Summary
Cataract is one of the most important causes of blindness worldwide, with age‐related cataract being the most common one. Agents preventing cataract formation are urgently required. Substantial evidences point out aggravated oxidative stress as a vital factor for cataract formation. Nuclear factor (erythroid‐derived 2)‐like 2 (Nrf2)/Kelch‐like erythroid‐cell‐derived protein with CNC homology (ECH)‐associated protein 1 (Keap1) system is considered as one of the main cellular defense mechanisms against oxidative stresses. This review discusses the role of Nrf2 pathway in the prevention of cataracts and highlights that Nrf2 suppressors may augment oxidative stress of the lens, and Nrf2 inducers may decrease the oxidative stress and prevent the cataract formation. Thus, Nrf2 may serve as a promising therapeutic target for cataract treatment.
Keywords: antioxidant response element, cataracts, Keap1, lenses, Nrf2, oxidative stress
Abbreviations
- AKT
serine–threonine kinase
- ALCAR
acetyl‐l‐carnitine
- ARCs
age‐related cataracts
- ARE
antioxidant response element
- ATF4
activating transcription factor 4
- ATF6
activating transcription factor 6
- BiP
immunoglobulin heavy chain binding protein
- CHOP
CCAAT/enhancer‐binding protein‐homologous protein
- eIF2α
eukaryotic initiation factor 2α
- ER
endoplasmic reticulum
- ERK
extracellular signal‐regulated kinase
- GR
glutathione reductase
- GSH
glutathione
- GST
glutathione‐S‐transferase
- H2O2
hydrogen peroxide
- Hcy
homocysteine
- HLECs
human lens epithelial cells
- HO‐1
heme oxygenase‐1
- IRE1α
Inositol‐requiring kinase 1α
- Keap1
Kelch‐like erythroid‐cell‐derived protein with CNC homology (ECH)‐associated protein 1
- LECs
lens epithelial cells
- MAPK
mitogen‐activated protein kinases
- MGO
methylglyoxal
- NBP
DL‐3‐n‐butylphthalide
- Nrf2
transcription factors like nuclear factor (erythroid‐derived 2)‐like 2
- O2
oxygen
- PERK
protein kinase RNA (PKR)‐like endoplasmic reticulum kinase
- PI3K
phosphatidylinositol 3‐kinase
- RLM
Rosa laevigata Michx
- ROS
reactive oxygen species
- SFN
sulforaphane
- STZ
streptozocin
- TrxR
thioredoxin reductase
- Trx
thioredoxin
- UPR
unfolded protein response
- VPA
Valproic acid
Introduction
Oxidative stress and cataracts
Oxidative stress
Oxidative stress is the outcome of instability of pro‐oxidants and antioxidants (Babizhayev & Yegorov, 2016). Decrease in molecular oxygen (O2) metabolites is considered as ‘reactive oxygen species’ (ROS) on account of the higher reactivity in relation to molecular O2. ROS are mainly short‐lived and highly reactive molecules, which are induced intracellularly through different pathways, such as by‐products during normal aerobic metabolism or as messengers in different cellular signaling pathways. In addition, ROS can also be produced by cells from exogenous sources, either being generated as a result of the cells’ experience with several environmental stimuli or absorbed instantly from the extracellular milieu (Babizhayev & Yegorov, 2016). Oxidative stress is important to regulate normal physiological functions associated with cell cycle, migration, and cell death (Redza‐Dutordoir & Averill‐Bates, 2016; Schumann et al., 2016). Oxidative stress has been involved in the activation of different transcription factors like nuclear factor (erythroid‐ derived 2)‐like 2 (Nrf2, also known as NFE2L2)/Kelch‐like erythroid‐cell‐derived protein with CNC homology (ECH)‐associated protein 1 (Keap1), mitogen‐activated protein kinases (MAPK), which are involved in the activation of cell survival and death processes. On the contrary, when cell oxidant production overwhelms the intrinsic antioxidant detoxification capacity, damage to cellular macromolecules like lipids, proteins, nucleic acids, membranes occurs. Oxidative stress is believed to involve in the pathogenesis of various aging‐related diseases, and sight‐threatening eye diseases (Babizhayev & Yegorov, 2016). Clinical evidence supports a connection role of oxidative stress with diseases, but the exact underlying molecular mechanisms are still not entirely understood.
Cataract vs. oxidative stress
Human lens is composed of lens capsule (outer layer), lens epithelium (middle layer), and lens fibers (inner layer). Human lens epithelium is composed of a monolayer of lens epithelial cells (LECs) with foremost metabolically activities (such as oxidation, cross‐linking). LECs migrate to the equator portion of the lens, transform into lens fibers, gradually compress centrally, and form the nuclear opacity with aging increase.
Cataracts, the opacification of eye lens, are the foremost cause of blindness worldwide. Aging is the foremost cause of the cataracts, followed by other causes such as environmental factors (trauma, radiation exposure etc.), genetic susceptibility, and ocular diseases (Liu et al., 2017b). Age‐related cataract can be divided into cortical (wedge‐shaped, starting from the cortex and extending to the center), posterior subcapsular (plaque‐like, locating in the axial posterior cortical layer), and nuclear cataracts according to the opacity location. Ultraviolet irradiation and oxidative damage are considered as predominant contributors for cataract formation. Aging eye seems to be at great risk to oxidative stress (Babizhayev & Yegorov, 2016), and LECs are inclined to ROS (Vinson, 2006; Bassnett et al., 2011). Human lens consists of α, β, and γ crystalline proteins, and oxidative stress may lead to aggregate of these proteins, developing clumps, and results in loss of transparency with increasing age, leading to cataract formation. Besides oxidation of crystalline proteins, DNA damage, membrane lipid peroxidation, and imbalance in calcium homeostasis all contribute to the oxidative stress‐induced cataract formation. Cataracts also are prevalent in diabetes where the level of superoxide in the mitochondria is increased owing to hyperglycemia (Vinson, 2006).
