Abstract
Arsenic trioxide (ATO) is used as a chemotherapeutic agent for the treatment of acute promyelocytic leukemia. However, increasing drug resistance is reducing its efficacy. Therefore, a better understanding of ATO resistance mechanism is required. In this study, we established an ATO-resistant human epidermoid carcinoma cell line, KB/ATO, from its parental KB-3-1 cells. In addition to ATO, KB/ATO cells also exhibited cross-resistance to other anticancer drugs such as cisplatin, antimony potassium tartrate, and 6-mercaptopurine. The arsenic accumulation in KB/ATO cells was significantly lower than that in KB-3-1 cells. Further analysis indicated that neither application of P-glycoprotein inhibitor, breast cancer resistant protein (BCRP) inhibitor, or multidrug resistance protein 1 (MRP1) inhibitor could eliminate ATO resistance. We found that the expression level of ABCB6 was increased in KB/ATO cells. In conclusion, ABCB6 could be an important factor for ATO resistance in KB/ATO cells. The ABCB6 level may serve as a predictive biomarker for the effectiveness of ATO therapy.
KEY WORDS: Arsenic trioxide, KB/ATO cells, Multidrug resistance, ABCB6, KB-3-1 cells, Biomarker
Graphical abstract
An ATO-resistant human epidermoid carcinoma cell line, KB/ATO, was established from its parental KB-3-1 cells. The arsenic accumulation in KB/ATO cells was significantly lower than that in KB-3-1 cells and the expression level of ABCB6 was increased. The upregulated ABCB6 may be important for ATO resistance in KB/ATO cells.
1. Introduction
As an ancient medicinal formula for over 2000 years, inorganic arsenic salts existed in three common forms, which were As4S4, As4S3, and As2O3 (ATO, arsenic trioxide)1. Historically, arsenic preparations were used to treat the serious diseases such as malaria and malignant diseases2. Acute promyelocytic leukemia (APL) is a malignancy of the bone marrow, which is characterized by abnormal accumulation of promyelocyte. The United States Food and Drug Administration (FDA) has approved the use of ATO as a treatment of APL to the patient who has relapsed after all-trans-retinoic acid (ATRA) and anthracycline-based chemotherapy3. ATO was previously described to be able to induce apoptosis and differentiation in APL cells4, 5. The therapeutic effect of ATO in APL cells is strongly associated with growth suppressor promyelocytic leukemia protein (PML)6. Disruption of nuclear bodies and related abnormal localization of PML was found in APL cells. ATO promoted the recruitment of PML and therefore induce apoptosis7, 8, 9.
Multidrug resistance (MDR) in cancer is a major impediment to successful chemotherapy10. Similar to other anticancer agents, arsenic resistance has been reported following treatment of ATO11. Many mechanisms can be related to ATO resistance, such as improved inorganic arsenic methylation12, differential synthesis of heat-shock proteins and ATO target protein13, and decreased the intracellular accumulation of ATO mediated by efflux pumps. Among these mechanisms, reduction of drug accumulation within cancer cells mediated by ATP-binding cassette (ABC) transporters is a very common mode of action for conferring multidrug resistance10. As previously reported, undiscovered efflux pumps may be a major contributor to arsenic elimination and arsenic resistance14, 15. ABC transporters belong to an important efflux pump superfamily. The energy derived from ATP hydrolysis drives transmembrane transport of various substrates, including heavy metals10. P-glycoprotein (P-gp/ABCB1/MDR1), breast cancer resistant protein (BCRP/ABCG2/MXR), and multidrug resistance protein 1 (MRP1/ABCC1) have all been reported to be putative heavy metal efflux pumps and may have physiological protection effects16.
To understand the molecular basis of ATO resistance, we established an ATO-induced resistant KB/ATO cell line for this study. Drug-resistant phenotype for the ATO-resistant cell line showed that the development of ATO resistance was accompanied by cross-resistance to antimony potassium tartrate (APT), 6-mercaptopurine (6-MP), cisplatin, and vincristine. In the present study, we investigated several promising candidates conducting ATO resistance by reversal studies and Western blot analysis. The increased expression of ABCB6 might serve as an efflux pump for mediating ATO resistance.
