Abstract
Background
Surgical sutures, wound tension, additional skin incisions and other factors may result in recurrence of tumor-like scar.
Objective
To investigate the role of wound natural healing therapy in tumor-like hypertrophic scar.
Methods
In this study, tumor-like hypertrophic scars of 47 cases were excised completely and the residual wounds were treated with natural healing. The short-term and long-term effects of treatment were evaluated.
Results
All cases were successfully cured by natural healing therapy. The healing time of the maximum wound (80mm × 20mm) and the minimal wound (5mm× 5mm) was 25 days and 7 days respectively. The size of new skin scars ranged from 3mm to 11 mm. Clinical followed-up was performed in 34 cases for 36 months. Among them, no recurrence happened in 31 cases and new scar size ranged from 2mm to 8mm, while local recurrence happened in 3 cases whose scar size were less than 5 mm.
Study Limitations
The cure rate of the therapy was 91.2%.
Conclusion
The wound natural healing therapy is effective in treating tumor-like hypertrophic scar, which can prevent recurrence and has good cosmetic results.
Keywords: Keloid, Wound closure techniques, Wound healing
INTRODUCTION
Tumor-like hypertrophic scar is a temporary name according to the characteristics of the type of scar, which used to be classified as keloid. The pathogenesis of keloids and hypertrophic scars are not well clear.1 Many traditional methods classified hypertrophic scars and keloids as different types of scars. 2 However, there are still many cases whose scar exhibited features of both hypertrophic scars and keloids.3
Tumor-like hypertrophic scars are characterized by continuous, expansive, slow growth, with spherical shape outward skin, clear margin, progressive expansion, no skin infiltration and high recurrence rate after surgery. The size of tumor-like hypertrophic scar is not very big. Conventional treatment usually employed the complete surgical resection of scar tissue, and then direct suture, local flap transplantation or skin transplantation performed to repair skin wounds.4,5 The history of skin grafting can be traced back to 2,000 years ago, as it is believed to have been performed by the natives of India.6 In some rare instances, the non-healing wound developed in a neoplasia that could be transformed into a malignancy.7
The above mentioned surgical repair process is quite complicated. Surgical sutures, wound tension, additional skin incisions and other factors may result in recurrence of tumor-like scar. 8 In order to avoid recurrence of the scar, after scar excision we employed wound natural healing therapy to treat the 47 cases with tumor-like hypertrophic scars, and achieved effective results.
METHODS
Ethics statement
This research was approved by the Ethics Committee of Jinan Military General Hospital and had been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. The informed consent was obtained from all individual participants included in the study.
Selection of cases
Forty-seven patients (33 men and 14 women; age range 8-78 years) were diagnosed with tumor-like hypertrophic scars. Among them, 15 cases had earring scar, 12 cases had infection scar, 10 cases had incision scar, and 4 cases had skin micro-injury scar. The area of scar ranged from 5mm × 5mm to 80mm × 20mm.
Scar resection
All patients were treated with tumescent local anesthesia. The skin was sterilized with 0.1% povidone iodine. Tumescent local anesthesia was performed by using 0.5% lidocaine injection (containing 1: 400,000 epinephrine). All the layers of skin were cut at a distance of 1 mm apart from the margins of scar. After removing the tumor-like hypertrophic scars, the wound was checked to ensure that there was no residual white scar tissue and then hemostasis was performed.
Natural healing therapy for wounds
After surgery, vaseline oil gauze was applied to cover on the wounds, with the addition of sterile gauze. After changing the dressing on day 4, the wound was sterilized by using 0.1% povidone iodine, sprayed by using basic fibroblast growth factor (bFGF) solution, and smeared with hyaluronic acid ointment. The dressing was changed twice daily in the same way until the wound had healed completely.
Treatment of scar modeling period
Anti-scar therapy was applied on one week after wound healing. The new scar was massaged with fingers, and then smeared with silicone ointment 2-3 times a day. The treatment was continued for more than 12 weeks.
