Abstract
Production of IFN-γ contributes to host defense against Mycobacterium tuberculosis (Mtb) infection. We previously demonstrated that Signaling lymphocytic activation molecule-associated protein (SAP) expression on cells from tuberculosis (TB) patients was inversely correlated with IFN-γ production. Here we first investigated the role of NK, T and B cell antigen (NTB-A)/SAP pathway in the regulation of Th1 response against Mtb. Upon antigen stimulation, NTB-A phosphorylation rapidly increases and afterwards modulates IFN-γ and IL-17 secretion. To sustain a healthy immune system, controlled expansion and contraction of lymphocytes, both during and after an adaptive immune response, is essential. Besides, restimulation-induced cell death (RICD) results in an essential homeostatic mechanism for precluding excess T-cell accumulation and associated immunopathology during the course of certain infections. Accordingly, we found that the NTB-A/SAP pathway was required for RICD during active tuberculosis. In low responder (LR) TB patients, impaired RICD was associated with diminished FASL levels, IL-2 production and CD25high expression after cell-restimulation. Interestingly, we next observed that SAP mediated the recruitment of the Src-related kinase FYNT, only in T cells from LR TB patients that were resistant to RICD. Together, we showed that the NTB-A/SAP pathway regulates T cell activation and RICD during human TB. Moreover, the NTB-A/SAP/FYNT axis promotes polarization to an unfavorable Th2-phenotype.
Introduction
Tuberculosis (TB) now ranks alongside HIV as the leading cause of death from an infectious disease. In fact, Mycobacterium tuberculosis (Mtb) causes nearly 10 million of new cases and 1.5 million deaths per year 1. Protective immunity against Mtb requires the generation of Th1 immune responses. Production of IFN-γ by CD4+ and CD8+ T cells is especially critical in order to fight the bacteria 2. Actually, reduced IFN-γ production by peripheral blood mononuclear cells (PBMCs) of TB patients is a marker of severe disease 3. Thus, cell-mediated immunity displays a key role in protection against TB 4.
An effective cell-mediated immunity is dependent on the strength of T cell stimulation signal, which regulates lymphocyte progression through activation, proliferation and death 5, 6. Furthermore, the cellular environment where the antigen is recognized, the length of T cell/antigen-presenting cell interaction and the immunoreceptors signaling modulate the levels and the pattern of cytokines produced by antigen-stimulated T cells. The signaling lymphocytic activation molecule (SLAM) family was found to modulate immune cells due to the capacity of these receptors to interact with SLAM associated protein (SAP)-related molecules, a group of SRC homology 2 domain adaptors 7. SAP adaptors couple SLAM family receptors during the immunological synapse to activate biochemical signals that promote T cells to produce a specific pattern of cytokines. The SH2D1A gene that encodes SAP is mutated or deleted in X-linked lymphoproliferative disease (XLP), a rare genetic disorder characterized by a fatal response to Epstein-Barr virus infection, hypogammaglobulinemia and malignant lymphomas. SAP deficiency leads to reduced CD4+ Th2 responses, deficient IgE production and increased Th1 cytokine secretion 8. Previously, we demonstrated that in the absence of SAP, SLAM contributed to induce Th1 cytokine responses during tuberculosis infection 9. Moreover, SAP expression interfered with Mtb-induced IFN-γ production, and the levels of SAP directly correlated with the severity of the disease 9.
Most members of SLAM receptors, including NK, T and B cell antigen (NTB-A or SLAMF6), are homophilic (self-ligands) receptors 7. In particular, NTB-A ligation triggers phosphorylation of its immunoreceptor tyrosine-based switch motifs in the cytoplasmic tail, regions that function as docking sites for SAP. In mice, NTB-A acts as a positive regulator of Th1 lymphocyte functions increasing CD4+ T cell proliferation and IFN-γ production, whereas NTB-A blockage delays the beginning of experimental autoimmune encephalomyelitis 10. Therefore, NTB-A increases or amplifies TCR signal, acting as a positive regulator of Th1 lymphocyte functions 10. In NK cells, NTB-A association with SAP induces cellular cytotoxicity and increases IFN-γ and TNF-α production 11. The study of NTB-A in NK cells from XLP patients demonstrated that the absence of functional SAP protein, decreased or abrogated cell cytotoxicity while IFN-γ secretion is not modified 11. Instead, the association of NTB-A with SAP in T cells induces tolerance and cellular homeostasis 12.
In order to sustain a healthy immune system, controlled expansion and contraction of lymphocytes, during and after an adaptive immune response, are critical 13. In the presence of high levels of antigen and IL-2, TCR restimulation of activated cycling T cells can lead to a self-regulatory process of apoptosis known as restimulation-induced cell death (RICD), an important physiologic mechanism mediated by death receptors expressed in T cells. RICD is induced when T cells previously activated under a particular cytokine microenvironment are restimulated through TCR signaling, upon which they undergo apoptosis 14. Moreover, RICD represents a negative feedback mechanism induced by restimulation of T cells to prevent an excessive expansion of T lymphocytes 14. This regulatory pathway of the T cell pool initiated by TCR signaling depends on cell death mechanisms mediated through FAS and BIM 15. Thus, RICD limits immunopathological and autoimmune manifestations that could emerge as a consequence of an extensive expansion of antigen-specific T cells 16, 17.
In addition to antigen-restimulation, triggering of RICD requires reaching a particular threshold 13. During lymphocyte activation, signaling through TCR and costimulatory molecules occurs. Therefore, the activation of these receptors will also influence RICD after a second encounter with an antigen-presenting cell. However, little is known about how those pathways modulate the threshold required for RICD 13. Besides, it has been recently demonstrated that T cells from XLP individuals are specifically resistant to apoptosis mediated by TCR restimulation 18. The authors showed that the NTB-A/SAP pathway participated in the fine tuning of the TCR signaling strength upon restimulation, regulating T cell susceptibility to apoptosis during immune responses 18. SAP displays a pro-apoptotic role increasing the TCR signal to reach an optimal RICD 18.
In this work, we evaluated the biological function and the molecular mechanisms of NTB-A/SAP signaling during T cell responses and homeostasis regulation against Mtb.
Results
Patient population
Because T cell responsiveness is critical for immunity against the pathogen, tuberculosis patients were classified as High responder (HR) and Low responder (LR) TB patients based on in vitro T cell responses to M. tuberculosis, as described in Methods and Table 1.
Table I.
Epidemiological and Demographic Characteristics of TB Patients.
| Characteristic | HR TB (n = 57) | LR TB (n = 42) | p Value |
|---|---|---|---|
| Proliferative responses (Proliferation index) | 17.76 ± 4.59 | 5.01 ± 0.93 | 0.0001a |
|
| |||
| IFN-γ production (fold-stimulation) | 548.89 ± 142.51 | 39.57 ± 18.41 | 0.0001a |
|
| |||
| Increase in the percentage of SLAM-positive cells | 11.04 ± 1.67 | 4.26 ± 1.12 | 0.0003a |
|
| |||
| Increase in the percentage of CD25-positive cells | 25.02 ± 5.89 | 6.12 ± 2.25 | 0.0079a |
|
| |||
| Age | 36.02 ± 2.31 | 34.36 ± 2.49 | 0.3953a |
|
| |||
| Sex | |||
| Male | 51 (89.47%) | 39 (92.86%) | 0.7293b |
| Female | 6 (10.53%) | 3 (7.14%) | |
|
| |||
| Ethnicity | |||
| Caucasian | 38 (66.67%) | 24 (57.14%) | 0.2972b |
| American Indian | 18 (31.58%) | 18 (42.86%) | |
| Asian | 1 (1.75%) | 0 (0.00%) | |
|
| |||
| Hematologic studies | |||
| Leukocytes (cells/mm3) | 10357.32 ± 441.17 | 8840.34 ± 382.83 | 0.0082a |
| Lymphocytes (cells/mm3) | 1759.33 ± 93.57 | 1382.77 ± 111.38 | 0.0057a |
| Monocytes (cells/mm3) | 904.16 ± 48.46 | 741.83 ± 34.36 | 0.0073a |
|
| |||
| Radiological Lesions | |||
| Mild | 6 (12.50%) | 0 | |
| Moderate | 15 (31.25%) | 7 (20.00%) | 0.0292c |
| Severe | 27 (56.25%) | 28 (80.00%) | |
| ND | 9 | 7 | |
|
| |||
| AFB in sputum smear | |||
| − | 5 (10.42%) | 2 (5.71%) | |
| + | 35 (72.92%) | 27 (77.14%) | |
| ++ | 4 (8.33%) | 2 (5.71%) | |
| +++ | 4 (8.33%) | 4 (11.43%) | 0.8079c |
| ND | 9 | 7 | |
|
| |||
| Extrapulmonary TB | |||
| Pleural effusion | 4 (7.02%) | 2 (4.76%) | 0.8703c |
| Milliary | 2 (3.51%) | 2 (4.76%) | |
| Other | 6 (10.53%) | 4 (9.52%) | |
Continuous data are expressed as mean ± SEM, and categorical data are expressed as number (percentages). High Responder Tuberculosis patients (HR TB) and Low Responder Tuberculosis patients (LR TB) were classified according to proliferation [proliferation index: (cpm after sonicated Mtb-Ag stimulation)/(cpm after culturing with medium)]; IFN-γ production [fold-stimulation: (ng/mL after sonicated Mtb-Ag stimulation)/(ng/mL after culturing with medium)]; and increase in the percentage of SLAM-positive cells in response to sonicated Mtb-Ag stimulation, as previously reported (see Materials and Methods). HR TB, Proliferation index ≥ 4, IFN-γ production ≥ 34, increase in % SLAM-positive cells ≥ 8; LR TB, proliferation index < 4, IFN-γ production < 34, increase in % SLAM-positive cells < 8. If a patient fulfilled two of three of these criteria, the patient was assigned to that group. Radiological lesions: mild, patients with a single lobe involved and without visible cavities; moderate, patients presenting unilateral involvement of two or more lobes with cavities, if present, reaching a total diameter no greater than 4 cm; severe, bilateral disease with massive affectation and multiple cavities.
p values were calculated by the Mann-Whitney test for unpaired samples.
p values were calculated by Fisher’s exact test for categorical variables.
p values were calculated by the χ2 for trend test for categorical variables. p values of < 0.05 were considered statistically significant.