2. Methods
2.1. Chemicals
Dulbecco׳s modified Eagle׳s Medium (DMEM, Catalog SH30022), fetal bovine serum (FBS, Catalog SH30071), penicillin/streptomycin (Catalog SV30010) and trypsin 0.25% (Catalog SH30042) were purchased from Hyclone (GE Healthcare Life Science, Pittsburgh, PA). ATO (Catalog A1010), 6-MP (Catalog 38171), APT (Catalog P6949), vincristine (Catalog V8879), cisplatin (Catalog 1134357), doxorubicin (Catalog D1515), mitoxantrone (Catalog M6545), paclitaxel (Catalog T7191), verapamil (Catalog V4629), and 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT, Catalog M2128) were purchased from Sigma–Aldrich, USA. Sipholenol A was obtained from Dr. El Sayed׳s laboratory (Univeristy of Louisiana). PAK-104P and Pranlukast (ONO-1078) were obtained from Dr. Akiyama׳s Lab in Japan. Sildenafil (Viagra) was purchased from Pfizer Pharmaceutical Co. Fumitremorgin C (FTC) was obtained from Dr. Susan Bates (NIH). Monoclonal antibody C219 (Catalog MA126528) against human P-gp, monoclonal antibody BXP21 (Catalog NB1002177) against human BCRP were purchased from Thermo Fisher Scientific Inc. (Rockford, IL, USA). Monoclonal antibody QCRL-1 (Catalog sc-18835) against human MRP1 was purchased from Santa Cruz Biotechnology. Antibody against ABCB6 was a gift generously provided by Dr. John D. Schuetz (St. Jude׳s Children׳s Hospital, TN, USA).
2.2. Cell lines and cell culture
Human epidermoid carcinoma KB-3-1 cells were obtained from the NIH and were used as the parental cell line in this study. The resistance cell line, KB/ATO, was created by continuously culturing KB-3-1 cells in ATO in gradually increasing concentrations. The final concentration of ATO was increased stepwise up to 18 μmol/L with a total of 8 months of induction. Established KB/ATO cells were cultured in ATO-free medium for at least four weeks before further experiments were performed. All the cell lines were cultured as adherent monolayers in flasks with DMEM supplemented with 10% FBS and 1% penicillin/streptomycin in a humidified incubator containing 5% CO2 at 37 °C.
2.3. Drug resistance profile of KB/ATO
A slightly modified MTT colorimetric assay was used to detect the sensitivity of the cells to various anticancer drugs as described previously17. Equal amount of cells (3000 cells/well for KB-3-1 and 6000 cells/well for KB/ATO) were seeded into 96-well plates and cultured overnight. Anticancer drugs were then added into the designated wells with a concentration gradient. After 72 h of incubation, 20 μL of MTT solution (4 mg/mL) was added into each well. The microplates were further incubated for 4 h. The medium was then aspirated and 100 μL of DMSO was added to dissolve the formazan crystal in each well. The absorbance was determined at 570 nm by the OPSYS microplate reader (Dynex Technology, Chantilly, VA, USA). The IC50 values were calculated from the survival curves following our previous protocol18. ATO, APT, 6-MP, cisplatin, vincristine, paclitaxel, doxorubicin and mitoxantrone were tested to acquire resistance profile of KB/ATO cells.
2.4. Intracellular ATO accumulation
KB-3-1 and KB/ATO cells were aliquoted and cultured until they were at the condition of around 80% confluence. Cells were further cultured in DMEM supplemented with 10, 20 or 40 μmol/L ATO for another 2 h. Cells were rinsed with cold phosphate-buffered saline (PBS) three times to remove excess extracellular ATO. Cells were trypsinized and suspended in PBS. All cell suspensions were centrifuged at 2400×g and cell pellets were lysed by 500 μL HNO3 for 4 h at 65 °C. The sample solution was evaporated at 85 °C and then dissolved with distilled deionized water. Arsenic levels in the sample solutions were then analyzed using flow injection inductively coupled plasma mass spectroscopy (ICPMS) protocol.
2.5. Reversal effects against ATO resistance
Several known inhibitors of ABC transporters were investigated to evaluate whether they had promising reversal effects against ATO resistance. After seeding cells into 96-well plates, these agents were added into designated wells at a final concentration of 5 μmol/L and 4 h prior anticancer drugs as described above. Verapamil, sildenafil, sipholenol A, PAK-104P, ONO-1078 and FTC were tested respectively.
2.6. Total cell lysates preparation and Western blot
Cell extracts were prepared by incubation on ice with lysis buffer (10 mmol/L Tris, 1 mmol/L EDTA, 0.1% SDS, 150 mmol/L NaCl, 1% Triton-X and protease inhibitor cocktail) for 20 min, followed by centrifugation at 12,000×g at 4 °C for 5 min. The supernatant containing total cell lysate was collected and protein concentration was determined by bicinchoninic acid (BCATM)-based protein assay (Thermo Scientific, Rockford, IL, USA). Equal amount of total cell lysates (40 μg) were loaded and separated by SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. Total cell lysates from HEK293, HEK/ABCB1, HEK/ABCC1 and HEK/ABCG2 were used as positive control for P-gp, MRP1 and BCRP, respectively.
2.7. RT-PCR
The RNA samples were extracted from KB-3-1 and KB/ATO cells by Prefect RNATM kit (Eppendorf) and 1 μg of total RNA were used for cDNA synthesis by using cMasterTM RTplusPCR system and cMaster RT Kit. The primer sequence of ABCB6 for amplification was forward: CAACCGCACCACCATCGTAGT; reverse: AATAAGCCAGGGAAAGGAGACACA.
