Abstract
Background
A number of studies reports reduced hippocampal volume in individuals who engage in problematic alcohol use. However, the magnitude of the difference in hippocampal volume between individuals with v. without problematic alcohol use has varied widely, and there have been null findings. Moreover, the studies comprise diverse alcohol use constructs and samples, including clinically significant alcohol use disorders and subclinical but problematic alcohol use (e.g. binge drinking), adults and adolescents, and males and females.
Methods
We conducted the first quantitative synthesis of the published empirical research on associations between problematic alcohol use and hippocampal volume. In total, 23 studies were identified and selected for inclusion in the meta-analysis; effects sizes were aggregated using a random-effects model.
Results
Problematic alcohol use was associated with significantly smaller hippocampal volume (d = −0.53). Moderator analyses indicated that effects were stronger for clinically significant v. subclinical alcohol use and among adults relative to adolescents; effects did not differ among males and females.
Conclusions
Problematic alcohol use is associated with reduced hippocampal volume. The moderate overall effect size suggests the need for larger samples than are typically included in studies of alcohol use and hippocampal volume. Because the existing literature is almost entirely cross-sectional, future research using causally informative study designs is needed to determine whether this association reflects premorbid risk for the development of problematic alcohol use and/or whether alcohol has a neurotoxic effect on the hippocampus.
Keywords: Alcohol use, hippocampal volume, meta-analysis
The last several decades of psychological research have seen increasing emphasis on identifying the neural underpinnings of psychopathology. There is growing evidence that psychiatric disorders are associated with aberrant brain structure and functioning, as well as neurocognitive impairment (see Honey et al. 2002; Miller, 2010). One brain region that has generated particular interest for psychological functioning is the hippocampus. The hippocampus is part of the limbic system; it is implicated in memory and learning processes, and, through interactions with other brain regions, including the prefrontal cortex, amygdala, and nucleus accumbens, in emotionality and emotion regulation (Cipolotti & Bird, 2006; La Bar & Cabeza, 2006). The hippocampus (specifically, the dentate gyrus) is one of just two brain regions as yet identified to show neurogenesis, or the generation of new neurons, past the prenatal period and into adulthood (Eriksson et al. 1998; van Praag et al. 2002). Thus, insults to the hippocampus have pervasive implications for the brain more broadly, and for key cognitive and emotional processes; impairment in these processes may further exacerbate psychopathology (Abrous et al. 2005).
Given its important role for memory, learning, and emotionality, there has been considerable research conducted on the hippocampus using animal models of psychopathology, as well as among individuals with varied forms of psychopathology characterized by cognitive impairments and emotional dysregulation. Experimental animal studies suggest that the hippocampus is disproportionately affected by exposure to environmental stressors (see Kim & Diamond, 2002; de Kloet et al. 2005). In addition, several meta-analytic syntheses of the human literature have shown significant reductions in hippocampal volume in schizophrenia, post-traumatic stress disorder, and unipolar depression (Wright et al. 2000; Campbell et al. 2004; Videbech & Ravnkilde, 2004; Kitayama et al. 2005; Smith, 2005; Karl et al. 2006). There is also a growing body of research examining problematic alcohol use and hippocampal volume, but this literature has not yet been synthesized in a meta-analytic review. Several studies find an association between problematic alcohol use and smaller hippocampal volume (e.g. De Bellis et al. 2000; Beresford et al. 2006; Gross et al. 2013), but the magnitude of effects varies, and some studies do not find an association at all (e.g. Fein et al. 2013). There is also considerable methodological variability across studies. Sample sizes vary widely; participants include patients, veterans, and community volunteers; participant ages range from adolescence to late adulthood; problematic alcohol use is defined using different clinical diagnoses, including alcohol abuse and/or alcohol dependence, and as problematic but subclinical alcohol use, such as binge drinking and drinking-related problems; and comparison groups include patients with other psychiatric disorders, minimally drinking healthy controls, and nondrinkers.
We conducted a quantitative meta-analysis of existing studies examining the association between problematic alcohol use and hippocampal volume. Aggregating across studies provides a more stable estimate of the magnitude of the population effect and its significance. We expected to find that problematic alcohol use was significantly associated with reduced hippocampal volume. Meta-analysis also allows for examination of potential moderators that may account for heterogeneity in study effect sizes. We considered several potential sample moderators, including the severity of alcohol use, participant age, and participant sex. Many of the existing studies have focused on associations for clinically significant alcohol use diagnoses, but several studies have also reported effects for subclinical drinking. Stronger associations for clinically significant alcohol use would be consistent with neurotoxic effects of alcohol on the brain, in that more severe alcohol use is associated with greater hippocampal reductions. They would also be consistent with the effects of a genetic predisposition to develop problematic alcohol use, in that individuals at higher genetic risk, indexed by larger hippocampal deviations, can be expected to drink more alcohol. The majority of existing studies has been conducted among adult samples, but several noteworthy studies have reported that alcohol use disorders in adolescents are also associated with reduced hippocampal volume (De Bellis et al. 2000; Nagel et al. 2005); however, other studies among adolescents have failed to find any association (Fein et al. 2013). Stronger associations among adult samples, who have presumably had greater alcohol exposure than adolescent samples, would be consistent with an alcohol exposure-related effect on the brain, in that alcohol exposure is associated with greater hippocampal reductions. Our examination of participant sex was exploratory (see Hommer, 2003). Stronger associations among males or females would be consistent with differential effects of alcohol exposure on the hippocampus as a function of sex. Finally, we considered several potential methodological moderators, including segmentation method, scanner strength, and the inclusion of covariates, which may help to guide future research.
Method
Inclusion and exclusion criteria
Inclusion criteria were (1) comparison of hippocampal volumes in an alcohol using group v. a no or minimal alcohol using group, or association between hippocampal volume and alcohol use; (2) alcohol use current or within the past year; (3) hippocampal volume assessed using MRI and segmented using hand tracing or automated software; (4) human; (5) empirical report; (6) published in a peer-reviewed journal; (7) English language; and (8) sufficient information given to calculate study effect sizes. Exclusion criteria were (1) serious medical conditions due to prenatal substance exposure (e.g. fetal alcohol syndrome); (2) >1 year abstinent from alcohol use; (3) hippocampal volume assessed using non-MRI methods (e.g. autopsy); (4) non-human (e.g. animal study); (5) non-empirical report (e.g. case study); (6) unpublished study or conference proceeding; (7) non-English language; or (8) information necessary for calculating study effect sizes not given.
