Summary
Cone-bipolar cells are interneurons that receive synaptic input from cone photoreceptor cells and provide the output of the first synaptic layer of the retina. These cells exhibit center-surround receptive fields, a prototype of lateral inhibition between neighboring sensory cells in which stimulation of the receptive field center excites the cell whereas stimulation of the surrounding region laterally inhibits the cell. This fundamental sensory coding mechanism facilitates spatial discrimination and detection of stimulus edges. However, although it is well established that the receptive field surround is strongest when ambient or background illumination is most intense, e.g., at midday, and that the surround is minimal following maintained darkness, the synaptic mechanisms that produce and modulate the surround have not been resolved. Using electrical recording of bipolar cells under experimental conditions in which the cells exhibited surround light responses, and light and electron microscopic immunocytochemistry, we show in the rabbit retina that bright light-induced activation of dopamine D1 receptors located on ON-center cone-bipolar cell dendrites increases the expression and activity of GABAA receptors on the dendrites of the cells and that surround light responses depend on endogenous GABAA receptor activation. We also show that maintained darkness and D1 receptor blockade following maintained illumination and D1 receptor activation result in minimal GABAA receptor expression and activity and greatly diminished surrounds. Modulation of the D1/GABAA receptor signaling pathway of ON-cBC dendrites by the ambient light level facilitates detection of spatial details on bright days and large dim objects on moonless nights.
Keywords: receptive field surround, lateral inhibition, dopamine D1 receptors, GABAA receptors, bipolar cells
Introduction
Many neurons in the visual, auditory, and somatosensory systems inhibit nearby cells via lateral connections. This lateral inhibition between neighboring sensory cells produces receptive fields (RFs) with a center-surround organization in which stimulation of the RF center excites a cell but stimulation of the surrounding region laterally inhibits the cell, a fundamental sensory coding characteristic that enhances spatial discrimination and the detection of stimulus edges.
Cone-bipolar cells (cBCs) receive input from cone photoreceptor cells (Fig. 1A) and provide excitatory signals to ganglion cells (GCs), the output neurons of the retina. Classic recordings of in vivo GCs and of cBCs in intact in vitro retinas have established that both cell types exhibit a center-surround RF organization with two characteristic features. First, following maintained (30 min) bright background illumination, stimulation of the RF surround alone using an annulus or ring of light produces a response that is opposite in polarity to that produced by center (spot) stimulation alone, a phenomenon called “surround activation,” and surround stimulation in the presence of center stimulation using spot and annulus together reduces the amplitude of the center response, a phenomenon called “surround antagonism” [1–8]. Second, although cBC and GC surround responses are strongest under maintained bright background illumination, surround strength progressively decreases as the light level decreases so that the surround is minimal following maintained darkness [7–12]. This adaptive process likely contributes to modulation of the spatial discrimination ability of human and monkey observers by the ambient light level [13]. In addition, evidence indicates that the greatly weakened surround that is observed following maintained darkness does not arise from the shift from cone to rod pathway function [9, 11, 14]. However, although these classic surround light responses depend on the ambient light level, the mechanisms and neural pathways (Fig. 1A) that produce cBC surrounds following maintained bright illumination and the light/dark adaptive processes that regulate surround strength remain unclear and controversial.
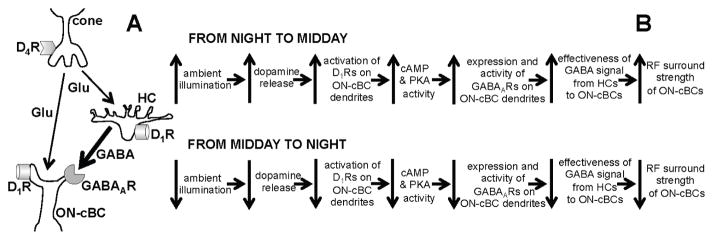
Figure 1. Schematic diagram showing signaling between cone, cone bipolar cell and horizontal cell (A) and model of how light/dark regulation of the dopamine D1R/GABAAR pathway of ON-cone bipolar cell dendrites modulates surround light responses (B).
(A) Cones use glutamate (Glu) to signal cone bipolar cells (cBCs) and horizontal cells (HCs). The dendrites of cBCs and HCs have both dopamine D1Rs and GABAARs. Evidence also suggests that HCs provide a feedforward GABA signal to cBC dendrites (shown) and an inhibitory feedback signal to cones (not shown), but the roles of these pathways in producing cBC surrounds remain unclear. (B) Model that accounts for how both the effectiveness of the GABA feedforward signal to ON-cBC dendrites and the strength of ON-cBC surround responses are modulated by gradual changes in the ambient (background) light level. As ambient illumination slowly increases during the morning, reaching a peak at midday, D1R activation increases, which in turn augments intracellular PKA, so that the expression and activity of GABAARs on ON-cBC dendrites - including dendritic tips - are enhanced. As a result, the effectiveness of the GABAAR-mediated feedforward signal from HC dendrites to ON-cBC dendrites increases, enhancing the strength of surround antagonism and activation. Conversely, as background illumination slowly decreases during the afternoon and evening, reaching darkness at night, D1R activation is reduced. This in turn decreases intracellular PKA, substantially lowering GABAAR expression and activity so that ON-cBC surround strength is minimal. Experiments and findings in this study primarily address whether light/dark regulation of ON-cBC dendritic D1Rs and GABAARs modulates the strength of ON-cBC surround light responses.
Intracellular cAMP and protein kinase A (PKA), by regulating the expression and activity of GABAA receptors (GABAARs) [15], modulate the effectiveness of GABA inhibition. Because in the retina 1) bright light increases the release of dopamine, which modulates a variety of cellular processes such as gap junction permeability, glutamate receptor sensitivity, and voltage-gated channels [16], 2) cBC dendrites express GABAARs [17–20] and dopamine D1 receptors (D1Rs), which increase intracellular cAMP and PKA when activated by dopamine [16, 21, 22], and 3) following maintained bright illumination D1, but not D2R-like antagonists reduce rabbit GC surround responses [23], we studied whether D1Rs and GABAARs on the dendrites of ON-center cBCs (ON-cBCs), a type of cBC, mediate light/dark modulation of the surround (Fig. 1B). Using a retinal preparation in which ON-cBCs exhibited surround light responses, we found that light-induced activation of ON-cBC dendritic D1Rs increases ON-cBC surround strength by enhancing the expression and activity of GABAARs on their dendrites (Fig. 1B). Our measurements also suggest that the dendrites of horizontal cells (HCs), which receive input from cones (Fig. 1A), provide a GABA feedforward signal that tonically depolarizes ON-cBC dendrites. As a result, ON-cBC hyperpolarizing surround light responses may reflect a reduction in GABAAR-mediated excitation.
RESULTS
Role of Dopamine D1Rs in ON-cBC Surround Light Responses
To study the mechanisms that underlie light/dark modulation of the ON-cBC RF surround, it is essential to utilize a preparation, such as intact retina, in which cells exhibit surround light responses. However, because BC somata are located in the middle of the retina, it is extremely difficult when using intact retinas to obtain electrical recordings from them and not feasible to selectively apply GABA onto their dendrites (see below). Therefore, we used retinal slices for BC recording experiments because they offered easy access to BC somata and dendrites. A limitation of slicing the retina is that it physically eliminates many neural connections, especially those involving lateral interactions, and limits neural and transmitter interactions. Moreover, we found that maintained bright background illumination greatly reduced the viability of rabbit retinal slices. Therefore, to study the mechanisms that underlie light/dark modulation of the surround, we maintained rabbit retinal slices in the dark. Furthermore, because increases in background light intensity enhance dopamine release in the retina [16], we added dopamine (5 μM) to the superfusate to mimic the effect of maintained bright illumination in the intact retina.
Whole-cell patch-clamp recordings of ON-cBCs (Fig. 2A-top traces, Fig. 2B) in this dopamine-bathed retinal slice preparation show that the cells exhibited surround antagonism (13 out of 18 cells) and surround activation (10 out of 18 cells). Moreover, the center and surround of ON-cBCs were relatively small in spatial extent (center diam < 150 μm; surround outer diam. < 350 μm). It is worth noting that following maintained bright illumination of monkey and amphibian eyecups, an in vitro preparation that includes intact neural retina and pigment epithelium (which enhances viability and light responsiveness), cone-driven BCs exhibited center and surround light responses - including both surround activation and antagonism - and their RF center and surround were also similarly small in spatial extent [6, 7]. The control data in Fig. 2 thus show that most rabbit ON-cBCs in dopamine-bathed slices exhibited center and surround responses that were similar in many respects to those of bright light-adapted cone-driven ON-BCs in more intact retinal preparations.