RESULTS
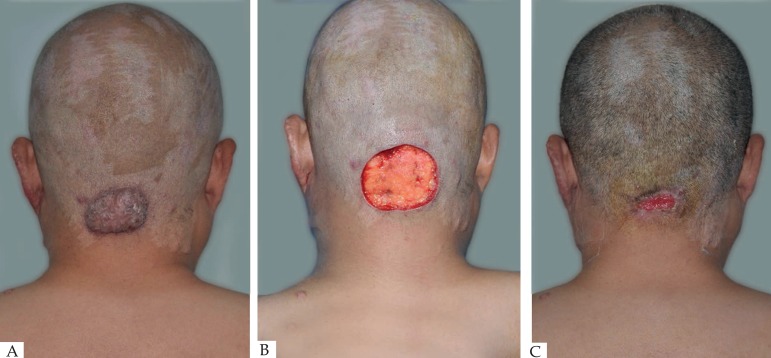
All of the 47 cases were cured by natural healing therapy. The healing time of the maximum wound (80mm × 20mm) and the minimal wound (5mm × 5mm) was 25 days and 7 days, respectively. After wound had healed completely, the size of new skin scars ranged from 3mm to 11mm, and the scars were flat. After 3 months, new scar exhibited slight hyperplasia with hard and reddish texture. Clinical followed-up was performed in 34 cases for 36 months. Among them, no recurrence happened in 31 cases. The healing area were flat or slightly bulge and ranged from 2mm to 8mm, which were much smaller than early stages of healing; 3 cases had local recurrence with less than 5mm of scar size, and were cured after reoperation (Figures 1-5). The cure rate of the therapy was 91.2%.
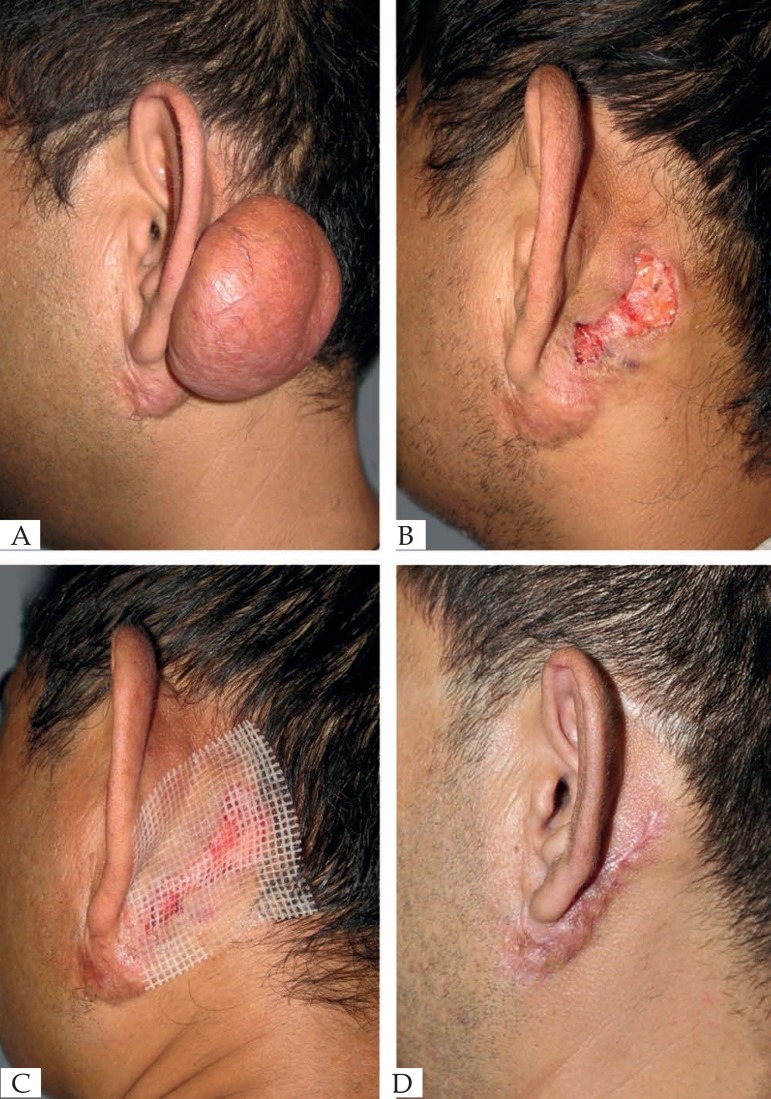
Figure 1.