No differences regarding age distribution, sex, ethnicity, acid-fast bacilli in sputum, or frequency of extra pulmonary forms of tuberculosis were found between HR and LR TB patients (Table 1). However, significant differences were detected regarding X-ray radiography severity and leukocyte count (Table 1). A considerable severity pattern was detected especially in LR TB, who presented significant lower leukocytes, lymphocytes and monocytes counts and evidenced more severe pulmonary lesions compared to HR TB patients (Table 1).
As expected, LR TB patients showed reduced proliferative responses, IFN-γ production and diminished SLAM, but also CD25 expression in response to Mtb-antigen (Mtb-Ag), as compared to HR TB patients (Table 1).
Phosphorylation profile of immunoreceptors in cells from tuberculosis patients: characterization of NTB-A
Previously, we demonstrated that SLAM and ICOS signaling increased Th1 responses against Mtb 9, 19, while SAP, CD31 and PD-1 interfered with IFN-γ production during human active TB 9, 19, 20. Here we performed a preliminary screening of phosphorylation levels of ITAM/ITIM-associated immunoreceptors and adaptor signaling molecules using a human phosphoimmunoreceptor array. Our initial data showed a differential pattern of immunoreceptors’ phosphorylation between a LR TB and a HD after stimulation with Mtb-Ag during 24h (Supplementary Fig. S1A, S1B, S1C). Quantitative image analysis from the preliminary screening depicted that PBMCs from a HD displayed increased phosphorylation of 2B4/SLAMF4, CD229/SLAMF3, NTB-A/SLAMF6, SHIP-1, SHP-1, SHP-2 and CD150/SLAMF1/SLAM molecules. In contrast, PBMCs from the LR TB patient showed an augmented phosphorylation of BLAME/SLAMF8, CRACC/SLAMF7 and CD3 epsilon (CD3 ε) (Supplementary Fig. S1A, S1B, S1C).
Given that SLAMF1 induces Th1 responses during active TB 9 and considering that NTB-A phosphorylation was clearly increased in HD as compared to LR TB patients, here we evaluated the role of this receptor in the immune response displayed by patients with active TB. Initially, we measured the surface expression of NTB-A on T cells from HD, HR TB and LR TB patients after Mtb-Ag stimulation. High levels of NTB-A expression were observed on the three groups of individuals under study (Fig. 1A and Supplementary Fig. S2A, S2B). No changes in NTB-A expression were observed on T cells from HR and LR TB patients after 5 days of Mtb-Ag stimulation (Fig. 1A and Supplementary Fig. S2A, S2B). On the contrary, Mtb-Ag stimulation significantly down-regulated NTB-A mean fluorescence intensity (MFI) on CD3+ T cells from HD (138.86 ± 13.63), as compared to non-stimulated PBMCs (174.97 ± 4.16) (Fig. 1A).
Figure 1. Functional characterization of NTB-A.
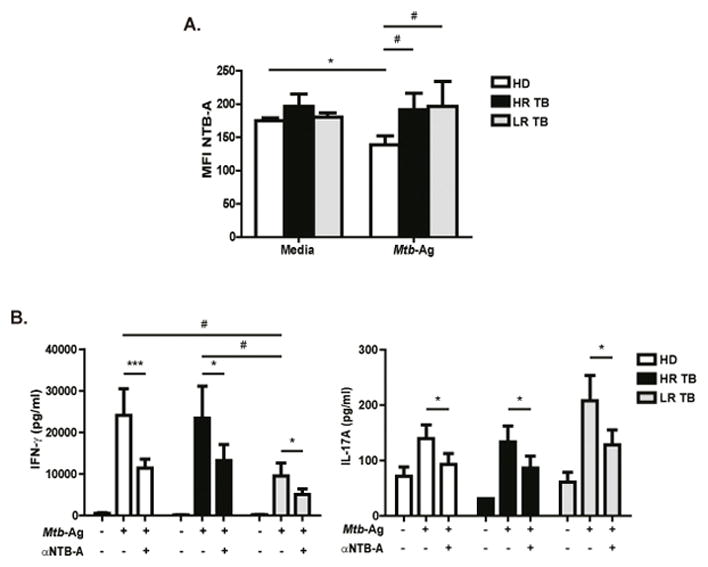
(A) PBMCs from HD (N=8), High Responder Tuberculosis patients (HR TB, N=5) and Low Responder Tuberculosis patients (LR TB, N=4) were stimulated with Mtb-Ag for 5 days. Then, NTB-A expression was determined on CD3+ T cells by flow cytometry, by first gating on lymphocytes by light scatter, and then on CD3+ cells. Bars represent the mean fluorescence intensity (MFI) ± Standard Error of the Mean (SEM) of NTB-A on CD3+ T cells. (B) PBMCs from HD (N=12), HR TB (N=7) and LR TB (N=5) were stimulated with Mtb-Ag for 5 days in the presence or absence of α-NTB-A blocking antibody. Cell free supernatants were collected and IFN-γ and IL-17A production was determined by ELISA. Bars represent the mean ± SEM of the values. Statistical differences were calculated using One way ANOVA-Uncorrected Fisher’s LSD. ***p < 0.001; *,#p < 0.05.
We next evaluated the potential role of NTB-A in modulating the cytokine microenvironment upon Mtb-Ag stimulation. PBMCs from HD and TB patients were cultured with Mtb-Ag ± anti-NTB-A monoclonal blocking antibody for 5 days, and IFN-γ and IL-17A production was determined. Blockage of NTB-A in Mtb-Ag stimulated cells down-regulated the production of both IFN-γ and IL-17A in all groups of individuals under study, indicating that signaling through NTB-A participates in the immune response of the host against Mtb (Fig. 1B).
IFN-γ production negatively correlates with SAP mRNA levels upon Mtb stimulation
SLAM-related receptors interact with SAP with high affinity and specificity 21. Actually, the SLAM receptors can amplify the signaling initiated by the TCR through SAP, which recruits kinases (FYN, LCK), modulating humoral and cellular immune responses 10, 22, 23. Accordingly, we previously demonstrated that LR TB patients’ cells exhibited high levels of SAP that interact with SLAMF1, selectively inhibiting Th1 immune responses during human active tuberculosis 9. Given the importance of SAP protein in Mtb infection, we further investigated the transcriptional and post-transcriptional regulation of this molecule. Detectable levels of SAP mRNA were observed at 48h in PBMCs from LR TB patients (Fig. 2A and Supplementary Fig. S3A). In contrast, in PBMCs from HR TB patients and HD, SAP mRNA levels were detectable after 5 days of Ag-stimulation (Fig. 2A). Furthermore, cells from LR TB patients displayed the highest levels of SAP mRNA and protein after 5 days of culture with Mtb-Ag (Fig. 2A, B). Additionally, an inverse correlation between SAP mRNA and IFN-γ levels was observed (Fig. 2C and Supplementary Fig. S3B), in agreement with our previous reports 9, 24. By using SAP siRNA, we observed an increase in Mtb-induced IFN-γ production by PBMCs from most LR TB patients, up to the levels secreted by PBMCs from HR TB patients (Fig. 2D and Supplementary Fig. S3C). Thus, inhibition of SAP expression improves the immune response in individuals with weak cell-mediated immunity against Mtb, confirming that SAP is a key molecule in attenuating Th1 immune responses.
Figure 2. SAP mRNA and protein levels are increased in patients with low IFN-γ production.
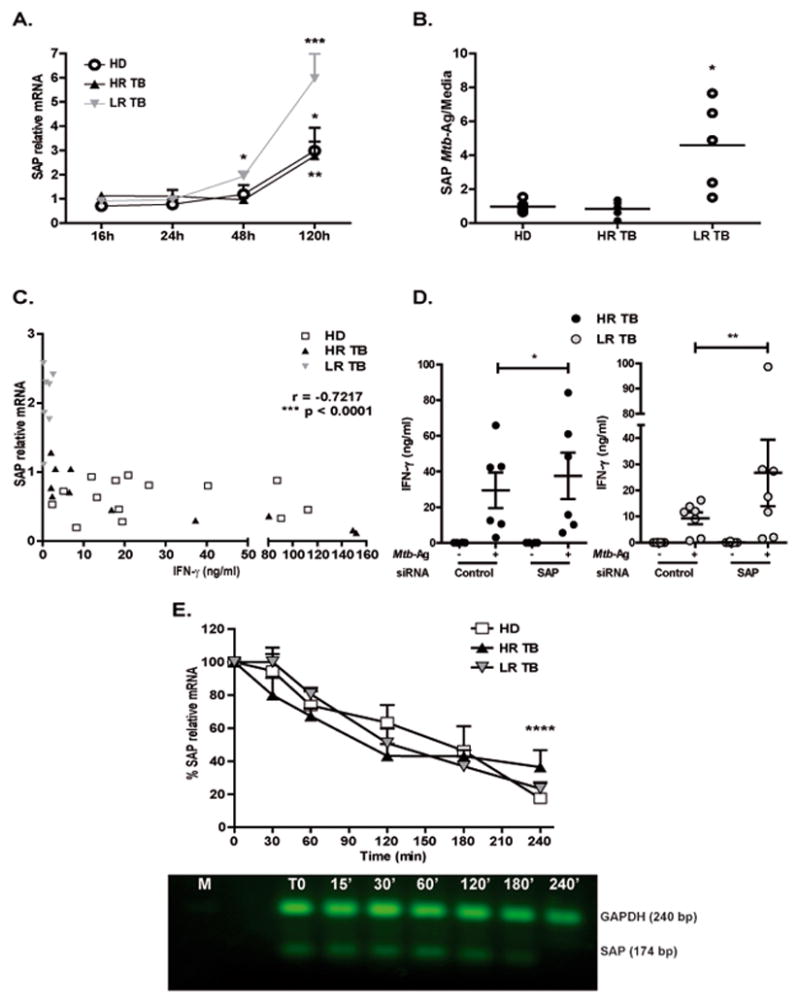
(A) PBMCs from Healthy Donors (HD, N=10), High Responder Tuberculosis patients (HR TB, N=8) and Low Responder Tuberculosis patients (LR TB, N=6) were stimulated with Mtb-Ag for different times. SAP mRNA expression was determined by Real time PCR. Values were calculated as fold of increase using the comparative method for relative quantification after normalization to GAPDH expression. Fold increase = 2(−ΔΔCt), where ΔCt = [Ct (SAP) – Ct (GAPDH)] and ΔΔCt [ΔCt Mtb − ΔCt Media]. Bars represent the mean ± SEM. (B) Total cell protein extracts were prepared from PBMCs stimulated with Mtb-Ag for 5 days and SAP protein expression were then measured by Western Blot. (C) Correlation between IFN-γ production and SAP mRNA expression. PBMCs from HD (N=16), HR TB (N=11) and LR TB (N=7) patients were stimulated for 48h with Mtb-Ag. Cell-free supernatants were then collected and assayed for IFN-γ by ELISA (X axis). SAP mRNA expression was determined by Real time PCR (Y axis). (D) PBMCs from HR TB (N=6, black circles) and LR TB (N=7, grey circles) patients were incubated in presence of SAP siRNA or unspecific control (GFP) for 48h. After 5 days of Mtb-Ag stimulation, cell-free supernatants were collected and assayed for IFN-γ by ELISA. Bars represent the mean ± SEM. (E) PBMCs from HD (N=5), HR (N=5) and LR (N=4) tuberculosis patients were stimulated with Mtb-Ag for 48 hours. Actinomycin D (actD) was added and cells were collected at different time points. SAP mRNA levels were determined by Real Time PCR. Values are expressed as the mean ± SEM of the Fold increase relative to time zero, as follows Fold increase = 2(−ΔΔCt), where ΔCt = [Ct (SAP) – Ct (GAPDH)] and ΔΔCt [ΔCt Mtb with actD − ΔCt Mtb without actD]. The image shows cropped gel corresponding to GAPDH and SAP mRNA decay. ****p <0.0001 Differences between time zero vs. Mtb with ActD at 240’ for each group of individuals. (A, B, D, E) One way ANOVA-Uncorrected Fisher’s LSD. *p <0.05, **p <0.01, ****p <0.0001. (C) Correlation factor (r) and p value were calculated by the non-parametric Spearman correlation test.
