Abstract
As indispensable protein cofactors, Fe, Mn, Cu and Zn are at the center of multifaceted acclimation mechanisms that have evolved to ensure extracellular supply meets intracellular demand. Starting with selective transport at the plasma membrane and ending in protein metalation, metal homeostasis in algae involves regulated trafficking of metal ions across membranes, intracellular compartmentalization by proteins and organelles, and metal-sparing/recycling mechanisms to optimize metal-use efficiency. Overlaid on these processes are additional circuits that respond to the metabolic state as well as to the prior metal status of the cell. In this review, we focus on recent progress made toward understanding the pathways by which the single-celled, green alga Chlamydomonas reinhardtii controls its cellular trace metal economy. We also compare these mechanisms to characterized and putative processes in other algal lineages. Photosynthetic microbes continue to provide insight into cellular regulation and handling of Cu, Fe, Zn and Mn as a function of the nutritional supply and cellular demand for metal cofactors. New experimental tools such as RNA-Seq and subcellular metal imaging are bringing us closer to a molecular understanding of acclimation to supply dynamics in algae and beyond.
Keywords: acidocalcisome, polyphosphate, flavodoxin, diatoms, transferrin, iron, copper, zinc
Graphical abstract
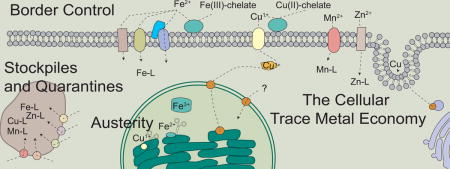
Introduction
Economics of metal ion metabolism
Homeostasis is the property of a biological system to regulate its internal environment at or near a steady-state as the external environment varies. For the trace metal nutrients Zn, Cu, Mn and Fe, homeostasis is the process by which organisms maintain a steady-state level of metal ion availability inside the cell as the abundance and availability of metal ions varies outside the cell. It is important to note that metal ion availability inside the cell is not synonymous with a cell’s metal quota, which is defined as the total metal content of a cell. Because of luxury consumption, the ability to compartmentalize excess ions, the presence of metal-sparing/recycling strategies during sustained metal limitation, and changing metabolic requirements for metal ions, the metal quota can vary [1–11]. Metal homeostasis embodies a multitude of mechanisms that each act to ensure the cellular demand for metal ions is met. Supply and demand is one of the most fundamental concepts of economics, and it forms the foundation of cellular metal economy.
Definitions and scenarios
Supply refers to the concentration/amount of a metal ion or metal-chelate that is nutritionally available for uptake. Demand refers to how many proteins in the cell require that metal ion to function – resulting in a baseline or minimal cellular quota for that metal (metal/cell). For microorganisms, the ideal situation of supply being equal to demand is rare. The availability of metal ions in the environment can increase or decrease as can the cellular demand for that metal. For instance, acclimation to a change in carbon or nitrogen source may involve de novo synthesis (or degradation) of abundant metalloproteins resulting in a change in cellular quota for that metal [12,13]. Therefore, metal economy involves consideration of the environmental variable (supply) and also the metabolic/developmental variable (demand). Maintaining homeostasis requires multiple regulatory loops involving selective uptake in response to changing nutrition (or in the real world, competition for nutrients), intracellular distribution of the metal nutrient, and expression of metalloproteins (demand). Overlaid on the regulatory scheme are additional circuits dependent on the prior history of the cell. For instance, if a prior period of selective storage occurred because supply exceeded demand, mobilization from such reserves might be the first level response. Without metal economy, organisms would lose the flexibility to occupy varied niches, respond to other organisms (such as host-pathogen or host-symbiont interactions), or develop in response to environmental or biotic cues (Figure 1A). Most organisms, but microbes in particular, have evolved mechanisms to thrive within the range of feast and famine scenarios (Figure 1B).
Figure 1. Controlled metal economy.
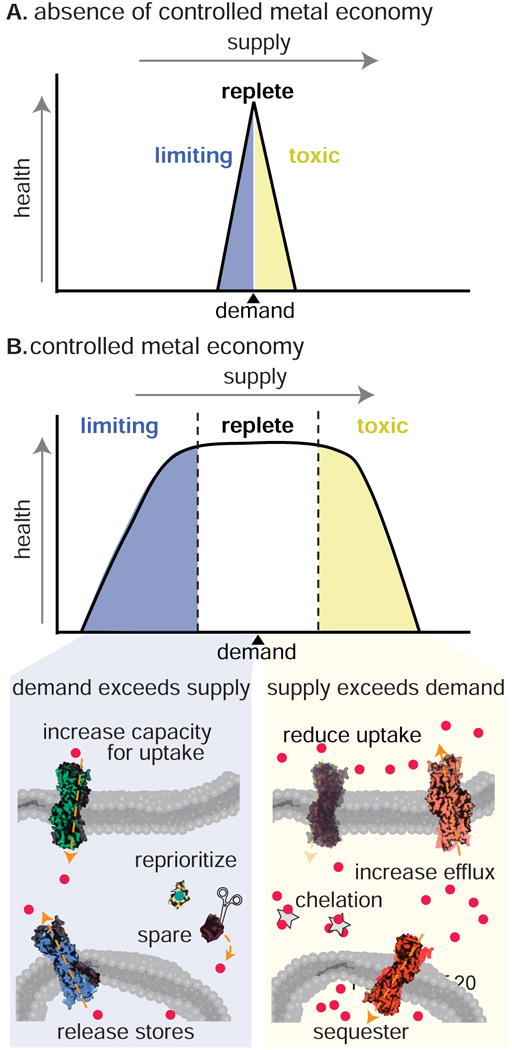
Organisms have evolved to respond to and control their interaction with nutrients supplied by the environment. Metal ions such as Cu, Fe, Zn and Mn provide a particular challenge in that they are often required as essential cofactors for indispensable functions within the cell. Therefore, each organism has to ensure that enough of these metals are concentrated within the cell in the face of supply fluctuations and possible starvation. Nevertheless, if allowed to accumulate in excess within the cell, these ions can cause damage through deleterious reactions with macromolecules, particularly in the presence of oxygen. Each cell must strike a balance between the essential and potentially toxic nature of these elements. In the absence of controlled metal economy (A), organisms would be restricted to life in a static utopian environment where supply of these nutrients perfectly matches the cellular requirement. Instead, organisms have evolved to control their metal economy (B) through a collection of processes that serves to tolerate nutrient fluctuations and quickly acclimate to cellular demands.
