Abstract
Background
Point-of-care (POC) CD4 T-cell counting is increasingly recognized as providing improved linkage-to-care during management of HIV infection, particularly in resource-limited settings where disease burden is highest. This study evaluated prototype POC CD4 T-cell counters from MBio Diagnostics in the context of low CD4 count, hospitalized patients in Mozambique. This study measured system performance when presented with challenging, low count samples from HIV/AIDS patients with acute illnesses resulting in hospitalization.
Methods
Forty whole blood samples were collected from donors on the medical service at Maputo Central Hospital and absolute CD4 counts were generated on the MBio CD4 system and a reference laboratory using flow cytometry.
Results
The mean and median CD4 counts by the flow cytometry reference were 173 and 80 cells/µL, respectively. Correlation between the MBio CD4 System and the reference was good. Bland-Altman analysis showed a mean bias of +15 cells/µL (+9 to +21 cells/µL, 95% CI), and limits of agreement of −47 to 77 cells/µL. For samples with counts >100 cells/µL (N = 14), the mean coefficient of variation was 7.3%. For samples with counts <50 cells/µL, mean absolute bias of replicate samples was 4.8 cells/µL. When two MBio readers were compared, Bland-Altman bias was −4 cells/µL (−13 to +6 cells/µL, 95% CI), and limits of agreement of −63 and +55 cells/µL.
Conclusions
The MBio System holds promise as a POC system for quantitation of CD4 T cells in resource-limited settings given system throughput (80–100 cartridges/day), design simplicity, and ease-of-use.
Key terms: HIV, CD4, T-cell, diagnostic, point-of-care
The absolute CD4 T cell count is a critical component of HIV disease management worldwide (1). For disease staging, particularly in resource-limited settings, the CD4 count is used with clinical evaluation to guide decisions on antiretroviral therapy (ART) initiation, with the World Health Organization (WHO) recommending ART for all HIV-infected individuals with CD4 T cell count below 500 cells/µL (2). Less attention has been given to the potential utility of timely CD4 cell counting in the context of acute hospitalizations. Physicians in areas of high HIV prevalence are not infrequently confronted with patients presenting with acute HIV-1 related illnesses, who have not had or are unaware of the results of recent CD4 cell counts. Early knowledge about the degree of immunodeficiency may have a profound impact on early management and triage decisions. The CD4 count is also used to monitor ART, particularly in settings where HIV viral load testing is unavailable or cost prohibitive.
CD4 testing is performed primarily using flow cytometry equipment in central laboratories. Although some health systems, particularly in South Africa, have successfully implemented broad access CD4 testing based on a highly automated, central laboratory model, it is widely recognized in the global health field that cost-effective, decentralized, higher throughput tools for absolute CD4 count have the potential to significantly improve HIV care. The introduction of point-of-care (POC) CD4 technologies in recent years has been shown to improve patient retention and time to initiation of ART (3,4). The Alere PIMA Analyzer is the most widely implemented of POC CD4 systems, and several other POC CD4 technologies are either available or are expected to be available soon. POC CD4 technologies for resource limited settings have been the subject of several recent reviews (5–10).
MBio Diagnostics is developing a portable CD4 T-cell counting system specifically for POC, near patient, and small laboratory implementation in resource-limited settings. MBio is focused on three important features. First, the MBio system is designed for batch operation, with a single-operator throughput of 80–100 cartridges/day on a single instrument. Barcodes can be read by the instrument to reduce errors due to identifying samples when large volumes of cartridges are used. Second, the system has been developed to minimize cost of the disposable cartridge, eliminating pumps, valves and make-break fluidic features. Third, the CD4 system will be merged with MBio’s multiplexed immunoassay analyzer (11), providing the ability to deliver CD4 count, HIV testing, and opportunistic infection diagnosis on a single platform.
An early prototype of the MBio system, called SnapCount™, has been described previously (12). An improved cartridge and reader, now called the MBio CD4 System, provides two major advances relative to SnapCount: the imaging system has been modified to be compatible with undiluted whole blood, eliminating front end sample processing, and all assay reagents have been incorporated as heat-stable dried reagents on-board the cartridge. As a result, the MBio CD4 System requires minimal user interactions, making it more compatible with busy POC testing settings. Although the system is designed for use in POC settings, reliable, POC diagnostics technologies may also facilitate patient management within in-patient hospital settings, where access to laboratory infrastructure may still be limited.