SAP gene expression is closely regulated at post-transcriptional levels 25. During post-transcriptional stage, the 3′ untranslated region of SAP displays a destabilizing effect on the transcripts 25. We then hypothesized that unresponsive LR TB patients could display an increased stability of SAP mRNA, explaining their increased SAP expression. In contrast to our hypothesis, by arresting the transcription process for 3 hours with Actinomycin D, we observed the degradation of more than 60% of SAP mRNA in Mtb-stimulated PBMCs from all groups of individuals (Fig. 2E). Furthermore, after 4 hours of arresting the transcription process, ≥ 80% of SAP mRNA degradation was detected in both PBMCs from LR TB patients and HD. Therefore, the high levels of SAP mRNA displayed by PBMCs from LR TB patients are not related to an increased stability of SAP mRNA compared to HD and HR TB patients. Nevertheless, our present findings suggest that a differential rate of transcription could account for the increased SAP mRNA levels in LR TB patients during the immune response against Mtb.
Signaling through NTB-A/SAP participates in RICD during the immune response to Mtb
Although SAP expression correlates with TB disease severity (Fig. 2A–C) 9, our data also showed increased SAP mRNA levels after 5 days of Mtb-Ag stimulation in all groups under study (Fig. 2A). Therefore, besides the inverse association between SAP expression and IFN-γ production in PBMCs from TB patients, SAP might be mediating other mechanisms during the immune response against Mtb. Accordingly, recent studies have demonstrated the role of SAP in TCR RICD, showing that T cells from XLP patients were resistant to RICD 18. Moreover, a direct relationship between hyperproliferation of SAP-deficient CD8+ T cells and both, impaired apoptosis and RICD, has been reported 26. Thus, to analyze whether SAP was involved in the homeostatic regulation of the immune response against Mtb, we investigated the induction of RICD in TB patients and HD. To this end, PBMCs were stimulated for 5 days with Mtb-Ag. Then, the cells were washed and cultured with hrIL-2 for 7 days. After lymphocyte enrichment, these cells were restimulated with α-CD3 + α-CD28 and apoptosis was determined by flow cytometry. T cells from HR TB patients and HD subjects displayed a markedly higher percentage of cell loss at 24h of TCR restimulation than XLP (Fig. 3A and Supplementary Fig. S4C). Surprisingly, we observed that LR TB patients’ T cells presented a reduced percentage of apoptosis after restimulation, similar to XLP individuals (Fig. 3A and Supplementary Fig. S4C). Therefore, both patients with the highest levels of SAP (LR TB) and patients with the lowest or null SAP expression (XLP), were resistant to RICD. In contrast, PBMCs from HD and HR TB patients displayed elevated levels of RICD (Fig. 3A and Supplementary Fig. S4A, S4C). Furthermore, when we analyzed the percentage of cells in each stage of the apoptotic process after 24h restimulation of HD and HR TB patients, individuals susceptible to RICD, evidenced higher percentages of late apoptotic cells (HD: 50.33% ± 6.57 and HR: 47.88% ± 8.55), as compared to those patients whose cells were resistant to the RICD process (LR: 31.21% ± 7.11 and XLP: 26.77% ± 9.66) (Fig. 3B).
Figure 3. NTB-A/SAP signaling mediates apoptosis after TCR restimulation.
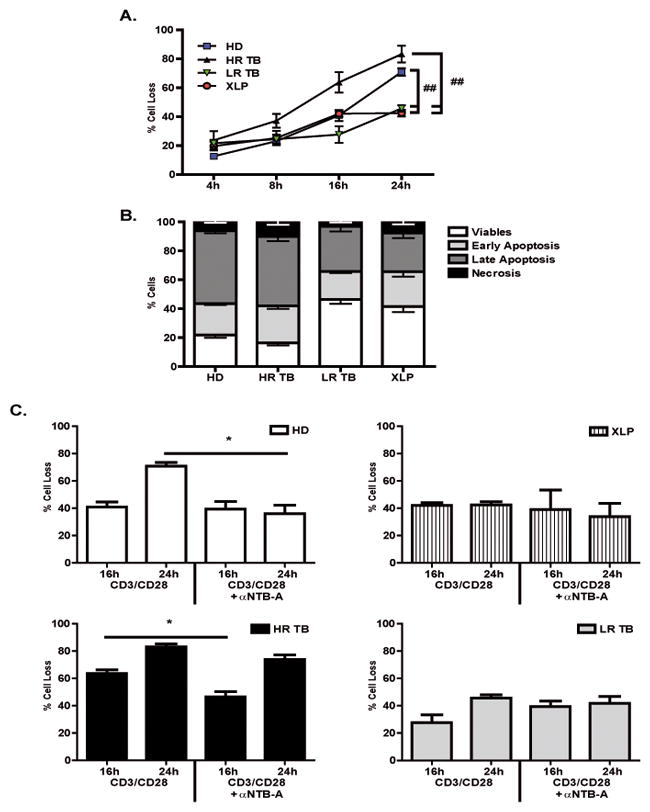
PBMCs from Healthy Donors (HD, N=11), High Responder Tuberculosis patients (HR TB, N=7), Low Responder Tuberculosis patients (LR TB, N=6) and SAP deficient (XLP, N=7) patients were stimulated with Mtb-Ag for 5 days. Then, the cells were washed and cultured with RPMI plus hrIL-2. After 7 days, lymphocyte enrichment was performed by centrifugation over Ficoll-Hypaque and cells were stimulated with α-CD3 + α-CD28 for different times. (A) Apoptosis was determined by evaluating Annexin V (A) and Propidium Iodide (IP) by first gating on lymphocytes by light scatter and then on CD3+ cells. Cell death was quantified as percentage of cell loss = (1 – [number of treated viable cells / number of untreated viable cells]) × 100. Viable cells correspond to A−IP−. Each bar represents the mean ± SEM of the % Cell Loss. (B) Percentage of cells in each stage of the apoptotic process after 24h of restimulation where: Early Apoptosis (A+IP−), Late Apoptosis (A+IP+) and Necrosis (A−IP+). (C) Cells were restimulated in the presence or absence of α-NTB-A blocking mAb for 24h. Apoptosis was determined by evaluating Annexin V (A) and Propidium Iodide (IP). Cell death was quantified as described in (A). Each bar represents the mean ± SEM of the % Cell Loss. One way ANOVA-Uncorrected Fisher’s LSD. *p <0.05, ##p <0.01.
It has been demonstrated that specific knockdown of NTB-A induced a decrease of RICD sensitivity 18. We then wondered whether NTB-A signaling could be differentially modulated in PBMCs from LR TB patients during TCR restimulation, leading to a defect in apoptosis induction. Supporting our results showing that Mtb-Ag stimulation did not modulate NTB-A levels in TB patients (Fig. 1A), almost all of CD3+ T cells expressed NTB-A after RICD (data not shown). Next, we investigated the potential role of the NTB-A pathway and its relationship to SAP during active TB. By blocking NTB-A, we observed that PBMCs from XLP patients were resistant to RICD both at 16h and 24h of restimulation (Fig. 3C). In contrast, blockage of NTB-A in PBMCs from HR TB patients significantly diminished the percentage of cell loss after 16h of restimulation (Fig. 3C), indicating that NTB-A participates in RICD in these patients. However, in LR TB patients, blocking NTB-A signaling had no effect on the percentage of cell loss (Fig. 3C). On the other hand, blockage of NTB-A did not modulate the proliferation induced after restimulation in any of the groups under study (Supplementary Fig. S4B). Together, these results would indicate that NTB-A signaling might be required for optimal TCR-induced apoptosis of T cells in responding individuals with controlled T cell responses.
