Abstract
Obesity and high fat intake induce alterations in vascular function and structure. Aberrant O-GlcNAcylation (O-GlcNAc) of vascular proteins has been implicated in vascular dysfunction associated with cardiovascular and metabolic diseases. In the present study, we tested the hypothesis that high-fat diet (HFD)-mediated increases in O-GlcNAc-modified proteins contribute to cerebrovascular dysfunction. O-GlcNAc-protein content was increased in arteries from male Wistar rats treated with a HFD (45% fat) for 12 weeks vs. arteries from rats on control diet (CD). HFD augmented body weight [(g) 550±10 vs. 502±10 CD], increased plasma triglycerides [(mg/dl) 160±20 vs. 95±15 CD] and increased contractile responses of basilar arteries to serotonin (5-HT) [(pD2) 7.0±0.1 vs. 6.7±0.09 CD] and the thromboxane analog U-46619 [(pD2) 7.2±0.1 vs. 6.8±0.09 CD]. Of importance, increased levels of O-GlcNAc [induced by 24 h-incubation of vessels with a potent inhibitor of O-GlcNAcase (PugNAc)] increased basilar artery contractions to U-46619 [(pD2) 7.4±0.07 vs. 6.8±0.08 CD] and 5-HT [(pD2) 7.5±0.06 vs. 7.1±0.1 CD]. Vessels from rats on the HFD for 12 weeks and vessels treated with PugNAc displayed increased phosphorylation of p38 (Thr180/182) and Erk1/2 (Ser180/221). Increased 5HT-induced contractions in arteries from rats on the HFD or arteries incubated with PugNAc were abrogated by mitogen-activated protein kinase (MAPK) inhibitors. Our data show that HFD augments cerebrovascular O-GlcNAcylation and this modification contributes to increased contractile responses and to the activation of the MAPK pathway in the rat basilar artery.
Keywords: high fat, O-GlcNAc, vascular contraction, cerebral artery, basilar artery
INTRODUCTION
The quantity and type of dietary fat have important effects on the development of cardiovascular diseases, including arterial hypertension, atherosclerosis and stroke [1,2]. Studies on blood vessels from humans and animal models indicate that vascular function is impaired during hypercholesterolemia [3–5]. In addition, a high-fat diet (HFD) negatively affects vascular function by increasing myogenic tone and favoring endothelial dysfunction in diet-induced as well as genetic models of obesity [4–8]. We have recently shown that a HFD impairs cerebrovascular reactivity and worsens outcome after cerebral ischemia, even before the development of obesity and metabolic disturbances [2].
O-GlcNAcylation (O-GlcNAc) is a dynamic posttranslational modification of nuclear and cytosolic proteins, first described by Torres and Hart in 1984 [9]. Recent evidence indicates that O-GlcNAc modulates vascular function, structure and development. Augmented O-GlcNAcylation increases vascular reactivity to constrictor stimuli [10–12], impairs nitric oxide (NO)-dependent arteriolar dilations [13], reduces neointima formation, prevents inflammation-induced vascular dysfunction [14] and modulates placental vasculogenesis [15]. Of importance, increased vascular O-GlcNAc-modified proteins, or O-GlcNAcylated proteins, have been reported in aging [16] and cardiovascular diseases such as arterial hypertension, diabetes and hyperlipidemia/obesity [11,17–20], reinforcing the concept that O-GlcNAcylation may play a role in vascular dysfunction associated with these pathological conditions.
Additionally, chronic ingestion of diets high in saturated fat and sugar increases O-GlcNAc protein modification in the rat heart, and this fact may contribute to the adverse effects of metabolic syndrome and diabetes by an O-GlcNAc-mediated process [21]. Furthermore increased vascular O-GlcNAc-protein content was found in HFD-treated animals after cerebral ischemia induced by middle cerebral artery occlusion [2], but it remained unknown whether a HFD impacts O-GlcNAc levels in the cerebral circulation in the absence of other complications (e.g. ischemia).
Brain is one of the tissues where O-GlcNAc is most highly expressed and disturbances in O-GlcNAc levels are implicated in neurodegenerative processes [22]. O-GlcNAcylation modulates protein phosphorylation, including the mitogen-activated protein kinase (MAPK) pathway, and regulates several cellular signaling and functions [23]. Besides the regulatory role of O-GlcNAc has been extensively described in the brain, few studies have addressed how this post-translational modification interferes with the cerebral vasculature.
Large arteries, including the basilar artery, importantly contribute to total cerebral vascular resistance and are major determinants of local microvascular pressure [24]. Basilar artery occlusion is associated with a high mortality rate [25] and the mortality trend for stroke appears to be similar to that of coronary heart disease [26]. However, our current understanding of the mechanisms that modulate basilar artery (dys)function remains incomplete. Therefore, the aim of the present study was to determine whether a HFD activates the O-GlcNAc pathway in cerebral vessels and whether increased O-GlcNAcylation augments contraction in the rat basilar artery. We hypothesized that a HFD increases O-GlcNAc-modified proteins in cerebral arteries leading to increased vascular contractile responses. To address the mechanisms by which O-GlcNAcylation changes cerebral vascular function, we determined whether proteins of the MAPK are modified by O-GlcNAc and whether MAPK activation contributes to increased contractile response in basilar arteries from HFD-treated rats.
METHODS
Animals
Male Wistar rats [4 weeks-old (high fat diet protocol) or 12 weeks-old (PugNAc-incubation protocol); Harlan Laboratories, Indianapolis, IN, USA] were used in this study. All procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals, approved by the Augusta University Committee on the Use of Animals in Research and Education and by the Ethics Committee on Animal Experiments of the Ribeirao Preto Medical School, University of São Paulo (protocol 052/2012). The animals were housed four per cage on a 12-h light/dark cycle and fed a standard chow diet with water ad libitum.
High fat diet and systolic blood pressure measurements
Wistar rats, 4 weeks-old, received either a control diet (CD, 10% fat) or a high fat diet (HFD, 45% fat, from Research Diets, Inc.), for 8 or 12 weeks. Systolic blood pressure (SBP) measurements were performed by tail cuff before and during the treatment period, as previously described [11].
Metabolic parameters
At the end of the 8 or 12 week-treatment, insulin was measured by an ELISA kit, from ALPCO Diagnostics (Windham, NH, USA). Plasma triglycerides and cholesterol were measured by spectrophotometry, using commercially available kits (Wako USA, Richmond, VA, USA).
Vascular functional studies
After euthanasia by decapitation, basilar, thoracic aorta and mesentery arteries from rats on the control diet or HFD were carefully removed and cleaned in an ice-cold physiological salt solution. Segments of basilar arteries from control rats were incubated in Eagle’s Minimum Essential Medium (EMEM) containing L-glutamine (1%), fetal bovine serum (10%), penicillin and streptomycin (0.5%), containing either vehicle (methanol) or PugNAc [O-(2-acetamido-2-deoxy-D-glucopyranosylidene) amino- N-phenylcarbamate; 100 µM], for 24h. Vessels from both sets of experiments (2 mm in length) were carefully mounted as ring preparations in standard organ chambers for isometric tension recordings, as previously described [27].
