Abstract
Liposarcomas correspond to the most common histological subtype of soft tissue sarcomas. They can be subdivided into: well differentiated or atypical lipoma, undifferentiated, myxoid, round, and pleomorphic cells. Atypical lipomas are the most prevalent and usually appear as asymptomatic softened tumors. They are locally aggressive but rarely lead to distant metastases. The diagnosis of this tumor is based on the imaging and histopathologic findings. Treatment consists of excision surgery with complete tumor removal. It has a good prognosis due to the low percentage of distant metastases. We report a rare case of giant atypical lipoma as well as the adopted therapy and evolution.
Keywords: Dermatology, Immunohistochemistry, Liposarcoma, Pathology
INTRODUCTION
Soft tissue sarcomas are a heterogeneous group of neoplasia, with over 50 histological subtypes, which correspond to 1% of all solid tumors.1 Liposarcomas were initially described by Virchow in 1857 and are considered to be the most common subtype, representing 17-25% of all sarcomas.2
Their incidence peaks during the sixth and seventh decades of life, with equal prevalence for men and women, except when they affect the inguinal area, where they are more common in male patients. The deep muscle tissue of extremities (especially thighs) is where 75% of the cases develop, whereas 20% of the cases develop in the retroperitoneum. The remaining cases are divided into inguinal area, spermatic cord, and other areas. Cases in the subcutaneous tissues are rare.3
Nowadays, the World Health Organization recognizes five histological sub-types of liposarcoma: well differentiated or atypical lipoma, undifferentiated, myxoid, round cells, and pleomorphic.4 This histological differentiation is important, as it determines the prognosis and guides the treatment.5
The purpose of this article is to report a rare case of atypical lipoma in the gluteal region of a young patient, as well as to review the literature.
CASE REPORT
A 30-year-old, male patient, born and residing in São Paulo, phototype 5, was referred to the Dermatology Department due to the presence of a tumor in the left gluteal region for the previous year. The examination revealed a soft, multilobed tumor, measuring 17 cm, with ulceration (Figure 1).
Figure 1.
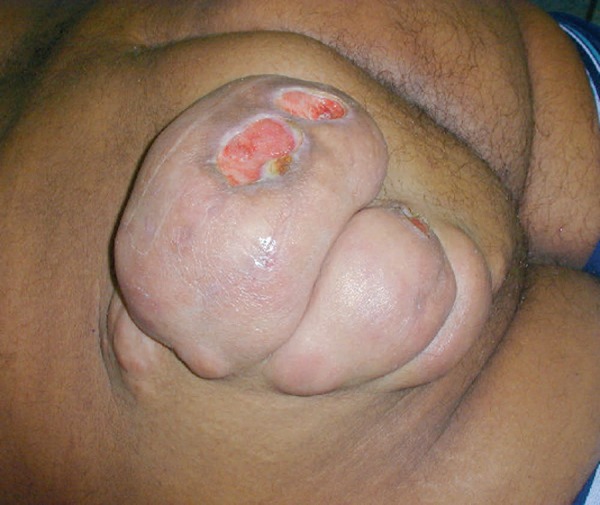
Soft, ulcerated tumor on the left buttock
A fusiform incisional biopsy was performed, whose anatomopathological examination a fibrolipoma. Due to its large size, the tumor was excised, and reconstruction was done with primary suture and approximation of the edges, whereas the central area was left for second intention healing (Figure 2). The patient was instructed to change the dressing daily, applying a powder containing magnesium silicate, zinc oxide, thymol iodide, and bismuth subgallate, and closing with gauze and micropore tape.
Figure 2.
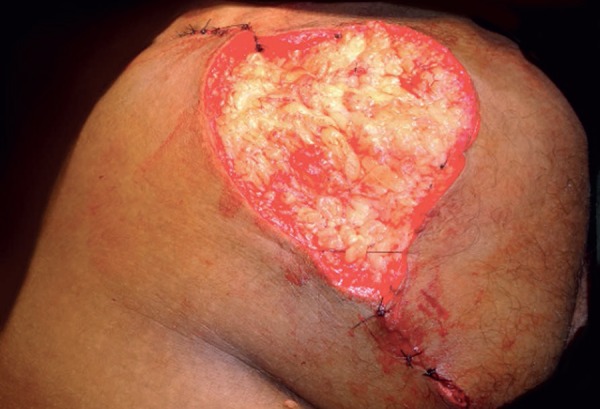
Immediately after surgery
After carefully analyzing the surgical piece, the anatomopathological examination revealed an atypical lipomatous neoplasm (Figures 3 and 4). Currently, two months after surgery, the patient has been monitored on a monthly basis, and has exhibited good healing (Figure 5).