Expression of IL-2, CD25 and FASL in T cells from patients with Tuberculosis
Dysregulation of production and response to the pivotal cytokine IL-2 may be critical in the defective cellular immune response of the host against Mtb. Actually, it was demonstrated that TB patients that produce low levels of IL-2 had a defect in PPD-stimulated IL-2 production, while responses to streptococcal antigens were comparable to those of healthy donors 27. Thus, disordered regulation of IL-2 metabolism may be a key feature in the depressed cellular immune response to TB. Moreover, a key role for IL-2 in RICD context has been reported 28. Anti-CD3 Abs combined with IL-2 induce γδ T cell apoptosis 29. Exogenous IL-2 addition to Mtb-stimulated cells displayed a synergistic effect that increased the apoptotic activity of γδ T cells 28. Then, we hypothesized that a deficiency in IL-2 production or IL-2 receptor expression could contribute to the observed defect in RICD, as suggested by other authors 29. By measuring the intracellular expression of IL-2, we observed markedly lower percentages of IL-2+CD4+T-secreting lymphocytes after restimulation of PBMCs from LR TB patients (resistant to RICD) as compared to PBMCs from HR TB patients and HD (Fig. 4A and Supplementary Fig. S5A). PBMCs from XLP individuals displayed the highest percentages of IL-2 secreting cells after restimulation, probably due to their lymphocytes’ hyper-responsiveness (Fig. 4A and Supplementary Fig. S5A).
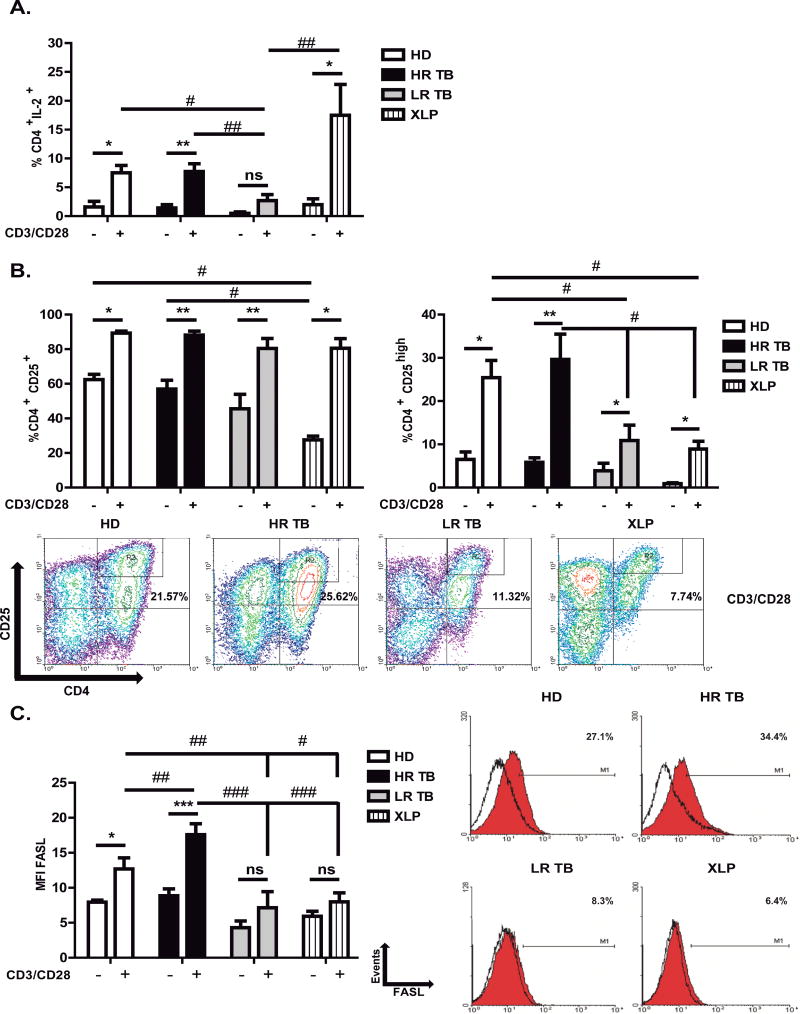
Figure 4. Association between expression of IL-2, IL-2 receptor (CD25) and FASL with RICD sensitivity.
(A–C) PBMCs from Healthy Donors (HD), High Responder Tuberculosis patients (HR TB), Low Responder Tuberculosis patients (LR TB), and SAP deficient (XLP) patients were stimulated with Mtb-Ag for 5 days. Then, the cells were washed and cultured with RPMI plus hrIL-2. After 7 days, lymphocyte enrichment was performed by centrifugation over Ficoll-Hypaque and cells were stimulated with α-CD3 + α-CD28 for 24h. Then, (A) intracellular IL-2 (HD N= 5, HR TB N= 9, LR TB N= 8 and XLP N= 6), (B) surface CD25 (HD N= 5, HR TB N= 9, LR TB N= 8 and XLP N= 5) and (C) surface FASL (HD N= 7, HR TB N= 10, LR TB N= 6 and XLP N= 5) expression were determined by flow cytometry on CD4+ T cells. Each bar represents the mean ± SEM of the percentages of CD4+IL-2+ (A), CD4+CD25+ (B) cells; and the mean fluorescence intensity (MFI) ± SEM of FASL+ CD4+ (C). IL-2, CD25 and FASL expression was determined by first gating on lymphocytes by light scatter and then on CD4+ cells. CD25high expression was determined by gating on lymphocytes by light scatter, then CD25 vs CD4 was plotted as shown in the representative dot plot. One way ANOVA-Uncorrected Fisher’s LSD. **,##p < 0.01; *,#p < 0.05. ns, differences not significant.
Upon T cell activation, IL-2 receptor α chain (CD25) is expressed and together with the β and γ chains constitute the high affinity IL-2 receptor. It is known that the IL-2 receptor participates in the modulation of the RICD process 30, 31. We observed a reduced expression of CD25 in XLP patients’ non-restimulated T cells as compared to TB patients and HD (Fig. 4B and Supplementary Fig. S5B). However, after restimulation, almost 90% of CD4+ T cells of the four groups of individuals under study expressed CD25, with no significant differences detected among the groups (Fig. 4B left panel and Supplementary Fig. S5B). It has been recently demonstrated that cells expressing low expression of CD25 (CD25lo) are less sensitive to IL-2, generating long lived memory cells 32. In contrast, CD25 high (CD25hi) cells display a higher response to IL-2, faster proliferation and are also more sensitive to undergo apoptosis 32. Considering those findings, we next evaluated the expression of CD4+CD25hi cells. Our results demonstrated that HD and HR TB patients’ cells in fact displayed the highest percentages of CD25hi cells (25.41% ± 3.97 and 29.65% ± 5.83, respectively, Fig. 4B right panel). Interestingly, although patients that are resistant to RICD (XLP and LR TB patients) positively modulate the expression of CD25hi cells after restimulation (10.90% ± 3.55, LR TB and 8.93% ± 1.78, XLP), they showed significantly lower levels of CD25hi cells, as compared to RICD-sensitive individuals (HD and HR TB, Fig. 4B right panel).
It has been previously reported that the ability of IL-2 to enhance the expression of the pro-apoptotic molecule FASL and its capacity to suppress the expression of the inhibitor of FAS signaling, cellular FLICE-like inhibitory protein, accounts for the role of IL-2 in T cell apoptosis 33. Therefore, we then determined FASL expression on CD4+ T cells. FASL levels were not significantly increased in LR TB and XLP patients’ restimulated cells (Fig. 4C). In contrast, HR TB patients and HD’s T cells showed a significant increase in the expression of FASL upon restimulation (Fig. 4C). Together, these data demonstrate that resistance to RICD is associated with low levels of IL-2, CD25high and FASL.
FYNT participates in the NTB-A pathway abrogating RICD in LR TB patients
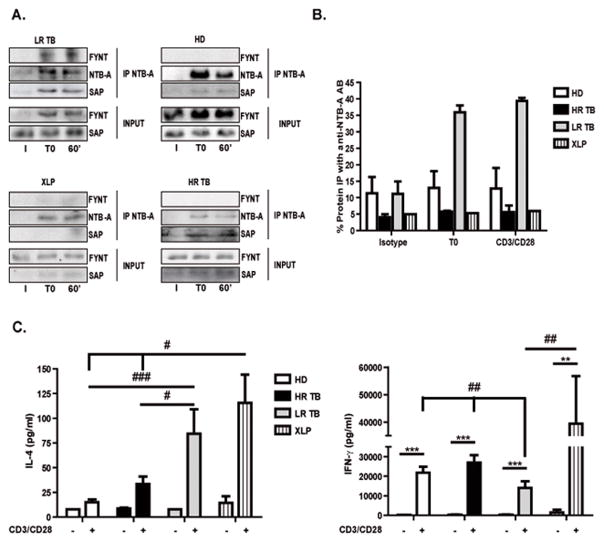
During the establishment of immune synapse, clustering of SLAMF receptors brings SAP to its cytoplasmic tails and mediates recruitment and activation of FYNT 34. Accordingly, we have shown that SAP mediates the recruitment of FYNT to SLAM in leprosy, inhibiting IFN-γ production in lepromatous leprosy patients 35. Snow et al. showed that RICD mechanism is independent of FYNT and that Src-family kinase LCK contributes to TCR signal transduction during RICD 18, 36. Then, we analyzed the events downstream the NTB-A pathway. We performed NTB-A immunoprecipitations in activated T cells from HD, TB patients and XLP individuals to test the potential interaction between SAP and FYNT in the regulation of the NTB-A signaling pathway that controls RICD. Association of SAP with NTB-A was detected before and after TCR restimulation in all groups under study, except for XLP patients that do not express SAP (Fig. 5A). Interestingly, we observed that FYNT was associated to NTB-A only in LR TB patients’ T cells (Fig. 5A). In fact, more than 35% of FYNT co-precipitated with NTB-A, both in restimulated and non-restimulated T cells (Fig. 5B). However, FYNT was not detected in NTB-A immunoprecipitates from HD and HR TB patients’ cells (Fig. 5A, B). Thus, these results demonstrate that FYNT is differentially recruited to SAP on T cells from LR TB patients resistant to RICD.
Figure 5. The NTB-A/SAP pathway impairs RICD in LR TB patients.