One step RT-PCR was carried out for 35 cycles as follows: reverse transcription at 50 °C for 30 min, initial denaturation at 94 °C for 2 min, template denaturation at 94 °C for 15 s, primer annealing at 52 °C for 20 s and primer extension/elongation at 68 °C for 30 s. The PCR products were separated by denaturing agarose gel electrophoresis. The gel was stained by ethidium bromide and the bands were visualized by the ECL chemiluminescence system.
2.8. Statistics
All data are expressed as mean±SD from three or more experiments and statistically evaluated by Student׳s t-test. Differences were considered significant when P < 0.05.
3. Results
3.1. Development of ATO resistant cancerous cell line and cross-resistance profile
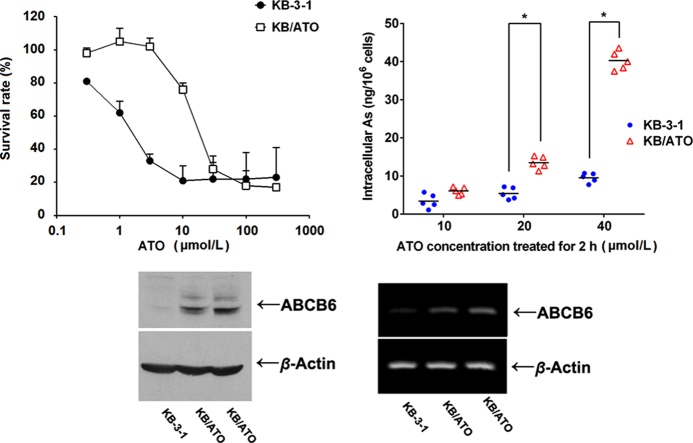
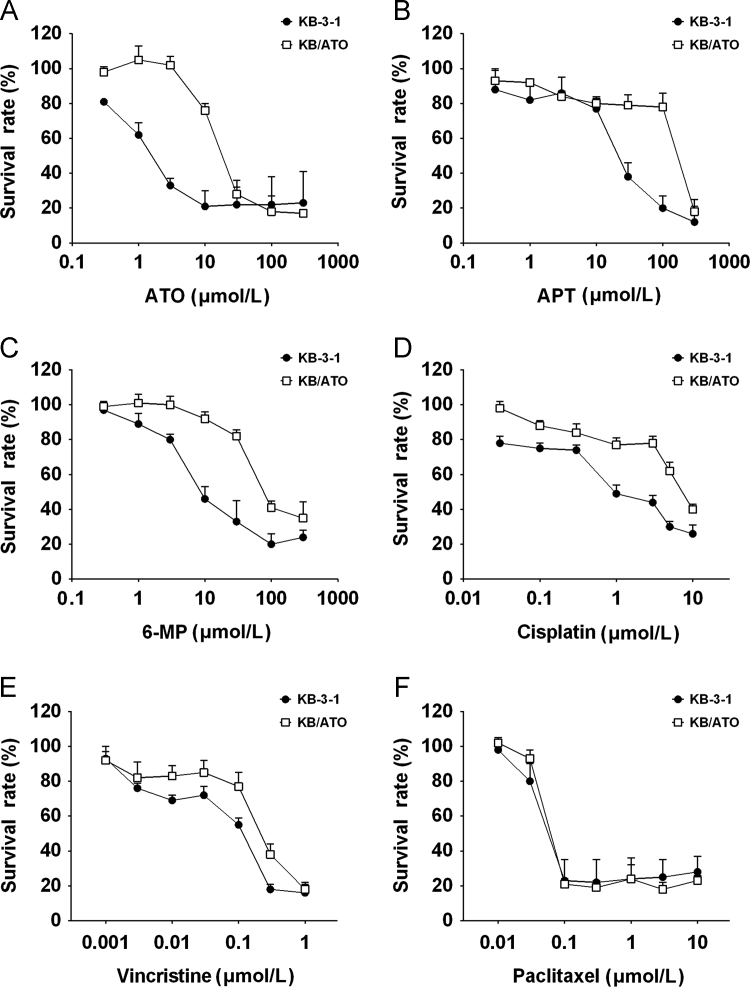
By adding ATO when culturing KB-3-1 cells, we eventually established an ATO-resistant cell line (KB/ATO) which could survive at ATO concentration up to 18 μmol/L. After cells were cultured in ATO-free media for 4 weeks, our MTT assays showed that the resistance level to ATO in KB/ATO cells was still maintained. As shown in Fig. 1A, the survival rate curve of KB/ATO towards ATO was significantly shifted to the right as compared with that of KB-3-1 cells, indicating a resistance-mediated improvement in survival.
Figure 1.
Cytotoxicity of different anticancer drugs towards KB-3-1 and KB/ATO cells. Cell survival rate was determined by the MTT assay as described in Section 2.3. Data points with error bars represent the mean±SD. Each above figure is a representative of three independent experiments, each done in triplicate.