Literature search
Studies were obtained using multiple search strategies, including (1) searches using 7 online databases (PsycINFO, MEDLINE, Embase Classic + Embase, Web of Science, ERIC, CINAHL, Cochrane Library); (2) examination of reference sections in studies selected for inclusion in the meta-analysis; and (3) examination of reference sections in relevant review articles and meta-analyses. Keywords used in the database searches included combinations of the terms ‘alcohol’ AND ‘magnetic resonance imaging’ OR ‘MRI’ OR ‘imaging’ AND ‘hippocamp*’. The search was limited to journal articles of human studies published in the English language through December 2015. In addition, reference sections for all studies included in the meta-analysis, as well as reference sections from relevant review articles and meta-analyses, obtained using searches of the above-listed databases (using the above search terms in combination with the terms ‘meta-analysis,’ ‘literature review,’ OR ‘systematic review’), were examined. These database and reference section searches yielded 597 nonoverlapping abstracts. Titles and abstracts for all potentially eligible studies were reviewed, and 190 studies that clearly did not meet the inclusion criteria were excluded. The full text of the remaining 407 studies was then reviewed. All told, these search efforts yielded a total of 23 studies that met inclusion criteria and were selected for inclusion in the meta-analysis. An overview of the literature search is presented in Fig. 1.
Fig. 1.
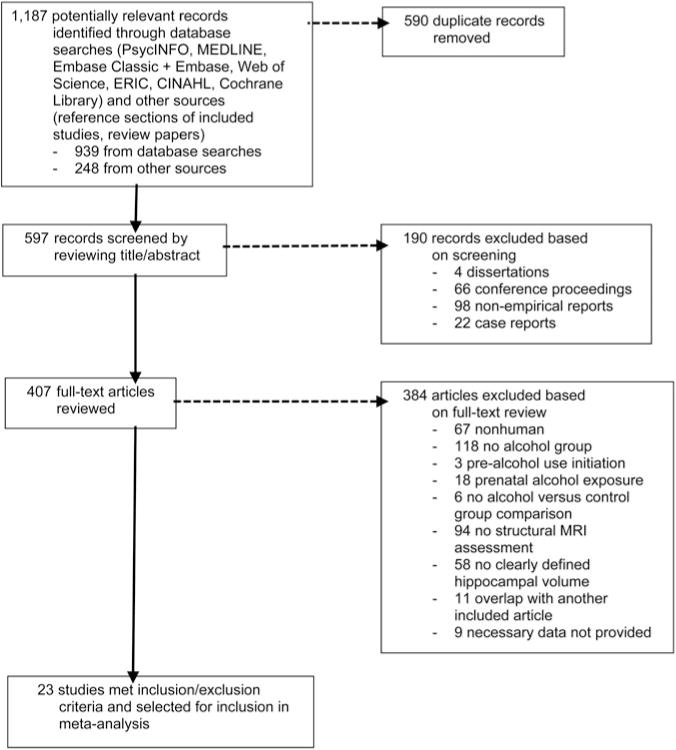
Flow diagram for the literature search.
Study coding
Studies that met inclusion criteria were coded for the following: (1) alcohol use and comparison group information, (2) hippocampal volume information, (3) descriptive study information and sample characteristics, and (4) data for the calculation of effect sizes. All studies were coded by the first and second authors (S. W. and J. L. B.) to assess reliability of study coding; interrater reliability coefficients for the first coding pass are provided below (any coding disagreements were resolved by discussion and consensus). An overview of all included studies, study and sample characteristics, MRI information, and study effect sizes is given in Table 1.
Table 1.
Overview of studies included in the meta-analysis
| Sample characteristics
|
MRI information
|
Effect size statistics
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohol use group
|
Comparison group
|
Slice thick (mm) |
Segment method | Cohen’s d
|
|||||||||||
| Study | Alcohol use | n | Age | Fem | Alcohol use | n | Age | Fem | Scanner | L | R | T | Covariates | ||
| Agartz et al. (2003) | DSM-III-R alcohol dependence | 54 | 38 | 26 | No DSM-III-R diagnosis | 20 | 39 | 15 | 1.5 T GE | 1.5 | Manual | −0.20 | −0.45 | −0.32 | |
| Beresford et al. (2006) | DSM-IV alcohol dependence, recent and chronic heavy drinking | 8 | 47 | 0 | No DSM-IV alcohol dependence, no or light drinking | 8 | 48 | 0 | 3 T GE | 3.0 | Software | −1.34 | −0.89 | −1.15 | |
| Bleich et al. (2003) | DSM-IV alcohol dependence | 52 | 47 | 35 | No DSM-IV diagnosis | 30 | 48 | 47 | 1.5 T Philips Gyroscan ACS | 1.3 | Manual | −2.58 | −2.43 | −2.50 | |
| Cheetham et al. (2014) | Alcohol-related problems | 38 | 17 | 47 | No alcohol-related problems | 60 | 17 | 53 | 3 T | 1.5 | Manual | 0.22 | 0.11 | 0.16 | |
| De Bellis et al. (2000) | DSM-IV alcohol abuse or dependence | 12 | 17 | 58 | No DSM-IV diagnosis | 24 | 17 | 58 | 1.5 T GE Signa | 1.5 | Manual | −0.97 | −0.80 | −0.97 | |
| den Heijer et al. (2004) | Moderate to heavy drinking | 229 | 72 | 37 | No, very light, or light drinking | 238 | 72 | 62 | 1.5 T Siemens | 1.5 | Manual | – | – | −0.50 | Age, sex, pack-years cigarette smoking |
| Durazzo et al. (2011) | DSM-IV alcohol abuse or dependence | 75 | 50 | 5 | No history of DSM-IV diagnosis | 43 | 47 | 9 | 1.5 T Siemens Vision | 1.5 | Software | −0.67 | −0.86 | −0.76 | Intracranial volume, age |
| Fein & Fein (2013) | DSM-IV alcohol dependence | 77 | 46 | 38 | No substance use | 74 | 47 | 50 | 3 T Siemens Trio | 1.0, 3.0 | Software | – | – | −0.40 | Intracranial volume |
| Fein et al. (2013) | DSM-IV alcohol abuse or dependence, lifetime excess of 100 alcohol units | 64 | 15 | 55 | No DSM-IV alcohol abuse or dependence | 64 | 15 | 55 | 3 T Siemens Magnetom Allegra | 1.0 | Software | – | – | 0.11 | |
| Gross et al. (2013) | DSM-IV alcohol dependence | 34 | 43 | 29 | Healthy controls | 16 | 46 | 56 | 1.5 T Siemens Magnetom Sonata | 1.0 | Manual | −1.54 | −1.73 | −1.78 | Age, sex |
| Laakso et al. (2000) | ICD-10/DSM-IV alcohol abuse or dependence | 36 | 39 | 0 | No history of substance abuse or somatic, psychiatric, neurological disorders | 34 | 35 | 0 | 1.0 T Impact or 1.5 T Siemens Magnetom or 1.5 T Siemens Vision | 2.0 | Manual | −0.43 | −0.74 | −0.60 | Intracranial volume |
| Le Berre et al. (2014) | DSM-IV alcohol dependence or RDC alcoholism | 191 | 48 | 32 | Social drinkers | 288 | 50 | 35 | 1.5 T GE Signa Advantage EchoSpeed | 1.3, 1.5 | Manual | – | – | −0.32 | Intracranial volume, age |
| Lee et al. (2007) | DSM-IV alcohol dependence | 13 | 34 | 0 | <14 drinks/week, <4 drinks/occasion | 18 | 33 | 0 | 1.5 T GE Signa | – | Manual | 0.33 | 0.33 | 0.33 | |
| Makris et al. (2008) | DSM-IV alcohol abuse or dependence | 21 | 54 | 0 | Healthy controls | 21 | 51 | 0 | 3 T Siemens Trio | 1.3 | Manual | −0.57 | −0.57 | −0.57 | Total brain volume |
| Malone et al. (2014) | Alcohol use | 93 | 16 | 50 | – | – | – | – | 3 T Siemens Trio | 1.0 | Software | – | – | 0.11 | Intracranial volume, age, sex |
| Medina et al. (2007) | Alcohol use | 42 | 17 | 29 | Limited experience with alcohol, no experience with any other substances | 21 | 18 | 35 | 1.5 T GE Signa | 1.3 | Manual | 0.01 | 0.27 | 0.13 | |
| Nagel et al. (2005) | DSM-IV alcohol abuse or dependence | 14 | 17 | 36 | Non-alcohol abusing controls | 17 | 17 | 41 | 1.5 T GE | 1.3 | Manual | −0.72 | 0.00 | −0.37 | |
