, two known stimuli of calcineurin, indeed stimulated mCherry-Crz1 in this overexpression strain to relocate from the cytosol into the nucleus (Fig 6A). As reported previously [75], NaCl induced granular localization of Crz1 in the cytosol (Fig 6A), indicating that the nuclear translocation of Crz1 is stimulus-specific. We then tested the effect of glucosamine on the subcellular localization of Crz1. Remarkably, greater than 90% of the cryptococcal population showed nuclear localization of Crz1 in the presence of glucosamine (Fig 6A and 6B). This indicates that glucosamine, like calcium, greatly increases the translocation of Crz1 from the cytosol into the nucleus. The translocation of Crz1 to the nucleus in response to calcium and glucosamine was not affected by Znf2 (Fig 6C and 6D), consistent with Crz1 functioning upstream of Znf2. To verify that the nuclear translocation effect of glucosamine was not an artifact due to CRZ1 overexpression, we tested Crz1-mCherry placed under the control of its native promoter that was used in a recent study [79]. Again, glucosamine greatly increased the population of cryptococcal cells with nucleus-localized Crz1, as did calcium and the exposure to high temperature (S7 Fig). This finding indicates that glucosamine enhances nuclear translocation of the Crz1 protein regardless of its gene expression level.
Fig 6. Glucosamine stimulates Crz1 translocation to the nucleus.
(A) Localization of mCherry-Crz1 under different the indicated conditions. To test temperature’s effect on the subcellular localization of mCrz1, the strain PGPD1-mCherry-CRZ1 was cultured in YPD liquid at 22°C (first row, or 37°C (2nd row) with shaking overnight. To test the effect of calcium or NaCl on the subcellular localization of mCrz1, cells of the strain PGPD1-mCherry-CRZ1 were collected from an overnight culture in liquid YPD at 22°C and then suspended in 100 mM CaCl2 or 1.5 M NaCl solution for 10–20 min (3rd and 5th rows). To test the effect of glucosamine on mCrz1’s localization, the strain PGPD1-mCherry-CRZ1 was cultured in YPGlcN liquid medium for 12 hours at 22°C (4th row). (B) Quantification of the percent of cells with mCherry-Crz1 located to the nucleus under the conditions used in panel A. (n≥60) (*** p<0.001). (C) Cells of the strain mCherry-CRZ1/znf2Δ were tested for the effect of glucosamine and CaCl2 on the localization of mCherry-Crz1 as described in panel A. (D) Quantification of the percentage of cells with mCherry-Crz1 located to the nucleus under the same conditions used in panel C.
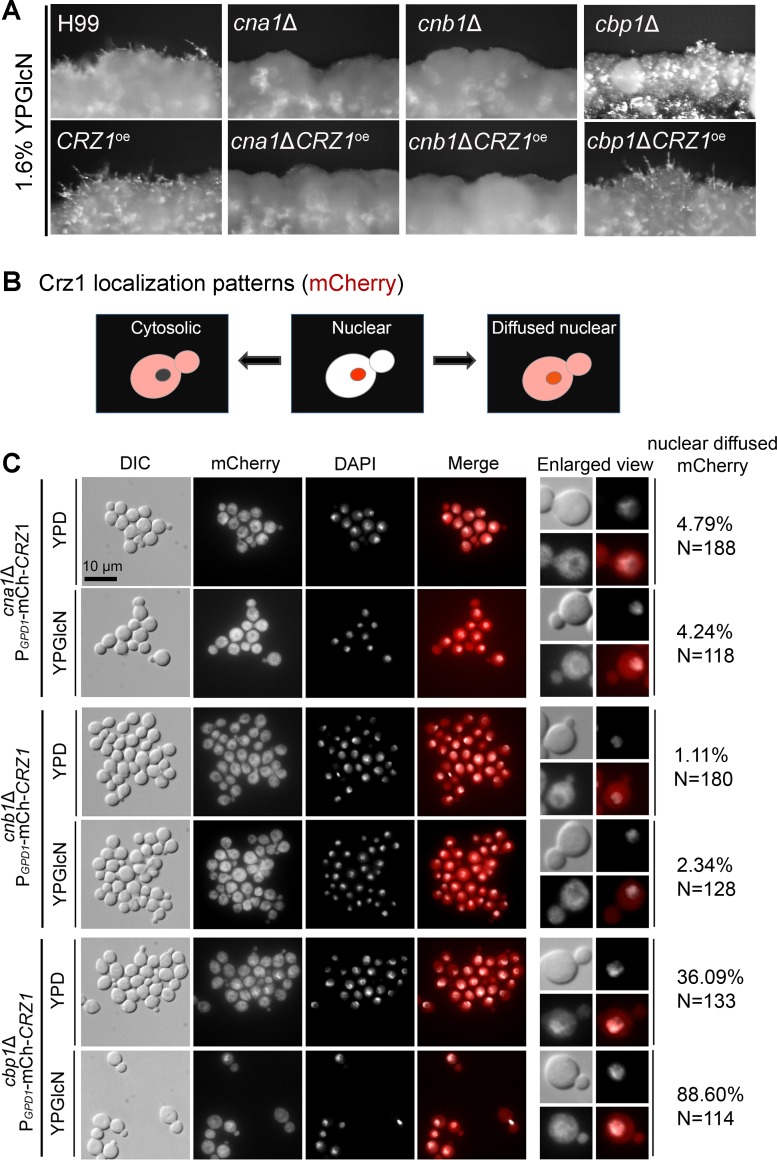
If glucosamine activates filamentation through its effect on the translocation of Crz1, we hypothesized that overexpression of CRZ1 would be futile in the absence of a functional calcineurin. Indeed, no filamentation was observed when CRZ1 was overexpressed in the cna1Δ mutant or the cnb1Δ mutant (Fig 7A). Similarly, overexpression of CRZ1 did not restore the temperature sensitivity of the cna1Δ mutant (S8 Fig). Consistent with our hypothesis, Crz1 showed only cytoplasmic localization in the calcineurin cna1Δ and cnb1Δ mutants, regardless whether the cells were cultured in YPD medium or in glucosamine medium (Fig 7B and 7C). The cbp1Δ mutant showed reduced filamentation and overexpression of CRZ1 in cbp1Δ restored filamentation (Fig 7A). Consistently, Crz1 was more concentrated in the nucleus in this mutant background (Fig 7B and 7C). The results demonstrate the essential role of calcineurin in controlling the nuclear translocation of Crz1, which correlates with robustness in filamentation.
Fig 7. Crz1 depends on calcineurin for its nuclear localization and its regulation of filamentation on glucosamine medium.
(A) Strains cna1Δ, cna1ΔPGPD1-mCherry-Crz1, cnb1Δ, cnb1ΔPGPD1-mCherry-Crz1, cbp1Δ, and cbp1ΔPGPD1-mCherry-Crz1 were cultured on YP+GlcN medium for 7 days. (B) a diagram depicting the different localization patterns of Crz1: diffused in the cytosol with nuclear exclusion (left); localized to the nucleus (middle); localized to both cytoplasm and the nucleus but more concentrated in the nucleus (right), (C) Strains cna1ΔPGPD1-mCherry-Crz1, cnb1ΔPGPD1-mCherry-Crz1, and cbp1ΔPGPD1-mCherry-Crz1 were cultured either in YPD medium or in YP+GlcN medium overnight. The mCherry-Crz1 signal showed diffused cytoplasmic localization in the cna1Δ and cnb1Δ mutants under both conditions. The mCherry-Crz1 signal showed diffused cytoplasmic localization but with more concentrated signals in the nucleus in the cbp1Δ mutant.