Although cataract surgery has been considered very successful, surgery‐associated complications unavoidably cause irreversible blindness. Thus, it is imperative to identify strategies and antioxidants that would prevent cataract formation based on the association between cataract and oxidative stress; natural compounds namely vitamin C, vitamin E, and curcumin have been considered as promising antioxidants in preventing the cataract formation.
Nrf2‐Keap1 system and cataracts
Classic Nrf2‐Keap1 system and oxidative stresses
Nrf2‐Keap1 system is known as one of the main cellular defense mechanisms against oxidative stresses. Nrf2 is a vital nuclear transcriptional inducer (Cullinan & Diehl, 2004), which binds to the antioxidant response element (ARE) in the DNA promoter and controls the transcriptions of many antioxidant genes, including glutathione‐S‐transferase (GST), glutathione reductase (GR), thioredoxin reductase (TrxR) (Rushmore et al., 1991; Yu et al., 2010). Regulated by protein kinase RNA (PKR)‐like endoplasmic reticulum kinase (PERK) (Cullinan & Diehl, 2004), MAPK (Yu et al., 2000), protein kinase C (Bloom & Jaiswal, 2003), phosphatidylinositol 3‐kinase (PI3K), etc. (Kang et al., 2002), Nrf2 serves as a molecular switch turning on/off of the Nrf2‐mediated antioxidant systems. Keap1 serves as an oxidative stress sensor and a primary Nrf2 inhibitor. The promoter of Keap1 gene contains a key CpG island, with 68 CpG dinucleotides in total, and locates between −460 and +341. DNA demethylation in the Keap1 promoter eventually disturbs the Nrf2/Keap1‐dependent antioxidant protection (Palsamy et al., 2012). Keap1 constantly targets Nrf2 for ubiquitination and subsequent 26S proteasomal degradation, maintaining Nrf2 at basal level. Under favorable conditions, Nrf2 location is restricted to the cytoplasm by binding with Keap1 in the form of Nrf2/Keap1 complex. Under stressed conditions, such as oxidative or endoplasmic reticulum (ER) stress, Nrf2 separates from Keap1, phosphorylates, and translocates into the nucleus, and induces the ARE‐controlled antioxidant gene transcription, which initiates detoxification of ROS through regulation of GSH (Nakagami, 2016).
Nrf2‐Keap1 system and cataracts
Aging is a key risk factor for most disorders such as cataracts and the causes remain elusive. ROS, which may cause the oxidative damage to proteins, lipids (e.g., lipid peroxidation), and DNA (e.g., oxidation, methylation), has been assigned to be one of the main damage concepts to the aging. Senescent cells those have lost the ability to divide contribute to age‐related diseases, and their selective removal increases physiological function and extends longevity (Naylor et al., 2013). Caloric restriction may enhance lifespan and delay age‐related diseases (Bishop & Guarente, 2007). As one of the major regulators of system defense against the oxidative stress, Nrf2 is also a vital regulator of the beneficial effects of calorie restriction, and it correlates with the increased autophagy and decreased induction of senescence cell. Nrf2 and its target genes showed a decrease with age in rats (Shih & Yen, 2007). The levels of total as well as nuclear Nrf2 protein were drastically lower in the liver of older vs. younger rats (Suh et al., 2004), indicating that Nrf2 is a protective factor during aging. Tomatidine, abundant in tomatoes, extended lifespan and healthspan in Caenorhabditis elegans (an animal aging model sharing vital longevity related pathways with mammals) via the Nrf2 pathway (Fang et al., 2017). Nrf2 activator protandim, a botanical extracts (Velmurugan et al., 2009), increased longevity in mice (Strong et al., 2016), and orally protandim supplements robustly elevated superoxide dismutase (SOD) and catalase level in erythrocyte of healthy humans (Nelson et al., 2006). Fumarate, the citric acid cycle metabolite provided cardioprotection via Nrf2 activation in mice (Ashrafian et al., 2012). Nrf2 also mediates cancer protection induced by caloric restriction (Pearson et al., 2008). The long‐lived species (e.g., naked mole rat) may rapidly respond and neutralize the stressors (e.g., endogenous ROS and environmental stressors), retarding the age‐related diseases (e.g., cataracts, neurodegeneration, cardiovascular diseases, cancers). Keap1 and β‐transducin repeat‐containing protein are both negative Nrf2 regulators which target Nrf2 for degradation, and their different expression profiles may contribute the divergent longevity of species (Lewis et al., 2015). These results indicate that Nrf2 activation may confer stress and extend lifespan, motivating researches into delaying and preventing aging.
Rapamycin is an mTOR inhibitor, and rapamycin‐based therapies have been studies for combating aging (Arriola Apelo & Lamming, 2016). Rapamycin extended lifespan in mice with retarded age‐associated activity declining and postponed cancer caused death (Harrison et al., 2009; Miller et al., 2011). RNA interference against mTOR in C. elegans extended worm lifespan (Vellai et al., 2003) and rapamycin enhanced stress resistance and longevity in C. elegans by regulating SKN‐1/Nrf pathway (Robida‐Stubbs et al., 2012). Rapamycin regulated cell cycle arrest in mouse fibroblasts via the upregulation of Nrf2, but inhibited the phenotype of senescent cells in a Nrf2‐independent mechanism (Wang et al., 2017), indicating that although Nrf2 possesses the ability to combat the senescence cell, cell senescence is a complicated process and further studies on new anti‐aging compounds are still needed.