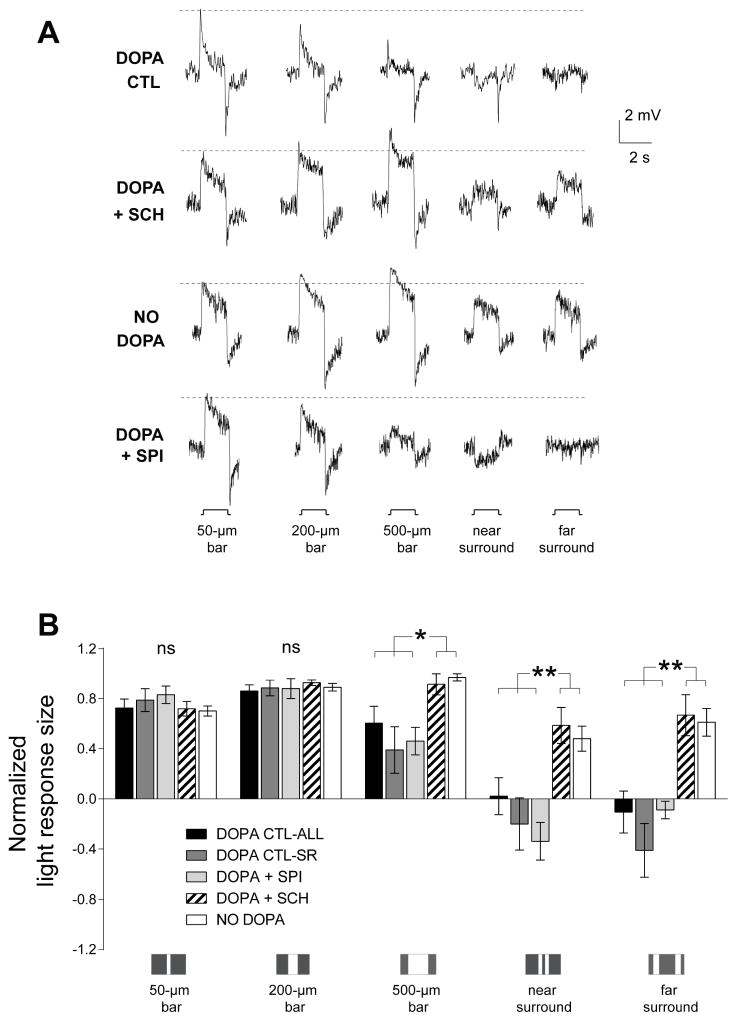
Figure 2. ON-cBC surround light responses depend on dopamine D1R activation.
(A) In dark-adapted rabbit retinal slices superfused with an Ames medium that contained dopamine (5 μM) to mimic the effect of maintained (30 min) bright illumination, ON-cBCs (top traces-control, DOPA CTL) exhibited both surround antagonism (i.e., the amplitude of center responses was reduced by simultaneous surround stimulation, as occurred in response to 500-μm (center and surround stimulation) vs. 50-μm (center stimulation only) wide bars), and surround activation (i.e., response to surround stimulation alone was opposite in polarity to the response produced by center stimulation alone, as occurred in response to near surround stimulation). In separate experiments, when the dopamine medium also contained SCH23390 (SCH; 5 μM) for ≥30 min (traces second row from top) or when slices were superfused without dopamine (NO DOPA, traces third row from top) to mimic the effect of maintained darkness, ON-cBCs exhibited minimal surround antagonism and activation. When the dopamine medium also contained spiperone (SPI; 5 μM) for ≥30 min (bottom traces), ON-cBCs exhibited both surround antagonism and activation. On-cBCs with sustained and transient light responses were observed under all four experimental conditions. (B) Average normalized peak response size of ON-cBCs to centered single bar stimuli of various widths (50-, 200- and 500-μm) and to near and far surround stimulation revealed that, with respect to both surround antagonism (p < 0.05) and surround activation (p < 0.01), the entire population of dopamine control cells (DOPA CTL-ALL, n=18), the sub-population of dopamine control cells that exhibited clear surround light responses (DOPA CTL-SR, n=10), and the entire population of cells bathed in both dopamine and spiperone (DOPA + SPI, n=5) were significantly different from the population of SCH-treated ON-cBCs (DOPA + SCH, n=5) and from the cells superfused without dopamine (NO DOPA, n=6). (A, B) Surround responses were evoked by two simultaneously flashed bar stimuli equidistant from the RF center. Near surround stimulation: distance between 50-μm wide bars = 100 μm. Far surround stimulation: distance between 100-μm wide bars = 500 μm. (A) The dotted horizontal lines adjacent to the response traces denote the peak response amplitude of the cells to the smallest centered stimulus (50-μm wide bar).
Separate experiments revealed that the strength of ON-cBC surround light responses depends on dopamine D1R – but not D2R-like - activation. When the superfusate contained both dopamine and SCH23390 (selective D1R antagonist), ON-cBCs exhibited depolarizing (RF center) responses to thin and wide centered bar stimuli and to bars flashed in the peripheral portion of their RFs, with little, if any, evidence of surround antagonism or surround activation (Fig. 2A-traces second row from top, Fig. 2B), as was observed when slices were superfused without dopamine to mimic the effect of maintained darkness (Fig. 2A-traces third row from top, Fig. 2B). When the superfusate contained both dopamine and spiperone (selective D2R family antagonist). ON-cBCs exhibited both surround antagonism and activation (Fig. 2A-bottom traces, Fig. 2B), and the spatial extent of center and surround were similar to those observed for dopamine control cells. These results show that most ON-cBCs exhibited both surround antagonism and activation when D1Rs were activated but not when D1Rs were blocked or not activated. In addition, the lack of effect of spiperone suggests that D2-like receptors, such as cone D4Rs, do not play a role in ON-cBC surround responses following maintained bright illumination.
Interestingly, the average peak amplitude of ON-cBC responses to light stimulation (intensity = 40 lux) was significantly (p < 0.05) larger (~60%) in SCH23390-treated slices (ave. 7.86 ± 2.13 (SEM) mV; n=5) and in slices superfused in the dark without dopamine (p < 0.01, ave. 8.30 ± 1.46 mV; n=6) than observed in slices in which D1Rs were strongly activated (i.e., containing dopamine alone or both dopamine and spiperone) and in which the cells exhibited surround light responses (4.62 ± 0.6 mV; n=14), suggesting that the light responses obtained during D1R activation were produced by opposing excitatory and inhibitory mechanisms. In addition, the average resting membrane potential of ON-cBCs was more hyperpolarized (p < 0.01) in both the presence of SCH23390 (−47.6 ± 1.4 mV, n=5) and in the absence of dopamine (−46.7 ± 1.1 mV, n=6) than in slices in which D1Rs were strongly activated (i.e., containing dopamine alone or both dopamine and spiperone) and in which the cells exhibited surround responses (−40.2 ± 1.0 mV; n=14) (see Discussion).
Activation of ON-cBC Dendritic D1Rs Increases GABAAR Activity of ON-cBC Dendrites
If maintained bright illumination – compared to maintained darkness - produces a sustained increase in dopamine activation of ON-cBC dendritic D1Rs so that GABAAR activity of the dendrites is strongly enhanced, there should be a clear difference in ON-cBC dendritic GABAAR activity following D1R activation compared to D1R blockade or to the absence of D1R activation. We therefore measured the GABAAR activity of ON-cBC dendrites by puffing GABA onto the dendrites following Co2+ (2 mM) blockade of synaptic transmission. Because mammalian ON-cBC dendrites may express GABACRs [19], the superfusate also included TPMPA (selective GABACR antagonist), so that GABA responses could be definitively attributed to GABAAR activity. We found that ON-cBC dendrites exhibited clear GABAAR activity when slices were maintained in dopamine-containing medium (Figs. 3A, D). Conversely, dendrites exhibited minimal GABAAR activity following > 30-min SCH23390-treatment (Figs. 3B, D) or in a medium without dopamine (Figs. 3C, D). Because GABA was puffed directly onto the dendrites following synaptic blockade and because light stimulation increases extracellular dopamine and D1R activation [16], the results suggest that light-evoked endogenous activation of D1Rs on ON-cBC dendrites increases GABAAR activity of the dendrites.
Figure 3. GABAAR activity of ON-cBC dendrites depends on endogenous activation of ON-cBC dendritic D1Rs.
