Cytoplasmic lipid droplets are now established as bona fide organelles, nearly ubiquitous in eukaryotic and some prokaryotic cells, with several known functions besides storage of fat (see other articles in this issue). Their importance has drawn attention to their assembly. Neutral lipid are produced in the endoplasmic reticulum, which has the capacity to store a small amount of it among the acyl chains of the bilayer. Beyond some threshold, however, neutral lipids will destabilize the bilayer. If unregulated, emulsion chemistry dictates that the neutral lipid, surrounded by an ER-derived phospholipid monolayer, will bleb off from the ER compartment [1].
However, mechanisms have evolved in nature to control this process, both to prevent excess neutral lipid accumulating in the ER where it might destabilize membrane functions, and to ensure that droplets of proper lipid and protein composition are assembled. In addition, proteins likely maintain connections between droplets and the ER, although the specific roles of these synapses are poorly understood. The frequency to which droplets separate from the ER is still unclear; in yeast they remain attached based on proximity from fluorescent live cell imaging [2], while lack of access of GPAT4 from the ER to a subset of droplets implies separation [3].
This review describes what we know of the roles of three proteins and one lipid in regulating droplet assembly. Proteins that synthesize or metabolize neutral lipids, or those which likely function indirectly from the ER, such as atlastin, ERAD proteins, or those involved in the ER stress response, are not discussed. There is now evidence in yeast for the collaboration of three of these proteins, seipin, perilipin, and Fit2 in droplet formation, and the review ends with a discussion of how they work together. The mechanisms by which cells control droplet formation is slowly becoming better understood, but there will undoubtedly be more twists and turns in this evolving story.
Seipin
Congenital generalized lipodystrophy, the absence of normal adipose tissue throughout the body, was first described in young children in Norway by Martin Seip and in Brazil by Waldemar Berardinelli in the 1950s [4–6]. The firsts genes responsible for the syndrome, known as Berardinelli-Seip Congenital Lipodystrophy (BSCL), or as it is better known now, Congenital Generalized Lipodystrophy (CGL), were discovered to code for acylglycerolphosphate acyltransferase (AGPAT) 2 (patients were termed to have BSCL1 disease) [7] and a gene coding for another protein in BSCL2 patients, named seipin to honor the Norwegian pediatrician [8]. Lipodystrophy is caused by loss-of-function of the corresponding proteins, with loss of seipin causing the more severe disease. Seipin contains a site for asparagine-linked glycosylation, and mutations in the site interfere with protein folding, manifest in patients as neuropathies, or “seipinopathies” [9].
Seipin is an ER protein, found in animals, plants, and fungi, that concentrates at lipid droplet/ER junctions [2, 10]. The protein spans the membrane twice, with both termini facing the cytosolic side. The membrane spans and intervening “loop” are evolutionarily conserved, while both termini vary widely in size and sequence among organisms [6]. Different mRNA splice forms and two translational start sites exist in mammals yielding proteins with extended amino and/or carboxy sequences.
Seipin self-associates, as demonstrated by co-immunoprecipations [11, 12]. In yeast, detergent-solubilized seipin (Sei1p) behaves as toroidal homo-nonamers (9-mers), as determined by hydrodynamics, gel exclusion chromatography, and EM negative staining [13]. Correspondingly, the human form exists as homo-dodecamers (12-mers) which are visualized by atomic force microscopy [14]. Seipin can also associate with other partners. In yeast, the product of the LDB16 gene is an important binding partner. This protein, of previous unknown function (the gene name refers to low dye binding to cell walls of the corresponding deletion strain), has two predicted transmembrane segments and no known homologs in high eukaryotes. Together with Sei1p (formerly Fld1p), they comprise core seipin [15]. Deletions of either, or both in combination, have a similar phenotype, and the double deletion is complemented by heterologous expression of human seipin. Ldb16p is stabilized by Sei1p. While these observations suggest that the two proteins operate as a unit, they do not always colocalize [15]. Overexpression of Ldb16p in the absence of Sei1p results in an increase in TG compared to the double deletion [16], suggesting a discrete function of that subunit.
Besides yeast Ldb16p, six other seipin-binding partners have been identified, three of which catalyze successive steps in glycerolipid synthesis: glycerophosphate acyltransferase (GPAT) [17], AGPAT2 [18], and the phosphatidate phosphatase, lipin [19]. Binding of seipin to both lipin and AGPAT is not mutually exclusive [18]. Binding of AGPAT2 to seipin, detected by co-IP, was independently confirmed by bimolecular fluorescence in HEK-293 and 3T3-L1 cells [18]. In addition, seipin localizes with stearoyl-CoA desaturase 1, SCD1 [20], and it is pulled down with the SERCA transporter [12]. Finally, seipin binds to 14-3-3β, a scaffolding protein with many known binding partners [21, 22]. These associations have been pursued to various extents to determine seipin function, leading to hypotheses discussed below.
Phenotypes of seipin knockout/knockdown and overexpression
Besides obtaining hints from binding partners, phenotypes resulting from knockdown/knockout and overexpression studies have proven valuable in suggesting seipin function.
The most obvious phenotype in seipin lipodystrophy patients is a near absence of white adipose tissue. The cause is a defective adipogenesis program. Central in this transcriptional cascade, which proceeds lipogenesis, is PPARγ. Early cell-culture studies have shown that seipin is crucial for white fat cell development by activating or maintaining this central transcription factor [23, 24].
Seipin patients also suffer from hepatic steatosis and hepatomegaly, as well as fat deposits in muscle, a result of both the absent capacity of adipose storage and possible cell autonomous effects on ectopic fat storage, based on studies in Drosophila [25]. Many patients have metabolic syndrome and diabetes. A subset of patients suffer from cardiomyopathy, which may be related more to hyperglycemia and mitochondrial defects than to fat storage [26]. Patients also display deficiencies in other systems, including nervous and reproductive. A depressionlike phenotype in knockout mice has been attributed to low levels of PPARγ and an inhibition of neural stem cell proliferation. Proliferation and the mental state can be ameliorated by the PPARγ agonist rosiglitazone [27]. Consistent with high levels of seipin mRNA in testes [8], the absence of this protein causes teratozoospermia [28].
SEIPIN (BSCL2) has been knocked out in several animal species including flies, mouse, and rat [25, 29–32]. In general, these animals phenocopy the human disease in the lack of white adipose tissue, although the degree of lipodystrophy is less severe than in humans. In mammals brown adipose is less severely affected, and recent studies indicate that the developmental pathway is near normal; rather, seipin-deficient animals prematurely activate PKA-dependent lipolysis, display apoptosis and atrophy of the tissue, and have defects in acclimation to cold temperatures under certain circumstances [33, 34].