Border Control
Some of the most important players in intracellular metal economy are membranes and the transporters embedded within them. Together they create a selective barrier that separates a potentially capricious source of nutrients in the environment or growth milieu and the controlled chemical reactions within the cell. Genes encoding candidate metal transporters for Cu, Zn, Fe and Mn have been identified in the C. reinhardtii genome by several techniques, including sequence similarity to previously characterized transporters, differential expression (Figure 3) and reverse genetics (reviewed in [14]). These transporters belong to a mix of protein families with origins in various lineages and unique phylogenetic occurrences (Figure 2). For instance, Fe uptake at the plasma membrane occurs by a reduction-oxidation-transport mechanism, involving FRE1 (Fe reductase), FOX1 (Fe oxidase) and FTR1 (permease) [15–19], which is found in other algae, such as rhodophytes and diatoms [20], but absent from most land plants and prasinophytes [21,22] (Figure 2A). Additional components of high-affinity Fe uptake include the soluble secreted proteins FEA1 and FEA2, which are only found in the algal lineages [17,23,24]. The Cu-ATPases encoded in the C. reinhardtii genome reflect the origin of metalloproteins to whom they supply Cu. For instance, in the green lineage two Cu-ATPases (PAA1 and PAA2 in Arabidopsis) and a Cu chaperone (PCH1 in Arabidopsis) supply Cu to plastocyanin within the chloroplast [25]. This intracellular Cu transport pathway only exists in algae that contain plastocyanin; red algae constitutively express cytochrome c6 (requires heme instead of Cu) and orthologs of PAA1, PAA2 and PCH1 are missing (Figure 4B). Interestingly, the diatoms Thalassiosira oceanica and Fragilariopsis cylindrus and the haptophyte Emiliana huxleyi have plastocyanin [26,27], but like red algae, which are the modern relatives of the engulfed alga that became their plastid, these algae are also missing orthologs of PAA1, PAA2 and PCH1, suggesting a different pathway exists to metalate plastocyanin.
Figure 3. Transporters involved in border control.
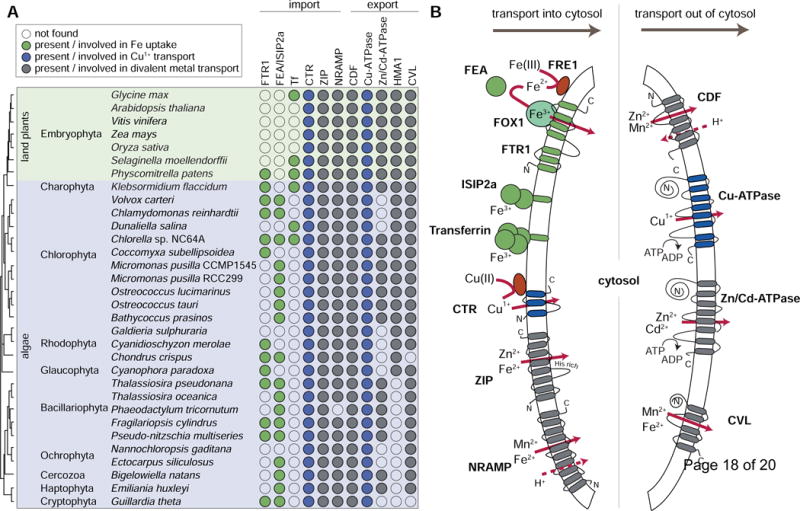
A, a filled circle signifies the presence of at least one homolog of the indicated protein family (FTR1, high-affinity iron permease; FEA/ISIP2a, Fe-assimilation proteins; Tf, transferrin; CTR, Cu assimilatory transport; ZIP, divalent metal transporter; NRAMP, divalent metal transporter; CDF, divalent metal transporter; Cu-ATPase, Cu-transporting subfamily of the P1B-type ATPases; Zn/Cd-ATPase, Zn/Cd-transporting subfamily of the P1B-type ATPases; HMA1, similar to Zn/Cd-subfamily of P1B-type ATPases but with unknown function; CVL, Ccc1/VIT1-like. A schematic species tree is shown at the left. B, predicted membrane topologies and common substrates for each transporter family are shown. The C. reinhardtii protein names are given for the high-affinity Fe-transport complex involving FRE1, FOX1 and FTR1. Solid arrows represent the direction of metal ion transport either into the cytosol across the plasma or organelle membrane (left hand side of figure) or out of the cytosol across the intracellular membranes of organelles (right hand side of figure). Metal reductases (FRE1 for Fe reduction in C. reinhardtii) and an as yet unidentified reductase for Cu2+ reduction before transport by CTR are represented as dark red ovals Abbreviations for importers: FRE1, ferric reductase 1 ; FOX1, ferroxidase 1, MCO (multi-copper oxidase) family; FTR1, Fe transporter 1; FEA, Fe-assimilation protein; ISIP2a, iron starvation inducible protein 2a, first identified in Phaeodactylum tricornutum; ZIP, Zrt-, Irt-like proteins; CTR, Cu transporter; NRAMP (natural resistance-associated macrophage proteins). Abbreviations for exporters: CDF, cation diffusion facilitator, named MTP in plants; CVL, Ccc1/VIT1, Ca(II)-sensitive cross-complementer 1/vacuolar iron transporter 1.
Figure 2. Metal-nutrition-specific transcript abundance of transporters involved in border control.