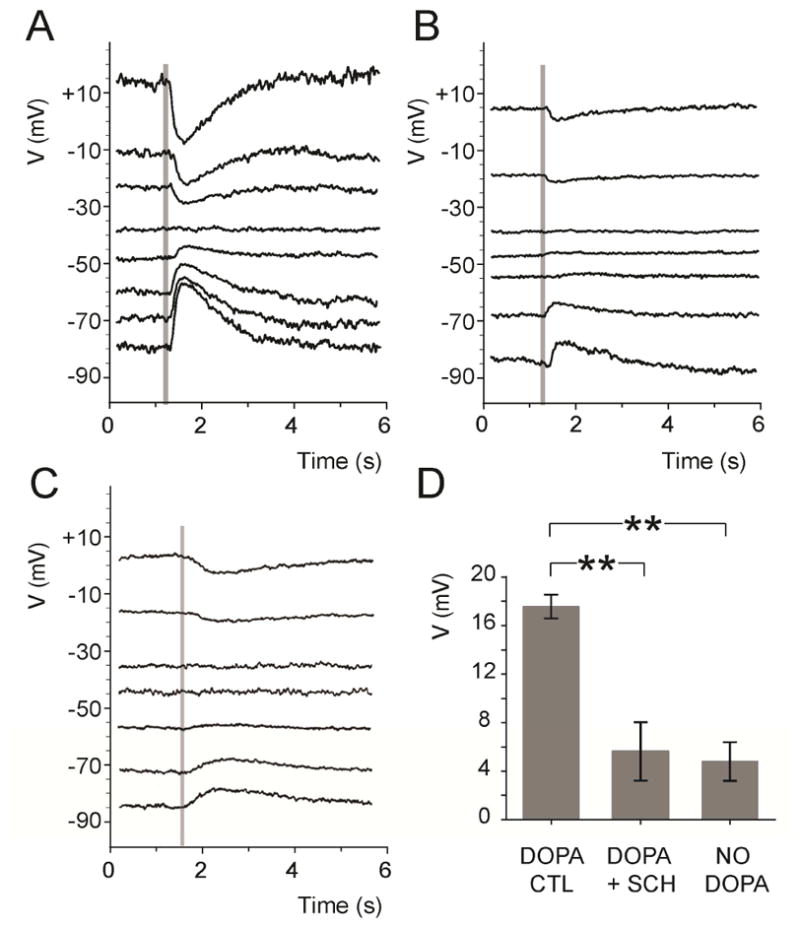
In rabbit retinal slices superfused with medium that contained dopamine (5 μM) and TPMPA (50 μM) and in which synaptic transmission was blocked with cobalt (2 mM), GABA (0.5 mM) was puffed onto BC dendrites (time of puff is indicated by shaded vertical lines) by pressure ejection. GABA responses were clearly evident under dopamine (control) conditions (A) with an average GABA reversal potential (EGABA) = −42.2 ± 2.6 (SEM) mV (n=5), but were much reduced in the presence of SCH (5 μM) (B) and when the superfusate did not contain dopamine (C). (D) When GABA was puffed at −70 mV, average peak GABAAR response size was significantly greater (p < 0.01) when the superfusate contained dopamine (DOPA CTL; 17.6 ± 1 mV; n=5) compared to when it contained dopamine and SCH (DOPA + SCH; 5.6 ± 2.4 mV; n=5) or did not contain dopamine (NO DOPA; 4.8 ± 1.6 mV; n=5).
A Reduction in Tonic GABAAR-mediated Excitation Underlies ON-cBC Hyperpolarizing Surround Light Responses
Because activation of D1Rs on ON-cBC dendrites modulates the surround light responses (Fig. 2) and GABAAR activity (Fig. 3) of the dendrites, we next determined whether endogenous GABAAR activity modulates the RF surround. Addition of APB, which selectively blocks cone to ON-BC signaling [24], revealed a hyperpolarizing response to wide centered bar stimuli (Fig. 4A, 14 out of 19 cells (= 74%)), as reported for cone-driven ON-BCs in the amphibian retina [7, 25]. This result suggests that most light-adapted mammalian ON-cBCs exhibit hyperpolarizing surround responses mediated by a neural pathway that does not involve direct input from cones or feedback to cones. Moreover, the opposite-polarity surround responses revealed by APB were greatly reduced by gabazine (GABAAR antagonist) (Figs. 4A, B), demonstrating for the first time in vertebrate retina that endogenous GABAAR activation mediates the surround of a majority of ON-cBCs. Interestingly, APB, but not SCH23390 (Fig. 2A), blocked the hyperpolarizing response waveform at light offset (11 out of 14 ON-cBCs), suggesting that hyperpolarizing OFF-responses of ON-cBCs [6, 7] originate in cones. As occurred with ON-cBCs, APB application eliminated the depolarizing responses of rod-BCs to centered bar stimuli. However, in contrast to ON-cBCs, when retinal slices were bathed in 5 μM dopamine APB did not reveal rod BC surround responses or an effect of gabazine (7 out of 7 recorded cells; Fig. 4C).
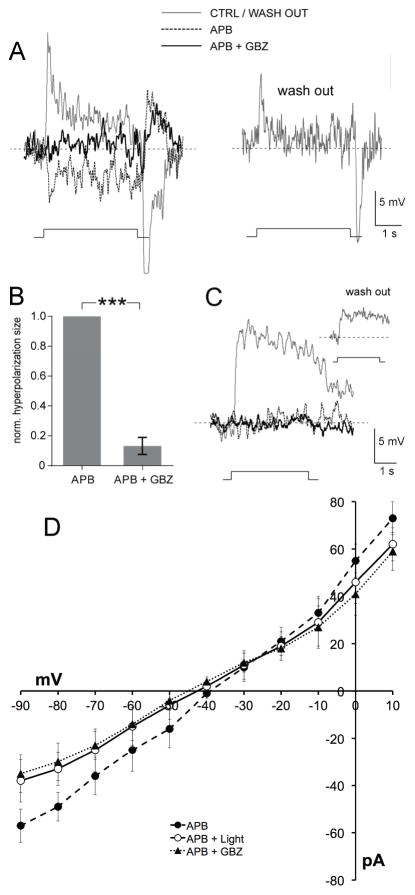
Figure 4. A reduction in tonic endogenous GABAAR excitation underlies the opposite-polarity, hyperpolarizing surround light responses of ON-cBCs.
In the presence of dopamine (5 μM) and the glutamate analogue APB (i.e., L-AP4; 50 μM), a selective agonist of the mGluR6-Rs on both ON-cBC and rod BC dendrites that blocks cone to ON-cBC and rod to rod BC signaling [24], ON-cBCs (A, B), but not rod BCs (C), produced opposite-polarity (i.e., hyperpolarizing) surround responses to large (500-μm wide) centered bar stimuli that were blocked by gabazine (GBZ; 50 μM; 9 out of 9 cells). Washout of APB and GBZ showed recovery from their effects (A, C) (see Fig. S1 and “Identification of Cell Types” in STAR Methods). (B) For each ON-cBC, addition of GBZ to the APB-containing medium greatly reduced the average normalized size of opposite-polarity hyperpolarizing surround light responses (p < 0.001; paired t-test; n=9). (D) Comparison of the current-voltage relationship of ON-cBCs (n=5) following applications of APB alone in the dark and during surround light responses, and following application of both APB and gabazine in the dark. Average steady-state current at each holding potential (i.e., current measured near the end of the voltage pulses and when the amplitude of the light responses was relatively steady) is shown for all three experimental conditions.
To study whether ON-cBC hyperpolarizing surround light responses revealed by APB result from an increase or decrease in GABAAR conductance, we made I–V measurements of ON-cBCs using a medium that contained APB (blocks glutamate-gated conductance of ON-cBCs) and dopamine. For each cell, we made measurements before and during light stimulation and before and during gabazine application (Fig. 4D). We found that light stimulation (880 ± 124 pS, n=5) and gabazine (840 ± 111 pS, n=5) significantly decreased (p < 0.05, paired t-test) the mean slope conductance of ON-cBCs compared to APB alone (i.e., before light stimulation and gabazine application) (1,180 ± 135 pS, n=5) (Fig. 4D). In every cell, the conductance in APB alone exceeded the conductances during hyperpolarizing light responses and following gabazine application. Moreover, light stimulation following gabazine application in the dark did not further decrease the cell conductance. These results suggest that GABA tonically excites ON-cBCs following maintained D1R activation and that hyperpolarizing surround light responses revealed by APB can be attributed to reduced GABAAR activity.
Light/dark Modulation of D1Rs Regulates GABAAR expression of ON-cBC Dendrites
We performed immunocytochemistry experiments to determine whether the background illumination conditions and D1Rs regulate ON-cBC dendritic GABAAR expression. As reported for mammalian retinas [8, 17–20], we found that ON-cBC dendrites express GABAARs following maintained bright illumination. We also report the novel findings that ON-cBC dendritic GABAAR expression is greatly diminished following maintained darkness due to minimal activation of their D1Rs and significantly enhanced following maintained bright illumination due to light-induced activation of their D1Rs (Figs. 5, 6).
Figure 5. GABAAR expression on rabbit ON-cBC dendrites is regulated by the illumination conditions and D1R activation.
(A, D) Projected images of several ON-BCs showing double immuno-staining of GABAARs and Goα, which labels both ON-cBCs and rod BCs in the rabbit retina (A), and double immuno-staining of GABAARs and PKCα, which labels rod BCs, but not ON-cBCs (D), revealed that GABAAR-IR was located more on the dendrites of ON-cBCs (but not rod-BCs) following maintained bright light adaptation (LA) than following maintained dark adaptation (DA) or following maintained bright illumination with SCH (5 μM) (See also Fig. S2). Little evidence of GABAAR-IR on rod-BC dendrites following maintained bright illumination (D) was observed. (B) Single confocal vertical optical sections (0.30-μm thick and at intervals of 0.60 μm) of the 3D-reconstructed rabbit ON-cBC dendrites shown in A indicate that GABAAR-IR was located on the dendrites of ON-cBCs following maintained bright illumination. Immunostaining pattern in the absence of primary antibody confirmed the specificity of the GABAAR labeling (data not shown). (C) Quantification showed that the average number of co-localized GABAAR-IR signals on ON-cBC dendrites was significantly greater (p < 0.01) following maintained bright illumination compared to following maintained darkness or following maintained bright illumination with SCH. In addition, quantitative colocalization analysis confirmed that the GABAAR-IR puncta were located on ON-BC dendrites following maintained bright illumination (Fig. S3). (A, B) GABAARs: cyan; ON-BCs (Goα): magenta; colocalized: white. (D) GABAARs: cyan; rod BCs (PKCα): magenta. (A, B, D) Arrowheads denote colocalized puncta in A and B but denote GABAAR-IR in D. (A) Asterisks denote ON-cBC somata. (A, B, D) Scale bars: 5 μm.