The cross-resistant profile of KB/ATO cells in summarized in Table 1. The IC50 values of KB-3-1 and KB/ATO cells׳ susceptibility to ATO are 2.07±0.92 and 21.07±2.47 μmol/L, respectively. KB/ATO exhibited a 10.04-fold resistance to ATO as compared to KB-3-1. In addition, KB/ATO cells conferred a 13.52-fold cross-resistance towards APT as compared to that in parental cells (Fig. 1B). Similarly, KB/ATO cells also showed 8.75-, 5.07- and 2.98-fold of cross resistance to 6-MP (Fig. 1C), cisplatin (Fig. 1D), and vincristine (Fig. 1E), respectively. Moderate increases in IC50 values were observed in KB/ATO cells towards paclitaxel (Fig. 1F) and doxorubicin; however, these moderate increases did not have significant difference as compared with that in parental KB-3-1 cells. KB/ATO cells did not show any resistance towards mitoxantrone.
Table 1.
The resistance profile of KB/ATO cell.
| Substrate | IC50 value ± SDa (μmol/L) |
Resistance foldb | |
|---|---|---|---|
| KB-3-1 | KB/ATO | ||
| ATO | 2.07 ± 0.92 | 21.07 ± 2.47 | 10.04* |
| APT | 16.29 ± 7.19 | 220.36 ± 40.41 | 13.52* |
| 6-MP | 7.86 ± 0.84 | 68.84 ± 2.88 | 8.75* |
| Cisplatin | 1.66 ± 1.46 | 8.43 ± 2.43 | 5.07* |
| Vincristine | 0.067 ± 0.036 | 0.20 ± 0.039 | 2.98* |
| Paclitaxel | 0.054 ± 0.019 | 0.080 ± 0.013 | 1.48 |
| Doxorubicin | 0.91 ± 1.14 | 1.56 ± 1.23 | 1.71 |
| Mitoxantrone | 0.25 ± 0.22 | 0.23 ± 0.18 | 0.92 |
IC50 values are represented as mean±SD of three independent experiments performed in triplicate.
Resistance fold was calculated by dividing the IC50 values of substrates by the IC50 of parental cells.
P < 0.05 versus parental cells.
3.2. ATO accumulation in KB-3-1 and KB/ATO cells
In order to evaluate whether the acquired ATO resistance in KB/ATO cells was due to the decrease of ATO accumulation, we investigate ATO accumulation levels in both KB-3-1 and KB/ATO cells. Cells were incubated at different concentrations of ATO and the intracellular arsenic was measured by ICPMS.
As shown in Fig. 2, the average intracellular arsenic accumulation for cells incubated with 10 μmol/L ATO were 6.0±1.1 and 2.8±2.9 ng/106 cells for KB-3-1 and KB/ATO cells, respectively. The average intracellular arsenic accumulation for cells incubated with 20 μmol/L ATO were 12.5±2.8 and 5.4±1.7 for KB-3-1 and KB/ATO cells, respectively. The average intracellular arsenic accumulation for cells incubated with 40 μmol/L ATO were 39.4±2.6 and 8.7±1.6 for KB-3-1 and KB/ATO, respectively. The intracellular arsenic accumulation in KB/ATO cells with the three ATO concentrations (10, 20, and 40 μmol/L) were 47%, 44%, and 22% of that observed in KB-3-1 cells, respectively. Significant differences between KB/ATO and KB-3-1 were acquired in groups treated with 20 and 40 μmol/L ATO.
Figure 2.
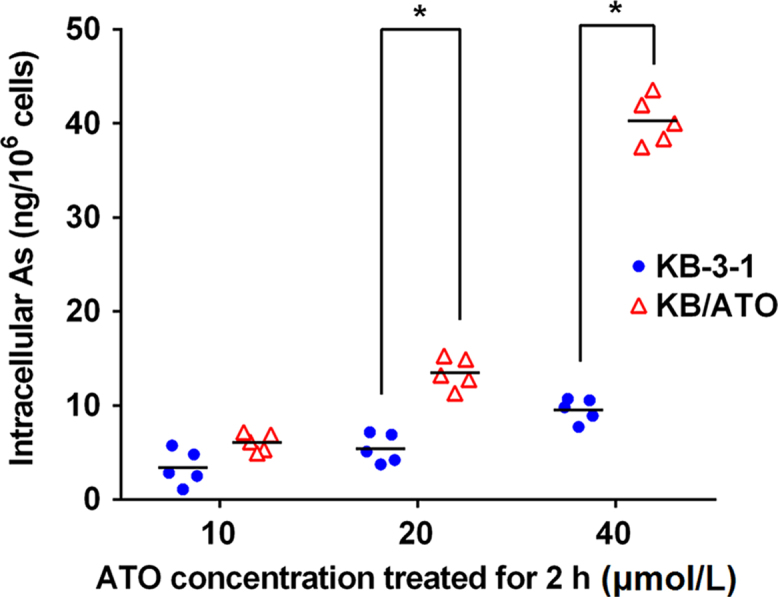
Intracellular accumulation of ATO in KB-3-1 and KB/ATO cells. The bar lines represent the mean valve. *P < 0.05 between groups. A representative result was shown here and similar results were obtained in other trials.
