Abstract
Fiberoptic bronchoscopy and transbronchial lung biopsy are currently the gold standard for detection of acute rejection following human lung transplantation (LTx). However, these surveillance procedures are expensive and invasive. Up to now, there are few new methods that have demonstrated clinical utility for detecting early stages of rejection following human lung transplantation. We optimized and technically validated a novel method to quantify donor-derived circulating cell free DNA (DcfDNA) that can be used as an early biomarker for lung allograft rejection. The method involves the initial development of a panel of probes in which each probe will specifically target a unique sequence on human leucocyte antigen (HLA) allele. After transplantation, donor/recipient specific probes are chosen based on the mismatched HLA loci, followed by droplet digital PCR (ddPCR) used as a quantitative assay to accurately track the trace amount of DcfDNA in an ample excess of recipient DNA background. The average false positive rate noted was about 1 per 800,000 molecules. Serially 2-fold diluted cfDNA, representing donor fractions of cfDNA, were spiked into a constant level of cfDNA representing the recipient cfDNA. The fraction of spiked cfDNA was measured and quantitative linearity was observed across seven serially diluted cfDNA samples. We were able to measure the minor portion of cfDNA as low as 0.2% of total cfDNA. We subsequently applied the method to a pilot set of 18 LTx recipients grouped into biopsy-proven acute rejection, bronchiolitis obliterans syndrome (BOS) or stable groups. Serial plasma samples were used to identify the percentage of DcfDNA over total cfDNA. The level of DcfDNA was significantly elevated in patients diagnosed with acute rejection (10.30 ± 2.80, n=18), compared to that from stable (1.71 ± 0.50, n=24) or from BOS patients (2.52 ± 0.62, n=20). In conclusion, we present results validating the application of digital PCR to quantify DcfDNA assay in primary clinical specimens, which demonstrate that DcfDNA can be used as an early non-invasive biomarker for acute lung allograft rejection.
Keywords: Lung Transplantation, HLA, Acute rejection, Donor cell free DNA, Droplet Digital PCR
1. Introduction
Lung transplantation (LTx) is the accepted treatment for advanced lung disease. The United Network for Organ Sharing (UNOS) reported 2057 lung transplants in the US in 2015 (www.unos.org/about/annual-report). Despite recent advances in medical management of LTx, the median 5-year survival of recipients remains the lowest among the major solid organ allografts [1]. Currently, fiberoptic bronchoscopy and transbronchial lung biopsy is the gold standard for detection of allograft dysfunction post LTx [2]. However, this surveillance procedure is expensive and invasive. Scott et al studied the sensitivity and specificity of transbronchial biopsy (TBB) in heart-lung and single LTx recipients and found eighteen samples per procedure were needed to reach 95% confidence of rejection detection [3]. In light of these limitations on the use of biopsy, new methods that aim to detect early rejection are needed. However, few studies have definitively demonstrated routine clinical utility at early clinical stages of presentation, since most methods rely on relatively non-specific and/or insensitive biomarkers [4].
Circulating cell free DNA, which is likely derived from apoptotic tissue or cells, is now considered as “liquid biopsy” in early detection of cancer and monitoring therapeutic effects [5]. Following allogeneic organ transplantation, DcfDNA has potential as a biomarker of graft dysfunction or rejection following renal and pancreas transplantation [6, 7]. Persistent increases in DcfDNA were detected in pancreas-kidney transplantation patients with biopsy-confirmed rejection [8]. It has been also reported that levels of DcfDNA reduced significantly (from 66% to <5%) when tacrolimus concentration was adjusted from under-therapeutic level to therapeutic level following liver transplantation [9], suggesting that DcfDNA can also be used to adjust the immunosuppression. Reports from Dr. Quake’s lab following human heart transplantation suggested that the percentage of DcfDNA was less than 1% at stable patients, whereas the level can increase up to 5% during a rejection episode [10, 11]. Recent report has shown that DcfDNA can be used in detection of rejection in LTx as well [12]. In their studies, single-nucleotide polymorphism (SNPs) markers were employed using shotgun sequencing to discriminate donor and recipient derived cfDNA. This approach however can be costly and time consuming. In addition, obtaining the donor DNA post-transplantation may not always be feasible. However, HLA phenotypic variation between donor and recipient are commonly seen in all of the solid organ transplantations. For instance, over 85% of LTx has at least 4 out 6 mismatched HLA alleles, and less than 0.1% of LTx are 6 out 6 matched [13]. The information of mismatched HLA alleles is available even prior to the transplantation since HLA typing from donor and recipients are a part of routine transplantation workup in most centers.
For this study, we developed a novel, rapid, cost-effective, genomics-based approach based on variation between mismatched HLA allele. We optimized and technically validated this method to quantify DcfDNA that can be used as personalized markers for rejection. The method involves the initial development of a probe panel in which each probe will specifically target a unique sequence on HLA alleles. After transplantation, donor/recipient specific probes are chosen based on the mismatched HLA loci, followed by ddPCR used as a quantitative assay to track the trace amount of DcfDNA in an extensive excess of recipient DNA background. Additionally, we applied the method to a pilot set of 18 LTx patients to test the clinical applicability. We demonstrate that this novel validated assay can be applied to routine clinical specimens for the detection of early graft rejection following human LTx using individual HLA variants making this as a non-invasive biomarker for acute rejection following solid organ transplantations.