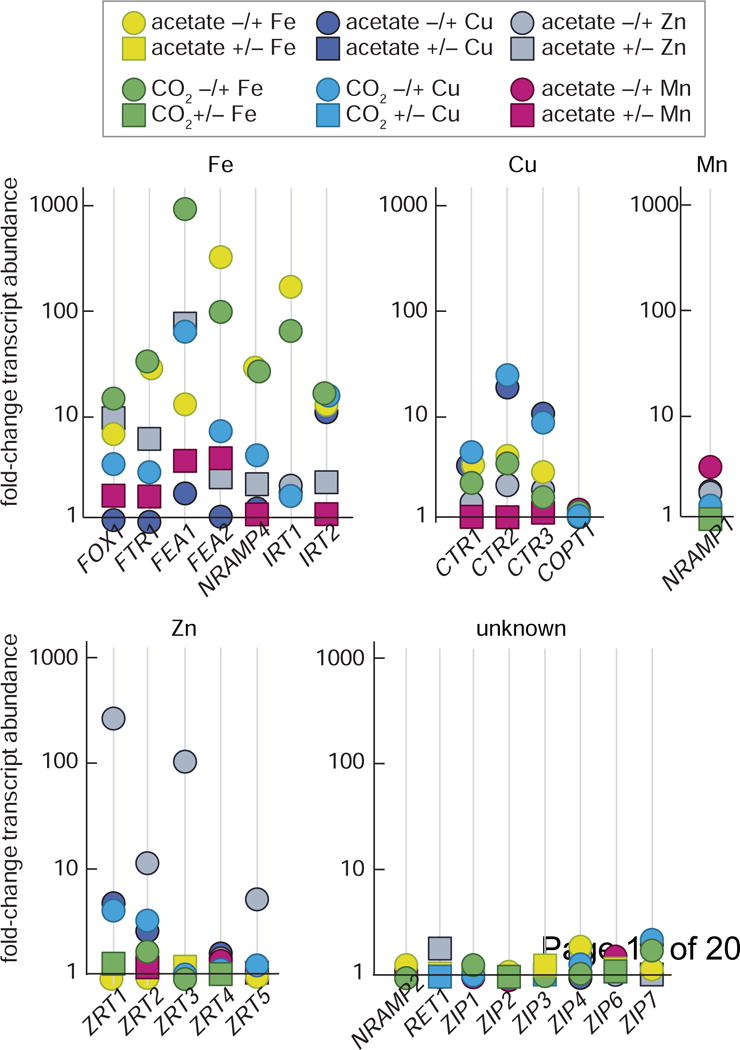
Fold-change in transcript abundance for known and candidate transition metal transporters encoded in the C. reinhardtii genome. Data compiled from Castruita, et al. [56], Urzica, et al. [70], Malasarn, et al. [44] and Castruita and Merchant, unpublished. Each data point represents the fold-change in transcript abundance as measured by RNA-Seq. Circles indicate that the corresponding transcript increased in abundance under the metal-limitation condition, while a square indicates that transcript abundance decreased under the metal-limitation condition. Where available, metal-responsive fold-changes in the presence of a fixed carbon source (acetate) or in the absence (CO2) is given.
Figure 4. Austerity measures in algae.
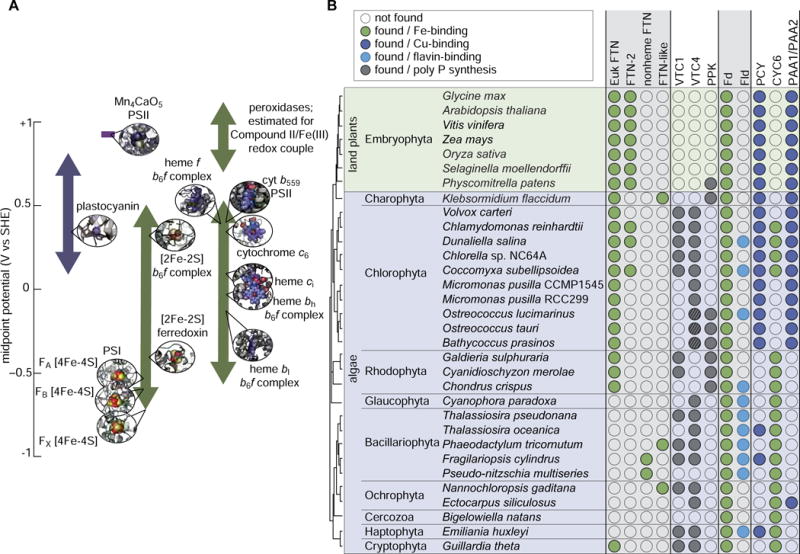
A, the same metal ion can catalyze different types of reactions, because the cognate apo-protein is able to tune the inherent chemistry of the metal ion. The range of midpoint potentials (measured in volts (V) vs. the Standard Hydrogen Electrode (SHE)) achieved by various combinations of metal ions, prosthetic groups and protein backbones illustrates both the functional versatility of metal ions and the control applied by the protein environment. The approximate midpoint potentials for specific metal-dependent redox centers in the oxygen-evolving electron transfer chain are given. The placement of the Mn cluster of photosystem II in this scheme is estimated. Illustrations and midpoint potentials for the photosynthetic redox centers are based on the following structures and references: plastocyanin (PDB entry 2PLT) [71], PSI (PDB entry 3LW5) [72,73], PSII (PDB entry 3ARC) [74], cytochrome c6 (PDB entry 1CYI) [75], cytochrome b6f (PDB entry 1Q90) [76,77]. B, identification of homologs and co-occurrence of selected protein families involved in metal storage or austerity measures. The identified ferritins are categorized based on the top domain hit in NCBI CDD: Euk FTN, cd01056; FTN-2 pfam13668; nonheme FTN, cd01055; FTN-like, cl00264. VTC1/VTC4 (vacuolar transport chaperone complex) and PPK (polyphosphate kinase) synthesize polyphosphate and are used as markers for acidocalcisome-like compartments. For the two Ostreococcus species and Bathycoccus prasinos, a protein similar to VTC4 is identifiable in their genomes, but these proteins lack conservation within the N-terminal portion of the SPX domain. The Thalassiosira pseudonana VTC4 homolog also appears to lack the full-length N-terminal domain, but the apparent truncation is likely the result of an incorrect gene model (specifically, the full-length SPX domain is recovered if an alternative upstream methionine is used). Other abbreviations: Fd, ferredoxin; Fld, flavodoxin; PCY, plastocyanin; CYC6, cytochrome c6; PAA1/PAA2, chloroplast-localized Cu-ATPases.
Stockpiles and quarantines
It is advantageous to bank or hoard a limiting resource when supplies of the resource wax and wane, and the same is true for the essential trace metals. They are essential because each metal has unique chemical properties that render it irreplaceable as a catalyst (Figure 4): for example, Mn in photosystem II, Fe in the Fe-S centers of photosystem I, and Cu in plastocyanin. If a metal ion is unavailable for synthesis of metalloproteins, and at least one of those metal-dependent proteins is essential [28] (such as RNA polymerase (Zn) [29,30], Rli1p (Fe) [31,32], the linear photosynthetic electron transport chain (Fe, Mn and Cu) during photoautotrophic growth, or respiratory electron transport chain (Fe and Cu) during heterotrophic growth), cell viability is lost. The capacity for storage offers a distinct selective advantage.
