Abstract
Falls are a serious health problem in the aging population. Because low levels of vitamin D have been associated with increased fall rates, many trials have been performed with vitamin D; two meta-analyses showed either a small effect or no effect of vitamin D on falls. We conducted a study of the effect of vitamin D on serum 25 hydroxyvitamin D (25OHD) and data on falls was collected as a secondary outcome. In a 12-month double blind randomized placebo trial, elderly women, mean age 66 years, were randomized to one of seven daily oral doses of vitamin D or placebo. The main inclusion criterion for study was a baseline serum 25OHD< 20ng/ml(50nmol/L). A history of falls was collected at baseline and fall events were collected every 3 months. Results showed that the effect of vitamin D on falls followed a U-shaped curve whether analyzed by dose or serum 25OHD levels. There was no decrease in falls on low vitamin D doses 400, 800 IU, a significant decrease on medium doses 1600, 2400,3200 IU (p=0.020) and no decrease on high doses 4000, 4800 IU compared to placebo(p=0.55). When compared to 12-month serum 25OHD quintiles, the faller rate was 60% in the lowest quintile < 25ng/ml(<50nmol/L), 21% in the low middle quintile 32–38 ng/ml (80–95nmo/L), 72% in the high middle quintile 38–46ng/ml(95–115nmo/L) and 45% in the highest quintile 46–66 ng/ml (115–165nmol/L). In the subgroup with a fall history, fall rates were 68% on low dose, 27% on medium doses and 100% on higher doses. Fall rates on high doses were increased compared to medium doses (Odds Ratio 5.6.95% CI: 2.1–14.8). In summary, the maximum decrease in falls corresponds to a 12- month serum 25OHD of 32–38 ng/ml(80–95nmol/L) and faller rates increase as serum 25OHD exceed 40–45 ng/ml (100–112.5nmol/l). The Tolerable upper limit (TUL) recently increased in 2010 from 2000 to 4000 IU/day may need to be reduced in elderly women especially in those with a fall history.
Keywords: Vitamin D supplementation, Falls, clinical trial
1. Introduction
Falls are a serious health problem of the aging population and start to increase after age 60 years old and increasing even more in the 70’s and 80’s (1). About 25–30% of elderly women fall at least once a year and in those with a recent fall history more than 50% will fall again during the next year (2). Falls have many consequences; injuries and fear of falling again restricts physical activity and social activities (3). Falls were responsible for more than 400,000 patients admitted to hospital emergency rooms in the USA, causing fractures, soft tissue injuries and open wounds in 90 percent of cases (4). In the United States in the year 2000 the costs of injurious falls were estimated at 19 billion dollars (5). Recurrent falls indicate more of a problem than single event falls because these subjects have twice the admission rate to nursing homes and twice the mortality rate (6). The most important risk factors for falling are a previous history of falls, lower extremity weakness, poor balance and gait abnormalities, decreased muscle strength, old age, cognitive impairment, medications, orthostatic hypotension, anemia, female gender, arthritis and psychological factors (7).
Prevention of falls can be effective with individualized programs such as physical therapy, vision correction, removal of home hazards, and hypnotics. In a meta-analysis, the risk of falls was reduced with preventive measures (RR=0.75, 95% CI: 0.58–0.99) (8). However, these interventions are time consuming and difficult to maintain over a long time. The idea of a medical treatment that can reduce falls has appeal and Vitamin D has been the most commonly studied option. An association between low serum 25-hydroxyvitamin D (25OHD) and lower physical performance was noted in some longitudinal studies (9–11). It is well recognized clinically that proximal myopathy and osteomalacia responds to vitamin D, but in these cases serum 25OHD levels are very low. The concept that vitamin D could improve physical performance and reduce falls has gained popularity and so far, more than 25 trials have been conducted. One of the problems in interpreting results of vitamin D on falls was the considerable variation in study design; different doses of vitamin D, type of vitamin D2 or D3, method of administration, oral or injection, frequency of administration, whether calcium was used, and length of the trial which ranged from 6 weeks to 5 years. Two meta-analyses of the effect of vitamin D on falls have been performed, one showing a significant effect of vitamin D with or without calcium with an odds ratio (OR) of 0.80 (95% CI: 0.69–0.93) (12) and the other showing a non-significant effect, (OR=0.96; 95% CI: 0.91–1.01) (13), but most of the trials included were single dose studies. In this paper, we report the incidence of falls as a pre-specified secondary outcome of a 12-month randomized clinical trial that tested 7 doses of vitamin D along with placebo.
2. Methods
2.1 Study design
Vitamin D supplementation in Older Subjects (ViDOS) was a 1-year, randomized, prospective, placebo-controlled clinical trial aimed at establishing the dose of vitamin D3 required to increase serum 25OHD levels above 30ng/ml in 97.5% of subjects and normalize serum PTH. The subjects were older Caucasian and African American women with vitamin D insufficiency. The inclusion criteria were postmenopausal women, age range of 57–90 years, and baseline serum 25OHD of 20 ng/mL or less indicating vitamin D insufficiency. Exclusion criteria included any significant conditions or medications that might affect calcium and vitamin D metabolism; more detail of exclusions are provided in the primary outcome paper (14). There were several pre-specified secondary outcomes, one of which was falls and is the subject of this paper.