| Ozsoy et al. (2013) | DSM-IV alcohol dependence | 21 | 43 | 0 | No history or family history of substance use disorders | 13 | 38 | 0 | 1.5 T Philips Gyroscan Intera | 2.0 | Manual | −0.15 | −0.11 | −0.43 | |
| Schuff et al. (2008) | DSM-IV alcohol abuse | 51 | 47 | 4 | DSM-IV PTSD, no DSM-IV alcohol abuse | 53 | 48 | 19 | 1.5 T Siemens Vision | 3.0 | Manual | – | – | −0.22 | Intracranial volume |
| Smith et al. (2011) | DSM-IV alcohol abuse or dependence | 16 | 39 | 25 | No current or remote DSM-IV substance use disorder | 56 | 38 | 29 | 1.5 T Siemens Magnetom | 1.0 | Software | −0.06 | −0.10 | −0.08 | |
| Starcevic et al. (2015) | ICD-10 alcohol abuse or dependence | 29 | 47 | 0 | Healthy volunteers | 25 | 45 | 0 | 3 T Philips | 1.2 | Software | – | – | −1.75 | |
| Sullivan et al. (1995) | RCD alcoholism | 47 | 44 | 0 | Community, <4 drinks/day | 72 | 44 | 0 | 1.5 T GE Signa | 3.0 | Manual | −0.42 | −2.26 | −0.66 | |
| Wrase et al. (2008) | ICD-10/DSM-IV alcohol dependence | 51 | 41 | 33 | Healthy controls | 52 | 40 | 38 | 1.5 T Siemens Magnetom | 1.0 | Manual | – | – | −0.49 | |
Notes: Sample characteristics include the number of participants in the alcohol using and comparison groups and their age and sex. Alcohol use = definition of alcohol use in the alcohol using group and the comparison group. Age = mean age. Fem = percentage female. MRI information includes the MRI scanner strength, the thickness of the acquired slices, and the method of hippocampal segmentation. Slice thick = slice thickness. Segment method = hippocampal segmentation method. DSM-III-R, -IV = Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised, Fourth Edition. ICD-10 = International Statistical Classification of Diseases and Related Health Problems, Tenth Edition. RDC = Research Diagnostic Criteria. PTSD = post-traumatic stress disorder. Effect size statistics are Cohen’s ds, given for left, right, and total hippocampal volumes. L = left. R = right. T = total. Covariates include variables used to adjust hippocampal volumes in analyses, if applicable. All effect size statistics were coded so that negative ds indicated smaller hippocampal volumes in the alcohol group relative to the comparison group, or reduced hippocampal volumes associated with alcohol use. By convention, ds greater than |0.20| are considered to be modest, greater than |0.50| moderate, and greater than |0.80| large (Cohen, 1988).
Alcohol use and hippocampal volume
Information coded for the alcohol use group included the alcohol use diagnosis or construct (κ = 0.94), coded as clinically significant (alcohol use disorder diagnosis) or subclinical (problematic but not diagnosed alcohol use) (κ = 1.00); information coded for the comparison group included the exclusionary diagnosis or construct (e.g. no psychiatric diagnosis) (κ = 1.00). Information coded about hippocampal volume included the hemisphere (κ = 1.00), segmentation method (hand tracing, automated software) (κ = 0.94), scanner strength (κ = 1.00), and whether the volumes were adjusted for covariates (e.g. intracranial volume, age, sex; κ = 0.88).
Study information and sample characteristics
Information coded for each study included participant age in years (κ = 0.94), also coded as child/adolescent (younger than 18 years) or adult (18 years and older) (κ = 1.00), and participant sex, coded as percentage male and female (κ = 0.94).
Effect size data
Information coded for the calculation of effect sizes included statistics for the comparison of hippocampal volumes between the alcohol use and comparison groups (means and S.D., t statistic) (κ = 1.00).
Data analysis
Effect sizes for the comparison of hippocampal volumes between the alcohol use and comparison groups, or for the association between hippocampal volumes and alcohol use, were derived from each study. All effect sizes were converted to Cohen’s ds prior to analyses using standard formulas (Lipsey & Wilson, 2001), and all effect sizes were coded so that negative ds indicated smaller hippocampal volumes in the alcohol group relative to the comparison group, or smaller hippocampal volumes associated with greater alcohol use. Following conventional guidelines, we considered ds >|0.20| to be modest, >|0.50| to be moderate, and >|0.80| to be large (Cohen, 1988). Several studies reported means and S.D. separately for the left and right hemispheres; in such instances, total hippocampal volumes were calculated by summing the means and using a standard formula for computing the pooled S.D. (see Koolschijn et al. 2009). Mean effect sizes across studies were calculated by weighting each individual effect size by the inverse of its variance. A random effects model, in which both random and systematic components are assumed to account for effect size variance, was used to fit the effect size data.
We examined variability among study effect sizes, as indexed by Cochran’s Q statistic; when the Q statistic indicated significant heterogeneity, we calculated I2 (the percentage of total variance across studies due to heterogeneity, rather than sampling error) to quantify the extent of heterogeneity, and conducted follow-up moderator analyses that attempted to account for variability in effect sizes. We considered several potential sample moderators, including alcohol use severity (clinically significant v. subclinical), sample age (mean age, adult v. adolescent), and sample sex (percentage male, female v. male). We also considered several potential methodological moderators, including segmentation method (hand tracing v. automated software), scanner strength (1.0 T/1.5 T v. 3.0 T), and covariate adjustment (unadjusted v. adjusted for common covariates). Moderator analyses for categorical variables followed the analog to the Analysis of Variance and examined whether effect sizes stratified by moderator variables differed significantly; meta-regression analyses were conducted for continuous moderator variables and examined whether effect sizes were linearly associated with the moderator variable.