Components of the HOG pathway suppress filamentation by regulating the subcellular localization of Crz1
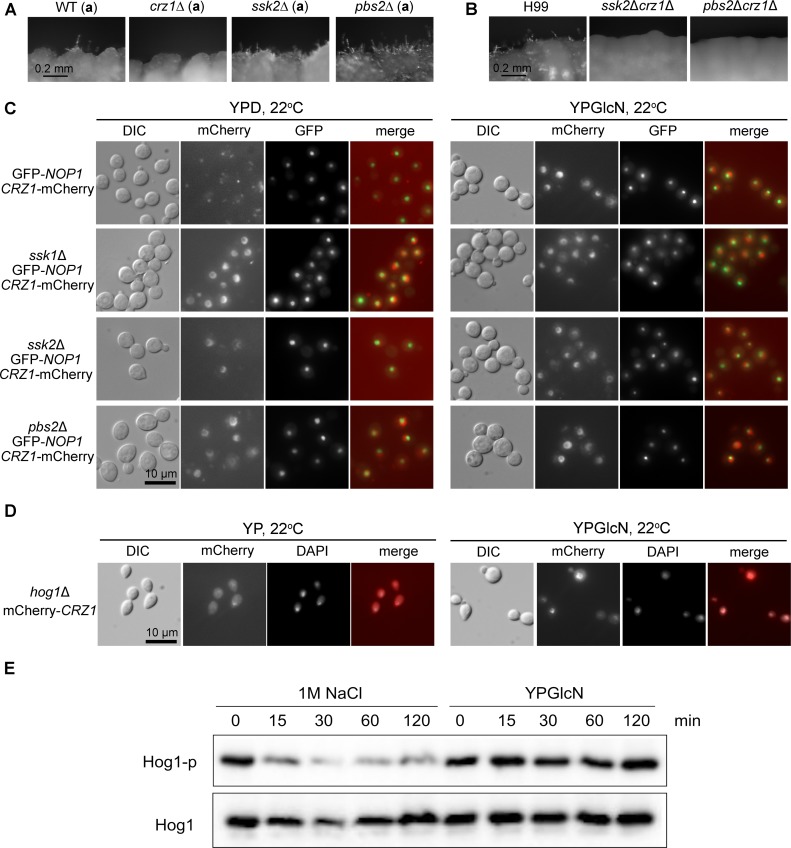
Multiple components of the HOG pathway, namely Tco1 (hybrid histidine kinase), Ssk1 (response regulator), Ssk2 (MAPKKK), and Pbs2 (MAPKK), suppress glucosamine-stimulated filamentation given that disruption of these components enhanced filamentation (Fig 3). We decided to examine if the HOG pathway components suppress filamentation through Crz1. For this purpose, we made double gene deletion mutants ssk2Δ crz1Δ and pbs2Δ crz1Δ and examined their phenotypes on glucosamine medium. The ssk2Δ and pbs2Δ single mutants showed enhanced filamentation on glucosamine medium (Fig 3, Fig 8A and 8B). The ssk2Δ crz1Δ and pbs2Δ crz1Δ double mutants were non-filamentous on glucosamine medium (Fig 8A), similar to the crz1Δ single mutant. This result suggests that Crz1 is essential in the regulation of filamentation in response to glucosamine and it functions downstream of Ssk2 and Pbs2.
Fig 8. The HOG components function upstream of Crz1 and suppress Crz1’s nuclear localization.
(A) Cells of the wild-type KN99a, crz1Δ (a), ssk2Δ (a), and pbs2Δ (a) were cultured on YP+GlcN (0.5%) medium for 7 days. (B) Cells of the wild-type H99, the ssk2Δcrz1Δ double mutant, and the pbs2Δcrz1Δ double mutant were cultured on YP+GlcN medium for 7 days. (C) The control strain XW252 (GFP-Nop1, PCRZ1-Crz1-mCherry) [79] and the corresponding ssk1Δ, ssk2Δ, and pbs2Δ mutants in the XW252 background were cultured in YPD or YP+GlcN medium overnight at 22°C. GFP-Nop1 labels the nucleolus within the nucleus [79]. (D) The strain JL408 (MATα, hog1Δ; PGPD1-mCherry-CRZ1) was generated from a cross between the hog1Δ mutant in the mating type a background and the strain JL410 (MATα, PGPD1-mCherry-CRZ1). JL408 was cultured in YP or YP+GlcN medium overnight at 22°C. (E) The wild-type strain H99 was grown to mid-logarithmic phase and then exposed to 1 M NaCl or 2% glucosamine (YPGlcN) for the indicated time points. Hog1 phosphorylation levels were monitored using anti-P-p38 antibody. The blot was stripped and then used for detection of Hog1 protein level with a polyclonal anti-Hog1 antibody as a loading control.
We postulate that the HOG pathway components may oppose the effect of calcineurin and suppress the nuclear translocation of Crz1. If this hypothesis is valid, then disruption of the HOG pathway components would increase the level of nucleus-localized Crz1. To test this hypothesis, we constructed mCherry labeled Crz1 in the ssk1Δ mutant, the ssk2Δ mutant, and the pbs2Δ mutant by crossing these strains to XW252 (PCRZ1-Crz1-mCherry, GFP-Nop1) [79]. We then examined the subcellular localization of Crz1-mCherry in the absence of these HOG pathway components. We found that most cells showed nuclear localized Crz1-mCherry next to the nucleolus marker GFP-Nop1 in the absence of Ssk1, Ssk2, or Pbs2 even when these cells were cultured in YPD medium at 22°C without any stimulus (Fig 8C). Upon induction with glucosamine, almost all cells showed nuclear localized Crz1, regardless whether the SSK1, SSK2, or PBS2 gene was intact or not (Fig 8C). Thus, the absence of the HOG pathway upstream components increased the basal level of nuclear localized Crz1, which may have enhanced the initiation of filamentation in the ssk1Δ, ssk2Δ, or pbs2Δ mutant on glucosamine medium. In contrast to the deletion of SSK1, SSK2, or PBS2, the deletion of HOG1 gene did not significantly enhance the basal level of nuclear-translocation in the absence of glucosamine compared to the wild type (generated by crossing PGPD1-mCherry-CRZ1 to hog1Δ) (left panel in Fig 8D). Nonetheless, the treatment of glucosamine stimulated the translocation of cytosolic Crz1 into the nucleus in the hog1Δ strain, just like the wild type (right panel in Fig 8D). Thus, Hog1, the downstream MAPK of the HOG pathway, appears to be dispensable for glucosamine-stimulated filamentation. In the wild-type H99 strain, Hog1 is known to be highly phosphorylated under normal growth conditions and it undergoes dephosphorylation in response to osmotic shock [81]. Indeed, we observed reduced level of phosphorylation of Hog1 in response to osmotic stress caused by NaCl (Fig 8E). However, no apparent change in Hog1 phosphorylation was observed in response to glucosamine (Fig 8E). This result indicates that Hog1 phosphorylation is not affected by glucosamine, which corroborates the dispensability of Hog1 in glucosamine-stimulated filamentation.
Discussion
C. neoformans could undergo yeast-to-hypha transition and this morphotype switch is linked to its virulence potential. Yeast is the virulent form, whereas the filamentous form is attenuated in virulence in mammalian models of cryptococcosis ([82] and references therein). Our previous studies demonstrated that upregulation of ZNF2 is sufficient to drive C. neoformans to undergo filamentation and to abolish/attenuate virulence [24–26]. Thus, activation of filamentation could potentially be used to mitigate cryptococcosis if suitable effectors that can trigger cryptococcal filamentation program in vivo can be identified. In addition, the filamentous form of Cryptococcus elicits protective immune-responses in a mammalian host [26], providing a platform for future vaccine development.
Because the pheromone pathway has no or minimal impact on virulence and C. neoformans infections are largely caused by serotype A α isolates (α >99% among serotype A isolates), it is of great value to identify conserved signals and pathways that control self-filamentation independent of the pheromone pathway. Self-filamentation in C. neoformans is mostly observed in serotype D isolates and rarely in serotype A isolates. The widely used and highly virulent serotype A reference strain H99, for instance, has not been observed to undergo self-filamentation under laboratory conditions despite numerous attempts. Here we found that H99 can undergo self-filamentation in response to glucosamine and this morphological transition is independent of the pheromone pathway. Why glucosamine, but not any other carbon-source tested, triggers self-filamentation in H99 remains mysterious. Glucosamine is the subunit of chitosan from Cryptococcus cell wall. Chitosan is the deacetylated form of chitin, and chitin is a common cell wall component in fungi and in the exoskeletons of arthropods, such as the shells of crustaceans and the outer coverings of insects. It is possible that the presence of glucosamine, rather than N-acetyl glucosamine, the subunit of chitin, serves as a unique danger signal to Cryptococcus. Alternatively, unknown secondary signals triggered by glucosamine are the real signals stimuli of filamentation. Regardless of the true biological meaning of glucosamine, the identification of pathways that control self-filamentation in natural serotype A strains like H99 represents an important advance in the endeavors to understand the regulation of cryptococcal dimorphism, which was primarily considered a response to pheromone.
By screening approximately 2500 gene deletion mutants for altered filamentation on glucosamine medium, we found that the transcription factor Crz1 was critical for glucosamine-induced filamentation: deletion of CRZ1 abolished filamentation and overexpression of CRZ1 enhanced filamentation on glucosamine medium. Crz1 appears to regulate filamentation specifically in response to glucosamine, as neither deletion nor overexpression of CRZ1 showed any effect on cryptococcal yeast-to-hypha transition during mating on V8 medium (S6 Fig)[38]. We found that the pheromone pathway responding to mating cues was overall dispensable for filamentation in response to glucosamine (Fig 2). Glucosamine strongly induced Crz1 to translocate from the cytosol to the nucleus, where it can exert its function as a transcription factor. Two pathways converged on Crz1 and play important but opposing roles. One pathway is the expected calcineurin pathway known to dephosphorylate Crz1, which required for its nuclear translocation [75, 79]. Indeed, Crz1 was retained in the cytoplasm in the absence of calcineurin catalytic subunit Cna1 or the regulatory subunit Cnb1 (Fig 7B and 7C). Interestingly, the absence of Cbp1 didn’t affect Crz1’s translocation into the nucleus (Fig 7C). This offers a plausible explanation for the lack of dramatic phenotype of the cbp1Δ mutant, in contrast to the non-filamentous phenotype of the cna1Δ and the cnb1Δ mutant on glucosamine medium (Fig 4B). The other pathway is the HOG components upstream of the Hog1 MAPK, which is known for their regulation of a variety of environmental stress responses. We found that the HOG components inhibited filamentation on glucosamine medium and suppressed the nuclear translocation of Crz1 (Fig 8C), likely through their direct or indirect effect on Crz1 phosphorylation that counter-balances the phosphatase activity of calcineurin (Fig 9).