PBMCs from Healthy Donors (HD, N=3), High Responder Tuberculosis patients (HR TB, N=3), Low Responder Tuberculosis patients (LR TB, N=3) and SAP deficient (XLP, N=1) patients were stimulated with Mtb-Ag for 5 days. Then, the cells were washed and cultured with RPMI plus hrIL-2. After 7 days, lymphocyte enrichment was performed by centrifugation over Ficoll-Hypaque and afterwards cells were stimulated with α-CD3 + α-CD28 for 1h. (A) Immunoprecipitation of NTB-A. The immunoprecipitates were separated by SDS-PAGE and immunoblotted for the presence of SAP, NTB-A and FYNT. SAP and FYNT expression in input lysates are shown for comparison (bottom) (I = Isotype, T0 = unstimulated cells and 60’ = stimulated cells with α-CD3 + α-CD28 for 1h). The images show cropped lines corresponding to NTB-A, SAP and FYNT. (B) Polyacrylamide gels were scanned, densitometry was performed, and the results were expressed as % FYNT IP with anti-NTB-A= ([FYNT AU Immunoprecipitated fraction (IP)/(FYNT AU IP + FYNT AU Non-Immunoprecipitated fraction])*100. (C) PBMCs from HD (N=6), HR (N=7), LR (N=5) tuberculosis patients and XLP (N=3) patients were stimulated with Mtb-Ag for 5 days. Then, cells were washed and cultured with RPMI plus hrIL-2. After 7 days, lymphocyte enrichment was performed by centrifugation over Ficoll-Hypaque and cells were stimulated with α-CD3 + α-CD28 for 24 h. Cell free supernatants were collected and IL-4 and IFN-γ production was determined by ELISA. Bars represent the mean ± Standard Error of the Mean (SEM) of the values. One way ANOVA-Uncorrected Fisher’s LSD. ***p <0.001, **,##p <0.01, #p <0,05.
To further investigate the role of NTB-A, SAP and FYNT during TB, we tested the cytokine microenvironment after restimulation of the TCR. The NTB-A/SAP signaling pathway promotes, among other processes, the formation of the B germinal center, T/B synapsis and RICD 22. However, when FYNT is recruited to SLAM, differentiation of T lymphocytes to a Th2 cytokine pattern is promoted 22. Interestingly, Th2 CD4+ cells induce cell loss by RICD through granzyme B, since those lymphocytes are less sensitive to FAS-mediated apoptosis, as compared to Th1 cells after TCR restimulation 37. Thus, we investigated IL-4 and IFN-γ levels in the context of restimulation. The results obtained showed that PBMCs from LR TB patients produced higher IL-4 levels as compared to PBMCs from HD and HR TB patients (Fig. 5C). These data indicate that the lack of response of LR TB patients is mediated, at least in part, by induction of a Th2 cytokine pattern, an unfavorable phenotype for TB resolution. In addition, our observations might explain why LR TB patients show resistance to the cellular homeostasis regulation process; given that Th2 lymphocytes show lower susceptibility to RICD. In contrast, when we measured IFN-γ production, we observed a similar IFN-γ secretion as that detected upon Mtb-Ag stimulation (Supplementary Fig. S3B), where PBMCs from HD and HR TB patients produce more elevated levels of IFN-γ than LR TB patients (Fig. 5C). Therefore, our results indicate that the immune response of HD and HR TB patients continues to be effective during TCR restimulation, where SLAM collaboration in IFN-γ secretion (Supplementary Fig. S5C–D) participates both in primary activation with Mtb and in restimulation. In contrast, LR TB patients are unable to secrete enough IFN-γ levels to fight the pathogen, indicating a possible defect downstream of the TCR in these individuals, which would involve both SAP and FYNT.
Discussion
Positive and negative signals delivered by the interaction of several costimulatory molecules with their receptors are required to mount an effective immune response during T cell activation. Previously, we demonstrated that SLAM/SLAM signaling increases cell-mediated immunity in response to Mtb 9; while SAP, through interaction with SLAM, interferes with IFN-γ production during mycobacterial infection 9, 35. NTB-A, another member of SLAM family, increases Th1 cytokine production 10 and participates, in association with SAP, in the induction of tolerance and cellular homeostasis 12, 38. Here, we evaluated the NTB-A/SAP pathway during the immune response against Mtb.
A pattern phosphorylation study including 59 immunoreceptors containing ITAM/ITIM/ITSM motifs showed that, in response to Mtb-Ag, NTB-A and SLAM were highly phosphorylated in a healthy individual but not in PBMCs from a LR TB patient, suggesting a decreased activation state in patients with impaired immunity (Supplementary Fig. S1A, S1B, S1C). In contrast to our previous results, where the expression of SLAM was positively modulated after Mtb stimulation in HD and HR TB patients 9, NTB-A showed a constitutively high expression on T cells from all the groups of individuals studied (Supplementary Fig. S2A). However, Mtb-Ag down-regulated the MFI of NTB-A compared to unstimulated HD’s T cells (Fig. 1A), as has been reported for Vpu-1 (ref. 39). Besides, we observed that signaling through NTB-A increased the production of IFN-γ and IL-17A during Mtb infection (Fig. 1B).
Previous studies in SAP-deficient mice demonstrated an increased activation and hyperproliferation of IFN-γ-producing CD4+ and CD8+ T cells in response to LCMV or T. gondii infection 40, 41. Our results showed that, in PBMCs from LR TB patients, SAP mRNA was positively regulated after 48h of Mtb-Ag stimulation, while no differences were observed for HD and HR TB patients (Fig. 2A). Furthermore, our data showed a negative correlation between SAP mRNA levels and IFN-γ production upon Mtb-Ag stimulation (Fig. 2C). Moreover, blockage of SAP using specific siRNA increased IFN-γ in PBMCs from LR TB patients up to the levels detected in Ag-stimulated cells from HR TB patients (Fig. 2D).
Okamoto et al. previously demonstrated that SAP mRNA stability is a key post-transcriptional mechanism that determines SAP levels of expression 25. However, we observed that SAP mRNA decay was similar in all the groups of individuals under study (Fig. 2E). Besides, SAP degradation occurred significantly faster than GAPDH (Fig. 2E and data not shown), in line with studies performed in Jurkat T cells 25. Therefore, our results indicate that the increased expression of SAP observed in LR TB patients could be related to a higher rate of mRNA transcription but not to an increased stability of the mRNA transcripts. Further experiments are required to determine how Mtb-Ag stimulation induces the transcription of SAP. It could be possible that transcription factors such as Ets-1 and Ets-2 cooperate with promoter activity of SAP, as previously demonstrated 25.
The maximum expression of SAP and SLAM is reached at 6 days of α-CD3 stimulation in PBMCs from HD 42. In agreement, in the late phases of T cell activation against Mtb-Ag, we observed a marked increase in SAP mRNA levels of HD and patients with active TB (Fig. 2A). Moreover, LR TB patients with a weak immune response to the antigen showed the highest expression of SAP mRNA and protein (Fig. 2A, B).
Interestingly, SAP was shown to regulate the contraction phase of the T cell response through regulation of RICD 18, 42, 43. During the caseation of human tuberculosis granulomas, CD68+ macrophages, T CD3+ and CD45RO+ cells undergo apoptosis as a result of restimulation with Mtb antigens 44–46. Snow et al. have recently demonstrated that NTB-A co-localizes with CD3 complex on activated T cells after restimulation, and that binding of SAP to NTB-A amplifies proximal TCR signaling in the synapse, increasing RICD susceptibility 18. Our present results revealed increased cell loss in HD and HR TB patients’ T cells as compared to LR TB patients and XLP individuals (Fig. 3A). Besides, expression of SAP protein during T cell reactivation was positively modulated in HD and HR TB patients (Supplementary Fig. S4A). Moreover, after TCR restimulation, the higher proportion of apoptotic cells found in HD and HR TB patients were late apoptotic/necrotic T cells (Fig. 3B), indicating that these cells might be entering an irreversible “cell suicide” as part of the homeostatic process of the immune response. Our findings in patients with a deficient (LR TB) or an exacerbated Th1 response (XLP) might indicate that SAP expression abnormalities could affect diverse homeostatic mechanisms.
The immune response to an antigen occurs in two stages involving different molecular events: an activation phase and a proliferation phase. Induction of IL-2 and CD25 expression during the first phase is essential for leading to the next proliferative stage, where T cells have a high susceptibility to cell death after TCR restimulation. At the site of infection, the high levels of IL-2 and antigen are critical to start the “self-regulation” program of RICD 13. We found that PBMCs from HD and HR TB patients, which showed the highest percentages of cell loss, upregulated IL-2 levels, whereas no significant changes in the amounts of this cytokine were observed in PBMCs from LR TB patients upon restimulation (Fig. 4A). Although our results did not show differences in the expression of CD25 among the individuals under study (Fig. 4B), we observed a higher percentage of CD4+ CD25high T cells in HD and HR TB patients as compared to LR TB patients and XLP subjects. These findings might explain, in part, the higher resistance to apoptosis displayed by LR TB patients (Fig. 4B).
The central role of the FAS/FASL pathway during RICD has been widely demonstrated 13. During FAS/FASL signaling, IL-2 plays a key role through the negative modulation of cellular FLICE-like inhibitory protein, which turns lymphocytes sensitive to the RICD process. In line with our results with IL-2 and CD25, CD4 T cells from HD and HR TB patients also presented the highest levels of FASL (Fig. 4C), in direct association with their percentages of cell loss upon restimulation (Fig. 3A). Moreover CD4 T cells from XLP and LR TB patients, individuals that are resistant to the RICD mechanism, did not modulate FASL expression after restimulation (Fig. 4C). Together, our results suggest that the induction of RICD, as part of a mechanism of homeostasis against Mtb, is finely regulated by a complex network of signaling proteins, including those mediated by FAS and IL-2 receptors.
RICD mechanism is mainly triggered by the NTB-A/SAP pathway in human peripheral blood lymphocytes restimulated with anti-CD3 18, 36. In this work, we demonstrated that blocking NTB-A negatively modulated the cell loss displayed by T cells from HD and HR TB patients (Fig. 3C). By studying the NTB-A/SAP signaling pathway, we showed that LR TB patients that were refractory to RICD mechanism showed a differential recruitment of FYNT (Fig. 5A, B).
