Abstract
Lipid droplets in chordates are decorated by two or more members of the perilipin family of lipid droplet surface proteins. The perilipins sequester lipids by protecting lipid droplets from lipase action. Their relative expression and protective nature is adapted to the balance of lipid storage and utilization in specific cells. Most cells of the body have tiny lipid droplets with perilipins 2 and 3 at the surfaces, whereas specialized fat-storing cells with larger lipid droplets also express perilipins 1, 4, and/or 5. Perilipins 1, 2, and 5 modulate lipolysis by controlling the access of lipases and co-factors of lipases to substrate lipids stored within lipid droplets. Although perilipin 2 is relatively permissive to lipolysis, perilipins 1 and 5 have distinct control mechanisms that are altered by phosphorylation. Here we evaluate recent progress toward understanding functions of the perilipins with a focus on their role in regulating lipolysis and autophagy.
Keywords: Perilipin, lipid droplet, triacylglycerol, lipolysis, adipose triglyceride lipase, hormone-sensitive lipase, monoacylglycerol lipase, ABHD5, autophagy
1. Introduction
Lipid droplets are found in the cytoplasm of nearly every type of cell in the tissues of mammals, as well as in cells of other chordates and many lower organisms. In mammals, lipid droplets play an important role in cellular lipid homeostasis, storing primarily either cholesterol esters to be used for membrane and steroid hormone synthesis, or triacylglycerols that serve as a source of energy substrates or precursors for signaling lipids or membrane phospholipid synthesis. The neutral lipid core of lipid droplets is covered by a monolayer of phospholipids and cholesterol into which proteins are embedded. Proteomics studies have revealed more than 200 protein components of lipid droplets, some of which localize solely to lipid droplets, while others are also found in other subcellular compartments. Some of the most abundant lipid droplet proteins include perilipins; to date, cytosolic lipid droplets lacking perilipins have not been identified in mammalian cells. Thus, wherever neutral lipids are stored, two or more members of the perilipin family of proteins are expressed and bind to lipid droplets. Five perilipin genes encode five major perilipin proteins (perilipins 1 through 5) in mammals, with additional splice variants that are expressed in lower amounts. Perilipins 2 and 3 are ubiquitously expressed, whereas perilipins 1, 4, and 5 have more limited tissue expression. The perilipins can be either exclusively associated with lipid droplets (perilipins 1 and 2) or exchangeable proteins that are stable in either the cytoplasm or when associated with lipid droplets (perilipins 3, 4, and 5). However, an interesting recent study provides evidence that the putative cytosolic forms of perilipin 3 and perilipin 5 are actually associated with lipoprotein particles or lipid micro-droplets with neutral lipid content, which are only 10–20 nm in diameter and thus smaller than structures easily identified as lipid droplets by light microscopy (1). Like apolipoproteins, some of the perilipins have specific preferences for the neutral lipid composition of lipid droplets. The major isoform of perilipin 1 (perilipin 1A), and perilipins 2 and 5 preferentially associate with lipid droplets enriched in triacylglycerols, whereas shorter isoforms of perilipin 1 (perilipins 1C and 1D) and perilipin 4 preferentially associate with lipid droplets enriched in cholesterol esters (2). Interestingly, perilipin 6, expressed only in teleost fish, associates with lipid droplets containing carotenoid pigments in skin xanthophores (3); in contrast, retinoid containing lipid droplets of mammalian hepatic stellate cells and retinal pigment epithelial cells are coated with perilipins 2 and 3 (4,5).
How the perilipin family members recognize and target to lipid droplets with specific lipid content and whether interactions with the surface phospholipid monolayer direct perilipin targeting remain largely unanswered questions, although a recent study has revealed that the acyl chain saturation of phospholipids affects the interaction of recombinant truncated forms of perilipin 3 with phospholipid monolayers of varying composition in vitro (6). To date, few studies have addressed the biophysics of the phospholipid and protein surface properties of lipid droplets. Several recent studies have suggested that phospholipid packing and surface protein crowding differ for lipid droplets relative to membrane bilayers (7–9), thus conferring unique properties that likely affect protein targeting to lipid droplets. The findings of a recent molecular dynamics computer simulation suggest that the interdigitation of core neutral lipids (triacylglycerols) into the phospholipid monolayer of a lipid droplet alters the biophysical properties of the surface relative to effects conferred by interactions between the hemi-leaflets of a phospholipid bilayer (7). Since there is little information regarding the biophysics of lipid interactions within a lipid droplet, this computational simulation used parameters determined by biophysical measurements made for individual pure lipids and combinations of pure lipids, including pure triacylglycerols and phospholipids and triacylglycerols at a water interface, both individually and as mixtures including a variety of phospholipid species and acyl chain compositions. Although the supporting measurements have been made under non-physiological conditions, the simulation used the experimentally determined lipid composition of a lipid droplet of physiologically relevant size to replicate the appropriate surface curvature. If this model is correct, the physical properties of the phospholipid monolayer of lipid droplets may confer specificity and selectivity to the binding of surface-associated proteins, with sensitivity to variations in the neutral lipid content of the lipid droplet core. These biophysical characteristics may underpin the selective targeting of the various perilipins to lipid droplets with differing core lipid compositions, and may also impact the distinct mechanisms by which different perilipins control lipolysis. Experimental testing is required to confirm the theoretical models for protein interactions with lipid droplets, a task which is neither straightforward nor easy, given the complexity of lipid droplets.
A common feature of many proteins that bind to lipid droplets, including perilipins, is the presence of amino acid sequences that are predicted to form amphipathic alpha helices (10–12). An amino acid sequence of 11-mer repeats predicted to form amphipathic helices is conserved in all members of the perilipin family of proteins, and has been shown to direct targeting of recombinant perilipins to lipid droplets in a heterologous yeast system (13). Perilipin 1 additionally has three sequences of hydrophobic amino acids with central proline residues that mediate targeting of mutated variants of perilipin 1 to lipid droplets in cultured cells (14,15), independent of the 11-mer repeat sequences. The former sequences likely form hydrophobic hairpin structures that embed into the core of the lipid droplet, and bear similarity to the predicted hairpin structures that mediate lipid droplet localization of other proteins, such as acyl CoA synthetase 3 and acyl-CoA:diacylglycerol acyltransferase 2 (DGAT21) (16). Future progress in obtaining high resolution structures of full-length perilipins, particularly in the lipid-bound form, will be useful in resolving the mechanisms governing the specificity of perilipin targeting to lipid droplets and revealing how perilipins function to control lipolysis.
Perilipins 1, 2, and 5 have been studied in the context of control of the metabolism of neutral lipids stored in lipid droplets, whereas less is known about the functions of perilipins 3 and 4. This review provides an update focused on assembling recent findings regarding perilipin function into models for perilipin control of lipolysis. For earlier reviews on the subject, see (17–21).
2. Evolution and tissue-specific expression of perilipins
The five mammalian perilipins have been numbered in the order of their discovery (22). Comparison of amino acid sequences reveals that perilipins 2 and 3 have the highest overall similarity (18,19,23); both are ubiquitously expressed in mammalian cells and tissues. The amino acid sequence of perilipin 5 bears similarity to perilipins 2 and 3 throughout its entirety. However, perilipin 5 is selectively expressed in tissues in which fatty acids released during lipolysis are transported to mitochondria for oxidation, including brown adipose tissue, cardiac and skeletal muscle, and, to a lesser extent, liver (24–26). The sequence of perilipin 1 shares similarity to perilipins 2, 3, and 5 predominantly in the amino terminus, whereas the carboxyl terminus lacks significant similarity to other family members. Perilipin 1 is abundantly expressed only in adipocytes in white and brown adipose tissue, with lower levels of expression in steroidogenic cells of the adrenal cortex, testis and ovaries (27). Perilipin 4 has the most divergent amino acid sequence with only limited similarity to other family members in an expanded amino terminal region of 11-mer repeat sequences that are predicted to fold into amphipathic alpha helices (10) and thought to direct targeting of the nascent protein specifically to lipid droplets. Perilipin 4 is most highly expressed in white adipose tissue, with lower levels of expression in heart and skeletal muscle (28,29).
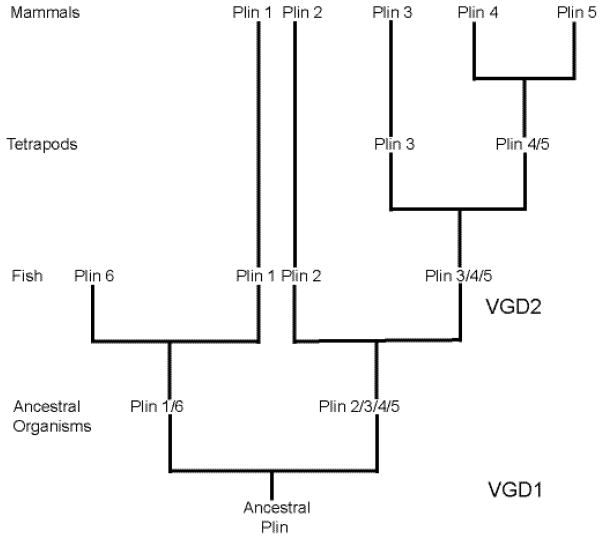
Comparative studies of species from key phylogenic lineages within chordates can be used to elucidate the functional evolution of a family of proteins. An important component of this type of research is the impact of gene duplication, particularly that arising from whole genome duplications during early stages of evolution. The increment of gene numbers followed by episodes of subfunctionalizations, neofunctionalizations, and gene loss, have all impacted vertebrate physiology, and, in the context of this review, the number and function of genes encoding the perilipins. A fascinating recent theoretical model for the molecular evolution of this gene family is based on the analysis of phylogenetic and conserved synteny (3); this analysis examines the conservation of segments of chromosomes containing multiple genes among closely related organisms. These regions of chromosomes are more highly conserved among evolutionarily related species than they are among distant ancestors. Genes are identified in diverse organisms by sequence similarity and the conservation of intron/exon boundaries. This model reveals that an evolutionary precursor of perilipin 1 (and the recently identified perilipin 6 found only in fish) and a precursor of perilipins 2, 3, 4, and 5 were derived from an ancestral perilipin gene during an early vertebrate genome duplication event (VGD) (Figure 1). Further, the model suggests that a second genome duplication event led to the divergence of perilipin 1 from perilipin 6 and separately, the divergence of perilipin 2 from an ancestral gene that eventually gave rise to perilipin 3, and then perilipins 4 and 5 during tetrapod evolution. Although mammals express perilipins 1 through 5, birds and reptiles express only perilipins 1, 2, 3, and a 5-like gene, and fish express only perilipins 1, 2, 3, and 6 (3). The more recent gene duplication and divergence of the sequences of perilipins 4 and 5 suggests that these perilipins gained specialized functions during the evolution of mammals. The phylogenetic dissection of the perilipin family, coupled with ongoing structural and biochemical analyses, may provide significant insight into the mechanisms by which perilipins control the mobilization of stored neutral lipids.
Figure 1. Theoretical model of perilipin molecular evolution.
The perilipins are predicted to have evolved from an ancestral perilipin (Plin) gene expressed in an early chordate. During the first vertebrate genome duplication (VGD) event, two precursor genes that eventually gave rise to perilipins 1 and 6 and perilipins 2, 3, 4, and 5 were formed, followed by a second VGD that gave rise to the individual perilipins 1, 6, 2, and the precursor gene for perilipins 3, 4, and 5. Duplication of the latter precursor gene during the evolution of tetrapods gave rise to perilipin 3 and the precursor gene for perilipins 4 and 5, which diverged during yet another gene duplication event. Perilipin 6 is only expressed in some species of fish, along with perilipins 1, 2, and a precursor gene that gave rise to perilipins 3, 4, and 5 during evolution. Birds and reptiles express perilipins 1, 2, 3, and a perilipin 5-like gene. Only mammals express perilipins 1, 2, 3, 4, and 5. This model was proposed by Granneman, et al. (3), and this figure is derived from Figure 1-figure supplement 2 of that paper. Lines in the model are not to scale and do not represent evolutionary timeframes.