The best understood storage mechanism in algae is ferritin for Fe. In C. reinhardtii, as in land plants, ferritin is located in the chloroplast, and this is likely true in other algae and diatoms as well [33,34]. Given the location of ferritin in the chloroplast and the abundance of Fe in the photosynthetic complexes, a physiological role for ferritin in mediating Fe availability to those complexes during various developmental stages and stress conditions occurs both on land and in the oceans (reviewed in [35]). However, the regulation and roles of ferritin in Fe homeostasis differs. In Arabidopsis, ferritin expression is induced by a supply of excess Fe, and a role in storage and protection has been prescribed [36]. In C. reinhardtii, expression of two ferritins Fer1 (recovered in soluble fraction) and Fer2 (recovered in particulate fraction) is increased during Fe limitation [34]. Instead of Fe-storage, these ferritins are proposed to mediate an Fe-sparing/recycling process involving degradation of the Fe-rich photosystem I (PSI) complex [37]. The Ostreococcus tauri ferritin appears to play a similar role in buffering Fe in the chloroplast (rather than storage) as a function of the day/night cycle [22,38]. Although ferritin is central to iron homeostasis in both green algae and diatoms [39], it is not found in all algal genomes (Figure 4B).
The acidic vacuole, or acidocalcisome, is less understood but potentially of broader relevance to metal metabolism in algae (Figure 4B). The acidocalcisome, first characterized in trypanosomatids, is a storage organelle that contains polyphosphate, pyrophosphate, calcium and divalent metal ions, such as Fe and Zn, and appears as an electron-dense granule in transmission electron micrographs [40]. Acidocalcisomes share properties with lysosomes and lysosome-related organelles from mammalian cells and, like the plant vacuole, contain both V-type ATPases and V-type pyrophosphatases, which acidify the interior. In addition to storage, these compartments can quarantine potentially toxic metals such as cadmium [41] and are expected to play a significant role in balancing intracellular accessibility of essential and non-essential (even deleterious) metal ions in C. reinhardtii and other algae [42]. Indeed, acidic vacuoles appear to be waypoints for Fe in Dunaliella salina [43] and recent investigation of unexpected Cu hyper-accumulation during zinc starvation [44] has revealed a role of lysosome-like organelles in C. reinhardtii in Cu homeostasis. These compartments trap Cu in a bio-unavailable state concurrent with the induction of the well-characterized Cu-deficiency response [45]. The Cu does not stay ensnared forever. When Zn is resupplied, the Cu-response is turned off, the size and number of bodies decreases and Cu is redistributed to Cu-dependent proteins [45], demonstrating that the acidocalcisome is not a trash can, but rather a storage silo. The purpose of this Cu stockpiling during Zn starvation is not yet known, but one attractive hypothesis is that this phenomenon serves to protect Zn-dependent proteins from mismetallation by the highly competitive Cu ion [46].
Austerity
When unable to import sufficient supply of a particular metal ion and if the stockpiles are depleted, C. reinhardtii and other algae initiate austerity measures. The best characterized austerity responses in algae are metal nutrition responsive accumulation of electron-transfer proteins in the chloroplast: plastocyanin (Cu-containing) replaced by cytochrome c6 (Fe-containing) as a function of Cu availability and ferredoxin (Fd, Fe-containing) replaced by flavodoxin (Fld, flavin-containing, Fe-independent) as a function of Fe availability [49] (Figure 4B). Note that the specific mechanism by which Cu-responsive regulation is achieved can differ between algae [47,48]. While these metal-sparing mechanisms permit a reduction in the cellular Cu and Fe quota, respectively, recently plastocyanin was found to serve as an intracellular stockpile of Cu [8], and suggests that in those algae that contain Fd and Fld, Fd is a reservoir for Fe that can be recycled after the switch to Fld. When faced with Cu starvation, Cu can be mobilized from plastocyanin and transferred to the mitochondrion, where it is used to sustain the activity of cytochrome c oxidase, an irreplaceable component of the respiratory electron transfer chain. If plastocyanin were lost in the green algal lineage and constitutively replaced by cytochrome c6, these organisms would also lose a copper reservoir. Plastocyanin therefore provides an ingenious mechanism for algae to compete with other copper-utilizing organisms in their environment.
Sometimes austerity and reduced quota takes place without direct functional replacement. During mixotrophic growth (when the cells are supplied with air, light and acetate), C. reinhardtii down-regulates the abundance of Fe-containing complexes of the photosynthetic chain, in particular PSI, cytochrome b6f and Fd [50–52]. The cell sacrifices photosynthetic capacity in favor of heterotrophic oxygen-dependent metabolism with a reduced Fe quota: Fe is recycled from the photosynthetic complexes to Fe-dependent superoxide dismutase within the chloroplast and to respiratory complexes in the mitochondria [37,52,53]. However, because Fd is involved in indispensable processes, a low level is maintained [51]. Transcriptome and proteome studies have provided evidence of similar metal-sparing events, whereby the demand for the limiting nutrient is decreased without necessarily providing a replacement [54].
Sensing and responding
At the center of a healthy metal economy is the ability to sense changes and respond quickly and appropriately to accommodate those changes. Understanding the mechanistic details of how C. reinhardtii (and other eukaryotic microbes) senses an imbalance between intracellular metal availability and the number of proteins requiring that metal ion is an active area of research with much to be discovered. Indeed, the mechanisms responsible for sensing metal status and adjusting gene expression or protein activity are largely unknown. A few exceptions do exist, which highlight the complexity of regulating a balance between supply and demand and underscore the regulatory mechanisms that remain to be discovered.
The Cu-response regulator CRR1
CRR1 is a transcription factor with a DNA binding domain (named SBP for the prototype identified as a SQUAMOSA promoter binding protein) that is unique to the plant lineage [55]. Its SBP domain recognizes the GTAC core of copper response elements (CuREs) found within the promoters of roughly 60 C. reinhardtii genes [56]. CRR1 regulates Cu-responsive processes such as the plastocyanin/cytochrome c6 switch, Cu salvage from plastocyanin, and Cu assimilation (CTR genes) [8,56]. Other targets are involved in subtle modifications of the photosynthetic apparatus, such as desaturation of thylakoid membrane galactolipids and changes in minor Chl-protein abundances, which may be required to accommodate the switch to Cyt c6 [56,57]. Remarkably, this transcription factor is also involved in the hypoxia-, Ni- and Zn-sensing pathways [58,59,60], but the mechanisms behind these regulatory pathways are yet to be discerned.