While adipose tissue development is blocked in the absence of seipin, the absence or overexpression of seipin has varying effects on fat accumulation in non-adipose cells. Several cultured cell lines gain neutral lipids upon seipin gene disruption, and lose fat upon seipin overexpression [11]. Overexpression of plant seipins results in an increase in triacylglycerol (TG), while knockdown of Arabidopsis SEIPIN1 causes less fat to accumulate in seeds where this gene is normally expressed [35]. Even in yeast, SEI1 knockout increases TG in some background strains but decreases it in others [10, 16]. Seipin deficiency causes ER stress in liver cells, as manifest by increased levels of CHOP, Grp78, ARTF4, and PERK [20], and N88S, a seipinopathy allele, is well known to induce this protective response as well in neurons [9, 36]. It is important to note that ER stress activates many lipogenic genes including ACC, FAS, SCD1, and SREBP-1c [20, 37] and leads to an increase in droplets [38]. Thus, is seems likely that an increase in TG in non-adipose tissue in the absence of seipin is due, at least in part, to the ER stress response.
There are changes in other lipids in SEIPIN-deleted cells. Degree of saturation of TG acyl groups (more saturated) and chain length of phospholipids (longer to shorter) accompany seipin loss-of-function [10, 39]. Several groups have found an increase in phosphatidic acid (PA) in seipin-deficient cells and the presence of “PA puncta” at droplet/ER junctions [10, 16, 40, 41]. PA puncta are normally not seen in wild type cells.
TG is stored in lipid droplets, but the phenotype of lipid droplets in seipin-deficient cells is due to more than would be expected from increased or decreased TG. Lipid droplet size is much more heterogeneous in knockout cells. In yeast, deletion of SEI1 results in clusters of small droplets often tangled in the ER, as well as “supersized droplets” [2, 10]. Deletion of its partner LDB16 has a similar phenotype [15]. The supersized droplets are suppressed in cells cultured in high inositol, a condition that stimulates phospholipid synthesis. Multiple small droplets are also seen in fibroblasts from BSCL2 patients [2, 42]. The droplets generated in the absence of seipin are not fully functional. In yeast, they cannot segregate well into budding cells, and do not contain a full set of droplet proteins: the lipase Tgl3p in yeast, and AGPAT2 and ASCL3 in mammalian cells, do not traffic well to droplets generated in the absence of seipin [42, 43]. Droplets in the mutant detach from the ER and can accumulate in the nucleus [40, 44].
The assembly of droplets is delayed in cells lacking seipin, as seen in a yeast system in which neutral lipid synthesis is turned on by culturing cells in galactose medium [44, 45]. In this situation cells lack the enzymes to generate TG and steryl ester, although the expression of one of these proteins is driven by the GAL1 promoter. Shortly after diacylglycerol acyltransferase Dga1p (for example) is expressed, droplets developed. Although expression of Dga1p was not affected by seipin, droplets were slow to develop in its absence, and instead accumulated in the ER. When droplets became apparent in the seipin-null strain, they had abnormal morphology, appearing dimmer with indistinct borders by fluorescence microscopy, revealed by electron microscopy to be a large collection of microdroplets with accompanying membrane fragments [44], as if these structures were a result of uncontrolled destabilization of the ER by neutral lipid.
The rate of droplet initiation events can be slowed by removing the amino-terminal 14 amino acids from yeast seipin. With fewer droplets formed without affecting lipid accumulation, each is larger but otherwise of normal morphology, suggesting the importance of the amino terminus in an early event in assembly [44]. Similarly, the amino terminus of Arabidopsis seipin controls droplet size and number [35].
Delay in droplet assembly in the absence of seipin has also been seen in Drosophila S2 cells [46]. A probe, termed LiveDrop, consisting of the droplet-associating domain of GPAT4 linked to Cherry, was developed. Upon incubation of cells with oleic acid-containing medium, LiveDrop formed puncta even before BODIPY spots became visible. The LiveDrop puncta were initially mobile in the ER. The authors showed that fluorescently-tagged seipin stabilized these puncta and allowed droplets to form. In the absence of seipin the LiveDrop puncta not stained with BODIPY proliferated and remained mobile. More mobile nascent droplets in the absence of seipin was also recently observed for human cells [42]. Similar to yeast, new droplets in the knockout detach from the ER, behavior not seen in wild type controls.
Besides indicating an important role in droplet assembly, phenotypes of seipin null strains suggest a role in droplet maintenance. Junctions normally had similar morphology, with a small “footprint” of ER making a synapse with a droplet. These footprints were much more irregular in the absence of seipin [42]. While a probe for protein targeting to droplets, HPos, could sort to droplets during their formation, it was only slowly and inefficiently targeted to pre-exisiting droplets in the absence of seipin. Interestingly, while alkyne-tagged oleic acid could be assimilated into TG and localized to newly forming droplets, these processes were inhibited in the absence of seipin, indicating that TG formation and assembly is coupled [42].
Based largely on the identification of binding partners and the phenotypes associated with changes in seipin expression as outlined above, several hypotheses of seipin function have been forwarded. They are far from mutually exclusive, each may all be true to some extent, but none to date yet fully explains the roles of seipin in both adipogenesis and lipid droplet biology.
(1) Seipin as a docking nexus
Based on the ability for seipin to bind simultaneously to two successive enzymes in the lipogenic pathway, AGPAT and PA phosphohydrolase (lipin), seipin may promote lipogenesis. The oligomeric nature of seipin would allow multiple copies of these enzymes to bind to a single oligomer. In this regard seipin would act stoichometrically rather than catalytically. The localization of seipin to sites of lipid droplet synthesis suggests that seipin provides enzymes in TG synthesis at the site of droplet synthesis. As it has been shown that both AGPAT and lipin are required for PPARγ activation [47, 48], and that overexpression of AGPAT and seipin causes increased expression of PPARγ [18], this model explains both the role of seipin in adipogenesis as well as droplet formation. However attractive this model, more data are required to show that such interactions are of physiological importance.
(2) Regulation of glycerol phosphate acyltransferases (GPAT)
GPAT, the rate limiting step in TAG synthesis, was recently described as a seipin-binding protein in yeast, Drosophila, and mammalian cells [17]. The absence of seipin resulted in higher GPAT activity but not protein levels, indicating that seipin negatively regulates its activity. This would explain the increase in TG levels in the absence of seipin. The authors also showed that overexpression of GPAT can phenocopy a seipin knockout, while inhibiting GPAT expression can rescue a seipin knockout in the 3T3-L1 system of adipogenesis. These results are indeed intriguing. The next step will be to determine whether inhibition of GPAT activity is directly an effect of seipin binding, and to confirm that the effect on PPARγ is due to an increase of a metabolite of GPAT such as PA. While this hypothesis elegantly links the supersized phenotype of droplets with a defect in adipogenesis, it does not explain the effect of seipin on the aberrant morphology of droplets such as the heterogeneity in size, ER tangles, or budding of droplets into the nucleus.