3. Lipolysis plays an essential role in lipid homeostasis
Tissues store triacylglycerols in lipid droplets when exogenous fatty acids are plentiful and available for import and esterification. The fed state increases fatty acid availability for delivery to adipocytes, while insulin promotes glucose uptake to support triacylglycerol synthesis. In contrast, triacylglycerol storage in other tissues, including liver, pancreas, and skeletal and cardiac muscle, increases during fasting, when fatty acids are exported from adipose tissue for transport to these tissues. When extracellular fatty acid supplies dwindle or energy is required to support exercise, hormones initiate signaling cascades that increase kinase activity to activate lipolytic pathways in adipocytes. The subsequent phosphorylation of perilipins, lipases, and cofactors for lipases initiates the translocation of lipases from the cytoplasm to lipid droplets and enables protein-protein interactions to assemble the lipolytic complex on the perilipin scaffold surrounding lipid droplets. Thus, lipases gain access to lipid substrates and lipolysis of stored triacylglycerols ensues.
Lipolysis is catalyzed by lipases that cycle between the cytoplasm or cytoplasmic surfaces of the endoplasmic reticulum and the surfaces of lipid droplets; for recent reviews on lipases, see (30–34). The first identified and most highly characterized lipase is hormone-sensitive lipase (HSL), an enzyme with strong diacylglycerol and cholesterol ester hydrolase activity, and weaker triacylglycerol, monoacylglycerol, and retinyl ester hydrolase activity in vitro. HSL is highly expressed in white and brown adipose tissue, and at lower levels in a variety of tissues including testis, ovaries, adrenal gland, skeletal muscle, heart, and mammary gland. The subcellular localization and activity of HSL is dynamically regulated by phosphorylation. The cAMP-dependent protein kinase (PKA)-mediated phosphorylation of HSL on 2 serine residues (Ser659 and Ser660 in rat HSL) is required for activating the lipase and promoting the translocation of HSL from the cytoplasm to the surfaces of lipid droplets to gain access to substrate lipids (35). An additional serine residue (Ser563 in rat HSL) is phosphorylated by PKA, although the consequence of this phosphorylation is unknown. The use of antibodies raised against these specific phosphorylated residues of HSL has revealed that the rapid PKA-mediated phosphorylation of Ser660 precedes the phosphorylation of Ser563 in hormonally stimulated 3T3-L1 adipocytes (36). Moreover, within 1 minute of the activation of PKA, HSL with phosphorylated Ser660 can be detected on lipid droplets, as well as in the cytoplasm, particularly near the periphery of cells. In contrast, phosphorylated Ser563 was detected only on lipid droplets at 10 minutes after the activation of PKA, suggesting both spatial and temporal differences in the phosphorylation of these serine residues by PKA. These data suggest that the phosphorylation of Ser660 (and likely Ser659) is required for translocation of HSL from the cytoplasm to lipid droplets. HSL is also phosphorylated by AMP kinase; a recent study has shown that the phosphorylation of Ser565 of murine HSL impedes the PKA-mediated phosphorylation of HSL at Ser563 and Ser660, thus attenuating translocation and activation of the lipase (37).
For many years, HSL was considered to be the major, and perhaps only, neutral lipid lipase in adipocytes; however, when HSL null mice showed relatively modest alterations in adipose triacylglycerol content, researchers searched for and identified a second major lipase, adipose triglyceride lipase (ATGL) (32,34). ATGL has strong triacylglycerol hydrolase activity and weak retinyl ester hydrolase activity (38), but lacks other lipase activities; catalytic activity is enhanced by a co-activator protein, ABHD5 (alpha beta hydrolase domain 5, also called CGI-58), and inhibited by G0S2. ATGL is most highly expressed in adipose tissue and at lower levels in the same tissues as HSL, as well as in several additional tissues. In adipose tissue, ATGL is thought to be the major triacylglycerol hydrolase, with diacylglycerol and monoacylglycerol hydrolysis catalyzed by HSL and monoacylglycerol lipase (MAGL), respectively. Studies using tissue specific knockout mice have established important roles for ATGL in triacylglycerol metabolism in several additional tissues. Recent studies have shown that lipolysis in multiple types of cells is dependent on COPI complex proteins that control retrograde transport of proteins between the Golgi and endoplasmic reticulum (39–41); specifically, the translocation of ATGL between cellular membranes and lipid droplets is dependent upon COPI complexes. Moreover, the activation of PKA increases the translocation of ATGL to lipid droplets in cultured 3T3-L1 adipocytes (42), dissected murine white adipose tissue (43), and adipocytes differentiated from human adipose tissue-derived stem cells (44), implying that phosphorylation events trigger ATGL movement in adipocytes, but perhaps not in other types of cells. Phosphorylation of ATGL has been reported by two groups (37,45–47), although the identity of the relevant kinase is controversial, with both AMP kinase and PKA being proposed as the kinase that phosphorylates Ser406 in murine ATGL (Ser404 in human ATGL); triacylglycerol hydrolase activity is diminished when this serine residue is mutated to alanine, providing evidence of the functional significance of phosphorylation. Moreover, an additional phosphorylated residue (Ser430 in mouse ATGL, Ser428 in human ATGL) has been identified by mass spectrometry studies (48,49); further investigation is needed to identify or confirm the relevant kinases and functional consequences of ATGL phosphorylation.
Although HSL and ATGL have been detected in tissues for which the lipolysis of neutral lipids plays an important role in the generation of energy or substrates for lipoprotein or steroid hormone synthesis, it is clear from studies of mice with genetic deletions of HSL or ATGL that many tissues express additional lipases that have not yet been identified. Moreover, neutral lipid turnover can be detected in many cultured cell lines that lack detectable protein levels of HSL or ATGL. Although additional cytosolic neutral lipid lipases have not been unequivocally established, several families of putative hydrolases may include these lipases. ATGL belongs to a family of nine patatin-like phospholipase domain containing (PNPLA) hydrolases (50). Although either triacylglycerol or retinyl ester hydrolase activity has been reported for several of these enzymes (PNPLA3, PNPLA4, PNPLA5), other studies either fail to find relevant activity or report alternate activities for these proteins, so it is unclear as to whether these enzymes have significant lipase activity against neutral lipids or play a role in cellular triacylglycerol homeostasis in vivo. Another extensive family of lipid hydrolases is the alpha beta hydrolase domain proteins that includes 19 putative hydrolases in mammals (51,52), several of which have been identified as cytosolic enzymes. Two of these hydrolases, ABHD6 and ABHD12, have been shown to have monoacylglycerol lipase activity against the signaling lipid 2-arachidonoylglycerol, as well as lysophospholipid hydrolase activity. The subcellular localization and enzyme activity for the majority of members of this family have not yet been identified, hence, it is possible that one or more of these proteins will prove to be a cytosolic neutral lipid lipase. The ATGL co-activator ABHD5 is a member of this family, although it lacks a serine residue in the conserved nucleophilic elbow sequence of GXSXG, and hence, lacks detectable hydrolase activity. Finally, the carboxylesterase family of proteins includes neutral lipid lipases (53,54). Although several of these enzymes have been shown to have triacylglycerol and/or cholesterol ester hydrolase activity, they localize to the lumen of the endoplasmic reticulum and have been demonstrated to play a role in the utilization of stored triacylglycerols for lipoprotein assembly in hepatocytes. It is unclear whether these lipases can gain access to cytosolic lipid droplets through membrane contact sites between the endoplasmic reticulum and lipid droplets, or whether their activity is limited to hydrolysis of lipids within the endoplasmic reticulum. Further study of as yet uncharacterized hydrolases will undoubtedly uncover additional cytosolic lipases that act on neutral lipids stored in lipid droplets.
4. Perilipins control lipolysis of stored neutral lipids by cytosolic lipases
Perilipins 1, 2, and 5 at the surfaces of lipid droplets coordinate the access of the cytoplasmic lipolytic machinery to substrate lipids through fundamentally distinct mechanisms, each controlled, in part, through PKA mediated phosphorylation of perilipins, lipases and co-factors for lipases.
4.1. Perilipin 1 control of lipolysis in adipocytes
Perilipin 1 plays a crucial role in restricting adipose lipolysis under basal (or fed) conditions, as demonstrated by experiments in cultured cells and perilipin null mice, and the study of rare human mutations. The expression of perilipin 1 in cultured cells that typically coat lipid droplets with perilipin 2, leads to perilipin 1-coated lipid droplets and increased triacylglycerol storage due to decreased triacylglycerol turnover (55,56). Consistently, in perilipin 1 null mice, adipose lipid droplets are coated with perilipin 2 and triacylglycerol turnover is accelerated, leading to an approximate 70% reduction in adipose tissue mass (57,58). Moreover, humans with frameshift mutations in perilipin 1 that lack carboxyl terminal amino acid sequences have lipodystrophy (59–61), and the expression of these and other truncated variants of perilipin in cultured cells reduces triacylglycerol storage in cells by increasing turnover (59–62). These studies support the concept that perilipin 1 promotes triacylglycerol storage under basal conditions at least in part by reducing the access of cytosolic lipases to triacylglycerol substrates stored in lipid droplets (Figure 2). This function is aided by the insulin-mediated reduction of PKA activity in the fed state. Insulin suppresses lipolysis by at least three mechanisms. The binding of insulin to its receptor initiates a signaling cascade that ultimately activates phosphodiesterase 3b (PDE3b) through Akt-mediated phosphorylation of serine 273 (63,64). PDE3b catalyzes the hydrolysis of cAMP to 5′AMP, thus reducing the activation of PKA and consequently, attenuating lipolysis. Secondly, insulin signals through AKT to reduce the PKA-mediated phosphorylation of hormone-sensitive lipase, while signaling through a PI3K-dependent, but AKT-independent, signaling pathway to decrease the PKA-mediated phosphorylation of perilipin 1 (65). Although the former mechanism may be through increases in PDE3b activity, the molecular mechanism for the decreased perilipin 1 phosphorylation is unknown. Finally, chronically elevated levels of insulin, such as those observed with Type II Diabetes, disrupt communication between β-adrenergic receptors and PKA, most likely by altering the activity of A-kinase anchoring proteins (AKAPs) (66) that provide a scaffold to guide PKA to its protein substrates. An additional factor that reduces lipolysis under basal or fed conditions is the sequestration of ABHD5 on the perilipin 1 scaffold surrounding lipid droplets (67,68), preventing the interaction of ABHD5 with ATGL (69,70) and reducing basal triacylglycerol hydrolase activity. Studies of recently identified human mutations in perilipin 1 provide support for this model; truncation of the perilipin 1 carboxyl terminal binding site for ABHD5 releases the cofactor from perilipin 1 on lipid droplets, enabling its interaction with ATGL, and increasing the rate of basal lipolysis (59,60).
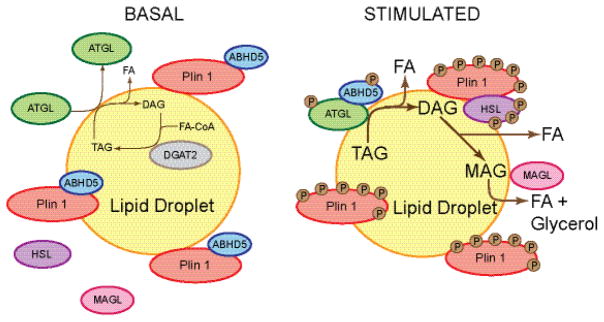
Figure 2. Schematic model of perilipin 1 function in adipocytes under basal and lipolytically stimulated conditions.
Under basal conditions, perilipin 1 (Plin 1) resides on lipid droplets in adipocytes and binds ABHD5, thus preventing ABHD5 interaction with and co-activation of ATGL. ATGL, HSL, and presumably MAGL are primarily cytosolic, although low levels of ATGL associate with lipid droplets permitting a low level of basal lipolysis. DAG released from TAG hydrolysis is likely re-esterified by DGAT2 (135). Under lipolytically stimulated conditions, PKA phosphorylates most protein components of the lipolytic machinery, including perilipin 1, ATGL, ABHD5, and HSL, and lipolysis increases markedly. The phosphorylation of perilipin 1 releases phosphorylated ABHD5, which interacts with phosphorylated ATGL to co-activate TAG hydrolysis. Phosphorylated ATGL is highly recruited to lipid droplets where it binds in a perilipin 1-independent manner. Phosphorylated HSL translocates from the cytoplasm to the surfaces of lipid droplets, where it binds to phosphorylated perilipin 1 and hydrolyzes primarily DAG. MAGL cleaves the remaining fatty acid; it is currently unknown whether or not perilipin 1 controls MAGL activity or localization to lipid droplets, or whether the localization of MAGL is altered under stimulated conditions.