Fig 9. Proposed model of genetic regulation of glucosamine-stimulated filamentation in Cryptococcus neoformans.
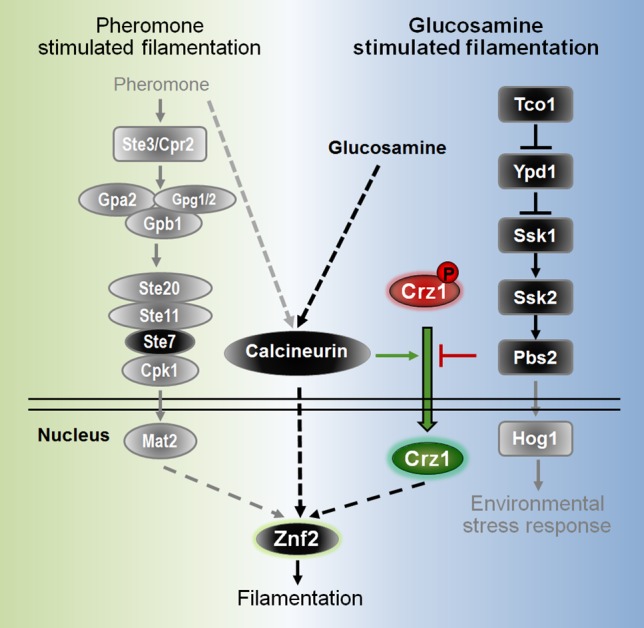
In another fungal pathogen Aspergillus fumigatus, CrzA (Crz1 homolog) translocates to the nucleus upon osmotic stress caused by NaCl or sorbitol [83]. CrzA also directly upregulates the expression of the histidine kinase PhkB and the MAPKKK SskB of the osmotic sensing pathway by binding to their promoters [83]. Thus in A. fumigatus, CrzA plays a role in osmotic stress response [83], and there appears to be a positive feedback regulation between the osmotic sensing pathway and CrzA in A. fumigatus. Unlike CrzA in A. fumigatus, Crz1 in Cryptococcus translocates to granule-like structures in the cytoplasm after osmotic stress [46] (Fig 6). Consistent with its cytoplasmic localization in response to osmotic stress, the crz1Δ mutant was as resistant to the osmotic stress caused by NaCl as the wild type (S8 Fig). Overexpression of CRZ1 in the pbs2Δ mutant also failed to restore pbs2Δ’s sensitivity to osmotic stress (S8 Fig). These findings are consistent with the idea that Crz1 is not critical for the osmotic stress response in C. neoformans (S8 Fig).
The calcineurin pathway is known to control growth, stress responses, morphogenesis and pathogenicity in various fungal species [70–72, 78, 84–90]. However, Crz1, the established downstream target of calcineurin, appears to be more specific in promoting hyphal growth than the adaptation to the general stresses based on previous studies in A. fumigatus [91, 92] and Candida species [85, 93]. Interestingly, the HOG pathway plays a more suppressive role in hyphal growth as demonstrated in Candida species [94–96]) and in Cryptococcus neoformans during bisexual mating [69, 81]. Thus the opposing effect between the calcineurin pathway and the HOG pathway on hyphal growth might be conserved in multiple fungal species. Whether Crz1 is the conserved conjunction of these two pathways in regulating filamentation in these fungal species is yet to be determined.
We believe that the upstream components of the HOG pathway normally suppress the translocation of Crz1 to the nucleus based on the elevated basal level of nuclear Crz1 in the corresponding deletion mutants in the absence of any stimuli (Fig 8C). This suggests that the upstream components of the HOG pathway inactivate Crz1, possibly by enabling the phosphorylation of Crz1 either directly or indirectly, and consequently opposing the activity of calcineurin. It is important to note that nuclear localization of Crz1 is necessary, but not sufficient to drive filamentation in the absence of glucosamine. This is evident given that some cells showed nuclear localized Crz1 even in YPD medium, but all cells grew in the yeast form under that condition. This is also consistent with the observation that heat-shock and calcium, although both stimulate nuclear translocation of Crz1, were unable to elicit filamentation in H99 in the absence of glucosamine. Thus, a yet unknown factor affected by glucosamine, in addition to the requirement of Crz1 nuclear translocation, has to be involved to enable filamentation.
One interesting observation is that not all components in a well-established pathway behave in the same fashion. For example, most key components in the pheromone pathway, including the transcription factor Mat2, are dispensable for self-filamentation induced by glucosamine. However, ste7Δ is non-filamentous on glucosamine medium. This finding is surprising given that ste7Δ and mat2Δ are both non-filamentous with identical transcriptomes under mating-inducing conditions during both bisexual and unisexual development [25, 32, 97]. Thus, the distinct phenotype of ste7Δ on glucosamine medium suggests that Ste7 might have additional functions besides its established role in pheromone sensing and response. Another example is Hog1 in the HOG pathway. Most upstream components of the HOG pathway suppress filamentation on glucosamine medium, but the MAPK Hog1 itself shows no or minimal involvement in this process. We postulate that there is a divergence in the downstream effectors of this phosphorelay system in response to osmotic stress or glucosamine. Hog1 is activated in response to osmotic stress when Crz1 is being concentrated in granules in the cytoplasm in Cryptococcus [75] (Fig 6A). In contrast, Crz1 is localized to the nucleus in response to glucosamine. How different effectors are activated by the same phosphorelay system, what controls the multiple distinct subcellular localizations of Crz1, and what prevents cross-activation of the downstream effectors remain to be investigated.
Materials and methods
Media and growth conditions
Strains were stored as glycerol stocks in -80°C. Freshly streaked cells were used for experiments. The three deletion sets made in the H99 background were obtained from the Fungal Genetics Stock Center (FGSC) and the information about these strains can be obtained from the FGSC website (http://www.fgsc.net/crypto/crypto.htm). Other strains were listed in S2 Table. Cryptococcal cells were maintained on YPD medium (20 peptone, 10 yeast extract, 20 glucose, 20 agar, gram/liter) unless stated otherwise.
Filamentation assay
For the filamentation assay, the YP medium (20 peptone, 10 yeast extract, 20 agar, gram/liter) was used as the base medium. All the different carbon sources tested were made to the final concentration of 2%. When testing filamentation on YPGlcN medium (20 peptone, 10 yeast extract, 20 glucosamine, 20 agar, gram/liter), 3 μl of cells (optical density OD600 = 1) of the tested strains were dropped onto the agar medium. Cells were cultured at 30°C for two days before being transferred to 22°C for additional incubation of 4 to 7 days in the dark. To test the effect of the calcineurin inhibitor FK506 on filamentation, FK506 was added to the YPGlcN medium at the final concentration of 1 μg/ml. To test the dose-dependent effects of glucosamine on filamentation, glucosamine were added to the YP base medium to the final concentration of 0, 0.2%, 0.5%, 1%, and 2%. To test the effect of the addition of another carbon source to the YPGlcN medium on filamentation, 2% galactose, glycerol, or xylose was added to the YPGlcN medium (2% glucosamine).
Phenotypical assays
To test thermo-tolerance, cells of the tested strains with 5x serial dilutions (OD600 = 10, 2, 0.4, 0.08, 0.016, and 0.0032) were dropped onto YPD medium and incubated at 30°C or 37°C for 2 days. To test the susceptibility to cell wall stress, cells of the indicated strains were serial diluted and spotted onto YPD medium, YPD with 0.2% Congo Red, or YPD medium with 10 μg/ml of Calcofluor white. Cells were then incubated at 30°C for 2 days.
RNA extraction and qPCR
RNA extraction and qPCR were performed as we described previously [24]. For the transcript measurements used in Fig 2, strains H99, mat2Δ, and znf2Δ were cultured on YPD medium or glucosamine medium at 30°C for 2 days, and then were transferred to 22°C for additional 2 days before cells were harvested. For the transcript measurements used in Fig 5, Strains H99, crz1Δ, and znf2Δ were cultured on the YP-glucosamine or YP base medium at 30°C for 2 days, and then incubated at 22°C for additional incubation. Cells were harvested at the time points (0, 2 days, 4 days, and 6 days) as indicated in the figures.