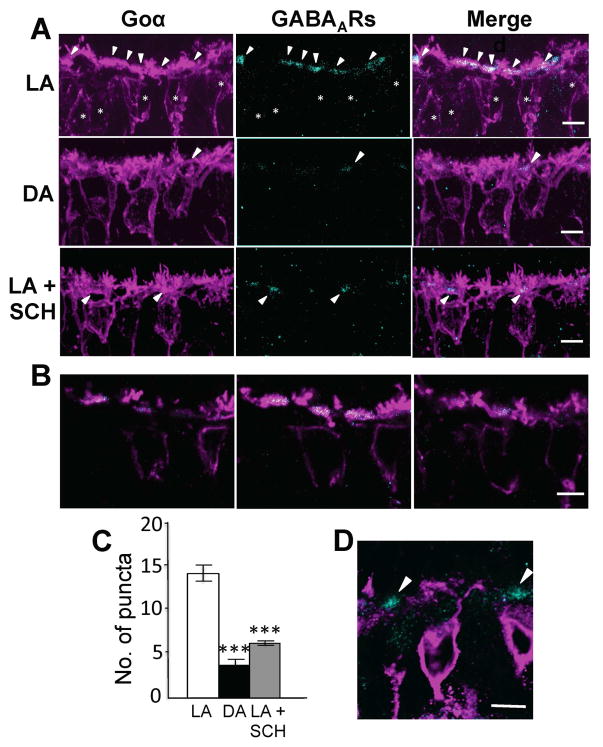
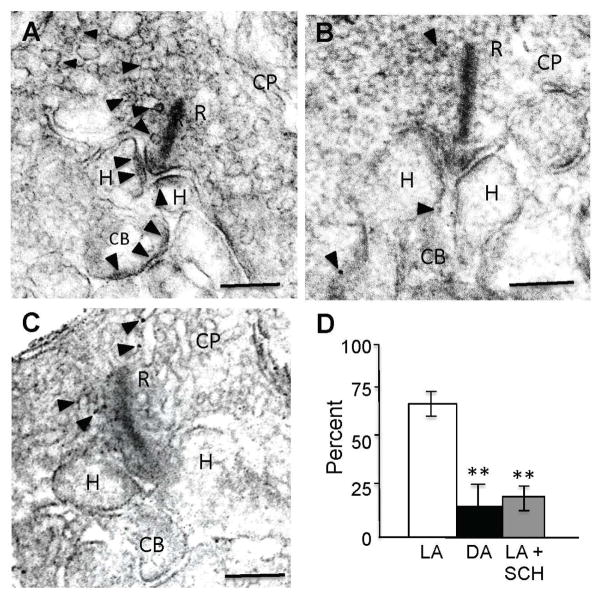
Figure 6. GABAAR expression on the dendritic tips of ON-cBCs is increased by bright light-evoked dopamine D1R activation.
(A–C) GABAARs were located at the plasma membrane of BC and HC dendritic tips following maintained bright illumination (A) but the number and size of clustered GABAAR-IR puncta were reduced following maintained darkness (B) and following maintained bright illumination when D1Rs were blocked with SCH (5 μM) (C). (D) Average percent of GABAAR-IR puncta in invaginations of ON-cBC dendritic tips at cone ribbon (triadic) synapses for each of the three experimental conditions. The average percent values shown were obtained by first determining the average percent value for each retina and then calculating the average value for all of the retinas in each experimental condition (1 retina/rabbit, 3 rabbits per experimental condition). GABAAR-IR was observed significantly more (p < 0.01; χ2 test for both LA vs. DA and LA vs. LA + SCH) in invaginated ON-cBC dendritic tips following maintained bright illumination (light-adapted, LA; total of 33 triadic synapses) than following maintained darkness (dark-adapted, DA; total of 27 triadic synapses) and following maintained bright illumination when D1Rs were blocked (LA + SCH; total of 23 triadic synapses). (A–C) Arrowheads: GABAAR-IR puncta; R: synaptic ribbons; CP: cone pedicles; CB: ON-cBC dendrites; H: HC dendrites; Scale bars: 100 nm. See also Figure S2.
Following maintained bright illumination, staining for the α1 and β2/3 subunits of GABAA Rs were similar [17–19], revealing narrow bands composed of clustered puncta just beneath cone pedicles (Fig. S2). Although staining for the β2/3-subunit was somewhat less intense than that of the α1 subunit, the following results concerning the effects of background illumination and D1R activation are based on β2/3-subunit immunoreactivity, because this subunit, and not the α1-subunit, contains phosphorylation and regulatory sites that modulate GABAAR expression and activity [15].
Double immunostaining of GABAARs with cell-type specific markers demonstrated that GABAAR-IR was located significantly more on the dendrites of ON-cBCs (Figs. 5A, 5B), but not rod BCs (Fig. 5D), following maintained bright illumination, than following maintained darkness or following maintained bright illumination with SCH23390. Quantification of co-localized signals supports these results (Figs. 5C, S3).
Immuno-electron microscopic data revealed that in the rabbit puncta of GABAAR-IR were located in the dendritic tips of ON-cBCs and HCs within the invagination of the cone synaptic (“triadic”) terminal following maintained bright illumination (Fig. 6A), as reported [17–19], but the number and size of GABAAR-IR puncta were significantly reduced following maintained darkness (Fig. 6B, 6D) and following maintained bright illumination when dopamine D1Rs were blocked (Fig. 6C, 6D). GABAARs were also found on vesicles within cone pedicles following maintained bright illumination (Fig. 6A), as reported in fish [26], and following maintained darkness and maintained bright illumination when D1Rs were blocked (Figs. 6B, C).
DISCUSSION
Roles of ON-cBC Dendritic GABAARs and D1Rs in Light/Dark Modulation of the RF Surround
The findings reported here show in the rabbit retina the following: (1) endogenous activation of dopamine D1Rs and GABAARs strengthens the RF surround of ON-cBCs (Figs. 2, 4); (2) activation of D1Rs on the dendrites of ON-cBCs increases the GABAAR activity of the dendrites (Fig. 3); (3) GABAAR expression increases on the dendrites - including dendritic tips - of ON-cBCs due to bright light-induced activation of D1Rs located on the dendrites (Figs. 5, 6); (4) following maintained darkness when D1R activation is minimal, ON-cBC surround light responses and ON-cBC dendritic GABAAR activity are strongly reduced (Figs. 2, 3) and GABAAR expression is significantly lower on the dendrites and dendritic tips of ON-cBCs (Figs. 5, 6); (5) the hyperpolarizing surround responses of most ON-cBCs are mediated by a neural pathway that does not involve feedback to cones (Fig. 4); (6) the input resistance of ON-cBCs increases during GABAAR-mediated hyperpolarizing surround light responses (Fig. 4D); and (7) following maintained bright illumination when D1R activation is strong, rod BCs do not produce surround light responses and their dendrites do not exhibit GABAAR activity or express GABAARs (Figs. 4C, 5D; STAR Methods). Together, these results show that the increase in GABAAR expression and activity of the dendrites – including dendritic tips - of ON-cBCs following maintained bright light-induced activation of ON-cBC dendritic D1Rs strengthens the RF surround of ON-cBCs (Fig. 1). Conversely, following maintained darkness when D1R activation is minimal, GABAAR function is substantially reduced and surround strength is greatly diminished.
The relatively slow time course of D1R-mediated regulation of GABAAR expression and activity, which likely involves receptor trafficking, is consistent with measurements of the time course of light/dark-induced changes in surround strength. Observations from in vivo mammalian GCs and from GCs in intact in vitro non-mammalian retinas indicate that light/dark-induced changes in GC surround strength occur slowly (up to 30 minutes or more) depending on the extent and rate of change of the background light [9, 10, 27, 28]. For example, following 30 minutes of bright illumination GC surround strength becomes minimal only after ~30 minutes of complete darkness, and following maintained darkness surround strength reaches full strength – including both surround activation and antagonism - following 30 minutes of subsequent bright illumination. We observed a change from full to minimal GABAAR expression and activity of ON-cBC dendrites ~30 min following SCH23390 application (see STAR Methods; Figs. 2, 3, 5, 6). These results are consistent with the idea that a sudden extreme change in background light level from bright to dark (or dark to bright) switches surround strength from robust - including both surround activation and antagonism - to minimal over the course of 30 min during which GABAAR expression and activity decrease from full strength to minimal.
However, unlike in the laboratory where sudden extreme changes in background light intensity are often used, ambient light in the natural outdoor environment typically changes gradually over the course of day and night. Experimental evidence suggests that changes in surround strength in response to gradual changes in background illumination require minutes, rather than tens of minutes, to reach steady state [8, 10, 27–29]. Thus, if surround strength signals the ambient light level as suggested [29, 30], then modulation of the surround from its strongest state during bright illumination (midday) until it is minimal (middle of the night) results from changes in GABAAR expression and activity that need to keep pace with the rotation of the Earth, i.e., that occur gradually over the course of ~12 hours (Fig. 1B).