Concentration-response curves to serotonin [5-hydroxytriptamine (5-HT), 1 nM to 10 µM], phenylephrine (PE, 1 nM to 100 µM), endothelin-1 (ET-1, 0.1 nM to 0.1 µM) or the thromboxane analog 9.11-dideoxy-9α.11α-methanoepoxyprostaglandin F2α (U-46619, 0.1 nM to 10 µM) were performed to determine vascular contractility. Concentration-response curves to 5-HT were performed in the presence and absence of PD98059 ([extracellular signal-regulated kinase (Erk1/2) inhibitor, 10 µM] or SB203580 (p38 MAPK inhibitor, 10 µM).
Western blot analysis
Proteins (60 µg) extracted from cerebral arteries were separated by electrophoresis, and Western blots were performed as previously described [28]. Antibodies were as follows: anti-O-GlcNAc antibody, CTD 110.6 (1:2000; Pierce Biotechnology, USA), total p38 MAPK and Erk1/2 [(1:800) Cell Signaling Technology, Inc]. Immunoblots for nonphosphoproteins were carried out in the same membranes used to evaluate their phosphorylated forms: phospho-p38 MAPK (Thr180/182) and phospho-Erk1/2 (Ser 180/221) [(1:300); Cell Signaling Technology, Inc].
Data analysis
The results are shown as mean ± SEM and “n” represents the number of animals used in the experiments. Contractile responses are calculated as a percentage of KCl (120 mM)-induced contraction. Concentration–response curves were fitted using a nonlinear interactive fitting program (Graph Pad Prism 5.0; GraphPad Software Inc., San Diego, CA, USA) and two pharmacological parameters were obtained: the maximal effect generated by the agonist (or Emax) and −log EC50 (or pD2). Statistical analyses of Emax and pD2 values were performed using one-way ANOVA or Student’s t-test. Post hoc comparisons were performed using Newman-Keuls’s test. Western blot data were analyzed by one-sample t test and the P value was computed from the t ratio and the numbers of degrees of freedom. Values of P<0.05 were considered statistically significant.
RESULTS
In order to determine the effects of O-GlcNAcylation on cerebral artery function, basilar arteries were incubated with PugNAc, a potent O-GlcNAcase inhibitor [29], in order to increase O-GlcNAc levels. Rat basilar arteries incubated with PugNAc (100 µM) for 24 hours displayed increased vascular content of O-GlcNAc-modified proteins (Figure 1A).
Figure 1. PugNAc increases the content of O-GlcNAc-proteins and enhances contraction to 5-HT in basilar arteries.
(A) - On the top, representative Western blot image of O-GlcNAc-proteins; on the bottom, corresponding bar graphs showing the relative O-GlcNAc-proteins after normalization to β-actin expression. (B) – Concentration-response curves to 5-HT in basilar arteries incubated with PugNAc (24h) or vehicle. Experimental values of contraction were calculated relatively to the contractile response produced by KCl 120 mM, which was taken as 100%. Results are presented as mean ± SEM for n=6–9 in each experimental group. *, P<0.05 vs. vehicle (methanol).
To determine whether increased levels of O-GlcNAc-proteins augment reactivity to contractile stimuli in the cerebral vasculature, concentration-response curves to contractile agonists were performed in basilar arteries incubated with vehicle or PugNAc. Accordingly, 5-HT-induced contraction was more sensitive in basilar arteries incubated with PugNAc, when compared with vehicle [(pD2) 7.0 ± 0.08 vehicle vs. 7.5 ± 0.06 PugNAc (p = 0,0005) and (Emax %) 122 ± 6 vehicle vs. 136 ± 3 PugNAc (p = 0.22); n=6, Figure 1B]. Responses to the thromboxane A2 receptor analog U-46619 were increased in basilar arteries incubated with PugNAc, vs. control arteries [(pD2) 6.8 ± 0.08 vehicle vs. 7.4 ± 0.07 PugNAc (p = 0,0002) and (Emax %) 84 ± 5 vehicle 101 ± 3 PugNAc (p = 0.01); n=6; Figure 1C]. KCl-induced contraction in basilar arteries elicited force development of 3.4 ± 0.4 mN in vehicle and 3.2 ± 0.3 mN in PugNAc group.
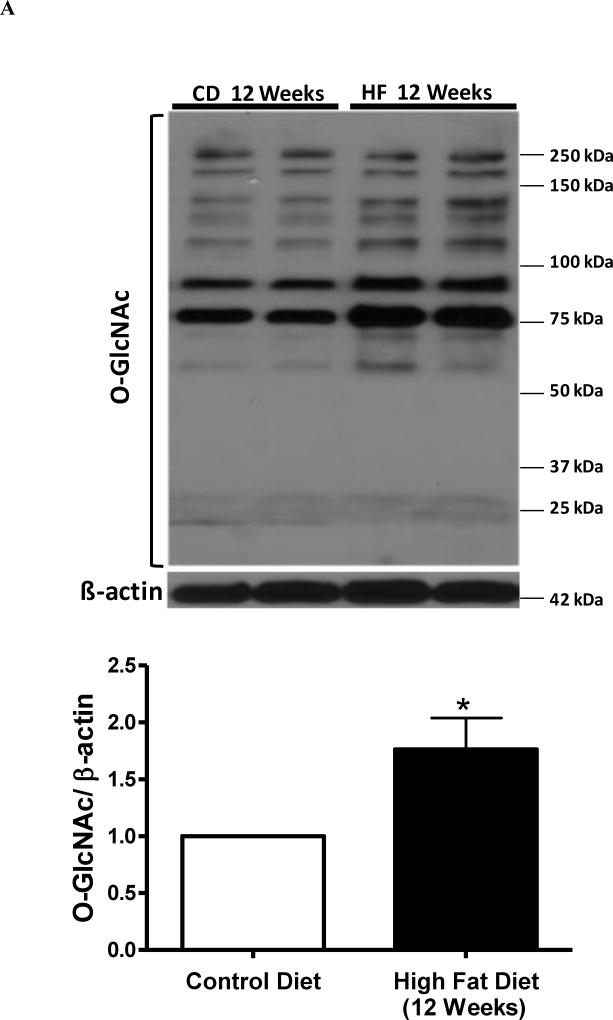
To test the hypothesis that a HFD increases O-GlcNAc levels in cerebral arteries leading to increased contractile response, we first determined the content of O-GlcNAc-modified proteins in cerebral arteries from rats fed either a control diet (10% fat) or a HFD (45% fat) for 8 or 12 weeks. O-GlcNAcylated-protein content was increased in cerebral arteries from male Wistar rats treated with HFD for 8 or 12 weeks (Figures 2A and 2B, respectivelly). The HFD augmented body weight afte 8 and 12 weeks, and plasma triglycerides after 12 weeks, compared to the control diet. There were no differences in blood glucose, plasma cholesterol, plasma insulin concentrations or systolic blood pressure values between the control and HFD groups (Table 1).