Figure 3.
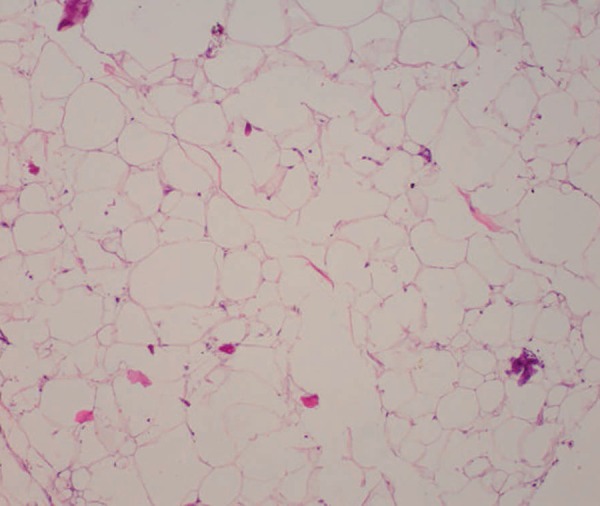
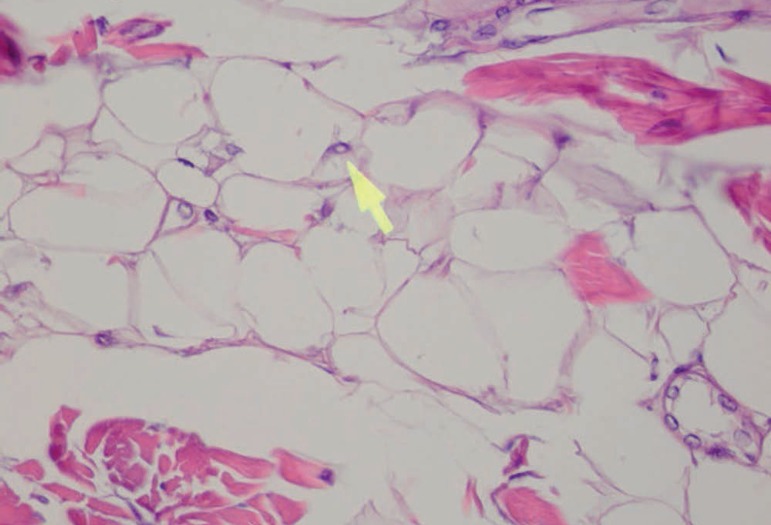
Adipocytes of different sizes, some more globose than others (Hematoxylin & eosin, X40)
Figure 4.
In some adipocytes, intranuclear pseudoinclusions are detected (arrow) (Hematoxylin & eosin, X400)
Figure 5.
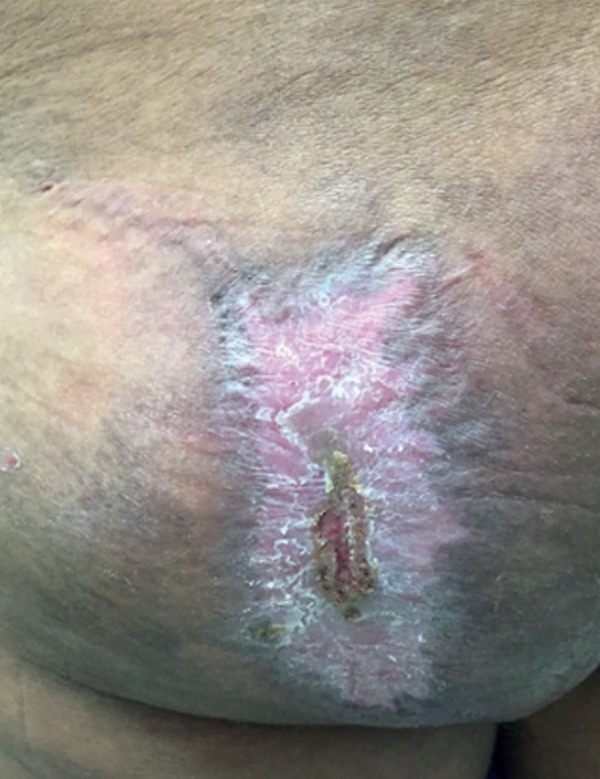
Four months after surgery
DISCUSSION
Liposarcomas correspond to a group of lipomatous neoplasms that vary from well-differentiated forms, with low potential for distant metastasis, to undifferentiated and pleomorphic, which are more aggressive and present a worse prognosis (Table 1). 6
Table 1.