Mechanisms of stimulated lipolysis are best understood for adipocytes from white adipose tissue, where perilipin 1 coats mature, generally unilocular lipid droplets. During fasting or exercise, epinephrine and norepinephrine bind to β-adrenergic receptors on the plasma membranes of adipocytes, initiating a Gαs-protein mediated signaling cascade that activates adenylyl cyclase, leading to increased cellular levels of cAMP. Elevated cAMP triggers the release of the regulatory subunits of tetrameric PKA and the consequent activation of the catalytic subunits (71). PKA then phosphorylates most of the components of the lipolytic complex. The most highly phosphorylated protein associated with lipid droplets in stimulated adipocytes is perilipin 1 (72). Examination of amino acid sequences predicts six consensus sites for the phosphorylation of serine residues by PKA (consensus R(R/K)XS) in mouse and rat perilipin 1, with five of these sites conserved in primates and dogs (18). Moreover, an AKAP called optic atrophy 1 has been shown to form a complex between perilipin 1 and PKA at the surfaces of lipid droplets in adipocytes and to control PKA-mediated phosphorylation of perilipin 1 (73). Interestingly, recent phosphoproteomics analysis of cultured murine 3T3-L1 adipocytes and primary mouse adipocytes from both white and brown adipose tissue has revealed as many as 27 phosphorylated serine or threonine residues in perilipin 1, with some of these sites being possible additional PKA targets, but other sites residing within consensus sequences for other kinases (74–77). The role of kinases other than PKA in the control of perilipin 1-mediated lipolysis has not been addressed.
Mutagenesis studies have established functional significance of the PKA-mediated phosphorylation of the originally identified six serine residues in murine perilipin 1 in the control of stimulated lipolysis. Mutation of the amino terminal-most three serine residues in PKA consensus sequences, either collectively or individually, eliminates recruitment of HSL to lipid droplets and correspondingly decreases stimulated lipolysis (56,78,79). A binding site for HSL has been identified in the amino terminus of perilipin 1 that encompasses the sequence that is most highly conserved in four out of five perilipins (79,80); HSL interactions with this conserved sequence in perilipins 2, 3, and 5 have also been reported (79). Thus, the PKA-mediated phosphorylation of both HSL and perilipin 1 triggers the translocation of HSL from the cytosol to lipid droplets, where the lipase docks in a protein-protein interaction with perilipin 1, and gains access to lipid substrates. It is thought that the phosphorylation of perilipin 1 is required to expose the amino terminal binding site for HSL (79).
Perilipin 1 binds ABHD5 on a carboxyl terminal sequence under basal conditions, attenuating lipolysis, but following the activation of PKA, ABHD5 is released from the perilipin 1 scaffold (67). The PKA-mediated phosphorylation of the two PKA-site serine residues closest to the carboxyl terminus of perilipin 1 (70) and ABHD5 on a single serine residue (81) induces the release of ABHD5, enabling the interaction of ABHD5 with ATGL and the activation of triacylglycerol hydrolysis. Additionally, the activation of PKA in adipocytes promotes a dramatically increased translocation of ATGL to perilipin 1-coated lipid droplets (42–44), but the mechanism is unknown. This hormonally-induced recruitment of ATGL to lipid droplets has not been reported for cells in which lipid droplets are coated with other perilipins, yet no specific interactions between perilipin 1 and ATGL have been reported. It is possible that the increased interaction between ABHD5 and ATGL forms a complex that is then more efficiently recruited to lipid droplets.
In adipocytes, ATGL cleaves a fatty acid from triacylglycerol to release diacylglycerol, then HSL cleaves diacylglycerol to release monoacylglycerol; the third fatty acid is released by monoacylglycerol lipase (MAGL) (82). Perilipin 1 modulates the activity of ATGL by controlling the access of the co-activator ABHD5 to ATGL, and the activity of HSL by providing a docking site on lipid droplets for lipase access under specific metabolic conditions that promote lipolysis. In contrast, how perilipin 1 may control MAGL activity has not been investigated. MAGL is thought to be a soluble cytosolic enzyme that associates peripherally with membranes (82,83), yet the factors that regulate subcellular localization and the access of the lipase to substrate lipids are unknown.
4.2. Perilipin 5 control of lipolysis in oxidative tissues
In oxidative tissues such as skeletal muscle, heart, and brown adipose tissue, lipolysis is coordinated by perilipin 5 (Figure 3). In myocytes, the surfaces of lipid droplets are decorated with both perilipin 2 and perilipin 5, whereas lipid droplets in adipocytes of brown adipose tissue are decorated with perilipin 1 and perilipin 5. Despite the mixture of perilipins on these lipid droplets, perilipin 5 plays a dominant role in the control of lipolysis. Studies of perilipin 5 null mice and perilipin 5 tissue specific transgenic mice reveal that perilipin 5 plays an important role in energy homeostasis, particularly in the heart, by controlling triacylglycerol storage and lipolysis (84–88). The whole body genetic deletion of perilipin 5 in mice leads to impaired triacylglycerol storage in the heart (84–86), particularly during fasting conditions, and increased fatty acid oxidation in isolated cardiomyocytes (85), suggesting more rapid turnover of triacylglycerol in the absence of perilipin 5. Despite lower lipid droplet storage capacity, chow-fed perilipin 5 null mice maintain nearly normal heart function, compensating for the absence of perilipin 5 by reducing fatty acid uptake and increasing glucose uptake (84). However, when cardiac function is challenged by inducing myocardial stress or ischemia, perilipin 5 deficiency reduces the availability of stored energy substrates to myocardial tissue, resulting in severely impaired heart function and increased mortality (84). In contrast, the overexpression of perilipin 5 in cardiomyocytes of mice massively increases the storage of triacylglycerols in lipid droplets (87,88), and the augmented perilipin 5 content renders lipid droplets that have been isolated from the hearts of the transgenic mice more resistant to lipolysis (87). To date, the relevance of perilipin 5 in human cardiac physiology has been explored in only one analysis performed on a small human cohort with suspected coronary artery disease. The results indicate the association of a common non-coding polymorphism, rs884164, with reduced heart function following myocardial ischemia, and decreased cardiac expression of the perilipin 5 gene (PLIN5) (84). Further studies are needed to confirm the role of perilipin 5 in the control of lipolysis in human cardiac dysfunction.
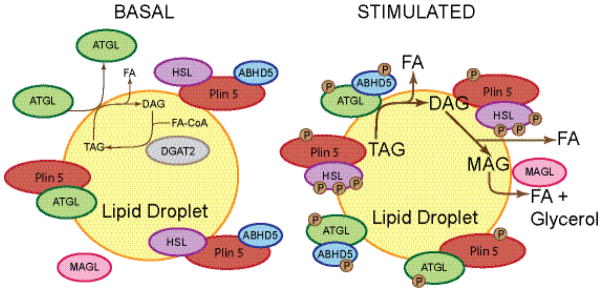
Figure 3. Schematic model of perilipin 5 function in myocytes under basal and lipolytically stimulated conditions.
Under basal conditions, perilipin 5 (Plin 5) resides primarily on lipid droplets, where it binds HSL, ATGL, and ABHD5, while attenuating lipolysis. Perilipin 5 binding of ATGL and ABHD5 at the carboxyl terminus of perilipin 5 is mutually exclusive; the binding sites overlap, preventing both proteins from binding to a single molecule of perilipin 5, whereas the binding site for HSL is distinct and at the amino terminus. The basal level of TAG hydrolysis catalyzed by ATGL is very low, and released DAG is likely re-esterified by DGAT2. The mechanism for perilipin 5-mediated inhibition of basal lipolysis is unknown, but may include the sequestration of ABHD5, with consequent reduced co-activation of ATGL. Under lipolytically stimulated conditions, PKA phosphorylates most components of the pathway, including perilipin 5, ATGL, CGI-58, and HSL, and lipolysis increases. How these phosphorylation events increase lipolysis is unknown, but may include the release of ABHD5 from the perilipin 5 scaffold to enable its interaction with and co-activation of ATGL. ATGL hydrolyzes TAG; HSL hydrolyzes DAG; MAGL hydrolyzes MAG. It is unknown whether ATGL or HSL remain associated with perilipin 5 under stimulated conditions or how perilipin 5 affects MAGL localization or activity.
In addition to a cardiac phenotype in response to stress, perilipin 5 null mice also have altered triacylglycerol storage in muscle and liver. In perilipin 5 null mice, triacylglycerol content is reduced in red (oxidative) quadriceps muscle, but not white (glycolytic) quadriceps muscle of perilipin 5 null mice relative to wild-type mice (86), and in liver, particularly following feeding of a high fat diet (89). In contrast, the overexpression of perilipin 5 in skeletal muscle increases triacylglycerol storage in intramyocellular lipid droplets (90,91). These findings complement results from studies conducted using cultured cell models with overexpressed perilipin 5 to demonstrate that perilipin 5 increases triacylglycerol storage by reducing turnover (24,25,43,92).
Perilipin 5 binds multiple components of the lipolytic complex while attenuating lipolysis. The amino terminal sequence of perilipin 5 includes a binding site for HSL that is also conserved in perilipins 1, 2, and 3 (79). Interestingly, perilipin 5 on lipid droplets binds HSL under basal conditions, while restricting the lipolysis of stored triacylglycerols (79). The carboxyl terminal sequence of perilipin 5 contains overlapping binding sites for ATGL and ABHD5 (43,93,94). In contrast, the carboxyl terminus of perilipin 1 binds only ABHD5, not ATGL; moreover, the amino acid sequence of the ABHD5 binding site is not conserved between perilipin 1 and perilipin 5. Competitive protein binding experiments have revealed that the binding of ABHD5 and ATGL to perilipin 5 is mutually exclusive (94); a single copy of perilipin 5 can bind only one of these proteins, not both. The outcome of these experiments suggests that the binding of ABHD5 to perilipin 5 precludes the interaction of ABHD5 with ATGL. Similarly, when ATGL binds perilipin 5, it cannot also bind ABHD5. These exclusive binding interactions are likely part of the mechanism by which perilipin 5 attenuates lipolysis under basal conditions. Although both ATGL and ABHD5 are recruited to perilipin 5 coated lipid droplets under basal conditions, they cannot interact with each other, thus reducing triacylglycerol hydrolysis.
Perilipin 5 effectively protects stored triacylglycerols from lipolysis under basal conditions while recruiting lipolytic effectors to lipid droplets containing substrate lipids; however, when hormonal signals activate PKA, perilipin 5 is phosphorylated and lipolysis increases (43,92). A serine residue within a PKA consensus sequence has been identified as a target for PKA-mediated phosphorylation; when serine 155 of murine perilipin 5 is mutated to alanine, PKA-mediated stimulation of lipolysis is attenuated (92). Phosphorylation of this site and a neighboring conserved site of RRSMS (including serine 161 and serine 163 in mouse perilipin 5; RRSVS in humans) have been identified in phosphoproteomics studies of proteins from skeletal muscle biopsies of exercising humans (95), cardiac muscle from mice treated with β-adrenergic agonists (96), and livers from fasted and re-fed mice (97), providing evidence for in vivo phosphorylation of these three serine residues. These studies identified three additional phosphorylated serine residues that are likely targets for other kinases; the functional consequences of the phosphorylation of perilipin 5 following the activation of other signaling pathways has not been addressed. It would be interesting to test whether the aforementioned optic atrophy 1 is an AKAP that facilitates the PKA-mediated phosphorylation of perilipin 5. Gene expression data reported by Bio-GPS (Biogps.org) indicates that opa1 is highly expressed in brown adipose tissue, heart and skeletal muscle of mice; hence, this AKAP may play an important role in facilitating the phosphorylation of perilipin 5.
The mechanism for how the phosphorylation of perilipin 5 enhances lipolysis is as yet unknown. Proposed mechanisms include the release of ABHD5 from the perilipin scaffold to enable interaction with and co-activation of ATGL (92), however, this remains to be proven. Additionally, it is unknown whether ATGL and HSL remain associated with perilipin 5 or are also released following the activation of PKA. Study of the association of ATGL with lipid droplets in white and brown adipose tissue has revealed that, under basal conditions, the ATGL content of lipid droplets from brown adipocytes is higher than that observed for white adipocytes. Moreover, unlike the situation in white adipocytes where perilipin 1 exerts major control over lipolysis, the activation of PKA in brown adipocytes does not increase ATGL recruitment to lipid droplets (43).