Although GABAAR activity itself, which increases the chloride conductance, can produce surround antagonism by reducing glutamate-mediated center responses (i.e., by shunting inhibition), opposite-polarity hyperpolarizing surround responses (i.e., surround activation) that are GABAAR-mediated require that the ECl of ON-cBC dendrites is more positive than that of the somata or synaptic terminals. The finding that the conductance of ON-cBCs decreased during GABAAR-mediated hyperpolarizing surround light responses (Fig. 4D) suggests that GABA tonically excites ON-cBCs following maintained D1R activation, i.e., following maintained illumination. As a result, brief surround light stimulation brighter than the background decreases this GABA excitation, producing a hyperpolarization. We found that the absence or blockade of D1R activation hyperpolarized the resting membrane potential of rabbit ON-cBCs by ~7 mV (Fig. 2), supporting the idea that GABA depolarizes ON-cBCs following maintained bright illumination when D1R activation is strongest but not following maintained darkness when D1R activation is minimal. This result is consistent with the observation that the resting membrane potential of cone-driven ON-BCs in amphibian eyecups became progressively more negative as the maintained background illumination decreased in intensity [31]. Our conductance measurements (Fig. 4D) are not consistent with a scenario in which light-evoked inhibitory GABAAR-mediated signals produce ON-cBC hyperpolarizing surround responses. If this were so, light stimulation and gabazine application in the presence of APB would increase and decrease the conductance of the cells, respectively.
Although some reports suggest that GABAAR activity depolarizes ON-cBC dendrites [32, 33], especially in the case of a specific ON-cBC subtype [34], other work does not support this view. However, the strong dependence of surround strength on the intensity and duration of background illumination, especially the finding that in vivo mammalian GC surround activation requires brighter maintained illumination than does surround antagonism [8, 10, 27. 29], and the findings here that the expression and activity of ON-cBC dendritic GABAARs and the GABAAR-mediated conductance of ON-cBCs increase as background illumination and D1R activation increase, suggest that previous studies did not find that GABA depolarizes ON-cBC dendrites because the experiments were performed in the dark [34, 35] when surround activation does not occur. We note that one study of ON-BCs in rat retinal slices [36], done with gramicidin perforated patch recording (to preserve ECl) under light-adapted conditions, found no evidence of GABA depolarizations. However, the data in that report came primarily from rod BCs, rather than ON-cBCs, and it was not documented that the ON-BCs exhibited surround activation or any light responses at all. As noted in Results, ON-cBCs in bright light-adapted rabbit retinal slices produced weak, if any, light responses and did not exhibit surround activation.
Neural Pathways that Contribute to the RF Surround of ON-cBCs
The cellular source of the GABA that activates ON-cBC dendritic GABAARs following maintained bright illumination is not yet resolved. However, the following findings together strongly suggest that HC dendrites (adjacent to ON-cBC dendrites within the cone synaptic terminal invagination [17–19]) contribute to ON-cBC surround responses [2, 3, 5, 37–40] by directly releasing GABA onto ON-cBC dendrites and activating their GABAARs [31, 32, 41]: 1) synaptic blocking experiments indicate that activation of ON-cBC dendritic D1Rs increases the GABAAR activity of ON-cBC dendrites (Fig. 3); and 2) APB experiments show that hyperpolarizing surround light responses of most ON-cBCs are mediated by a reduction in tonic GABA excitation and a pathway that does not involve direct cone input to ON-cBCs or feedback to cones (Fig. 4). Moreover, the observation that HC dendritic tips, which express D1Rs [16], express GABAARs following maintained bright illumination but not following maintained bright illumination during D1R blockade or maintained darkness (Fig. 6) suggests that the background light level and D1R activation modulate the expression and activity of HC dendritic and ON-cBC dendritic GABAARs in a similar fashion. In addition, because GABA depolarizes HCs [42], it seems possible that the light-evoked D1R/GABAAR pathway in HC dendrites enhances the tonic release of GABA onto ON-cBC dendrites via GABAAR-mediated excitation of HCs. According to this view, HC light responses initiate ON-cBC surround responses but the effectiveness of the HC signal is strongly modulated by dopamine regulation of ON-cBC dendritic GABAARs (Fig. 1B). Moreover, the D1R-mediated decrease in HC coupling that occurs when background illumination increases [8, 16] may account for the decrease in RF surround size that has been observed for GCs [8, 9, 29] and ON-cBCs (Fig. 2 here) when the background light level increases. Further HC studies are needed to test these ideas.
The data in Fig. 4 suggest that following maintained bright illumination most (~70%) rabbit ON-cBCs exhibit a GABAAR-mediated surround that is not blocked by APB (i.e., driven by a pathway independent of direct cone input). This is consistent with the observation in amphibian retina that following maintained bright illumination ~67% of BCs receive surround input via a pathway that does not involve direct cone input (i.e., is APB-insensitive) [7]. Based on these findings, and also because in vivo immunoEM has shown that virtually all mammalian ON-cBC dendrites express GABAARs [18], it seems plausible that in in vivo retina most (> 70%) ON-cBC dendrites express functional GABAARs that mediate surround responses following maintained bright illumination. However, whereas our findings suggest that ON-cBC dendritic GABAAR activity plays a key role in ON-cBC surround responses following maintained bright illumination, our results do not reveal the relative weight of this surround mechanism. Non-GABAAR mechanisms, such as protons and ephaptic communication [8, 43–46], and other neural pathways, such as HC feedback to cones (Fig. 1A) [8, 47, 48], have been reported to contribute to ON-cBC surrounds. However, evidence is still needed to address whether these other mechanisms and neural pathways strongly contribute to ON-cBC surround activation and antagonism following maintained bright illumination but are weakly active following maintained darkness when surround responses are minimal [8].
Electrical recordings of in vivo mammalian ON-GCs and ON-BCs in intact in vitro retinas, as well as the findings here, have shown that the classic antagonistic RF surround: 1) originates in the outer retina; 2) is strongest following maintained bright illumination and minimal following maintained darkness; 3) is relatively sustained [8, 10, 11, 49, 50]; and 4) is D1R-dependent (Figs. 2, 3, 5, 6 here) [23, 49]. The similarity in the characteristics of the classic antagonist surround of ON-cBCs and ON-GCs, post-synaptic targets of ON-cBCs, as well as the finding that D1Rs mediate light/dark modulation of GABAAR expression of in vivo ON-cBC dendrites (Figs. 5, 6), suggest that dopamine regulation of ON-cBC dendritic GABAARs contributes to light/dark modulation of the surrounds of in vivo ON-cBCs and ON-GCs. It is worth noting that some GC subtypes exhibit additional diverse surround mechanisms that may be produced in the inner retina. However, in contrast to the classic antagonist surround, inner retinal surrounds are relatively transient and D1R-independent [8, 49–52].
Function of Light/dark Adaptive Regulation of the RF Surround
Because the RF surround enhances spatial discrimination and edge detection, differentiating regions of the visual scene that are brighter or dimmer than the background [8, 29], the bright light driven increase in D1R activation, and the resultant increase in ON-cBC dendritic GABAAR expression and activity, may enhance the spatial discrimination ability of ON-center cBCs and their downstream targets such as ON-center GCs. Conversely, the greatly diminished function of D1Rs/GABAARs following maintained darkness weakens the surround of ON-cBCs, reducing their spatial discrimination ability. Moreover, our finding (Fig. 2) and reports that the RF centers and surrounds of cone-driven BCs and GCs shrink as the maintained background light level increases [6–9, 29] suggest that the interaction between center and surround spatially differentiates progressively smaller regions of the visual scene as ambient illumination gradually increases, thereby enhancing the ability of BCs and GCs to discriminate fine spatial detail and edges. Light/dark adaptive modulation of the strength and size of the ON-cBC surround thus may contribute to modulation of the spatial discrimination ability of human and monkey observers by ambient illumination, as we and others suggested [8, 13, 29, 53]. The D1R/GABAAR signaling pathway of ON-cBC dendrites therefore serves as a light/dark adaptive regulatory mechanism that tunes synaptic and neural pathway function in the retina to visual performance needs under different ambient light levels, increasing the ability of animals to see fine spatial details on bright days and to see large dim objects on moonless nights.
STAR★METHODS
CONTACT FOR REAGENTS AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Stuart Mangel (mangel.1@osu.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
Experiments were performed on adult pigmented Dutch-belted rabbits (2.5–4.0 kg) of either sex. Rabbits were maintained on a 12h/12h light-dark schedule (lights-ON at 6 am and lights-OFF at 6 pm) at a temperature of 22°C with food and water available ad libitum. Retinal eyecups were obtained following deep general (urethane, induction dose: 2.0 g/kg, 31% solution, i.p.) and local intraorbital (2% Xylocaine) anesthesia. Following enucleations, deeply anaesthetized rabbits were killed by injection of 3.5 M KCl into the heart. Animal care and use complied with NIH regulations and all USA guidelines, and all procedures involving the care and use of rabbits in this study were reviewed and approved by the Ohio State University Institutional Animal Care and Use Committee (PHS Animal Welfare Assurance No. A3261-01). All light and dark adaptation electrophysiological and immunocytochemical experiments in this study were carried out at midday.
METHOD DETAILS
Tissue preparation
Rabbits were kept dark-adapted for at least 1 hour before surgery and retinal slice preparation. Following enucleation, under dim-red illumination, a central rectangular portion of retina was cut close to the visual streak, placed photoreceptor side down on filter paper (Millipore, Billerica, MA) before isolating the retina. Thick (250-μm) slices were prepared with a tissue chopper (Stoelting, Wood Dale, IL) and placed in a custom electrical recording chamber for use over the course of 3 hours. The retinal slices were continuously superfused at 3 mL/min with bicarbonate-based Ames medium and maintained at 34°C and pH 7.4 by bubbling it with 95% O2 and 5% CO2. The osmolality was adjusted to 285 mOsm/kg.