This article describes an evaluation of the improved MBio CD4 System in the context of low CD4 count HIV patients at Maputo Central Hospital in Mozambique. The goal was to assess analytical performance of the MBio CD4 System relative to a flow cytometry reference, and to establish system functionality in terms of cartridge and reader reproducibility. The authors note that this is a small study designed to give a preliminary assessment of a new technology. Conclusions must be confirmed with larger clinical evaluations.
METHODS
Study Participants and Sample Collection
Blood donors were recruited by a study nurse from the adult in-patient medicine ward at Maputo Central Hospital (MCH) under an informed consent protocol approved by ethical review boards in Mozambique and at the University of California, San Diego (UCSD). Donors with suspected or confirmed HIV-infection were selected based on clinical evaluation by admitting physicians and on ancillary information available in the inpatient medical record. Exclusion criteria included contraindication to venipuncture. No participants were excluded on the basis of gender, race, or ethnicity, or socioeconomic status. Samples were deidentified by study staff prior to testing on the MBio System.
Upon informed consent, 2 separate 3 ml whole blood specimens were collected via venipuncture into evacuated K2EDTA Vacutainer® tubes. One tube was delivered to the Instituto Nacional De Saude (INS) for reference flow cytometry testing. The other tube was delivered to the Universidade Eduardo Mondlane (UEM) laboratory that hosted the prototype MBio system and study technical staff. Both the INS and UEM laboratories are walking distance from the MCH medical ward, and all CD4 testing was performed the same day as sample collection.
Reference Testing
Reference flow cytometry CD4 counts for each sample were measured using a single platform BD FACSCalibur flow cytometer with BD Multitest™ kits at the INS Cellular Immunology Laboratory. Reference CD4 counts were reported to the study team after completion of the analysis on the MBio CD4 System.
MBio CD4 System and Protocol
The MBio CD4 System consists of single-use disposable cartridges and a simple reader. Absolute CD4 count is generated using two-color fluorescent immunostaining and imaging cytometry. The disposable cartridge consists of a single fluid channel with passive fluidics based on capillary flow. A proprietary antibody formulation is dried on the cartridge. When rehydrated with a 15 µl blood sample, CD3+ cells and CD4+ cells are stained with two different fluorophores. When the cartridge is analyzed on the reader, software automatically processes the fluorescence images and generates a CD3+/CD4+ T cell count using a proprietary image analysis algorithm. For this study, each cartridge was loaded and run at room temperature for 20 min, followed by a ~5 min read.
Two MBio CD4 readers were installed in the laboratory of Dr. Emilia Noormahomed in the UEM Division of Parasitology. Fifteen microliters of whole blood were transferred from the Vacutainer® tube to the cartridge inlet port with an adjustable pipette. The test is insensitive to the volume of blood added to the cartridge between 10 and 20 µl. The blood was manually aspirated and dispensed on the cartridge to promote dried reagent rehydration and mixing. A vent on the cartridge was then manually punctured to allow the blood to flow into the imaging region of the cartridge. After the fluid flowed down the channel in the cartridge, an adhesive seal was placed over the cartridge inlet to minimize biohazard. The cartridge was then allowed to incubate for 20 min on the bench top before insertion into an MBio CD4 Reader for analysis. All 40 samples were analyzed on two MBio readers. Thirty-two of the samples were measured in triplicate with two measurements made on one reader and one on the other.
Statistical Analysis
Absolute CD4 count results from the MBio CD4 System were compared to flow cytometry using Bland-Altman analysis, providing mean bias with 95% confidence intervals and limits of agreement (LOA).
Cartridge reproducibility was assessed by running the same blood sample on multiple cartridges. Percent coefficient of variation (%CV, ratio of the sample standard deviation to the mean value) is used as the reproducibility metric. %CV was calculated when three or more sample replicates were available. %CV was not calculated for samples with absolute counts <50 cells/µl, as clinically insignificant absolute count variations can lead to misleadingly high %CVs. For samples with CD4 counts <50 cells/µl, the mean absolute count bias is reported as an assessment of reproducibility. Instrument-to-instrument reproducibility was assessed using a Bland-Altman analysis of the same blood sample run on both MBio readers. This study did not attempt to address operator variability.
RESULTS
Study Participants and Sample Characteristics
A total of 40 whole blood samples were provided by HIV-infected individuals at Maputo Central Hospital. Counts ranged from 7 to 692 cells/µl. The mean and median CD4 counts for the sample set were 173 and 80 cells/µl, respectively.