Identifying proteins involved in Fe-responsive regulation
A multitude of enzymes in C. reinhardtii require Fe as a catalytic cofactor [61] and, as discussed above, Fe-responsive gene regulation is different depending on trophic status. Therefore, whereas a single central transcription factor has been found at the heart of Cu-dependent gene regulation, Fe sensing and signaling is expected to be more complex. Some aspects are being uncovered, such as the identification of TAA1 (TRANSLATION OF psaA1), which is proposed to be involved in down-regulation of PSI during Fe starvation [62]. How TAA1, in turn, is regulated by Fe status is unknown. Proteins potentially involved in FOX1 expression have also been identified through a genetic screen assaying absence of FOX1-promoter-arylsulfatase fusion activity during Fe starvation. The corresponding loci encode a putative protein kinase [63] and a RING-type Zn finger protein that contains a Ran-binding domain (Ran regulates receptor-mediated transport between the nucleus and cytoplasm) [64]. However, it remains unknown whether these two proteins are directly involved in Fe-responsive signaling. Indeed, the experimentally determined consensus sequence for the RING-finger protein is not found in the promoter region of FOX1.
Identifying proteins involved in Zn- and Mn-responsive regulation
Transcriptome sequencing of C. reinhardtii cells grown with or without Zn suggests a specific transcriptional Zn response involving ZIP-family transporters and members of the COG0523 family of putative Zn chaperones [44]. While CRR1 is clearly involved at some level based on analysis of CRR1 mutants [58], how CRR1 regulates Zn-responsive gene expression and the identity of other transcription factors is currently unknown. In contrast, transcriptome sequencing of Mn-limited cells has not revealed a specific Mn-response bringing to question whether a Mn-specific transcription factor exists in C. reinhardtii (Castruita and Merchant, unpublished).
Conclusions
While significant progress has been made in identifying the means by which C. reinhardtii and other algae have evolved to regulate their cellular trace metal economy, many questions remain. Transporters encoded in the genome are routinely identified by homology-based techniques and the response of those transporters’ expression to metal status is easily quantified, but the intracellular location of most metal transporters is unknown. This information is central to understanding function. For instance, an importer localized to the plasma membrane has a different function (assimilate metal ions) from a homologous transporter in the vacuolar membrane (liberate metal store). Ongoing development of a genome-wide library of C. reinhardtii gene disruptants [65] and the characterization of mutants defective in metal homeostasis are expected to yield considerable insight, especially for those proteins, such as membrane-bound transporters, that are notoriously difficult to characterize in vitro. Reverse genetics by inducible artificial miRNAs [66] and fluorescent protein tags [67,68] combined with subcellular metal imaging by live cell imaging and imaging mass spectrometry also promises to generate insight into the molecular mechanisms underlying subcellular metal speciation and compartmentalization. For instance, given the disparate observations of vacuole-like compartmentalization for both essential and non-essential metal ions, how is selectivity determined? Do different compartments have different compositions in terms of transporter profile, ligands and element content? In addition, recent studies with Ostreococcus have revealed diel rhythms for Fe and Zn quotas [38,69], further highlighting our lack of understanding concerning how these metals are sensed and regulated. In general, (with the exception of CRR1) the proteins responsible for linking metal status to gene/protein expression are unknown. In some cases, these networks are expected to be complex as is the case for regulatory circuits that enable Fe-responsive gene expression as a function of carbon metabolism in C. reinhardtii.
Highlights.
Trace metal economy: border control, stockpiles, quarantines, and austerity
Compartmentalized metal sequestration and storage is dynamic
The molecular details of metal-status sensing in algae remain to be detailed
Acknowledgments
This work was supported by the Office of Biological and Environmental Research of the Department Of Energy (CBB) and by grants (to SM) from the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S Department of Energy (DE-FD02-04ER15529) and the National Institutes of Health (GM42143).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- 1.Raja MR, Waterman SR, Qiu J, Bleher R, Williamson PR, O’Halloran TV. A copper hyperaccumulation phenotype correlates with pathogenesis in Cryptococcus neoformans. Metallomics. 2013;5:363–371. doi: 10.1039/c3mt20220h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simm C, Lahner B, Salt D, LeFurgey A, Ingram P, Yandell B, Eide DJ. Saccharomyces cerevisiae vacuole in zinc storage and intracellular zinc distribution. Eukaryot Cell. 2007;6:1166–1177. doi: 10.1128/EC.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peers G, Price NM. A role for manganese in superoxide dismutases and growth of iron-deficient diatoms. Limnology and Oceanography. 2004;49:1774–1783. [Google Scholar]
- 4.Rodriguez IB, Lin S, Ho J, Ho TY. Effects of Trace Metal Concentrations on the Growth of the Coral Endosymbiont Symbiodinium kawagutii. Front Microbiol. 2016;7:82. doi: 10.3389/fmicb.2016.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho T-Y, Quigg A, Finkel ZV, Milligan AJ, Wyman K, Falkowski PG, Morel FMM. The elemental composition of some marine phytoplankton. Journal of Phycology. 2003;39:1145–1159. [Google Scholar]
- 6.Li CX, Gleason JE, Zhang SX, Bruno VM, Cormack BP, Culotta VC. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proc Natl Acad Sci U S A. 2015;112:E5336–5342. doi: 10.1073/pnas.1513447112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkel Z, Quigg A, Raven J, Reinfelder J, Schofield O, Falkowski P. Irradiance and the elemental stoichiometry of marine phytoplankton. Limnology and Oceanography. 2006;51:2690–2701. [Google Scholar]