Finally, we considered potential publication bias due to the underrepresentation of studies with small samples and subsequently lower power to detect significant effects by examining funnel plots for the expected shape of the distribution of study effect sizes (Light & Pillemer, 1984; Egger et al. 1997). This approach assumes that, when all relevant studies are included in a meta-analysis, a scatterplot of effect sizes will be symmetrically dispersed on either side of the overall mean effect; when effect sizes are dispersed asymmetrically, the missing studies are assumed to be those that report small, nonsignificant effects, and a trim-and-fill method (Duval & Tweedie, 2000) can be used to impute missing studies to the analyses and then recompute the mean effect. All analyses were conducted using the Comprehensive Meta-Analysis 3.0 software (Borenstein et al. 2014).
Results
Overall effects for problematic alcohol use and hippocampal volume
A total of 23 studies was included in the meta-analysis. The weighted mean effect size, aggregated across these 23 studies, for the association between problematic alcohol use and total hippocampal volume was negative, moderate in magnitude, and significantly different from zero, d = −0.53, p < 0.001, indicating that problematic alcohol use was associated with significantly smaller total hippocampal volume (see Table 2). Effects computed separately for left and right hippocampal volumes (k = 17) were likewise negative, moderate, and significant, d = −0.61, p < 0.001, and d = −0.73, p < 0.001, respectively; the 95% confidence intervals for effect sizes for left and right hippocampal volumes were almost completely overlapping, indicating that the association between problematic alcohol use and hippocampal volume did not differ laterally (see Table 2).
Table 2.
Summary of meta-analytic results
| k | Cohen’s d | 95% CI | p | ||||
|---|---|---|---|---|---|---|---|
| Overall analyses | |||||||
| Hippocampal volume | QW (df) | p | I2 | ||||
|
|
|||||||
| Total | 23 | −0.53 | −0.74 to −0.31 | <0.001 | 124.83 (22) | <0.001 | 0.82 |
| Left | 17 | −0.61 | −0.96 to −0.26 | <0.001 | 112.97 (16) | <0.001 | 0.86 |
| Right | 17 | −0.73 | −1.15 to −0.32 | <0.001 | 152.65 (16) | <0.001 | 0.90 |
| Moderator analyses: sample characteristics | |||||||
| Alcohol use | QB (df) | p | |||||
|
|
|||||||
| Clinical | 19 | −0.65 | −0.90 to −0.39 | <0.001 | |||
| Subclinical | 4 | −0.07 | −0.47 to 0.34 | 0.752 | |||
| 5.64 (1) | 0.018 | ||||||
| Age | |||||||
| Adult | 17 | −0.68 | −0.92 to −0.44 | <0.001 | |||
| Adolescent | 6 | −0.04 | −0.31 to 0.23 | 0.763 | |||
| 11.84 (1) | <0.001 | ||||||
| Age (clinical alcohol use) | |||||||
| Adult | 16 | −0.70 | −0.98 to −0.42 | <0.001 | |||
| Adolescent | 3 | −0.35 | −1.00 to 0.30 | 0.295 | |||
| 0.93 (1) | 0.335 | ||||||
| Sex | |||||||
| Male | 11 | −0.69 | −1.05 to −0.32 | <0.001 | |||
| Female | 4 | −0.83 | −2.07 to 0.42 | 0.193 | |||
| 0.05 (1) | 0.832 | ||||||
| Moderator analyses: methodological variables | |||||||
| Segmentation method | QB (df) | p | |||||
|
|
|||||||
| Hand tracing | 14 | −0.62 | −0.92 to −0.32 | <0.001 | |||
| Automated | 9 | −0.38 | −0.70 to −0.07 | 0.016 | |||
| 1.15 (1) | 0.283 | ||||||
| Scanner strength | |||||||
| 1.0 T/1.5 T | 16 | −0.58 | −0.82 to −0.33 | <0.001 | |||
| 3 T | 7 | −0.42 | −0.86 to 0.02 | 0.062 | |||
| 0.37 (1) | 0.543 | ||||||
| Covariate adjustment | |||||||
| Unadjusted | 14 | −0.55 | −0.94 to −0.16 | 0.006 | |||
| Adjusted | 9 | −0.49 | −0.70 to −0.28 | <0.001 | |||
| 0.07 (1) | 0.791 | ||||||
Notes: k = number of studies. Negative Cohen’s ds indicate reduced hippocampal volumes associated with alcohol use. By convention, ds > |0.20| are considered to be modest, >|0.50| moderate, and >|0.80| large (Cohen, 1988). CI, confidence interval. QW = heterogeneity within a set of studies. QB = heterogeneity between two sets of studies. df = degrees of freedom. I2 = percentage of total variance across studies due to heterogeneity.
Moderator analyses
The study effect sizes included in the overall meta-analysis were significantly heterogeneous, as indicated by the significant QW statistic; the I2 statistic indicated that there was substantial heterogeneity (see Table 2). Thus, follow-up moderator analyses were conducted that attempted to account for this variability.
Sample characteristics
Clinically significant v. subclinical alcohol use
We examined the potentially moderating effect of the severity of alcohol use by stratifying effect sizes for studies examining clinically significant (k = 19) and subclinical alcohol use (k = 4). The effect was moderate and significant for clinically significant alcohol use, d = −0.65, p < 0.001, but was nonsignificant for subclinical alcohol use, d = −0.07, p = 0.752; the significant QB statistic indicated that there was a significant difference in effect sizes for clinically significant and subclinical alcohol use (see Table 2). Thus, the meta-analytic results for the included studies indicates that the association between problematic alcohol use and reduced hippocampal volume is larger for clinically significant levels of alcohol use relative to subclinical levels, and the effect for subclinical alcohol use is trivial.
Adults v. adolescents
We examined the potentially moderating effect of sample age using both categorical and continuous indicators. First, we stratified effect sizes for studies examining alcohol use among adult (k = 17) and adolescent samples (k = 6). The effect was moderate and significant among adults, d = −0.68, p < 0.001, but was nonsignificant among adolescents, d = −0.04, p = 0.763; the significant QB statistic indicated that there was a significant difference in effect sizes for adults and adolescents (see Table 2). Second, we conducted meta-regression analyses that examined the linear association between effect sizes and the age of the sample (k = 23). The association was significant and negative, b = −0.02, p = 0.015, indicating that effect sizes were larger among older samples (i.e. greater reductions in hippocampal volume). Thus, the meta-analytic results for the included studies indicates that the association between problematic alcohol use and reduced hippocampal volume is larger among adults relative to adolescents, is trivial among adolescents, and increases with sample age.