(A) Before operation. (B) Scar removed immediately. (C) Wound healing progressed. (D) 3 months after operation
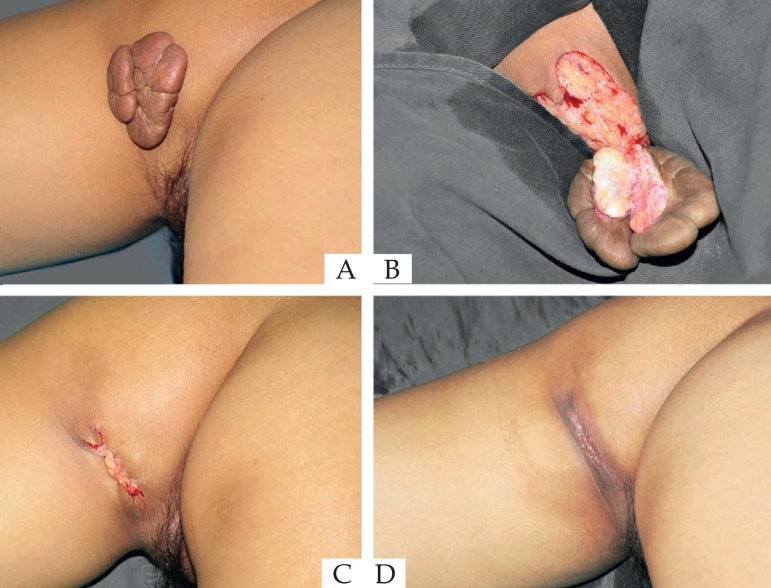
Figure 5.
(A) Before operation. (B) Scar removed immediately. (C) 7 days after operation. (D) 36 months after operation.
DISCUSSION
The causes of scar are various, and the treatment of scar is also extremely complicated, which is always a challenge to plastic surgery. Surgeons, especially plastic surgeons, prefer efficient closure for long surgical incisional wounds and expect effective scar outcomes. However, if wound closure proceeds too quickly, the adjacent wound margin will be ignored, resulting in inevitable scarring. In contrast, if closure is performed carefully, the surgery will last long. 9 Hypertrophic scars and keloids begin with a similar morphology, with hypertrophic scars phasing through proliferative to the static state with collagen, which is increasingly organized. Clinically, keloids are characterized as scars that outgrow the original wound edges, invade adjacent normal tissue and rarely regress over time.10 Differences in shape, foci of increased fibroblast and vascular density, and the presence of hyalinized collagen nodules in the core of keloid lesions distinguish keloids from hypertrophic scars at the histological level.11-14 Hypertrophic scars are hard, red or pink raised scars, elevated but remaining within the limits of the original wound. They usually emerge within the first month after injury and may regress over time. In contrast, keloids are raised, reddish-purple, nodular scars, firmer than hypertrophic scars, extending beyond the margins of the original wound and not regressing over time.15 The comparison of the two scars is described in table 1. The feature of tumor-like hypertrophic scars is that the body of scar grows expansively with spherical shape outward skin, and the scar root grows expansively, which results in distortion of local tissue without skin infiltration. All tumor-like hypertrophic scars have small roots and large bodies. Therefore, we consider that tumor-like hypertrophic scars and keloids do not belong to the same class.