Oxidative stress and failure of the antioxidants’ protection are considered as 2 key contributors to age‐related cataract formation. LECs have powerful defense mechanisms against oxidation, with Nrf2 as a vital one, which can eliminate ROS, maintaining the redox balance. The basic level of Nrf2 in the lens is very low (von Otter et al., 2010). Overproduction of ROS leads to the suppression of Nrf2‐dependent antioxidant protection in LECs (Elanchezhian et al., 2012b); 2 haplotype alleles of NFE2L2 were found to be associated with cataract, indicating that NFE2L2 gene variation may affect cataract progression, although not convinced enough to support NFE2L2 or KEAP1 as cataract susceptibility genes (von Otter et al., 2010). A dramatically decreased level of Nrf2 (protein and gene), a significantly increased level of Keap1 (protein and gene), and an highly elevated levels of DNA demethylation in the Keap1 promoter were found in cultured HLECs, human aging lenses, and diabetic cataractous lenses (Palsamy et al., 2012). On the contrary, DNA methylation was found in the clear human lens and cultured HLECs (SRA01/04), indicating that DNA promoter demethylation of Keap1 gene is an age‐dependent behavior, and it is also a crucial mechanism for cataract formation (Gao et al., 2015). The demethylation of the Keap1 promoter activates the expression of Keap1 protein, accelerates Nrf2 proteasomal degradation (Palsamy et al., 2012), and abolishes Nrf2 activity, leading to the failure of Nrf2‐dependent antioxidant system and resulting in the cataract formation eventually (Gao et al., 2015). These results further indicate that Nrf2 inducers might also serve as an anticataract formation compounds.
For better understanding the mechanism of cataract formation and well supporting the exploration of the anti‐aging medications in treating cataracts, here, we review recent scientific developments illustrating the relationship between Nrf2 and cataracts and discuss the future insight in this field. Sodium selenite, homocysteine (Hcy), valproic acid (VPA), lack/excessive O2, hypoglycemia under hypoxia, methylglyoxal (MGO) are reported as Nrf2 suppressors (Fig. 1), which aggravate cataract formation by inhibiting the Nrf2‐dependent antioxidant protection in lenses (Table 1). Hyperoside, morin, Acetyl‐l‐carnitine (ALCAR), sulforaphane (SFN), DL‐3‐n‐butylphthalide (NBP), and Rosa laevigata Michx. (RLM) extracts are reported as Nrf2 inducers (Fig. 1), which activate Nrf2 and protect lenses from oxidation (Table 1). These results indicate that Nrf2 serves as a promising therapeutic target for preventing and delaying the cataract formation.
Figure 1.
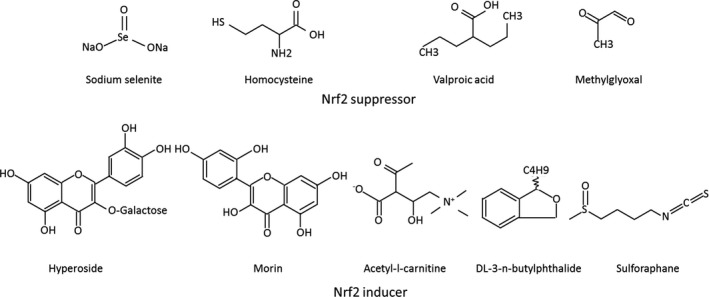
Chemical structure of typical Nrf2 suppressors and inducers tested in cataracts.
Table 1.
Studies on the relationship between Nrf2 inducer/suppressor and cataract models
| Chemical | Type | Level | Models | Results | References |
|---|---|---|---|---|---|
| Hcy (−) | In vitro | Cell | HLECs treated with Hcy | High Hcy‐induced ROS overproduction, ER stress, UPR activation, Ca2+ releases and Nrf2 degradation in LECs | Elanchezhian et al. (2012a) |
| VPA (−) | In vitro | Cell | HLECs treated with VPA | VPA‐induced ROS overproduction, UPR activation, Ca2+ releases and suppressed the Nrf2/Keap1‐dependent catalase and GR expressions, resulting in HLECs death | Palsamy et al. (2014c) |
| Lack/excessive O2 (−) | In vitro | Cell | HLECs cultured under different O2 | ROS, ER stress, keap1, cell death were increased and Nrf2 was decreased in HLECs cultured in 0 or 20% O2 than in 1 or 4% O2 | Zheng et al. (2015) |
| Sodium selenite (−) | In vitro | Cell | HLECs treated with sodium selenite | Sodium selenite‐induced ROS overproduction, ER stress, UPR activation, Ca2+ releases, Keap1 overexpression and Nrf2 degradation, resulting in HLECs death | Palsamy et al. (2014b) |
| Animal | Lenses enucleated from sodium selenite injected rats | Overproduction of ROS in LECs and newly formed lens fiber cells resulted in HLECs death | |||
| Hypoglycemia under hypoxia (−) | In vitro | Cell | HLECs cultured under different O2 | Hypoglycemia under hypoxia induced the UPR, promote cell proliferation and cause loss of GSH in HLECs | Elanchezhian et al. (2012b) |
| Animal | Rat lenses organ cultured under different O2 | UPR was activated by ischemia in rat lenses | |||
| MGO (−) | In vitro | Cell | HLECs treated with MGO | MGO‐induced ROS overproduction, ER stress, UPR activation, Ca2+ releases, Keap1 overexpression and Nrf2 degradation, resulting in HLECs death | Palsamy et al. (2014a) |
| Animal | Nrf2−/−, Nrf2+/+ diabetic mice lenses cultured with MGO | Greater ROS production and more cell death were found in Nrf2−/− diabetic mouse LECs than those of Nrf2+/+ mouse | |||
| In vivo | Human | Human clear lenses and diabetic cataractous lenses | Clear lenses slowly lose 5‐methylcytosine in the Keap1 promoter at a rate of 1% per year. Diabetic cataractous lenses lost 90% of the 5 methylcytosine | ||