2.2 Randomization and interventions
Caucasian or African American women who met the eligibility criteria were randomly assigned to one of seven vitamin D dose groups or placebo at the university clinical center. The study design was double blind randomized placebo controlled and only the statistician had access to the treatment code. The randomization method was randomized blocks, stratified by screening serum 25OHD level < 15 vs 15 ng/mL or greater for Caucasians and < 12 vs 12 ng/mL or greater for African American women. Screening occurred throughout the late winter and early spring months from January 2008 to January 2010 in a city that is at a latitude of 41 degrees. Vitamin D3 400-, 800-, 1600-, 2400-, 3200-, 4000-, and 4800-IU and matching placebo capsules were custom manufactured for the study (Douglas Labs, Pittsburgh, PA). The actual vitamin D3 concentrations in the capsules were measured independently every 6 months over 3 years and there was no significant change in potency over the time (14).
A pre-study 7-day dietary record was used to estimate dietary intake of calcium and vitamin D. Subjects were asked to maintain a total calcium intake between 1200–1400 mg/d and if they were not able to increase dietary calcium to those levels, they were given calcium citrate as a supplement (Citracal 200mg elemental; Bayer Health Care). Subjects were not allowed to take other vitamin D supplements during study, those who wanted to take multivitamins were provided multivitamins without vitamin D (Kirkman multivitamin without vitamins A and D). A central medication log was maintained of all study drugs dispensed to the subjects. At the 3, 6, 9, and 12-month visits, compliance was calculated by counting the pills returned and dispensing new study pills. Questionnaires were used for smoking history, alcohol use, caffeine intake, sun exposure, physical activity, and previous fall and fracture history. Fasting blood samples were collected at all visits. Questionnaires including fall and fracture history were collected every 3 months by an interviewer.
2.3 Laboratory analysis
Serum 25OHD was measured by RIA after an acetonitrile extraction in our laboratory using Diasorin kits. The minimum detection range is 5 ng/Mr. The inter assay variation is 9.8% and in our laboratory, the intra assay variation is 9.2%. The Bone Metabolism Laboratory participates in the vitamin D External Quality Assessment Scheme (DEQAS) and our results were within one standard deviation (SD) of the all-laboratory trimmed mean. The National Institute of Standards and Technology standards (NIST) were also used in the assays (14). The interassay variations for NIST standards were as follows: level 1; 8.4%; level 2; 9%; level 3; 7.7%; and level 4; 10.5%. Serum 25OHD3 and 25OHD2 were measured in triplicate by Liquid Chromatography Mass spectrophotometry (LCMS) in the laboratory of Glenville Jones. Serum intact PTH was measured by the Diasorin immunoradiometric assay. The limit for serum PTH detection range in our laboratory is 1.0 pg/Mr. The inter assay variation is 4.1% and the intra assay variation is 2.9%.
2.4 Outcomes
Primary outcomes of this study were serum 25OHD and serum PTH levels at 12 months. Serum 25OHD and serum PTH were measured at baseline and 6 and 12 months. The dose-response effect of vitamin D3 400, 800, 1600, 2400, 3200, 4000, and 4800 IU/d plus calcium was compared with a placebo plus calcium control group and these results were published in detail earlier (14). Secondary outcomes. Falls was one of the pre-specified secondary outcomes. A fall was defined as a sudden unintentional change in position causing an individual to land on the ground, floor or at a lower level, with or without injury. Excluded are falls where the subject came to rest on a chair or bed. A faller was defined as any subject that had one or more falls during the 12-month study. The faller rate is the proportion of subjects who had one or more falls during the 12-month study out of those who completed the study. Details on the number of falls and whether a fall resulted in a break or fracture were collected by an interviewer every 3 months. Prior to study entry a history of falls and fractures were collected regarding the previous 12- months.
2.5 Statistical analysis
Subject characteristics at baseline are compared by faller status at 12 months (faller vs. non-faller) using t-tests for continuous variables and chi-square or Fisher’s exact test for categorical variables. Chi-square test were used to test the association between faller status and vitamin D dose, serum 25OHD quintiles, serum PTH quintiles, both at 12 months. Logistic regression was used to look at faller rate by vitamin D dose, race and vitamin D dose*race interaction. The race*dose interaction was not significant (p=0.18), but there is little power for this test. Since their faller rate is very different by race, we stratified the analyses by race. Logistic regression evaluated the quadratic response of falls by serum 25OHD quintiles. Multivariate logistic regression examined whether vitamin D dose was associated with faller status at 12 months, adjusting for age, BMI, alcohol use, smoking status, and history of falls. An interaction between vitamin D dose and history of falls was explored. Multivariate Poisson regression was used to assess the relationship between number of falls and vitamin D dose, history of falls, while adjusting for covariates. If the overall tests were statistically significant pairwise comparisons were conducted, with adjustment made for multiple comparisons using the simulation approach of Westfall et al (15). P-values less than 0.05 are statistically significant. SAS software was used for statistical analysis (SAS Institute Inc., Cary, NC).
3. Results
One hundred sixty-three Caucasian and 110 African American women were randomized to placebo or one of 7 daily vitamin D dose groups. Sixteen of 163 Caucasian women and 19 of 110 African American women withdrew from the study. The incidence of fallers in the Caucasian women was 49% and in African American women was 22% at 12 months.
Compliance of the study drug was very high and over 12 months averaged 94% for vitamin D and 91% for calcium. The mean total daily calcium intake at baseline was 69 2mg and 1272 mg at 12 months, the mean calcium supplement was 580 mg. The effect of the vitamin D doses on serum 25OHD showed a quadratic curve with a flattening of the curve between the dose 3200–4000 IU daily at a serum level of ~ 45 ng/ml (14), there was no significant difference in the dose response curves between those with or without a pre-study fall history.