We conducted follow-up moderator analyses of sample age just among studies examining clinically significant alcohol use. We stratified effect sizes for clinically significant alcohol use among adult (k = 16) and adolescent samples (k = 3). The effect for clinically significant alcohol use was moderate and significant among adults, d = −0.70, p < 0.001. However, although the effect for clinically significant alcohol use was moderate in magnitude among adolescents, it failed to reach significance, d = −0.35, p = 0.295, perhaps due to the relatively small number of studies in the analysis; notably, the nonsignificant QB statistic (p = 0.335) indicated a lack of significant differences in effect sizes among adults and adolescents for clinically significant alcohol use (see Table 2). Thus, the meta-analytic results for the included studies indicates that the association between problematic alcohol use and reduced hippocampal volume increases with sample age, but there is also suggestive evidence that more severe alcohol use is associated with reduced hippocampal volume, even among adolescents.
Males v. females
We examined the potentially moderating effect of sample sex using both categorical and continuous indicators. First, we stratified effect sizes for studies among male (k = 11) and female samples (k = 4). The effect was moderate and significant among males, d = −0.69, p < 0.001, but, though large in magnitude, the effect failed to reach significance among females, d = −0.83, p = 0.193, perhaps due to the relatively small number of studies in the analysis; the nonsignificant QB statistic (p = 0.832) indicated a lack of significant differences in effect sizes among males and females (see Table 2). Second, we conducted meta-regression analyses that examined the linear association between effect sizes and the percentage of males in the sample (k = 23). The association was nonsignificant, b = −0.01, p = 0.275, indicating that effect sizes did not differ significantly as a function of sample sex. Thus, the meta-analytic results for the included studies indicate that the association between problematic alcohol use and reduced hippocampal volume is statistically comparable among males and females.
Methodological variables
Hand tracing v. automated software
We examined the potentially moderating effect of segmentation method by stratifying effect sizes for studies that used hand tracing (k = 14) and automated software (k = 9) to segment the hippocampus. The effect for hand tracing was moderate and significant, d = −0.62, p < 0.001, and the effect for automated software was modest and significant, d = −0.38, p = 0.016; the non-significant QB statistic (p = 0.283) indicated a lack of significant differences in effect sizes as a function of segmentation method (see Table 2). Thus, the meta-analytic results for the included studies indicate that the association between problematic alcohol use and reduced hippocampal volume is statistically comparable whether hand tracing or automated software is used to segment the hippocampus.
1.0 T/1.5 T v. 3 T
We examined the potentially moderating effect of scanner strength by stratifying effect sizes for studies that used 1.0 T/1.5 T scanners (k = 16) and 3 T scanners (k = 7). The effect for 1.0 T/1.5 T scanners was moderate and significant, d = −0.58, p < 0.001, and the effect for 3 T scanners was modest and approached significance, d = −0.42, p = 0.062; the nonsignificant QB statistic (p = 0.543) indicated a lack of significant differences in effect sizes as a function of scanner strength (see Table 2). Thus, the meta-analytic results for the included studies indicate that the association between problematic alcohol use and reduced hippocampal volume is statistically comparable across 1.0 T/1.5 T and 3 T scanners.
Unadjusted v. adjusted for covariates
We examined the potentially moderating effect of covariate adjustment by stratifying unadjusted effect sizes (k = 14) and effect sizes that had been adjusted for covariates (k = 9). The unadjusted effect was moderate and significant, d = −0.55, p = 0.006, and the adjusted effect was moderate and significant, d = −0.49, p < 0.001; the nonsignificant QB statistic (p = 0.791) indicated a lack of significant differences in effect sizes as a function of covariate adjustment (see Table 2). Thus, the meta-analytic results for the included studies indicate that the association between problematic alcohol use and reduced hippocampal volume is statistically comparable whether effects are unadjusted or adjusted for common covariates.
Publication bias
Examination of a funnel plot for effect sizes for the association between problematic drinking and hippocampal volume indicated that effect sizes were asymmetrically dispersed around the overall mean effect – several studies with small samples and large effects were located to the left of the mean effect size (i.e. these studies reported very large associations between problematic alcohol use and reduced hippocampal volumes) (see Fig. 2). Duval & Tweedie’s (2000) trim-and-fill method was used to impute three missing studies to the right of the mean effect, which resulted in an imputed mean effect of d = −0.34, p = 0.009 (reduced from d = −0.53, p < 0.001), Thus, there was some evidence of publication bias, in that studies with small samples but large effects were present in the published literature, whereas studies with small samples and small effects were missing from the published literature.
Fig. 2.
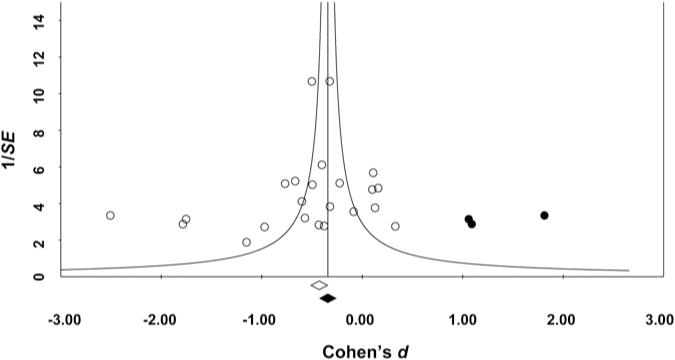
Funnel plot of effect sizes (Cohen’s ds) for each study plotted against study precision (the inverse of the sample’s standard error (S.E.)). White circles indicate effect sizes for published studies included in the meta-analysis; the white diamond indicates the aggregated mean effect for the included studies. Black circles indicate ‘missing’ effect sizes imputed using Duval and Tweedie’s trim-and-fill method to account for asymmetry in included studies; the black diamond indicates the recomputed aggregated mean effect, which includes the missing studies.
Discussion
Alcohol-related effects are evident in the physiology of the brain, including its structure and functioning, and in the learning, memory, and emotional impairments observed among individuals evidencing problematic alcohol use (see White et al. 2000; Oscar-Berman & Marinković, 2007). The present meta-analysis indicates that problematic alcohol use is associated with significant, moderate reductions in hippocampal volume, which may account for the impairments in neurocognitive and emotional functioning observed in individuals with problematic alcohol use (see White & Swartzwelder, 2005; Zeigler et al. 2005). Moreover, more severe alcohol use, indexed as clinically significant alcohol use disorders characterized by the consumption of large amounts of alcohol despite negative consequences, is associated with reduced hippocampal volume relative to less severe alcohol use, indexed as problematic but subclinical alcohol use. These results are consistent with greater alcohol exposure leading to greater reductions in hippocampal volume. Effects are also larger with increasing age – to the extent that age is a proxy for the amount of alcohol consumed over time, this result also suggests that greater alcohol exposure leads to greater reductions in hippocampal volume (though an alternative explanation may be that older brains are more susceptible to alcohol effects). Notably, although the association between problematic alcohol use and reduced hippocampal volume was larger among adults relative to adolescents, when we restricted our analyses to clinically significant alcohol use, this difference was no longer significant, again suggesting that it is the greater alcohol exposure associated with clinically significant alcohol use disorders that leads to reduced hippocampal volume, even among adolescents.