Harvested cells were washed with cold water, frozen in liquid nitrogen, and then lyophilized. Lyophilized cells were broken into fine powder with glass beads and total RNA was extracted with the PureLink RNA Mini Kit (life technology) according to the manufacture’s instruction. First strand cDNA was synthesized with Superscript III cDNA synthesis kit (Invitrogen) according to the manufacture’s instruction. The house-keeping gene TEF1 was used as the endogenous control. The relative transcript levels were determined using the comparative ΔΔCt method as described previously [24]. Three biological replicates were performed for each sample and their values were used to calculate the mean and the standard error. Primers used for realtime PCR were listed in S3 Table.
Genetic screen of the gene deletion mutants on glucosamine medium
All the gene deletion mutants in the serotype A background used in this study generated by the Lin’s group or Bahn’s group were made in the same H99 background (see S2 Table for strains used in this study). The 2015 gene deletion set deposited by Dr. Hiten Madhani’s lab and the transcription factor and kinase gene deletion sets deposited by Dr. Bahn’s lab are available from the Fungal Genetics Stock Center. http://www.fgsc.net/crypto/crypto.htm). These mutants were also generated in the same H99 background. The mutants were screened on the YP-glucosamine (2%) medium after replicating from 96 well plates as described earlier for the filamentation assays. Strains with altered filamentation were selected based on comparison with other strains on the same plate during the initial screen. These mutant phenotypes were further confirmed in the secondary screen with the wild type H99 control. For the genes and pathways that were further characterized in this study, including the pheromone pathway, the calcineurin pathway, and the Hog1 pathway, separate mutants were obtained from the original sources where the mutations were verified in the previously published work. These strains and their sources/references were listed in S2 Table.
Gene deletion and gene overexpression
To generate the knockout construct, 1 kb of the 5’ and 3’ flanking sequences bordering the open reading frame of the gene of interest were amplified using the genomic DNA of the wild-type strain as the template. They were then fused with the NEO or NAT dominant drug marker amplified from the plasmid pAI1 or pJAF1 by overlap PCR as we described previously [98]. The knockout constructs were introduced into appropriate recipient strains by biolistic transformation as described previously [99]. The transformants grown on selective medium (YPD+NAT or YPD+G418/NEO) were then screened for gene replacement via homologous recombination events by diagnostic PCR as described previously [98]. To generate CRZ1 or ZNF2 overexpression strains, the open reading frame of the CRZ1 or the ZNF2 gene were first amplified by PCR with specifically designed primers with FseI/PacI cut sites at the ends. After digestion, the digested products were ligated into the PGPD1 vector or the PCTR4 vector where the ORF was placed downstream of the GPD1 or the CTR4 promoter, as we described previously [58, 59]. The resulting plasmids were then linearized and introduced into the recipient strains as indicated in the text by biolistic transformation. All the primers used for constructing or confirming gene deletion or gene overexpression were listed in S3 Table.
Mating and genetic crosses
Yeast cells of α and a mating partners were mixed together on V8 juice agar medium (5% V8 juice, 0.5 g/L KH2PO4, 4% agar, pH adjusted to 5). The mixed culture was then incubated for 2 weeks at 22°C in the dark until spores were produced following filamentation. Cells from V8 medium were transferred to fresh YPD agar medium and spores were micro-manipulated with a dissecting microscope. The mating type of the germinated spores was determined by successful mating of their derived colonies with either JEC20a or JEC21α. Genetic linkage between the presence of the drug marker and the observed mutant phenotype was established by analyzing the dissected spores as we described previously [42].
Protein tagging
To characterize the subcellular localization of Znf2 and Crz1, mCherry was fused to the N-terminus of Znf2 or Crz1 in frame. The ORF of CRZ1 or ZNF2 with PacI recognition site at 3’ end was amplified by PCR and then fused at the N-terminus with mCherry carrying FseI recognition site at its 5’end. The fragment mCherry-CRZ1 and mCherry-ZNF2 was digested with FseI and PacI and then ligated into the PGPD1 vector or the PCTR4 vector. The construct of the mCherry tagged protein controlled by the GPD1 or the CTR4 promoter (PGPD1-mCherry-CRZ1 and PCTR4-mCherry-ZNF2) were then introduced into the recipient strains by biolistic transformation as described previously [99]. The N-terminal tagged Crz1 and Znf2 are functional based on the observation that they could restore the filamentation defect observed in the corresponding gene deletion mutants.
Microscopic examination
Colony morphology was examined with a SZX16 stereoscope (Olympus). Colony images were captured with a GO-21camera and acquired using the QIMAGINE software. To determine the subcellular localization of mCherry-Crz1 or mCherry-Znf2, cells were observed with a Zeiss M2 epi-fluorescence microscope and images were acquired with the AxioCam MRm camera and processed with the software Zen 11 (Carl Zeiss Microscopy). The filter used for visualizing mCherry was the FL filter set 43 HE cy3 (Carl Zeiss Microscopy). GFP was visualized using the filter FL filter set 38 HE GFP (Carl Zeiss Microscopy). To visualize the nuclei, cells were fixed in a fixer solution (3.7% formaldehyde; 1X PBS; 1% Triton X) for 10 min and then stained with DAPI (0.4 μg/ml) for 15 min. The filter used to visualizing DAPI was FL Filter Set 49 DAPI (Carl Zeiss Microscopy).
Crz1-mCherry translocation assay
To examine the effect of temperature on Crz1 localization, cells of the tested strains were cultured in the YPD medium at 22°C, 30°C, or 37°C overnight. To test the effects of different conditions or mutations on the subcellular localization of mCherry tagged Crz1, Cryptococcus cells were grown in liquid media at 22°C for 10 hours. To examine the impact of glucosamine on Crz1’s subcellular localization, cells were cultured in the YP-glucosamine (2%) liquid medium at 22°C for 10 hrs. To examine the impact of calcium or salt on the localization of Crz1, cells were cultured first in YPD at 22°C, centrifuged, washed with PBS, and then suspended in 100 mM CaCl2 or 1.5 M NaCl for 10–30 minutes. To test the impact on Crz1’s subcellular localization by the deletion of SSK1, SSK2, PBS2, CNA1, CNB1 or CBP1, the corresponding Cryptococcus strains were cultured in either YPD or glucosamine medium at 22°C for 10 hours. To quantify the percentage of cells with Crz1 localized to the nucleus, the numbers of cells with Crz1 in the nucleus and the total cells with fluorescence signals were determined and the ratio was calculated in three replicated samples. The data were used to calculate the mean value of the population with nuclear localization and the standard errors.
Western blot analysis of Hog1 phosphorylation
The overnight culture of wild-type strain H99 was inoculated in fresh YPD liquid medium (250 ml) and incubated at 30°C until the culture reached the optical density of approximately 0.9–1.0 at 600 nm (OD600). Cells were harvested by centrifugation, washed two times in PBS, and resuspended in YPD medium containing 1 M NaCl or in YP medium containing 2% glucosamine. At each designated time point, an aliquot of 50 ml of the culture was mixed with an equal volume of ice-cold stop solution (0.9% NaCl, 1 mM NaN3, 10 mM EDTA, 50 mM NaF). The cells were then collected by centrifugation and resuspended in lysis buffer [50 mM Tris-Cl (pH 7.5), 1% sodium deoxycholate, 5 mM sodium pyrophosphate, 0.2 mM sodium orthovanadate, 50 mM NaF, 0.1% SDS, 1% Triton X-100, 0.5 mM phenylmethylsulfonyl fluoride, and 2.5× protease inhibitor cocktail solution (Calbiochem)]. The resuspended cells were disrupted using a bead-beater for 6 cycles (30 sec bead beating with 2 min rest intervals). Protein concentrations were determined with the Pierce BCA protein assay kit (Thermo Fisher Scientific). A total of 5 μg of proteins were loaded into 10% SDS-polyacrylamide gel and analyzed by western blot using a primary antibody of rabbit P-p38 MAPK specific antibody (Cell Signalling Technology) to detect phosphorylated Hog1 and polyclonal anti-Hog1 antibody (Santa Cruz) for the detection of Hog1 as a loading control. Anti-rabbit IgG horseradish peroxidase-conjugated antibody (Santa Cruz) was used as a secondary antibody. The blot was developed using the ECL western blotting detection system according to the instruction of the manufacture (Bio-Rad).
Statistical analysis
Statistical significance of different groups in terms of Crz1 localization was assessed by the t-test. The statistical analyses were performed using the Graphpad Prism 5 program, with p values lower than 0.05 considered statistically significant.
Supporting information
Cells (optical density of OD600 = 1.0) were dropped onto the indicated medium and cultured at 30°C for 2 days followed by additional incubation at 22°C for 4 days.
