Abstract
While the World Health Organization included Epstein-Barr virus (EBV)–positive diffuse large B-cell lymphoma (DLBCL) as a provisional entity of a lymphoma occurring in older individuals without any known immunodeficiency in 2008, it has since been recognized that this entity may occur in younger individuals. As a result, the 2016 revision has substituted the modifier “elderly” with “not otherwise specified” (NOS). The NOS highlights that there are more specific entities with neoplastic EBV-positive large B cells such as lymphomatoid granulomatosis. Diagnosis requires that there be no other cause of immunodeficiency and that other more specific entities with neoplastic EBV plus large B cells be excluded. We present the case of an 81-year-old woman hospitalized for generalized weakness, increasing confusion, unexplained weight loss, and intermittent fevers. Examination showed lymphadenopathy, lesions in the liver and small intestine, and a very high EBV viral load. She experienced a rapid demise and at autopsy was found to have EBV+ DLBCL, NOS.
CASE PRESENTATION
An 81-year-old white woman presented to the emergency department after increased confusion and generalized weakness followed by an unwitnessed fall at home. She had a 20-pound weight loss over 2 months and intermittent fevers during the same time. Her past medical history was notable for a cerebrovascular accident 4 years prior, vascular dementia, diabetes mellitus type 2, paroxysmal atrial fibrillation, hyperlipidemia, gastroesophageal reflux disease, and hypothyroidism. On admission, she had a temperature of 97.7°, blood pressure of 118/58 mm Hg, and heart rate of 100 beats/min. Her blood work showed a white blood cell count of 3.2 K/µL and a platelet count of 94 K/µL. Cultures and testing for HIV, hepatitis B, hepatitis C, cytomegalovirus, and parvovirus B19 were all negative. Her Epstein-Barr virus (EBV) viral load was >1,100,000 IU/mL by polymerase chain reaction. A computed tomography (CT) scan of the chest, abdomen, and pelvis found multiple prominent lymph nodes. The abdominal ultrasound showed thickening of the gallbladder, but no overt cholecystitis. A CT of the head was negative. The patient was started on broad-spectrum antibiotics for continued fever and leukopenia, but treatment was discontinued because no source of infection was identified. The patient continued to decline and did not desire invasive life-sustaining measures; thus, comfort measures were started on hospital day 13, and she died shortly thereafter.
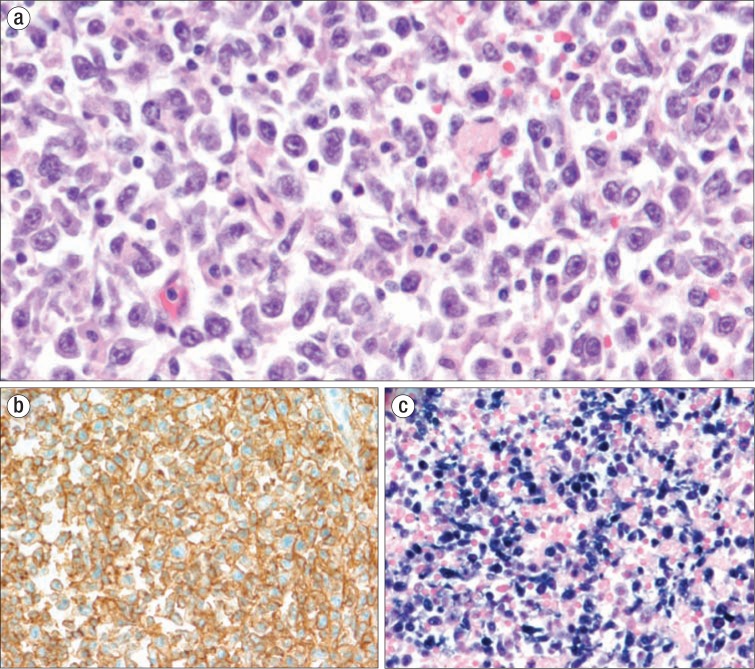
An autopsy was performed and revealed diffuse lymphadenopathy, especially in the paratracheal, periaortic, and peripancreatic areas. Microscopically, the lymph nodes showed massive effacement with infiltrates of large lymphoid cells (Figure 1a). These cells had scant to moderate amounts of basophilic cytoplasm, large irregular nuclei, and prominent nucleoli, and they focally extended through the capsules into the surrounding adipose tissue and into lymphatic vessels. Similar collections of atypical lymphoid cells were found in the lung interstitium, liver portal tracts and sinusoids, splenic white and red pulp, gastric submucosal vessels, epicardium, left atrium, and bone marrow. Immunohistochemistry testing showed that the tumor was positive for CD20 (Figure 1b), MUM1, and BCL2, had a proliferative index (Ki-67) of 60%, and was negative for BCL6, CD3, cMYC, and CD10. In situ hybridization for EBV (EBER-ISH) was positive (Figure 1c). The morphologic and immunophenotypic results represent a diffuse large B-cell lymphoma (DLBCL), post–germinal center type, consistent with EBV-positive DLBCL, not otherwise specified (NOS).
Figure 1.