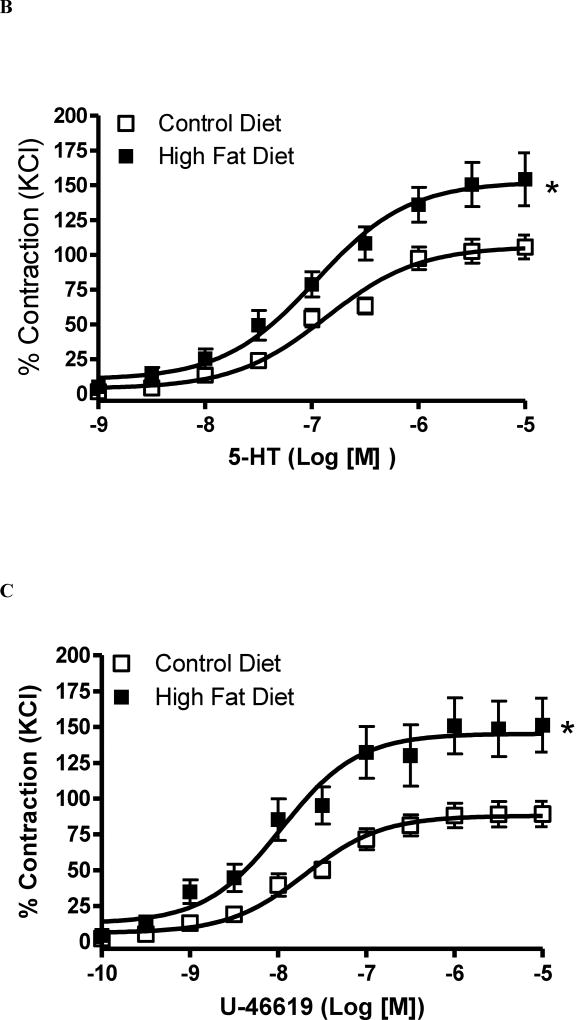
Figure 2. High fat diet for 12 weeks augments O-GlcNAc levels and reactivity to 5-HT in cerebral arteries.
(A-B) - On the top, representative Western blot images of O-GlcNAc-proteins in cerebral arteries after 8 weeks (A) or 12 weeks (B) of high-fat diet (HFD); on the bottom, corresponding bar graphs showing the relative expression of O-GlcNAc-proteins after normalization to β-actin expression. (C-F) - Concentration-response curves performed in basilar arteries from rats treated with HFD for 8 weeks: 5-HT (C), U-46619 (D); or treated with HFD for 12 weeks: 5-HT (E), U-46619 (F). Experimental values of contraction were calculated relatively to the contractile response produced by KCl 120 mM, which was taken as 100%. Results are presented as mean ± SEM for n=6–9 in each experimental group. *, P<0.05 vs. control diet.
Table 1.
Plasma glucose, insulin concentration, triglycerides, cholesterol, body weight and systolic blood pressure in rats treated with a high-fat diet or control diet for 12 weeks.
| Control diet 8 weeks (n=12) |
HF diet 8 weeks (n=12) |
Control diet 12 weeks (n=6) |
HF diet 12 weeks (n=6) |
|
|---|---|---|---|---|
| Body weight (g) | 410±8 | 441±12* | 502±10 | 550±10* |
| Triglycerides (mg/dl) | 56±3 | 69±9 | 95±15 | 160±20* |
| Cholesterol (mg/dl) | 50±3 | 68±7 | 57±4 | 69±4 |
| Blood glucose (mg/dl) | 111±3 | 115±2 | 111±4 | 110±2 |
| Insulin (ng/mL) | 1.3±0.7 | 1.2±0.6 | 1.3±0.6 | 1.0±0.6 |
| SBP (mmHg) | 112±2 | 115±3 | 118±3 | 113±2 |
HFD, high fat diet; SBP, systolic blood pressure.
P < 0.05 vs. respective control diet, Values are mean ± SEM.
Basilar arteries from rats treated with HFD treated for 8 weeks did not change contraction to 5-HT [(pD2) 7.3 ± 0.1 CD vs. 7.2 ± 0.1 HFD (p = 0.49) and (Emax %) 107 ± 4 CD vs. 117 ± 4 HFD, (p = 0.08); n=6, Figures 2C] or U-46619 [(pD2) 8.3 ± 0.08 CD vs. 8.5 ± 0.1 HFD (p = 0.14) and (Emax %) 93 ± 5 CD vs. 107 ± 6 HFD, (p < 0. 1); n=6,; Figures 2D]. KCl-induced contraction in basilar arteries elicited force development of 2.9 ± 0.3 mN in CD-8 weeks and 2.8 ± 0.2 mN in HFD-8 weeks group.
After 12 weeks, basilar arteries from rats submitted to HFD displayed increased vascular reactivity to 5-HT [(pD2) 6.8 ± 0.08 CD vs. 7.0 ± 0.1 HFD (p = 0.15) and (Emax %) 106 ± 4 CD vs. 152 ± 7 HFD, (p = 0.0002); n=6, Figures 2E], U-46619 [(pD2) 7.6 ± 0.1 CD vs. 7.9 ± 0.1 HFD (p = 0.06) and (Emax %) 88 ± 3 CD vs. 145 ± 7 HFD, (p < 0.0001); n=6,; Figures 2F] and ET-1 [(pD2) 8.3 ± 0.2 CD vs. 8.7 ± 0.1 HFD (p = 0,10) and (Emax %) 93 ± 6 CD vs. 167 ± 6 HFD, (p < 0.0001); n=6], developing force levels that were similar to those exhibited by basilar arteries incubated with PugNAc for 24 hours, as described above (Figure 1B and 1C, respectively). KCl-induced contraction in basilar arteries elicited force development of 2.9 ± 0.4 mN in CD-12 weeks and 2.5 ± 0.5 mN in HFD-12 weeks group.
Concentration-response curves to contractile agonists were also performed in aorta and second order-mesenteric arteries from rats on the HFD and control diet. No differences in 5-HT-, ET-1- or PE-induced contractions were observed between the groups in these blood vessels (Table 2).
Table 2.
pD2 and Emax values for agonists-induced contraction in aorta and second-order mesenteric arteries from rats treated with a high-fat diet or control diet for 12 weeks.
| Control diet 12 weeks (n=6) |
HFD 12 weeks (n=6) |
|||
|---|---|---|---|---|
|
| ||||
| pD2 | Emax | pD2 | Emax | |
| Aorta | ||||
| PE | 7.37 ± 0.07 | 103.1 ± 2.7 | 7.35 ± 0.10 | 106.8 ± 8.5 |
| 5-HT | 5.91 ± 0.13 | 115.1 ± 3.0 | 5.87 ± 0.05 | 118.9 ± 11.5 |
| ET-1 | 8.31 ± 0.08 | 125.8 ± 3.0 | 8.32 ± 0.09 | 132.9 ± 11.6 |
| Mesenteric artery | ||||
| PE | 5.79 ± 0.08 | 121.4 ± 2.8 | 5.92 ± 0.14 | 117.8 ± 2.9 |
| 5-HT | 6.45 ± 0.14 | 116.7 ± 2.5 | 6.49 ± 0.11 | 118.3 ± 2.0 |
| ET-1 | 7.87 ± 0.18 | 116.9 ± 4.6 | 7.93 ± 0.11 | 120.2 ± 1.6 |
The results are presented as mean ± SEM of n = 6 in each experimental group. The pD2 values are −logEC50 and Emax values are represented as the percentage of contraction induced by 120 mM KCl. HFD, high fat diet.