(PDF)
The wild-type serotype D strain XL280 and the corresponding mat2Δ and znf2Δ mutants were cultured on V8 juice agar medium for 6 days at 22°C or on glucosamine medium for 2 days at 30°C followed by additional 4 days of incubation at 22°C. (upper two panels). The wild-type serotype A strain H99 and the corresponding mat2Δ and znf2Δ mutants were cultured on V8 juice agar medium at 22°C for 4 days.
(PDF)
(A) A diagram of the hexamine metabolism pathway. HK: Hexose Kinase, GNAD: Glucosamine Deacetylase, GND: Glucosamine Deaminase, GNAT: Glucosamine N-acetyl transferase. (B) The growth of the gnd1Δ mutant is hypersensitive to glucosamine. Wild-type H99 and the gnd1Δ mutant were cultured at 30°C for 2 days on YP medium containing glucosamine of different concentrations with or without the addition of other carbon sources (galactose, glycerol, or xylose). (C) The gnd1Δ mutant filamented similarly as the wild-type strain H99. The wild-type H99 and the gnd1Δ mutant were cultured on glucosamine medium for 2 days at 30°C followed by additional 4 days of incubation at 22°C.
(PDF)
(PDF)
Cells from the wild-type H99, the cna1Δ mutant, the cnb1Δ mutant, the cbp1Δ mutant, and the crz1Δ mutant were serial diluted (5x) and spotted onto YPD medium or YPD medium with Calcofluor white/CFW (10 μg/ml) or Congo Red (0.2%). The cells on YPD medium were incubated at 30°C or 37°C as indicated. Cells on medium with Calcofluor white or Congo red were cultured at 30°C.
(PDF)
(A) The wild-type H99, the crz1Δ mutant, and the CRZ1oe strain all in the mating type α were cultured alone on V8 juice agar medium at 22°C for 9 days. 3 μl of cells at the density of OD600 = 3 were used to inoculate. (B) The wild-type H99α, the crz1Δ α mutant, and the CRZ1oe α strain were mixed with the mating partner KN99a of the opposite mating type. The mixed co-cultures were inoculated and cultured on V8 medium at 22°C in the dark for 9 days.
(PDF)
To test temperature’s effect on the subcellular localization of Crz1-mCherry, the strain XW252 (PCRZ1- CRZ1-mCherry, GFP-Nop1) was cultured in YPD liquid at 37°C with shaking for 9 hours. To test the effect of calcium, cells of the strain P CRZ1-mCherry-CRZ1 were first collected from the culture in liquid YPD at 22°C for 9 hours and then suspended in YPD with 100 mM of CaCl2 for 10–20 min. To test the effect of glucosamine, the strain PCRZ1-mCherry-CRZ1 was cultured in YP-glucosamine liquid medium at 22°C for 9 hours. For the examination of a shift in carbon-source, cells were incubated in YP-glucosamine liquid medium at 22°C for 9 hours and then shifted to glucose medium at 22°C for 90 minutes. (A) Images of the cells under the conditions tested. (B) Quantification of cells with nuclear localization of Crz1.
(PDF)
Cells of the following strains (wild-type H99, crz1Δ, cna1Δ, cna1ΔCRZ1oe, CRZ1oe, pbs2Δ, pbs2Δcrz1Δ, and pbs2ΔCRZ1oe) were serial diluted and spotted onto YPD medium with or without the addition of NaCl at 1M, or 1.5M. Cells were then incubated at 30°C or 37°C for 3 days before images were taken.
(PDF)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Dr. Heitman (Duke) and Dr. Madhani (UCSF) for their generous gifts of strains, and the fungal genetics stock center (FGSC, http://www.fgsc.net/) for providing the service for the storage and maintenance of the deletion sets.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by National Institutes of Health (http://www.niaid.nih.gov) (R01AI097599 to XL). Dr. Lin holds an Investigator Award in the Pathogenesis of Infectious Disease from the Burroughs Wellcome Fund (http://www.bwfund.org/) (#1012445). Additional financial support came from the Biology department of Texas A&M University (http://www.bio.tamu.edu/) and the Microbiology department of the University of Georgia (http://mib.uga.edu/). YSB is supported by the General International Collaborative R&D program funded by Ministry of Trade, Industry and Energy (MOTIE) in Republic of Korea (N0001720). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
References
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–30. doi: 10.1097/QAD.0b013e328322ffac . [DOI] [PubMed] [Google Scholar]
- 2.Casadevall A, Perfect JR. Cryptococcus neoformans. Washington, D.C.: ASM Press; 1998. [Google Scholar]
- 3.Belay T, Cherniak R, O'Neill EB, Kozel TR. Serotyping of Cryptococcus neoformans by dot enzyme assay. Journal of clinical microbiology. 1996;34(2):466–70. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon-Chung KJ, Bennett JE. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am J Epidemiol. 1984;120(1):123–30. . [DOI] [PubMed] [Google Scholar]
- 5.Franzot SP, Salkin IF, Casadevall A. Cryptococcus neoformans var. grubii: separate varietal status for Cryptococcus neoformans serotype A isolates. Journal of clinical microbiology. 1999;37(3):838–40. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010;50(3):291–322. doi: 10.1086/649858 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhein J, Morawski BM, Hullsiek KH, Nabeta HW, Kiggundu R, Tugume L, et al. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis. 2016;16(7):809–18. doi: 10.1016/S1473-3099(16)00074-8 ; PubMed Central PMCID: PMCPMC4927382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Day JN, Chau TT, Wolbers M, Mai PP, Dung NT, Mai NH, et al. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med. 2013;368(14):1291–302. doi: 10.1056/NEJMoa1110404 ; PubMed Central PMCID: PMCPMC3978204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarvis JN, Bicanic T, Loyse A, Namarika D, Jackson A, Nussbaum JC, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated Cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis. 2014;58(5):736–45. doi: 10.1093/cid/cit794 ; PubMed Central PMCID: PMCPMC3922213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith KD, Achan B, Huppler Hullsiek K, McDonald T, Okagaki LH, Akampurira A, et al. Increased antifungal drug resistance in Ugandan clinical isolates of Cryptococcus neoformans. Antimicrobial agents and chemotherapy. 2015. doi: 10.1128/AAC.01299-15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sionov E, Chang YC, Kwon-Chung KJ. Azole heteroresistance in Cryptococcus neoformans: emergence of resistant clones with chromosomal disomy in the mouse brain during fluconazole treatment. Antimicrobial agents and chemotherapy. 2013;57(10):5127–30. doi: 10.1128/AAC.00694-13 ; PubMed Central PMCID: PMCPMC3811407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Wyk M, Govender NP, Mitchell TG, Litvintseva AP. Multilocus sequence typing of serially collected isolates of Cryptococcus from HIV-infected patients in South Africa. Journal of clinical microbiology. 2014;52(6):1921–31. Epub 2014/03/22. doi: 10.1128/JCM.03177-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Bijie H, Dzierzanowska D, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: 10.5-year analysis of susceptibilities of noncandidal yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. Journal of clinical microbiology. 2009;47(1):117–23. doi: 10.1128/JCM.01747-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YC, Chang TY, Liu JW, Chen FJ, Chien CC, Lee CH, et al. Increasing trend of fluconazole-non-susceptible Cryptococcus neoformans in patients with invasive cryptococcosis: a 12-year longitudinal study. BMC Infect Dis. 2015;15(1):277 doi: 10.1186/s12879-015-1023-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powderly WG, Saag MS, Cloud GA, Robinson P, Meyer RD, Jacobson JM, et al. A controlled trial of fluconazole or amphotericin B to prevent relapse of cryptococcal meningitis in patients with the acquired immunodeficiency syndrome. The NIAID AIDS Clinical Trials Group and Mycoses Study Group. N Engl J Med. 1992;326(12):793–8. doi: 10.1056/NEJM199203193261203 . [DOI] [PubMed] [Google Scholar]
- 16.Sil A, Andrianopoulos A. Thermally dimorphic human fungal pathogens-polyphyletic pathogens with a convergent pathogenicity trait. Cold Spring Harbor perspectives in medicine. 2014. doi: 10.1101/cshperspect.a019794 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell AP. Dimorphism and virulence in Candida albicans. Current opinion in microbiology. 1998;1(6):687–92. . [DOI] [PubMed] [Google Scholar]
