Abstract
A 56-year-old Texas rancher with a prior diagnosis of acquired erythropoietic protoporphyria secondary to an underlying myelodysplastic disorder developed an uncommon tumor, blastic plasmacytoid dendritic cell neoplasm (BPDCN). During his initial disease, analysis revealed a TET2 mutation, which is the most common mutation associated with BPDCN. This article discusses this unusual hematopoietic neoplasm, the possible evolution from erythropoietic protoporphyria, and the underlying myelodysplastic process.
CASE REPORT
A 56-year-old Texas rancher was diagnosed with erythropoietic protoporphyria secondary to an underlying myelodysplastic disorder, refractory anemia with ring sideroblasts, in 2014 (1). His chromosome analysis revealed no abnormalities, but a mutational analysis revealed a TET2 mutation. His skin disease was well controlled with the high-dose beta-carotene supplement Lumitene and sun avoidance. The patient's blood counts were monitored regularly and remained within normal parameters.
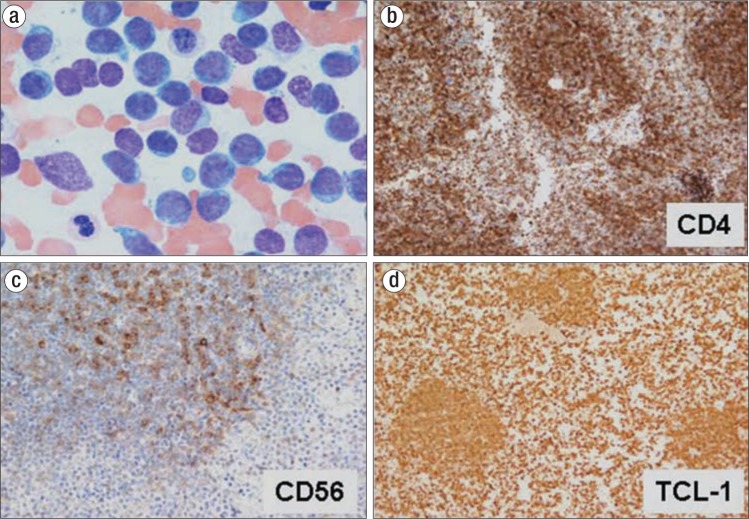
In January 2017, he presented to the emergency room with severe abdominal pain. He stated that he had also noted slowly enlarging lymph nodes in the prior 2 weeks. Physical exam revealed a diffuse violaceous rash across his trunk and upper extremities with diffuse adenopathy in his neck, axillae, and groin. Blood work revealed an elevated white blood cell count of 250 × 109/L with 70% blasts, a hemoglobin of 8.5 g/dL, a hematocrit of 24.7%, and a platelet count of 134 × 109/L. Flow cytometry revealed a population of cells positive for CD4, CD56, and CD123 consistent with a blastic plasmacytoid dendritic cell neoplasm (BPDCN). The diagnosis was confirmed by bone marrow aspirate and biopsy with appropriate immunostains (Figure 1).
Figure 1.
The patient's bone marrow biopsy results: (a) hematoxylin and eosin, 100×; (b) CD4, 40×; (c) CD56, 40×; and (d) TCL-1, 40×.
The patient's karyotype was diploid, and an acute myeloid leukemia fluorescent in situ hybridization panel was normal. CKit was negative. A TET2 mutation was detected on sequencing. The patient was initiated on a Hyper CVAD regimen (cyclophosphamide, vincristine, doxorubicin, and dexamethasone). He developed severe disseminated intravascular coagulation, respiratory distress, and renal insufficiency but slowly recovered without serious morbidity. The plans are to continue his chemotherapy regimen with an eventual allogeneic bone marrow transplant.
DISCUSSION
BPDCN is a rare malignant hematological neoplasm characterized by the clonal population of immature plasmacytoid dendritic cells (2–4). This entity has been known under various names including agranular CD4+ natural killer (NK) cell leukemia, blastic NK-cell lymphoma, blastic NK leukemia, and agranular CD4+, CD56+ hematodermic neoplasm. In the 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia, this entity is classified under the acute myeloid leukemias (5). This is a clinically aggressive tumor derived from precursors of plasmacytoid dendritic cells, also known as professional type I interferon-producing cells or plasmacytoid monocytes (3).