Among the Caucasian subjects, 147 completed the study, 71 (49%) were classified as fallers and 75 (51%) were classified as non-fallers during the 12 months (1-no fall data available). Among the 71 fallers, there were 96 total falls recorded during the 12-month study; 32.2% subjects had 1 fall, 15.8% had 2 falls and 0.7% had 3 or more falls. There were 91 African American women who completed the study, 20 (22%) were fallers and 71 (78%) were non-fallers during the 12 months. Among the 20 African American fallers, there were 27 total falls recorded; 15 (16%) subjects had 1 fall, 3 (3%) had 2 falls and 2 (2%) had 3 falls. Those who completed the study were similar to those who withdrew from the study in terms of baseline characteristics (data not shown). Among Caucasian women, age, BMI, smoking status, alcohol use, dietary calcium/vitamin D intake, diuretic use, baseline serum 25OHD, 1,25(OH)2D and PTH were not associated with faller status at 12 months (Table 1). There was no difference in total calcium intake at 12 months between the groups; 1266mg in fallers and 1231mg in non-fallers (p=0.17). A history of a falls in the 12-month before the study occurred in 32% of the 147 women who completed the study; 39% of women who were fallers at 12 months had a history of falls compared to 25% who were non-fallers at 12 months (p=0.068). There was a marginal association between faller status and the Short Physical Performance Battery (SPPB) at baseline, fallers tended to have higher (better) SPPB scores on average compared to non-fallers (p=0.070). African American women showed differences by faller status in baseline serum 25OHD, serum PTH, and fall history. African American fallers at 12 months had lower levels of serum 25OHD, higher levels of serum PTH at baseline and had a higher proportion of history of falls (50% vs. 18%, p=0.004) compared to non-fallers. All other baseline variables were similar between fallers and non-fallers in African American women. Vitamin D intake is unavailable and serum 1,25(OH)2D has limited availability for African American women at baseline.
Table 1.
Baseline characteristics compared between fallers and non-fallers
| Caucasian women | African American women | |||
|---|---|---|---|---|
|
| ||||
| Non-Faller (n=75) Mean (SD) |
Faller (n=71) Mean (SD) |
Non-Faller (n=71) Mean (SD) |
Faller (n=20) Mean (SD) |
|
|
| ||||
| Age, years | 65.9 (6.4) | 66.5 (8.2) | 67.0 (7.8) | 65.6 (7.7) |
|
| ||||
| Weight, kg | 77.9 (14.2) | 80.4 (17.9) | 84.6 (19.4) | 84.8 (15.9) |
|
| ||||
| BMI, kg/m2 | 30.1 (5.7) | 30.6 (6.2) | 32.3 (7.6) | 32.4 (5.9) |
|
| ||||
| Smoking status, n (%) | ||||
| Current | 9 (12%) | 6 (8%) | 13 (18%) | 2 (10%) |
| Former | 25 (33%) | 28 (39%) | 31 (44%) | 5 (25%) |
| Never | 41 (55%) | 37 (52%) | 27 (38%) | 13 (65%) |
|
| ||||
| Alcohol use, n (%) | 41 (55%) | 42 (59%) | 38 (54%) | 8 (40%) |
|
| ||||
| Serum creatinine, mg/dl | 0.78 (0.13) | 0.77 (0.12) | 0.87 (0.21) | 0.96 (0.23) |
|
| ||||
| Serum calcium, mg/dl | 9.5 (0.3) | 9.4 (0.4) | 9.4 (0.3) | 9.5 (0.4) |
|
| ||||
| Serum 25-(OH)D, ng/ml | 15.5 (3.9) | 15.2 (3.6) | 14.0 (4.1) | 11.5 (4.4) * |
|
| ||||
| Serum 1,25(OH)2D, pg/ml | 43.5 (14.6) | 43.1 (14.9) | 43.1 (10.4) | 41.3 (16.4) |
|
| ||||
| Serum PTH, pg/ml | 35.5 (12.5) | 38.0 (14.9) | 40.1 (16.6) | 52.7 (20.0) * |
|
| ||||
| Calcium intake^, mg/day | 710 (216) | 677 (241) | 555 (224) | 546 (206) |
|
| ||||
| Vitamin D intake^, IU/day | 116 (71) | 123 (83) | - | - |
|
| ||||
| History of Falls, n (%) | 19 (25%) | 28 (39%) | 13 (18%) | 10 (50%)* |
|
| ||||
| Medication use, n (%) | ||||
| Thiazide diuretic | 19 (25%) | 16 (23%) | 29 (41%) | 7 (35%) |
| Loop diuretic | 6 (8%) | 3 (4%) | 3 (4%) | 3 (15%) |
|
| ||||
| Anti-depressants | 7 (9%) | 10 (14%) | 4 (6%) | 2 (10%) |
|
| ||||
| GDS | 4.1 (4.5) | 3.2 (3.3) | 2.9 (3.6) | 3.8 (4.4) |
|
| ||||
| SPPB | 9.9 (1.3) | 10.3 (1.2) | 9.5 (1.2) | 9.9 (1.1) |
25-(OH) D = 25-hydroxyvitamin D, BMI = body mass index, PTH = parathyroid hormone, GDS=Geriatric Depression Score, SPPB=Short Physical Performance Battery, Vitamin D intake baseline is unavailable and Serum 1,25(OH)2D available for only 28 African American women.