2. Methods and Materials
2.1. HLA-specific primers and probes
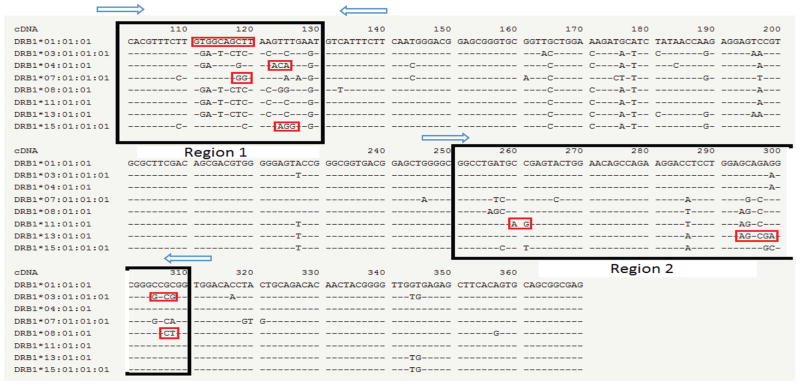
A semi-personalized panel of HLA-specific targeting primers and probes were designed and synthesized. Two pairs of primers flanking two regions containing eight of the most common HLA-DR alleles, including HLA-DRB1*01, HLA-DRB1*03/DRB3*01, HLA-DRB1*04, HLA-DRB1*07 HLA-DRB1*08 HLA-DRB1*11 HLA-DRB1*13 HLA-DRB1*15/*16 were synthesized (Fig. 1). Produced amplicon sizes were designed to be less than 70 bp giving that circulating cfDNA is largely fragmented [14]. Additionally, eight probes targeting each allele specifically were generated (Red boxes Fig. 1). Each probe was generated in two versions containing either a HEX or a FAM fluorophore and they are now commercially available at BioRad (ID: dHsaEXD29156242, dHsaEXD93426015, dHsaEXD67695788, dHsaEXD41965561, dHsaEXD16235334, dHsaEXD80505107, dHsaEXD54774880, dHsaEXD29044653). The DcfDNA and recipient cfDNA give signals in different channels, allowing the ratio between donor and recipient cfDNA to be calculated.
Figure 1.
Primers and probes of digital PCR targeting to HLA-DRB1 region. Primers are shown by the blue arrow and the amplicons are highlighted in the black box. The specific probes are specifically designed to target a unique sequence shown in red.
2.2. Patients selection
Sixty-two serum samples at multiple time points from 18 adult bilateral LTx recipients (LTxR) were selected and subjected to cfDNA extraction and measurement, respectively. All transplantations were performed at Barnes-Jewish Hospital (St. Louis, MO) between 2002 and 2014. Twenty four samples from 7 stable LTxR, 20 samples from 5 LTxR diagnosed with Bronchiolitis Obliterans Syndrome (BOS) and 18 samples from 6 LTxR with biopsy-proven acute rejection were included in the study. We excluded the samples collected within two weeks post-transplantation, as it has been shown that the donor contributed cfDNA was elevated for a short period of time after transplantation probably due to ischemia-reperfusion injury [12]. All of the LTxR underwent surveillance bronchoscopy with broncheoalveolar lavage (BAL) collection and TBB during episodes of clinical deterioration. The allograft status was determined based on microbiologic cultures, radiographic studies and biopsy notes. Acute and chronic rejection was defined by pathologic diagnosis on TBB and graded according to the standard International Society for Heart and Lung Transplantation (ISHLT) criteria [15]. The HLA typing for the donors and recipients were performed at the HLA laboratory at Barnes-Jewish Hospital. Informed consent was obtained on all LTxR and was approved by the human studies institutional review board (IRB#201103312) Washington University Human Studies Committee.
2.3. Isolation of cfDNA and pre-amplification
The cfDNA isolation protocol was modified from a well-established procedure [9, 10]. Briefly, the plasma was separated from peripheral blood cells within four hours of sample collection by centrifugation at 1600g for 10min. The serum was stored at −80°C until DNA extraction. cfDNA was extracted from 2ml aliquots (of patient plasma using the QIAamp circulating nucleic acid kit (Qiagen) according to the manufacturer’s instructions. To increase the total number of DNA molecules tested, isolated cfDNA was amplified by utilizing an unbiased pre-amplification method from Bio-Rad (SSo PreAmp Assays). Each 50ul reaction included: 25 ul of SSoAdvanced PreAmp Supermix; 12ul of PreAmp assay pool (BioRad commercial ID dHsaEXD93314426 for region 1 and dHsaEXD67584199 for region 2); 12 ul of water and 8ul of isolated cfDNA. The PCR was conducted as: 95 °C for 3 min, 10 × (95°C for 15s, 58°C for 4 min). 2ul of the product was used in the following Droplet digital PCR assay.