(3) Regulation of PKA-dependent lipolysis
The PKA-dependent phosphorylation of cAMP response element binding protein (CREB) is essential for initiation of adipogenesis [49]. However, Chen et al. reported that MEFs from seipin-knockout mice had hyperactivated hormone-sensitive lipase [31]. Blocking lipase activity with the drug E600 caused a rescue of the adipogenic pathway in these cells. The authors hypothesized that lipolysis and loss of TAG and droplets short-circuited differentiation to adipocytes, leading to lipodystrophy in the animals. This is supported by older data that chronic activation of cAMP/PKA negatively regulates adipogenesis [50]. This is an attractive model to explain the defect in adipogenesis in CGL2 but does not address changes in droplet morphology.
(4) Sensing lipids and regulation of cytoskeleton
Seipin was reported to interact with the scaffolding protein 14-3-3β. The seipin carboxy-terminus was sufficient for binding, although the amino-terminus may also play a role [21]. Knockdown of 14-3-3β attenuated lipid storage during lipogenesis (about 30%) in the 3T3-L1 model, implicating this protein as an intermediate in seipin action. The 14-3-3β protein then binds to cofilin-1 (a known binding partner) through phosphorylation, and the binding of these two proteins increased during adipogenesis in this model. Moreover, cofilin-1, an actin-remodeling protein, was determined to be important for adipogenesis. The authors hypothesized that during adipogenesis there is influx of excess lipids that seipin can detect. It then uses 14-3-3β to activate cofilin which in turn modifies actin filiaments from stress fibers to cortical actin, which they show accompanies adipogenesis. Although not stated, the “excess lipids” could be a result of insulin-mediated adipogenesis, which would cause glucose influx and lipogenesis. While this is an attractive hypothesis to explain the role of seipin in adipogenesis, it does not address the issue of lipid droplet synthesis.
(5) Regulation of ER calcium flux
Seipin immunoprecipates contain the sarco/ER calcium-ATPase (SERCA) influx pump [12], suggesting that the functions of seipin and the ER calcium flux are related. Two main observations are consistent with this hypothesis: (1) Similar to many seipin KO cells, knockdown of SERCA resulted in increased TG levels. (2) SERCA activity was decreased about 30% in animals lacking seipin, and these animals had lower ER calcium stored. Interestingly, reducing calcium release from the ER by knockdown of the ryanodine receptor rescued the seipin phenotype. The authors speculated that seipin controls lipogenesis through maintaining ER calcium homeostasis [12]. This is an attractive hypothesis. Consistent with this is the downregulation of SERCA upon knockdown of lipin-1 or its activator, NEP1-R1 [51]. Reduction of SERCA would tend to counteract the decrease in TG produced by low lipin levels. However, the knockdown of both SERCA and seipin had an additive effect on lipogenesis, suggesting that there may be some independent contributions to a common pathway. Reduction of either SERCA or seipin causes ER stress [12, 20], and it is also possible that ER stress, known to upregulate lipogenesis [38], may be driving the increase in droplets seen with either the seipin or SERCA down-regulation.
(6) Biogenesis of lipid droplets
As noted earlier, droplet formation is retarded without seipin, and droplets, when they are generated, can be either much smaller than normal, often enwrapped in the ER, or supersized [2, 10]. Facilitation may involve interfering with the leaflet, such as creating a bend or kink to allow neutral lipid to flow outward, generating a nascent bud. At the same time, seipin uncouples phospholipid synthesis from droplet size, such that an increase in phospholipid synthesis does not result in smaller droplets [52]. It must tightly control the amount of phospholipid that accompanies the neutral lipid into the bud, perhaps by sensing and regulating the surface tension at the droplet face of the ER/droplet junction, a property dependent on phospholipid packing [1]. Perhaps this sensing involves communication with other ER or droplet factors. Seipin also influences the droplet proteome, but whether this is a direct or indirect effect (through the surface properties of the droplet bud) is another unresolved question.
(7) Maintaining existing droplets
Seipin is found at virtually all ER/droplet junctions in yeast, suggesting it plays a role throughout the life of the organelle [2]. Indeed, seipin has been shown to be important for maintaining the segregation between droplet and ER components [41]. In the absence of seipin yeast droplets can separate from the ER, which normally doesn’t occur in these cells [2, 44]. When connected, knock-out cells have broader ER/droplet junctions, suggesting either that seipin organizes the junction, or is important for interorganellar communication such that its absence elicits an adaptive response to increase junctional size [42].
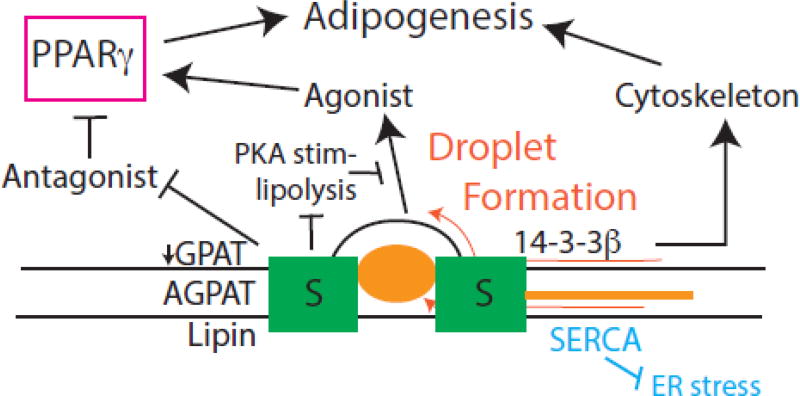
Three principles have emerged from seipin studies in animals, plants, and fungi: (1) Without ruling out catalytic function, seipin likely behaves as a structural or regulatory protein. (2) Seipin likely has independent domains to elicit its function. The core conserved domain likely functions in all cells to regulate lipogenesis and droplet physiology, while the mammalian-specific carboxy terminus is important for adipogenesis. (3) The way seipin regulates lipogenesis is specific to cell type. Hypotheses of seipin function are summarized in Fig. 1.
Fig. 1. Function of seipin.
In orange, seipin promotes formation of lipid droplets by controling the flow of neutral lipid, phospholipid and certain proteins in the nascent bud. In blue, seipin prevents ER stress, perhaps by minimizing the accumulation of neutral lipid in the ER bilayer. Seipin interacts with SERCA, which may also promote ER homeostasis. Black, pathways by which seipin allows adipogenesis through PPARgamma. This includes promotion of the cytoskeleton through 14-3-3β, controlling GPAT levels and perhaps other lipogenic enzymes to prevent accumulation of a PPARγ antagonist, and prevention of PKA-stimulated lipolysis (perhaps stimulated by a disorganized droplet leaflet), which may allow a fatty acid-related PPAR© agonist to be stored and later available for adipogenesis.
Perilipins
The view that droplets were organelles and not simply coalesced fat began with the discovery of an abundant protein, perilipin (now Plin1), on their surface [53]. Four other mammalian perilipinlike proteins have since been identified by their sequence similarities [54]. Most share a conserved PAT domain [55, 56]. While all perilipins are considered barriers guarding the droplet from access of lipases to the neutral lipid core, Plin1 undergoes phosphorylation in response to hormones that drives lipase activity [55–57]. The expression of Plin proteins has been documented in virtually all types of mammalian tissues under various physiological and pathological states [55, 56]. The list of Plin proteins has exploded rapidly in evolutionarily distant species from yeast to human [54, 58, 59].