The present meta-analytic results for human studies of problematic alcohol use and hippocampal volume are consistent with the results of experimental animal studies, which show exposure-related effects of alcohol on the hippocampus (see Nixon, 2006; Crews & Nixon, 2009). Research on animal models of alcoholism highlights the effect of alcohol exposure on neurogenesis – ethanol-exposed animals (rodents, nonhuman primates) show reduced survival of newly formed neurons (Nixon & Crews, 2002; Herrera et al. 2003; He et al. 2005; Ieraci & Herrera, 2007; Taffe et al. 2010). Notably, both animal (Nixon & Crews, 2004) and human studies (Bartels et al. 2007; Cardenas et al. 2007; Gazdzinski et al. 2008) indicate substantial neuronal recovery, increased hippocampal volume, and improved neuropsychological functioning with long-term abstinence from alcohol exposure.
Taken together, the results of the meta-analysis, along with these lines of research, are consistent with the notion that the association between problematic alcohol use and reduced hippocampal volume reflects a neurotoxic effect of alcohol exposure on the brain. This is consistent with the results of experimental animal studies, which show a causal exposure effect of alcohol on reduced hippocampal volume (see Nixon, 2006; Crews & Nixon, 2009). However, because the existing human studies are necessarily correlational in study design – random assignment to an alcohol v. control group is not possible – these studies, and, thus, the present meta-analysis, cannot determine the causal relationship between problematic alcohol use and hippocampal volume reductions. It is possible that alcohol exposure has a neurotoxic effect on the hippocampus, but it is also possible that hippocampal deviations reflect underlying vulnerability toward problematic alcohol use and/or lead to the development of problematic alcohol use.
Future research using causally informative study designs is needed to determine the causal relationship between problematic alcohol use and reduced hippocampal volume in humans. There is increasing evidence, from longitudinal studies that prospectively assess alcohol use and brain morphometry over time, high-risk family studies that compare substance-naïve offspring at high and low familial risk for problematic alcohol use, and co-twin control studies that compare twins who vary in their alcohol use, that at least some of the brain deviations observed among individuals with problematic alcohol use reflect premorbid abnormalities (see Jacobus & Tapert, 2013; Wilson et al. 2015a, b). However, this body of research is as yet relatively small, and the few studies on the hippocampus have yielded inconsistent findings. To our knowledge, only two longitudinal studies have examined alcohol use and hippocampal volume over time. Cheetham et al. (2014) found no association between hippocampal volume at age 12 and alcohol-related problems at age 16 in a sample of 98 adolescents. Similarly, Hanson et al. (2010) found no association between hippocampal volume at age 13 and alcohol and other substance use at age 17 in a sample of 30 adolescents. Three high-risk family studies have examined hippocampal volumes among adolescents and young adults at high and low risk for developing problematic alcohol use. Hill et al. (2001) and Hanson et al. (2010) found no significant differences in hippocampal volume between offspring with and without a family history of alcohol use disorders (total N = 30 and 34, respectively), but Benegal et al. (2007) did find reduced hippocampal volume among high-risk relative to low-risk offspring (total N = 41). Thus, there is limited evidence that reduced hippocampal volume may reflect premorbid deviation, but additional research is needed to clarify the discrepant findings.
We found little evidence that the methodological variables we considered (segmentation method, scanner strength, the inclusion of covariates) moderated the association between problematic alcohol use and hippocampal volume. One aspect of the covariates in the included studies warrants note – only seven (30%) of the studies included an indicator of intracranial or total brain volume as a covariate in analyses. Alcohol exposure has been found to be associated with generally smaller brain volume (Bjork et al. 2003; Hommer, 2003; Cardenas et al. 2005). Although we found no significant difference in effect sizes for studies that did v. did not include common covariates, because both intracranial volume and other common covariates (e.g. age, sex) were typically included in those studies that included covariates, we cannot definitively say whether the association between problematic alcohol use and hippocampal volume differs depending on whether hippocampal volume is measured as absolute (unadjusted for intracranial volume) or proportional (adjusted for intracranial volume).
We found some evidence of a bias toward the publication of studies reporting larger, significant effects for problematic alcohol use and hippocampal volume reductions. Sample sizes for the majority of the included studies were quite small. Although this is perhaps not surprising, given the clinical nature of many of the samples and the resources required for conducting MRI research, it is likely that many of the existing studies in the literature are underpowered to detect significant effects and/or that effects reported as significant are spurious. A post hoc power analysis (using G*Power 3.1; Faul et al. 2007) indicated that a total sample of 120 (with 60 participants in the alcohol use group and 60 in the comparison group) is needed to have 80% power to detect an effect size of d = |0.53|, the magnitude of the overall effect identified in the present meta-analysis; a total sample of 274 (137 in the alcohol group and 137 in the comparison group) is needed to detect an effect size of d = |0.34|, the magnitude of the overall effect after imputing studies missing due to publication bias. Only four (17%) of the studies included in the present meta-analysis had samples sizes equal to or greater than 120 participants, and many had sample sizes that were considerably smaller. The present meta-analysis informs future research in quantifying the magnitude of the expected effect, and suggests that larger sample sizes than are typically used are needed in this research.