2.4. Droplet digital PCR (ddpcR)
Corresponding probes were selected based on the mismatched HLA-DR alleles (Table 1). The ddPCR was set up according to manufacturer instructions (Bio-Rad). The Bio-Rad QX200 ddPCR system was used, which partitions each sample and PCR assay mixture, including Supermix (Bio-Rad), primers and specific probes, into approximately 20,000 water-oil emulsion droplets. Thermal cycling was carried out within each droplet to amplify the target of interest followed by binding of sequence-specific probes labeled with fluorescent dye. A reaction with non-target molecule was counted as fluorescent negative and a reaction with one target molecule was counted positive. All reactions were carried out and read on a 96 well plate. The condition of ddPCR was optimized: 95 °C for 3 min, 40 × (94°C for 10s, 55°C for 30 sec), then 72°C for 5 min. Droplets were read in QX200 droplets reader with rare event detection settings and analyzed using the Quantasoft software.
Table 1.
Demographic of the patients and probes selected in the assay.
| Patient # | Age at Tx. | Gender | Disease | HLA mismatch (A, B, DR) | Recipient HLA-DR | Donor HLA-DR | Probe Target (Reci/Donor) | # of samples |
|---|---|---|---|---|---|---|---|---|
| 1 | 42 | M | ILD | 2--2--2 | 03, 15 | 01,04 | 03, 01 | 3 |
| 2 | 52 | M | IPF | 1--2--2 | 04,13 | 07,13 | 04, 07 | 4 |
| 3 | 45 | F | COPD | 1--1--1 | 04,14 | 01,04 | 04, 01 | 5 |
| 4 | 59 | F | IPF | 2--2--2 | 04,08 | 07,12 | 04, 07 | 3 |
| 5 | 57 | F | COPD | 2--2--2 | 12,15 | 11,14 | 15, 11 | 4 |
| 6 | 69 | M | IPF | 2--2--2 | 04,14 | 01,13 | 04, 13 | 3 |
| 7 | 66 | M | IPF | 0--2--1 | 04,15 | 04,13 | 15, 13 | 2 |
| 8 | 50 | M | A1AD | 1--2--2 | 04,07 | 04,11 | 07, 11 | 3 |
| 9 | 22 | F | CF | 2--2--2 | 01,04 | 01,07 | 04, 07 | 5 |
| 10 | 62 | M | COPD | 1--2--1 | 03,13 | 11,13 | 03, 11 | 5 |
| 11 | 62 | F | IPF | 2--2--1 | 03,04 | 04,07 | 03, 07 | 3 |
| 12 | 66 | F | IPF | 1--2--1 | 11,13 | 03,04 | 11, 04 | 4 |
| 13 | 39 | F | MCTD | 2--2--1 | 07,07 | 07,11 | 07, 11 | 2 |
| 14 | 56 | M | IPF | 2--2--2 | 07,16 | 03,15 | 07, 03 | 2 |
| 15 | 57 | M | IPF | 1--1--2 | 04, 13 | 07,11 | 13, 07 | 2 |
| 16 | 53 | M | IPF | 2--1--2 | 04,15 | 03,08 | 15, 08 | 5 |
| 17 | 70 | M | ILD | 2--2--1 | 15,16 | 13,15 | 15, 13 | 5 |
| 18 | 68 | M | COPD | 2--1--2 | 01,03 | 11,15 | 01, 11 | 2 |
A1AD: Alpha 1-antitrypsin deficiency; MCTD: Mixed connective tissue disease; CF: Cystic fibrosis; ILD: Interstitial lung disease; IPF: Idiopathic pulmonary fibrosis; COPD: Chronic obstructive pulmonary disease. Patient 1–7 are stable patients. 8–12 are BOS positive patients and 13–18 are rejection patients.
2.5. Fraction calculation and Statistical analysis
Donor and recipient cfDNA was measured using duplicate samples; the average was used in the statistical analysis. The donor contributed cell free DNA is quantified as previously described [14]: DcfDNA = donor copy number/total copy number (donor copy number + recipient copy number). If there was one shared allele between donor and recipient the calculation was altered assuming each allele contributes equally [14]. Also, the HLA-DRB1* 03 probe co-hybridizes HLA-DRB3*01, the calculation was modified accordingly.
For statistical analysis, the Mann-Whitney test was performed using GraphPad Prism version 6.0. All tests were 2-sided and p value less than 0.05 were considered statistically significant.