There is at least one Plin on the surface of lipid droplets [60]; fluorescently tagged Plins are generally seen surrounding droplets [58], and they co-migrate with them during centrifugation [60].
There are gaps to our understanding of the function of Plin proteins. While Plin1 regulates lipases in white adipose tissue, other Plin proteins expressed in other tissues also serve as barrier guarding against lipolysis and autophagy activities [56, 61]. Plin5, a protein found largely in oxidative tissues, facilitates the interaction of droplets with mitochondria and presumably regulates lipolysis at these sites, although the nature of the regulation is still not clear [62]. Plin3 and Plin4, are attracted to newly formed droplets, implying a possible function in their assembly [60, 63]. Plin3 knockdown affects droplet maturation although the underlining mechanism is not clear [64].
Recently two PAT-containing proteins, Pet10p and Sps4p were identified in yeast [59]. The expression of Sps4p is normally difficult to detect but is greatly induced during sporulation, although it is not necessary for that process [65, 66]. Pet10p is more constitutively expressed but is unstable in the absence of triacylglycerol-containing droplets. Pet10p is considered the yeast perilipin as loss of function can be largely complemented by human Plin2 and Plin3 [59]. Unlike other systems, the single expressed perilipin in yeast, Pet10p, may shed light on the ancestral and most conserved function of this class of proteins. As expected for an assembly factor, Pet10p binds to nascent droplets very early, usually before or concomitant with BODIPY staining [59].
As an example, the absence of PET10 leads to fragile droplets. This is manifest after isolation of the organelle. Droplets from the pet10Δ strain readily aggregate in vitro and lyse when observed under a microscope. Similarly, droplets in intact cells fuse, often into one mega-droplet, when cultures are grown in oleic acid-containing medium. This result suggests that a basic function of perilipins, beyond protection of the surface from adventitious lipases, is to physically stabilize the droplet structure.
FIT2
The upregulation of Fat Storage-inducing Transmembrane (FIT) proteins, FIT1 and FIT2 were identified in mouse liver when animals were fed fenofibrate, a PPARα agonist. Both FIT1 and FIT2 reside in the ER and share about 50% sequence similarity. Tissue distribution profiling showed that FIT1 is more restricted in oxidative tissues such as heart and skeletal muscle, whereas FIT2 is ubiquitously expressed, having the highest expression in adipose tissues [67]. FIT proteins are evolutionarily conserved from yeast to mammals [67]. Most lower organisms only have one FIT protein, the sequences of which align more closely with FIT2. There are two FIT2 proteins in Saccharomyces cerevisiae, Scs3p and Yft2p.
There are several lines of evidence suggesting that FIT2 regulates droplet formation. FIT2 proteins are not abundant in cells and it has been difficult to localize the endogenous forms. When overexpressed they localize to the ER [67–69], although a small fraction of mouse FIT2 expressed in plant leaves localizes to the ER/droplet junction [69].
Similar to seipin, FIT2 is believed to regulate droplet formation per se although the protein also has a modest effect on TG accumulation when overexpressed in HEK2934 cells [67]. Overexpression of FIT2 in mouse liver, human HEK293 cells, insect cells, and plant leaf cells promotes droplet formation [67, 69, 70]. Conversely, knockdown of FIT2 in 3T3-L1 cells, zebrafish and pathogenic yeast significantly decreased droplet formation [67, 71]. When the sole FIT2 gene of C. elegans is knocked out, the number and size of droplets are smaller and the worm dies during late larval development [68]. Postnatal deletion of FIT2 in the mouse is lethal. Upon acute oil feeding, neutral lipids within enterocytes in these animals accumulate in the ER and fail to make droplets for TG storage [72].
The conserved function of FIT2 proteins was demonstrated after swapping human and yeast analogs. Expression of the two yeast genes in human embryonic kidney cell line promotes LD formation, and the expression of human FIT2 in yeast rescues inositol auxotrophy, a behavior of the yeast knockouts [73].
There are six predicted transmembrane spans in FIT2, with both of its amino- and carboxyl-termini facing the cytosol. The protein specifically binds TG and DG in vitro, although the binding, performed in detergent micelles, is considerably substoichiometric [70]. Importantly, site-directed mutagenesis of 3 amino acids (to alanine) in a conserved sequence in span 4 led to an increase in both TG binding affinity and the size of droplets [70, 74]. Therefore FIT2 might concentrate TG in the ER as a precursor to droplet budding.
Besides concentrating TG, FIT2 also affects the directionality of droplet budding. This was first suggested by data showing that droplets in cells expressing the triple-A mutant FIT2 were surrounded by an ER marker, is if droplets budded into the ER lumen [74]. An initial report indicated that FIT2 knockouts in yeast had no apparent droplet phenotype although it participated in control of protein and phospholipid synthesis [73]. A more recent study demonstrated that nascent droplets forming in yeast in which both FIT2 genes are deleted often bud into the ER instead of toward the cytosol [68], as the previous report suggested.
Diacylglycerol
Lipids contained within the ER bilayer can influence droplet formation. Blocking the formation of diacylglycerol (DG) by knocking out Pah1p (lipin) in yeast prevented budding of droplets and led to accumulation of neutral lipids within the ER bilayer [75]. However, the absence of lipin would create an increase in phosphatidic acid (PA), its substrate, which could be the cause of the droplet defect, and, in fact, the absence of seipin leads to a local increase in PA, as was described above. However, droplet formation has been shown to be stimulated by addition to cells of a DG analog [63]. Therefore, while DG is important for droplet budding, the exact role of PA in prevention of this process is still not clear. Furthermore, properties of other phospholipids, not yet defined, may also prove important in the initiation, development, or maintenance of droplets.
Functional interactions of seipin, FIT2, and perilipin
Experiments to probe interactions among seipin, FIT2, or perilipins are rare at best. In a recently submitted paper our group found functional interactions among Sei1p/Ldb16p (seipin), Scs3p/Yft2 (FIT2), and Pet10p (perilipin) [59], but the interactions appear complex. The host strain used was a standard one (W303) in which only one acyltransferase providing neutral lipid, Dga1, remained. (A 3KO strain in which genes for both steryl acytransferases, ARE1 and ARE2, were deleted as well as the gene for the other DG acyltransferase, LRO1.) Seipin, FIT2, and perilipin were found to interact in promoting TG accumulation and normal droplet size. First, deletions of any of these genes affected cellular TG: Deletion of seipin (sei1Δ or ldb16Δ or sei1Δ ldb16Δ) resulted in a 50% reduction of TG as previously shown [16], deletion of the two FIT2 proteins (scs3Δyft2Δ) a 15% reduction, and that of perilipin (pet10Δ) a 35% reduction. Surprisingly, all combinations of deletions that included pet10Δ yielded behaved as the single PET10 deletion, resulting in a 35% loss of TG. This result indicates that perilipin is epistatic to (i.e., functions upstream of) seipin and FIT2 with regards to TG homeostasis.