- 18.Klein BS, Tebbets B. Dimorphism and virulence in fungi. Current opinion in microbiology. 2007;10(4):314–9. doi: 10.1016/j.mib.2007.04.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibrahim AS, Luo G, Gebremariam T, Lee H, Schmidt CS, Hennessey JP Jr., et al. NDV-3 protects mice from vulvovaginal candidiasis through T- and B-cell immune response. Vaccine. 2013;31(47):5549–56. doi: 10.1016/j.vaccine.2013.09.016 ; PubMed Central PMCID: PMCPMC3866209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Filler SG. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryotic cell. 2011;10(2):168–73. Epub 2010/12/01. doi: 10.1128/EC.00279-10 ; PubMed Central PMCID: PMC3067396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532(7597):64–8. doi: 10.1038/nature17625 ; PubMed Central PMCID: PMCPMC4851236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staab JF, Bradway SD, Fidel PL, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science (New York, NY. 1999;283(5407):1535–8. Epub 1999/03/05. . [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim AS, Spellberg BJ, Avanesian V, Fu Y, Edwards JE Jr. The anti-Candida vaccine based on the recombinant N-terminal domain of Als1p is broadly active against disseminated candidiasis. Infection and immunity. 2006;74(5):3039–41. Epub 2006/04/20. doi: 10.1128/IAI.74.5.3039-3041.2006 ; PubMed Central PMCID: PMC1459699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Zhai B, Lin X. The link between morphotype transition and virulence in Cryptococcus neoformans. PLoS pathogens. 2012;8(6):e1002765 Epub 2012/06/28. doi: 10.1371/journal.ppat.1002765 ; PubMed Central PMCID: PMC3380952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin X, Jackson JC, Feretzaki M, Xue C, Heitman J. Transcription factors Mat2 and Znf2 operate cellular circuits orchestrating opposite- and same-sex mating in Cryptococcus neoformans. PLoS genetics. 2010;6(5):e1000953 Epub 2010/05/21. doi: 10.1371/journal.pgen.1000953 ; PubMed Central PMCID: PMC2869318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhai B, Wozniak KL, Masso-Silva J, Upadhyay S, Hole C, Rivera A, et al. Development of protective inflammation and cell-mediated immunity against Cryptococcus neoformans after exposure to hyphal mutants. MBio. 2015;6(5):e01433–15. doi: 10.1128/mBio.01433-15 ; PubMed Central PMCID: PMCPMC4611043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P, Heitman J. Signal transduction cascades regulating mating, filamentation, and virulence in Cryptococcus neoformans. Current opinion in microbiology. 1999;2(4):358–62. doi: 10.1016/S1369-5274(99)80063-0 . [DOI] [PubMed] [Google Scholar]
- 28.Chung S, Karos M, Chang YC, Lukszo J, Wickes BL, Kwon-Chung KJ. Molecular analysis of CPRalpha, a MATalpha-specific pheromone receptor gene of Cryptococcus neoformans. Eukaryotic cell. 2002;1(3):432–9. Epub 2002/11/29. doi: 10.1128/EC.1.3.432-439.2002 ; PubMed Central PMCID: PMC118017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen WC, Davidson RC, Cox GM, Heitman J. Pheromones stimulate mating and differentiation via paracrine and autocrine signaling in Cryptococcus neoformans. Eukaryotic cell. 2002;1(3):366–77. doi: 10.1128/EC.1.3.366-377.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waugh MS, Vallim MA, Heitman J, Alspaugh JA. Ras1 controls pheromone expression and response during mating in Cryptococcus neoformans. Fungal Genet Biol. 2003;38(1):110–21. . [DOI] [PubMed] [Google Scholar]
- 31.Hsueh YP, Shen WC. A homolog of Ste6, the a-factor transporter in Saccharomyces cerevisiae, is required for mating but not for monokaryotic fruiting in Cryptococcus neoformans. Eukaryotic cell. 2005;4(1):147–55. Epub 2005/01/12. doi: 10.1128/EC.4.1.147-155.2005 ; PubMed Central PMCID: PMC544149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidson RC, Nichols CB, Cox GM, Perfect JR, Heitman J. A MAP kinase cascade composed of cell type specific and non-specific elements controls mating and differentiation of the fungal pathogen Cryptococcus neoformans. Molecular microbiology. 2003;49(2):469–85. . [DOI] [PubMed] [Google Scholar]
- 33.Clarke DL, Woodlee GL, McClelland CM, Seymour TS, Wickes BL. The Cryptococcus neoformans STE11a gene is similar to other fungal mitogen-activated protein kinase kinase kinase (MAPKKK) genes but is mating type specific. Molecular microbiology. 2001;40(1):200–13. . [DOI] [PubMed] [Google Scholar]
- 34.Lengeler KB, Davidson RC, D'Souza C, Harashima T, Shen WC, Wang P, et al. Signal transduction cascades regulating fungal development and virulence. Microbiol Mol Biol Rev. 2000;64(4):746–85. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozubowski L, Heitman J. Profiling a killer, the development of Cryptococcus neoformans. FEMS microbiology reviews. 2012;36(1):78–94. Epub 2011/06/11. doi: 10.1111/j.1574-6976.2011.00286.x ; PubMed Central PMCID: PMC3318972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin X, Huang JC, Mitchell TG, Heitman J. Virulence attributes and hyphal growth of C. neoformans are quantitative traits and the MATalpha allele enhances filamentation. PLoS genetics. 2006;2(11):e187 doi: 10.1371/journal.pgen.0020187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kruzel EK, Giles SS, Hull CM. Analysis of Cryptococcus neoformans sexual development reveals rewiring of the pheromone response network by a change in transcription factor identity. Genetics. 2012. Epub 2012/04/03. doi: 10.1534/genetics.112.138958 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gyawali R, Zhao Y, Lin J, Fan Y, Xu X, Upadhyay S, et al. Pheromone Independent Unisexual Development in Cryptococcus neoformans. PLoS genetics. 2017;13(5):e1006772 doi: 10.1371/journal.pgen.1006772 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung S, Karos M, Chang YC, Lukszo J, Wickes BL, Kwon-Chung KJ. Molecular Analysis of CPRα, a MATα-Specific Pheromone Receptor Gene of Cryptococcus neoformans. Eukaryotic Cell. 2002;1(3):432–9. doi: 10.1128/EC.1.3.432-439.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsueh Y-P, Shen W-C. A Homolog of Ste6, the a-Factor Transporter in Saccharomyces cerevisiae, Is Required for Mating but Not for Monokaryotic Fruiting in Cryptococcus neoformans. Eukaryotic Cell. 2005;4(1):147–55. doi: 10.1128/EC.4.1.147-155.2005 PMC544149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsueh Y-P, Xue C, Heitman J. G protein signaling governing cell fate decisions involves opposing Gα subunits in Cryptococcus neoformans. Mol Biol Cell. 2007;18(9):3237–49. doi: 10.1091/mbc.E07-02-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhai B, Zhu P, Foyle D, Upadhyay S, Idnurm A, Lin X. Congenic strains of the filamentous form of Cryptococcus neoformans for studies of fungal morphogenesis and virulence. Infection and immunity. 2013;81(7):2626–37. Epub 2013/05/15. doi: 10.1128/IAI.00259-13 ; PubMed Central PMCID: PMC3697605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin X, Hull CM, Heitman J. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature. 2005;434(7036):1017–21. doi: 10.1038/nature03448 . [DOI] [PubMed] [Google Scholar]
- 44.Yan Z, Li X, Xu J. Geographic distribution of mating type alleles of Cryptococcus neoformans in four areas of the United States. Journal of clinical microbiology. 2002;40(3):965–72. doi: 10.1128/JCM.40.3.965-972.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frazzitta AE, Vora H, Price MS, Tenor JL, Betancourt-Quiroz M, Toffaletti DL, et al. Nitrogen source-dependent capsule induction in human-pathogenic Cryptococcus species. Eukaryotic cell. 2013;12(11):1439–50. doi: 10.1128/EC.00169-13 ; PubMed Central PMCID: PMC3837930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nielsen K, Cox GM, Wang P, Toffaletti DL, Perfect JR, Heitman J. Sexual cycle of Cryptococcus neoformans var. grubii and virulence of congenic a and alpha isolates. Infection and immunity. 2003;71(9):4831–41. doi: 10.1128/IAI.71.9.4831-4841.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janbon G, Ormerod KL, Paulet D, Byrnes EJ 3rd, Yadav V, Chatterjee G, et al. Analysis of the genome and transcriptome of Cryptococcus neoformans var. grubii reveals complex RNA expression and microevolution leading to virulence attenuation. PLoS genetics. 2014;10(4):e1004261 Epub 2014/04/20. doi: 10.1371/journal.pgen.1004261 ; PubMed Central PMCID: PMC3990503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perfect JR, Lang SD, Durack DT. Chronic cryptococcal meningitis: a new experimental model in rabbits. Am J Pathol. 1980;101(1):177–94. . [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon-Chung KJ, Fraser JA, Doering TL, Wang Z, Janbon G, Idnurm A, et al. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harbor perspectives in medicine. 2014;4(7). Epub 2014/07/06. doi: 10.1101/cshperspect.a019760 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Idnurm A, Bahn YS, Nielsen K, Lin X, Fraser JA, Heitman J. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nature reviews. 2005;3(10):753–64. doi: 10.1038/nrmicro1245 . [DOI] [PubMed] [Google Scholar]