Derived from 7-day food diary.
p-value<0.05 comparing non-fallers and fallers within race.
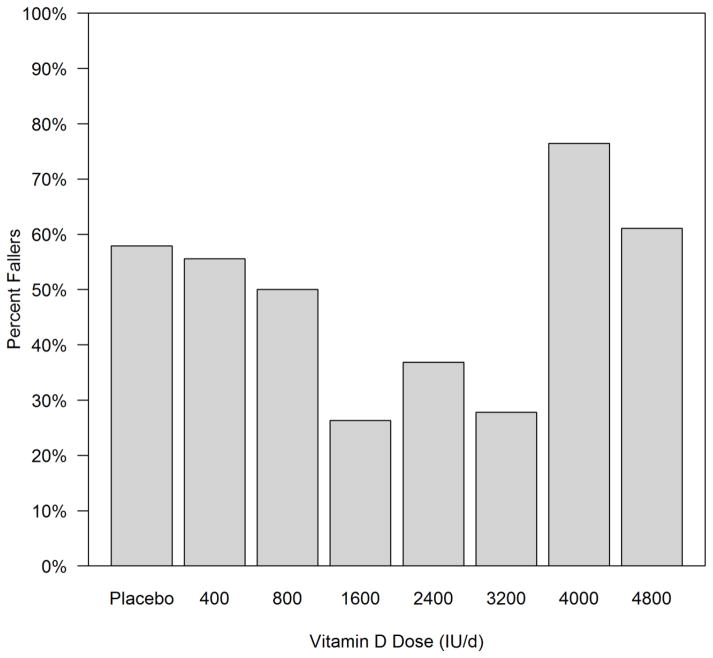
Of the 146 Caucasian women with fall status at 12 months, n=19 subjects were in the placebo group, n=18, 18, 19, 19, 18, 17, and 18 were in 400, 800, 1600, 2400, 3200, 4000, and 4800 IU/day respectively. The faller rate (proportion of subjects with one or more falls by 12 months) was analyzed in all 8 groups and showed the lowest incidence of fallers on the doses 1600–3200 IU daily compared to all other doses and placebo (figure 1).
Figure 1.
Faller rate amongst the different Vitamin D dose groups and placebo in Caucasian women. N=19, 18, 18, 19, 19, 18, 17, and 18 subject were in placebo, 400, 800, 1600, 2400, 3200, 4000, and 4800 IU/day dose groups respectively.
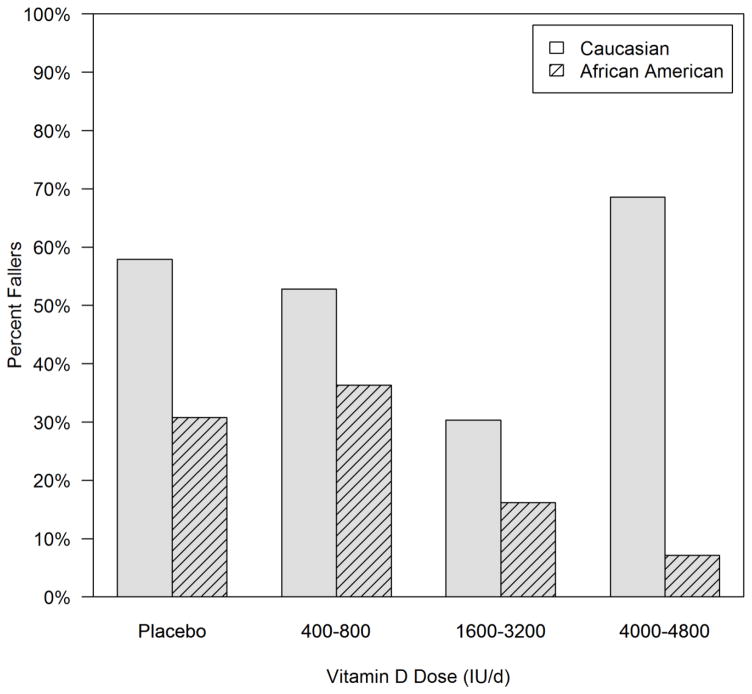
To increase the sample size in each group for both Caucasian and African American women, we combined the 7 vitamin D groups into 3 groups; a low dose group consisting of 400 and 800 IU/d, a medium dose group (1600, 2400, 3200IU/d), a high dose group (4000 and 4800 IU/d), and the placebo group. The faller rates were 58% on placebo, 53% on low dose, 30% on medium dose, and 69% on high dose. There is a significant association between dose and faller rate in the Caucasian women (p=0.0030). The medium dose group has a significantly lower faller rate than all the other groups (p<0.05 for all), but none of the other groups differ significantly from each other (p>0.05). In contrast, the African American women see a decreasing trend in faller rate with dose group, though the difference is not significant (figure 2).
Figure 2.