Considering that augmented O-GlcNAcylation levels lead to increased phosphorylation of proteins, including mitogen-activated protein kinases (MAPKs) [18,30], and that augmented MAPK activity increases vascular contractile responses [31], we determined whether changes in vascular reactivity, following treatment with PugNAc and HFD, are associated with changes in the expression and activity (indicated by phosphorylation levels) of MAPK enzymes.
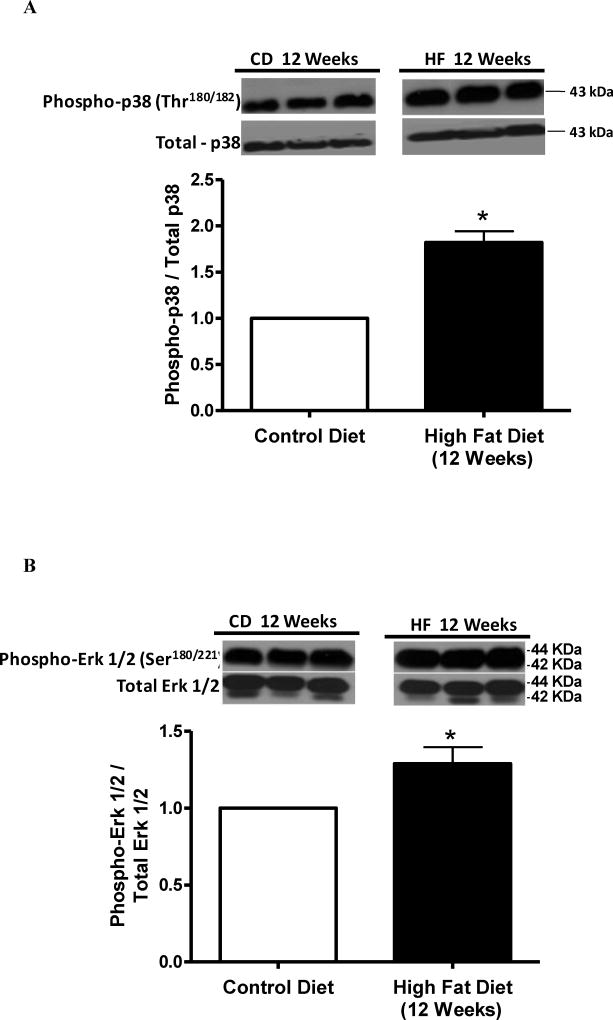
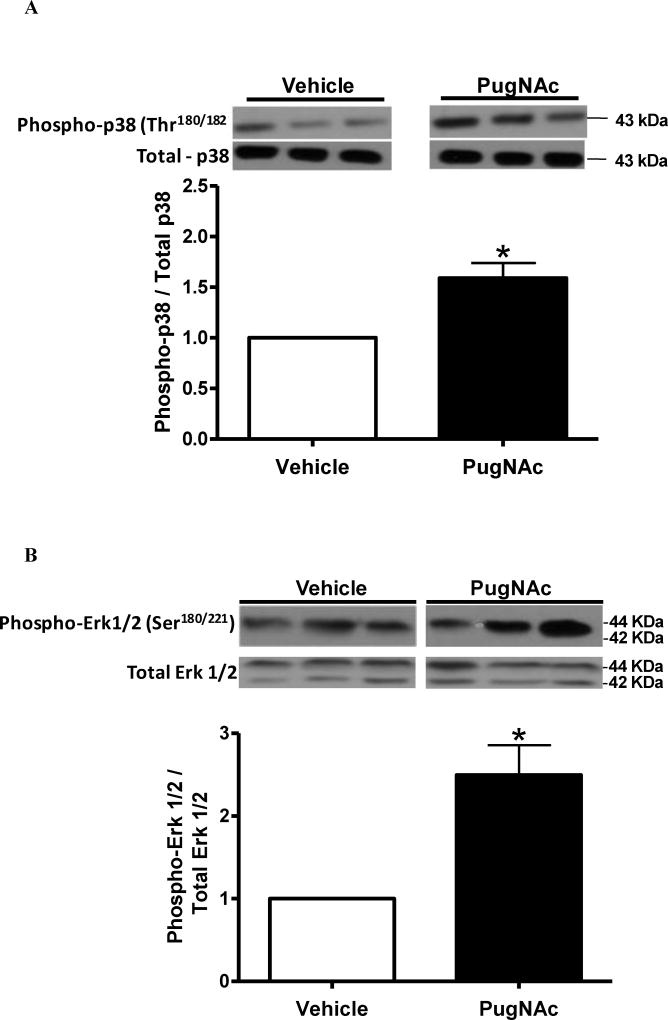
Cerebral arteries from rats on the HFD for 12 weeks, but not for 8 weeks, displayed increased phosphorylation of p38 MAPK at Thr180/182 (Figure 3A and 3C). Increased phosphorylation of Erk1/2 at Ser180/221 was also observed in the HFD group treated for 12 weeks, but not for 8 weeks (Figure 3B and 3D). Total expression of p38 MAPK and Erk1/2 was not modified by the HFD treatment. Similarly, increased O-GlcNAcylation induced by treatment with PugNAc increased phosphorylation of p38 MAPK Thr180/182 and Erk1/2 Ser180/221, but did not change the total expression levels of p38 MAPK or Erk1/2 in cerebral arteries, when compared to vehicle incubation (Figures 4A and 4B, respectively).
Figure 3. Cerebral arteries from rats treated for 12 weeks with high fat diet display increased phosphorylation of p38 MAPK (Thr180/182) and Erk1/2 (Ser180/221).
Bar graphs show the relative expression of phosphorylated forms of (A-C) - phospho-p38 MAPK (Thr180/182) and (B-D) - phospho-Erk1/2 (Ser180/221) in cerebral arteries from rats on control and HFD for 8 (A-B) or 12 weeks (C-D) of treatment, after normalization to corresponding total protein expression (n=6). Results are presented as mean ± SEM in each experimental group. *, P<0.05 vs. control diet.
Figure 4. PugNAc-incubation induces phosphorylation of p38 MAPK Thr180/182 and Erk1/2 Ser180/221 in cerebral arteries.
Incubation of cerebral arteries with PugNAc (100 µM) did not change the total expression levels of p38 MAPK or Erk1/2. PugNAc increased expression of the phosphorylated forms of (A) p38 MAPK Thr180/182 (Thr38) and (B) Erk1/2 Ser180/221. Bar graphs show the relative expression of phosphorylated forms of phospho-p38 MAPK (Thr180/182) and phospho-Erk1/2 (Ser180/221) after normalization to corresponding total protein expression (n=6). Results are presented as mean ± SEM in each experimental group. *, P<0.05 vs. vehicle (methanol).