In addition to increasing triacylglycerol storage, the overexpression of perilipin 5 increases fatty acid oxidation in intact cultured cells (25,90). In contrast, mitochondria isolated from the cardiomyocytes of mice with transgenic overexpression of perilipin 5 showed impaired fatty acid oxidation (92). Several groups have proposed that perilipin 5 helps to channel fatty acids released during lipolysis toward mitochondria for oxidation (25,90,98). Perilipin 5 has been shown to associate with both lipid droplets and mitochondria (90,98), and hence, may assist with fatty acid transfer. Moreover, the expression of perilipin 5 in cells increases the localization of mitochondria to the immediate neighborhood of lipid droplets; the final twenty amino acids of the carboxyl terminus of perilipin 5 are essential for this recruitment of mitochondria to lipid droplets (98). It is unknown whether this sequence of perilipin 5 interacts with mitochondrial proteins or lipids, but the increased contact between lipid droplets and mitochondria is likely important for chaperoning or transport of fatty acids released during lipolysis to mitochondria. Additional experimentation is needed to provide evidence for this function. It is also currently unknown how the phosphorylation of perilipin 5 during stimulated lipolysis affects the interaction of perilipin 5 with mitochondria and channeling of fatty acids to mitochondria for oxidation. Perilipin 5 may also promote efficient coupling between triacylglycerol hydrolysis and mitochondrial fatty acid oxidation by altering the transcription of genes crucial for mitochondrial biogenesis and oxidative function (99); the activation of PKA triggers the translocation of perilipin 5 to the nucleus, where it forms complexes with PGC-1α and SIRT1 and increases transcription of PGC-1α target genes (100). The biology of perilipin 5 and its dual functions to control lipolysis and impact mitochondrial function appear to be complex and as yet incompletely understood, involving both transcriptional and post-transcriptional mechanisms at two cellular locales, the lipid droplet and the nucleus. Given these recent findings, it is surprising that perilipin 5 null mice have a relatively mild phenotype under normal, chow-fed conditions. To fully unravel the complexity of the role of perilipin 5 in energy homeostasis, it may be necessary to study mice with tissue specific deletions of perilipin 5 under energetically challenging conditions, with careful examination of potential compensatory roles played by other perilipins.
4.3. Perilipin 2 exerts minimal control over lipolysis
Perilipin 2 is ubiquitously expressed and is the major lipid droplet-associated perilipin in cells in which perilipin 1 or perilipin 5 are not also expressed. While perilipins 1 and 5 exert specific and distinct control over lipolysis that is modulated by PKA-mediated phosphorylation of perilipins, lipases, and co-factors for lipases, perilipin 2 attenuates lipolysis only moderately and is not phosphorylated by PKA. Perilipin 2 is substantially more permissive to lipolysis than either perilipin 1 or perilipin 5 (43,79), and does not effectively recruit lipases to lipid droplets through protein binding interactions under either basal or hormonally stimulated conditions that activate PKA (Figure 4). Instead, the overexpression of perilipin 2 in cells reduces the access of ATGL to lipid droplets, thus attenuating lipolysis (101,102). At first glance, the phenotype of perilipin 2 null mice appears to be relatively mild, but significant changes in lipid homeostasis in liver and white adipose tissue are revealed when the mice are fed a high fat (Western) diet (Teklad TD88137; 42% fat with cholesterol). Perilipin 2 null mice have reduced triacylglycerol storage in liver, particularly when the mice are fed a high fat diet, altered mammary gland development and lactation, and protection against obesity with browning of white adipose tissue in mice fed a high fat (103–107). Interestingly, the consequent reduction in liver triacylglycerol has no major effect on the packaging or secretion of very low density lipoproteins (103), although the loss of perilipin 2 in hepatocytes promotes large scale changes in the expression of lipogenic genes that are the targets of SREBP-1 and SREBP-2 (104). Complicating our understanding of perilipin 2 function, a recent study reported that mice with liver-specific deletion of perilipin 2 are not protected against hepatic steatosis when fed a high fat (Western) diet2, implying a systemic mechanism of hepatic protection. Most of these data are consistent with perilipin 2 serving a role to attenuate lipolysis; when this barrier function is reduced, increased flux of fatty acids due to increased lipolysis alters gene expression leading to the observed phenotype that is exacerbated when fatty acid influx is increased with high fat diet feeding.
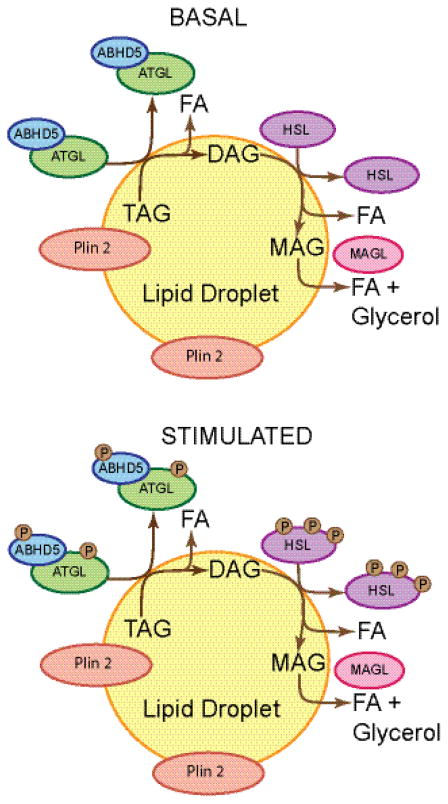
Figure 4. Schematic model of perilipin 2 function in cells under basal and lipolytically stimulated conditions.
Perilipin 2 localizes almost exclusively to lipid droplets and does not significantly recruit ATGL, ABHD5, or HSL to lipid droplets under either basal or lipolytically stimulated conditions. Perilipin 2 is relatively permissive to lipolysis; lipolysis under basal conditions is approximately comparable to lipolysis when PKA is activated. Perilipin 2 is not a substrate for PKA, although HSL, ATGL, and ABHD5 are substrates. The localization of ATGL with ABHD5 and HSL to perilipin 2 coated lipid droplets is likely transient under both conditions. It is unknown whether or not perilipin 2 affects MAGL localization or activity.
4.4 The functions of perilipins 3 and 4 in controlling lipolysis have been minimally studied
Perilipin 3 is ubiquitously expressed in tissues and cultured cell lines. When cultured adipocytes are incubated with oleic acid, glucose and insulin, perilipin 3 localizes to tiny nascent lipid droplets, but is displaced by perilipin 2 over time as the sizes of the lipid droplets increase (108). Additionally, the majority of non-adipose cells have tiny lipid droplets coated with perilipin 3, perilipin 2, or both proteins. The function of perilipin 3 is poorly understood, and the possible involvement of perilipin 3 in the control of lipolysis has not been directly tested. A knockout mouse model for perilipin 3 has not yet been reported; however, when anti-sense oligonucleotides are used to knockdown perilipin 3 expression in mice, hepatic and serum levels of triacylglycerol are reduced (109), suggesting that perilipin 3 plays a role in stabilizing stored triacylglycerol and thus affects the availability of lipid substrates for hepatic lipoprotein assembly and secretion. This observation is supported by studies in cultured cells; when perilipin 3 is knocked down in HeLa cells, triacylglycerol storage is reduced, while fatty acid import is unaffected (110). Interestingly, the knockdown of perilipin 3 in mouse AML12 hepatoma cells does not decrease triacylglycerol storage, but the lipid droplets are smaller in size and coated with perilipin 2. Knockdown of both perilipin 2 and perilipin 3 decreases triacylglycerol storage, and the residual lipid droplets are fewer and larger in size (101). These morphological changes suggest that perilipins may have surfactant properties, and in their absence, fusion of lipid droplets minimizes surface area in contact with the surrounding aqueous cytosol. Overall, the studies suggest that perilipin 3 may modulate lipolysis in cells that lack perilipin 1 or perilipin 5, but definitive experiments are needed to specifically examine the physiological function of perilipin 3 in animal models.
Perilipin 4 is associated with tiny nascent lipid droplets of cultured adipocytes (29,108), and the lipid droplets of white, but not brown, adipose tissue, heart, and skeletal muscle (28). Little is known about the function of perilipin 4. The perilipin 4 gene is located next to the perilipin 5 gene in the mouse genome. The genetic deletion of perilipin 4 in mice reduced the triacylglycerol content of the heart, but not other tissues, including adipose tissue; however, the levels of both mRNA and protein of perilipin 5 were also strikingly reduced (28). Consequently, it is not possible to determine whether the observed phenotype is due to the absence of perilipin 4 or perilipin 5. Fasting and high fat diet failed to increase cardiac triacylglycerol accumulation in these mice, and cardiac function was comparable to that of wild-type mice. Surprisingly, there were no major changes in white adipose tissue function in perilipin 4 null mice when compared to wild-type mice, suggesting that perilipin 4 is nonessential in this tissue; since white adipose tissue does not express perilipin 5, these findings are specific for perilipin 4. Further study is needed to identify the specific functions of perilipin 4.
5. Beyond the role of perilipins in the classical lipolytic cascade: the role of perilipins in autophagic lipolysis
The importance of perilipin control of lipolysis through interactions of perilipins with cytoplasmic lipases and regulatory proteins is well established. Moreover, the involvement of cytosolic lipases in mobilizing adipose triacylglycerol stores is unquestionable, since the deletion, knockdown, or pharmacological inhibition of ATGL and HSL abolishes the majority of lipase activity and severely impairs the release of fatty acids into circulation (reviewed in (31–34)). However, in non-adipose tissues, the contribution of additional neutral lipid lipases to the catabolism of triacylglycerols stored in lipid droplets is likely, but relatively unstudied. For example, in the livers of fasted mice, ATGL accounts for less than 50% of triacylglycerol hydrolase activity (111), suggesting the existence of additional lipases and possibly different molecular mechanisms for the mobilization of fatty acids from hepatic triacylglycerol stores. Autophagy, initially identified as a pathway for protein turnover and recycling of amino acids during starvation, is now recognized to contribute to lipid metabolism and the catabolism of lipid droplets. While the ubiquitin-proteasome system degrades solely proteins, the lysosome can degrade both proteins and lipids. Autophagy and lipolysis are both evolutionarily conserved pathways central to cell survival during periods of nutrient deprivation, and both provide energy substrates. Both processes are suppressed during conditions of sufficient or excessive nutrient supply (as in the fed state or with obesity) and both processes are increased in response to a limited supply of energy (during fasting or starvation), providing free fatty acids as fuel for mitochondrial β-oxidation to meet cellular energy requirements (112,113). The remarkable similarities in the regulation of both autophagy and “classical” lipolysis mechanisms suggest that both contribute to the mobilization of intracellular lipid stores and share common molecular machinery, although direct fusion of lipid droplets with lysosomes has not been reported. To date, two types of autophagic processes have been identified to recycle components of lipid droplets, macroautophagy and chaperone-mediated autophagy (CMA) (114–116); both processes can occur in the same cell. These forms of autophagy differ in the mode of substrate delivery to autophagic vesicles and utilize distinct molecular mediators that are specific to the type of cell and environmental stressor. Both types of autophagy require recognition of lipid droplets and reorganization of the lipid droplet surface to facilitate docking of components used in the process of autophagy. Proteomics characterization of lipid droplets has revealed that the lipid droplet surface provides a dynamic interface harboring proteins involved in lipid metabolism and protein and lipid trafficking (reviewed in (117–119)). Based on the abundance of perilipins on lipid droplets, their relatively specific localization to this organelle, and their defined roles in orchestrating “classical” lipolysis, it is logical to speculate that perilipins play a major role in the regulation of autophagic lipolysis. Here, we highlight recent studies that provide evidence for a regulatory function of perilipins 1, 2 and 3 in autophagic lipolysis.
5.1 Perilipins and macroautophagy
A mechanistic connection between lysosomes and lipid droplets was initially established in studies investigating the cause of excessive hepatic accumulation of lipid droplets in murine models with liver-specific knockdown of autophagic genes (120). This was the first demonstration that mobilization of neutral lipids in mouse liver and cultured hepatocytes can occur through sequestration of perilipin 2-coated lipid droplets in autophagosomes, with subsequent delivery of the lipid droplets to lysosomes where triacylglycerols are then hydrolyzed by lysosomal lipases. This process has been termed macrolipophagy or lipophagy; the physiological importance of lipophagy has recently been demonstrated in other types of mammalian cells (114–116).