Faller rate according to Vitamin D dose groups placebo, low, medium and high in Caucasian and African American women. Caucasian women N=19, 36, 56, and 35 subject were in placebo, low, medium, and high dose groups respectively; African Americans: N=13, 22, 42, and 14 respectively. Faller rate was significantly associated with dose group in Caucasian (p=0.003) but not African American women (p=0.14)
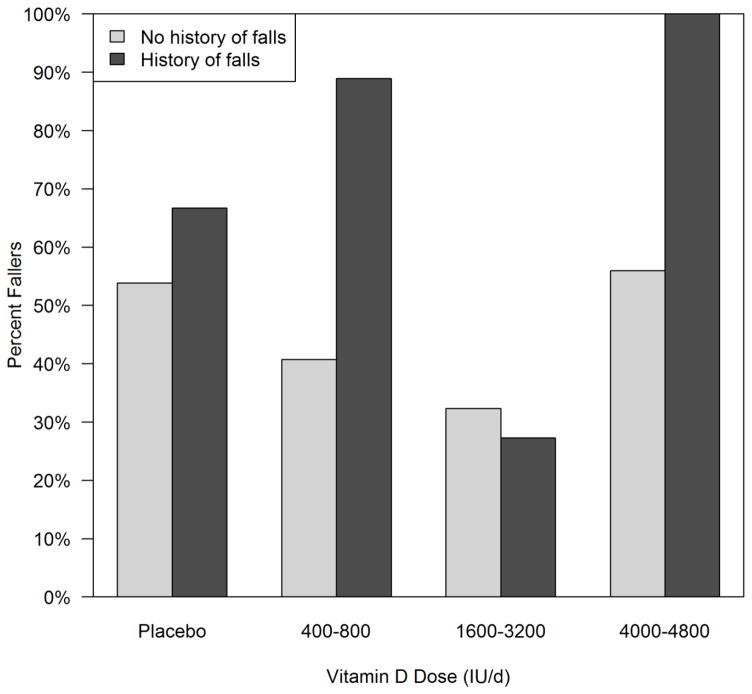
The remainder of the paper is focused on the Caucasian women due to sample size limitations in the African American subjects. Faller rates are compared by dose group in those with a history of falls pre-study (n=47). Faller rates were higher in those with a history of fall versus no fall, except in the medium dose group. Placebo group 67% versus 54%, low dose group 88% versus 41%, medium dose group 27% versus 32% and high dose group 100% versus 56% respectively. There is a significant association between faller rate and dose group among those with a history of falls, with those in the medium dose group having a much lower rate of falls than the other groups, p< 0.001(figure 3). In women without a history of falls, the association between dose and fallers at 12 months is not significant (p=0.27).
Figure 3.
Faller rates according to vitamin D dose groups placebo, low, medium and high subdivided into those with or without a previous fall history in Caucasian women. In those without a history of falls, there are n=13, 27, 34, and 25 in the placebo, low, medium, and high dose groups respectively. In those with a history of falls, there are n=6, 9, 32, and n=10 in the placebo, low, medium, and high dose groups respectively.
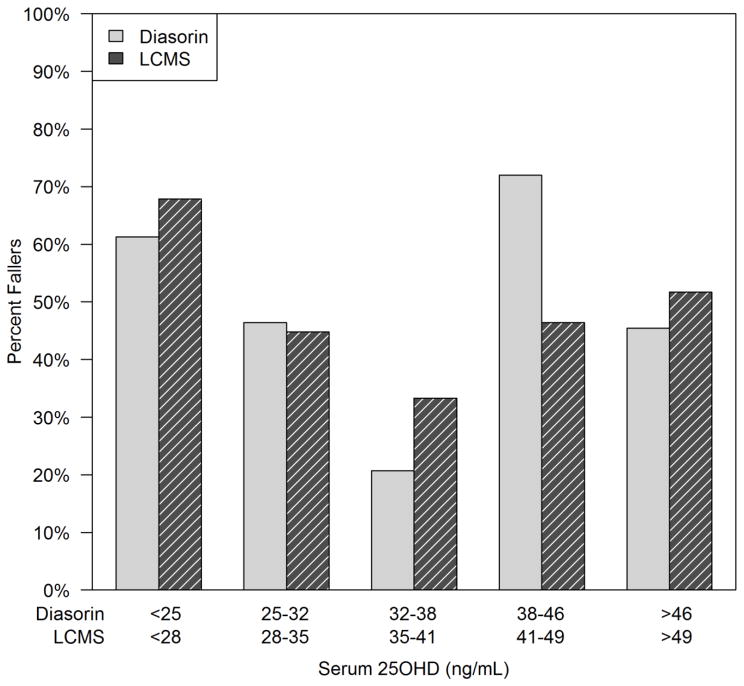
Faller rates were compared also according to the serum 25OHD reached at 12 month, divided into quintiles. Serum 25OHD was measured by two different assays and quintiles were computed by each method. From the Diasorin assay, the lowest faller rate of 21% was in the 25OHD quintile 32–37.9 ng/ml and the highest faller rate of 72% was in the quintile range 38–45.9 ng/ml; the faller rate in the highest quintile range 46–66 ng/ml was 45%. The association between faller rate and serum 25OHD quintile at 12 months was statistically significant by chi-square test (p=0.0022) and there is a significant quadratic effect with quintile as well (p=0.033). By the LCMS assay there is an even stronger quadratic trend observed in faller rate (p=0.005) with faller rates of 68%, 45%, 33%, 46%, and 52% in quintiles 1–5 respectively (figure 4). When looking at serum PTH at 12 months divided into quintiles, there was no difference by fallers status (p=0.43), and no serum PTH association with faller status by history of falls subgroups (data not shown).
Figure 4.
Faller rates by serum 25OHD quintiles measured by Diasorin and LCMS assay. Serum 25OHD quintiles measured by Diasorin and LCMS assays showed a significant quadratic trend in faller rate (p=0.033 and p=0.005 respectively).