3.3. Investigation of reversal effects of ATO resistance
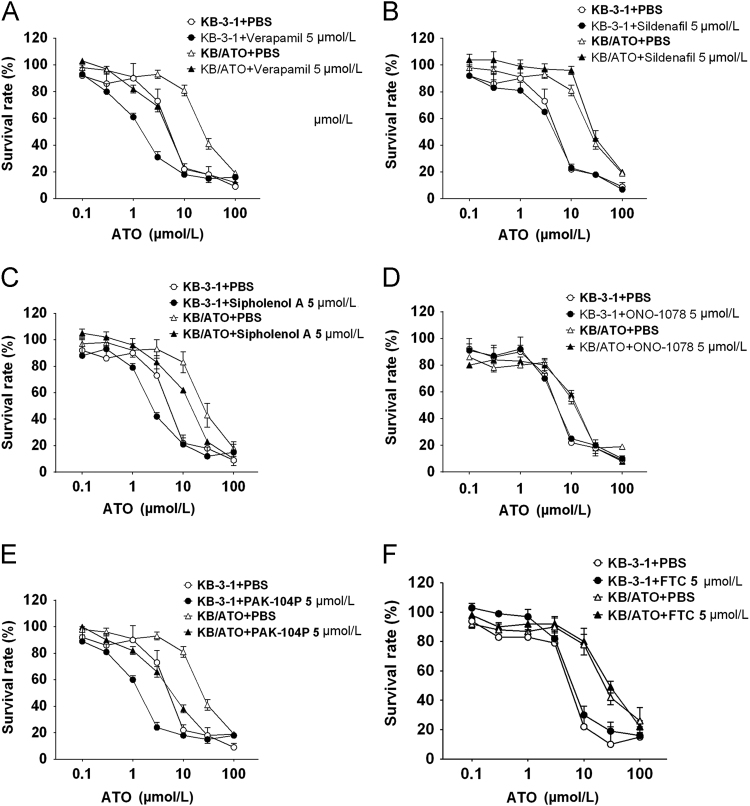
Several compounds were investigated to inspect whether any of them can be a promising reversal agent in ATO-resistance. We first investigate the reversal effect of ATO resistance by using P-gp inhibitors verapamil, sildenafil and sipholenol A. As shown in Table 2, verapamil increased the sensitivity of KB-3-1 and KB/ATO to ATO. However as shown in Fig. 3A, the increased sensitivity does not overcome ATO resistance. A significantly higher IC50 value of ATO was still observed in verapamil-pretreated KB/ATO cells as compared to that in verapamil-pretreated KB-3-1 cells. A similar trend was observed in the sipholenol A-pretreated group. Pretreatment with sildenafil did not significantly alter the IC50 values of ATO in KB-3-1 and KB/ATO cells.
Table 2.
The effects of reversal agents in KB-3-1 and KB/ATO cells to the cytotoxicity of ATO.
| Treatment | IC50 value±SDa (μmol/L) |
Resistant Foldb | |
|---|---|---|---|
| KB-3-1 | KB/ATO | ||
| ATO | 3.71 ± 2.89 | 26.24 ± 8.77 | 7.07* |
| + Verapamil 5 μmol/L | 1.95 ± 1.33 | 7.45 ± 5.09 | 3.82 |
| + Sildenafil 5 μmol/L | 5.77 ± 5.23 | 26.72 ± 9.23 | 4.63* |
| + Sipholenol A 5 μmol/L | 3.31 ± 2.22 | 16.32 ± 10.29 | 4.93 |
| + PAK-104P 5 μmol/L | 2.44 ± 2.13 | 6.34 ± 5.24 | 2.59 |
| + ONO-1078 5 μmol/L | 3.69 ± 3.13 | 27.13 ± 5.12 | 7.35* |
| + FTC 5 μmol/L | 4.28 ± 1.72 | 26.43 ± 4.33 | 6.18* |
*P < 0.05 versus parental cells.
IC50 values are represented as mean ± SD of three independent experiments performed in triplicate.
Resistance fold was calculated by dividing the IC50 values of KB/ATO cells by the IC50 of KB-3-1 cells after treatment.
Figure 3.
Reversal effect of several ABC transporter inhibitors in KB-3-1 and KB/ATO. (A) Verapamil; (B) sildenafil; (C) sipholenol A; (D) pranlukast (ONO-1078); (E) PAK-104P; (F) fumitremorgin C (FTC). Cell survival rate was determined by the MTT assay as described in Section 2.3. Data points with error bars represent the mean ± SD. Each above figure is a representative of three independent experiments, each done in triplicate.