The mechanism by which lipid droplets are recognized as a substrate and sequestered by autophagosomes is not well understood, but association of a small GTPase, Rab7, with the surfaces of lipid droplets has been identified as an early step to initiate lipophagy in adipocytes and hepatocytes (121). In cultured 3T3-L1 adipocytes, Rab7 associates minimally with perilipin 1 coated lipid droplets under unstimulated (basal) conditions, but increases upon the stimulation of β-adrenergic receptors, with subsequent activation of PKA and phosphorylation of perilipin 1 (122); however, this recruitment of Rab7 to lipid droplets is not mediated by protein-protein interactions with perilipin 1. Rather, the knockdown of perilipin 1 increases the association of both Rab7 and the lysosomal integral membrane protein Lamp-1 with lipid droplets under basal conditions, concomitant with the enhancement of lipolysis; moreover, the stimulation of β-adrenergic receptors causes no further increases of Lamp-1 recruitment (122). These data suggest that unphosphorylated perilipin 1 blocks the docking of Rab7 on lipid droplets, and hence, plays a protective role in inhibiting lipophagy, and that phosphorylation-induced conformational changes in perilipin 1 enable Rab7 recruitment to lipid droplets, with the ensuing association of lipid droplets with lysosomes.
Protection of stored neutral lipids from cytosolic lipases is a function shared by perilipins 1, 2, and 5, so it is interesting to speculate that the perilipins may also protect lipid droplets from autophagic lipolysis. A recent study provides the first evidence that overexpression of perilipin 2 protects hepatic lipid droplets from autophagic lipolysis, whereas perilipin 2 deficiency reduces lipid droplet content by enhancing autophagic lipolysis in both the livers of mice and cultured hepatoma cells (123). Reduced levels of perilipin 2 lead to reduced triacylglycerol storage in the livers of perilipin 2 null mice relative to wild-type mice, and cultured hepatoma cells treated with shRNA to knockdown perilipin 2 expression or CRISPR/Cas to delete the perilipin 2 gene relative to control cells (123). Since perilipin 2 has been shown to impair the access of cytosolic lipases to lipid droplets (102), the observed decrease in triacylglycerol storage may be due to increased lipase activity by “classical” lipolysis. However, the observed decrease in triacylglycerol in perilipin 2 deficient cells and liver was attenuated by a specific inhibitor (Lalistat2) of lysosomal lipases, indicating autophagic lipolysis as the most relevant pathway (123). Moreover, the autophagy inhibitor bafilomycin A1, which prevents lysosome acidification and fusion of autophagosomes with lysosomes, blocked the increased turnover of triacylglycerols in cells with reduced perilipin 2 (123). Interestingly, adenoviral-shAtg7-mediated knockdown of hepatic Atg7, an autophagy mediator, is insufficient to rescue decreased levels of hepatic triacylglycerol in perilipin 2 null mice despite reducing levels of protein markers of autophagy (123), suggesting that additional processes may be involved.
In contrast to the role of perilipins in recruiting lipases and co-factors to lipid droplets when “classical” lipolysis is stimulated, perilipins 1 and 2 downregulate autophagic lipolysis by acting as a physical barrier, rather than recruiting key mediators of autophagy to the lipid droplet. Therefore, removal of perilipins from the surfaces of lipid droplets is likely a critical first step to promote autophagic lipolysis.
5.2 Removing the perilipin barrier to promote cytosolic and autophagic lipolysis
Due to the lipid-protective functions of perilipins, protein stability of lipid droplet-associated perilipins is a major impediment to both “classical” and autophagic lipolysis. Perilipins 1 and 2 are stabilized when binding to lipid droplets (124–126); lipid droplet-associated perilipin 1 has a very long half-life, longer than 72 hours (127). However, when perilipins 1 or 2 are translated in excess of available lipid droplet binding sites, they are unstable and rapidly degraded through either proteasomal or lysosomal pathways (126–130), depending upon the type of cell and environmental factors. Interestingly, although levels of perilipin 2 mRNA increase during adipocyte differentiation, protein levels of perilipin 2 are very low in fully differentiated adipocytes (124,131). It is thought that perilipin 1 has a higher binding affinity for lipid droplets than perilipin 2, and hence, outcompetes perilipin 2 to become the major lipid droplet-associated perilipin in mature adipocytes, leading to effective protection of stored triacylglycerols from cytosolic lipases, and rapid degradation of perilipin 2. However, when adipocytes are lipolytically stimulated, PKA-mediated phosphorylation of perilipin 1 attenuates the barrier function, promoting massive triacylglycerol hydrolysis. The consequent release of fatty acids and diacylglycerols leads to the re-esterification of a portion of these products of lipolysis and the formation of new lipid droplets. Perilipin 2 binds to these nascent lipid droplets, thus escaping proteasomal degradation (128,131) and likely adding stability to the lipid droplets by keeping lipolysis in check.
For most cells of the body, lipid droplets are coated with perilipins 2 and 3; autophagic lipolysis requires the removal of these perilipins from lipid droplets. Recent studies have revealed that, under conditions of limited nutrient availability, chaperone-mediated autophagy (CMA) is the major mechanism for removal of perilipins 2 and 3 from lipid droplets (132,133). CMA was originally described as a response of hepatocytes to nutrient scarcity (134). In contrast to macroautophagy, the process of bulk recycling of organelles, including lipid droplets, CMA degrades a specific subset of proteins that cross the lysosomal membrane through the CMA receptor, lysosome-associated membrane protein type 2A (LAMP-2A). CMA substrate proteins contain a pentapeptide motif, KFERQ, that is selectively recognized and bound by the cytosolic heat shock cognate protein of 70 kDa (hsc70), that interacts with LAMP-2A to import the proteins into lysosomes for degradation. Murine perilipin 2 and perilipin 3 contain related pentapeptide sequences of SLKVQ and LDRLQ, respectively (132). When the perilipin 2 pentapeptide sequence is mutated to SLKAA, hsc70 binding to perilipin 2 on lipid droplets is nearly eliminated, and perilipin 2 remains stably associated with lipid droplets of cultured mouse fibroblasts under starvation conditions (132). The consequent retention of the mutated perilipin 2 on lipid droplets reduces both ATGL association with lipid droplets, hence, classical lipolysis, and the recruitment of multiple protein mediators of macroautophagy to lipid droplets, hence, autophagic lipolysis. Conversely, the removal of perilipins 2 and 3 from lipid droplets via CMA promotes both classical lipolysis and autophagic lipolysis. Additionally, a recent study found that perilipin 2 is phosphorylated by AMP kinase, but only after the co-localization of perilipin 2 and hsc70 on lipid droplets; this phosphorylation of perilipin 2 is required to prime the protein for CMA (133). Thus, excess perilipin 2 that remains unbound to lipid droplets is rapidly degraded by the proteasome, whereas lipid droplet-bound perilipin 2 must be phosphorylated prior to extraction from lipid droplets and lysosomal degradation via CMA. These novel and exciting studies were conducted in mouse fibroblasts; it will be important to confirm the findings in additional types of cells such as hepatocytes to determine whether this is a universal mechanism for the control of classical and autophagic lipolysis. More importantly, it is imperative to determine whether the CMA depletion of perilipins 2 and 3 is an adaptive mechanism for survival of prolonged starvation applicable only to rodent physiology, since the two proposed CMA motif sequences for perilipins 2 and 3 are conserved in rat, but not other species, including humans. Further studies of these pathways as well as the cross talk between the pathways of classical and autophagic lipolysis in different cell and tissue types from a variety of species will hopefully provide answers to remaining questions and insight into human disease pathology, like non-alcoholic fatty liver disease.
6. Perspectives on the future
Studies over the past 25 years have begun to unravel the complicated mechanisms by which perilipins control lipolysis of stored triacylglycerols, yet much is still unknown. Most mechanistic studies have focused on perilipin 1; in contrast, little is known about how the other perilipins act to coordinate the activities of lipases. Better definition of the functions of the other members of the perilipin family will likely provide a greater understanding of the role of non-adipose lipid droplets in whole body energy homeostasis and metabolic disease. Given the critical role that perilipins play in cellular lipid homeostasis, it is not surprising that their function in the mobilization of stored lipids is complex, involving multiple molecular mechanisms, and perhaps, multiple intracellular sites, to optimize cell-specific responses to energy demands. Moreover, for many tissues, there are undoubtedly additional unstudied lipases that contribute to the maintenance of lipid and energy homeostasis. Future work will likely reveal mechanisms by which perilipins control lipolysis by other lipases and perhaps MAGL. Recent phosphoproteomics studies have revealed that perilipins 1 and 5 as well as the known cytosolic lipases are multiply phosphorylated in vivo, yet there is a paucity of information regarding the consequences of most of these phosphorylation events, or the identity of the kinases that modify these proteins. The most well studied signaling pathways that promote lipolysis lead to the activation of PKA and subsequent phosphorylation of perilipins 1 and 5, lipases, and cofactors for lipases, yet additional signaling pathways in adipose tissue, muscle, and other tissues also enhance lipolysis, but have not been studied in the context of the control of lipolysis by perilipins. The known role of AMP kinase as a key regulator of cellular and whole-body energy homeostasis, and the recent discovery that perilipin 2 is a substrate of this kinase provide a new incentive to investigate AMP kinase function in phosphorylating other perilipins and controlling lipolysis in non-adipose tissues. Finally, it will be exciting to unravel additional mechanisms at the intersection of the pathways of classical lipolysis and the more recently defined autophagic lipolysis.
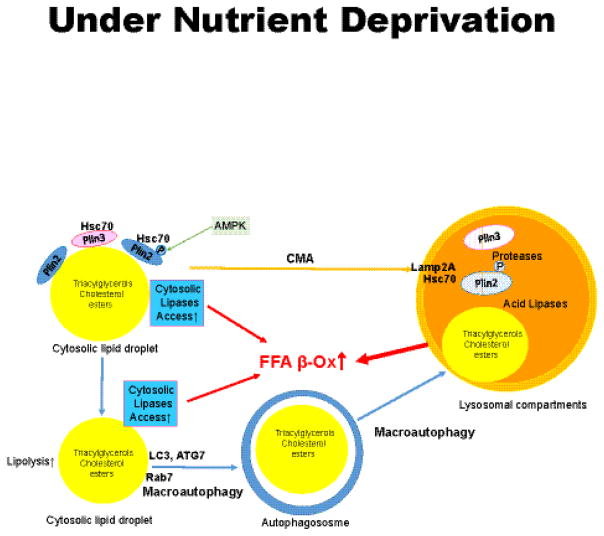
Figure 5. Schematic model of macroautophagy and chaperone-mediated autophagy (CMA) under conditions of nutrient deprivation.
Perilipins 2 and 3 provide a barrier to both cytosolic lipolysis and autophagy. Removal of perilipin (Plin) 2 and Plin 3 from the surfaces of lipid droplets is mediated by CMA and precedes lipolysis by both pathways. Plin 2 is phosphorylated by AMP kinase (AMPK) after associating with Hsc70. Plin 2 and Plin 3 are removed as complexes with Hsc70, followed by recognition by Lamp-2A on lysosomes, import into the lumen of the lysosome and degradation. Reduction of Plin 2 and Plin 3 on lipid droplets enables increased binding of cytosolic lipases (such as ATGL), Rab7 and protein effectors of macroautophagy (such as LC3-II) to lipid droplets. This leads to increased release of fatty acids by cytosolic lipases and the engulfment of the lipid droplet by the growing autophagosome. Autophagosomes fuse with lysosomes to form autolysosomes, wherein lysosomal acid lipases hydrolyze triacylglycerols and cholesterol esters, releasing fatty acids and cholesterol. Fatty acids are recycled back into the cytosol to mix with fatty acids generated by cytosolic lipolysis and support mitochondrial β-oxidation.
Acknowledgments
The research programs of the authors have recently been supported by the National Institutes of Health NIH R01 DK054797 (DLB) and NIH R01 DK075017 (CS), and the Geriatric Research, Education and Clinical Center, Baltimore Veterans Affairs Health Care Center and the Clinical Nutrition Research Unit of Maryland NIH DK072488 (to CS). The authors apologize for any unintentional omissions of references.