- 51.Santiago-Tirado FH, Doering TL. All about that fat: Lipid modification of proteins in Cryptococcus neoformans. J Microbiol. 2016;54(3):212–22. doi: 10.1007/s12275-016-5626-6 ; PubMed Central PMCID: PMCPMC4851765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srikanta D, Santiago-Tirado FH, Doering TL. Cryptococcus neoformans: historical curiosity to modern pathogen. Yeast. 2014;31(2):47–60. doi: 10.1002/yea.2997 ; PubMed Central PMCID: PMCPMC3938112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alvarez FJ, Konopka JB. Identification of an N-acetylglucosamine transporter that mediates hyphal induction in Candida albicans. Molecular biology of the cell. 2007;18(3):965–75. doi: 10.1091/mbc.E06-10-0931 ; PubMed Central PMCID: PMCPMC1805087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castilla R, Passeron S, Cantore ML. N-acetyl-D-glucosamine induces germination in Candida albicans through a mechanism sensitive to inhibitors of cAMP-dependent protein kinase. Cell Signal. 1998;10(10):713–9. . [DOI] [PubMed] [Google Scholar]
- 55.Huang G, Yi S, Sahni N, Daniels KJ, Srikantha T, Soll DR. N-acetylglucosamine induces white to opaque switching, a mating prerequisite in Candida albicans. PLoS pathogens. 2010;6(3):e1000806 doi: 10.1371/journal.ppat.1000806 ; PubMed Central PMCID: PMCPMC2837409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simonetti N, Strippoli V, Cassone A. Yeast-mycelial conversion induced by N-acetyl-D-glucosamine in Candida albicans. Nature. 1974;250(464):344–6. Epub 1974/07/26. . [DOI] [PubMed] [Google Scholar]
- 57.Gilmore SA, Naseem S, Konopka JB, Sil A. N-acetylglucosamine (GlcNAc) triggers a rapid, temperature-responsive morphogenetic program in thermally dimorphic fungi. PLoS genetics. 2013;9(9):e1003799 doi: 10.1371/journal.pgen.1003799 ; PubMed Central PMCID: PMCPMC3778022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gyawali R, Upadhyay S, Way J, Lin X. Characterizing a family of secretory proteins associated with different morphotypes in Cryptococcus neoformans. Applied and environmental microbiology. 2016. doi: 10.1128/AEM.02967-16 PMID: 28039134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L, Tian X, Gyawali R, Lin X. Fungal adhesion protein guides community behaviors and autoinduction in a paracrine manner. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(28):11571–6. Epub 2013/06/27. doi: 10.1073/pnas.1308173110 ; PubMed Central PMCID: PMC3710841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karos M, Chang YC, McClelland CM, Clarke DL, Fu J, Wickes BL, et al. Mapping of the Cryptococcus neoformans MATa locus: presence of mating type-specific mitogen-activated protein kinase cascade homologs. Journal of bacteriology. 2000;182(21):6222–7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang YC, Miller GF, Kwon-Chung KJ. Importance of a developmentally regulated pheromone receptor of Cryptococcus neoformans for virulence. Infection and immunity. 2003;71(9):4953–60. doi: 10.1128/IAI.71.9.4953-4960.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsueh YP, Xue C, Heitman J. A constitutively active GPCR governs morphogenic transitions in Cryptococcus neoformans. The EMBO journal. 2009;28(9):1220–33. doi: 10.1038/emboj.2009.68 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alspaugh JA, Perfect JR, Heitman J. Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes & development. 1997;11(23):3206–17. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang P, Perfect JR, Heitman J. The G-protein beta subunit Gpb1 is required for mating and haploid fruiting in Cryptococcus neoformans. Mol Cell Biol. 2000;20(1):352–62. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang P, Nichols CB, Lengeler KB, Cardenas ME, Cox GM, Perfect JR, et al. Mating-type-specific and nonspecific PAK kinases play shared and divergent roles in Cryptococcus neoformans. Eukaryotic cell. 2002;1(2):257–72. doi: 10.1128/EC.1.2.257-272.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jung KW, Yang DH, Maeng S, Lee KT, So YS, Hong J, et al. Systematic functional profiling of transcription factor networks in Cryptococcus neoformans. Nature communications. 2015;6:6757 doi: 10.1038/ncomms7757 ; PubMed Central PMCID: PMCPMC4391232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee KT, So YS, Yang DH, Jung KW, Choi J, Lee DG, et al. Systematic functional analysis of kinases in the fungal pathogen Cryptococcus neoformans. Nature communications. 2016;7:12766 doi: 10.1038/ncomms12766 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bahn YS, Geunes-Boyer S, Heitman J. Ssk2 mitogen-activated protein kinase kinase kinase governs divergent patterns of the stress-activated Hog1 signaling pathway in Cryptococcus neoformans. Eukaryotic cell. 2007;6(12):2278–89. doi: 10.1128/EC.00349-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bahn YS, Kojima K, Cox GM, Heitman J. A unique fungal two-component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans. Molecular biology of the cell. 2006;17(7):3122–35. doi: 10.1091/mbc.E06-02-0113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cruz MC, Fox DS, Heitman J. Calcineurin is required for hyphal elongation during mating and haploid fruiting in Cryptococcus neoformans. The EMBO journal. 2001;20(5):1020–32. doi: 10.1093/emboj/20.5.1020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. The EMBO journal. 1997;16(10):2576–89. doi: 10.1093/emboj/16.10.2576 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fox DS, Cruz MC, Sia RA, Ke H, Cox GM, Cardenas ME, et al. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans. Molecular microbiology. 2001;39(4):835–49. . [DOI] [PubMed] [Google Scholar]
- 73.Moranova Z, Virtudazo E, Hricova K, Ohkusu M, Kawamoto S, Husickova V, et al. The CRZ1/SP1-like gene links survival under limited aeration, cell integrity and biofilm formation in the pathogenic yeast Cryptococcus neoformans. Biomedical papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia. 2014;158(2):212–20. doi: 10.5507/bp.2013.024 . [DOI] [PubMed] [Google Scholar]
- 74.Adler A, Park YD, Larsen P, Nagarajan V, Wollenberg K, Qiu J, et al. A novel specificity protein 1 (SP1)-like gene regulating protein kinase C-1 (Pkc1)-dependent cell wall integrity and virulence factors in Cryptococcus neoformans. J Biol Chem. 2011;286(23):20977–90. Epub 2011/04/14. doi: 10.1074/jbc.M111.230268 ; PubMed Central PMCID: PMC3121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lev S, Desmarini D, Chayakulkeeree M, Sorrell TC, Djordjevic JT. The Crz1/Sp1 transcription factor of Cryptococcus neoformans is activated by calcineurin and regulates cell wall integrity. PloS one. 2012;7(12):e51403 doi: 10.1371/journal.pone.0051403 ; PubMed Central PMCID: PMCPMC3520850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gorlach J, Fox DS, Cutler NS, Cox GM, Perfect JR, Heitman J. Identification and characterization of a highly conserved calcineurin binding protein, CBP1/calcipressin, in Cryptococcus neoformans. The EMBO journal. 2000;19(14):3618–29. doi: 10.1093/emboj/19.14.3618 ; PubMed Central PMCID: PMCPMC313974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fox DS, Heitman J. Calcineurin-binding protein Cbp1 directs the specificity of calcineurin-dependent hyphal elongation during mating in Cryptococcus neoformans. Eukaryotic cell. 2005;4(9):1526–38. doi: 10.1128/EC.4.9.1526-1538.2005 ; PubMed Central PMCID: PMCPMC1214203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chow EW, Clancey SA, Billmyre RB, Averette AF, Granek JA, Mieczkowski P, et al. Elucidation of the calcineurin-Crz1 stress response transcriptional network in the human fungal pathogen Cryptococcus neoformans. PLoS genetics. 2017;13(4):e1006667 doi: 10.1371/journal.pgen.1006667 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park HS, Chow EW, Fu C, Soderblom EJ, Moseley MA, Heitman J, et al. Calcineurin targets involved in stress survival and fungal virulence. PLoS pathogens. 2016;12(9):e1005873 doi: 10.1371/journal.ppat.1005873 ; PubMed Central PMCID: PMCPMC5017699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L, Tian X, Gyawali R, Upadhyay S, Foyle D, Wang G, et al. Morphotype transition and sexual reproduction are genetically associated in a ubiquitous environmental pathogen. PLoS pathogens. 2014;10(6):e1004185 Epub 2014/06/06. doi: 10.1371/journal.ppat.1004185 ; PubMed Central PMCID: PMC4047104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bahn YS, Kojima K, Cox GM, Heitman J. Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Molecular biology of the cell. 2005;16(5):2285–300. doi: 10.1091/mbc.E04-11-0987 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin X. Cryptococcus neoformans: morphogenesis, infection, and evolution. Infect Genet Evol. 2009;9(4):401–16. doi: 10.1016/j.meegid.2009.01.013 [DOI] [PubMed] [Google Scholar]