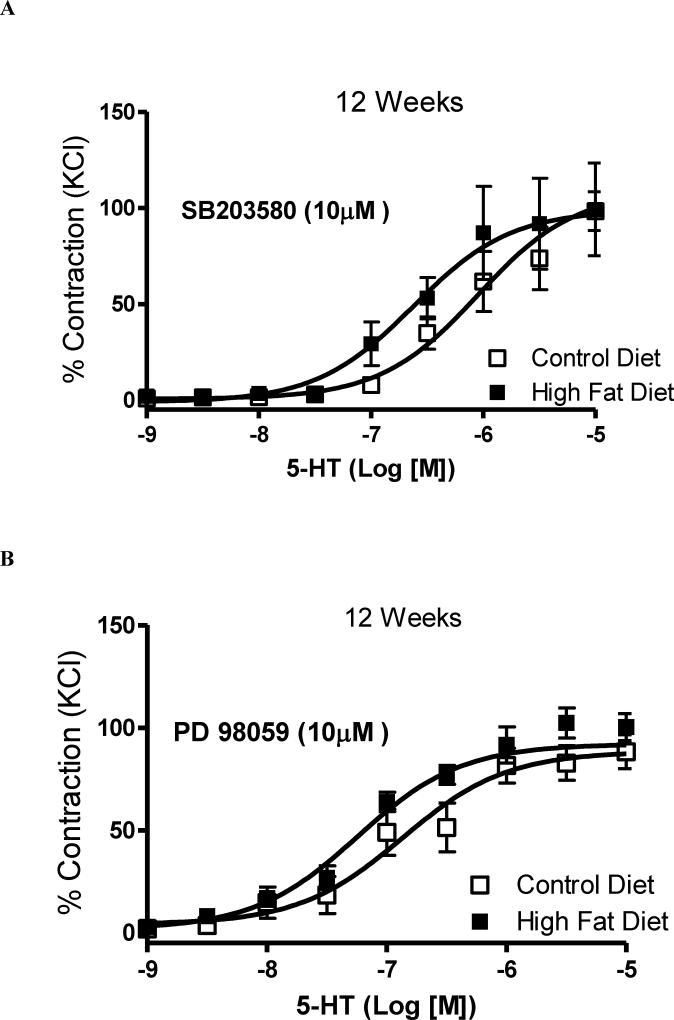
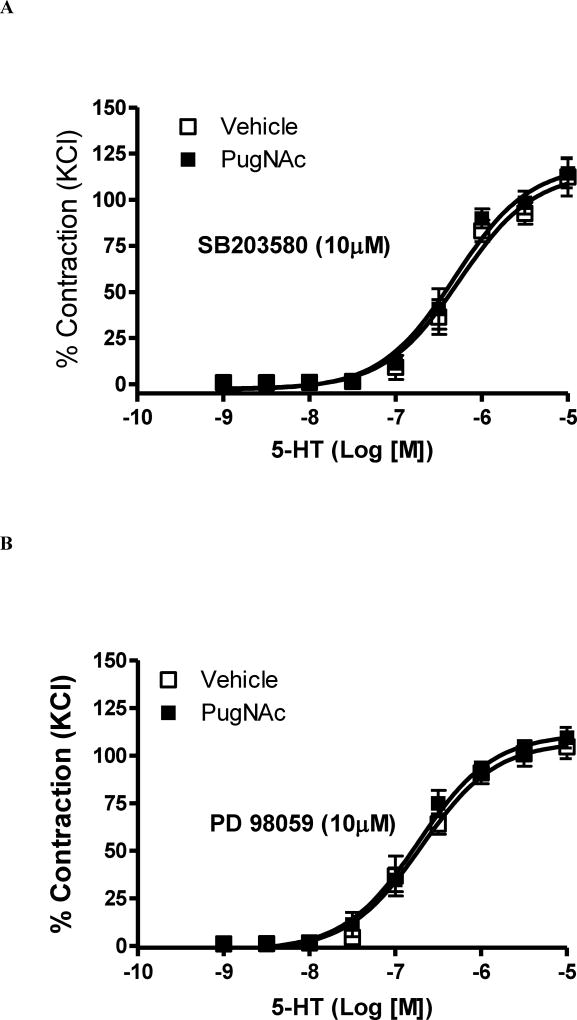
MAPK inhibitors were used to evaluate the contribution of p38 MAPK and Erk 1/2 to the increased vascular contractile-response observed in basilar arteries from HFD rats. Incubation with SB203580 (p38 MAPK inhibitor, 10 µM, 45 minutes) or with PD98059 (Erk1/2 inhibitor, 10 µM, 45 minutes) abolished the differences in 5-HT-response between arteries from rats on the HFD and their controls [(pD2) 6.04 ± 0.2 CD vs. 6.6 ± 0.2 HFD (p = 0.31) and (Emax %) 108 ± 5 CD vs. 98 ± 8 HFD, (p = 0.07); n=6, Figures 5A; and (pD2) 6.8 ± 0.2 CD vs. 7.2 ± 0.2 HFD (p = 0.18) and (Emax %) 88 ± 4 CD vs. 92 ± 3 HFD, (p = 0.44); n=6, Figures 5B, respectively]. Similarly, in the presence of the MAPK inhibitors, no differences in 5-HT reactivity were observed between arteries incubated with PugNAc and vehicle [(pD2) 6.3 ± 0.08 CD vs. 6.3 ± 0.07 HFD (p = 1) and (Emax %) 113 ± 8 CD vs. 118 ± 6 HFD, (p = 0.62); n=6, Figures 6A; and (pD2) 6.7 ± 0.07s CD vs. 6.7 ± 0.006 HFD (p = 1) and (Emax %) 106 ± 6 CD vs. 111 ± 5 HFD, (p = 0,53); n=6, Figures 6B, respectively].
Figure 5. Augmented contraction to 5-HT in basilar arteries from rats on high fat diet for 12 weeks is abolished by MAPK inhibitors.
Concentration-responses curves to 5-HT in basilar arteries from rats on control and HFD were performed in the presence of (A) SB203580 (p38 MAPK inhibitor, 10 µM, 45 minutes) or (B) PD98059 (Erk1/2 inhibitor, 10 µM, 45 minutes). Experimental values of contraction were calculated relatively to the contractile response 18 produced by KCl 120 mM, which was taken as 100% (n=6). Results are presented as mean ± SEM in each experimental group. *, P<0.05 vs. control diet.
Figure 6. Augmented contraction to 5-HT after PugNAc incubation was normalized by MAPK inhibitors in basilar artery.
Concentration-responses curves to 5-HT in basilar arteries incubated with vehicle or PugNAc were performed le in the presence of (A) SB203580 (p38 MAPK inhibitor, 10 µM, 45 minutes) or (B) PD98059 (Erk1/2 inhibitor, 10 µM, 45 minutes). Experimental values of contraction were calculated relatively to the contractile response produced by KCl 120 mM, which was taken as 100% (n=9). Results are presented as mean ± SEM in each experimental group. *, P<0.05 vs. vehicle (methanol).
DISCUSSION
Obesity and high fat intake induce both functional and structural changes in the vasculature [1,5]. Vascular dysfunction induced by high intake of fat occurs either in the presence [32] or absence of obesity [33], indicating that high fat, per se, leads to vascular dysfunction. In the present study, we show that a 12-week HFD augmented corporal weight and plasma triglycerides while cholesterol, blood glucose and insulin concentrations were not changed. The HFD also augmented cerebral artery, but not aortic or mesenteric arteries, reactivity to contractile stimuli, suggesting that short term HFD, without markedly obesity or metabolic imbalance, may be detrimental to regulate cerebral vascular function. Our data also show that the high intake of fat augments the vascular content of O-GlcNAc-modified proteins, which is associated with vascular dysfunction and molecular changes similar to those induced by the HFD. All together these data indicate that O-GlcNAcylation in cerebral arteries may represent an additional mechanism underlying vascular dysfunction in hyperlipidemia and obesity.