MBio CD4 System Performance Relative to Flow Cytometry
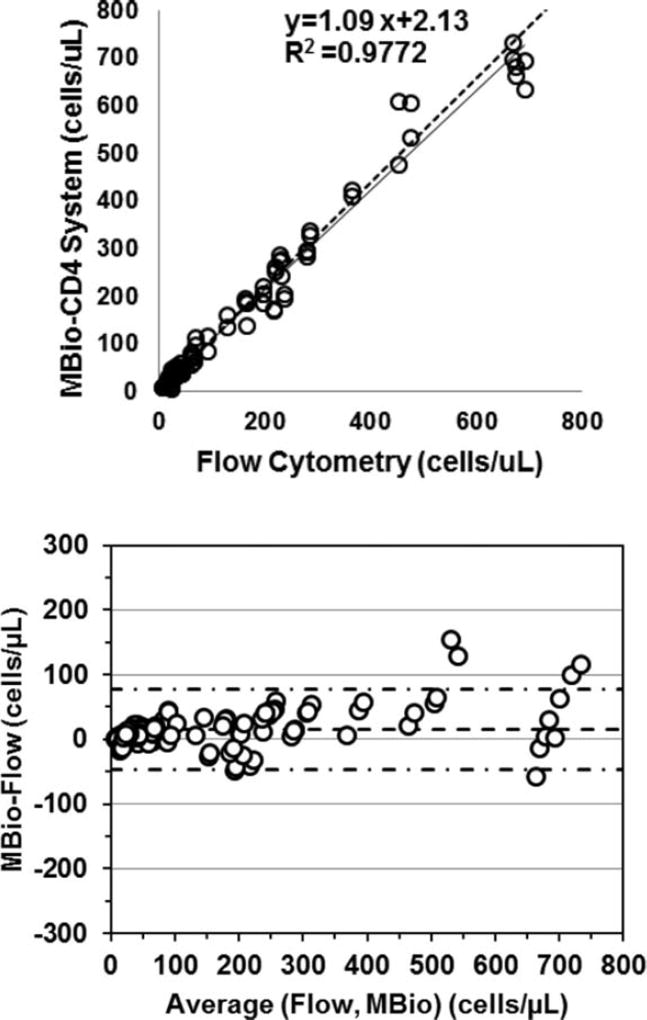
The MBio CD4 System showed good correlation with reference flow cytometry (Fig. 1). When all samples and cartridges are considered as independent measurements, Bland-Altman analysis resulted in a mean bias of +14.9 cells/µl (9–21 cells/µl at 95% confidence). The limits of agreement (LOA) were from −47 to 76 cells/µl. The Passing-Bablok regression was performed using the first two replicate measurements of each sample and yields a slope of 1.09 (1.05–1.13 95% CI) and intercept 2.13 (−0.32 to 4.70 cells/µl, 95% CI).
Fig. 1.
(Top) Scatterplot comparing MBio CD4 system cell counts to the flow cytometry reference (N = 40 blood samples, NC = 112 cartridges). Linear regression results and fit (dashed line) are shown on the plot. Additional data regression is described in the text. (Bottom) Bland-Altman plot of all cartridges processed in the study (NC = 112). Mean bias (dashed line) is +14.9 cells/µl (+9 to +21 cells/µl, 95% CI). The limits of agreement (LOA, dash-dot-dash lines) are −47 and 77 cells/µl.
WHO currently recommends initiating ART when CD4 counts are below 500 cells/µl (2). Because of resource constraints and lack of access to ARTs, many endemic settings still use a lower threshold of 350 cells/µl. The MBio CD4 System had no high or low misclassifications at either the 350 or 500 cells/µl thresholds, although the number of samples around the cutoff points was low and the misclassification rate analysis will need to be repeated in a larger study. In this particular study, we focused on Maputo Central Hospital inpatients. Maputo Central typically admits 15–20 new patients daily to its medical services and ~70% of these are HIV infected. Although the median CD4 cell count in our sample set was 80 cells/µl, we encountered a wide range of counts (7–692 cells/µl) in this convenience sample of inpatients. The platform performed extremely well in distinguishing those with <50 CD4 cells/µl and 50–200 CD4 cells/µl from those with higher counts. Patients in these lower CD4 cell count strata would be expected to be at risk from a much broader array of opportunistic pathogens and might benefit from broader use of empiric antimicrobials while the differential diagnosis is refined by the patient clinical evolution and the return of traditional microbiological tests.