3. Results
3.1. Quantification with Genomic DNA
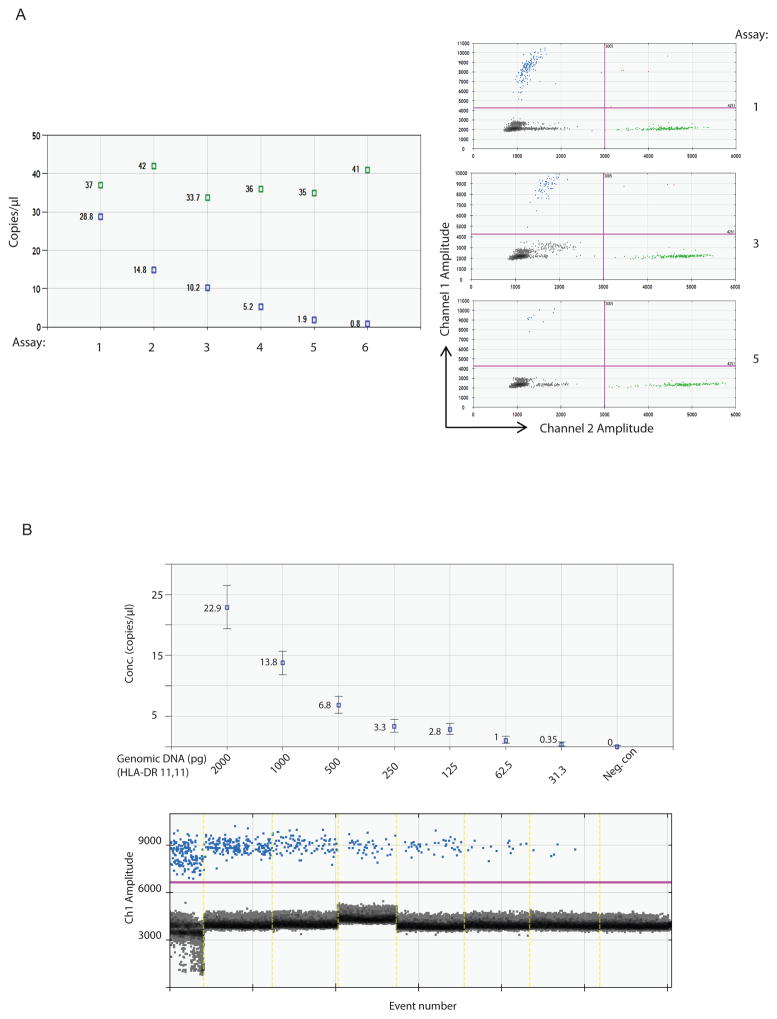
To determine technical feasibility we initially carried out a ddPCR assay targeting the high frequency alleles using genomic DNA from healthy individuals with known HLA typing (Fig. 1). By using different dilutions, we measured the absolute copy number of the target DNA molecule from the isolated DNA with the probes designed. When the genomic DNA from two different individuals were mixed and surveyed by the specific probes, FAM and HEX fluorescence were separated as distinct mutually exclusive signals representing donor and recipient (Fig. 2A).
Figure 2.
(A). The ddPCR reactions were carried out with one isolated genomic DNA containing DRB1*03 at a fixed concentration shown in green, spiked with another isolated genomic DNA sample containing DRB1*04 shown in blue that are serially diluted. The copy number of targeted alleles was measured. Scatterplots of three representing assays (1, 3, 5) with corresponding probes (HEX 03 and FAM 04) showing the segregating droplets. (B). The sensitivity of ddPCR was tested using genomic DNA (HLA-DRB1*11/11). The copy number of targeted allele (DR11) was measured at each sample with indicated concentration.
In order to test the sensitivity of this method, we quantified the level of the DNA molecule of interest. We serially diluted one genomic DNA (HLA-DR*11/11) with known concentration and the two fold dilution series demonstrated the linearity of the assay. We were able to accurately measure the absolute copy number of the target HLA-DR molecule down to approximately 30pg of genomic DNA (Fig. 2B). We also demonstrated sensitivity of the method using synthesized gblock (Integrated DNA Technologies, Inc. Coralville, Iowa) as well as the cfDNA from healthy individuals whose HLA typing is known (data not shown).
3.2. Specificity and CV (Coefficient of Variation) of the probes
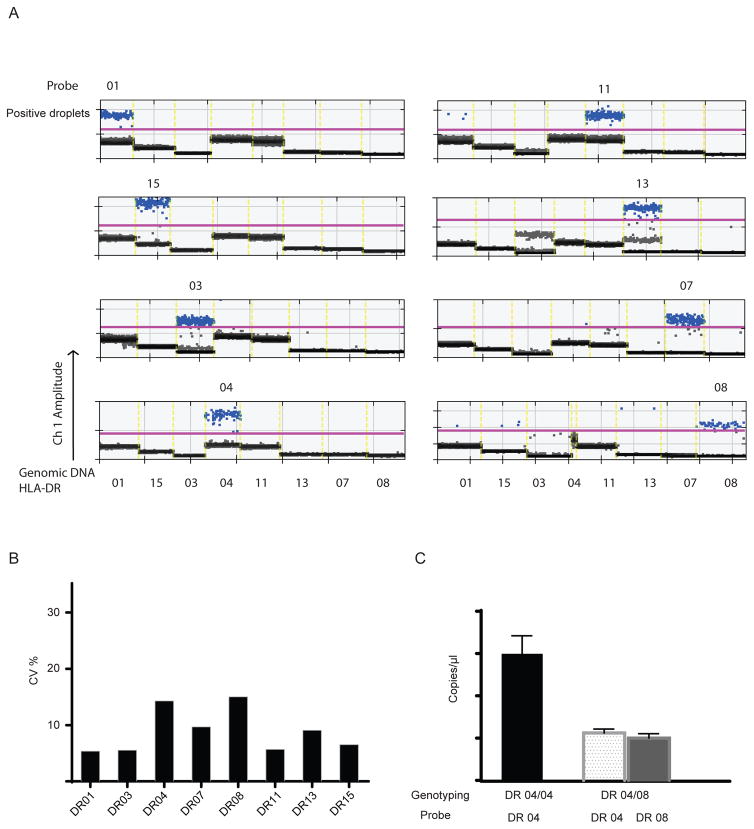
Given the close sequence homology between each HLA antigen, we set out to test the specificity of each probe we prepared. The ddPCR reactions were carried out with the individual probe against a panel of eight highly concentrated genomic DNA with known HLA-DR typing. Each DNA sample contains the specific allele corresponding to the designed probes. As shown in Figure 3, one probe only recognized the template containing the specific sequence, and not any other templates sharing homologous sequences despite the extremely high concentration of genomic DNA used in the experiment. The average false positive rate for all eight probes was about 1 per 800,000 reads in our tests.