Liposarcoma classification
| Clinical | Image (CT/NRM) | Anatomopathological | Molecular characteristics | Global survival rate | Relapse rate / Metastasis rate | |
|---|---|---|---|---|---|---|
| Atypical lipoma | Painless, soft tumor. Slow growth. Most common locations: extremities and retroperitoneum. Most common subtype | Encapsulated lipomatous mass (hyperintense in T1 and in T2 in NMR) with thick septations | Proliferation of mature adipocytes, with size variation, focal nuclear atypia, and/or fusiform cells | Amplification of MDM-2 and CDK-4 | 76-93% | (R) - 13-46% (extremities) (R) - 91% (retroperitoneum) (M) - almost zero |
| Undifferentiated LPS | May result from an atypical lipoma (10%). More frequent in retroperitoneum and extremities | Loss of signal intensity in images in T1 and focal nodules (>1 cm is suggestive) | Abrupt transition between mature adipocytes and high degree non-lipogenic sarcoma areas | Amplification of MDM-2 and CDK-4 | 54-64% | (R) - 18-57% (M) - 13-47% |
| Myxoid/ round cell LPS | Tumors more common in lower limbs. Affects young adults more frequently | Hypointensity of the signal in T1, and hyperintensity in T2 are pathognomonic | Predominance of round cell myxoid stroma. There is vascular system of thin walls (plexiform pattern) | FUS-CHOP gene fusion. Mutations PI3K (20%) | 40-75% | (R) - 7-28% (M) - 10-58% |
| Pleomorphic LPS | Rapid growth tumor. More frequent in old people and on extremities | Nonspecific mass, with frequent necrosis and hemorrhage necrosis | Intense cell atypia, irregular lipoblast. Hyperchromatic nuclei | Complex structural rearrangements | 0-63% | (R) - 16-45% (M) - 32-44% |
Abbreviations: LPS=Liposarcoma; CT= Computed tomography; NMR= Nuclear magnetic resonance; RT=Radiotherapy
Well-differentiated liposarcomas (WDL) or atypical lipomas (AL) represent 40-45% of all liposarcomas, corresponding to the most common subgroup. The current recommendation is to call them atypical lipomas (when they affect surface tissues) or atypical lipomatous neoplasms (when they affect the deeper tissues). However, the terms are still used as synonyms, since, depending on the location (such as retroperitoneum or mediastinum), the term liposarcoma is more appropriate.7 AL appears as a slow-growth, asymptomatic tumor. It is locally aggressive, but rarely leads to distant metastasis. The most common locations are extremities and retroperitoneum. The retroperitoneal forms do not metastasize either, unless they progress to undifferentiated forms.8
The pathogenesis is related to chromosomal aberrations. In WDL, giant markers and ring chromosomes are found in cytogenetic studies. These "neochromosomes" contain the amplification of oncogenes MDM-2 and CDK-4 from the 12q 13-15 chromosome region. These oncogenes are responsible for regulating the cell cycle, leading to the disordered proliferation of atypical cells.5,8,9
The diagnosis is based on image and histopathological examinations. Examinations such as computed tomography (CT) and magnetic resonance (NMR) may be performed before the biopsy. An NMR with or without contrast is usually recommended for lesions on extremities, and thorax, abdomen, and pelvic CT for patients with retroperitoneal/abdominal lesions. Atypical lipomas appear as lipomatous masses, encapsulated with internal septations, and may exhibit focal nodes. When these nodes are not lipogenic, and are larger than 1 cm, the presence of an undifferentiated area is suggested. This, therefore, will be the preferred site for a biopsy. 8.10