On the other hand, deletions of PET10 in combination with those of seipin or FIT2 are synergistic with respect to generation of large (supersized droplets) [59]. Under standard conditions, deletion of SEI1 or PET10 alone resulted in supersized droplets in 30% or 4% of cells, respectively, while 80% of cells were affected in the double deletion. While deletion of both FIT2 genes resulted in supersized droplets in 2.5% of cells, further deletion of PET10 yielded supersized droplets in 24% of cells. Over 89% of cells had supersized droplets when genes for seipin, FIT2, and Pet10p were all disrupted.
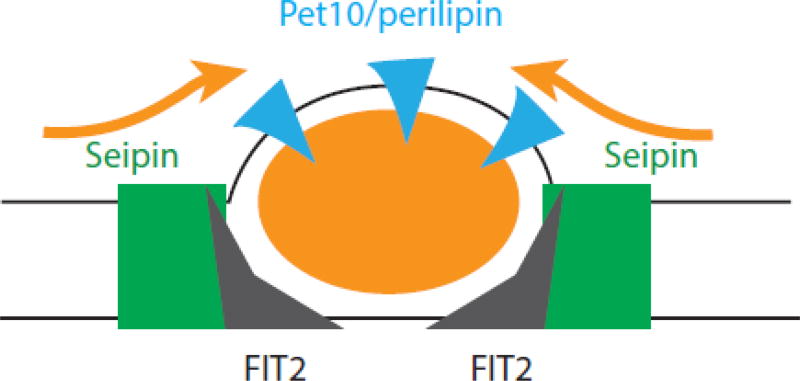
These results suggest that seipin, FIT2, and perilipin interact independently to ensure TG homeostasis and normal droplet size (Fig. 2). A model that explains these data is shown in Fig. 1. Dga1p, can localize both to the ER and droplets, similar to mammalian DGAT2 [3]. However, in our strains, Dga1p is mostly on the ER in the presence of Pet10p but is shifted to droplets in its absence. Our working model proposes interactions of unknown nature between Dga1p and both seipin and FIT2. However, in the absence of Pet10p, Dga1p migrates to the droplet and loses its association with seipin and FIT2. As Pet10p is always present in cells, this affect may not be of physiological consequence.
Fig. 2. Collaboration in Droplet Formation.
Seipin promotes the entry of lipids and some droplet proteins into the nascent droplet. FIT2 prevents droplets from budding into the ER lumen. Note that the localization of FIT2 specifically to the ER-droplet junction has yet to be shown. While seipin and FIT2 promote droplet assembly from the ER, Pet10/perilipin permits droplets to bud in a controlled way towards the cytosolic face.
The interaction among seipin, FIT2 and perilipin in preventing supersized droplets may be more interesting in providing hints on function. There is ample evidence that both seipin and FIT2 promote droplet formation by regulating the entry of neutral lipid (and perhaps phospholipids) into the nascent bud. We hypothesize that perilipin is permissive for this budding but also effectively stiffens the monolayer to control the rate of droplet filling. Loss of filling control from either the ER (by lack of seipin or FIT2) or the bud itself (by lack of perilipin) will lead to formation of supersized droplets, but loss of control at both sites would synergistically increase this activity.
Highlights.
Seipin is important for lipid droplet assembly but it’s mechanism is obscure
This review discusses several hypotheses of seipin function
It also describes the roles of Fit2 proteins and perilipins in droplet biogenesis
Acknowledgments
This research was supported by NIH grant GM084210 and The American Heart Association Grant-in-Aid 27540010. We are grateful for discussion with other members of the Goodman lab.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- This work is supported by the NIH grant GM084210 and the AHA grant-in-aid 27540010.
- I have no financial relationships relevant to my research.
- All sources of review for research come from the above grants. My salary is paid only by the University of Texas.
- I have not interactions with the sponsor.
- I have no patents or copyrights that are relevant.
- I have no other relationships relevant to this research or this manuscript.
References
- 1.Thiam AR, Farese RV, Jr, Walther TC. The biophysics and cell biology of lipid droplets. Nat Rev Mol Cell Biol. 2013;14:775–786. doi: 10.1038/nrm3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szymanski KM, Binns D, Bartz R, Grishin NV, Li WP, Agarwal AK, Garg A, Anderson RG, Goodman JM. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc Natl Acad Sci U S A. 2007;104:20890–20895. doi: 10.1073/pnas.0704154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilfling F, Wang H, Haas JT, Krahmer N, Gould TJ, Uchida A, Cheng JX, Graham M, Christiano R, Frohlich F, Liu X, Buhman KK, Coleman RA, Bewersdorf J, Farese RV, Jr, Walther TC. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev Cell. 2013;24:384–399. doi: 10.1016/j.devcel.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berardinelli W. An undiagnosed endocrinometabolic syndrome: report of 2 cases. The Journal of clinical endocrinology and metabolism. 1954;14:193–204. doi: 10.1210/jcem-14-2-193. [DOI] [PubMed] [Google Scholar]
- 5.Seip M. Lipodystrophy and gigantism with associated endocrine manifestations. A new diencephalic syndrome? Acta paediatrica. 1959;48:555–574. [PubMed] [Google Scholar]
- 6.Cartwright BR, Goodman JM. Seipin: from human disease to molecular mechanism. J Lipid Res. 2012;53:1042–1055. doi: 10.1194/jlr.R023754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal AK, Arioglu E, De Almeida S, Akkoc N, Taylor SI, Bowcock AM, Barnes RI, Garg A. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet. 2002;31:21–23. doi: 10.1038/ng880. [DOI] [PubMed] [Google Scholar]
- 8.Magre J, Delepine M, Khallouf E, Gedde-Dahl T, Jr, Van Maldergem L, Sobel E, Papp J, Meier M, Megarbane A, Bachy A, Verloes A, d'Abronzo FH, Seemanova E, Assan R, Baudic N, Bourut C, Czernichow P, Huet F, Grigorescu F, de Kerdanet M, Lacombe D, Labrune P, Lanza M, Loret H, Matsuda F, Navarro J, Nivelon-Chevalier A, Polak M, Robert JJ, Tric P, Tubiana-Rufi N, Vigouroux C, Weissenbach J, Savasta S, Maassen JA, Trygstad O, Bogalho P, Freitas P, Medina JL, Bonnicci F, Joffe BI, Loyson G, Panz VR, Raal FJ, O'Rahilly S, Stephenson T, Kahn CR, Lathrop M, Capeau J. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet. 2001;28:365–370. doi: 10.1038/ng585. [DOI] [PubMed] [Google Scholar]
- 9.Ito D, Suzuki N. Seipinopathy: a novel endoplasmic reticulum stress-associated disease. Brain : a journal of neurology. 2009;132:8–15. doi: 10.1093/brain/awn216. [DOI] [PubMed] [Google Scholar]