Table 1.
Comparison of two types of scars
| Type | Growth pattern | Shape | Margin | skin infiltration | Surgical effect |
|---|---|---|---|---|---|
| Keloid | Continued intermittent growth | Flat | Unclear | Yes | Easy recurrence |
| Tumor-likehypertrophicscar | Continued slow growth | Spherical | Clear | No | Easy recurrence |
Many treatment options and modalities have been applied for reduction and prevention of scar formation, including excision and intralesional corticosteroid injections. However, the therapeutic efficacy is still not satisfactory due to the high recurrence rate. 10,16 In order to avoid recurrence of the tumor-like hypertrophic scars, we must emphasize two important steps: on the one hand, scar resection should be clean and exhaustive; on the other hand, the surgical procedure should be simplified with no additional repair surgery and natural healing therapy should be performed after scar resection for small wound.
CONCLUSION
This study indicated that natural healing therapy is efficient in treatment of tumor-like hypertrophic scar, which could effectively prevent the recurrence after scar resection; also the cure rate reached 91.2%.
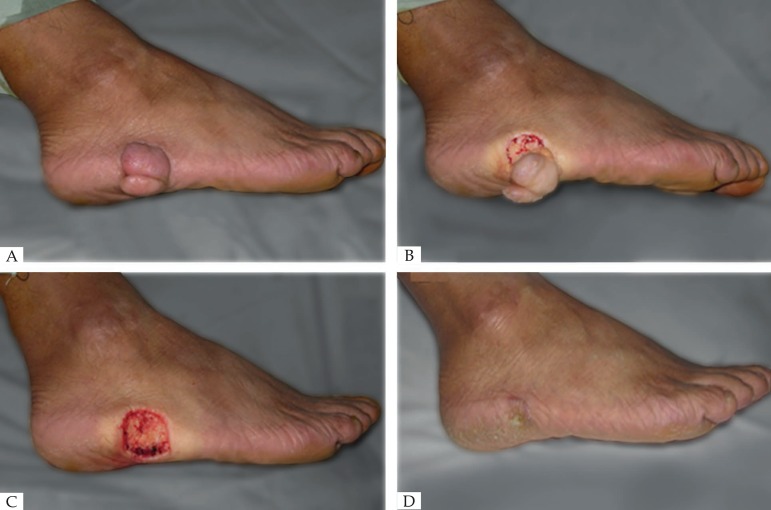
Figure 2.
(A) Before operation. (B) Scar removed immediately. (C) Wound healing progressed. (D) 3 months after operation
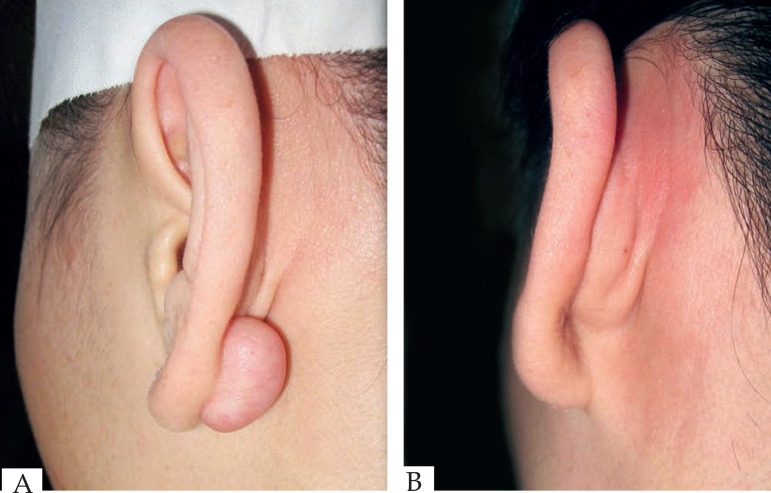
Figure 3.