Electrophysiology
The light and GABAAR-mediated responses of cone and rod BCs in the superfused rabbit retina slice preparation were monitored using patch pipettes in the whole-cell configuration. The patch electrodes had resistances of 7–12 MΩ and were filled with a solution that included (in mM): 91.3 K-Gluconate, 22.7 KCl, 4.1 KHCO3, 5 HEPES, 5.0 (H+)EGTA, 0.5 CaCl2, 0.5 Na3-GTP, 3 Mg-ATP, and 10 Na2-Phosphocreatine. The pH was adjusted to 7.4 with 1N KOH, and the osmolality of the pipette solution was adjusted to 270 mOsm/kg. All membrane potential values in this study include a −10 mV correction for liquid junction potential. All chemicals and test drugs were purchased from Sigma-Aldrich (St. Louis, MO), except for gabazine, SCH23390 and TPMPA (all from Tocris, Bristol, UK), and Alexa Fluor 488 and peanut agglutinin (Invitrogen, Carlsbad, CA).
The GABAAR activity and EGABA of ON-cBC dendrites were measured by pressure ejecting (6 psi, 100 msec) GABA (0.5 mM) from 6–8 MΩ pipettes [54]. GABA was applied onto ON-cBC dendrites in the OPL with synaptic transmission blocked with cobalt (2 mM) and in the presence of the GABACR antagonist, TPMPA (50 μM), while the membrane potential of the cells was shifted between −90 and +10 mV using constant current pulses. The potential at which GABA did not evoke a response corresponded to EGABA. In these experiments, the superfusate flowed over the slices toward the outer segments of cones and rods, so that GABA puffed onto ON-cBC dendrites did not reach the axon terminals of the cells. Dye included in the puff pipettes confirmed this (n = 4).
The I–V relationship of ON-cBCs was measured by clamping the cells at a holding potential of −40 mV and then stepping from −90 mV to +10 mV in 10-mV increments. This procedure was repeated for each cell (n = 5) following APB application in the dark and during light stimulation, and during application of both APB and GBZ. Steady-state current at each holding potential was then compared for the experimental conditions. Because of the presence of an outwardly rectifying current at step potentials more positive than −20 mV [55, 56], conductance calculations were restricted to holding potentials between −80 mV and −30 mV, the physiological range of the cells in which the relationship between current and voltage was relatively linear.
Identification of cell types
ON-cBCs and rod-BCs, both of which depolarize to small centered stimuli, were distinguished based on their different light response waveforms (Figs. 2, 4) and on differences in the characteristic morphology and location of Alexa488-injected axon terminals [57] (see Fig. S1 in Supplemental Information) and immunostained dendritic arbors (see Fig. 5),
Because we were able to distinguish ON-cBCs from rod BCs, our results show that the dendrites of ON-cBCs – but not rod BCs – express GABAAR-IR and exhibit GABAAR activity following maintained bright illumination when D1Rs are strongly activated. The finding that rabbit rod BCs do not exhibit surround responses under D1R activation is consistent with previous indirect evidence in wholemount rabbit retina that AII amacrine cells, which receive direct synaptic input from rod BCs, do not exhibit surround responses under maintained illumination [58]. However, recent observations in mouse wholemount retina suggest that following maintained background illumination rod BCs produce hyperpolarizing surround responses to large (800-μm diam) spot stimuli due to cone-driven HC input to rods [59]. Further work is needed to determine whether the discrepancy has a technical basis or is due to a species difference.
Although we could distinguish ON-cBCs and rod BCs, it was more difficult to distinguish the 4–5 different rabbit ON-cBC subtypes [57] based on their light response waveforms recorded in a retinal slice preparation and the morphology and location of their Alexa488 fluorescent cell bodies and axon terminals. As a result, it is not possible at this time to address whether GABAAR- and D1R-dependent surround responses are characteristic of specific ON-cBC subtypes or rather characteristic of most ON-cBCs irrespective of subtype. In addition, it is worth noting that our ON-cBC results do not address whether OFF-cBC dendritic GABAARs mediate OFF-cBC surround responses.
Light stimuli
Light stimuli, which were delivered to the retinal slices from a 2.54 cm-wide CRT monitor (green (545 nm light) phosphor) (Lucivid, MicroBrightField, Colchester, VT) that was positioned in a port of an Olympus Upright microscope (Model BX51WI), were used to map the sizes of the RF center and surround of ON-cBCs and to determine whether surround antagonism and activation contributed to ON-cBC light responses under control conditions when dopamine D1Rs were activated, as well as following D1R blockade. Because the light pathway to the slices was perpendicular to the direction of incident light to in vivo retinas, bar stimuli, rather than spots and annuli, were used. Centered single bars of various widths were used to assess the size and strength of the RF center and the size and strength of the antagonistic surround (i.e., difference in response size to wide compared to narrow bars). Two simultaneously flashed bars, which were equidistant from the RF center and separated by various distances, were used to stimulate different portions of the RF surround, thereby providing a measure of the strength of surround activation. Individual stimuli, which were non-saturating, were spaced apart by at least 15 sec and the entire series of stimuli were presented every 2 minutes to limit light adaptation. The stimuli were programmed with VisionWorks for Electrophysiology software (Vision Research Graphics, Durham, NH).
Immunocytochemical labeling
Rabbits were bright light (intensity = ~1,000 lux)- or dark (intensity = ~0.0001 lux)-adapted for 60 min. Following deep general and local anesthesia, as described above, physiological saline with or without SCH23390 (10 μM) was injected intraocularly under light-adapted conditions or physiological saline without SCH23390 was injected under dark-adapted conditions. Thirty minutes later rabbits were perfused via the cardiac circulation with fixative solution that contained 4% paraformaldehyde and 0.01% glutaraldehyde in 0.1 M phosphate buffer (PB). Their eyecups were then immersed in 4% paraformaldehyde or in a combination of 4% paraformaldehyde and 0.01% glutaraldehyde for light microscopic or electron microscopic immunocytochemistry, respectively. The retinas were cryoprotected by immersing them in increasing concentrations (10, 20 and 30%) of sucrose in 0.1 M PB, embedded and frozen. Vertical cryosections (20-μm thick) were then obtained and processed for immunostaining using the indirect fluorescence method. When two different monoclonal antibodies from the same species (mouse) were used [60], intrinsic IgG in the tissue was blocked with mouse IgG F(ab)2 monoclonal antibody (1:100, 115-006-006, Jackson Immunoresearch, West Grove, PA) following blocking with normal mouse serum at a dilution of 1:10 and normal goat serum at a dilution of 1:5. A mouse monoclonal antibody that specifically recognizes the β2/3-subunit of GABAARs (clone BD17, MAB341, Millipore) was used at a dilution of 1:100 to label GABAARs. Double-labeling with this GABAAR antibody and antibodies that label both ON-cBCs and rod-BCs (1:500, mouse anti-Gαo, clone 2A, MAB3073. Millipore) or only rod-BCs (1:100, anti-PKCα mc5, Sigma) revealed whether GABAARs are located on ON-cBC dendrites under various experimental conditions. After incubation in primary antiserum, the sections were washed in 0.1 M PB and incubated for 2 h in one of the following fluorescent secondary antisera as appropriate: Alexa fluor(R) 488 (1:200, A21202) donkey anti-mouse IgG antibody and Alexa Fluor(R) 647 donkey anti-mouse IgG antibody (1:200, A31571),(Invitrogen, Carlsbad, CA). Retinas in all experimental conditions were always processed in parallel and in an identical manner.
Immuno-electron microscopy
Immuno-EM was performed as previously described [61]. Briefly, after cryoprotection, 3×3-mm pieces of retina were repeatedly frozen with liquid nitrogen and thawed to permeabilize membranes in order to enhance antibody penetration. Tissue was then blocked with 30% normal donkey serum and 1% bovine serum albumin in 5% sucrose PB (0.1 M). Next, the GABAAR antibody was applied and incubated overnight for 3 d in 4°C, after which the biotinylated secondary antibody (Biotin-SP AffiniPure Donkey Anti-Mouse IgG, 715-065-150, Jackson Immnoresearch) for primary mouse IgG was applied at a dilution of 1:200 for 1 h at RT. The tissue was then washed with Tris buffer (0.1 M, pH 7.3) and quenched with 30% hydrogen peroxide and 5% methanol in 5% PBS. Next, ABC and DAB reactions were carried out sequentially (ABC and DAB kits, Vector Labs, Burlingame, CA). The DAB reaction product was silver-intensified the next day, and then gold-substituted, after which the gold particles were fixed with thio-sulfate and ammonium-tetraamine. OsO4 in cacodylate buffer was then used for post-fixation. The tissue was then dehydrated by immersion in a graded (30–99.9%) acetone series and embedded in resin (Ted Pella, Redding, CA) overnight at 60°C.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data collection and analysis
Values are expressed as mean ± standard error of the mean. Electrophysiological data were acquired using Clampex Software and analyzed using Clampfit 10.1 (Molecular Devices), GraphPad Prism (GraphPad Software, Inc.), and R (R Foundation for Statistical Computing, Vienna, Austria). One-tailed t-tests (Figs. 2, 3, 5) or paired t-tests (Fig. 4) with the assumption of normality were used in all statistical analyses, except where noted. Details of the specific statistical tests used and the meaning of “n” for each experiment can be found within figure legends and associated text in Results. Data were considered significantly different at p < 0.05.