- **8.Kropat J, Gallaher SD, Urzica EI, Nakamoto SS, Strenkert D, Tottey S, Mason AZ, Merchant SS. Copper economy in Chlamydomonas: Prioritized allocation and reallocation of copper to respiration vs. photosynthesis. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1422492112. The authors demonstrate recycling of Cu from plastocyanin in the chloroplast to cytochrome c oxidase in the mitochondrion medaited by CRR1-dependent degradation of plastocyanin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kropat J, Hong-Hermesdorf A, Casero D, Ent P, Castruita M, Pellegrini M, Merchant SS, Malasarn D. A revised mineral nutrient supplement increases biomass and growth rate in Chlamydomonas reinhardtii. Plant J. 2011;66:770–780. doi: 10.1111/j.1365-313X.2011.04537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King AL, Sañudo-Wilhelmy SA, Leblanc K, Hutchins DA, Fu F. CO2 and vitamin B12 interactions determine bioactive trace metal requirements of a subarctic Pacific diatom. ISME J. 2011;5:1388–1396. doi: 10.1038/ismej.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page MD, Kropat J, Hamel PP, Merchant SS. Two Chlamydomonas CTR copper transporters with a novel cys-met motif are localized to the plasma membrane and function in copper assimilation. Plant Cell. 2009;21:928–943. doi: 10.1105/tpc.108.064907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito MA, Bertrand EM, Dutkiewicz S, Bulygin VV, Moran DM, Monteiro FM, Follows MJ, Valois FW, Waterbury JB. Iron conservation by reduction of metalloenzyme inventories in the marine diazotroph Crocosphaera watsonii. Proc Natl Acad Sci U S A. 2011;108:2184–2189. doi: 10.1073/pnas.1006943108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuit C, Waterbury J, Ravizzaz G. Diel variation of molybdenum and iron in marine diazotrophic cyanobacteria. Limnology and Oceanography. 2004;49:978–990. [Google Scholar]
- 14.Blaby-Haas CE, Merchant SS. The ins and outs of algal metal transport. Biochim Biophys Acta. 2012;1823:1531–1552. doi: 10.1016/j.bbamcr.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terzulli A, Kosman DJ. Analysis of the high-affinity iron uptake system at the Chlamydomonas reinhardtii plasma membrane. Eukaryot Cell. 2010;9:815–826. doi: 10.1128/EC.00310-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Fontaine S, Quinn JM, Nakamoto SS, Page MD, Göhre V, Moseley JL, Kropat J, Merchant S. Copper-dependent iron assimilation pathway in the model photosynthetic eukaryote Chlamydomonas reinhardtii. Eukaryot Cell. 2002;1:736–757. doi: 10.1128/EC.1.5.736-757.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen MD, del Campo JA, Kropat J, Merchant SS. FEA1, FEA2, and FRE1, encoding two homologous secreted proteins and a candidate ferrireductase, are expressed coordinately with FOX1 and FTR1 in iron-deficient Chlamydomonas reinhardtii. Eukaryot Cell. 2007;6:1841–1852. doi: 10.1128/EC.00205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herbik A, Bölling C, Buckhout TJ. The involvement of a multicopper oxidase in iron uptake by the green algae Chlamydomonas reinhardtii. Plant Physiol. 2002;130:2039–2048. doi: 10.1104/pp.013060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen JC, Hsieh SI, Kropat J, Merchant SS. A ferroxidase encoded by FOX1 contributes to iron assimilation under conditions of poor iron nutrition in Chlamydomonas. Eukaryot Cell. 2008;7:541–545. doi: 10.1128/EC.00463-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groussman RD, Parker MS, Armbrust EV. Diversity and Evolutionary History of Iron Metabolism Genes in Diatoms. PLoS One. 2015;10:e0129081. doi: 10.1371/journal.pone.0129081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glaesener AG, Merchant SS, Blaby-Haas CE. Iron economy in Chlamydomonas reinhardtii. Front Plant Sci. 2013;4:337. doi: 10.3389/fpls.2013.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **22.Lelandais G, Scheiber I, Paz-Yepes J, Lozano JC, Botebol H, Pilátová J, Žárský V, Léger T, Blaiseau PL, Bowler C, et al. Ostreococcus tauri is a new model green alga for studying iron metabolism in eukaryotic phytoplankton. BMC Genomics. 2016;17:319. doi: 10.1186/s12864-016-2666-6. The authors present an overview of the transcriptional Fe-response in marine picoeukaryotic green alga O. tauri, a reference organism, which will likely accelerate attainment of algal-specific metal metabolism because it is relatively easy to culture and targeted gene knock-out mutants can be obtained through homolgous recombination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narayanan NN, Ihemere U, Chiu WT, Siritunga D, Rajamani S, Singh S, Oda S, Sayre RT. The iron assimilatory protein, FEA1, from Chlamydomonas reinhardtii facilitates iron-specific metal uptake in yeast and plants. Frontiers in Plant Science. 2011;2 doi: 10.3389/fpls.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Morrissey J, Sutak R, Paz-Yepes J, Tanaka A, Moustafa A, Veluchamy A, Thomas Y, Botebol H, Bouget FY, McQuaid JB, et al. A novel protein, ubiquitous in marine phytoplankton, concentrates iron at the cell surface and facilitates uptake. Curr Biol. 2015;25:364–371. doi: 10.1016/j.cub.2014.12.004. The authors characterize an Fe-assimilation protein unique to marine algae that shares a common ancestor with the FEA proteins first described in green algae. [DOI] [PubMed] [Google Scholar]
- 25.Blaby-Haas CE, Padilla-Benavides T, Stübe R, Argüello JM, Merchant SS. Evolution of a plant-specific copper chaperone family for chloroplast copper homeostasis. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1421545111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lommer M, Specht M, Roy AS, Kraemer L, Andreson R, Gutowska MA, Wolf J, Bergner SV, Schilhabel MB, Klostermeier UC, et al. Genome and low-iron response of an oceanic diatom adapted to chronic iron limitation. Genome Biol. 2012;13:R66. doi: 10.1186/gb-2012-13-7-r66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peers G, Price NM. Copper-containing plastocyanin used for electron transport by an oceanic diatom. Nature. 2006;441:341–344. doi: 10.1038/nature04630. [DOI] [PubMed] [Google Scholar]
- 28.Hänsch R, Mendel RR. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl) Curr Opin Plant Biol. 2009;12:259–266. doi: 10.1016/j.pbi.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Scrutton MC, Wu CW, Goldthwait DA. The presence and possible role of zinc in RNA polymerase obtained from Escherichia coli. Proc Natl Acad Sci U S A. 1971;68:2497–2501. doi: 10.1073/pnas.68.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falchuk KH, Mazus B, Ulpino L, Vallee BL. Euglena gracilis DNA dependent RNA polymerase II: a zinc metalloenzyme. Biochemistry. 1976;15:4468–4475. doi: 10.1021/bi00665a021. [DOI] [PubMed] [Google Scholar]