Figure 3.
(A). 1-D plot of individual probe binding to the panel of different HLA-DR alleles. The ddPCR reactions were carried out with the individual probe against a panel of highly concentrated genomic DNA containing all eight alleles (HLA-DR 01, 03, 04, 07, 08, 11, 13 and 15). (B). CV (%) of each assay with individual probe. The CV is obtained using 8 repetitions within the same run. (C). The absolute copy number of each allele measured from homozygous (HLA-DR04/04) and heterozygous (HLA-DR04/08) genomic DNA with similar amount (2ng).
The inter-assay imprecision was also determined in a series of eight repetitions to calculate a CV. The CV profiles for all eight probes are shown here with range from 15 – 4% (Fig. 3B), which is comparable to a published cell-free DNA studies using SNPs [16]. In order to confirm that each allele contributed equally in our assay, the copy number of target molecules from homozygous or heterozygous genomic DNA was measured. With roughly similar amount of genomic DNA, the copy number of each allele in heterozygous DNA is about half of that from homozygous DNA that carries two copies of the same allele (Fig. 3C)
3.3. Quantification with DcfDNA
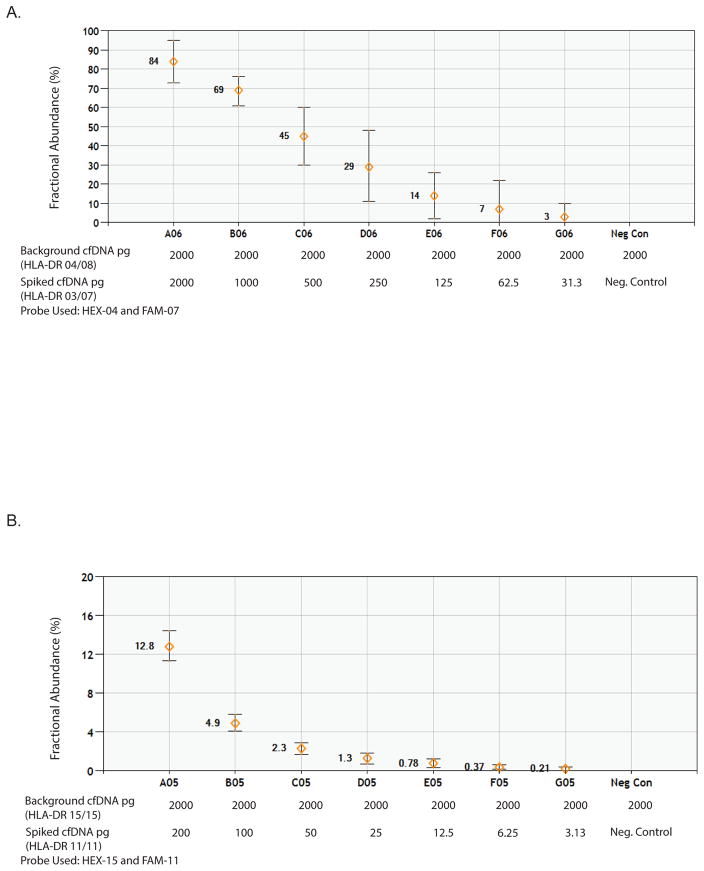
We then tested if the method allowed us to track the trace amount of DcfDNA in an extensive excess of recipient cfDNA background. To this end, isolated cfDNA from healthy individuals with known HLA typing was utilized. HLA-DR mismatched surrogate “donor” cfDNA was serially 2-fold diluted into a constant level of background cfDNA representing recipient cfDNA. The fraction of serially “donor” cfDNA to “recipient’ DNA was calculated and linearity was observed across seven serially diluted cfDNA samples at both low (Fig. 4A) and high levels of background recipient cfDNA (Fig. 4B). We demonstrated that one can measure the minor portion of cfDNA as low as 0.2% of total cfDNA.
Figure 4.
Quantification with cfDNA. Serially diluted cfDNA representing donor DNA, was spiked into a constant level of cfDNA representing the recipient DNA. The fraction of the spiked DNA was tested and spotted at high (A) and low levels (B) of background cfDNA.