Table 2 shows the results of a multivariate logistic regression of faller status at 12 months by dose category (placebo, low, medium, high), adjusting for age, BMI, alcohol use, smoking status and history of falls in the 12 months prior to the study. There was no interaction between history of falls and dose category, though a trend toward an interaction was observed (p=0.11). A subject with a history of falls was 2.6 time more likely to be a faller at 12 months than those without history of falls (p=0.018). Dose category was a significant predictor of being a faller after adjusting for covariates (p=0.0024). A subject in the 4000–4800 dose category was 5.6 (95% CI: 2.14–14.85) times more likely to be a faller at 12 months than a subject in the 1600–3200 dose category (adjusted padj=0.0027). Subjects in the 1600–3200 group were less likely to have fallen by 12 months than those in the placebo group and the 400–800 group as well, but after adjusting for multiple comparisons, the result is marginally significant (padj=0.063 and padj=0.062 respectively). No differences were observed between the other groups (placebo vs. low or placebo vs. high).
Table 2.
Multivariate logistic regression model of faller status at 12 months.
| OR | 95% CI | p-value | ||
|---|---|---|---|---|
| Age | 1 year increase | 1.02 | 0.97–1.07 | 0.40 |
| BMI | 1 unit increase | 1.01 | 0.95–1.08 | 0.75 |
| Smoking status | Currently vs. Never | 0.95 | 0.27–3.34 | 0.87 |
| Formerly vs. Never | 1.21 | 0.56–2.61 | ||
| Alcohol use | Yes vs. No | 1.25 | 0.59–2.67 | 0.56 |
| History of Falls | Yes vs. No | 2.61 | 1.18–5.74 | 0.018 |
| Vitamin D Dose groups | Placebo vs. 1600–3200 | 3.86 | 1.24–12.04 | 0.0024 |
| 400–800 vs. 1600–3200 | 3.15 | 1.24–7.99 | ||
| 4000–4800 vs. 1600–3200 | 5.63 | 2.14–14.85 |
BMI = body mass index, OR = odds ratio, CI = confidence interval.
Multivariate Poisson regression was used to look at the total number of falls during the 12-month study by dose group and history of falls, while adjusting for age, BMI, smoking status, and alcohol use (Table 3). The interaction between history of falls and dose group is not significant (p=0.27). History of falls was marginally predictive of the total number of falls with 0.9 falls on average per person in the history of falls group and 0.6 in the no history falls group (p=0.054). Dose was significantly predictive of the total number of falls after adjusting for covariates (p=0.030). In pairwise comparisons, the 1600–3200 group had fewer falls than all the other groups, but after adjusting for multiple comparisons, the results are marginally significant (vs. placebo padj=0.058, vs. 400–800 padj=0.058, vs. 4000–4800 padj=0.094). None of the other dose groups were different from each other in terms of number of falls.
Table 3.
Multivariate Poisson regression of the total number of falls
| Mean falls | SE | p-value | ||
|---|---|---|---|---|
| History of falls | No | 0.58 | 0.10 | 0.054 |
| Yes | 0.89 | 0.16 | ||
| Dose group | Placebo | 0.94 | 0.23 | 0.030 |
| Vitamin D | 400–800 | 0.85 | 0.19 | |
| 1600–3200 | 0.41 | 0.09 | ||
| 4000–4800 | 0.79 | 0.19 |
Model adjusted for age, body mass index, smoking status and alcohol use
4. Discussion
The results of this 12-month vitamin D intervention study show a U- shaped response curve in the incidence of Fallers with no effect on low doses of vitamin D 400–800 IU daily, a significant reduction in the faller rate on medium doses 1600, 2400 and 3200 IU daily and then an increase in the faller rate on the higher doses 4000 and 4800 IU daily compared to the medium group. The fall rate at the higher doses is not significantly different from placebo. Faller rates were much higher in those with a pre-study history of falls and 100 percent of this group fell in the two high dose groups. Because the variance in the serum 25OHD response to vitamin D doses is large (14) it is better to compare faller rates to the final 12-month serum 25OHD level When final serum 25OHD levels are divided into quintiles (both by immunoassay and LCMS) the decrease in faller rate to was lowest in the serum 25OHD middle quintile 32–41 ng/ml. As serum 25OHD levels increase above 40 ng/ml there is a subsequent increase in Faller rate. There were no interactions of falls with serum PTH, calcium or vitamin D intake.
The effect of vitamin D on falls has been controversial. There have been 30 studies most of them summarized in two meta analyses, one meta-analysis showed a positive effect of vitamin D with or without calcium compared to controls in reducing falls, OR 0.86 (95% CI: 0.77–0.96) (12). In a later meta-analysis discrepancies in results were analyzed further and showed that selection of subjects and the use of raw data or imputed data could cause issues in interpretation (13). When the same selection criteria for data and subjects was combined in this meta-analyses there was a marginal benefit in favor of vitamin D. The overall relative risk was 0.95 (95% CI: 0.90–1.00), but most of the effect came from the subgroup of vitamin D + calcium versus the calcium control group (RR 0.84; 95% CI: 0.76–0.92). When comparing vitamin D to controls the RR was 1.00 (95% CI: 0.92–1.08) and comparing vitamin D + calcium to controls the RR was 0.95 (95% CI: 0.88–1.02). It may not be valid to use calcium supplements as a control group since calcium could have an adverse effect on falls by reducing levels of serum PTH and 1,25 dihydroxyvitamin D. If vitamin D were to be effective, it should be through conversion to the active hormone 1,25 dihydroxyvitamin D. In an analysis of calcium supplement cohort trials there was an estimated 64% increase in hip fractures suggesting that calcium supplementation may have an adverse effect on falls since nearly all hip fractures are due to falls (16).