- 10.Fei W, Shui G, Gaeta B, Du X, Kuerschner L, Li P, Brown AJ, Wenk MR, Parton RG, Yang H. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J Cell Biol. 2008;180:473–482. doi: 10.1083/jcb.200711136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fei W, Li H, Shui G, Kapterian TS, Bielby C, Du X, Brown AJ, Li P, Wenk MR, Liu P, Yang H. Molecular characterization of seipin and its mutants: implications for seipin in triacylglycerol synthesis. J Lipid Res. 2011;52:2136–2147. doi: 10.1194/jlr.M017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bi J, Wang W, Liu Z, Huang X, Jiang Q, Liu G, Wang Y, Huang X. Seipin promotes adipose tissue fat storage through the ER Ca(2)(+)-ATPase SERCA. Cell Metab. 2014;19:861–871. doi: 10.1016/j.cmet.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 13.Binns D, Lee S, Hilton CL, Jiang QX, Goodman JM. Seipin is a discrete homooligomer. Biochemistry. 2010;49:10747–10755. doi: 10.1021/bi1013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sim MF, Talukder MM, Dennis RJ, O'Rahilly S, Edwardson JM, Rochford JJ. Analysis of naturally occurring mutations in the human lipodystrophy protein seipin reveals multiple potential pathogenic mechanisms. Diabetologia. 2013;56:2498–2506. doi: 10.1007/s00125-013-3029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang CW, Miao YH, Chang YS. Control of lipid droplet size in budding yeast requires the collaboration between Fld1 and Ldb16. J Cell Sci. 2014;127:1214–1228. doi: 10.1242/jcs.137737. [DOI] [PubMed] [Google Scholar]
- 16.Han S, Binns DD, Chang YF, Goodman JM. Dissecting seipin function: the localized accumulation of phosphatidic acid at ER/LD junctions in the absence of seipin is suppressed by Sei1p(DeltaNterm) only in combination with Ldb16p. BMC Cell Biol. 2015;16:29. doi: 10.1186/s12860-015-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagac M, Cooper DE, Qi Y, Lukmantara IE, Mak HY, Wu Z, Tian Y, Liu Z, Lei M, Du X, Ferguson C, Kotevski D, Sadowski P, Chen W, Boroda S, Harris TE, Liu G, Parton RG, Huang X, Coleman RA, Yang H. SEIPIN Regulates Lipid Droplet Expansion and Adipocyte Development by Modulating the Activity of Glycerol-3-phosphate Acyltransferase. Cell Rep. 2016;17:1546–1559. doi: 10.1016/j.celrep.2016.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talukder MM, Sim MF, O'Rahilly S, Edwardson JM, Rochford JJ. Seipin oligomers can interact directly with AGPAT2 and lipin 1, physically scaffolding critical regulators of adipogenesis. Mol Metab. 2015;4:199–209. doi: 10.1016/j.molmet.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sim MF, Dennis RJ, Aubry EM, Ramanathan N, Sembongi H, Saudek V, Ito D, O'Rahilly S, Siniossoglou S, Rochford JJ. The human lipodystrophy protein seipin is an ER membrane adaptor for the adipogenic PA phosphatase lipin 1. Mol Metab. 2012;2:38–46. doi: 10.1016/j.molmet.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lounis MA, Lalonde S, Rial SA, Bergeron KF, Ralston JC, Mutch DM, Mounier C. Hepatic BSCL2 (Seipin) Deficiency Disrupts Lipid Droplet Homeostasis and Increases Lipid Metabolism via SCD1 Activity. Lipids. 2017;52:129–150. doi: 10.1007/s11745-016-4210-5. [DOI] [PubMed] [Google Scholar]
- 21.Yang W, Thein S, Wang X, Bi X, Ericksen RE, Xu F, Han W. BSCL2/seipin regulates adipogenesis through actin cytoskeleton remodelling. Hum Mol Genet. 2014;23:502–513. doi: 10.1093/hmg/ddt444. [DOI] [PubMed] [Google Scholar]
- 22.Wee K, Yang W, Sugii S, Han W. Towards a mechanistic understanding of lipodystrophy and seipin functions. Biosci Rep. 2014;34 doi: 10.1042/BSR20140114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Payne VA, Grimsey N, Tuthill A, Virtue S, Gray SL, Dalla Nora E, Semple RK, O'Rahilly S, Rochford JJ. The human lipodystrophy gene BSCL2/seipin may be essential for normal adipocyte differentiation. Diabetes. 2008;57:2055–2060. doi: 10.2337/db08-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Yechoor VK, Chang BH, Li MV, March KL, Chan L. The human lipodystrophy gene product Berardinelli-Seip congenital lipodystrophy 2/seipin plays a key role in adipocyte differentiation. Endocrinology. 2009;150:4552–4561. doi: 10.1210/en.2009-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian Y, Bi J, Shui G, Liu Z, Xiang Y, Liu Y, Wenk MR, Yang H, Huang X. Tissue-autonomous function of Drosophila seipin in preventing ectopic lipid droplet formation. PLoS Genet. 2011;7:e1001364. doi: 10.1371/journal.pgen.1001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joubert M, Jagu B, Montaigne D, Marechal X, Tesse A, Ayer A, Dollet L, Le May C, Toumaniantz G, Manrique A, Charpentier F, Staels B, Magre J, Cariou B, Prieur X. The Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Cardiomyopathy in a Diabetic Lipodystrophic Mouse Model. Diabetes. 2017;66:1030–1040. doi: 10.2337/db16-0733. [DOI] [PubMed] [Google Scholar]
- 27.Li G, Zhou L, Zhu Y, Wang C, Sha S, Xian X, Ji Y, Liu G, Chen L. Seipin knockout in mice impairs stem cell proliferation and progenitor cell differentiation in the adult hippocampal dentate gyrus via reduced levels of PPARgamma. Dis Model Mech. 2015;8:1615–1624. doi: 10.1242/dmm.021550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang M, Gao M, Wu C, He H, Guo X, Zhou Z, Yang H, Xiao X, Liu G, Sha J. Lack of testicular seipin causes teratozoospermia syndrome in men. Proc Natl Acad Sci U S A. 2014;111:7054–7059. doi: 10.1073/pnas.1324025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebihara C, Ebihara K, Aizawa-Abe M, Mashimo T, Tomita T, Zhao M, Gumbilai V, Kusakabe T, Yamamoto Y, Aotani D, Yamamoto-Kataoka S, Sakai T, Hosoda K, Serikawa T, Nakao K. Seipin is necessary for normal brain development and spermatogenesis in addition to adipogenesis. Hum Mol Genet. 2015;24:4238–4249. doi: 10.1093/hmg/ddv156. [DOI] [PubMed] [Google Scholar]