Footnotes
Abbreviations: ABHD5, alpha beta hydrolase domain protein 5; AKAP, A-kinase anchoring protein; ATGL, adipose triglyceride lipase; CMA, chaperone mediated autophagy; DAG, diacylglycerol; DGAT2, acyl-CoA:diacylglycerol acyltransferase 2; FA, fatty acid; hsc70, heat shock cognate protein of 70 kDa; HSL, hormone-sensitive lipase; MAG, monoacylglycerol; MAGL, monoacylglycerol lipase; PDE3b, phosphodiesterase 3b; PKA, cAMP-dependent protein kinase; plin, perilipin; TAG, triacylglycerol; VGD, vertebrate genome duplication
Griffin, J.D., Salter, D.M., Bowman, T., and Greenberg, A.S. 2017. Role of Hepatic PLIN2 and PLIN4 in the development of Western type diet induced hepatosteatosis. FASEB J. 31, no. 1 supplement, Abstract 458.3
Conflict of Interest
The authors, Dawn Brasaemle and Carole Stalryd have no conflicts to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bartholomew SR, Bell EH, Summerfield T, Newman LC, Miller EL, Patterson B, Niday ZP, Ackerman WE, Tansey JT. Distinct cellular pools of perilipin 5 point to roles in lipid trafficking. Biochim Biophys Acta. 2012;1821:268–278. doi: 10.1016/j.bbalip.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsieh K, Lee YK, Londos C, Raaka BM, Dalen KT, Kimmel AR. Perilipin family members preferentially sequester to either triacylglycerol-specific or cholesteryl-ester-specific intracellular lipid storage droplets. J Cell Sci. 2012;125:4067–4076. doi: 10.1242/jcs.104943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granneman JG, Kimler VA, Zhang H, Ye X, Luo X, Postlethwait JH, Thummel R. Lipid droplet biology and evolution illuminated by the characterization of a novel perilipin in teleost fish. Elife. 2017;6:e21771. doi: 10.7554/eLife.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaner WS, O’Byrne SM, Wongsiriroj N, Kluwe J, D’Ambrosio DM, Jiang H, Schwabe RF, Hillman EM, Piantedosi R, Libien J. Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage. Biochim Biophys Acta. 2009;1791:467–473. doi: 10.1016/j.bbalip.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orban T, Palczewska G, Palczewski K. Retinyl ester storage particles (retinosomes) from the retinal pigmented epithelium resemble lipid droplets in other tissues. J Biol Chem. 2011;286:17248–17258. doi: 10.1074/jbc.M110.195198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirheydari M, Rathnayake SS, Frederick H, Arhar T, Mann EK, Cocklin S, Kooijman EE. Insertion of perilipin 3 into a glycero(phospho)lipid monolayer depends on lipid headgroup and acyl chain species. J Lipid Res. 2016;57:1465–1476. doi: 10.1194/jlr.M068205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacle A, Gautier R, Jackson CL, Fuchs PF, Vanni S. Interdigitation between Triglycerides and Lipids Modulates Surface Properties of Lipid Droplets. Biophys J. 2017;112:1417–1430. doi: 10.1016/j.bpj.2017.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kory N, Thiam AR, Farese RV, Jr, Walther TC. Protein Crowding Is a Determinant of Lipid Droplet Protein Composition. Dev Cell. 2015;34:351–363. doi: 10.1016/j.devcel.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiam AR, Antonny B, Wang J, Delacotte J, Wilfling F, Walther TC, Beck R, Rothman JE, Pincet F. COPI buds 60-nm lipid droplets from reconstituted water-phospholipid-triacylglyceride interfaces, suggesting a tension clamp function. Proc Natl Acad Sci U S A. 2013;110:13244–13249. doi: 10.1073/pnas.1307685110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bussell R, Jr, Eliezer D. A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J Mol Biol. 2003;329:763–778. doi: 10.1016/s0022-2836(03)00520-5. [DOI] [PubMed] [Google Scholar]
- 11.Drin G, Antonny B. Amphipathic helices and membrane curvature. FEBS Lett. 2010;584:1840–1847. doi: 10.1016/j.febslet.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Krahmer N, Guo Y, Wilfling F, Hilger M, Lingrell S, Heger K, Newman HW, Schmidt-Supprian M, Vance DE, Mann M, Farese RV, Jr, Walther TC. Phosphatidylcholine Synthesis for Lipid Droplet Expansion Is Mediated by Localized Activation of CTP:Phosphocholine Cytidylyltransferase. Cell Metab. 2011;14:504–515. doi: 10.1016/j.cmet.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowe ER, Mimmack ML, Barbosa AD, Haider A, Isaac I, Ouberai MM, Thiam AR, Patel S, Saudek V, Siniossoglou S, Savage DB. Conserved Amphipathic Helices Mediate Lipid Droplet Targeting of Perilipins 1–3. J Biol Chem. 2016;291:6664–6678. doi: 10.1074/jbc.M115.691048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia A, Sekowski A, Subramanian V, Brasaemle DL. The central domain is required to target and anchor perilipin A to lipid droplets. J Biol Chem. 2003;278:625–635. doi: 10.1074/jbc.M206602200. [DOI] [PubMed] [Google Scholar]
- 15.Subramanian V, Garcia A, Sekowski A, Brasaemle DL. Hydrophobic sequences target and anchor perilipin A to lipid droplets. J Lipid Res. 2004;45:1983–1991. doi: 10.1194/jlr.M400291-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Kory N, Farese RV, Jr, Walther TC. Targeting Fat: Mechanisms of Protein Localization to Lipid Droplets. Trends Cell Biol. 2016;26:535–546. doi: 10.1016/j.tcb.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brasaemle DL. Thematic review series: Adipocyte Biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Kimmel AR, Sztalryd C. The Perilipins: Major Cytosolic Lipid Droplet-Associated Proteins and Their Roles in Cellular Lipid Storage, Mobilization, and Systemic Homeostasis. Annu Rev Nutr. 2016;36:471–509. doi: 10.1146/annurev-nutr-071813-105410. [DOI] [PubMed] [Google Scholar]
- 20.Sztalryd C, Kimmel AR. Perilipins: lipid droplet coat proteins adapted for tissue-specific energy storage and utilization, and lipid cytoprotection. Biochimie. 2014;96:96–101. doi: 10.1016/j.biochi.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–5491. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 22.Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res. 2010;51:468–471. doi: 10.1194/jlr.R000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miura S, Gan JW, Brzostowski J, Parisi MJ, Schultz CJ, Londos C, Oliver B, Kimmel AR. Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J Biol Chem. 2002;277:32253–32257. doi: 10.1074/jbc.M204410200. [DOI] [PubMed] [Google Scholar]
- 24.Dalen KT, Dahl T, Holter E, Arntsen B, Londos C, Sztalryd C, Nebb HI. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim Biophys Acta. 2007;1771:210–227. doi: 10.1016/j.bbalip.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Croce MA, Gropler MC, Varma V, Yao-Borengasser A, Rasouli N, Kern PA, Finck BN, Bickel PE. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 2006;55:3418–3428. doi: 10.2337/db06-0399. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi T, Matsushita S, Motojima K, Hirose F, Osumi T. MLDP, a novel PAT family protein localized to lipid droplets and enriched in the heart, is regulated by peroxisome proliferator-activated receptor alpha. J Biol Chem. 2006;281:14232–14240. doi: 10.1074/jbc.M601682200. [DOI] [PubMed] [Google Scholar]
- 27.Servetnick DA, Brasaemle DL, Gruia-Gray J, Kimmel AR, Wolff J, Londos C. Perilipins are associated with cholesteryl ester droplets in steroidogenic adrenal cortical and Leydig cells. J Biol Chem. 1995;270:16970–16973. doi: 10.1074/jbc.270.28.16970. [DOI] [PubMed] [Google Scholar]
- 28.Chen W, Chang B, Wu X, Li L, Sleeman M, Chan L. Inactivation of Plin4 downregulates Plin5 and reduces cardiac lipid accumulation in mice. Am J Physiol Endocrinol Metab. 2013;304:E770–E779. doi: 10.1152/ajpendo.00523.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolins NE, Skinner JR, Schoenfish MJ, Tzekov A, Bensch KG, Bickel PE. Adipocyte protein s3–12 coats nascent lipid droplets. J Biol Chem. 2003;278:37713–37721. doi: 10.1074/jbc.M304025200. [DOI] [PubMed] [Google Scholar]
- 30.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009;48:275–297. doi: 10.1016/j.plipres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie. 2016;125:259–266. doi: 10.1016/j.biochi.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 34.Young SG, Zechner R. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev. 2013;27:459–484. doi: 10.1101/gad.209296.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans. 2003;31:1120–1124. doi: 10.1042/bst0311120. [DOI] [PubMed] [Google Scholar]
- 36.Martin S, Okano S, Kistler C, Fernandez-Rojo MA, Hill MM, Parton RG. Spatiotemporal regulation of early lipolytic signaling in adipocytes. J Biol Chem. 2009;284:32097–32107. doi: 10.1074/jbc.M109.002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SJ, Tang T, Abbott M, Viscarra JA, Wang Y, Sul HS. AMPK Phosphorylates Desnutrin/ATGL and Hormone-Sensitive Lipase To Regulate Lipolysis and Fatty Acid Oxidation within Adipose Tissue. Mol Cell Biol. 2016;36:1961–1976. doi: 10.1128/MCB.00244-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eichmann TO, Grumet L, Taschler U, Hartler J, Heier C, Woblistin A, Pajed L, Kollroser M, Rechberger G, Thallinger GG, Zechner R, Haemmerle G, Zimmermann R, Lass A. ATGL and CGI-58 are lipid droplet proteins of the hepatic stellate cell line HSC-T6. J Lipid Res. 2015;56:1972–1984. doi: 10.1194/jlr.M062372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beller M, Sztalryd C, Southall N, Bell M, Jackle H, Auld DS, Oliver B. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 2008;6:e292. doi: 10.1371/journal.pbio.0060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo Y, Walther TC, Rao M, Stuurman N, Goshima G, Terayama K, Wong JS, Vale RD, Walter P, Farese RV. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soni KG, Mardones GA, Sougrat R, Smirnova E, Jackson CL, Bonifacino JS. Coatomer-dependent protein delivery to lipid droplets. J Cell Sci. 2009;122:1834–1841. doi: 10.1242/jcs.045849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Lu X, Lombes M, Rha GB, Chi YI, Guerin TM, Smart EJ, Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H, Bell M, Sreenevasan U, Hu H, Liu J, Dalen K, Londos C, Yamaguchi T, Rizzo MA, Coleman R, Gong DW, Brasaemle D, Sztalryd C. Unique Regulation of Adipose Triglyceride Lipase (ATGL) by Perilipin 5, a Lipid Droplet-associated Protein. Journal of Biological Chemistry. 2011;286:15707–15715. doi: 10.1074/jbc.M110.207779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bezaire V, Mairal A, Ribet C, Lefort C, Girousse A, Jocken J, Laurencikiene J, Anesia R, Rodriguez AM, Ryden M, Stenson BM, Dani C, Ailhaud G, Arner P, Langin D. Contribution of Adipose Triglyceride Lipase and Hormone-sensitive Lipase to Lipolysis in hMADS Adipocytes. J Biol Chem. 2009;284:18282–18291. doi: 10.1074/jbc.M109.008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, Wang Y, Duncan RE, Kang C, Sul HS. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mason RR, Meex RC, Lee-Young R, Canny BJ, Watt MJ. Phosphorylation of adipose triglyceride lipase Ser(404) is not related to 5′-AMPK activation during moderate-intensity exercise in humans. Am J Physiol Endocrinol Metab. 2012;303:E534–E541. doi: 10.1152/ajpendo.00082.2012. [DOI] [PubMed] [Google Scholar]
- 47.Pagnon J, Matzaris M, Stark R, Meex RC, Macaulay SL, Brown W, O’Brien PE, Tiganis T, Watt MJ. Identification and functional characterization of protein kinase A phosphorylation sites in the major lipolytic protein, adipose triglyceride lipase. Endocrinology. 2012;153:4278–4289. doi: 10.1210/en.2012-1127. [DOI] [PubMed] [Google Scholar]