Earlier evidence showed that bolus administration of vitamin D caused increased fall and fracture rates. A study from Australia of 2256 women randomized to an annual oral dose of vitamin D3 500,000 IU or placebo showed an increase in falls and fractures that peaked at about 3 months after the dose and this pattern was repeated in the second year (RR=1.31; 95% CI: 1.12–1.54). A subset that had testing of serum 25OHD showed a level of 60 ng/ml one month after dosing and levels averaging~ 30 ng/ml at 12 months just before the next annual dose (17). Another study showed that 100,000 IU given monthly to elderly residents increased falls (RR 2.33;1.49–3.63) when compared to a group given routine care of 400–1,000 IU daily) (18)
In a recent study where vitamin D was given as a monthly dose there was an increased incidence of fallers when analyzed by serum 25OHD levels (19). In this study, men and women average age 78 years, were given a monthly bolus of 24,000 and 60,000 IU equivalent to daily doses of 800 and 2,000 IU daily. The mean serum 25OHD levels at the end of the month just before the next dose were 31ng/ml on the 24,000 IU dose, ~39ng/ml on 60,000IU and ~44 ng/ml on the combination of 60,000 IU + calcifediol 300 mcg (synthetic 25 hydroxyvitamin D). Serum 25OHD levels in the highest quartile group > 45ng/ml were associated with an increase in faller rates and faller rates that were 5.5 times higher compared to the lower quartile of 21–30ng/ml. These results are similar to our results in that the highest fall rate was associated with the highest quartile of serum 25OHD. Thus, the association of increased falls with higher serum 25OHD levels greater than 40–45 ng/ml in three separate studies is a new observation and represents an adverse response to vitamin D. Although the 60,000 IU monthly bolus is equivalent to 2,000 IU daily the results on the bolus doses show an increase in falls (67%) whereas in our study on a daily dose of ~ 2000 IU the faller rate was much lower (30%) suggesting that bolus doses are harmful probably because they produce higher sustained levels of serum 25OHD and possibly serum1,25 dihydroxyvitamin D. Since there is no change in physical performance on high doses (19) it is suggested that the effect represents a form of toxic encephalopathy.
The American Geriatrics Society recommended 4,000 IU daily for Fall prevention especially in those with a history of falls, yet in our study 100 percent of women with a history of falls fell in the 4,000/4800 IU doses, so in the light of new data this recommendation may need to be reconsidered (20).
There are limitations to our study; fall history was collected by study coordinator at 3 month intervals and may underestimate the number of falls. In an older group of women and men with a mean age 78 years a quarterly telephone call compared to a daily log was 100 percent accurate in non-fallers, but in fallers the quarterly telephone call underestimated falls by 25% compared to the daily calendar. In this study the Mini Mental score was in the range 18–23 in 19% and between 20–29 in 40% thus there was significant cognitive impairment in the group and memory would be a problem (21). The women in our study were 10 years younger and likely had better recall. In the high dose group the fall rate was 100% in those with a previous fall history thus cannot be underestimated. Another limitation was that fallers rates were a secondary outcome and power calculations were not based on the secondary outcome. The sample sizes in each group were relatively small, especially in those women with a history of falls. In order to increase power, the dose groups were combined into placebo, low, medium and high, causing some imbalance in sample sizes. A strength however was the number of vitamin D dose groups and the ability to stratify faller data among serum 25OHD quintiles extending from <25–68 ng/ml showing both the lowest and highest faller rates. These results should be regarded as preliminary and need to be confirmed in a larger study.
The Tolerable Upper limit (TUL) for vitamin D was recently increased to 4,000 IU daily from 2,000 IU daily by the Institutes of Medicine (22) and the Endocrine Society guidelines recommended a TUL of 10,000 IU daily (23). Much of the reasoning was based on the lack of occurrence of hypercalcemia on high doses of vitamin D. The Institutes of Medicine was more conservative than the Endocrine society because of the association of an increase in all-cause mortality related to serum 25OHD levels greater than 40–50ng/ml (22). The results from these 3 faller studies suggest that higher serum 25OHD levels are associated with higher fall and fracture rates and the TUL may need to be reduced below 4,000 IU daily in older people.
Highlights.
One year double blind trial, 7 daily oral doses of vitamin D or placebo, on incidence Fallers
Faller rate was a U shaped curve, maximum decrease on doses 1600–3200 IU or serum 25OHD of 32–38 ng/ml
High vitamin D dose 4,000–4800 IU increases Faller incidence in those with previous Fall history
Acknowledgments
Supported by the National Institute on Aging (RO1-AG28168) and the Office of Dietary Supplements, and LM Smith was supported by Great Plains IDEA-CTR Network (1U54GM115458-01). Presented at Endocrine Society 2015 by Shervin Yousefian MD. Thanks to Dr. Glenville Jones and Martin Kaufmann, PhD for LCMS measurements.