Fluorescent image acquisition and analysis of histological data
Immunolabeled images were acquired with a Zeiss 510 confocal microscope at the highest resolution in tiff format. ZEN software (Carl Zeiss, Germany) was used to reconstruct 3D images from a z-stack series of contiguous optical sections obtained at 0.30 μm intervals. When collecting images of different fluorophores, each fluorophore was excited sequentially and each emission channel was acquired independently to minimize bleed-through artifacts and dye cross-reactivity. This procedure resulted in z-stack sets of different fluorophores that contained the same number of sections. All of these images were then imported into NIH-ImageJ software to assess the relative strength of co-localized signals. Co-localized signals were extracted and converted into a binary code by the Colocalization plugin of ImageJ. Using the same thresholding procedure for all experimental conditions, intensity measurements were obtained sequentially from the corresponding sections of the z-stack sets of different fluorophores. Images of labeled cells were divided manually into regions of interest (i.e., ON-BC dendrites), so that the number of co-localized pixels in each region of interest, which were more intense than a predefined threshold value, could be counted. Use of the JACoP plugin for ImageJ confirmed that the GABAAR-IR puncta were located on ON-BC dendrites (Fig. S3) [62]. Final images were adjusted in intensity using Adobe Photoshop CS5 (Adobe, CA).
DATA AND SOFTWARE AVAILABILITY
Data are available on request from the Lead Contact, Stuart Mangel (mangel.1@osu.edu).
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal antibody anti-β2/3 subunit of GABAA receptor; clone BD-17 | Millipore | Cat#MAB341; RRID: AB_2109419 |
| Anti-G-protein Goα; clone 2A | Millipore | Cat#MAB3073; RRID: AB_94671 |
| Anti-PKCα, mc5 | Sigma-Aldrich | Cat#P4334; RRID: AB_477345 |
| Alexa Fluor®488 Donkey anti-mouse IgG antibody | Invitrogen | Cat#A21202; RRID: AB_141607 |
| Alexa Fluor®647 Donkey anti-mouse IgG antibody | Invitrogen | Cat#A31571; RRID: AB_162542 |
| Mouse IgG F(ab)2 monoclonal antibody | Jackson Immunoresearch | CAT#115-006-006 |
| Biotin-SP AffinityPure Donkey anti-mouse IgG | Jackson Immunoresearch | Cat#715-065-150; RRID: AB_2307438 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| SCH23390; dopamine D1 receptor antagonist | Tocris | Cat#0925 |
| Spiperone, dopamine D2-like receptor antagonist | Sigma-Aldrich | Cat#S7395 |
| Gabazine, GABAA receptor antagonist | Tocris | Cat#1262 |
| TPMPA, GABAC receptor antagonist | Tocris | Cat#1040 |
| Alexa Fluor 488 Hydrazide | Invitrogen | Cat#A10436 |
| Lectin Peanut Agglutinin (PNA) from Arachis Hypogaea; Alexa Fluor ® conjugate | Invitrogen | Cat#L21409 |
| Osmium tetroxide | Fisher Scientific | Cat#AC19118-0010 |
| Gold Chloride Trihydrate | Fisher Scientific; MP Biomedicals | Cat#0215252601 |
| Silver Nitrate | Fisher Scientific | Cat#BP2546100 |
| Critical Commercial Assays | ||
| Vectastain Standard Elite ABC Kit | Vector Labs | Cat#3333333 |
| DAB Substrate Kit | Vector Labs | Cat#SK-4100 |
| Epon812 Embedding kit | Electron Microscopy Science | Cat#50-980-391 |
| Experimental Models: Organisms/Strains | ||
| Dutch-belted rabbits | Myrtle’s; Covance | N/A |
| Software and Algorithms | ||
| VisionWorks for Electrophysiology | Vision Research Graphics, Durham, NH | http://www.vrg.com |
| Clampfit 10.1 | Molecular Devices | https://www.moleculardevices.com/systems/conventional-patch-clamp/pclamp-10-software |
| R | R Foundation for Statistical Computing, Vienna, Austria | https://www.r-project.org/about.html |
| GraphPad Prism, Version 6.01 | GraphPad Software, Inc. | https://www.graphpad.com |
| ZEN Software | Carl Zeiss, Germany | https://www.zeiss.com/microscopy/us/products/microscope-software/zen.html |
| JACoP plugin for ImageJ | NIH | https://imagej.nih.gov/ij/plugins/track/jacop.html |
Acknowledgments
We thank Drs. John Dowling and Karin Dedek for providing comments on an early version of the manuscript and Drs. Richard Burry, Ulrike Janssen-Bienhold, Arndt Meyer, and Christophe Ribelayga for helpful discussions. This work was supported in part by grants to S.C.M from the National Institutes of Health (R01-EY005102 and R01-EY014235) and the Plum Foundation (Studio City, CA).
Footnotes
AUTHOR CONTRIBUTIONS
A.C., M.I. Y.C. and S.C.M. performed the experiments and analyzed the data. A.C., M.I., Y.C. and S.C.M. conceptualized the study, designed experiments, and discussed results and implications. A.C., M.I. and S.C.M. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953;16:37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- 2.Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II Intracellular recording. J Neurophysiol. 1969;32:339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970;207:623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dacheux RF, Miller RF. An intracellular electrophysiological study of the ontogeny of functional synapses in the rabbit retina. I Receptors, horizontal, and bipolar cells. J Comp Neurol. 1981;198:307–326. doi: 10.1002/cne.901980209. [DOI] [PubMed] [Google Scholar]
- 5.Mangel SC. Analysis of the horizontal cell contribution to the receptive field surround of ganglion cells in the rabbit retina. J Physiol. 1991;442:211–234. doi: 10.1113/jphysiol.1991.sp018790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dacey D, Packer OS, Diller L, Brainard D, Peterson B, Lee B. Center surround receptive field structure of cone bipolar cells in primate retina. Vision Res. 2000;40:1801–1811. doi: 10.1016/s0042-6989(00)00039-0. [DOI] [PubMed] [Google Scholar]
- 7.Fahey PK, Burkhardt DA. Center-surround organization in bipolar cells: symmetry for opposing contrasts. Vis Neurosci. 2003;20:1–10. doi: 10.1017/s0952523803201012. [DOI] [PubMed] [Google Scholar]
- 8.Thoreson WB, Mangel SC. Lateral interactions in the outer retina. Prog Retinal Eye Res. 2012;32:1–35. doi: 10.1016/j.preteyeres.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barlow HB, Fitzhugh R, Kuffler SW. Change of organization in the receptive fields of the cat’s retina during dark adaptation. J Physiol. 1957;137:338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barlow HB, Levick WR. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969;202:699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werblin FS. Control of retinal sensitivity II. Lateral interactions at the outer plexiform layer. J Gen Physiol. 1974;63:62–87. doi: 10.1085/jgp.63.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller JF, Dacheux RF. Alpha ganglion cells of the rabbit retina lose antagonistic surround responses under dark adaptation. Visual Neurosci. 1997;14:395–401. doi: 10.1017/s0952523800011512. [DOI] [PubMed] [Google Scholar]
- 13.Meister M, Tessier-Lavigne M. Low-level visual processing: the retina. In: Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ, editors. Principles of Neural Science. 5. Chapter 26 New York: McGraw-Hill; 2013. pp. 577–601. [Google Scholar]
- 14.Chan LH, Freeman AW, Cleland BG. The rod–cone shift and its effect on ganglion cells in the cat’s retina. Vision Res. 1992;32:2209–2219. doi: 10.1016/0042-6989(92)90085-w. [DOI] [PubMed] [Google Scholar]
- 15.Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- 17.Greferath U, Grunert U, Muller F, Wassle H. Localization of GABAA receptors in the rabbit retina. Cell Tissue Res. 1994;276:295–307. doi: 10.1007/BF00306115. [DOI] [PubMed] [Google Scholar]
- 18.Vardi N, Sterling P. Subcellular localization of GABAA receptor on bipolar cells in macaque and human retina. Vision Res. 1994;34:1235–1246. doi: 10.1016/0042-6989(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 19.Haverkamp S, Grünert U, Wässle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- 20.Shields CR, Tran MN, Wong RO, Lukasiewicz PD. Distinct ionotropic GABA receptors mediate presynaptic and post-synaptic inhibition in retinal bipolar cells. J Neurosci. 2000;20:2673–2682. doi: 10.1523/JNEUROSCI.20-07-02673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC, Chaurassia SS. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog Retin Eye Res. 2005;24:433–456. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Zhang DQ, Tong-Rong Z, McMahon DG. Functional heterogeneity of retinal dopaminergic neurons underlying their multiple roles in vision. J Neurosci. 2007;27:692–699. doi: 10.1523/JNEUROSCI.4478-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen RJ, Daw NW. Effects of dopamine antagonists on receptive fields of brisk cells and directionally selective cells in the rabbit retina. J Neurosci. 1984;4:2972–2985. doi: 10.1523/JNEUROSCI.04-12-02972.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slaughter MM, Miller RF. 2-Amino-4-phosphonobutyric acid: A new pharmacological tool for retina research. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- 25.Yang XL, Wu SM. Feedforward lateral inhibition in retinal bipolar cells: input-output relation of the horizontal cell-depolarizing bipolar cell synapse. Proc Natl Acad Sci USA. 1991;88:3310–3313. doi: 10.1073/pnas.88.8.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yazulla S, Studholme KM. Light adaptation affects synaptic vesicle density but not the distribution of GABAA receptors in goldfish photoreceptor terminals. Microsc Res Tech. 1997;36:43–56. doi: 10.1002/(SICI)1097-0029(19970101)36:1<43::AID-JEMT4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Hammond P. Receptive field mechanisms of sustained and transient retinal ganglion cells in the cat. Exp Brain Res. 1975;23:113–128. doi: 10.1007/BF00235454. [DOI] [PubMed] [Google Scholar]