3.4. cfDNA levels increased in LTxR diagnosed with rejection
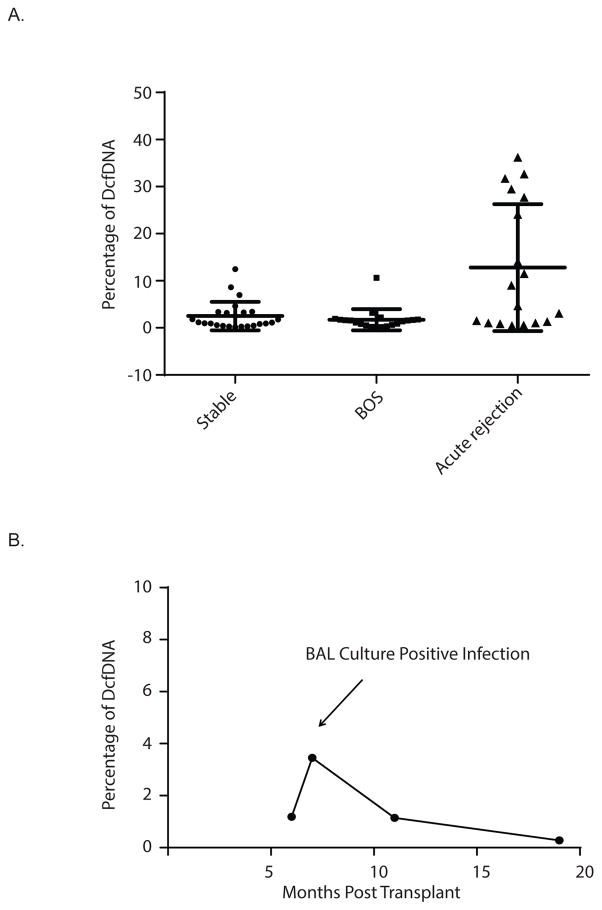
To further validate this method, 62 serum samples at multiple time points from 18 LTxR were selected. As described above, all specimens were subjected to cfDNA extraction and measurement. The fraction of DcfDNA was calculated and categorized according to rejection status. Levels of DcfDNA from each group were compared. No significant difference was observed between the percentage of DcfDNA from stable and from BOS positive patients (2.52 ± 0.62 vs 1.71 ± 0.50). In contrast, the levels of DcfDNA were significantly elevated in patients who developed acute rejection (10.30 ± 2.80) compared to that from stable or BOS patients (Fig. 5A, p<0.01). Out of six patients with rejection, four had at least one sample with high levels of DcfDNA (>10%). Consistent with a previous study in LTx [12], transient elevations of DcfDNA were observed at infection events. As shown in Fig. 5B, a temporary and relatively small elevation of DcfDNA was observed during a bronchial wash contemporaneous with a culture positive infection, suggesting a higher level of turnover rate in the grafted organ during infection. The levels of DcfDNA returned to the base line level when the infection was under control.
Figure 5.
(A). The levels of DcfDNA in patient who developed acute rejection compared to that from stable or BOS positive patients. B. The time course of the level of donor cfDNA in stable patient who developed BAL culture positive infection (B).
4. Discussion
In this communication, we describe a novel, rapid, cost-effective, HLA genomics-based diagnostic tool for early detection of acute rejection following human lung transplantation using peripheral blood specimens. This approach takes advantage of individual HLA phenotypic variation between donor and recipient, which is present in nearly all solid organ transplantations. We established a panel of DNA probes that recognize the unique allele specific sequences in the HLA-DRB1 region in the 6th chromosome, which allows detection and quantitation of the portion of DcfDNA from the recipient cfNDA by using ddPCR.
Several studies demonstrated that DcfDNA can be used as a potential biomarker for integrity of grafted organ. The methods utilized in previous studies are limited by certain factors. Some studies analyzed DcfDNA utilized Y-chromosomal markers on male donor, which can only be used in male to female transplant [6, 17]. Compared to the studies using different homozygous SNPs between donor and recipient [10, 11], our method is more cost-efficient with a quicker turnaround time. It only requires recipient serum and donor/recipient HLA phenotype, which has already been obtained in all solid organ transplant cases. The ddPCR method employed in our study has been utilized successfully for the detection of other disease biomarkers given its highly sensitive and precise performance [18].
This study has several limitations. Previous study demonstrated that infections especially CMV infection, which are commonly seen post lung transplantation, could also contribute to DcfDNA as a result of graft injury. In our study only 3 out 18 samples from rejection group were collected within two weeks prior or after any infection and only one sample was collected two weeks post CMV PCR positive test. We therefore believe that the impact of infection is less significant compared to rejection in our study cohort. However, given the small sample size and lack of longitudinal data, we cannot make any conclusion of the impact of infection on the level of cfDNA from this study. In the future, use of the combination of DcfDNA and one or several specific marker(s) of infection such as CMV PCR, would improve the diagnostic specificity. Higher levels of DcfDNA is observed during first a few weeks post lung Tx. Therefore, DcfDNA may not be a specific biomarker of rejection at early stage post Tx when ischemia caused graft injury could also contribute DcfDNA. Additionally, we relied on biopsy result as our gold standard in this study given the fact it still remains as corner stone in diagnosing acute rejections in current stage. It should be noted that the reported interobserver variability was significant in diagnosing and grading transbronchial biopsy specimens after lung transplantation and concordance among pathologist need to be improved [19].