The pathological examination reveals the proliferation of mature adipocytes, exhibiting cell size variation, focal nuclear atypia and/or fusiform cells. The presence of lipoblasts is not essential. Finding hyperchromatic stromal cells among the fibrous septa is an important clue for diagnosis. This finding, along with immunohistochemistry (positive for markers MDM-2 and CDK-4), helps to differentiate benign lipomas. The anatomopathological examination enables one to sub-classify the WDL into four types: lipoma-like, sclerosing, inflammatory, and fusiform cells.9,10 The main histopathological and differential diagnosis for AL are: benign lipomas, fatty atrophy, reaction to silicone, diffuse lipomatosis, angiolipoma, and lipomatous hemangiopericytoma.3
The histological subtype and the location are the main prognostic factors. Survival of the WDL located on the torso and on the extremities is 95%, compared to the 70% undifferentiated with the same location. Survival of WDL in the retroperitoneum is 70%. Relapse rates are also higher for retroperitoneal tumors (91%) in comparison to those on the extremities (43%).8
Complete surgical removal of atypical lipomas is the prime treatment. If the surgical margins are impaired, the expectant treatment may be chosen, especially in cases when amplification may lead to patient morbidity. The WDL should not be subjected to radiotherapy, as they are not radiosensitive and have a very low risk of metastasis. Intermediate or high degree LPS require excision of at least 10 mm of margin. If the surgical margins are necessarily small, adjuvant, or neoadjuvant, radiotherapy may be chosen. This therapy lowers relapse rates but does not alter the global survival of the disease.8,10 Performing adjuvant/neoadjuvant chemotherapy on well-differentiated liposarcomas and on undifferentiated liposarcomas is still controversial.10
In short, this work presents a literature review and a rare case of exuberant well-differentiated liposarcoma on a young patient, in order to offer medical advice in relation to the diagnosis of this neoplasm, which is similar to benign lipomas and angiolipomas, tumors that are very frequently present in a dermatologist's routine.
Footnotes
Conflict of interest: none.
Work conducted at Universidade Federal de São Paulo (UNIFESP), São Paulo, SP, Brazil.
Financial support: none.
REFERENCES
- 1.Hoffman A, Lazar AJ, Pollock RE, Lev D. New frontiers in the treatment of liposarcoma, a therapeutically resistant malignant cohort. Drug Resist Updat. 2011;14:52–66. doi: 10.1016/j.drup.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Kim YB, Leem DH, Baek JA, Ko SO. Atypical Lipomatous Tumor/Well-Differentiated Liposarcoma of the Gingiva: A Case Report and Review of Literature. J Oral Maxillofac Surg. 2014;72:431–439. doi: 10.1016/j.joms.2013.06.222. [DOI] [PubMed] [Google Scholar]
- 3.Weiss SW, Goldblum JR. Enziger and Weiss's Soft Tissue Tumors. 5th ed. St. Louis, Mo: Mosby; 2008. [Google Scholar]
- 4.Fleury LFF Jr, Sanches JA Jr. Sarcomas cutâneos primários. An Bras Dermatol. 2006;81:207–221. [Google Scholar]
- 5.Dodd LG. Update on Liposarcoma: a Review for Cytopathologists. Diagn Cytopathol. 2012;40:1122–1131. doi: 10.1002/dc.21794. [DOI] [PubMed] [Google Scholar]
- 6.Zagars GK, Goswitz MS, Pollack A. Liposarcoma: outcome and prognostic factors following conservation surgery and radiotin therapy. Int J Radiat Oncol Biol Phys. 1996;36:311–319. doi: 10.1016/s0360-3016(96)00265-9. [DOI] [PubMed] [Google Scholar]
- 7.Katenkamp K, Katenkamp D. Soft tissue tumors: new perspectives on classification and diagnosis. Dtsch Arztebl Int. 2009;106:632–636. doi: 10.3238/arztebl.2009.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henze J, Bauer S. Liposarcomas. Hematol Oncol Clin North Am. 2013;27:939–955. doi: 10.1016/j.hoc.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Tos APD. Liposarcomas: diagnostic pitfalls and new insights. Histopathology. 2014;64:38–52. doi: 10.1111/his.12311. [DOI] [PubMed] [Google Scholar]
- 10.Crago AM, Singer S. Clinical and molecular approaches to well-differentiated and dedifferentiated liposarcoma. Curr Opin Oncol. 2011;23:373–378. doi: 10.1097/CCO.0b013e32834796e6. [DOI] [PMC free article] [PubMed] [Google Scholar]