MBio cartridge reproducibility was good. For the 18 samples with a CD4 count >50 and with three or more replicates per sample, the mean %CV was 8.1%, a clinically acceptable reproducibility for a sample collection in this low count range. When only samples with a CD4 count >100 cells/µl are considered (N = 14), the %CV is 7.3%. Reproducibility was also good for the very low count samples; for the samples with CD4 counts <50 cells/µl (N = 16), the average absolute bias was 4.8 cells/µl.
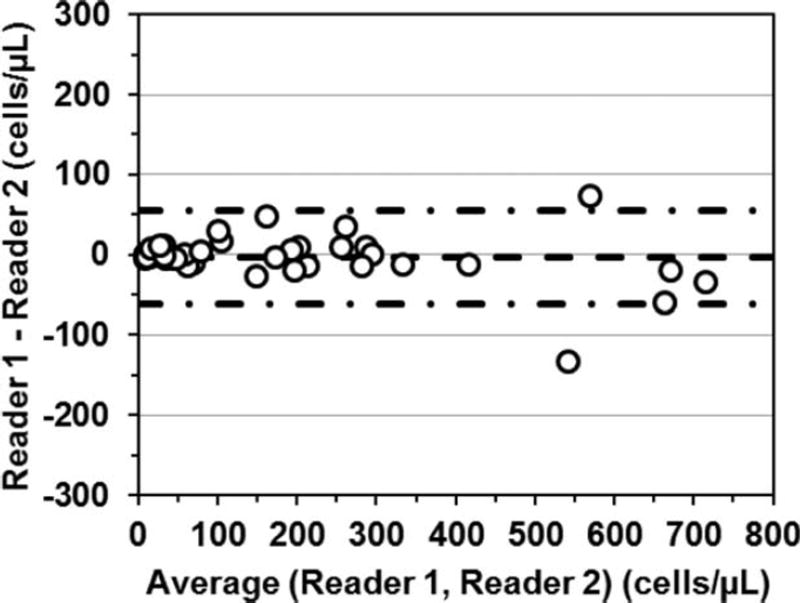
Comparison of the two MBio Readers is provided in Figure 2. When the same blood sample was run on the two instruments the mean bias is −3.6 cells/µl (−13 to +6 cells/µl 95% CI) with LOAs of −62 and +55 cells/µl.
Fig. 2.
Bland-Altman analysis for blood samples run on two MBio Readers, with Reader 1 as compared to Reader 2. Mean bias (dashed line) is −3.6 cells/µl (−13.2 to +6.0 cells/µl 95% CI). LOAs (dash-dot-dash lines) are −62.5 and +55.3 cells/µl.
DISCUSSION
Moving CD4 counting from a small number of reference laboratories to district hospitals, health clinics, and rural POC settings in high disease burden countries has been a major advance in the last 5 years. Jani et al. showed that POC CD4 testing in rural and periurban settings in Mozambique reduced pretreatment loss to follow-up, with more patients identified as eligible and initiated on ART (4,13). This observation has been confirmed in multiple subsequent linkage-to-care studies (3). Technologies that deliver CD4 count at the point of care have been the subject of significant investment and development, with a pipeline of products expected to be available in the near term. Yet despite the success of technologies such as the Alere PIMA Analyzer, there is still a need for higher throughput, lower cost, easy-to-use near patient CD4 systems in resource-limited settings (8) that ideally deliver additional features while providing lab quality performance in challenging operational environments.
An important factor to consider in POC and near-patient CD4 testing environments is sample throughput. For example, it is not uncommon for HIV clinics across sub-Saharan Africa to see >50 patients/day. Even in clinics with lower overall patient visit numbers, it is common to have clinical hours clustered in a short time period. Throughput of POC testing and the ability to run batch processes are therefore of paramount importance for practical implementation. The current leading POC CD4 system (PIMA), has a throughput of only 2–3 cartridges per hour per instrument, run serially, (instrument is occupied ~20 min for each test). The MBio CD4 System has been designed to handle all cartridge incubations off-instrument, with an ~3 min analysis on the reader. As a result a single operator can process 10 to 15 cartridges per hour, and 80–100 cartridges per shift using a single reader. Cartridges can be read up to 1 h after the sample is added to the cartridge, and can be reread multiple times.