The present meta-analysis makes an important contribution to the study of problematic alcohol use and hippocampal volume in quantifying the magnitude of the association and identifying several important sample and methodological moderators. However, there are a number of limitations that must be noted. Meta-analysis is necessarily limited to the existing empirical research. We identified 23 studies that were included in the present meta-analysis, which is comparable with or larger than the number of studies included in previous meta-analyses of schizophrenia, post-traumatic stress disorder, and unipolar depression and hippocampal volume (Wright et al. 2000; Campbell et al. 2004; Videbech & Ravnkilde, 2004; Kitayama et al. 2005; Smith, 2005; Karl et al. 2006). Even so, the number of studies available for moderator analyses was relatively small and analyses may consequently have been underpowered to detect effects as significant – as such, additional research is needed to determine whether the moderate but nonsignificant effects for adolescents with clinically significant alcohol use and for females reflects a lack of power. Although the studies included in the meta-analysis all examined associations between problematic alcohol use and hippocampal volumes, there was considerable variability in how alcohol use was defined, study inclusion and exclusion criteria, the nature of the comparison group, and how and whether other substance use and psychiatric comorbidity was addressed. Many studies defined problematic alcohol use as alcohol dependence (Laakso et al. 2000; Bleich et al. 2003; Beresford et al. 2006; Lee et al. 2007; Wrase et al. 2008; Fein & Fein, 2013; Gross et al. 2013; Ozsoy et al. 2013; Le Berre et al. 2014; Starcevic et al. 2015), but some studies defined it as a diagnosis of either alcohol abuse or dependence (De Bellis et al. 2000; Nagel et al. 2005; Makris et al. 2008; Durazzo et al. 2011; Smith et al. 2011; Fein et al. 2013), others defined it as alcohol abuse (Schuff et al. 2008), and one study included participants with alcohol dependence but excluded those with alcohol abuse (Agartz et al. 2003). Many studies included a ‘super healthy’ comparison group (i.e. no diagnosis of any psychiatric disorder; Laakso et al. 2000; Agartz et al. 2003; Bleich et al. 2003), but other studies included a psychiatric comparison group (Schuff et al. 2008). Some studies included participants with current use of substances other than alcohol (Medina et al. 2007; Fein & Fein, 2013), but most studies excluded participants with either a current or history of other substance use (Sullivan et al. 1995; Laakso et al. 2000; Agartz et al. 2003; Nagel et al. 2005; Lee et al. 2007; Makris et al. 2008; Schuff et al. 2008; Wrase et al. 2008; Durazzo et al. 2011; Smith et al. 2011; Fein et al. 2013; Gross et al. 2013; Ozsoy et al. 2013; Le Berre et al. 2014; Starcevic et al. 2015); several studies did not report whether other substance use was assessed or used as an inclusion or exclusion criterion. Of particular importance, given that other forms of psychopathology have been found to be associated with reduced hippocampal volume, as noted above, several studies included participants with comorbid psychiatric disorders (e.g. schizophrenia, post-traumatic stress disorder, antisocial personality disorder, or varied disorders; De Bellis et al. 2000; Laakso et al. 2000; Agartz et al. 2003; Schuff et al. 2008; Smith et al. 2011; Cheetham et al. 2014; Starcevic et al. 2015), whereas other studies excluded participants with any comorbid psychiatric disorder (Sullivan et al. 1995; Lee et al. 2007; Durazzo et al. 2011; Gross et al. 2013; Ozsoy et al. 2013). Because the comorbid psychiatric disorders varied so much across studies, and because half of the included studies did not report on comorbidity, we did not examine comorbidity as a potential moderator in the present meta-analysis. However, there is suggestive evidence that the association between problematic alcohol use and reduced hippocampal volume may be driven by other substance use and externalizing psychopathology (Fein et al. 2013); future research should, thus, assess and report on comorbidity and its effects on hippocampal volume. Additional research is also needed that further explicates the finding that varied forms of psychopathology show reduced hippocampal volume – this may reflect, for example, a nonspecific effect of psychopathology-related stress on the hippocampus (e.g. McEwen, 2007). Finally, given that the existing literature is as yet relatively small, we were unable to examine specific aspects of alcohol use that may be particularly relevant, such as binge drinking or hangover symptoms; evidence from animal studies indicates that chronic intermittent alcohol exposure (an analog to human binge drinking) leads to reduced hippocampal neurogenesis (Nixon & Crews, 2002), and speaks to the importance of considering such factors as quantity and frequency of alcohol use, and hangover and withdrawal symptoms in future research.
In conclusion, the present meta-analysis indicates that problematic alcohol use is associated with reduced hippocampal volume. Effects are stronger for clinically significant v. subclinical alcohol use and among adults relative to adolescents; effects did not differ among males and females, or as a function of segmentation method, scanner strength, or covariate adjustment. The cross-sectional nature of the existing studies limits the conclusions that can be drawn regarding the causal nature of the association between problematic alcohol use and hippocampal volume. Future research using causally informative study designs will help to determine whether this association reflects premorbid risk for the development of problematic alcohol use and/or whether alcohol has a neurotoxic effect on the hippocampus.
Acknowledgments
Research reported in this article was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers K01DA037280 (S. W.), R37DA05147 (W. G. I.), and R01DA036216 (W. G. I.), and the National Institute of Mental Health of the National Institutes of Health under Award Number T32MH15755 (S. W.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiological Reviews. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Agartz I, Shoaf S, Rawlings RR, Momenan R, Hommer DW. CSF monoamine metabolites and MRI brain volumes in alcohol dependence. Psychiatry Research: Neuroimaging. 2003;122:21–35. doi: 10.1016/s0925-4927(02)00084-7. [DOI] [PubMed] [Google Scholar]
- Bartels C, Kunert H-J, Stawicki S, Kröner-Herwig B, Ehrenreich H, Krampe H. Recovery of hippocampus-related functions in chronic alcoholics during monitored long-term abstinence. Alcohol and Alcoholism. 2007;42:92–102. doi: 10.1093/alcalc/agl104. [DOI] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addiction Biology. 2007;12:122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Lie YDD, Shen D, Davatzikos C. Hippocampus volume loss due to chronic heavy drinking. Alcoholism: Clinical and Experimental Research. 2006;30:1866–1870. doi: 10.1111/j.1530-0277.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Grant SJ, Hommer DW. Cross-sectional volumetric analysis of brain atrophy in alcohol dependence: effects of drinking history and comorbid substance use disorder. American Journal of Psychiatry. 2003;160:1–8. doi: 10.1176/appi.ajp.160.11.2038. [DOI] [PubMed] [Google Scholar]