Increased flux through the hexosamine biosynthesis pathway (HBP) leads to rapid changes in the production of UDP-GlcNAc, the immediate donor substrate for O-GlcNAc, and increases O-GlcNAcylation of many proteins. HBP flux and UDP-GlcNAc availability are affected directly by many different nutrients, such as glucose, amino acids and fatty acids [30,34,35]. Free fatty acids can increase HBP flux by inhibiting glycolysis, resulting in elevated fructose-6-phosphate levels. Acetyl-CoA, produced by fatty acid metabolism, serves as the donor for glucosamine acetylation in the formation of UDP-GlcNAc [30,34–36]. Our data show that although the HFD for 12 weeks did not change blood glucose, plasma cholesterol or plasma insulin concentrations, it significantly increased plasma triglycerides. The HFD also increased O-GlcNAcylation of proteins in the cerebral vasculature, indicating that increased fatty acids may indeed culminate in augmented levels of O-GlcNAcylated-proteins.
Augmented O-GlcNAc-protein levels affect vascular function in several vascular beds [12,27,37], but this is the first study to show the effects of this post-translational modification in the cerebral vasculature using two different approaches. First, cerebral arteries incubated with PugNAc (a potent inhibitor of O-GlcNAcase) exhibited augmented O-GlcNAc levels, as well as increased responses to contractile stimuli. Second, cerebral arteries from rats fed a HFD display increased O-GlcNAc-protein levels and increased reactivity to several contractile stimuli. Increased reactivity was only observed in the cerebral vasculature from rats on the HFD; vascular responsiveness to various agonists was not changed in aorta or resistance mesenteric arteries. These results suggest that the cerebral vasculature responds earlier to the effects of high fat intake, compared to other vascular beds.
Several proteins can either be directly modified by O-GlcNAc or their activity is modified in response to increased levels of O-GlcNAc [38,39]. Targets for the O-GlcNAc modification include cytokines, transcription factors, proteins involved in calcium handling, insulin responses, glucose metabolism as well as intracellular signaling pathways [18]. An extensive crosstalk between O-GlcNAcylation and phosphorylation, resulting from competition by the same or proximal attachment sites (Serine or Threonine residues on a given protein) has been extensively described [30,37,40]. In addition, O-GlcNAc regulates an increasing number of kinases [34,41].
MAPKs are a family of serine/threonine kinases which are classically associated with vascular smooth muscle cell contraction, migration, adhesion, collagen deposition, growth, differentiation, and survival [42]. Of the major MAPKs, Erk1/2, p38 MAPK, and stress-activated protein kinase/c-Jun N-terminal kinases (SAPK/JNK) are the best characterized [42]. Considering that the effects of PugNAc as well as HFD on 5-HT reactivity were not observed in the presence of the p38 MAPK and Erk1/2 inhibitors, we determined whether increased levels of O-GlcNAcylation by HFD or PugNAc increases signaling via the MAPKs pathway. Vessels from rats treated with the HFD as well as vessels incubated with PugNAc displayed increased phosphorylation of p38 MAPK (Thr180/182) and Erk1/2 (Ser180/221). In agreement with our data, MAPKs (p38 MAPK and Erk1/2) are phosphorylated in response to increased O-GlcNAc levels [18,30]. A positive correlation between phosphorylation of the MAPK cascade (Erk1/2 and p38 MAPK) and nuclear O-GlcNAcylation was observed in fetal human cardiac myocytes exposed to high glucose [43]. In addition, exposure of neutrophils to PugNAc or glucosamine also stimulates the small GTPase Rac, which is an important upstream regulatory element in p38 MAPK and Erk1/2 signaling in neutrophils [44]. Therefore, one potential mechanism by which O-GlcNAcylation may change vascular reactivity includes the complex interplay between O-GlcNAcylation and phosphorylation [30,34]. Accordingly, augmented O-GlcNAcylation levels, as a result of OGA inhibition, result in lower phosphorylation at 280 sites and increased phosphorylation at 148 sites, which implies that the interaction between these two translational modifications is large [30,34]. All together these results support the hypothesis that increased levels of O-GlcNAcylation may increase contractile responses in rat basilar artery via activation of the MAPKs pathway and, therefore, may contribute to abnormal vascular reactivity associated with a HFD.
Another important observation is that vascular responsiveness to various agonists was not changed in aorta or mesenteric arteries from rats on the HFD after 8 or 12 weeks, but in basilar arteries, vascular contractility was enhanced after 12 weeks, but not 8-weeks HFD treatment. Besides we observed higher levels of O-GlcNAc modification in basilar arteries from 8-weeks HFD treated rats, we were not able to see vascular dysfunction in these arteries. These results are interesting, because it seems that increased O-GlcNAc modification elicits vascular dysfunction in basilar arteries earlier than in mesenteric arteries or aorta. Therefore, it seems that basilar arteries functionality is more susceptive to alterations elicited by a HFD, compared to aorta or mesenteric artery. This may be important if we consider that humans, who are submitted to diets rich in fat, such as the Western diet, are more susceptive to complications in the cerebral vasculature.
Interestingly, we have recently shown that although a HFD for 8 weeks does not change cerebral artery reactivity under physiological conditions, it does increase cerebral artery contraction after focal ischemic injury. When basilar arteries were tested at 24 h after middle cerebral artery occlusion (MCAO), the concentration-response curves to several vasoconstrictors including 5-HT, ET-1, and U-46619 were left-shifted, indicating enhanced sensitivity, as well as greater maximum responses [2]. These results indicate that although vascular dysfunction is not present after 8 weeks of high fat intake, the cerebral vasculature is already primed to display an abnormal function, especially upon a deleterious event. The same effect may be occurring in other vascular beds, such as aorta or mesenteric arteries, but this remains to be evaluated.
One important issue to be remembered here is that blood pressure and other metabolic parameters, such as glucose or cholesterol, seems to not be involved in the process of impaired vascular function observed in basilar arteries after HFD treatment for 12 weeks, since no differences in these parameters were observed between the groups.
One potential limitation of the present work is that we were not able to inhibit O-GlcNacylation during the whole period of high fat intake, in order to determine whether vascular dysfunction would be prevented by such approach. Unfortunately, orally and selective OGT inhibitors for chronic treatment are unavailable.
CLINICAL PERSPECTIVES
O-GlcNAc levels are regulated in part by the metabolism of glucose via the hexosamine biosynthesis pathway (HBP), and the metabolic abnormalities associated with insulin resistance and diabetes, such as hyperglycemia, hyperlipidemia and hyperinsulinemia, are all associated with increased HBP flux and increased O-GlcNAc levels [30,35,36]. Consequently, these pathways may play a critical role in the pathogenesis of many cerebrovascular events, including cerebral ischemia, vertebro-basilar transient attacks and stroke. Recent studies have demonstrated that many proteins important in vascular function are targets for O-GlcNAcylation. We showed here, for the first time, that high fat diet is associated with increased of O-GlcNAc levels in cerebral arteries leading to increased vascular contractile responses, along with activation of the MAPK pathway. Taken together with our previous finding that high fat intake impairs cerebrovascular reactivity and worsens outcomes after cerebral ischemia, prevention of O-GlcNAcylation and associated cerebrovascular dysfunction, may represent a novel strategy to target new therapies to improve vascular function, especially when this function is disrupted by a high fat intake.