(A) Before operation. (B) 36 months after operation
Figure 4.
(A) Before operation. (B) Scar removed immediately. (C) 36 months after operation.
Footnotes
Conflict of interest: None.
Study performed at the Jinan Military General Hospital - Sichuan Province, China.
Financial support: None
REFERENCES
- 1.Ogawa R, Chin MS. Animal models of keloids and hypertrophic scars. J Burn Care Res. 2008;29:1016–1017. doi: 10.1097/BCR.0b013e31818ba189. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg. 2010;125:557–568. doi: 10.1097/PRS.0b013e3181c82dd5. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa R, Akaishi S, Izumi M. Histologic analysis of keloids and hypertrophic scars. Ann Plast Surg. 2009;62:104–105. doi: 10.1097/SAP.0b013e3181855172. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Z, Shan Y, Ying L, Zheng J, Mohamed S, Ma Z. Complete earlobe keloid resection with fistulectomy. Dermatol Surg. 2015;41:83–86. doi: 10.1097/DSS.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 5.Hamnerius N, Wallin E, Svensson Å, Stenström P, Svensjö T. Fast and Standardized Skin Grafting of Leg Wounds With a New Technique: Report of 2 Cases and Review of Previous Methods. Eplasty. 2016;16:e14. [PMC free article] [PubMed] [Google Scholar]
- 6.Hauben DJ, Baruchin A, Mahler A. On the history of the free skin graft. Ann Plast Surg. 1982;9:242–245. doi: 10.1097/00000637-198209000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Cohen IK, Die-gelmann RF, Lindblad WJ, Hugo NE. Wound Healing: biochemical e clinical aspects. Philadelphia: WB Saunders Co; 1992. pp. 541–561. [Google Scholar]
- 8.Stier MF, Glick SA, Hirsch RJ. Laser treatment of pediatric vascular lesions: Port wine stains and hemangiomas. J Am Acad Dermatol. 2008;58:261–285. doi: 10.1016/j.jaad.2007.10.492. [DOI] [PubMed] [Google Scholar]
- 9.Han HH, Kim SY, Lee YJ, Moon SH, Oh DY. Donor-site closure using absorbable dermal staple for deep inferior epigastric artery perforator flaps: its efficacy and cosmetic outcomes. Springerplus. 2016;5:363–363. doi: 10.1186/s40064-016-1988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bran GM, Goessler UR, Hormann K, Riedel F, Sadick H. Keloids: current concepts of pathogenesis (review) Int J Mol Med. 2009;24:283–293. doi: 10.3892/ijmm_00000231. [DOI] [PubMed] [Google Scholar]
- 11.Tuan TL, Nichter LS. The molecular basis of keloid and hypertrophic scar formation. Mol Med Today. 1998;4:19–24. doi: 10.1016/S1357-4310(97)80541-2. [DOI] [PubMed] [Google Scholar]
- 12.Ehrlich HP, Desmoulière A, Diegelmann RF, Cohen IK, Compton CC, Garner WL, et al. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1994;145:105–113. [PMC free article] [PubMed] [Google Scholar]
- 13.Bux S, Madaree A. Keloids show regional distribution of proliferative and degenerate connective tissue elements. Cells Tissues Organs. 2010;191:213–234. doi: 10.1159/000231899. [DOI] [PubMed] [Google Scholar]
- 14.Jumper N, Paus R, Bayat A. Functional histopathology of keloid disease. Histol Histopathol. 2015;30:1033–1057. doi: 10.14670/HH-11-624. [DOI] [PubMed] [Google Scholar]
- 15.Wolfram D, Tzankov A, Pülzl P, Piza-Katzer H. Hypertrophic scars and keloids--a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35:171–181. doi: 10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- 16.Love PB, Kundu RV. Keloids: an update on medical and surgical treatments. J Drugs Dermatol. 2013;12:403–409. [PubMed] [Google Scholar]