- 48.Bartz R, Zehmer JK, Zhu M, Chen Y, Serrero G, Zhao Y, Liu P. Dynamic Activity of Lipid Droplets: Protein Phosphorylation and GTP-Mediated Protein Translocation. J Proteome Res. 2007;6:3256–3265. doi: 10.1021/pr070158j. [DOI] [PubMed] [Google Scholar]
- 49.Kim SC, Chen Y, Mirza S, Xu Y, Lee J, Liu P, Zhao Y. A clean, more efficient method for in-solution digestion of protein mixtures without detergent or urea. J Proteome Res. 2006;5:3446–3452. doi: 10.1021/pr0603396. [DOI] [PubMed] [Google Scholar]
- 50.Kienesberger PC, Oberer M, Lass A, Zechner R. Mammalian patatin domain containing proteins: a family with diverse lipolytic activities involved in multiple biological functions. J Lipid Res. 2009;50(Suppl):S63–S68. doi: 10.1194/jlr.R800082-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lord CC, Thomas G, Brown JM. Mammalian alpha beta hydrolase domain (ABHD) proteins: Lipid metabolizing enzymes at the interface of cell signaling and energy metabolism. Biochim Biophys Acta. 2013;1831:792–802. doi: 10.1016/j.bbalip.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas G, Brown AL, Brown JM. In vivo metabolite profiling as a means to identify uncharacterized lipase function: recent success stories within the alpha beta hydrolase domain (ABHD) enzyme family. Biochim Biophys Acta. 2014;1841:1097–1101. doi: 10.1016/j.bbalip.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quiroga AD, Lehner R. Role of endoplasmic reticulum neutral lipid hydrolases. Trends Endocrinol Metab. 2011;22:218–225. doi: 10.1016/j.tem.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Quiroga AD, Lehner R. Liver triacylglycerol lipases. Biochim Biophys Acta. 2012;1821:762–769. doi: 10.1016/j.bbalip.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Brasaemle DL, Rubin B, Harten IA, Gruia-Gray J, Kimmel AR, Londos C. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J Biol Chem. 2000;275:38486–38493. doi: 10.1074/jbc.M007322200. [DOI] [PubMed] [Google Scholar]
- 56.Tansey JT, Huml AM, Vogt R, Davis KE, Jones JM, Fraser KA, Brasaemle DL, Kimmel AR, Londos C. Functional studies on native and mutated forms of perilipins. A role in protein kinase A-mediated lipolysis of triacylglycerols. J Biol Chem. 2003;278:8401–8406. doi: 10.1074/jbc.M211005200. [DOI] [PubMed] [Google Scholar]
- 57.Martinez-Botas J, Anderson JB, Tessier D, Lapillonne A, Chang BH, Quast MJ, Gorenstein D, Chen KH, Chan L. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat Genet. 2000;26:474–479. doi: 10.1038/82630. [DOI] [PubMed] [Google Scholar]
- 58.Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR, Londos C. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci U S A. 2001;98:6494–6499. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gandotra S, Lim K, Girousse A, Saudek V, O’Rahilly S, Savage DB. Human frame shift mutations affecting the carboxyl terminus of perilipin increase lipolysis by failing to sequester the adipose triglyceride lipase (ATGL) coactivator AB-hydrolase-containing 5 (ABHD5) J Biol Chem. 2011;286:34998–35006. doi: 10.1074/jbc.M111.278853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gandotra S, Le DC, Bottomley W, Cervera P, Giral P, Reznik Y, Charpentier G, Auclair M, Delepine M, Barroso I, Semple RK, Lathrop M, Lascols O, Capeau J, O’Rahilly S, Magre J, Savage DB, Vigouroux C. Perilipin deficiency and autosomal dominant partial lipodystrophy. N Engl J Med. 2011;364:740–748. doi: 10.1056/NEJMoa1007487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kozusko K, Tsang VH, Bottomley W, Cho YH, Gandotra S, Mimmack M, Lim K, Isaac I, Patel S, Saudek V, O’Rahilly S, Srinivasan S, Greenfield JR, Barroso I, Campbell LV, Savage DB. Clinical and molecular characterization of a novel PLIN1 frameshift mutation identified in patients with familial partial lipodystrophy. Diabetes. 2015;64:299–310. doi: 10.2337/db14-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garcia A, Subramanian V, Sekowski A, Bhattacharyya S, Love MW, Brasaemle DL. The amino and carboxyl termini of perilipin a facilitate the storage of triacylglycerols. J Biol Chem. 2004;279:8409–8416. doi: 10.1074/jbc.M311198200. [DOI] [PubMed] [Google Scholar]
- 63.Ahmad F, Degerman E, Manganiello VC. Cyclic nucleotide phosphodiesterase 3 signaling complexes. Horm Metab Res. 2012;44:776–785. doi: 10.1055/s-0032-1312646. [DOI] [PubMed] [Google Scholar]
- 64.Degerman E, Ahmad F, Chung YW, Guirguis E, Omar B, Stenson L, Manganiello V. From PDE3B to the regulation of energy homeostasis. Curr Opin Pharmacol. 2011;11:676–682. doi: 10.1016/j.coph.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi SM, Tucker DF, Gross DN, Easton RM, DiPilato LM, Dean AS, Monks BR, Birnbaum MJ. Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Mol Cell Biol. 2010;30:5009–5020. doi: 10.1128/MCB.00797-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J, Hupfeld CJ, Taylor SS, Olefsky JM, Tsien RY. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature. 2005;437:569–573. doi: 10.1038/nature04140. [DOI] [PubMed] [Google Scholar]
- 67.Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, Bhattacharyya S, Shapiro L, Dolios G, Wang R, Lisanti MP, Brasaemle DL. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J Biol Chem. 2004;279:42062–42071. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- 68.Yamaguchi T, Omatsu N, Matsushita S, Osumi T. CGI-58 Interacts with Perilipin and Is Localized to Lipid Droplets: POSSIBLE INVOLVEMENT OF CGI-58 MISLOCALIZATION IN CHANARIN-DORFMAN SYNDROME. J Biol Chem. 2004;279:30490–30497. doi: 10.1074/jbc.M403920200. [DOI] [PubMed] [Google Scholar]
- 69.Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem. 2007;282:5726–5735. doi: 10.1074/jbc.M610580200. [DOI] [PubMed] [Google Scholar]
- 70.Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) J Biol Chem. 2009;284:34538–34544. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taylor SS, Ilouz R, Zhang P, Kornev AP. Assembly of allosteric macromolecular switches: lessons from PKA. Nat Rev Mol Cell Biol. 2012;13:646–658. doi: 10.1038/nrm3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–11346. [PubMed] [Google Scholar]
- 73.Pidoux G, Witczak O, Jarnaess E, Myrvold L, Urlaub H, Stokka AJ, Kuntziger T, Tasken K. Optic atrophy 1 is an A-kinase anchoring protein on lipid droplets that mediates adrenergic control of lipolysis. EMBO J. 2011;30:4371–4386. doi: 10.1038/emboj.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Humphrey SJ, Yang G, Yang P, Fazakerley DJ, Stockli J, Yang JY, James DE. Dynamic adipocyte phosphoproteome reveals that Akt directly regulates mTORC2. Cell Metab. 2013;17:1009–1020. doi: 10.1016/j.cmet.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villen J, Haas W, Sowa ME, Gygi SP. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143:1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kanshin E, Wang S, Ashmarina L, Fedjaev M, Nifant’ev I, Mitchell GA, Pshezhetsky AV. The stoichiometry of protein phosphorylation in adipocyte lipid droplets: analysis by N-terminal isotope tagging and enzymatic dephosphorylation. Proteomics. 2009;9:5067–5077. doi: 10.1002/pmic.200800861. [DOI] [PubMed] [Google Scholar]
- 77.Shinoda K, Ohyama K, Hasegawa Y, Chang HY, Ogura M, Sato A, Hong H, Hosono T, Sharp LZ, Scheel DW, Graham M, Ishihama Y, Kajimura S. Phosphoproteomics Identifies CK2 as a Negative Regulator of Beige Adipocyte Thermogenesis and Energy Expenditure. Cell Metab. 2015;22:997–1008. doi: 10.1016/j.cmet.2015.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Souza SC, Muliro KV, Liscum L, Lien P, Yamamoto MT, Schaffer JE, Dallal GE, Wang X, Kraemer FB, Obin M, Greenberg AS. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J Biol Chem. 2002;277:8267–8272. doi: 10.1074/jbc.M108329200. [DOI] [PubMed] [Google Scholar]
- 79.Wang H, Hu LP, Dalen K, Dorward H, Marcinkiewicz A, Russell D, Gong DW, Londos C, Yamaguchi T, Holm C, Rizzo MA, Brasaemle D, Sztalryd C. Activation of Hormone-sensitive Lipase Requires Two Steps, Protein Phosphorylation and Binding to the PAT-1 Domain of Lipid Droplet Coat Proteins. Journal of Biological Chemistry. 2009;284:32116–32125. doi: 10.1074/jbc.M109.006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen WJ, Patel S, Miyoshi H, Greenberg AS, Kraemer FB. Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. J Lipid Res. 2009;50:2306–2313. doi: 10.1194/jlr.M900176-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sahu-Osen A, Montero-Moran G, Schittmayer M, Fritz K, Dinh A, Chang YF, McMahon D, Boeszoermenyi A, Cornaciu I, Russell D, Oberer M, Carman GM, Birner-Gruenberger R, Brasaemle DL. CGI-58/ABHD5 is phosphorylated on Ser239 by protein kinase A: control of subcellular localization. J Lipid Res. 2015;56:109–121. doi: 10.1194/jlr.M055004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grabner GF, Zimmermann R, Schicho R, Taschler U. Monoglyceride lipase as a drug target: At the crossroads of arachidonic acid metabolism and endocannabinoid signaling. Pharmacol Ther. 2017;175:35–46. doi: 10.1016/j.pharmthera.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Drevinge C, Dalen KT, Mannila MN, Tang MS, Stahlman M, Klevstig M, Lundqvist A, Mardani I, Haugen F, Fogelstrand P, Adiels M, Asin-Cayuela J, Ekestam C, Gadin JR, Lee YK, Nebb H, Svedlund S, Johansson BR, Hulten LM, Romeo S, Redfors B, Omerovic E, Levin M, Gan LM, Eriksson P, Andersson L, Ehrenborg E, Kimmel AR, Boren J, Levin MC. Perilipin 5 is protective in the ischemic heart. Int J Cardiol. 2016;219:446–454. doi: 10.1016/j.ijcard.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 85.Kuramoto K, Okamura T, Yamaguchi T, Nakamura TY, Wakabayashi S, Morinaga H, Nomura M, Yanase T, Otsu K, Usuda N, Matsumura S, Inoue K, Fushiki T, Kojima Y, Hashimoto T, Sakai F, Hirose F, Osumi T. Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J Biol Chem. 2012;287:23852–23863. doi: 10.1074/jbc.M111.328708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mason RR, Mokhtar R, Matzaris M, Selathurai A, Kowalski GM, Mokbel N, Meikle PJ, Bruce CR, Watt MJ. PLIN5 deletion remodels intracellular lipid composition and causes insulin resistance in muscle. Mol Metab. 2014;3:652–663. doi: 10.1016/j.molmet.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pollak NM, Schweiger M, Jaeger D, Kolb D, Kumari M, Schreiber R, Kolleritsch S, Markolin P, Grabner GF, Heier C, Zierler KA, Ruelicke T, Zimmermann R, Lass A, Zechner R, Haemmerle G. Cardiac-specific overexpression of perilipin 5 provokes severe cardiac steatosis via the formation of a lipolytic barrier. J Lipid Res. 2013;54:1092–1102. doi: 10.1194/jlr.M034710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang H, Sreenivasan U, Gong DW, O’Connell KA, Dabkowski ER, Hecker PA, Ionica N, Konig M, Mahurkar A, Sun Y, Stanley WC, Sztalryd C. Cardiomyocyte specific perilipin 5 over expression leads to myocardial steatosis, and modest cardiac dysfunction. J Lipid Res. 2013;54:953–965. doi: 10.1194/jlr.M032466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang C, Zhao Y, Gao X, Li L, Yuan Y, Liu F, Zhang L, Wu J, Hu P, Zhang X, Gu Y, Xu Y, Wang Z, Li Z, Zhang H, Ye J. Perilipin 5 improves hepatic lipotoxicity by inhibiting lipolysis. Hepatology. 2015;61:870–882. doi: 10.1002/hep.27409. [DOI] [PubMed] [Google Scholar]