Although our panel of probes allowed us to cover approximated 90% of our Caucasian patient population (//optn.transplant.hrsa.gov/resources/allocation-calculators/cpra-calculator/), additional primers and probes are needed in order to cover other races including African Americans and Hispanics. In cases where donor and recipient are completely HLA-DR matched which occurs infrequently, certain probes that target other HLA regions outside of HLA-DR may be necessary. We are currently expanding our panel to cover greater than 95% of the population with less than 30 probes. The method described here will not be applicable in solid organ transplantations in which donor and recipient are HLA-identical. However, this occurs very infrequently and the chances of acute rejection episodes occurring in this scenario will be quite low.
Since this was a retrospective study, biopsy and BAL were not performed on a regular basis when blood samples were collected. Therefore, we were unable to perform longitudinal analysis of DcfDNA at a given time point with biopsy confirmation. This may explain why we observed a number of samples with low levels of DcfDNA in the rejection group as those samples may have been collected after the acute rejection was treated successfully. The variation from each sample from same patient is significant as they are collected at different stage of rejections. Additionally, the samples were not subjected to DNA extraction immediately. Although it has been demonstrated that the total, maternal and fetal cfDNA copies present in single – spun plasma did not change significantly after two weeks storage at −80 °C [20], further studies are needed to fully assess the stability of cell free DNA in the plasma samples stored at −80 °C for a longer time. As we measured the donor fraction of cfDNA relative to the recipient cfDNA used as internal control DNA, the variation resulting from extraction, elution and amplification efficiency can be minimized assuming levels of both donor and recipient’s cfDNA changed similarly during the process.
The number of total molecules screened determines the limit of detection in low levels of DcfDNA by ddPCR. The expected genomic copies recovered from 2ml serum is approximately 4000–6000. However, the measurement of less than 1% of donor DNA with the sequence of interest is extremely difficult and will be technically challenging. Therefore, we utilized an unbiased pre-amplification method to increase the total number of DNA molecules tested. Consistent with another study [16], we demonstrated that the fractional abundance of the minor allele was not significantly changed by unbiased pre-amplification using PreAmp Assays from Bio-Rad (data not shown). Further study is needed to address whether less serum could be used to obtain a sufficient amount of DcfDNA.
Early detection of acute rejection is extremely important as it has been reported that 93% of recipients developed at least one episode of acute rejection in the first 2 years after lung transplantation [21]. Acute rejection is also a crucial risk factor for the development of chronic rejection which is the leading cause of graft failure following lung transplantation [1]. It is also likely that early intervention and successful treatment of acute rejection could potentially prevent the graft form further injury leading to increased risk for developing chronic rejection. Consequently, it is important to detect the early acute rejection even when the patient is asymptomatic [22, 23]. We propose that DcfDNA as a noninvasive and sensitive biomarker, has the potential to detect early acute rejection prior to clinical manifestations.
The method and approach could be equally applicable for the detection of cfDNA from other solid organ transplantations (heart, pancreas and liver). Once a probe is validated and included in the panel, it can be used in any mismatched patient/donor assay and used repeatedly across the entire clinical course. In our experiment, the measurement of cfDNA takes about 8 hours and the reagent costs less than 40 dollars per sample once the panel is established. With a sufficient coverage of alleles it is possible to identify multiple probes and multiplex their employment in a single assay to further increase assay specificity and sensitivity.
Acknowledgments
This work was supported in part by NIH HL 056643 to TM. We thank Todd E. Druley for providing essential technical resources.
Abbreviations
- BAL
Broncheoalveolar Lavage
- BOS
Bronchiolitis Obliterans Syndrome
- DcfDNA
Donor cell free DNA
- ddPCR
Droplet Digital PCR
- HLA
Human Leucocyte Antigen
- LTx
Lung Transplantation
- LTxR
Lung Transplantation Recipient
- qPCR
Quantitative PCR
- SNP
Single-Nucleotide Polymorphism
- TBB
Transbronchial Biopsy
Footnotes
JZ, BD, and TM participated in research design; JZ, BD, NS and AY participated in performance of the research; JZ, BD, RH and MS participated in data analysis and the writing of the paper.