- 83.de Castro PA, Chen C, de Almeida RS, Freitas FZ, Bertolini MC, Morais ER, et al. ChIP-seq reveals a role for CrzA in the Aspergillus fumigatus high-osmolarity glycerol response (HOG) signalling pathway. Molecular microbiology. 2014;94(3):655–74. doi: 10.1111/mmi.12785 . [DOI] [PubMed] [Google Scholar]
- 84.Yu SJ, Chang YL, Chen YL. Calcineurin signaling: lessons from Candida species. FEMS yeast research. 2015;15(4):fov016 doi: 10.1093/femsyr/fov016 . [DOI] [PubMed] [Google Scholar]
- 85.Chen YL, Brand A, Morrison EL, Silao FG, Bigol UG, Malbas FF Jr., et al. Calcineurin controls drug tolerance, hyphal growth, and virulence in Candida dubliniensis. Eukaryotic cell. 2011;10(6):803–19. Epub 2011/05/03. doi: 10.1128/EC.00310-10 ; PubMed Central PMCID: PMC3127677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steinbach WJ, Cramer RA Jr., Perfect BZ, Asfaw YG, Sauer TC, Najvar LK, et al. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryotic cell. 2006;5(7):1091–103. doi: 10.1128/EC.00139-06 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tamuli R, Deka R, Borkovich KA. Calcineurin Subunits A and B Interact to Regulate Growth and Asexual and Sexual Development in Neurospora crassa. PloS one. 2016;11(3):e0151867 doi: 10.1371/journal.pone.0151867 ; PubMed Central PMCID: PMCPMC4809485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bader T, Schroppel K, Bentink S, Agabian N, Kohler G, Morschhauser J. Role of calcineurin in stress resistance, morphogenesis, and virulence of a Candida albicans wild-type strain. Infection and immunity. 2006;74(7):4366–9. doi: 10.1128/IAI.00142-06 ; PubMed Central PMCID: PMCPMC1489686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kontoyiannis DP, Lewis RE, Alexander BD, Lortholary O, Dromer F, Gupta KL, et al. Calcineurin inhibitor agents interact synergistically with antifungal agents in vitro against Cryptococcus neoformans isolates: correlation with outcome in solid organ transplant recipients with cryptococcosis. Antimicrobial agents and chemotherapy. 2008;52(2):735–8. doi: 10.1128/AAC.00990-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen YL, Lehman VN, Lewit Y, Averette AF, Heitman J. Calcineurin governs thermotolerance and virulence of Cryptococcus gattii. G3 (Bethesda). 2013;3(3):527–39. Epub 2013/03/02. doi: 10.1534/g3.112.004242 ; PubMed Central PMCID: PMC3583459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cramer RA Jr., Perfect BZ, Pinchai N, Park S, Perlin DS, Asfaw YG, et al. Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryotic cell. 2008;7(7):1085–97. doi: 10.1128/EC.00086-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soriani FM, Malavazi I, da Silva Ferreira ME, Savoldi M, Von Zeska Kress MR, de Souza Goldman MH, et al. Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Molecular microbiology. 2008;67(6):1274–91. doi: 10.1111/j.1365-2958.2008.06122.x . [DOI] [PubMed] [Google Scholar]
- 93.Karababa M, Valentino E, Pardini G, Coste AT, Bille J, Sanglard D. CRZ1, a target of the calcineurin pathway in Candida albicans. Molecular microbiology. 2006;59(5):1429–51. doi: 10.1111/j.1365-2958.2005.05037.x . [DOI] [PubMed] [Google Scholar]
- 94.Alonso-Monge R, Roman E, Arana DM, Prieto D, Urrialde V, Nombela C, et al. The Sko1 protein represses the yeast-to-hypha transition and regulates the oxidative stress response in Candida albicans. Fungal Genet Biol. 2010;47(7):587–601. doi: 10.1016/j.fgb.2010.03.009 . [DOI] [PubMed] [Google Scholar]
- 95.Alonso-Monge R, Navarro-Garcia F, Molero G, Diez-Orejas R, Gustin M, Pla J, et al. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. Journal of bacteriology. 1999;181(10):3058–68. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Day AM, Smith DA, Ikeh MA, Haider M, Herrero-de-Dios CM, Brown AJ, et al. Blocking two-component signalling enhances Candida albicans virulence and reveals adaptive mechanisms that counteract sustained SAPK activation. PLoS pathogens. 2017;13(1):e1006131 doi: 10.1371/journal.ppat.1006131 ; PubMed Central PMCID: PMCPMC5300278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feretzaki M, Heitman J. Genetic circuits that govern bisexual and unisexual reproduction in Cryptococcus neoformans. PLoS genetics. 2013;9(8):e1003688 Epub 2013/08/24. doi: 10.1371/journal.pgen.1003688 ; PubMed Central PMCID: PMC3744442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin X, Chacko N, Wang L, Pavuluri Y. Generation of stable mutants and targeted gene deletion strains in Cryptococcus neoformans through electroporation. Med Mycol. 2015;53(3):225–34. doi: 10.1093/mmy/myu083 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Toffaletti DL, Rude TH, Johnston SA, Durack DT, Perfect JR. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. Journal of bacteriology. 1993;175(5):1405–11. . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cells (optical density of OD600 = 1.0) were dropped onto the indicated medium and cultured at 30°C for 2 days followed by additional incubation at 22°C for 4 days.
(PDF)
The wild-type serotype D strain XL280 and the corresponding mat2Δ and znf2Δ mutants were cultured on V8 juice agar medium for 6 days at 22°C or on glucosamine medium for 2 days at 30°C followed by additional 4 days of incubation at 22°C. (upper two panels). The wild-type serotype A strain H99 and the corresponding mat2Δ and znf2Δ mutants were cultured on V8 juice agar medium at 22°C for 4 days.
(PDF)
(A) A diagram of the hexamine metabolism pathway. HK: Hexose Kinase, GNAD: Glucosamine Deacetylase, GND: Glucosamine Deaminase, GNAT: Glucosamine N-acetyl transferase. (B) The growth of the gnd1Δ mutant is hypersensitive to glucosamine. Wild-type H99 and the gnd1Δ mutant were cultured at 30°C for 2 days on YP medium containing glucosamine of different concentrations with or without the addition of other carbon sources (galactose, glycerol, or xylose). (C) The gnd1Δ mutant filamented similarly as the wild-type strain H99. The wild-type H99 and the gnd1Δ mutant were cultured on glucosamine medium for 2 days at 30°C followed by additional 4 days of incubation at 22°C.
(PDF)
(PDF)
Cells from the wild-type H99, the cna1Δ mutant, the cnb1Δ mutant, the cbp1Δ mutant, and the crz1Δ mutant were serial diluted (5x) and spotted onto YPD medium or YPD medium with Calcofluor white/CFW (10 μg/ml) or Congo Red (0.2%). The cells on YPD medium were incubated at 30°C or 37°C as indicated. Cells on medium with Calcofluor white or Congo red were cultured at 30°C.
(PDF)
(A) The wild-type H99, the crz1Δ mutant, and the CRZ1oe strain all in the mating type α were cultured alone on V8 juice agar medium at 22°C for 9 days. 3 μl of cells at the density of OD600 = 3 were used to inoculate. (B) The wild-type H99α, the crz1Δ α mutant, and the CRZ1oe α strain were mixed with the mating partner KN99a of the opposite mating type. The mixed co-cultures were inoculated and cultured on V8 medium at 22°C in the dark for 9 days.
(PDF)
To test temperature’s effect on the subcellular localization of Crz1-mCherry, the strain XW252 (PCRZ1- CRZ1-mCherry, GFP-Nop1) was cultured in YPD liquid at 37°C with shaking for 9 hours. To test the effect of calcium, cells of the strain P CRZ1-mCherry-CRZ1 were first collected from the culture in liquid YPD at 22°C for 9 hours and then suspended in YPD with 100 mM of CaCl2 for 10–20 min. To test the effect of glucosamine, the strain PCRZ1-mCherry-CRZ1 was cultured in YP-glucosamine liquid medium at 22°C for 9 hours. For the examination of a shift in carbon-source, cells were incubated in YP-glucosamine liquid medium at 22°C for 9 hours and then shifted to glucose medium at 22°C for 90 minutes. (A) Images of the cells under the conditions tested. (B) Quantification of cells with nuclear localization of Crz1.
(PDF)
Cells of the following strains (wild-type H99, crz1Δ, cna1Δ, cna1ΔCRZ1oe, CRZ1oe, pbs2Δ, pbs2Δcrz1Δ, and pbs2ΔCRZ1oe) were serial diluted and spotted onto YPD medium with or without the addition of NaCl at 1M, or 1.5M. Cells were then incubated at 30°C or 37°C for 3 days before images were taken.
(PDF)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.