Additionally, we demonstrated that LR TB patients displayed an increased IL-4 secretion after TCR restimulation (Fig. 5C). In T lymphocytes, SAP binding displaces SHP1 and SHP2. Furthermore, SAP SH2 domain binds to the SH3 domain of Src family kinases like FYNT and LCK directly couples Src proteins to SLAM receptors 47. Moreover, it has been demonstrated that FYNT promotes the differentiation of T CD4+ cells to Th2 22 and Th17 48 profiles, two cell populations that showed increased resistance to RICD 13, 49. Thus, the role of FYNT during the NTB-A signaling might explain the resistance to RICD as well as the polarization of T cells toward Th2 unfavorable profiles (Fig. 6). In contrast to LR, T cells from HD and HR TB patients showed no recruitment of FYNT, a positive modulation of SLAM expression (Supplementary Fig. S5C), increased production of IFN-γ and reduced secretion of IL-4 after TCR restimulation (Fig. 5C).
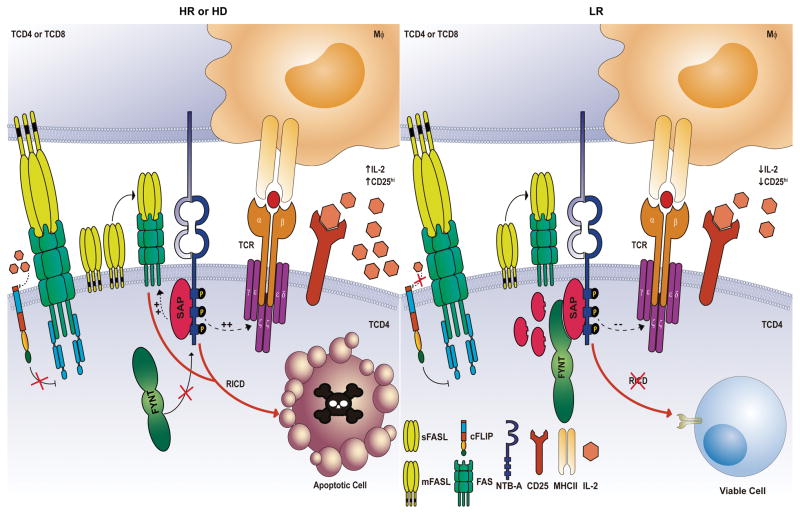
Figure 6. Model of NTB-A/SAP signaling that induces RICD during active tuberculosis.
In Healthy Donors (HD, left panel) and High Responder Tuberculosis patients (HR TB, left panel), SAP is associated with NTB-A, dislodging SHP-1 and inducing the expression of target genes such as FASL, increasing the TCR signal strength that triggers the RICD process after TCR restimulation. The exclusion of FYNT and the involvement of proteins like IL-2 and its receptor CD25, ensure the homeostasis of T cells in response to M. tuberculosis. On the other hand, Low Responder Tuberculosis patients (LR TB, right panel) suppress the induction of apoptosis by the recruitment of FYNT to NTB-A cytoplasmic tail. This association abrogates NTB-A signaling to sustain the viability of T cells. Furthermore, cellular FLICE-like inhibitory protein could lead to an inhibition of FAS receptor as a consequence of the low expression of IL-2. Similar to LR TB, SAP-deficient T cells of XLP patients show a resistance to RICD, inhibiting NTB-A signaling and allowing cells to escape from apoptosis. Solid arrows represent regulation based on experimental evidence; dotted arrows represent inferred regulation.
We have previously shown that at the site of infection, M. tuberculosis expanded and regulated CD4+IFN-γ+IL-17+ lymphocytes in the same way as in peripheral blood 50. Consequent to PBMCs, Pleural Fluid Mononuclear Cells from LR TB patients bared a superior proportion of CD4+IFN-γ+IL-17+ lymphocytes expanded against Mtb-Ag as compared to HR individuals. Moreover the presence of higher proportions of CD4+IFN-γ+IL-17+ lymphocytes was correlated directly with more extensive lung affectation and a higher number of pulmonary lesions 50. Taking these previous results into account it might be speculated that this pathogenic T cell population could be more resistant to RICD, which might explain the increased proportion in LR TB patients observed in our previous study 50. Moreover, it has been previously shown that Th1/Th17 cells are more resistant to cell death in patients with multiple sclerosis 49. In fact, the mechanism that mediates resistance to cell death in Th1/Th17 cells of patients with multiple sclerosis is related to reduced Fas ligand expression 49, in agreement with our results for LR TB patients (Fig. 4C).
Together our results suggest that T cells from individuals that display a protective Th1 response against Mtb are capable of undergoing apoptosis after TCR restimulation through the NTB-A/SAP signaling pathway (Fig. 6). Moreover, in these individuals FYNT is not recruited, which leads to a favorable Th1 cytokine microenvironment that influences the homeostasis of the immune response. This signaling pathway could also limit the uncontrolled expansion of T cells, controlling immunopathology in HR TB patients. Thus, together we found that the NTB-A/SAP pathway regulates T cell activation and RICD during human TB, and that the NTB-A/SAP/FYNT axis promotes polarization to an unfavorable Th2-phenotype. In conclusion, our present results contribute to elucidate the role of NTB-A/SAP and FYNT in the generation of a protective immune response against the intracellular pathogen Mtb.
Methods
Patients
HIV-seronegative patients with active tuberculosis (TB) were evaluated at Dr. F. J. Muñiz (Buenos Aires, Argentina). Diagnosis of disease was established based on clinical and radiological data, identification of acid-fast bacilli in sputum, and isolation of M. tuberculosis in culture. Patients included in this study had received less than one week of anti-tuberculosis therapy and were classified on the basis of lymphocyte responses to M. tuberculosis-antigen (Mtb-Ag), as previously reported 9,20,50. Briefly, PBMCs from High Responder tuberculosis patients (HR TB) showed significant lymphocyte proliferation, a high level of IFN-γ production, and a high level of SLAM expression in response to the antigen; whereas PBMCs from Low Responder tuberculosis patients (LR TB) exhibited low proliferative responses, IFN-γ secretion, and percentages of SLAM+ cells 9. LR TB patients had a more severe pulmonary disease, lower leukocyte counts and a more prolonged illness compared to HR TB individuals. Healthy donors (HD), who had received BCG vaccination at birth and lacked a history of TB, participated in the study, excluding those subjects with latent tuberculosis (determined by QuantiFERON-TB Gold In-Tube, Qiagen). The control group included individuals who were matched in terms of sex, age and ethnicity with TB patients included in the study. Two subjects with X-linked lymphoproliferative disease (XLP), confirmed at the International XLP Registry Headquarters, University of Nebraska Medical Center (Omaha, NE, USA) were included in this study 51. The experiments were performed and the results were analyzed without previous knowledge of patients-classification. Peripheral blood was collected in heparinized tubes from all individuals participating in the study, after receiving informed consent. All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by a licensing committee from Dr. F. J. Muñiz Hospital (Buenos Aires, Argentina).
Antigen
Throughout the study, in vitro stimulation of cells was performed with a cell lysate from the virulent M. tuberculosis H37Rv strain prepared by probe sonication (Mtb-Ag), obtained through BEI Resources, NIAID, NIH: Mycobacterium tuberculosis, Strain H37Rv, Whole cell lysate, NR-14822 (Bethesda, MD, USA).
Cell Preparation and Reagents
Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation over Ficoll-Hypaque (GE Healthcare, Chicago, IL, USA) and cultured (1 X 106 cells/mL) in complete media (RPMI 1640 medium (Gibco, Thermo Scientific, Waltham, MA, USA) supplemented with L-glutamine (Sigma Aldrich, St Louis, MO, USA), 10% Fetal Bovine Serum (Gibco), 100 U/mL of Penicillin and 100 μg/mL of Streptomycin (Gibco)). PBMCs were stimulated with or without Mtb-Ag (10 μg/mL) in the presence/absence of anti-NTB-A blocking antibody (5 μg/mL, NT-7, Biolegend, San Diego, CA, USA) for different time points.
In some experiments, cells were activated with Mtb-Ag and restimulated with anti-CD3 plus anti-CD28 mAbs, in the presence/absence of anti-NTB-A, as described below (Apoptosis assays and flow cytometry).
IFN-γ and IL-17A production was determined by enzyme-linked immunosorbent assay following the manufactured instructions (eBioscience, San Diego, CA, USA).
Phosphorylation profile of immunoreceptors
PBMCs from a HD and a LR TB were stimulated with Mtb-Ag for 24 hours. Then, cells were resuspended in PBS and lysed for 30 minutes on ice in a buffer containing protease and phosphatase inhibitors. Cell suspensions were subject to centrifugation, then supernatants were collected as total protein extracts and then applied on a human phospho-immunoreceptor array (Human Phospho-Immunoreceptor Array Kit, R&D Systems, Minneapolis, MN, USA). Phosphorylation levels of individual analytes were determined by calculating the average of the pixel density of the spots in duplicate; values were obtained after subtracting background signals.
Real-Time PCR
Total RNA was isolated from PBMCs stimulated with or without Mtb-Ag for 16, 24, 48 and 120 hours using TRIzol (Ambion, Thermo Scientific, Waltham, MA, USA). cDNA was obtained by reverse transcription according to the manufacturer’s instructions (Promega, Fitchburg, WI, USA). Real-time PCR was performed with FastStart SYBR Green Master (Roche Applied Science, Penzberg, Upper Bavaria, Germany) and specific primers for human SAP (Integrated DNA Technologies, Coralville, IA, USA ). Relative mRNA expression was normalized to the expression of GAPDH (encoding glyceraldehyde-3-phosphate dehydrogenase), calculated by the change-in-threshold 2(−ΔΔCT)-method. The primers used for SAP and GAPDH were as follows: 5′-AAATCAGCAGGGAAACCG-3′ (forward) and 5′-CTCAGCACTCCAAGAACC-3′ (reverse); and 5′-TGATGACATCAAGAAGGTGGTGAAG-3′ (forward) and 5′-TCCTTGGAGGCCATGTAGGCCAT-3′ (reverse), respectively.