All authors have no financial disclosure related to the study described in the submitted manuscript. The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report--2013; focus theme: age. J Heart Lung Transplant. 2013;32:965. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Glanville AR. Bronchoscopic monitoring after lung transplantation. Semin Respir Crit Care Med. 2010;31:208. doi: 10.1055/s-0030-1249117. [DOI] [PubMed] [Google Scholar]
- 3.Scott JP, Fradet G, Smyth RL, Mullins P, Pratt A, Clelland CA, et al. Prospective study of transbronchial biopsies in the management of heart-lung and single lung transplant patients. J Heart Lung Transplant. 1991;10:626. [PubMed] [Google Scholar]
- 4.Berastegui C, Roman J, Monforte V, Bravo C, Lopez-Meseguer M, Montero MA, et al. Biomarkers of pulmonary rejection. Transplant Proc. 2013;45:3163. doi: 10.1016/j.transproceed.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10:472. doi: 10.1038/nrclinonc.2013.110. [DOI] [PubMed] [Google Scholar]
- 6.Garcia Moreira V, Prieto Garcia B, Baltar Martin JM, Ortega Suarez F, Alvarez FV. Cell-free DNA as a noninvasive acute rejection marker in renal transplantation. Clin Chem. 2009;55:1958. doi: 10.1373/clinchem.2009.129072. [DOI] [PubMed] [Google Scholar]
- 7.Gadi VK, Nelson JL, Guthrie KA, Anderson CC, Boespflug ND, Redinger JW, et al. Soluble donor DNA and islet injury after transplantation. Transplantation. 2011;92:607. doi: 10.1097/TP.0b013e318228d799. [DOI] [PubMed] [Google Scholar]
- 8.Gadi VK, Nelson JL, Boespflug ND, Guthrie KA, Kuhr CS. Soluble donor DNA concentrations in recipient serum correlate with pancreas-kidney rejection. Clin Chem. 2006;52:379. doi: 10.1373/clinchem.2005.058974. [DOI] [PubMed] [Google Scholar]
- 9.Kanzow P, Kollmar O, Schutz E, Oellerich M, Schmitz J, Beck J, et al. Graft-derived cell-free DNA as an early organ integrity biomarker after transplantation of a marginal HELLP syndrome donor liver. Transplantation. 2014;98:e43. doi: 10.1097/TP.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 10.Snyder TM, Khush KK, Valantine HA, Quake SR. Universal noninvasive detection of solid organ transplant rejection. Proc Natl Acad Sci U S A. 2011;108:6229. doi: 10.1073/pnas.1013924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vlaminck I, Valantine HA, Snyder TM, Strehl C, Cohen G, Luikart H, et al. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med. 2014;6:241ra77. doi: 10.1126/scitranslmed.3007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Vlaminck I, Martin L, Kertesz M, Patel K, Kowarsky M, Strehl C, et al. Noninvasive monitoring of infection and rejection after lung transplantation. Proc Natl Acad Sci U S A. 2015;112:13336. doi: 10.1073/pnas.1517494112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valapour M, Skeans MA, Smith JM, Edwards LB, Cherikh WS, Callahan ER, et al. Lung. Am J Transplant. 2016;16(Suppl 2):141. doi: 10.1111/ajt.13671. [DOI] [PubMed] [Google Scholar]
- 14.Zheng YW, Chan KC, Sun H, Jiang P, Su X, Chen EZ, et al. Nonhematopoietically derived DNA is shorter than hematopoietically derived DNA in plasma: a transplantation model. Clin Chem. 2012;58:549. doi: 10.1373/clinchem.2011.169318. [DOI] [PubMed] [Google Scholar]
- 15.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Beck J, Bierau S, Balzer S, Andag R, Kanzow P, Schmitz J, et al. Digital droplet PCR for rapid quantification of donor DNA in the circulation of transplant recipients as a potential universal biomarker of graft injury. Clin Chem. 2013;59:1732. doi: 10.1373/clinchem.2013.210328. [DOI] [PubMed] [Google Scholar]
- 17.Lo YM, Tein MS, Pang CC, Yeung CK, Tong KL, Hjelm NM. Presence of donor-specific DNA in plasma of kidney and liver-transplant recipients. Lancet. 1998;351:1329. doi: 10.1016/s0140-6736(05)79055-3. [DOI] [PubMed] [Google Scholar]
- 18.Zec H, Shin DJ, Wang TH. Novel droplet platforms for the detection of disease biomarkers. Expert Rev Mol Diagn. 2014;14:787. doi: 10.1586/14737159.2014.945437. [DOI] [PubMed] [Google Scholar]
- 19.Bhorade SM, Husain AN, Liao C, Li LC, Ahya VN, Baz MA, et al. Interobserver variability in grading transbronchial lung biopsy specimens after lung transplantation. Chest. 2013;143:1717. doi: 10.1378/chest.12-2107. [DOI] [PubMed] [Google Scholar]
- 20.Barrett AN, Thadani HA, Laureano-Asibal C, Ponnusamy S, Choolani M. Stability of cell-free DNA from maternal plasma isolated following a single centrifugation step. Prenat Diagn. 2014;34:1283. doi: 10.1002/pd.4468. [DOI] [PubMed] [Google Scholar]
- 21.Burton CM, Iversen M, Scheike T, Carlsen J, Andersen CB. Minimal acute cellular rejection remains prevalent up to 2 years after lung transplantation: a retrospective analysis of 2697 transbronchial biopsies. Transplantation. 2008;85:547. doi: 10.1097/TP.0b013e3181641df9. [DOI] [PubMed] [Google Scholar]
- 22.Yousem SA. Significance of clinically silent untreated mild acute cellular rejection in lung allograft recipients. Hum Pathol. 1996;27:269. doi: 10.1016/s0046-8177(96)90068-4. [DOI] [PubMed] [Google Scholar]
- 23.Hachem RR. Lung allograft rejection: diagnosis and management. Curr Opin Organ Transplant. 2009;14:477. doi: 10.1097/MOT.0b013e32832fb981. [DOI] [PubMed] [Google Scholar]