- 31.Kispal G, Sipos K, Lange H, Fekete Z, Bedekovics T, Janáky T, Bassler J, Aguilar Netz DJ, Balk J, Rotte C, et al. Biogenesis of cytosolic ribosomes requires the essential iron-sulphur protein Rli1p and mitochondria. EMBO J. 2005;24:589–598. doi: 10.1038/sj.emboj.7600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khoshnevis S, Gross T, Rotte C, Baierlein C, Ficner R, Krebber H. The iron-sulphur protein RNase L inhibitor functions in translation termination. EMBO Rep. 2010;11:214–219. doi: 10.1038/embor.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seckbach J. Ferreting out the secrets of plant ferritin‐a review. J ournal of Plant Nutrition. 1982;5:369–394. [Google Scholar]
- 34.Long JC, Sommer F, Allen MD, Lu SF, Merchant SS. FER1 and FER2 encoding two ferritin complexes in Chlamydomonas reinhardtii chloroplasts are regulated by iron. Genetics. 2008;179:137–147. doi: 10.1534/genetics.107.083824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley JM, Le Brun NE, Moore GR. Ferritins: furnishing proteins with iron. J Biol Inorg Chem. 2016;21:13–28. doi: 10.1007/s00775-016-1336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briat JF, Duc C, Ravet K, Gaymard F. Ferritins and iron storage in plants. Biochim Biophys Acta. 2010;1800:806–814. doi: 10.1016/j.bbagen.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Busch A, Rimbauld B, Naumann B, Rensch S, Hippler M. Ferritin is required for rapid remodeling of the photosynthetic apparatus and minimizes photo-oxidative stress in response to iron availability in Chlamydomonas reinhardtii. Plant J. 2008;55:201–211. doi: 10.1111/j.1365-313X.2008.03490.x. [DOI] [PubMed] [Google Scholar]
- *38.Botebol H, Lesuisse E, Šuták R, Six C, Lozano JC, Schatt P, Vergé V, Kirilovsky A, Morrissey J, Léger T, et al. Central role for ferritin in the day/night regulation of iron homeostasis in marine phytoplankton. Proc Natl Acad Sci U S A. 2015;112:14652–14657. doi: 10.1073/pnas.1506074112. The authors demonstrate a role for ferritin in diurnal cycling of Fe in Ostreococcus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchetti A, Parker MS, Moccia LP, Lin EO, Arrieta AL, Ribalet F, Murphy ME, Maldonado MT, Armbrust EV. Ferritin is used for iron storage in bloom-forming marine pennate diatoms. Nature. 2009;457:467–470. doi: 10.1038/nature07539. [DOI] [PubMed] [Google Scholar]
- 40.Docampo R, de Souza W, Miranda K, Rohloff P, Moreno SN. Acidocalcisomes - conserved from bacteria to man. Nat Rev Microbiol. 2005;3:251–261. doi: 10.1038/nrmicro1097. [DOI] [PubMed] [Google Scholar]
- 41.Docampo R. Inclusions in prokaryotes. Springer; 2006. Acidocalcisomes and polyphosphate granules; pp. 53–70. [Google Scholar]
- 42.Blaby-Haas CE, Merchant SS. Lysosome-related organelles as mediators of metal homeostasis. J Biol Chem. 2014;289:28129–28136. doi: 10.1074/jbc.R114.592618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paz Y, Shimoni E, Weiss M, Pick U. Effects of iron deficiency on iron binding and internalization into acidic vacuoles in Dunaliella salina. Plant Physiol. 2007;144:1407–1415. doi: 10.1104/pp.107.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malasarn D, Kropat J, Hsieh SI, Finazzi G, Casero D, Loo JA, Pellegrini M, Wollman FA, Merchant SS. Zinc Deficiency Impacts CO2 Assimilation and Disrupts Copper Homeostasis in Chlamydomonas reinhardtii. J Biol Chem. 2013;288:10672–10683. doi: 10.1074/jbc.M113.455105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hong-Hermesdorf A, Miethke M, Gallaher SD, Kropat J, Dodani SC, Chan J, Barupala D, Domaille DW, Shirasaki DI, Loo JA, et al. Subcellular metal imaging identifies dynamic sites of Cu accumulation in Chlamydomonas. Nat Chem Biol. 2014;10:1034–1042. doi: 10.1038/nchembio.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nat Rev Microbiol. 2009;7:25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- 47.Li HH, Merchant SS. Two metal-dependent steps in the biosynthesis of Scenedesmus obliques plastocyanin. Differential mRNA accumulation and holoprotein formation. J Biol Chem. 1992;267(13):9368–9375. [PubMed] [Google Scholar]
- 48.Nakamura M, Yoshizaki F, Sugimura Y. Accumulation of plastocyanin mRNA lacking 5′ region in the green alga Pediastrum boryanum grown under copper-deficient conditions. Plant Cell Phys. 2000;41(1):33–41. doi: 10.1093/pcp/41.1.33. [DOI] [PubMed] [Google Scholar]
- 49.La Roche J, Geider RJ, Graziano LM, Murray H, Lewis K. Induction of specific proteins in eukaryotic algae grown under iron-, phosphorus-, or nitrogen-deficient conditions. Journal of Phycology. 1993;29:767–777. [Google Scholar]
- 50.Moseley JL, Allinger T, Herzog S, Hoerth P, Wehinger E, Merchant S, Hippler M. Adaptation to Fe-deficiency requires remodeling of the photosynthetic apparatus. EMBO J. 2002;21:6709–6720. doi: 10.1093/emboj/cdf666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terauchi AM, Peers G, Kobayashi MC, Niyogi KK, Merchant SS. Trophic status of Chlamydomonas reinhardtii influences the impact of iron deficiency on photosynthesis. Photosynth Res. 2010;105:39–49. doi: 10.1007/s11120-010-9562-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Page MD, Allen MD, Kropat J, Urzica EI, Karpowicz SJ, Hsieh SI, Loo JA, Merchant SS. Fe sparing and Fe recycling contribute to increased superoxide dismutase capacity in iron-starved Chlamydomonas reinhardtii. Plant Cell. 2012 doi: 10.1105/tpc.112.098962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Höhner R, Barth J, Magneschi L, Jaeger D, Niehues A, Bald T, Grossman A, Fufezan C, Hippler M. The metabolic status drives acclimation of iron deficiency responses in Chlamydomonas reinhardtii as revealed by proteomics based hierarchical clustering and reverse genetics. Mol Cell Proteomics. 2013;12:2774–2790. doi: 10.1074/mcp.M113.029991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsieh SI, Castruita M, Malasarn D, Urzica E, Erde J, Page MD, Yamasaki H, Casero D, Pellegrini M, Merchant SS, et al. The proteome of copper, iron, zinc, and manganese micronutrient deficiency in Chlamydomonas reinhardtii. Mol Cell Proteomics. 