| RLM extract (+) | In vitro | Cell | HLECs cultured under high glucose | RLM decreased ROS, elevated MMP protein in HLECs cultured under high glucose through the induction of HO‐1 expression via PI3K/AKT and Nrf2/ARE pathways | Liu et al. (2015) |
| Hyperoside (+) | In vitro | Cell | HLECs treated with hyperoside | Hyperoside increased the HO‐1 expression by activating ERK/Nrf2 pathway | Park et al. (2016) |
| Morin (+) | In vitro | Cell | HLECs treated with Morin | Morin increased the HO‐1 expression by activating ERK/Nrf2 pathway | Park et al. (2013) |
| ALCAR (+) | In vitro | Cell | HLECs treated with Hcy | ALCAR prevented Hcy‐induced‐ER stress, ROS overproduction, UPR activation and cell death in HLECs by activating Nrf2/Keap1 controlled catalase, superoxide dismutase, GPx, GSH expression | Yang et al. (2015) |
| SFN (+) | In vitro | Cell | HLECs treated with H2O2 | SFN inhibited H2O2‐induced apoptosis in FHL124 cells by inducing Nrf2 nucleus translocation | Liu et al. (2013) |
| Animal | Organ cultured porcine lenses | SFN protected against H2O2‐induced opacification | |||
| Animal | Organ cultured mouse lenses | SFN enhanced TrxR activity in mouse lens | Varma et al. (2013) | ||
| NBP (+) | In vivo | Animal | STZ‐induced diabetic cataract rat | NBP improved the cataract scores, increased 2, 4‐dinitrophenylhydrazone, 4‐hydroxynonenal, malondialdehyde, Nrf2, thioredoxin and catalase expression in the lens, and decreased blood glucose, serum malondialdehyde and 8‐Hydroxydeovexyguanosine | Wang et al. (2016) |
Hcy, homocysteine; VPA, valproic acid; MGO, methylglyoxal; RLM, Rosa laevigata Michx; ALCAR, acetyl‐l‐carnitine; SFN, sulforaphane; NBP, DL‐3‐n‐butylphthalide. (−) indicates Nrf2 suppressor and (+) indicates Nrf2 inducer.
The PubMed database was searched on January 19, 2017 with the terms ‘cataract*’, ‘lens*’, ‘nuclear factor erythroid 2‐related factor 2’, ‘Nrf2’, either alone and in combination. All relevant articles were manually picked out, with the most related 24 papers covering the period of 2002–2017.
Nrf2 suppressors and cataracts
Sodium selenite
Selenium is an essential micronutrient that involves in various important biological activities. Superfluous selenium, exposure at high levels (>1 μm), was considered to pose a great toxic threat to organisms for its pro‐oxidant effect. As elucidated in our previous review (Liu et al., 2017a), selenium‐ or sodium selenite‐induced cataract in neonatal rats is the well‐known cataract animal models. In lenses enucleated from sodium selenite‐treated rats, ROS overproduction caused considerable LECs death and cataract formation (Palsamy et al., 2014b). In HLECs, exposure to sodium selenite elevated ER stress activated unfolded protein response (UPR), increased endoplasmic reticulum (ER)‐Ca2+‐release, generated ROS, and demethylated Keap1 DNA promoter, leading to the cell death. Keap1 DNA demethylation of Keap1 promoter increased the Keap1 protein transcription. Overexpression Keap1 protein inhibited the Nrf2 protein via ER‐associated degradation, resulting in the inhibition of Nrf2/Keap1‐dependent antioxidant systems in the sodium selenite‐treated HLECs. Eventually, cataract formed, as the cellular redox status in lens, is altered toward oxidation (Palsamy et al., 2014b). However, further studies should be designed to determine the basis of the putative effect of ER stressors merely on immature LECs as well as explore the mechanism by which the DNA in these cells degenerate following selenite exposure.
VPA
Known as an anticonvulsant drug, VPA, in utero exposure, was also found to induce congenital cataracts (Glover et al., 2002). Promoter DNA demethylation of Keap1 was involved in human cataract formation by suppressing Nrf2‐dependent antioxidant system (Gao et al., 2015). VPA could stimulate ROS production, leading to the transcription of ARE‐driven genes (Kawai & Arinze, 2006). VPA induced ER stress and unfolded protein response (UPR) by activation the ER stress sensor proteins and transcription factor 6 in HLECs, resulting in DNA demethylation of the Keap1 promoter (Palsamy et al., 2014c), which suppresses the Nrf2‐dependent antioxidant system and results in cataract formation.
Lack/excessive O2
The lens is located in a hypoxic environment (0.5–2.3% O2) under normal circumstance (Shui et al., 2006). O2 level with the range of 0.1–1.0% can be defined as severe hypoxia, and the O2 level in lens can effortlessly fall into the severe hypoxia range even in a normal (13–15%) atmospheric O2 supply (Shui & Beebe, 2008), especially for diabetic patients (Holekamp et al., 2006). Either 0 or 20% atmospheric O2 induced significant ROS production and cell death in cultured HLECs, accompanied by increased UPR protein and inhibition of the Nrf2/Keap1‐dependent antioxidant protection in LECs, and the results showed that 1% O2 was appropriate for culturing LECs (Zheng et al., 2015).