- 90.Bosma M, Minnaard R, Sparks LM, Schaart G, Losen M, de Baets MH, Duimel H, Kersten S, Bickel PE, Schrauwen P, Hesselink MK. The lipid droplet coat protein perilipin 5 also localizes to muscle mitochondria. Histochem Cell Biol. 2012;137:205–216. doi: 10.1007/s00418-011-0888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harris LA, Skinner JR, Shew TM, Pietka TA, Abumrad NA, Wolins NE. Perilipin 5-Driven Lipid Droplet Accumulation in Skeletal Muscle Stimulates the Expression of Fibroblast Growth Factor 21. Diabetes. 2015;64:2757–2768. doi: 10.2337/db14-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pollak NM, Jaeger D, Kolleritsch S, Zimmermann R, Zechner R, Lass A, Haemmerle G. The interplay of protein kinase A and perilipin 5 regulates cardiac lipolysis. J Biol Chem. 2015;290:1295–1306. doi: 10.1074/jbc.M114.604744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Granneman JG, Moore HP, Mottillo EP, Zhu Z. Functional interactions between Mldp (LSDP5) and Abhd5 in the control of intracellular lipid accumulation. J Biol Chem. 2009;284:3049–3057. doi: 10.1074/jbc.M808251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Granneman JG, Moore HP, Mottillo EP, Zhu Z, Zhou L. Interactions of perilipin-5 (Plin5) with adipose triglyceride lipase. J Biol Chem. 2011;286:5126–5135. doi: 10.1074/jbc.M110.180711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoffman NJ, Parker BL, Chaudhuri R, Fisher-Wellman KH, Kleinert M, Humphrey SJ, Yang P, Holliday M, Trefely S, Fazakerley DJ, Stockli J, Burchfield JG, Jensen TE, Jothi R, Kiens B, Wojtaszewski JF, Richter EA, James DE. Global Phosphoproteomic Analysis of Human Skeletal Muscle Reveals a Network of Exercise-Regulated Kinases and AMPK Substrates. Cell Metab. 2015;22:922–935. doi: 10.1016/j.cmet.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lundby A, Andersen MN, Steffensen AB, Horn H, Kelstrup CD, Francavilla C, Jensen LJ, Schmitt N, Thomsen MB, Olsen JV. In vivo phosphoproteomics analysis reveals the cardiac targets of beta-adrenergic receptor signaling. Sci Signal. 2013;6:rs11. doi: 10.1126/scisignal.2003506. [DOI] [PubMed] [Google Scholar]
- 97.Wilson-Grady JT, Haas W, Gygi SP. Quantitative comparison of the fasted and re-fed mouse liver phosphoproteomes using lower pH reductive dimethylation. Methods. 2013;61:277–286. doi: 10.1016/j.ymeth.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 98.Wang H, Sreenivasan U, Hu H, Saladino A, Polster BM, Lund LM, Gong DW, Stanley WC, Sztalryd C. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J Lipid Res. 2011;52:2159–2168. doi: 10.1194/jlr.M017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bosma M, Sparks LM, Hooiveld GJ, Jorgensen JA, Houten SM, Schrauwen P, Kersten S, Hesselink MK. Overexpression of PLIN5 in skeletal muscle promotes oxidative gene expression and intramyocellular lipid content without compromising insulin sensitivity. Biochim Biophys Acta. 2013;1831:844–852. doi: 10.1016/j.bbalip.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 100.Gallardo-Montejano VI, Saxena G, Kusminski CM, Yang C, McAfee JL, Hahner L, Hoch K, Dubinsky W, Narkar VA, Bickel PE. Nuclear Perilipin 5 integrates lipid droplet lipolysis with PGC-1alpha/SIRT1-dependent transcriptional regulation of mitochondrial function. Nat Commun. 2016;7:12723. doi: 10.1038/ncomms12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bell M, Wang H, Chen H, McLenithan JC, Gong DW, Yang RZ, Yu D, Fried SK, Quon MJ, Londos C, Sztalryd C. Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes. 2008;57:2037–2045. doi: 10.2337/db07-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Listenberger LL, Ostermeyer-Fay AG, Goldberg EB, Brown WJ, Brown DA. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J Lipid Res. 2007;48:2751–2761. doi: 10.1194/jlr.M700359-JLR200. [DOI] [PubMed] [Google Scholar]
- 103.Chang BH, Li L, Paul A, Taniguchi S, Nannegari V, Heird WC, Chan L. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol Cell Biol. 2006;26:1063–1076. doi: 10.1128/MCB.26.3.1063-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Libby AE, Bales E, Orlicky DJ, McManaman JL. Perilipin-2 Deletion Impairs Hepatic Lipid Accumulation by Interfering with Sterol Regulatory Element-binding Protein (SREBP) Activation and Altering the Hepatic Lipidome. J Biol Chem. 2016;291:24231–24246. doi: 10.1074/jbc.M116.759795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McManaman JL, Bales ES, Orlicky DJ, Jackman M, MacLean PS, Cain S, Crunk AE, Mansur A, Graham CE, Bowman TA, Greenberg AS. Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J Lipid Res. 2013;54:1346–1359. doi: 10.1194/jlr.M035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Russell TD, Palmer CA, Orlicky DJ, Bales ES, Chang BH, Chan L, McManaman JL. Mammary glands of adipophilin-null mice produce an amino-terminally truncated form of adipophilin that mediates milk lipid droplet formation and secretion. J Lipid Res. 2008;49:206–216. doi: 10.1194/jlr.M700396-JLR200. [DOI] [PubMed] [Google Scholar]
- 107.Russell TD, Schaack J, Orlicky DJ, Palmer C, Chang BH, Chan L, McManaman JL. Adipophilin regulates maturation of cytoplasmic lipid droplets and alveolae in differentiating mammary glands. J Cell Sci. 2011;124:3247–3253. doi: 10.1242/jcs.082974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wolins NE, Quaynor BK, Skinner JR, Schoenfish MJ, Tzekov A, Bickel PE. S3–12, adipophilin, and TIP47 package lipid in adipocytes. J Biol Chem. 2005;280:19146–19155. doi: 10.1074/jbc.M500978200. [DOI] [PubMed] [Google Scholar]
- 109.Carr RM, Patel RT, Rao V, Dhir R, Graham MJ, Crooke RM, Ahima RS. Reduction of TIP47 improves hepatic steatosis and glucose homeostasis in mice. Am J Physiol Regul Integr Comp Physiol. 2012;302:R996–1003. doi: 10.1152/ajpregu.00177.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bulankina AV, Deggerich A, Wenzel D, Mutenda K, Wittmann JG, Rudolph MG, Burger KN, Honing S. TIP47 functions in the biogenesis of lipid droplets. J Cell Biol. 2009;185:641–655. doi: 10.1083/jcb.200812042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reid BN, Ables GP, Otlivanchik OA, Schoiswohl G, Zechner R, Blaner WS, Goldberg IJ, Schwabe RF, Chua SC, Jr, Huang LS. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J Biol Chem. 2008;283:13087–13099. doi: 10.1074/jbc.M800533200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Finn PF, Dice JF. Proteolytic and lipolytic responses to starvation. Nutrition. 2006;22:830–844. doi: 10.1016/j.nut.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 113.Gibbons GF, Islam K, Pease RJ. Mobilisation of triacylglycerol stores. Biochim Biophys Acta. 2000;1483:37–57. doi: 10.1016/s1388-1981(99)00182-1. [DOI] [PubMed] [Google Scholar]
- 114.Cingolani F, Czaja MJ. Regulation and Functions of Autophagic Lipolysis. Trends Endocrinol Metab. 2016;27:696–705. doi: 10.1016/j.tem.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jaishy B, Abel ED. Lipids, lysosomes, and autophagy. J Lipid Res. 2016;57:1619–1635. doi: 10.1194/jlr.R067520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tasset I, Cuervo AM. Role of chaperone-mediated autophagy in metabolism. FEBS J. 2016;283:2403–2413. doi: 10.1111/febs.13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hodges BDM, Wu CC. Proteomic insights into an expanded cellular role for cytoplasmic lipid droplets. Journal of Lipid Research. 2010;51:262–273. doi: 10.1194/jlr.R003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Na H, Zhang P, Ding Y, Yang L, Wang Y, Zhang H, Xie Z, Yang F, Cichello S, Liu P. Proteomic studies of isolated lipid droplets from bacteria, C. elegans, and mammals. Methods Cell Biol. 2013;116:1–14. doi: 10.1016/B978-0-12-408051-5.00001-2. [DOI] [PubMed] [Google Scholar]
- 119.Yang L, Ding Y, Chen Y, Zhang S, Huo C, Wang Y, Yu J, Zhang P, Na H, Zhang H, Ma Y, Liu P. The proteomics of lipid droplets: structure, dynamics, and functions of the organelle conserved from bacteria to humans. J Lipid Res. 2012;53:1245–1253. doi: 10.1194/jlr.R024117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schroeder B, Schulze RJ, Weller SG, Sletten AC, Casey CA, McNiven MA. The small GTPase Rab7 as a central regulator of hepatocellular lipophagy. Hepatology. 2015;61:1896–1907. doi: 10.1002/hep.27667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lizaso A, Tan KT, Lee YH. beta-adrenergic receptor-stimulated lipolysis requires the RAB7-mediated autolysosomal lipid degradation. Autophagy. 2013;9:1228–1243. doi: 10.4161/auto.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tsai TH, Chen E, Li L, Saha P, Lee HJ, Huang LS, Shelness GS, Chan L, Chang BH. The constitutive lipid droplet protein PLIN2 regulates autophagy in liver. Autophagy. 2017:1–15. doi: 10.1080/15548627.2017.1319544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997;38:2249–2263. [PubMed] [Google Scholar]
- 125.Brasaemle DL, Barber T, Kimmel AR, Londos C. Post-translational regulation of perilipin expression. Stabilization by stored intracellular neutral lipids. J Biol Chem. 1997;272:9378–9387. doi: 10.1074/jbc.272.14.9378. [DOI] [PubMed] [Google Scholar]
- 126.Xu G, Sztalryd C, Lu X, Tansey JT, Gan J, Dorward H, Kimmel AR, Londos C. Post-translational regulation of adipose differentiation-related protein by the ubiquitin/proteasome pathway. J Biol Chem. 2005;280:42841–42847. doi: 10.1074/jbc.M506569200. [DOI] [PubMed] [Google Scholar]
- 127.Kovsan J, Ben Romano R, Souza SC, Greenberg AS, Rudich A. Regulation of adipocyte lipolysis by degradation of the perilipin protein: Nelfinavir enhances lysosome-mediated perilipin proteolysis. J Biol Chem. 2007;282:21704–21711. doi: 10.1074/jbc.M702223200. [DOI] [PubMed] [Google Scholar]
- 128.Gross DN, Miyoshi H, Hosaka T, Zhang HH, Pino EC, Souza S, Obin M, Greenberg AS, Pilch PF. Dynamics of lipid droplet-associated proteins during hormonally stimulated lipolysis in engineered adipocytes: stabilization and lipid droplet binding of adipocyte differentiation-related protein/adipophilin. Mol Endocrinol. 2006;20:459–466. doi: 10.1210/me.2005-0323. [DOI] [PubMed] [Google Scholar]
- 129.Masuda Y, Itabe H, Odaki M, Hama K, Fujimoto Y, Mori M, Sasabe N, Aoki J, Arai H, Takano T. ADRP/adipophilin is degraded through the proteasome-dependent pathway during regression of lipid-storing cells. J Lipid Res. 2006;47:87–98. doi: 10.1194/jlr.M500170-JLR200. [DOI] [PubMed] [Google Scholar]
- 130.Xu G, Sztalryd C, Londos C. Degradation of perilipin is mediated through ubiquitination-proteasome pathway. Biochim Biophys Acta. 2006;1761:83–90. doi: 10.1016/j.bbalip.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 131.Takahashi Y, Shinoda A, Kamada H, Shimizu M, Inoue J, Sato R. Perilipin2 plays a positive role in adipocytes during lipolysis by escaping proteasomal degradation. Sci Rep. 2016;6:20975. doi: 10.1038/srep20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kaushik S, Cuervo AM. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol. 2015;17:759–770. doi: 10.1038/ncb3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kaushik S, Cuervo AM. AMPK-dependent phosphorylation of lipid droplet protein PLIN2 triggers its degradation by CMA. Autophagy. 2016;12:432–438. doi: 10.1080/15548627.2015.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schneider JL, Suh Y, Cuervo AM. Deficient chaperone-mediated autophagy in liver leads to metabolic dysregulation. Cell Metab. 2014;20:417–432. doi: 10.1016/j.cmet.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eichmann TO, Kumari M, Haas JT, Farese RV, Jr, Zimmermann R, Lass A, Zechner R. Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone-sensitive lipase, and diacylglycerol-O-acyltransferases. J Biol Chem. 2012;287:41446–41457. doi: 10.1074/jbc.M112.400416. [DOI] [PMC free article] [PubMed] [Google Scholar]