- Bleich S, Sperling W, Degner D, Graesel E, Bleich K, Wilhelm J, Havemann-Reinecke U, Javaheripour K, Kornhuber J. Lack of association between hippocampal volume reduction and first-onset alcohol withdrawal seizure: a volumetric MRI study. Alcohol and Alcoholism. 2003;38:40–44. doi: 10.1093/alcalc/agg017. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-Analysis Version 3. Biostat; Englewood, NJ: 2014. [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. American Journal of Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. NeuroImage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Research: Neuroimaging. 2005;138:115–130. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons J, Yücel M, Lubman DI. Volumetric differences in the anterior cingulate cortex prospectively predict alcohol-related problems in adolescence. Psychopharmacology. 2014;231:1731–1742. doi: 10.1007/s00213-014-3483-8. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Bird CM. Amnesia and the hippocampus. Current Opinion in Neurology. 2006;19:593–598. doi: 10.1097/01.wco.0000247608.42320.f9. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol and Alcoholism. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, van Duijn CM, Hofman A, Breteler MMB. Alcohol intake in relation to brain magnetic resonance imaging findings in older persons without dementia. American Journal of Clinical Nutrition. 2004;80:992–997. doi: 10.1093/ajcn/80.4.992. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcoholism: Clinical and Experimental Research. 2011;35:1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn A-M, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fein G, Fein D. Subcortical volumes are reduced in short-term and long-term abstinent alcoholics but not those with a comorbid stimulant disorder. NeuroImage: Clinical. 2013;3:47–53. doi: 10.1016/j.nicl.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Greenstein D, Cardenas VA, Cuzen NL, Fouche J-P, Ferrett H, Thomas K, Stein DJ. Cortical and subcortical volumes in adolescents with alcohol dependence but without substance or psychiatric comorbidities. Psychiatry Research: Neuroimaging. 2013;214:1–8. doi: 10.1016/j.pscychresns.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Yeh P-H, Hardin D, Banys P, Meyerhoff DJ. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Research: Neuroimaging. 2008;162:133–145. doi: 10.1016/j.pscychresns.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CM, Spiegelhalder K, Mercak J, Feige B, Langosch JM. Predictability of alcohol relapse by hippocampal volumetry and psychometric variables. Psychiatry Research: Neuroimaging. 2013;212:14–18. doi: 10.1016/j.pscychresns.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Nagel BJ, Spadoni AD, Gorlick A, Tapert SF. Hippocampal volumes in adolescents with and without a family history of alcoholism. American Journal of Drug and Alcohol Abuse. 2010;36:161–167. doi: 10.3109/00952991003736397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. European Journal of Neuroscience. 2005;21:2711–2720. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yagüe AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, García-Verdugo JM. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proceedings of the National Academy of Sciences. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biological Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hommer DW. Male and female sensitivity to alcohol-induced brain damage. Alcohol Research and Health. 2003;27:181–185. [PMC free article] [PubMed] [Google Scholar]
- Honey GD, Fletcher PC, Bullmore ET. Functional brain mapping of psychopathology. Journal of Neurology, Neurosurgery and Psychiatry. 2002;72:432–439. doi: 10.1136/jnnp.72.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Single alcohol exposure in early life damages hippocampal stem/progenitor cells and reduces adult neurogenesis. Neurobiology of Disease. 2007;26:597–605. doi: 10.1016/j.nbd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF. Neurotoxic effects of alcohol in adolescence. Annual Review of Clinical Psychology. 2013;9:703–721. doi: 10.1146/annurev-clinpsy-050212-185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dörfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neuroscience and Biobehavioral Reviews. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews Neuroscience. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. Journal of Affective Disorders. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Koolschijn P, van Haren NE, Lensvelt-Mulders GJ, Pol H, Hilleke E, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Human Brain Mapping. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Savolainen L, Repo E, Soininen H, Aronen HJ, Tiihonen J. A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behavioural Brain Research. 2000;109:177–186. doi: 10.1016/s0166-4328(99)00172-2. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Le Berre A-P, Pitel A-L, Chanraud S, Beaunieux H, Eustache F, Martinot J-L, Reynaud M, Martelli C, Rohlfing T, Sullivan EV, Pfefferbaum A. Chronic alcohol consumption and its effect on nodes of frontocerebellar and limbic circuitry: comparison of effects in France and the United States. Human Brain Mapping. 2014;35:4635–4653. doi: 10.1002/hbm.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Jang D-P, Kim J-J, An SK, Park S, Kim I-Y, Kim SI, Yoon K-J, Namkoong K. Alteration of brain metabolites in young alcoholics without structural changes. NeuroReport. 2007;18:1511–1514. doi: 10.1097/WNR.0b013e3282ef7625. [DOI] [PubMed] [Google Scholar]
- Light R, Pillemer DB. Summing Up. Harvard University Press; Cambridge, MA: 1984. [Google Scholar]
- Lipsey MW, Wilson DB. Practical Meta-analysis. Sage; Thousand Oaks, CA: 2001. [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone SM, Luciana M, Wilson S, Sparks JC, Hunt RH, Thomas KM, Iacono WG. Adolescent drinking and motivated decision-making: a cotwin-control investigation with monozygotic twins. Behavior Genetics. 2014;44:407–418. doi: 10.1007/s10519-014-9651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratology. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. Beyond DSM: seeking a brain-based classification of mental illness. Science. 2010;327:1437–1437. doi: 10.1126/science.327.5972.1437. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research: Neuroimaging. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K. Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16:287–295. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. Journal of Neurochemistry. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. Journal of Neuroscience. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinković K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychology Review. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsoy S, Durak AC, Esel E. Hippocampal volumes and cognitive functions in adult alcoholic patients with adolescent-onset. Alcohol. 2013;47:9–14. doi: 10.1016/j.alcohol.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Schuff N, Neylan TC, Fox-Bosetti S, Lenoci M, Samuelson KW, Studholme C, Kornak J, Marmar CR, Weiner MW. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry Research: Neuroimaging. 2008;162:147–157. doi: 10.1016/j.pscychresns.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Wang L, Cronenwett W, Goldman MB, Mamah D, Barch DM, Csernansky JG. Alcohol use disorders contribute to hippocampal and subcortical shape differences in schizophrenia. Schizophrenia Research. 2011;131:174–183. doi: 10.1016/j.schres.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcevic A, Dimitrijevic I, Aksic M, Stijak L, Radonjic V, Aleksic D, Filipovic B. Brain changes in patients with posttraumatic stress disorder and associated alcoholism: MRI based study. Psychiatria Danubina. 2015;27:78–83. [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proceedings of the National Academy of Sciences. 2010;107:11104–11109. doi: 10.1073/pnas.0912810107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. American Journal of Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- White AM, Matthews DB, Best PJ. Ethanol, memory, and hippocampal function: a review of recent findings. Hippocampus. 2000;10:88–93. doi: 10.1002/(SICI)1098-1063(2000)10:1<88::AID-HIPO10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Age-related effects of alcohol on memory and memory-related brain function in adolescents and adults. In: Galanter M, editor. Recent Developments in Alcoholism. Springer; New York, NY: 2005. pp. 161–176. [DOI] [PubMed] [Google Scholar]
- Wilson S, Malone SM, Thomas KM, Iacono WG. Adolescent drinking and brain morphometry: a co-twin control analysis. Developmental Cognitive Neuroscience. 2015a;16:130–138. doi: 10.1016/j.dcn.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S, Thomas KM, Iacono WG. Neurological risk factors for the development of problematic substance use. In: Wilson SJ, editor. Handbook of Cognitive Neuroscience. Wiley-Blackwell; Hoboken, NJ: 2015b. pp. 269–291. [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Caviness VS, Hodge SM, Tang L, Albaugh M. Amygdala volume associated with alcohol abuse relapse and craving. American Journal of Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. American Journal of Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Zeigler DW, Wang CC, Yoast RA, Dickinson BD, McCaffree MA, Rominowitz CB, Sterling ML. The neurocognitive effects of alcohol on adolescents and college students. Preventive Medicine. 2005;40:23–32. doi: 10.1016/j.ypmed.2004.04.044. [DOI] [PubMed] [Google Scholar]