Furthermore, the effect of MRP1 inhibitor ONO-1078 and PAK-104P was evaluated. ONO-1078 did not alter the IC50 values of ATO in both parental and ATO-resistant cells, while PAK-104P decreased the IC50 values of ATO but failed to reverse the ATO-resistance. Specific BCRP inhibitor FTC did not alter the IC50 of ATO in both cell lines. Overall, P-gp, MRP1, and BCRP may not be the main factors of ATO resistance in KB/ATO cells.
3.4. Western blot and RT-PCR analysis in KB-3-1 and KB/ATO cells
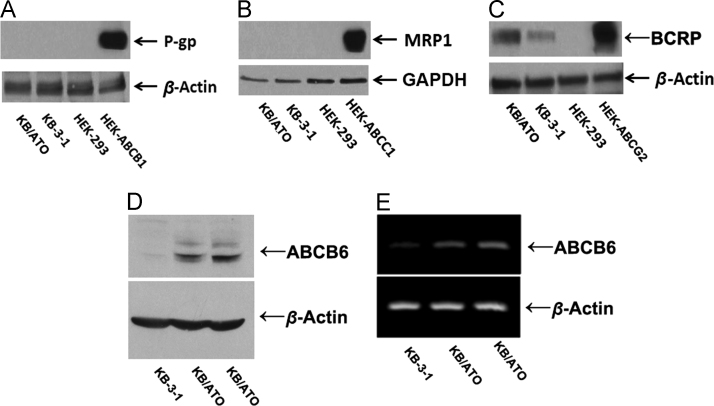
The expressions of ABC transporters including P-gp, MRP1, BCRP, and ABCB6 were analyzed by Western blotting. While the expression levels of P-gp and MRP1 were strongly observed in the positive control HEK/ABCB1 and HEK/ABCC1 cells, respectively, the expression levels of these two transporters were not observed in KB-3-1 or KB-ATO cells (Fig. 4A and B). Low levels of BCRP expression were observed in KB-ATO cells, which were shown to be much lower than the positive control HEK-ABCG2 cells (Fig. 4C). Increased expressions of ABCB6 were detected in both KB-ATO cells, but not in KB-3-1 cells (Fig. 4D). RT-PCR results indicated that ABCB6 are upregulated at mRNA level in KB/ATO cells (Fig. 4E).
Figure 4.
Protein expression profile in KB/ATO cells and parental KB-3-1 cells. Expression level of (A) P-gp, (B) MRP1, (C) BCRP and (D) ABCB6 were shown in western blot results. (E) RT-PCR was performed to confirm the mRNA levels of ABCB6. Cell lysate from HEK/ABCB1, HEK/ABCC1 and HEK/ABCG2 was used as positive control for P-gp, MRP1 and BCRP respectively.
4. Discussion
Drug resistance is one of the major problems that deter successful treatment for cancers. Resistance may occur due to several mechanisms such as decrease in accumulation of drugs inside the cells via reducing the inward transport or increasing drug efflux; decrease of apoptosis of cancer cells; enhanced inactivation or detoxification of drugs; inability of converting prodrugs to their active forms; enhanced ability of DNA repair19. In order to understand the mechanisms of ATO resistance, we established an ATO resistant cell line by culturing parental KB-3-1 cells with gradually increasing concentration of ATO, and termed them as the KB/ATO cells. Our hypothesis that this ATO resistance may be related to ABC transporters is supported by the following reasons. Firstly, the significantly lower accumulation of ATO in KB/ATO cells suggested that ATO might be being pumped out from the cells by ABC transporters. Secondly, KB/ATO cell line confers not only resistance to ATO, but also resistance to other structurally unrelated drugs, such as APT, 6-MP, cisplatin, and vincristine. This multi-drug resistance also suggested an existence of ABC transporter in KB/ATO cells.
P-gp, MRP1 and BCRP are the three ABC transporters that are broadly expressed in multidrug resistant cells. Therefore, we first examined the function of these transporters in ATO resistance in our KB/ATO cells. However, P-gp is unlikely related to ATO resistance based on our results. Firstly, resistant KB/ATO cells did not develop cross-resistance against paclitaxel, which is a known substrate of P-gp. Secondly, although P-gp inhibitors verapamil and sipholenol A sensitized KB/ATO cells, similar sensitization effects were also observed in parental KB-3-1 cells. Therefore, these sensitization effects are not generated from P-gp inhibition. Moreover, P-gp inhibitor sildenafil did not alter the efficacy of ATO in both parental and ATO-resistance cells. Thirdly and most directly, our Western blotting analysis did not show any expression of P-gp in KB/ATO cells. P-gp therefore is not the factor for conferring ATO resistance. Similarly, MRP1 was also excluded from contribution to ATO resistance in KB/ATO cells. PAK-104P and verapamil might be clinically useful as a combination with ATO for improving efficacy. However, the mechanism of ATO resistance needs to be further determined.