- 28.Donner K. Receptive fields of frog retinal ganglion cells: response formation and light-dark adaptation. J Physiol. 1981;319:131–142. doi: 10.1113/jphysiol.1981.sp013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Troy JB, Shou T. The receptive fields of cat retinal ganglion cells in physiological and pathological states: where we are after half a century of research. Prog Retinal Eye Res. 2002;21:263–302. doi: 10.1016/s1350-9462(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 30.Attick JJ, Redlich AN. What does the retina know about natural scenes? Neural Comput. 1992;4:196–210. [Google Scholar]
- 31.Fahey PK, Burkhardt DA. Effects of light adaptation on contrast processing in bipolar cells in the retina. Vis Neurosci. 2001;18:581–597. doi: 10.1017/s0952523801184087. [DOI] [PubMed] [Google Scholar]
- 32.Miller RF, Dacheux RF. Intracellular chloride in retinal neurons: measurement and meaning. Vision Res. 1983;23:399–411. doi: 10.1016/0042-6989(83)90087-1. [DOI] [PubMed] [Google Scholar]
- 33.Vardi N, Zhang LL, Payne JA, Sterling P. Evidence that different cation chloride cotransporters in retinal neurons allow opposite responses to GABA. J Neurosci. 2000;20:7657–7663. doi: 10.1523/JNEUROSCI.20-20-07657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duebel J, Haverkamp S, Kuner T, Euler T. Two-photon imaging reveals somatodendritic chloride gradient in retinal ON-type bipolar cells expressing the biosensor clomeleon. Neuron. 2006;49:81–94. doi: 10.1016/j.neuron.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 35.Satoh H, Kaneda M, Kaneko A. Intracellular chloride concentration is higher in rod bipolar cells than in cone bipolar cells of the mouse retina. Neurosci Lett. 2001;310:161–164. doi: 10.1016/s0304-3940(01)02120-6. [DOI] [PubMed] [Google Scholar]
- 36.Billups D, Attwell D. Control of intracellular chloride concentration and GABA response polarity in rat retinal ON bipolar cells. J Physiol. 2002;545:183–198. doi: 10.1113/jphysiol.2002.024877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naka KI, Nye PW. Role of horizontal cells in organization of the catfish retina receptive field. J Neurophysiol. 1971;34:785–801. doi: 10.1152/jn.1971.34.5.785. [DOI] [PubMed] [Google Scholar]
- 38.Toyoda JL, Kujiraoka T. Analysis of bipolar cell responses elicited by polarization of horizontal cells. J Gen Physiol. 1982;79:131–145. doi: 10.1085/jgp.79.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakuranaga M, Naka KI. Signal transmission in the catfish retina. I Transmission in the outer retina. J Neurophysiol. 1985;53:373–389. doi: 10.1152/jn.1985.53.2.373. [DOI] [PubMed] [Google Scholar]
- 40.Mangel SC, Miller RF. Horizontal cells contribute to the receptive field surround of ganglion cells in the rabbit retina. Brain Res. 1987;414:182–186. doi: 10.1016/0006-8993(87)91344-8. [DOI] [PubMed] [Google Scholar]
- 41.Puller C, Haverkamp S, Neitz M, Neitz J. Synaptic elements for GABAergic feed-forward signaling between HII horizontal cells and blue cone bipolar cells are enriched beneath primate S-cones. PLoS One. 2014;9:e88963. doi: 10.1371/journal.pone.0088963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valera C, Rivera L, Blanco R, de la Villa P. Depolarizing effect of GABA in horizontal cells of the rabbit retina. Neurosci Res. 2005;53:257–264. doi: 10.1016/j.neures.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Trenholm S, Baldridge WH. The effect of aminosulfonate buffers on the light responses and intracellular pH of goldfish retinal horizontal cells. J Neurochem. 2010;115:102–111. doi: 10.1111/j.1471-4159.2010.06906.x. [DOI] [PubMed] [Google Scholar]
- 44.Crook JD, Manookin MB, Packer OS, Dacey DM. Horizontal cell feedback without cone type-selective inhibition mediates “red-green” color opponency in midget ganglion cells of the primate retina. J Neurosci. 2011;31:1762–1772. doi: 10.1523/JNEUROSCI.4385-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klaassen LJ, et al. Synaptic transmission from horizontal cells to cones is impaired by loss of connexin hemichannels. PLoS Biol. 2011;9:e1001107. doi: 10.1371/journal.pbio.1001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kramer RH, Davenport CM. Lateral inhibition in the vertebrate retina: the case of the missing neurotransmitter. PLoS Biol. 2015;13:e1002322. doi: 10.1371/journal.pbio.1002322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lukasiewicz PD. Synaptic mechanisms that shape visual signaling at the inner retina. Prog Brain Res. 2005;147:205–218. doi: 10.1016/S0079-6123(04)47016-2. [DOI] [PubMed] [Google Scholar]
- 48.Zhang AJ, Wu SM. Receptive fields of retinal bipolar cells are mediated by heterogeneous synaptic circuitry. J Neurosci. 2009;29:789–797. doi: 10.1523/JNEUROSCI.4984-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cook PB, McReynolds JS. Modulation of sustained and transient lateral inhibitory mechanisms in the mudpuppy retina during light adaptation. J Neurophysiol. 1998;79:197–204. doi: 10.1152/jn.1998.79.1.197. [DOI] [PubMed] [Google Scholar]
- 50.Flores-Herr N, Protti DA, Wassle H. Synaptic currents generating the inhibitory surround of ganglion cells in the mammalian retina. J Neurosci. 2001;21:4852–4863. doi: 10.1523/JNEUROSCI.21-13-04852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thibos LN, Werblin FS. The properties of surround antagonism elicited by spinning windmill patterns in the mudpuppy retina. J Physiol. 1978;278:101–116. doi: 10.1113/jphysiol.1978.sp012295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farrow K, Teixeira M, Szikra T, Viney TJ, Balint K, Yonehara K, Roska B. Ambient illumination toggles a neuronal circuit switch in the retina and visual perception at cone threshold. Neuron. 2013;78:325–338. doi: 10.1016/j.neuron.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 53.De Valois RL, Morgan H, Snodderly DM. Psychophysical studies of monkey vision – III. spatial luminance contrast sensitivity tests of macaque and human observers. Vision Res. 1974;14:75–81. doi: 10.1016/0042-6989(74)90118-7. [DOI] [PubMed] [Google Scholar]
- 54.Gavrikov KE, Nilson JE, Dmitriev AV, Zucker CL, Mangel SC. Dendritic compartmentalization of chloride cotransporters underlies directional responses of starburst amacrine cells in retina. Proc Natl Acad Sci USA. 2006;103:18793–18798. doi: 10.1073/pnas.0604551103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tessier_Lavigne M, Attwell D, Mobbs P, Wilson M. Membrane currents in retinal bipolar cells of the axolotl. J Gen Physiol. 1988;91:49–72. doi: 10.1085/jgp.91.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tian N, Slaughter MM. Functional properties of a metabotropic glutamate receptor at dendritic synapses of ON bipolar cells in the amphibian retina. Vis Neurosci. 1995;12:755–765. doi: 10.1017/s0952523800009019. [DOI] [PubMed] [Google Scholar]
- 57.MacNeil MA, Heussy JE, Dacheux RF, Raviola E, Masland RH. The population of bipolar cells in the rabbit retina. J Comp Neurol. 2004;472:73–86. doi: 10.1002/cne.20063. [DOI] [PubMed] [Google Scholar]
- 58.Xin D, Bloomfield SA. Comparison of the responses of AII amacrine cells in dark- and light-adapted rabbit retina. Visual Neurosci. 1999;16:653–665. doi: 10.1017/s0952523899164058. [DOI] [PubMed] [Google Scholar]
- 59.Szikra T, et al. Rods in daylight act as relay cells for cone-driven horizontal cell-mediated surround inhibition. Nature Neurosci. 2014;17:1728–1735. doi: 10.1038/nn.3852. [DOI] [PubMed] [Google Scholar]
- 60.Burry RW. Immunocytochemistry: A Practical Guide for Biomedical Research. 1. 65–73. New York: Springer; 2010. pp. 119–130. [Google Scholar]
- 61.Sassoè-Pognetto M, Wassle H, Grunert U. Glycinergic synapses in the rod pathway of the rat retina: cone bipolar cells express the α1 subunit of the glycine receptor. J Neurosci. 1994;14:5131–5146. doi: 10.1523/JNEUROSCI.14-08-05131.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hilgen G, von Maltzahn J, Willecke K, Weiler R, Dedek K. Subcellular distribution of connexin45 in OFF bipolar cells of the mouse retina. J Comp Neurol. 2011;519:433–450. doi: 10.1002/cne.22526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.