Transfection of SAP Small-Interfering RNA (siRNA)
PBMCs were transfected (Lipofectamine, Invitrogen, Carlsbad, CA, USA) with SAP or control (GFP) siRNA for 48 hours (15 pmoles/106 cells/mL or 250 ng/106 cells/mL, respectively). Then cells were washed and incubated for 5 days, with or without Mtb-Ag. IFN-γ production was determined by ELISA.
mRNA Turnover
RNA transcription in PBMCs was arrested by adding Actinomycin D (10 μg/mL, Gibco) to the culture media. Then, cells were recovered at the indicated time points. Total RNA and SAP transcript expression was examined by Real-Time PCR, as described above.
Apoptosis assays and flow cytometry
To evaluate RICD, PBMCs were activated with Mtb-Ag (10 μg/mL). After 5 days, cells were exhaustively washed and then cultured in complete RPMI-1640 medium supplemented with 100 U/mL of rhIL-2 (Biolegend) for 7 days. Viable cells were isolated and restimulated with 1 μg/mL of anti-CD3ε and anti-CD28 mAbs (OKT3 and CD28.2, respectively, BioLegend) for 16 and 24 hours, in the presence or absence of 5 μg/mL anti-NTB-A blocking mAb (NT-7, Biolegend).
Cells were then stained with Annexin V-FITC and propidium iodide using the Annexin V FITC Apoptosis Detection KiT I (BD Biosciences, San Jose, CA, USA). Cell death was quantified as percentage of cell loss = (1 − [number of viable cells (treated) / number of viable cells (untreated)]) × 100.
For flow cytometry determinations cells were stained with fluorochrome conjugated Abs against CD3 (FITC, cat. 11-0037, eBioscience or PerCPCy5.5, cat. 356108, Biolegend), CD4 (FITC, cat. 300506, Biolegend or PercPCy5.5, cat. 317428, Biolegend), CD25 (PeCy7, cat. 356108, Biolegend), SLAMF1 (FITC, cat. 11.1509, eBioscience), FASL (PE, cat. 306407, Biolegend) and NTB-A (NT-7, cat. 317204, Biolegend and anti-mouse PE). Intracellular staining was performed to determine IL-2 expression on CD4 T cells (anti-IL-2 PE, cat. 500307, BioLegend). For cytokine staining, PBMCs were incubated with Golgi Stop reagent containing monensin (1 μL/mL, BD Biosciences) for the final 5 hours of culture and BD Cytofix/Cytoperm and BD Perm/Wash buffers were used following the manufacturer’s instructions. Negative control samples were incubated with irrelevant isotype-matched monoclonal antibodies in parallel with all experimental samples, and all samples were analyzed on a FACS ARIA II flow cytometer (BD Biosciences). Data analysis was performed using FlowJo 7.6.2 (Tree Star Inc., OR, USA).
Proliferation
PBMCs were restimulated as described above, in the presence or absence of 5 μg/mL anti-NTB-A blocking mAb. Then, the cells were pulsed with [3H] TdR and harvested 16 hours later. [3H] TdR incorporation was measured in a liquid scintillation counter.
Immunoblotting
Total cell protein extracts were prepared from PBMCs stimulated with Mtb-Ag for 5 days. In different experiments, PBMCs stimulated with Mtb-Ag for 5 days were exhaustively washed and then cultured in complete RPMI-1640 medium supplemented with 100 U/mL of rhIL-2 for at least 7 days before obtaining total cell protein extracts. Western blotting was performed by standard methods. Each nitrocellulose membrane was blotted with mouse monoclonal antibodies to SH2D1a (SAP, cat. 14-9888-82, eBioscience), stripped, and reblotted with GAPDH (Ambion) or β-actin (BD Biosciences). Bound antibodies were detected with an anti-mouse HRP-conjugated antibody (BioRad, Hercules, CA, USA) using Amersham ECL PLUS reagent (GE Healthcare). Images were obtained with an Intelligent Dark Box (Fujifilm LAS1000) and analyzed with ImageJ Analysis software (NIH, Bethesda, MA, USA). The intensity of each band was expressed as arbitrary units (AU).
Immunoprecipitation
PBMCs were restimulated with anti-CD3 plus anti-CD28 (as described before) for 1 hour. Afterwards, cells were washed with PBS and incubated in RIPA lysis buffer (Cell signaling Technology, Danvers, MA, USA) supplemented with proteases and phosphatases inhibitors (Protease Inhibitor Cocktail P8340 plus 100 mM of PMSF, Sigma Aldrich). Immunoprecipitation assays were then performed using specific antibodies: anti-NTB-A (NT-7, Biolegend) or irrelevant mAbs following the manufacturer’s instructions (Santa Cruz Biotechnology, Dallas, TX, USA).
Briefly, anti-NTBA or anti-IgG1 Abs were coupled to Protein A/G PLUS-Agarose (Santa Cruz Biotechnology). Cell extracts from stimulated cells were pre-incubated with Protein A/G PLUS-Agarose for 2 hours, to eliminate nonspecific binding. Cells extracts where then incubated with 50 μl of Protein A/G PLUS-Agarose coupled antibodies for 16 hours at 4°C. Immunoprecipitates were obtained by centrifugation at 2000xg for 5 minutes and washed repeatedly with RIPA buffer and PBS. Immunoprecipitates were finally incubated at 95°C to detach the Protein A/G PLUS-Agarose from the protein complexes. Next, NTB-A (NT-7, Biolegend), SAP (FL-128, cat. sc-8333, Santa Cruz Biotechnology) and FYNT (cat. 4023, Cell signaling Technology) expression in the immunoprecipitates was assessed by Western blot analysis. The intensity of each band was expressed as arbitrary units (AU) and the percentage of FYNT bound to NTB-A was calculated as: ([AU of Immunoprecipated fraction (IP)/(IP+ Non immunoprecipated fraction (NO IP Fr)])*100.
Statistical Analysis
One-way ANOVA with Uncorrected Fisher’s LSD post hoc test were used for comparison between experimental groups as indicated in the figure legends. Correlation analyses were performed using the non-parametric Spearman correlation test. The Mann-Whitney test was used to analyze differences between unpaired samples. Fisher’s exact test and X2 for trend test were used for categorical variables. P values of < 0.05 were considered statistically significant. Data were analyzed using GraphPad Prism 6.0 software (San Diego, CA, USA).
Supplementary Material
Acknowledgments
We thank Nancy L. Tateosian and Nicolás O. Amiano for helpful discussions. We also thank Guillermo Risso for technical assistance in immunoprecipitacion experiments.
This work was supported by project grants from the National Institutes of Health, National Institutes of Allergy and Infectious Diseases (R01 AI079007 to V. E. G.); Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, PICT-0240 and PICT-1762 to V. E. G.); University of Buenos Aires (20020100100221 and 20020130100236BA to V. E. G.), and Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET; PIP 0961 to V. E. G.). R.E.H.D.P. and G.I.A. are fellows of Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT). J.M.P. and A.R. are fellows of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). V.P. and V.E.G. are members of the Researcher Career of CONICET.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Competing financial interests. The authors have no financial conflicts of interest.
Author contributions statement
VEG, VP and REHDP designed the study. AM, RMM and DJP were in charge of patient recruitment, diagnosis and sample collection. REHDP, JMP and AIR were responsible for processing samples and performing ELISA and flow cytometry analysis. DP, GIA and AR contributed with standard laboratory work. REHDP, JMP, VP and VEG work on data management and analysis. REHDP and VEG wrote the manuscript. All authors contributed to data gathering and interpretation, and revision of the report.