- 30.Cui X, Wang Y, Tang Y, Liu Y, Zhao L, Deng J, Xu G, Peng X, Ju S, Liu G, Yang H. Seipin ablation in mice results in severe generalized lipodystrophy. Hum Mol Genet. 2011;20:3022–3030. doi: 10.1093/hmg/ddr205. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Chang B, Saha P, Hartig SM, Li L, Reddy VT, Yang Y, Yechoor V, Mancini MA, Chan L. Berardinelli-seip congenital lipodystrophy 2/seipin is a cell-autonomous regulator of lipolysis essential for adipocyte differentiation. Mol Cell Biol. 2012;32:1099–1111. doi: 10.1128/MCB.06465-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prieur X, Dollet L, Takahashi M, Nemani M, Pillot B, Le May C, Mounier C, Takigawa-Imamura H, Zelenika D, Matsuda F, Feve B, Capeau J, Lathrop M, Costet P, Cariou B, Magre J. Thiazolidinediones partially reverse the metabolic disturbances observed in Bscl2/seipin-deficient mice. Diabetologia. 2013;56:1813–1825. doi: 10.1007/s00125-013-2926-9. [DOI] [PubMed] [Google Scholar]
- 33.Dollet L, Magre J, Joubert M, Le May C, Ayer A, Arnaud L, Pecqueur C, Blouin V, Cariou B, Prieur X. Seipin deficiency alters brown adipose tissue thermogenesis and insulin sensitivity in a non-cell autonomous mode. Sci Rep. 2016;6:35487. doi: 10.1038/srep35487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou H, Black SM, Benson TW, Weintraub NL, Chen W. Berardinelli-Seip Congenital Lipodystrophy 2/Seipin Is Not Required for Brown Adipogenesis but Regulates Brown Adipose Tissue Development and Function. Mol Cell Biol. 2016;36:2027–2038. doi: 10.1128/MCB.01120-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai Y, Goodman JM, Pyc M, Mullen RT, Dyer JM, Chapman KD. Arabidopsis SEIPIN Proteins Modulate Triacylglycerol Accumulation and Influence Lipid Droplet Proliferation. Plant Cell. 2015;27:2616–2636. doi: 10.1105/tpc.15.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yagi T, Ito D, Nihei Y, Ishihara T, Suzuki N. N88S seipin mutant transgenic mice develop features of seipinopathy/BSCL2-related motor neuron disease via endoplasmic reticulum stress. Hum Mol Genet. 2011;20:3831–3840. doi: 10.1093/hmg/ddr304. [DOI] [PubMed] [Google Scholar]
- 37.Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, Katze MG, Hussain MM, Song B, Swathirajan J, Wang J, Yau GD, Kaufman RJ. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fei W, Wang H, Fu X, Bielby C, Yang H. Conditions of endoplasmic reticulum stress stimulate lipid droplet formation in Saccharomyces cerevisiae. Biochem J. 2009;424:61–67. doi: 10.1042/BJ20090785. [DOI] [PubMed] [Google Scholar]
- 39.Boutet E, El Mourabit H, Prot M, Nemani M, Khallouf E, Colard O, Maurice M, Durand-Schneider AM, Chretien Y, Gres S, Wolf C, Saulnier-Blache JS, Capeau J, Magre J. Seipin deficiency alters fatty acid Delta9 desaturation and lipid droplet formation in Berardinelli-Seip congenital lipodystrophy. Biochimie. 2009;91:796–803. doi: 10.1016/j.biochi.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Wolinski H, Hofbauer HF, Hellauer K, Cristobal-Sarramian A, Kolb D, Radulovic M, Knittelfelder OL, Rechberger GN, Kohlwein SD. Seipin is involved in the regulation of phosphatidic acid metabolism at a subdomain of the nuclear envelope in yeast. Biochim Biophys Acta. 2015;1851:1450–1464. doi: 10.1016/j.bbalip.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Grippa A, Buxo L, Mora G, Funaya C, Idrissi FZ, Mancuso F, Gomez R, Muntanya J, Sabido E, Carvalho P. The seipin complex Fld1/Ldb16 stabilizes ER-lipid droplet contact sites. J Cell Biol. 2015;211:829–844. doi: 10.1083/jcb.201502070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salo VT, Belevich I, Li S, Karhinen L, Vihinen H, Vigouroux C, Magre J, Thiele C, Holtta-Vuori M, Jokitalo E, Ikonen E. Seipin regulates ER-lipid droplet contacts and cargo delivery. EMBO J. 2016;35:2699–2716. doi: 10.15252/embj.201695170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolinski H, Kolb D, Hermann S, Koning RI, Kohlwein SD. A role for seipin in lipid droplet dynamics and inheritance in yeast. J Cell Sci. 2011;124:3894–3904. doi: 10.1242/jcs.091454. [DOI] [PubMed] [Google Scholar]
- 44.Cartwright BR, Binns DD, Hilton CL, Han S, Gao Q, Goodman JM. Seipin performs dissectible functions in promoting lipid droplet biogenesis and regulating droplet morphology. Mol Biol Cell. 2015;26:726–739. doi: 10.1091/mbc.E14-08-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacquier N, Choudhary V, Mari M, Toulmay A, Reggiori F, Schneiter R. Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae. Journal of cell science. 2011;124:2424–2437. doi: 10.1242/jcs.076836. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Becuwe M, Housden BE, Chitraju C, Porras AJ, Graham MM, Liu XN, Thiam AR, Savage DB, Agarwal AK, Garg A, Olarte MJ, Lin Q, Frohlich F, Hannibal-Bach HK, Upadhyayula S, Perrimon N, Kirchhausen T, Ejsing CS, Walther TC, Farese RV. Seipin is required for converting nascent to mature lipid droplets. Elife. 2016;5 doi: 10.7554/eLife.16582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subauste AR, Das AK, Li X, Elliott BG, Evans C, El Azzouny M, Treutelaar M, Oral E, Leff T, Burant CF. Alterations in lipid signaling underlie lipodystrophy secondary to AGPAT2 mutations. Diabetes. 2012;61:2922–2931. doi: 10.2337/db12-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang P, Takeuchi K, Csaki LS, Reue K. Lipin-1 phosphatidic phosphatase activity modulates phosphatidate levels to promote peroxisome proliferator-activated receptor gamma (PPARgamma) gene expression during adipogenesis. J Biol Chem. 2012;287:3485–3494. doi: 10.1074/jbc.M111.296681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reusch JE, Colton LA, Klemm DJ. CREB activation induces adipogenesis in 3T3-L1 cells. Mol Cell Biol. 2000;20:1008–1020. doi: 10.1128/mcb.20.3.1008-1020.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, Fong C, Chen Y, Cai G, Yang M. Beta-adrenergic signals regulate adipogenesis of mouse mesenchymal stem cells via cAMP/PKA pathway. Mol Cell Endocrinol. 2010;323:201–207. doi: 10.1016/j.mce.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 51.Han S, Bahmanyar S, Zhang P, Grishin N, Oegema K, Crooke R, Graham M, Reue K, Dixon JE, Goodman JM. Nuclear envelope phosphatase 1-regulatory subunit 1 (formerly TMEM188) is the metazoan Spo7p ortholog and functions in the lipin activation pathway. J Biol Chem. 2012;287:3123–3137. doi: 10.1074/jbc.M111.324350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fei W, Shui G, Zhang Y, Krahmer N, Ferguson C, Kapterian TS, Lin RC, Dawes IW, Brown AJ, Li P, Huang X, Parton RG, Wenk MR, Walther TC, Yang H. A role for phosphatidic acid in the formation of "supersized" lipid droplets. PLoS Genet. 2011;7:e1002201. doi: 10.1371/journal.pgen.1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–11346. [PubMed] [Google Scholar]