SUMMARY STATEMENT.
Increased O-GlcNAcylation in cerebral arteries, as a result of a high fat diet, augments reactivity to constrictor stimuli as well as increases MAPKs activity. Increased O-GlcNAc levels may represent a new mechanism to cerebral vasculature dysfunction under pathological conditions.
Acknowledgments
SOURCE OF FUNDINGS
This study was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [(CAPES) grant number 23038009165/2013-48 (to VVL)], Fundação de Amparo à Pesquisa do Estado de Mato Grosso [(FAPEMAT) grant number 211917/2015 (to VVL) and 151371/2014 (to FRG)], Fundação de Amparo à Pesquisa do Estado de São Paulo [(FAPESP) grant number 2008/58142-7, 2011/51317-9 and 2013/08216-2 - CRID (to RCT)], Conselho Nacional de Desenvolvimento Cientifico e Tecnologico [(CNPq) 471675/2013-0 (to FRG), 45777/2014-1 (to VVL) and 401463/2012-5 (to RCT and RCW) in Brazil, and the Diabetes and Obesity Discovery Institute at Augusta University (to AE and RCT), VA Merit Award (BX000347 to AE), and NIH (R01NS083559 to AE) in USA. AE is a Research Career Scientist at the Charlie Norwood Veterans Affairs Medical Center in Augusta, Georgia.
ABBREVIATIONS LIST
- O-GlcNAc
O-GlcNAcylation
- OGT
O-GlcNAc transferase
- OGA
O-GlcNAcase
- NO
Nitric oxide
- HFD
High fat diet
- MAPK
Mitogen-activated protein kinase
- CD
Control diet
- SBP
Systolic blood pressure
- EMEM
Eagle’s Minimum Essential Medium
- PugNAc
O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate
- 5-HT
5-hydroxytriptamine
- PE
Phenylephrine
- ET-1
Endothelin-1
- U-46619
Thromboxane analog 9.11-dideoxy-9α.11α-methanoepoxyprostaglandin F2α
- Emax
Maximal effect generated by the agonist
- HBP
Hexosamine biosynthesis pathway
- SAPK/JNK
Stress-activated protein kinase/c-Jun N-terminal kinases
- MCAO
Middle cerebral artery occlusion
Footnotes
AUTHORS CONTRIBUTION STATEMENT
Victor Lima and Takayuki Matsumoto performed the vascular reactivity studies. Victor Lima, Fernanda Giachini, Alecsander Bressan and Dhruv Chawla conducted metabolic parameters and western blot analysis. Victor Lima wrote the paper. Rita Tostes and Adviye Ergul designed the hypothesis and supervised the study. Rita Tostes provided the animals used in the study and, along with Clinton Webb, continuously provided ideas and expertise for the project and revisions for the paper. All of the authors had full access to the data and take responsibility for its integrity and the accuracy of the analysis. All authors have read and agree to the paper as written.
DISCLOSURES
The authors declared no disclosures. The contents do not represent the views of the Department of Veterans Affairs or the United States Government
References
- 1.Wilde DW, Massey KD, Walker GK, Vollmer A, Grekin RJ. High-fat diet elevates blood pressure and cerebrovascular muscle Ca(2+) current. Hypertension. 2000;35:832–837. doi: 10.1161/01.hyp.35.3.832. [DOI] [PubMed] [Google Scholar]
- 2.Li W, Prakash R, Chawla D, Du W, Didion SP, Filosa JA, Zhang Q, Brann DW, Lima VV, Tostes RC, et al. Early effects of high-fat diet on neurovascular function and focal ischemic brain injury. Am J Physiol Regul Integr Comp Physiol. 2013;304:R1001–1008. doi: 10.1152/ajpregu.00523.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.d'Uscio LV, Smith LA, Katusic ZS. Hypercholesterolemia impairs endothelium-dependent relaxations in common carotid arteries of apolipoprotein e-deficient mice. Stroke. 2001;32:2658–2664. doi: 10.1161/hs1101.097393. [DOI] [PubMed] [Google Scholar]
- 4.Osmond JM, Mintz JD, Dalton B, Stepp DW. Obesity increases blood pressure, cerebral vascular remodeling, and severity of stroke in the Zucker rat. Hypertension. 2009;53:381–386. doi: 10.1161/HYPERTENSIONAHA.108.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitayama J, Faraci FM, Lentz SR, Heistad DD. Cerebral vascular dysfunction during hypercholesterolemia. Stroke. 2007;38:2136–2141. doi: 10.1161/STROKEAHA.107.481879. [DOI] [PubMed] [Google Scholar]
- 6.Langdon KD, Clarke J, Corbett D. Long-term exposure to high fat diet is bad for your brain: exacerbation of focal ischemic brain injury. Neuroscience. 2011;182:82–87. doi: 10.1016/j.neuroscience.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Deutsch C, Portik-Dobos V, Smith AD, Ergul A, Dorrance AM. Diet-induced obesity causes cerebral vessel remodeling and increases the damage caused by ischemic stroke. Microvasc Res. 2009;78:100–106. doi: 10.1016/j.mvr.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sachidanandam K, Hutchinson JR, Elgebaly MM, Mezzetti EM, Wang MH, Ergul A. Differential effects of diet-induced dyslipidemia and hyperglycemia on mesenteric resistance artery structure and function in type 2 diabetes. J Pharmacol Exp Ther. 2009;328:123–130. doi: 10.1124/jpet.108.142612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259:3308–3317. [PubMed] [Google Scholar]
- 10.Lima VV, Giachini FR, Carneiro FS, Carneiro ZN, Saleh MA, Pollock DM, Fortes ZB, Carvalho MH, Ergul A, Webb RC, et al. O-GlcNAcylation contributes to augmented vascular reactivity induced by endothelin 1. Hypertension. 2010;55:180–188. doi: 10.1161/HYPERTENSIONAHA.109.143818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lima VV, Giachini FR, Choi H, Carneiro FS, Carneiro ZN, Fortes ZB, Carvalho MH, Webb RC, Tostes RC. Impaired vasodilator activity in deoxycorticosterone acetate-salt hypertension is associated with increased protein O-GlcNAcylation. Hypertension. 2009;53:166–174. doi: 10.1161/HYPERTENSIONAHA.108.116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim do H, Seok YM, Kim IK, Lee IK, Jeong SY, Jeoung NH. Glucosamine increases vascular contraction through activation of RhoA/Rho kinase pathway in isolated rat aorta. BMB Rep. 2011;44:415–420. doi: 10.5483/BMBRep.2011.44.6.415. [DOI] [PubMed] [Google Scholar]