Hypoglycemia under hypoxia
Besides hypoxia, the lens was also located in a hypoglycemic environment with the glucose detected in the aqueous fluid of 2–4 mm and 1.6 mm in the vitreous fluid of human eyes (Lundquist & Osterlin, 1994). The lens can develop nuclear cataract rapidly, if glucose is deprived for 48 h (Chylack & Schaefer, 1976). In opposite, extracellular glucose accumulation may also trigger the hyperglycemic toxic effects, and aging lens with diabetes might aggravate these stresses with increased incidence for cataract formation and progression (Vinson, 2006). Considering the fact that lens is an insulin‐independent tissue and not capable to downregulate the glucose transport, the strict application of insulin unavoidably increases the incidence of severe hypoglycemia fourfold to sixfold (Lacherade et al., 2009). Low level of glucose under severe hypoxia induced the production of ROS, UPR activation and inhibited Nrf2/Keap1‐dependent antioxidant enzymes, resulting the LECs apoptosis, revealing a new connection between hypoglycemia under hypoxia and LECs function impairment (Elanchezhian et al., 2012b).
MGO
DNA methylation was found to be completely methylated in HLEC line and clear human lens (Palsamy et al., 2012), while the demethylation of Keap1 promoter DNA was found to be increased in diabetic ARCs (Palsamy et al., 2014a), indicating DNA demethylation of keap1 may be involved in cataract formation. Streptozocin (STZ)‐induced diabetic Nrf2−/− mice exhibited more cell death in lenses and developed cataract earlier than Nrf2−/− mice (Palsamy et al., 2014a). As one of the abnormal reactive aldehydes that generated by hyperglycemia (Palsamy et al., 2014a), MGO induced oxidative stress in cultured HLECs and inhibited Nrf2/Keap1‐regulated GR, catalase protein expression, which eventually changed cellular redox balance toward lens oxidation and resulted in cataract formation (Palsamy et al., 2014a).
Hcy
Hcy is biosynthesized from methionine in a multiple step pathway. An elevated level of serum Hcy is related to the formation of juvenile cataracts and ARCs (Sen et al., 2008). Recently, several studies have illustrated significant increase of Hcy in the plasma of ARCs’ patients (Sen et al., 2008). Elevated level of Hcy also increased protein misfolding in the ER, which led to the UPR (Ron & Walter, 2007; Todd et al., 2008). In agreement with these studies, it was also confirmed that Hcy (100 μm) could induce the generation of ROS and cell death in HLECs (Yang et al., 2015). This study revealed Hcy had the ability to induce the activation of UPR regulated by ER stress, overproduction of ROS, and inhibition of Nrf2/Keap1‐mediated antioxidant protection via demethylation of Keap1 promoter, resulting in cell death in HLECs (Elanchezhian et al., 2012a; Yang et al., 2015).
Nrf2 inducers and cataracts
ALCAR
Beside the well‐known antioxidant and anti‐inflammation effects, ALCAR (the acetyl ester of the trimethylated amino acid L‐carnitine) has also been reported to possess the ability to prevent apoptosis and aging in yeast (Palermo et al., 2010). Moreover, ALCAR has been discovered to prevent the cataract formation in rat models (Elanchezhian et al., 2007). Elanchezhian et al. (2007) also found the protective function of ALCAR in selenite‐induced cataracts in rat. ALCAR increased Nrf2‐regulated antioxidant proteins and decreased ER stress‐mediated proteins in Hcy‐treated cells. A similar effect was also found in expression of the Nrf2 and Keap1 gene (Yang et al., 2015). These results implied that ALCAR is a promising agent in defending the human lens against oxidative stress‐induced injury, thus preventing the cataract formation (Yang et al., 2015). However, further advanced studies are still needed for the development of ALCAR as an anticataract component.
Hyperoside
Hyperoside (quercetin‐3‐D‐galactoside) generally found in Hypericum perforatum L., is a kind of flavonoid, which can inhibit the oxidative stress. Heme oxygenase‐1 (HO‐1), also known as 32‐kDa heat‐shock protein, is one of important enzymatic antioxidants involved in defense systems. Several signaling molecules are regulating the initiation of HO‐1, and Nrf2 is a key transcription factor of HO‐1. Hyperoside increased the expression of HO‐1, Nrf2, and its antioxidant response by upregulating extracellular signal‐regulated kinase (ERK) activity in hydrogen peroxide (H2O2)‐treated HLECs. Although not any anti‐aging function reported yet, hyperoside is an effective flavonoid to protect HLECs against oxidative stress through the induction of HO‐1 (Park et al., 2016).
Morin
Morin is a type of flavonoids, also known as 3, 5, 7, 2′, 4′‐pentahydroxyflavone, and has been widely used as herbal medicines. Morin has many biological functions, including antioxidant (Zhang et al., 2009, 2011), anti‐inflammatory (Lee et al., 2016), antidiabetic osteopenia (Abuohashish et al., 2013), and neuroprotective effects (Lee et al., 2016). It has been reported that morin could protect cells against oxidative stress stimulated by H2O2 and γ‐ray radiation (Zhang et al., 2009, 2011). Recently, Park et al. confirmed that morin could stimulate ERK‐Nrf2 signaling pathway in HLECs (HLE‐B3), resulting in the upregulation of HO‐1 and cytoprotective effects against oxidative stress. Morin increased the protein level of Nrf2, which enhances the expression of HO‐1 via binding to the ARE. Besides, morin activated ERK and triggered transport from cytosol to nucleus (Park et al., 2013).