Footnotes
Conflict of Interest: The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. WHO global report on falls prevention in older age. 2007 [Google Scholar]
- 2.Close J, Ellis M, Hooper R, Glickman E, Jackson S, Swift C. Prevention of falls in the elderly trial (PROFET): a randomized controlled trial. Lancet. 1999 Jan 9;353(9147):93–7. doi: 10.1016/S0140-6736(98)06119-4. [DOI] [PubMed] [Google Scholar]
- 3.Tinetti ME, Williams CS. The effect of falls and fall injuries on functioning in community-dwelling older persons. J Gerontol A Biol Sci Med Sci. 1998 Mar;53(2):M112–9. doi: 10.1093/gerona/53a.2.m112. [DOI] [PubMed] [Google Scholar]
- 4.Owens PL, Russo CA, Spector W, Mutter R. Healthcare Cost and Utilization Project (HCUP)Statistical Briefs. Rockville (MD): Agency for Health Care Policy and Research (US); 2009. Emergency Department Visits for Injurious Falls among the Elderly, 2006: Statistical Brief #80. [PubMed] [Google Scholar]
- 5.Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. Injury Prevention. 2006;12:290–295. doi: 10.1136/ip.2005.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donald IP, Bulpitt CJ. The prognosis of falls in elderly people living at home. Age Ageing. 1999;28:121–125. doi: 10.1093/ageing/28.2.121. [DOI] [PubMed] [Google Scholar]
- 7.Gillespie LD, Robertson MC, Gillespie WJ, Lamb SE, Gates S, Cumming RG, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009 Apr 15;(2):CD007146. doi: 10.1002/14651858.CD007146.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Interventions to Prevent Falls in Older Adults: An Updated Systematic Review, AHRQ Publication No. 11-05150-EF-1, December 2010.
- 9.Flicker L, Mead K, MacInnis RJ, et al. Serum vitamin D and falls in older women in residential care in Australia. J Am Geriatr Soc. 2003;51(11):1533–1538. doi: 10.1046/j.1532-5415.2003.51510.x. [DOI] [PubMed] [Google Scholar]
- 10.Snijder MB, van Schoor NM, Pluijm SM, van Dam RM, Visser M, Lips P. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J Clin Endocrinol Metab. 2006 Aug;91(8):2980–5. doi: 10.1210/jc.2006-0510. [DOI] [PubMed] [Google Scholar]
- 11.Bearne LM, Moniz C, Hurley MV, Jackson SH, Swift CG, Allain TJ. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res. 2002 May;17(5):891–7. doi: 10.1359/jbmr.2002.17.5.891. [DOI] [PubMed] [Google Scholar]
- 12.Murad MH, Elamin KB, Abu Elnour NO, Elamin MB, Alkatib AA, Fatourechi MM, Almandoz JP, Mullan RJ, Lane MA, Liu H, Erwin PJ, Hensrud DD, Montori VM. Clinical review: The effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011 Oct;96(10):2997–3006. doi: 10.1210/jc.2011-1193. [DOI] [PubMed] [Google Scholar]
- 13.Bolland MJ, Grey A, Reid IR. Differences in overlapping meta-analyses of vitamin D supplements and falls. J Clin Endocrinol Metab. 2014 Nov;99(11):4265–72. doi: 10.1210/jc.2014-2562. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher JC, Sai A, Templin T, 2nd, Smith L. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med. 2012 Mar 20;156(6):425–37. doi: 10.7326/0003-4819-156-6-201203200-00005. [DOI] [PubMed] [Google Scholar]
- 15.Westfall PH, Tobias RD, Rom D, Wolfinger RD, Hochberg Y. Multiple Comparisons and Multiple Tests Using the SAS System. Cary, NC: SAS Institute Inc; 1999. [Google Scholar]
- 16.Bischoff-Ferrari HA1, Dawson-Hughes B, Baron JA, Burckhardt P, Li R, Spiegelman D, Speck B, Orav JE, Wong JB, Staehelin HB, O’Reilly E, Kiel DP, Willett WC. Calcium intake and hip fracture risk in men and women: a meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr. 2007 Dec;86(6):1780–90. doi: 10.1093/ajcn/86.5.1780. [DOI] [PubMed] [Google Scholar]
- 17.Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010 May 12;303(18):1815–22. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 18.Ginde AA, Blatchford P, Breese K, Zarrabi L, Linnebur SA, Wallace JI, Schwartz RS. High-Dose Monthly Vitamin D for Prevention of Acute Respiratory Infection in Older Long-Term Care Residents: A Randomized Clinical Trial. J Am Geriatr Soc. 2016 Nov 16; doi: 10.1111/jgs.14679. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Stähelin HB, Meyer O, Theiler R, Dick W, Willett WC, Egli A. Effect of monthly high dose vitamin D for the prevention of functional decline. A double-blind randomized controlled trial. JAMA Intern Med. 2016;176:175–183. doi: 10.1001/jamainternmed.2015.7148. [DOI] [PubMed] [Google Scholar]
- 20.Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for Prevention of Falls and Their Consequences. J Am Geriatr Soc. 2014;62:147–52. doi: 10.1111/jgs.12631. [DOI] [PubMed] [Google Scholar]
- 21.Hannan MT, Gagnon MM, Aneja J, Jones RN, Cupples LA, Lipsitz LA, Samelson EJ, Leveille SG, Kiel DP. Optimizing the tracking of falls in studies of older participants: comparison of quarterly telephone recall with monthly falls calendars in the MOBILIZE Boston Study. Am J Epidemiol. 2010 May 1;171(9):1031–6. doi: 10.1093/aje/kwq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.IOM (Institute of Medicine) Dietary reference intakes for calcium and vitamin. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 23.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011 Jul;96(7):1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]