- 13.Beleznai T, Bagi Z. Activation of hexosamine pathway impairs nitric oxide (NO)-dependent arteriolar dilations by increased protein O-GlcNAcylation. Vascul Pharmacol. 2012;56:115–121. doi: 10.1016/j.vph.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilgers RH, Xing D, Gong K, Chen YF, Chatham JC, Oparil S. Acute O-GlcNAcylation prevents inflammation-induced vascular dysfunction. Am J Physiol Heart Circ Physiol. 2012;303:H513–522. doi: 10.1152/ajpheart.01175.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang YR, Jang HJ, Lee YH, Kim IS, Lee H, Ryu SH, Suh PG. O-GlcNAc cycling enzymes control vascular development of the placenta by modulating the levels of HIF-1α. Placenta. 2015;15:1–6. doi: 10.1016/j.placenta.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Vosseller K. O-GlcNAc and aging: C. elegans as a genetic model to test O-GlcNAc roles in type II diabetic insulin resistance. Aging (Albany NY) 2010;2:749–751. doi: 10.18632/aging.100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li SY, Liu Y, Sigmon VK, McCort A, Ren J. High-fat diet enhances visceral advanced glycation end products, nuclear O-Glc-Nac modification, p38 mitogen-activated protein kinase activation and apoptosis. Diabetes Obes Metab. 2005;7:448–454. doi: 10.1111/j.1463-1326.2004.00387.x. [DOI] [PubMed] [Google Scholar]
- 18.Laczy B, Hill BG, Wang K, Paterson AJ, White CR, Xing D, Chen YF, Darley-Usmar V, Oparil S, Chatham JC. Protein O-GlcNAcylation: a new signaling paradigm for the cardiovascular system. Am J Physiol Heart Circ Physiol. 2009;296:H13–28. doi: 10.1152/ajpheart.01056.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dias WB, Hart GW. O-GlcNAc modification in diabetes and Alzheimer's disease. Mol Biosyst. 2007;3:766–772. doi: 10.1039/b704905f. [DOI] [PubMed] [Google Scholar]
- 20.Fulop N, Marchase RB, Chatham JC. Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res. 2007;73:288–297. doi: 10.1016/j.cardiores.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medford HM, Chatham JC, Marsh SA. Chronic ingestion of a Western diet increases O-linked-beta-N-acetylglucosamine (O-GlcNAc) protein modification in the rat heart. Life Sci. 2012;90:883–888. doi: 10.1016/j.lfs.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagerlof O, Hart GW. O-GlcNAcylation of Neuronal Proteins: Roles in Neuronal Functions and in Neurodegeneration. Adv Neurobiol. 2014;9:343–366. doi: 10.1007/978-1-4939-1154-7_16. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Li X, Yu Y, Shi J, Liang Z, Run X, Li Y, Dai CL, Grundke-Iqbal I, Iqbal K, et al. Developmental regulation of protein O-GlcNAcylation, O-GlcNAc transferase, and O-GlcNAcase in mammalian brain. PLoS One. 2012;7:e43724. doi: 10.1371/journal.pone.0043724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- 25.Devuyst G, Bogousslavsky J, Meuli R, Moncayo J, de Freitas G, van Melle G. Stroke or transient ischemic attacks with basilar artery stenosis or occlusion: clinical patterns and outcome. Arch Neurol. 2002;59:567–573. doi: 10.1001/archneur.59.4.567. [DOI] [PubMed] [Google Scholar]
- 26.Renaud SC. Diet and stroke. J Nutr Health Aging. 2001;5:167–172. [PubMed] [Google Scholar]
- 27.Lima VV, Giachini FR, Carneiro FS, Carneiro ZN, Fortes ZB, Carvalho MHC, Webb RC, Tostes RC. Increased vascular O-GlcNAcylation augments reactivity to constrictor stimuli. JASH. 2008;2:410–417. doi: 10.1016/j.jash.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lima VV, Giachini FR, Carneiro FS, Carvalho MH, Fortes ZB, Webb RC, Tostes RC. O-GlcNAcylation contributes to the vascular effects of ET-1 via activation of the RhoA/Rho-kinase pathway. Cardiovasc Res. 2011;89:614–622. doi: 10.1093/cvr/cvq338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitworth GE, Macauley MS, Stubbs KA, Dennis RJ, Taylor EJ, Davies GJ, Greig IR, Vocadlo DJ. Analysis of PUGNAc and NAG-thiazoline as transition state analogues for human O-GlcNAcase: mechanistic and structural insights into inhibitor selectivity and transition state poise. J Am Chem Soc. 2007;129:635–644. doi: 10.1021/ja065697o. [DOI] [PubMed] [Google Scholar]
- 30.Hu P, Shimoji S, Hart GW. Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS Lett. 2010;584:2526–2538. doi: 10.1016/j.febslet.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 31.Chen QW, Edvinsson L, Xu CB. Role of ERK/MAPK in endothelin receptor signaling in human aortic smooth muscle cells. BMC Cell Biol. 2009;10:52. doi: 10.1186/1471-2121-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naderali EK, Brown MJ, Pickavance LC, Wilding JP, Doyle PJ, Williams G. Dietary obesity in the rat induces endothelial dysfunction without causing insulin resistance: a possible role for triacylglycerols. Clin Sci (Lond) 2001;101:499–506. doi: 10.1042/cs1010499. [DOI] [PubMed] [Google Scholar]
- 33.Naderali EK, Williams G. Effects of short-term feeding of a highly palatable diet on vascular reactivity in rats. Eur J Clin Invest. 2001;31:1024–1028. doi: 10.1046/j.1365-2362.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Gucek M, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc. Proc Natl Acad Sci U S A. 2008;105:13793–13798. doi: 10.1073/pnas.0806216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buse MG, Robinson KA, Gettys TW, McMahon EG, Gulve EA. Increased activity of the hexosamine synthesis pathway in muscles of insulin-resistant ob/ob mice. Am J Physiol. 1997;272:E1080–1088. doi: 10.1152/ajpendo.1997.272.6.E1080. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393:684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- 37.Lima VV, Rigsby CS, Hardy DM, Webb RC, Tostes RC. O-GlcNAcylation: a novel post-translational mechanism to alter vascular cellular signaling in health and disease: focus on hypertension. Journal of the American Society of Hypertension. 2009;3:374–387. doi: 10.1016/j.jash.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 39.Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. doi: 10.1093/glycob/12.4.43r. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Yan T, Wang Q, Wang W, Xu J, Wu X, Ji H. PKC-dependent extracellular signal-regulated kinase 1/2 pathway is involved in the inhibition of Ib on AngiotensinII-induced proliferation of vascular smooth muscle cells. Biochem Biophys Res Commun. 2008;375:151–155. doi: 10.1016/j.bbrc.2008.07.137. [DOI] [PubMed] [Google Scholar]
- 41.Dias WB, Cheung WD, Wang Z, Hart GW. Regulation of calcium/calmodulin-dependent kinase IV by O-glcnac modification. J Biol Chem. 2009 doi: 10.1074/jbc.M109.007310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 43.Gross BJ, Kraybill BC, Walker S. Discovery of O-GlcNAc transferase inhibitors. J Am Chem Soc. 2005;127:14588–14589. doi: 10.1021/ja0555217. [DOI] [PubMed] [Google Scholar]
- 44.Li SY, Sigmon VK, Babcock SA, Ren J. Advanced glycation endproduct induces ROS accumulation, apoptosis, MAP kinase activation and nuclear O-GlcNAcylation in human cardiac myocytes. Life Sci. 2007;80:1051–1056. doi: 10.1016/j.lfs.2006.11.035. [DOI] [PubMed] [Google Scholar]