SFN
SFN, a plant‐extracted isothiocyanate 1‐isothiocyanato‐4‐methylsulfinylbutane, induced the transcription of some antioxidant enzymes and stimulates cellular antioxidant defenses. Nevertheless, the mechanism involved is still not fully understood. TrxR is efficient in inhibiting oxidative stress with accumulating evidence of its value in preventing cataract formation, when consumed nutritionally. Recently, SFN has been detected to increase the activity of TrxR in the mouse lens, and prevents the tissue against oxidative stress that contributes to cataract formation. Further researches on the behaviors of other antioxidant enzymes like quinone oxidoreductase are still in progress (Varma et al., 2013). SFN was found to significantly inhibit the increased cytotoxicity/apoptosis induced by H2O2 in the human lens epithelial cell line FHL124 (Liu et al., 2013). Experimental evidence suggested that SFN could shield against H2O2‐stimulated opacification in cultured porcine lenses. Nrf2 translocated to the nucleus followed by the exposure of 0.5–2.0 μm SFN. Dietary SFN presents the capability to guard human lens cells against ROS via induction of the Keap1‐Nrf2‐ARE pathway and might postpone the formation of cataract (Liu et al., 2013). Furthermore, SFN supplement also reduced aging‐related oxidative stress and neuro‐inflammation in microglia cells both in vitro and in vivo (Townsend & Johnson, 2016), as well as protected the UV‐induced extrinsic skin aging (Sikdar et al., 2016). These results indicate that SFN may serve as a very promising anti‐aging compound in future.
NBP
NBP, a multiple‐target neuroprotective drug, is widely used clinically for the treatment of ischemic stroke, which greatly decreases oxidative damages, increases mitochondrial function, reduces inflammation, and attenuates neuronal apoptosis. NBP has been indicated to upregulate transcriptional activity and subsequently exert an antioxidant activity in the model of amyotrophic lateral sclerosis (Feng et al., 2012). In a diabetic rat model, NBP was found to increase the expression of Nrf2 in the lens of diabetic rats and decrease levels of blood glucose, serum MDA, and 8‐OHdG. NBP‐treated mice were also shown improvement in oxidative stress, as indicated by the levels of DNP, 4‐HNE, and MDA in lens. Even though the precise mechanisms of NBP in preventing diabetic cataract are still needed to investigate, NBP might be a promising therapeutic drug to prevent or treat diabetic cataract (Wang et al., 2016).
RLM
Diabetic cataract is one of the most serious complications of diabetes. Accumulating evidence reveals that ROS overproduced in mitochondria can induce metabolic abnormality, which might be a significant factor in the formation of diabetic cataract. RLM, belonging to the Rosaceae family, has been used as medicinal plants in China for centuries. RLM extract possess antioxidant and free radical‐scavenging capacity. The antioxidant effect of RLM on diabetic cataract was investigated in immortalized LEC lines (SRA01/04) cultured with 5.5 mm high glucose (Liu et al., 2015). This study indicated that RLM could reduce the production of ROS and improve mitochondrial membrane potential (MMP), via the stimulation of HO‐1 expression, in SRA01/04 cells in the hyperglycemic state. Moreover, the protective effects of RLM are regulated through the PI3K/serine–threonine kinase (AKT) and Nrf2/ARE signaling pathways (Liu et al., 2015). Nevertheless, additional studies should explore the mechanism of HO‐1 against the oxidative stress in diabetic cataract.
Conclusions
Accumulating evidence indicates that oxidative stress increases the injuries in the lens cell through oxidation of proteins, DNA damage, membrane lipid peroxidation, ER stress, UPR activation, and imbalance in calcium homeostasis, etc., all of which contribute to cataractogensis. The Nrf2/Keap1/ARE signaling system is now regarded as one of the major cell defense mechanisms against oxidative stresses. Hence, the stimulation of the Nrf2‐ARE signaling pathway has been evaluated as a crucial target for the design and synthesis of new agents for cataracts. Recently, natural compounds are a key source of Nrf2 inducers, which have been extensively studied. Agents in this article reviewed have illustrated most remarkable effects in vitro and in vivo models, protecting HLECs against oxidative stress, reducing the aberrant proteins and preventing the formation of cataract (Fig. 2). All those promising effects have been associated with the antioxidant and Nrf2 inducing effects of the compounds studied. In conclusion, the Nrf2/Keap1/ARE signaling pathway is a promising preventive and therapeutic target against oxidative stress for cataracts. Nrf2 inducers should be considered as an excellent target to start clinical trials.
Figure 2.