The clinical features of BPDCN consist of two main patterns (6). One is characterized by an indolent onset (70%–90% of cases) dominated by skin lesions followed by tumor dissemination. The other (10%–30% of cases) has features of acute leukemia and systemic involvement from the beginning. BPDCN is characterized by a diffuse monomorphic blastic infiltrate of cells that can resemble lymphoblasts or myeloblasts. The diagnosis is based on immunophenotyping and relies on expression of CD4 and CD56 along with antigens more specific for plasma dendritic cells, including CD123, TCL1, CD303, CD2AP, BCL11a, and SPIB. Currently there is no consensus as to the minimal phenotype necessary to establish the diagnosis, but it is proposed that a confident diagnosis may be established when four of the five principal markers (CD4, CD56, CD123, TCL1, and CD303) are expressed (7, 8).
T-cell and B-cell receptor genes are usually germline with clonal bystander T cells responsible for the rare cases of reported T-cell receptor gene rearrangements. There are no specific karyotypic abnormalities, but complex aberrations may be present with six major recurrent targets, namely 5q (72%), 12p (64%), 13q (64%), 6q (50%), 15q (43%), and loss of chromosome 9 (28%) (9). Next-generation sequencing shows that TET2 is the most common mutated gene (8). Gene expression profiling studies have shown a signature distinct from myeloid and lymphoid acute leukemia (10). The presence of TET in the patient's original diagnosis along with the presence of TET2 in BPDCN raises the possibility that this current lesion may have arisen from his prior underlying myelodysplastic lesion. BPDCN appears to be commonly associated with myelodysplastic features (8).
Despite the deceptively indolent clinical presentation with initial response in most cases to a variety of intensive chemotherapy regimens, the course is most always invariably aggressive, with median survival times from 10 to 16.4 months (7, 11). Occasional reports have noted longer survival/remission times following allogeneic hematopoietic stem cell transplantation (12, 13).
References
- 1.Lindsey K, Burch M, Krause JR. A case of erythropoietic protoporphyria. Proc (Bayl Univ Med Cent) 2016;29(3):311–312. doi: 10.1080/08998280.2016.11929448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaperot L, Bendriss N, Manches O, Gressin R, Maynadie M, Trimoreau F, Orfeuvre H, Corront B, Feuillard J, Sotto JJ, Bensa JC, Briere F, Plumas J, Jacob MC. Identification of a leukemic counterpart of the plasmacytoid dendritic cells. Blood. 2001;97(10):3210–3217. doi: 10.1182/blood.v97.10.3210. [DOI] [PubMed] [Google Scholar]
- 3.Petrella T, Comeau MR, Maynadié M, Couillault G, De Muret A, Maliszewski CR, Dalac S, Durlach A, Galibert L. Agranular CD4+ CD56+ hematodermic neoplasm (blastic NK-cell lymphoma) originates from a population of CD56+ precursor cells related to plasmacytoid monocytes. Am J Surg Pathol. 2002;26(7):852–862. doi: 10.1097/00000478-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Marafioti T, Paterson JC, Ballabio E, Reichard KK, Tedoldi S, Hollowood K, Dictor M, Hansmann ML, Pileri SA, Dyer MJ, Sozzani S, Dikic I, Shaw AS, Petrella T, Stein H, Isaacson PG, Facchetti F, Mason DY. Novel markers of normal and neoplastic human plasmacytoid dendritic cells. Blood. 2008;111(7):3778–3792. doi: 10.1182/blood-2007-10-117531. [DOI] [PubMed] [Google Scholar]