Our Western blotting results showed that BCRP was induced in resistant KB/ATO as compared to parental KB-3-1 cells. However, the expression level of BCRP may be too low to serve as an effective pump as shown in Fig. 3C. Besides that, KB/ATO cells did not acquire resistance against BCRP substrate mitoxantrone. Moreover, specific BCRP inhibitor FTC had no effect on cytotoxicity of ATO against KB-3-1 and KB/ATO cells. The induced BCRP might have indirect effect in cell signaling network or have other physiological effects in KB/ATO, in which further experiments are required.
Several half-type ABC transporters were reported to be related to heavy metal transport and iron metabolism in cells, such as Atm1p located in the mitochondrial inner membrane20. ABCB6 is also a half-type ABC transporter related to porphyrin transport. As previously reported by Krishnamurthy et al.21. ABCB6 is located in the outer mitochondrial membrane and is required for mitochondrial porphyrin uptake. Further study by Paterson et al.22 suggested that ABCB6 can exist in two molecular weight forms and is located on both outer mitochondrial membrane and the plasma membrane, and plays functional role in the plasma membrane. Moreover, ABCB6 is reported to be functionally related to ATO transport23. Therefore, ABCB6 may be a possible contributor for ATO resistance in KB/ATO cells. Our Western blot result showed that ABCB6 was over-expressed in KB/ATO cells. This result further suggested that ABCB6 is a promising candidate responsible for ATO resistance in KB/ATO cells. The result of RT-PCR showed some expression of ABCB6 mRNA in parental KB-3-1 cells, although resistant KB/ATO cells demonstrated a stronger expression level of ABCB6 mRNA. These results indicate that ATO may induce the transcription of ABCB6 in KB/ATO cells, while it may also have post-transcriptional regulation mechanism in ABCB6 expression. The discrepancy between the magnitude of resistance to ATO and the reduction of ATO accumulation in the MDR KB/ATO cells may be due, at least in part, to the expression pattern of ABCB6. ABCB6 may be expressed in both cytoplasmic membrane and mitochondria outer membrane. The apoptotic effect of ATO may be eliminated in KB/ATO cells by either being pumped out of cells or being transferred into mitochondria for metabolism. Both functions decrease the subcellular concentration of ATO in nuclear zone.
5. Conclusions
In conclusion, the ATO-induced resistant cell line, KB/ATO, confers resistance to ATO, APT, 6-MP, cisplatin, and vincristine. The multidrug resistance in KB/ATO cells compared to parental KB-3-1 cells may be due to the overexpression of ABCB6, however further work may still be needed to confirm the role of ABCB6 in KB/ATO cells. It will be also important to evaluate whether overexpression of ABCB6 as a mechanism of ATO resistance is clinically relevant. ABCB6 level may serve as a predictive biomarker for the effectiveness of ATO therapy.
Acknowledgments
This work was supported by funds from NIH (No. 1R15CA143701) and St. John׳s University Research Seed Grant (No. 579–1110-7002) to Zhe-Sheng Chen. We also thank Dr. Akiyama (Kagoshima University, Japan) for kindly providing PAK-104P and Pranlukast (ONO-1078), Drs. Schuetz and Fukuda (St. Jude Children׳s Research Hospital, Memphis, USA) for kindly provide ABCB6 antibody and technical supports. We thank Dr. El Sayed (Univeristy of Louisiana, USA) for the Sipholenol A. We also thank Dr. Yangmin Chen (ICON plc, North Wales, PA, USA) for critical editing of the manuscript.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Konkola K. More than a coincidence? The arrival of arsenic and the disappearance of plague in early modern europe. J Hist Med Allied Sci. 1992;47:186–209. doi: 10.1093/jhmas/47.2.186. [DOI] [PubMed] [Google Scholar]
- 2.Kwong Y.L., Todd D. Delicious poison: arsenic trioxide for the treatment of leukemia. Blood. 1997;89:3487–3488. [PubMed] [Google Scholar]
- 3.Wang Z.Y., Chen Z. Differentiation and apoptosis induction therapy in acute promyelocytic leukaemia. Lancet Oncol. 2000;1:101–106. doi: 10.1016/s1470-2045(00)00017-6. [DOI] [PubMed] [Google Scholar]
- 4.Muto A., Kizaki M., Kawamura C., Matsushita H., Fukuchi Y., Umezawa A. A novel differentiation-inducing therapy for acute promyelocytic leukemia with a combination of arsenic trioxide and GM-CSF. Leukemia. 2001;15:1176–1184. doi: 10.1038/sj.leu.2402162. [DOI] [PubMed] [Google Scholar]
- 5.Wood K., Swade C., Harwood J., Eccleston E., Bishop M., Coppen A. Comparison of methods for the determination of total and free tryptophan in plasma. Clin Chim Acta. 1977;80:299–303. doi: 10.1016/0009-8981(77)90037-7. [DOI] [PubMed] [Google Scholar]