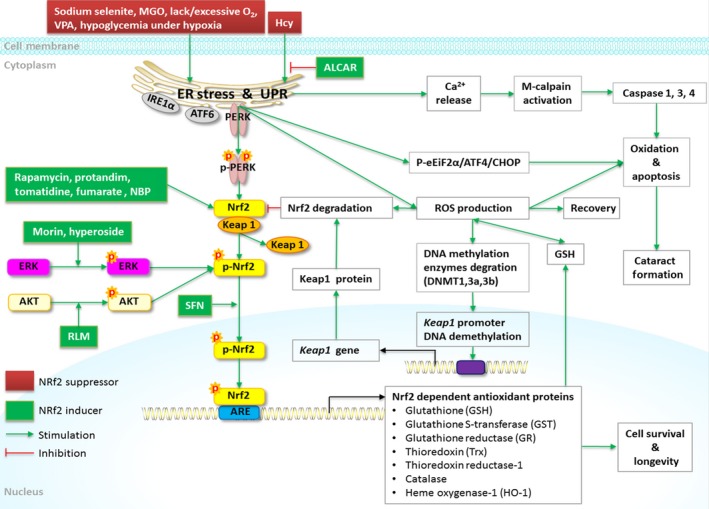
Nrf2 signaling and regulation in the lens. Various cataractogenic stressors induce ER stress, UPR activation, P‐PERK expression, and Nrf2 phosphorylation. The phosphorylated Nrf2 separates from Keap1, binds with ARE in the nucleus, and initiates the antioxidant enzymes transcriptions (GSH, GST, GR, Trx, thioredoxin reductase‐1, catalase, HO‐1), which help eliminating ROS by regenerating GSH. Severe or prolonged ER stressors (sodium selenite, Hcy, VPA, lack/excessive O2, hypoglycemia under hypoxia) cause chronic apoptotic UPR and ROS overproduction, ER‐Ca2+ release, calpain overexpression, and caspase 1,3,4 pathways’ activation, leading to the lens oxidation and cell death. Chronic apoptotic UPR also suppresses the Nrf2‐dependent antioxidant protection resulting in cataract formation. Hyperoside, morin, acetyl‐l‐carnitine, DL‐3‐n‐butylphthalide, RLM activate Nrf2 and protect lenses from oxidation. Excessive ROS also inhibit the Nrf2‐dependent antioxidant system via accelerating the DNA methylation enzymes degradation, triggering demethylation of DNA in the Keap1 promoter and overexpression of Keap1, which enhances the Nrf2 proteasomal degradation. Rapamycin, protandim, tomatidine, fumarate activate the Nrf2 signaling and extend the life longevity. Green solid line indicates direct stimulation. Red solid line indicates direct inhibition. ROS, reactive oxygen species; Nrf2, transcription factors like nuclear factor (erythroid‐ derived 2)‐like 2; Keap1, Kelch‐like erythroid‐cell‐derived protein with CNC homology (ECH)‐associated protein 1; ARE, antioxidant response element; GST, glutathione‐S‐transferase; GR, glutathione reductase; p‐PERK, phosphorylated protein kinase RNA (PKR)‐like endoplasmic reticulum kinase; UPR, unfolded protein response; ER, endoplasmic reticulum; Hcy, homocysteine; Trx, thioredoxin; GSH, glutathione; VPA, valproic acid; ALCAR, acetyl‐l‐carnitine; p‐eIF2α, phosphorylated eukaryotic initiation factor 2α; IRE1α, Inositol‐requiring kinase 1α; ATF6, transcription factor 6; ATF4, activating transcription factor 4; CHOP, CCAAT/enhancer‐binding protein‐homologous protein; Dnmt3a, DNA methyltransferase 3a; Dnmt3b, DNA methyltransferase 3b; Dnmt1, DNA methyltransferase 1; HO‐1, heme oxygenase‐1; ERK, extracellular signal‐regulated kinase; SFN, sulforaphane; NBP, DL‐3‐n‐butylphthalide; MGO, methylglyoxal; RLM, Rosa laevigata Michx.; AKT, serine–threonine kinase.
Author contributions
The topic was conceptualized by CWL. XFL, and DDZ, and JLH contributed to the literature database search, and writing of the manuscript. TX, CBL, CL, and CS contributed to vital revising. THM contributes to English Polishing.
Funding
It was funded by Development and Reform Commission of Jilin Province [2015Y031‐1] and The First Hospital of Jilin University Grant [JDYY72016055].
Conflicts of interest
None declared.
Contributor Information
Cheng‐Wei Lu, Email: lcwchina800@sina.com.
Dan‐Dan Zhou, Email: zhoudan0928@sohu.com.
References
- Abuohashish HM, Al‐Rejaie SS, Al‐Hosaini KA, Parmar MY, Ahmed MM (2013) Alleviating effects of morin against experimentally‐induced diabetic osteopenia. Diabetol. Metab. Syndr. 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriola Apelo SI, Lamming DW (2016) Rapamycin: an inhibiTOR of aging emerges from the soil of Easter Island. J. Gerontol. A Biol. Sci. Med. Sci. 71, 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafian H, Czibik G, Bellahcene M, Aksentijevic D, Smith AC, Mitchell SJ, Dodd MS, Kirwan J, Byrne JJ, Ludwig C, Isackson H, Yavari A, Stottrup NB, Contractor H, Cahill TJ, Sahgal N, Ball DR, Birkler RI, Hargreaves I, Tennant DA, Land J, Lygate CA, Johannsen M, Kharbanda RK, Neubauer S, Redwood C, de Cabo R, Ahmet I, Talan M, Gunther UL, Robinson AJ, Viant MR, Pollard PJ, Tyler DJ, Watkins H (2012) Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab. 15, 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babizhayev MA, Yegorov YE (2016) Reactive oxygen species and the aging eye: specific role of metabolically active mitochondria in maintaining lens function and in the initiation of the oxidation‐induced maturity onset cataract–a novel platform of mitochondria‐targeted antioxidants with broad therapeutic potential for redox regulation and detoxification of oxidants in eye diseases. Am. J. Ther. 23, e98–e117. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Shi Y, Vrensen GF (2011) Biological glass: structural determinants of eye lens transparency. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 1250–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Guarente L (2007) Two neurons mediate diet‐restriction‐induced longevity in C. elegans . Nature 447, 545–549. [DOI] [PubMed] [Google Scholar]
- Bloom DA, Jaiswal AK (2003) Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element‐mediated NAD(P)H:quinone oxidoreductase‐1 gene expression. J. Biol. Chem. 278, 44675–44682. [DOI] [PubMed] [Google Scholar]