- 5.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 6.Feuillard J, Jacob MC, Valensi F, Maynadie M, Gressin R, Chaperot L, Arnoulet C, Brignole-Baudouin F, Drenou B, Duchayne E, Falkenrodt A, Garand R, Homolle E, Husson B, Kuhlein E, Le Calves G, Sainty D, Sotto MF, Timoreau F, Bene MC. Clinical and biologic features of CD4+ CD56+ malignancies. Blood. 2002;99(5):1556–1563. doi: 10.1182/blood.v99.5.1556. [DOI] [PubMed] [Google Scholar]
- 7.Julia F, Dalle S, Duru G, Balme B, Vergier B, Ortonne N, Vignon-Pennamen MD, Costes-Martineau V, Lamant L, Dalac S, Dellattre C, Dechelotte P, Courville P, Carlotti A, De Muret A, Fraitag S, Levy A, Mitchell A, Petrella T. Blastic plasmacytoid dendritic cell neoplasms: clinico-immunohistochemical correlations in a series of 91 patients. Am J Surg Pathol. 2014;38(5):673–680. doi: 10.1097/PAS.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 8.Alayed K, Patel KP, Konoplev S, Singh RR, Routbort MJ, Reddy N, Pemmaraju N, Zhang L, Shaikj AA, Aladily TN, Jain N, Luthra R, Medeiros LJ, Khoury JD. TET 2 mutations, myelodysplastic features and a distinct immunoprofile characterize blastic plasmacytoid dendritic cell neoplasm in the bone marrow. Am J Hematol. 2013;88(12):1055–1061. doi: 10.1002/ajh.23567. [DOI] [PubMed] [Google Scholar]
- 9.Leroux D, Mugneret F, Callanan M, Radford-Weiss I, Dastugue N, Feuillard J, Le Mee F, Plessis G, Talmant P, Gachard N, Uettwiller F, Pages MP, Mozzoconacci MJ, Eclache V, Sibille C, Avet-Loiseau H, Lafage-Pochitaloff M. CD4+ CD56+ DC2 acute leukemia is characterized by recurrent clonal chromosomal changes affecting 6 major targets: a study of 21 cases by the Groupe Français de Cytogénétique Hématologique. Blood. 2002;99(11):4154–4159. doi: 10.1182/blood.v99.11.4154. [DOI] [PubMed] [Google Scholar]
- 10.Sapienza MR, Fuligni F, Agostinelli C, Tripodo C, Righi S, Laginestra MA, Pileri A, Jr, Mancini M, Rossi M, Ricci F, Gazzola A, Melle F, Mannu C, Ulbar F, Arpinati M, Paulli M, Maeda T, Gibellini D, Pagano L, Pimpinelli N, Santucci M, Cerroni L, Croce CM, Facchetti F, Piccaluga PP, Pileri SA AIRC 5xMille Consortium Genetics-Driven Targeted Management of Lymphoid Malignancies and the Italian Registry on Blastic Plasmacytoid Dendritic Cell Neoplasm. Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia. 2014;28(8):1606–1616. doi: 10.1038/leu.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccaluga PP, Paolini S, Sapienza MR, Pileri SA. Blastic plasmacytoid dendritic cell neoplasm: is it time to redefine the standard of care. Expert Rev Hematol. 2012;5(4):353–355. doi: 10.1586/ehm.12.33. [DOI] [PubMed] [Google Scholar]
- 12.Roos-Weil D, Dietrich S, Boumendi A, Polge E, Bron D, Carreras E, Iriondo A, Arcese W, Beelen DW, Cornelissen JJ, Kroger NS, Milone G, Rossi G, Jardin F, Peters C, Rocha V, Sureda A, Mohty M, Dreger P European Group for Blood and Marrow Transplantation Lymphoma, Pediatric Diseases, and Acute Leukemia Working Parties. Stem cell transplantation can provide durable disease control in blastic plasmacytoid dendritic cell neoplasm: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2013;121(3):440–446. doi: 10.1182/blood-2012-08-448613. [DOI] [PubMed] [Google Scholar]
- 13.Unteregger M, Valentin A, Zinke-Cerwenka W, Troppan K, Deutsch A, Cerroni L, Linkesch W, Neumeister P. Unrelated SCT induces long-term remission in patients with blastic plasmacytoid dendritic cell neoplasm. Bone Marrow Transplant. 2013;48(6):799–802. doi: 10.1038/bmt.2012.242. [DOI] [PubMed] [Google Scholar]