2013;12:65–86. doi: 10.1074/mcp.M112.021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riese MHS, Saedler H, Münster T, Huijser P. Comparative analysis of the SBP-box gene families in P. patens and seed plants. Gene. 2007;401:28–37. doi: 10.1016/j.gene.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Castruita M, Casero D, Karpowicz SJ, Kropat J, Vieler A, Hsieh SI, Yan W, Cokus S, Loo JA, Benning C, et al. Systems biology approach in Chlamydomonas reveals connections between copper nutrition and multiple metabolic steps. Plant Cell. 2011;23:1273–1292. doi: 10.1105/tpc.111.084400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strenkert D, Limso CA, Fatihi A, Schmollinger S, Basset GJ, Merchant SS. Genetically Programmed Changes in Photosynthetic Cofactor Metabolism in Copper-deficient Chlamydomonas. J Biol Chem. 2016;291:19118–19131. doi: 10.1074/jbc.M116.717413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sommer F, Kropat J, Malasarn D, Grossoehme NE, Chen X, Giedroc DP, Merchant SS. The CRR1 nutritional copper sensor in Chlamydomonas contains two distinct metal-responsive domains. Plant Cell. 2010;22:4098–4113. doi: 10.1105/tpc.110.080069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hemschemeier A, Casero D, Liu B, Benning C, Pellegrini M, Happe T, Merchant SS. COPPER RESPONSE REGULATOR1–dependent and–independent responses of the Chlamydomonas reinhardtii transcriptome to dark anoxia. Plant Cell. 2013;25:3186–211. doi: 10.1105/tpc.113.115741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blaby-Haas CE, Castruita M, Fitz-Gibbon ST, Kropat J, Merchant SS. Ni induces the CRR1-dependent regulon revealing overlap and distinction between hypoxia and Cu deficiency responses in Chlamydomonas reinhardtii. Metallomics. 2016;8:679–91. doi: 10.1039/c6mt00063k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blaby-Haas C, Merchant S. Sparing and salvaging metals in chloroplasts. In: John Wiley & Sons Ltd, editor. Encyclopedia of Inorganic and Bioinorganic Chemistry. 2013. Scott B, Culotta V (Series Editor), vol Metals in Cells. [Google Scholar]
- 62.Lefebvre-Legendre L, Choquet Y, Kuras R, Loubéry S, Douchi D, Goldschmidt-Clermont M. A nucleus-encoded chloroplast protein regulated by iron availability governs expression of the photosystem I subunit PsaA in Chlamydomonas reinhardtii. Plant Physiol. 2015;167:1527–1540. doi: 10.1104/pp.114.253906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, Deng X, Yang J, Fei X. A putative protein kinase Femu9p contributes to the iron deficiency-inducible expression of FOX1 gene in Chlamydomonas reinhardtii. Australian Journal of Crop Science. 2012;6:1724–1731A. [Google Scholar]
- 64.Deng X, Yang J, Wu X, Li Y, Fei X. A C2H2 zinc finger protein FEMU2 is required for fox1 expression in Chlamydomonas reinhardtii. PLoS One. 2014;9:e112977. doi: 10.1371/journal.pone.0112977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *65.Li X, Zhang R, Patena W, Gang SS, Blum SR, Ivanova N, Yue R, Robertson JM, Lefebvre PA, Fitz-Gibbon ST, et al. An Indexed, Mapped Mutant Library Enables Reverse Genetics Studies of Biological Processes in Chlamydomonas reinhardtii. Plant Cell. 2016;28:367–387. doi: 10.1105/tpc.15.00465. Functional discovery of metal homeostasis genes in algae is expected to accelerate as genome-wide disruptant libraries in C. reinahrdtii become available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmollinger S, Strenkert D, Schroda M. An inducible artificial microRNA system for Chlamydomonas reinhardtii confirms a key role for heat shock factor 1 in regulating thermotolerance. Curr Genet. 2010;56:383–389. doi: 10.1007/s00294-010-0304-4. [DOI] [PubMed] [Google Scholar]
- 67.Neupert JaKDaBR. Generation of Chlamydomonas strains that efficiently express nuclear transgenes. The Plant Journal. 2009;57:1140–1150. doi: 10.1111/j.1365-313X.2008.03746.x. [DOI] [PubMed] [Google Scholar]
- 68.Rasala BA, Barrera DJ, Ng J, Plucinak TM, Rosenberg JN, Weeks DP, Oyler GA, Peterson TC, Haerizadeh F, Mayfield SP. Expanding the spectral palette of fluorescent proteins for the green microalga Chlamydomonas reinhardtii. Plant J. 2013;74:545–556. doi: 10.1111/tpj.12165. [DOI] [PubMed] [Google Scholar]
- 69.Feeney KA, Hansen LL, Putker M, Olivares-Yañez C, Day J, Eades LJ, Larrondo LF, Hoyle NP, O’Neill JS, van Ooijen G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature. 2016;532:375–379. doi: 10.1038/nature17407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Urzica EI, Casero D, Yamasaki H, Hsieh SI, Adler LN, Karpowicz SJ, Blaby-Haas CE, Clarke SG, Loo JA, Pellegrini M, Merchant SS. Systems and trans-system level analysis identifies conserved iron deficiency responses in the plant lineage. Plant Cell. 2012;24:3921–48. doi: 10.1105/tpc.112.102491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malkin R, Knaff DB, Bearden AJ. The oxidation-reduction potential of membrane-bound chloroplast plastocyanin and cytochrome f. Biochim Biophys Acta. 1973;305:675–678. doi: 10.1016/0005-2728(73)90091-1. [DOI] [PubMed] [Google Scholar]
- 72.Ke B, Hansen RE, Beinert H. Oxidation-reduction potentials of bound iron-sulfur proteins of photosystem I. Proc Natl Acad Sci U S A. 1973;70:2941–2945. doi: 10.1073/pnas.70.10.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ke B, Dolan E, Sugahara K, Hawkridge F, Demeter S, Shaw ER. Electrochemical and kinetic evidence for a transient electron acceptor in the photochemical charge separation in Photosystem I. Plant Cell Physiol (Special Issue) 1977:187–199. [Google Scholar]
- 74.Wada K, Arnon DI. Three forms of cytochrome b559 and their relation to the photosynthetic activity of chloroplasts. Proc Natl Acad Sci U S A. 1971;68:3064–3068. doi: 10.1073/pnas.68.12.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gorman DS, Levine RP. Photosynthetic electron transport chain of Chlamydomonas reinhardi. V. Purification and properties of cytochrome 553 and ferredoxin. Plant Phys. 1966;41:1643–7. doi: 10.1104/pp.41.10.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alric J, Pierre Y, Picot D, Lavergne J, Rappaport F. Spectral and redox characterization of the heme ci of the cytochrome b6f complex. Proc Natl Acad Sci U S A. 2005;102:15860–15865. doi: 10.1073/pnas.0508102102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malkin R, Aparicio PJ. Identification of a g equals 1.90 high-potential iron-sulfur protein in chloroplasts. Biochem Biophys Res Commun. 1975;63:1157–1160. doi: 10.1016/0006-291x(75)90690-7. [DOI] [PubMed] [Google Scholar]