Supplementary information is available at Immunology & Cell Biology’s website
References
- 1.World Health Organization (WHO) Global tuberculosis report 2014. WHO Library; Geneva S: [Google Scholar]
- 2.Bold TD, Ernst JD. CD4+ T cell-dependent IFN-gamma production by CD8+ effector T cells in Mycobacterium tuberculosis infection. Journal of immunology. 2012;189(5):2530–6. doi: 10.4049/jimmunol.1200994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sodhi A, Gong J, Silva C, Qian D, Barnes PF. Clinical correlates of interferon gamma production in patients with tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1997;25(3):617–20. doi: 10.1086/513769. [DOI] [PubMed] [Google Scholar]
- 4.Dorman SE, Holland SM. Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine & growth factor reviews. 2000;11(4):321–33. doi: 10.1016/s1359-6101(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 5.Saito T. Negative regulation of T cell activation. Current opinion in immunology. 1998;10(3):313–21. doi: 10.1016/s0952-7915(98)80170-2. [DOI] [PubMed] [Google Scholar]
- 6.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290(5489):92–7. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 7.Veillette A. Immune regulation by SLAM family receptors and SAP-related adaptors. Nature reviews Immunology. 2006;6(1):56–66. doi: 10.1038/nri1761. [DOI] [PubMed] [Google Scholar]
- 8.Morra M, Howie D, Grande MS, Sayos J, Wang N, Wu C, et al. X-linked lymphoproliferative disease: a progressive immunodeficiency. Annu Rev Immunol. 2001;19:657–82. doi: 10.1146/annurev.immunol.19.1.657. [DOI] [PubMed] [Google Scholar]
- 9.Pasquinelli V, Quiroga MF, Martinez GJ, Zorrilla LC, Musella RM, Bracco MM, et al. Expression of signaling lymphocytic activation molecule-associated protein interrupts IFN-gamma production in human tuberculosis. Journal of immunology. 2004;172(2):1177–85. doi: 10.4049/jimmunol.172.2.1177. [DOI] [PubMed] [Google Scholar]
- 10.Valdez PA, Wang H, Seshasayee D, van Lookeren Campagne M, Gurney A, Lee WP, et al. NTB-A, a new activating receptor in T cells that regulates autoimmune disease. The Journal of biological chemistry. 2004;279(18):18662–9. doi: 10.1074/jbc.M312313200. [DOI] [PubMed] [Google Scholar]
- 11.Falco M, Marcenaro E, Romeo E, Bellora F, Marras D, Vely F, et al. Homophilic interaction of NTBA, a member of the CD2 molecular family: induction of cytotoxicity and cytokine release in human NK cells. European journal of immunology. 2004;34(6):1663–72. doi: 10.1002/eji.200424886. [DOI] [PubMed] [Google Scholar]
- 12.Kumar KR, Li L, Yan M, Bhaskarabhatla M, Mobley AB, Nguyen C, et al. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006;312(5780):1665–9. doi: 10.1126/science.1125893. [DOI] [PubMed] [Google Scholar]
- 13.Snow AL, Pandiyan P, Zheng L, Krummey SM, Lenardo MJ. The power and the promise of restimulation-induced cell death in human immune diseases. Immunological reviews. 2010;236:68–82. doi: 10.1111/j.1600-065X.2010.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353(6347):858–61. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 15.Snow AL, Oliveira JB, Zheng L, Dale JK, Fleisher TA, Lenardo MJ. Critical role for BIM in T cell receptor restimulation-induced death. Biology direct. 2008;3:34. doi: 10.1186/1745-6150-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bidere N, Su HC, Lenardo MJ. Genetic disorders of programmed cell death in the immune system. Annual review of immunology. 2006;24:321–52. doi: 10.1146/annurev.immunol.24.021605.090513. [DOI] [PubMed] [Google Scholar]
- 17.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, et al. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annual review of immunology. 1999;17:221–53. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 18.Snow AL, Marsh RA, Krummey SM, Roehrs P, Young LR, Zhang K, et al. Restimulation-induced apoptosis of T cells is impaired in patients with X-linked lymphoproliferative disease caused by SAP deficiency. The Journal of clinical investigation. 2009;119(10):2976–89. doi: 10.1172/JCI39518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quiroga MF, Pasquinelli V, Martinez GJ, Jurado JO, Zorrilla LC, Musella RM, et al. Inducible costimulator: a modulator of IFN-gamma production in human tuberculosis. Journal of immunology. 2006;176(10):5965–74. doi: 10.4049/jimmunol.176.10.5965. [DOI] [PubMed] [Google Scholar]
- 20.Jurado JO, Alvarez IB, Pasquinelli V, Martinez GJ, Quiroga MF, Abbate E, et al. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. Journal of immunology. 2008;181(1):116–25. doi: 10.4049/jimmunol.181.1.116. [DOI] [PubMed] [Google Scholar]
- 21.Veillette A, Latour S. The SLAM family of immune-cell receptors. Current opinion in immunology. 2003;15(3):277–85. doi: 10.1016/s0952-7915(03)00041-4. [DOI] [PubMed] [Google Scholar]
- 22.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annual review of immunology. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 23.Ma CS, Nichols KE, Tangye SG. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu Rev Immunol. 2007;25:337–79. doi: 10.1146/annurev.immunol.25.022106.141651. [DOI] [PubMed] [Google Scholar]
- 24.Quiroga MF, Jurado JO, Martinez GJ, Pasquinelli V, Musella RM, Abbate E, et al. Cross-talk between CD31 and the signaling lymphocytic activation molecule-associated protein during interferon- gamma production against Mycobacterium tuberculosis. The Journal of infectious diseases. 2007;196(9):1369–78. doi: 10.1086/522522. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto S, Ji H, Howie D, Clarke K, Gullo C, Manning S, et al. Expression of the SH2D1A gene is regulated by a combination of transcriptional and post-transcriptional mechanisms. European journal of immunology. 2004;34(11):3176–86. doi: 10.1002/eji.200324755. [DOI] [PubMed] [Google Scholar]
- 26.Chen G, Tai AK, Lin M, Chang F, Terhorst C, Huber BT. Increased proliferation of CD8+ T cells in SAP-deficient mice is associated with impaired activation-induced cell death. European journal of immunology. 2007;37(3):663–74. doi: 10.1002/eji.200636417. [DOI] [PubMed] [Google Scholar]
- 27.Toossi Z, Kleinhenz ME, Ellner JJ. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. The Journal of experimental medicine. 1986;163(5):1162–72. doi: 10.1084/jem.163.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li B, Bassiri H, Rossman MD, Kramer P, Eyuboglu AF, Torres M, et al. Involvement of the Fas/Fas ligand pathway in activation-induced cell death of mycobacteria-reactive human gamma delta T cells: a mechanism for the loss of gamma delta T cells in patients with pulmonary tuberculosis. Journal of immunology. 1998;161(3):1558–67. [PubMed] [Google Scholar]
- 29.Janssen O, Wesselborg S, Heckl-Ostreicher B, Pechhold K, Bender A, Schondelmaier S, et al. T cell receptor/CD3-signaling induces death by apoptosis in human T cell receptor gamma delta + T cells. Journal of immunology. 1991;146(1):35–9. [PubMed] [Google Scholar]
- 30.Dennett NS, Barcia RN, McLeod JD. Age associated decline in CD25 and CD28 expression correlate with an increased susceptibility to CD95 mediated apoptosis in T cells. Experimental gerontology. 2002;37(2–3):271–83. doi: 10.1016/s0531-5565(01)00193-0. [DOI] [PubMed] [Google Scholar]
- 31.Richter GH, Mollweide A, Hanewinkel K, Zobywalski C, Burdach S. CD25 blockade protects T cells from activation-induced cell death (AICD) via maintenance of TOSO expression. Scandinavian journal of immunology. 2009;70(3):206–15. doi: 10.1111/j.1365-3083.2009.02281.x. [DOI] [PubMed] [Google Scholar]
- 32.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32(1):91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8(5):615–23. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 34.Detre C, Keszei M, Romero X, Tsokos GC, Terhorst C. SLAM family receptors and the SLAM-associated protein (SAP) modulate T cell functions. Semin Immunopathol. 2010;32(2):157–71. doi: 10.1007/s00281-009-0193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quiroga MF, Martinez GJ, Pasquinelli V, Costas MA, Bracco MM, Malbran A, et al. Activation of signaling lymphocytic activation molecule triggers a signaling cascade that enhances Th1 responses in human intracellular infection. Journal of immunology. 2004;173(6):4120–9. doi: 10.4049/jimmunol.173.6.4120. [DOI] [PubMed] [Google Scholar]
- 36.Katz G, Krummey SM, Larsen SE, Stinson JR, Snow AL. SAP facilitates recruitment and activation of LCK at NTB-A receptors during restimulation-induced cell death. Journal of immunology. 2014;192(9):4202–9. doi: 10.4049/jimmunol.1303070. [DOI] [PubMed] [Google Scholar]
- 37.Devadas S, Das J, Liu C, Zhang L, Roberts AI, Pan Z, et al. Granzyme B is critical for T cell receptor-induced cell death of type 2 helper T cells. Immunity. 2006;25(2):237–47. doi: 10.1016/j.immuni.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Wandstrat AE, Nguyen C, Limaye N, Chan AY, Subramanian S, Tian XH, et al. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 2004;21(6):769–80. doi: 10.1016/j.immuni.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Shah AH, Sowrirajan B, Davis ZB, Ward JP, Campbell EM, Planelles V, et al. Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell host & microbe. 2010;8(5):397–409. doi: 10.1016/j.chom.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, et al. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(13):7449–54. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu C, Nguyen KB, Pien GC, Wang N, Gullo C, Howie D, et al. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nature immunology. 2001;2(5):410–4. doi: 10.1038/87713. [DOI] [PubMed] [Google Scholar]
- 42.Mehrle S, Frank S, Schmidt J, Schmidt-Wolf IG, Marten A. SAP and SLAM expression in anti-CD3 activated lymphocytes correlates with cytotoxic activity. Immunology and cell biology. 2005;83(1):33–9. doi: 10.1111/j.1440-1711.2004.01302.x. [DOI] [PubMed] [Google Scholar]
- 43.Nagy N, Matskova L, Kis LL, Hellman U, Klein G, Klein E. The proapoptotic function of SAP provides a clue to the clinical picture of X-linked lymphoproliferative disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(29):11966–71. doi: 10.1073/pnas.0905691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fayyazi A, Eichmeyer B, Soruri A, Schweyer S, Herms J, Schwarz P, et al. Apoptosis of macrophages and T cells in tuberculosis associated caseous necrosis. The Journal of pathology. 2000;191(4):417–25. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH664>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 45.Hirsch CS, Toossi Z, Vanham G, Johnson JL, Peters P, Okwera A, et al. Apoptosis and T cell hyporesponsiveness in pulmonary tuberculosis. The Journal of infectious diseases. 1999;179(4):945–53. doi: 10.1086/314667. [DOI] [PubMed] [Google Scholar]
- 46.Soruri A, Schweyer S, Radzun HJ, Fayyazi A. Mycobacterial antigens induce apoptosis in human purified protein derivative-specific alphabeta T lymphocytes in a concentration-dependent manner. Immunology. 2002;105(2):222–30. doi: 10.1046/j.0019-2805.2001.01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nichols KE, Koretzky GA, June CH. SAP: natural inhibitor or grand SLAM of T cell activation? Nature immunology. 2001;2(8):665–6. doi: 10.1038/90595. [DOI] [PubMed] [Google Scholar]
- 48.Ueda A, Zhou L, Stein PL. Fyn promotes Th17 differentiation by regulating the kinetics of RORgammat and Foxp3 expression. Journal of immunology. 2012;188(11):5247–56. doi: 10.4049/jimmunol.1102241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cencioni MT, Santini S, Ruocco G, Borsellino G, De Bardi M, Grasso MG, et al. FAS-ligand regulates differential activation-induced cell death of human T-helper 1 and 17 cells in healthy donors and multiple sclerosis patients. Cell death & disease. 2015;6:e1785. doi: 10.1038/cddis.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jurado JO, Pasquinelli V, Alvarez IB, Pena D, Rovetta AI, Tateosian NL, et al. IL-17 and IFN-gamma expression in lymphocytes from patients with active tuberculosis correlates with the severity of the disease. Journal of leukocyte biology. 2012;91(6):991–1002. doi: 10.1189/jlb.1211619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malbran A, Belmonte L, Ruibal-Ares B, Bare P, Massud I, Parodi C, et al. Loss of circulating CD27+ memory B cells and CCR4+ T cells occurring in association with elevated EBV loads in XLP patients surviving primary EBV infection. Blood. 2004;103(5):1625–31. doi: 10.1182/blood-2003-07-2525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.