Lymph node examination at autopsy. (a) Sheets of large lymphoid cells with irregular vesicular nuclei and prominent nucleoli, H&E ×400 (b) B-cell lineage demonstrated by diffuse positive CD20 staining, ×200. (c) Epstein-Barr virus involvement demonstrated by in situ hybridization for EBER, ×200.
DISCUSSION
EBV is a double-stranded, enveloped virus that belongs to the Herpesviridae family. EBV shows tropism for epithelial cells as well as B-cell lymphocytes (1, 2). Almost all humans are exposed to EBV at some point in their life, and after exposure EBV confers a lifelong latency. This can cause problems in the aging population. With age, the immune system enters a state of immunosenescence characterized by a decrease in the diversity of B cells, causing an in vivo clonal expansion. At the same time, T-cell lymphocytes are decreasing in number with a decline in naive T cells and T-cell receptor diversity (3). These changes result in more circulating cells with EBV-specific receptors. Cells with latent EBV infection express EBER protein and may express other proteins such as EBNA and LMP proteins (3). EBV also induces the NFκB pathway, which may be required for survival of the cells in DLBCL (2).
According to the 2016 World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, EBV+ DLBCL, NOS is diagnosed in apparent immunocompetent patients, usually over 50 years of age (4). This lymphoma was a provisional entity in the 2008 World Health Organization classification, entitled EBV+ DLBCL of the elderly, but the “elderly” designation was substituted with “not otherwise specified” with the recognition that this entity occurs in younger patients (4, 5). The NOS designation highlights that the lymphoma must be excluded from more specific entities with neoplastic EBV-positive large B cells, such as lymphomatoid granulomatosis, DLBCL associated with chronic inflammation, and the newly designated entity EBV-positive mucocutaneous ulcer (4).
EBV-positive DLBCL accounts for 8% to 15% of DLBCL in the Asian population (2–4). Within Western populations, the percentage is lower (<5%) (2, 3). Some studies found a median age of 71 and a slight male predominance. Lymph node involvement is seen in about 70% of cases. Microscopically, the lymph node architecture is effaced and consists of a uniform population of large cells with extensive necrosis, mitoses, and apoptoses. If minimal to no reactive component (small lymphocytes, plasma cells, or histiocytes) is seen, the disease is subclassified as monomorphic. The disease is classified as polymorphic if a reactive component is present (2, 3). This morphologic subclassification has not been shown to have prognostic implications (3). The immunohistochemical profile is generally positive for B-cell markers CD20, CD19, CD79a, and PAX-5. CD10 and BCL6 are usually negative, while MUM1 is commonly positive. Cases with immunoblastic or plasmablastic features may lack CD20 expression (5). In situ hybridization for EBER is positive and is considered the most important test in diagnosis, with the highest diagnostic sensitivity (2).
EBV+ DLBCL has a poor response to treatment, so rapid detection is a necessity. Detection relies on clinical suspicion and looking for EBV in every case of DLBCL. The prognosis of EBV+ DLBCL is worse than that of EBV-negative tumors, with a median survival of 2 years (5, 6). Prognosis is worse in patients 70 years or older and in those with B symptoms. Currently, there is no uniformly accepted treatment for EBV+ DLBCL beyond the current standard therapy for DLBCL (2, 6). The standard treatment for DLBCL is the combination of rituximab, a chimeric anti-CD20 monoclonal antibody, with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) (2). Some studies suggest improved prognosis with more intensive regimens, such as combination rituximab, etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (R-EPOCH) (7).
References
- 1.Mandell GL. Principles and Practice of Infectious Diseases. 6th ed. Vol. 2. Philadelphia, PA: Elsevier; 2005. [Google Scholar]
- 2.Castillo JJ, Beltran BE, Miranda RN, Paydas S, Winer ES, Butera JN. Epstein-Barr virus–positive diffuse large B-cell lymphoma of the elderly: what we know so far. Oncologist. 2011;16(1):87–96. doi: 10.1634/theoncologist.2010-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ok CY, Papathomas TG, Medeiros LJ, Young KH. EBV-positive diffuse large B-cell lymphoma of the elderly. Blood. 2013;122(3):328–340. doi: 10.1182/blood-2013-03-489708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura S, Campo E, Swerdlow S. EBV positive diffuse large B-cell of the elderly. In: Swerdlow S, Campo E, Harris NL, Jaffee ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. pp. 243–244. [Google Scholar]
- 6.Lu TX, Liang JH, Miao Y, Fan L, Wang L, Qu XY, Cao L, Gong QX, Wang Z, Zhang ZH, Xu W, Li JY. Epstein-Barr virus positive diffuse large B-cell lymphoma predicts poor outcome, regardless of the age. Sci Rep. 2015;5(1):12168. doi: 10.1038/srep12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song CG, Huang JJ, Li YJ, Xia Y, Wang Y, Bi XW, Jiang WQ, Huang HQ, Lin TY, Li ZM. Epstein-Barr virus–positive diffuse large B-cell lymphoma in the elderly: a matched case-control analysis. PLoS One. 2015;10(7):e0133973. doi: 10.1371/journal.pone.0133973. [DOI] [PMC free article] [PubMed] [Google Scholar]