Ease-of-use is another key factor for practical implementation. The premarketing version of the MBio CD4 System described here eliminated the volumetric sample dilution and transfer steps described in an earlier version (12), and moved to direct addition of whole blood to the assay cartridge containing all assay reagents. The assay protocol used to generate data in this article relied on minimal manual steps and is designed for use with capillary (i.e., finger stick) blood. The small footprint system is shown in Figure 3.
Fig. 3.
MBio CD4 System showing cartridge being inserted into the Reader. The MBio Rack used for cartridge and timing management is shown in the back.
In conclusion, this study showed the MBio CD4 System can provide reliable CD4 counts on low CD4 count samples. This is promising technology for near patient and point of care CD4 testing in resource-limited settings, particularly in settings where a rapid turnaround and high sample throughput is desired.
Acknowledgments
The authors are grateful to Nádia Sitoe, Cellular Immunology Laboratory, Instituto Nacional de Saúde for performing the flow cytometry analysis. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under award number R44AI070052. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
MBio Diagnostics, Inc. is a private, for-profit organization. Authors affiliated with MBio Diagnostics are paid employees. Authors from UCSD and UEM have no financial relationship with MBio Diagnostics, Inc.
LITERATURE CITED
- 1.UNITAID. HIV/AIDS Diagnostics Technology Landscape. 3. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 2.WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva, Switzerland: World Health Organization; 2013. [PubMed] [Google Scholar]
- 3.Wynberg E, Cooke G, Shroufi A, Reid SD, Ford N. Impact of point-of-care CD4 testing on linkage to HIV care: A systematic review. J Int AIDS Soc. 2014;17:18809. doi: 10.7448/IAS.17.1.18809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM, Lehe JD, Peter TF. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: An observational cohort study. Lancet. 2011;378:1572–1579. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- 5.Glynn MT, Kinahan DJ, Ducree J. CD4 counting technologies for HIV therapy monitoring in resource-poor settings–state-of-the-art and emerging microtechnologies. Lab Chip. 2013;13:2731–2748. doi: 10.1039/c3lc50213a. [DOI] [PubMed] [Google Scholar]
- 6.Setty MKHG, Hewlett IK. Point of care technologies for HIV. AIDS Res Treat. 2014;2014:497046. doi: 10.1155/2014/497046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varughese JK, Rosenberg MG, Kim K. HIV in the tropics: Staging in the resource-limited setting. Curr Opin Infect Dis. 2012;25:477–483. doi: 10.1097/QCO.0b013e3283567b00. [DOI] [PubMed] [Google Scholar]
- 8.Reid SD, Fidler SJ, Cooke GS. Tracking the progress of HIV: The impact of point-of-care tests on antiretroviral therapy. Clin Epidemiol. 2013;5:387–396. doi: 10.2147/CLEP.S37069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle DS, Hawkins KR, Steele MS, Singhal M, Cheng X. Emerging technologies for point-of-care CD4 T-lymphocyte counting. Trends Biotechnol. 2012;30:45–54. doi: 10.1016/j.tibtech.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peter T, Badrichani A, Wu E, Freeman R, Ncube B, Ariki F, Daily J, Shimada Y, Murtagh M. Challenges in implementing CD4 testing in resource-limited settings. Cytometry B: Clin Cytom. 2008;74(Suppl 1):S123–S130. doi: 10.1002/cyto.b.20416. [DOI] [PubMed] [Google Scholar]
- 11.Lochhead MJ, Todorof K, Delaney M, Ives JT, Greef C, Moll K, Rowley K, Vogel K, Myatt C, Zhang XQ, et al. Rapid multiplexed immunoassay for simultaneous serodiagnosis of HIV-1 and coinfections. J Clin Microbiol. 2011;49:3584–3590. doi: 10.1128/JCM.00970-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logan C, Givens M, Ives JT, Delaney M, Lochhead MJ, Schooley RT, Benson CA. Performance evaluation of the MBio diagnostics point-of-care CD4 counter. J Immunol Methods. 2013;387:107–113. doi: 10.1016/j.jim.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jani IV, Sitoe NE, Chongo PL, Alfai ER, Quevedo JI, Tobaiwa O, Lehe JD, Peter TF. Accurate CD4 T-cell enumeration and antiretroviral drug toxicity monitoring in primary healthcare clinics using point-of-care testing. AIDS. 2011;25:807–812. doi: 10.1097/QAD.0b013e328344f424. [DOI] [PubMed] [Google Scholar]