- 54.Lu X, Gruia-Gray J, Copeland NG, Gilbert DJ, Jenkins NA, Londos C, Kimmel AR. The murine perilipin gene: the lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm Genome. 2001;12:741–749. doi: 10.1007/s00335-01-2055-5. [DOI] [PubMed] [Google Scholar]
- 55.Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochimica et biophysica acta. 2009;1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kimmel AR, Sztalryd C. The Perilipins: Major Cytosolic Lipid Droplet-Associated Proteins and Their Roles in Cellular Lipid Storage, Mobilization, and Systemic Homeostasis. Annu Rev Nutr. 2016;36:471–509. doi: 10.1146/annurev-nutr-071813-105410. [DOI] [PubMed] [Google Scholar]
- 57.Londos C, Brasaemle DL, Schultz CJ, Adler-Wailes DC, Levin DM, Kimmel AR, Rondinone CM. On the control of lipolysis in adipocytes. Ann N Y Acad Sci. 1999;892:155–168. doi: 10.1111/j.1749-6632.1999.tb07794.x. [DOI] [PubMed] [Google Scholar]
- 58.Miura S, Gan JW, Brzostowski J, Parisi MJ, Schultz CJ, Londos C, Oliver B, Kimmel AR. Functional conservation for lipid storage droplet association among perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. Journal of Biological Chemistry. 2002;277:32253–32257. doi: 10.1074/jbc.M204410200. [DOI] [PubMed] [Google Scholar]
- 59.Gao Q, Binns DD, Kinch LN, Grishin NV, Ortiz N, Chen X, Goodman JM. Pet10p is a yeast perilipin that stabilizes lipid droplets and promotes their assembly. J Cell Biol. doi: 10.1083/jcb.201610013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolins NE, Quaynor BK, Skinner JR, Schoenfish MJ, Tzekov A, Bickel PE. S3-12, Adipophilin, and TIP47 package lipid in adipocytes. J Biol Chem. 2005;280:19146–19155. doi: 10.1074/jbc.M500978200. [DOI] [PubMed] [Google Scholar]
- 61.Kaushik S, Cuervo AM. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol. 2015;17:759–770. doi: 10.1038/ncb3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mason RR, Watt MJ. Unraveling the roles of PLIN5: linking cell biology to physiology. Trends Endocrinol Metab. 2015;26:144–152. doi: 10.1016/j.tem.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 63.Skinner JR, Shew TM, Schwartz DM, Tzekov A, Lepus CM, Abumrad NA, Wolins NE. Diacylglycerol enrichment of endoplasmic reticulum or lipid droplets recruits perilipin 3/TIP47 during lipid storage and mobilization. J Biol Chem. 2009;284:30941–30948. doi: 10.1074/jbc.M109.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bulankina AV, Deggerich A, Wenzel D, Mutenda K, Wittmann JG, Rudolph MG, Burger KN, Honing S. TIP47 functions in the biogenesis of lipid droplets. J Cell Biol. 2009;185:641–655. doi: 10.1083/jcb.200812042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garber AT, Segall J. The SPS4 gene of Saccharomyces cerevisiae encodes a major sporulation-specific mRNA. Mol Cell Biol. 1986;6:4478–4485. doi: 10.1128/mcb.6.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hepworth SR, Ebisuzaki LK, Segall J. A 15-base-pair element activates the SPS4 gene midway through sporulation in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:3934–3944. doi: 10.1128/mcb.15.7.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kadereit B, Kumar P, Wang WJ, Miranda D, Snapp EL, Severina N, Torregroza I, Evans T, Silver DL. Evolutionarily conserved gene family important for fat storage. Proc Natl Acad Sci U S A. 2008;105:94–99. doi: 10.1073/pnas.0708579105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choudhary V, Ojha N, Golden A, Prinz WA. A conserved family of proteins facilitates nascent lipid droplet budding from the ER. J Cell Biol. 2015;211:261–271. doi: 10.1083/jcb.201505067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cai Y, McClinchie E, Price A, Nguyen TN, Gidda SK, Watt SC, Yurchenko O, Park S, Sturtevant D, Mullen RT, Dyer JM, Chapman KD. Mouse fat storage-inducing transmembrane protein 2 (FIT2) promotes lipid droplet accumulation in plants. Plant Biotechnol J. 2016 doi: 10.1111/pbi.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gross DA, Zhan CY, Silver DL. Direct binding of triglyceride to fat storage-inducing transmembrane proteins 1 and 2 is important for lipid droplet formation. P Natl Acad Sci USA. 2011;108:19581–19586. doi: 10.1073/pnas.1110817108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nguyen LN, Hamari Z, Kadereit B, Trofa D, Agovino M, Martinez LR, Gacser A, Silver DL, Nosanchuk JD. Candida parapsilosis fat storage-inducing transmembrane (FIT) protein 2 regulates lipid droplet formation and impacts virulence Microbes Infect. 2011;13:663–672. doi: 10.1016/j.micinf.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 72.Goh VJ, Tan JS, Tan BC, Seow C, Ong WY, Lim YC, Sun L, Ghosh S, Silver DL. Postnatal Deletion of Fat Storage-inducing Transmembrane Protein 2 (FIT2/FITM2) Causes Lethal Enteropathy. J Biol Chem. 2015;290:25686–25699. doi: 10.1074/jbc.M115.676700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moir RD, Gross DA, Silver DL, Willis IM. SCS3 and YFT2 link transcription of phospholipid biosynthetic genes to ER stress and the UPR. PLoS Genet. 2012;8:e1002890. doi: 10.1371/journal.pgen.1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gross DA, Snapp EL, Silver DL. Structural Insights into Triglyceride Storage Mediated by Fat Storage-Inducing Transmembrane (FIT) Protein 2. Plos One. 2010;5 doi: 10.1371/journal.pone.0010796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adeyo O, Horn PJ, Lee S, Binns DD, Chandrahas A, Chapman KD, Goodman JM. The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. J Cell Biol. 2011;192:1043–1055. doi: 10.1083/jcb.201010111. [DOI] [PMC free article] [PubMed] [Google Scholar]