- 6.Chen B., Liu Q., Popowich A., Shen S., Yan X., Zhang Q. Therapeutic and analytical applications of arsenic binding to proteins. Metallomics. 2015;7:39–55. doi: 10.1039/c4mt00222a. [DOI] [PubMed] [Google Scholar]
- 7.Shao W., Fanelli M., Ferrara F.F., Riccioni R., Rosenauer A., Davison K. Arsenic trioxide as an inducer of apoptosis and loss of PML/RARα protein in acute promyelocytic leukemia cells. J Natl Cancer Inst. 1998;90:124–133. doi: 10.1093/jnci/90.2.124. [DOI] [PubMed] [Google Scholar]
- 8.Zhu J., Koken M.H., Quignon F., Chelbi-Alix M.K., Degos L., Wang Z.Y. Arsenic-induced pml targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 1997;94:3978–3983. doi: 10.1073/pnas.94.8.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J., Lallemand-Breitenbach V., de Thé H. Pathways of retinoic acid- or arsenic trioxide-induced PML/RARα catabolism, role of oncogene degradation in disease remission. Oncogene. 2001;20:7257–7265. doi: 10.1038/sj.onc.1204852. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y.K., Wang Y.J., Gupta P., Chen Z.S. Multidrug resistance proteins (MRPS) and cancer therapy. AAPS J. 2015;17:802–812. doi: 10.1208/s12248-015-9757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomita A., Kiyoi H., Naoe T. Mechanisms of action and resistance to all-trans retinoic acid (ATRA) and arsenic trioxide (As2O3) in acute promyelocytic leukemia. Int J Hematol. 2013;97:717–725. doi: 10.1007/s12185-013-1354-4. [DOI] [PubMed] [Google Scholar]
- 12.Vahter M. Methylation of inorganic arsenic in different mammalian species and population groups. Sci Prog. 1999;82:69–88. doi: 10.1177/003685049908200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghoshal S., Rao I., Earp J.C., Jusko W.J., Wetzler M. Down-regulation of heat shock protein 70 improves arsenic trioxide and 17-DMAG effects on constitutive signal transducer and activator of transcription 3 activity. Cancer Chemother Pharmacol. 2010;66:681–689. doi: 10.1007/s00280-009-1210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tachiwada T., Chen Z.S., Che X.F., Matsumoto M., Haraguchi M., Gotanda T. Isolation and characterization of arsenite-resistant human epidermoid carcinoma KB cells. Oncol Rep. 2007;18:721–727. [PubMed] [Google Scholar]
- 15.Banerjee M., Carew M.W., Roggenbeck B.A., Whitlock B.D., Naranmandura H., Le X.C. A novel pathway for arsenic elimination: human multidrug resistance protein 4 (MRP4/ABCC4) mediates cellular export of dimethylarsinic acid (DMAV) and the diglutathione conjugate of monomethylarsonous acid (MMAIII) Mol Pharmacol. 2014;86:168–179. doi: 10.1124/mol.113.091314. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari A.K., Sodani K., Dai C.L., Ashby C.R., Jr, Chen Z.S. Revisiting the abcs of multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12:570–594. doi: 10.2174/138920111795164048. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y.K., Zhang H., Zhang G.N., Wang Y.J., Kathawala R.J., Si R. Semi-synthetic ocotillol analogues as selective ABCB1-mediated drug resistance reversal agents. Oncotarget. 2015;6:24277–24290. doi: 10.18632/oncotarget.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y.K., Zhang G.N., Wang Y.J., Patel B.A., Talele T.T., Yang D.H. Bafetinib (INNO-406) reverses multidrug resistance by inhibiting the efflux function of ABCB1 and ABCG2 transporters. Sci Rep. 2016;6:25694. doi: 10.1038/srep25694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Q., Yang Z., Nie Y., Shi Y., Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014;347:159–166. doi: 10.1016/j.canlet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Lill R., Kispal G. Mitochondrial ABC transporters. Res Microbiol. 2001;152:331–340. doi: 10.1016/s0923-2508(01)01204-9. [DOI] [PubMed] [Google Scholar]
- 21.Krishnamurthy P.C., Du G., Fukuda Y., Sun D., Sampath J., Mercer K.E. Identification of a mammalian mitochondrial porphyrin transporter. Nature. 2006;443:586–589. doi: 10.1038/nature05125. [DOI] [PubMed] [Google Scholar]
- 22.Paterson J.K., Shukla S., Black C.M., Tachiwada T., Garfield S., Wincovitch S. Human ABCB6 localizes to both the outer mitochondrial membrane and the plasma membrane. Biochemistry. 2007;46:9443–9452. doi: 10.1021/bi700015m. [DOI] [PubMed] [Google Scholar]
- 23.Chavan H., Oruganti M., Krishnamurthy P. The ATP-binding cassette transporter ABCB6 is induced by arsenic and protects against arsenic cytotoxicity. Toxicol Sci. 2011;120:519–528. doi: 10.1093/toxsci/kfr008. [DOI] [PMC free article] [PubMed] [Google Scholar]