- Chylack LT Jr, Schaefer FL (1976) Mechanism of ‘hypoglycemic’ cataract formation in the rat lens. II. Further studies on the role of hexokinase instability. Invest. Ophthalmol. 15, 519–528. [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA (2004) PERK‐dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 279, 20108–20117. [DOI] [PubMed] [Google Scholar]
- Elanchezhian R, Ramesh E, Sakthivel M, Isai M, Geraldine P, Rajamohan M, Jesudasan CN, Thomas PA (2007) Acetyl‐L‐carnitine prevents selenite‐induced cataractogenesis in an experimental animal model. Curr. Eye Res. 32, 961–971. [DOI] [PubMed] [Google Scholar]
- Elanchezhian R, Palsamy P, Madson CJ, Lynch DW, Shinohara T (2012a) Age‐related cataracts: homocysteine coupled endoplasmic reticulum stress and suppression of Nrf2‐dependent antioxidant protection. Chem. Biol. Interact. 200, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elanchezhian R, Palsamy P, Madson CJ, Mulhern ML, Lynch DW, Troia AM, Usukura J, Shinohara T (2012b) Low glucose under hypoxic conditions induces unfolded protein response and produces reactive oxygen species in lens epithelial cells. Cell Death Dis. 3, e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Waltz TB, Kassahun H, Lu Q, Kerr JS, Morevati M, Fivenson EM, Wollman BN, Marosi K, Wilson MA, Iser WB, Eckley DM, Zhang Y, Lehrmann E, Goldberg IG, Scheibye‐Knudsen M, Mattson MP, Nilsen H, Bohr VA, Becker KG (2017) Tomatidine enhances lifespan and healthspan in C. elegans through mitophagy induction via the SKN‐1/Nrf2 pathway. Sci. Rep. 7, 46208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Peng Y, Liu M, Cui L (2012) DL‐3‐n‐butylphthalide extends survival by attenuating glial activation in a mouse model of amyotrophic lateral sclerosis. Neuropharmacology 62, 1004–1010. [DOI] [PubMed] [Google Scholar]
- Gao Y, Yan Y, Huang T (2015) Human age‐related cataracts: epigenetic suppression of the nuclear factor erythroid 2‐related factor 2‐mediated antioxidant system. Mol. Med. Rep. 11, 1442–1447. [DOI] [PubMed] [Google Scholar]
- Glover SJ, Quinn AG, Barter P, Hart J, Moore SJ, Dean JC, Turnpenny PD (2002) Ophthalmic findings in fetal anticonvulsant syndrome(s). Ophthalmology 109, 942–947. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holekamp NM, Shui YB, Beebe D (2006) Lower intraocular oxygen tension in diabetic patients: possible contribution to decreased incidence of nuclear sclerotic cataract. Am. J. Ophthalmol. 141, 1027–1032. [DOI] [PubMed] [Google Scholar]
- Kang KW, Choi SH, Kim SG (2002) Peroxynitrite activates NF‐E2‐related factor 2/antioxidant response element through the pathway of phosphatidylinositol 3‐kinase: the role of nitric oxide synthase in rat glutathione S‐transferase A2 induction. Nitric Oxide 7, 244–253. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Arinze IJ (2006) Valproic acid‐induced gene expression through production of reactive oxygen species. Cancer Res. 66, 6563–6569. [DOI] [PubMed] [Google Scholar]
- Lacherade JC, Jacqueminet S, Preiser JC (2009) An overview of hypoglycemia in the critically ill. J. Diabetes Sci. Technol. 3, 1242–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Lee Y, Chun HJ, Kim AH, Kim JY, Lee JY, Ishigami A, Lee J (2016) Neuroprotective and anti‐inflammatory effects of morin in a murine model of Parkinson's disease. J. Neurosci. Res. 94, 865–878. [DOI] [PubMed] [Google Scholar]
- Lewis KN, Wason E, Edrey YH, Kristan DM, Nevo E, Buffenstein R (2015) Regulation of Nrf2 signaling and longevity in naturally long‐lived rodents. Proc. Natl Acad. Sci. USA 112, 3722–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Smith AJ, Lott MC, Bao Y, Bowater RP, Reddan JR, Wormstone IM (2013) Sulforaphane can protect lens cells against oxidative stress: implications for cataract prevention. Invest. Ophthalmol. Vis. Sci. 54, 5236–5248. [DOI] [PubMed] [Google Scholar]
- Liu Y, Luo W, Luo X, Yong Z, Zhong X (2015) Effects of Rosa laevigata Michx. extract on reactive oxygen species production and mitochondrial membrane potential in lens epithelial cells cultured under high glucose. Int. J. Clin. Exp. Med. 8, 15759–15765. [PMC free article] [PubMed] [Google Scholar]
- Liu XF, Hao JL, Xie T, Mukhtar NJ, Zhang W, Malik TH, Lu CW, Zhou DD (2017a) Curcumin, a potential therapeutic candidate for anterior segment eye diseases: a review. Front. Pharmacol. 8, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Wilkins M, Kim T, Malyugin B, Mehta JS (2017b) Cataracts. Lancet https://doi.org/10.1016/S0140-6736(17)30544-5. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Lundquist O, Osterlin S (1994) Glucose concentration in the vitreous of nondiabetic and diabetic human eyes. Graefes Arch. Clin. Exp. Ophthalmol. 232, 71–74. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R (2011) Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 66, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami Y (2016) Nrf2 is an attractive therapeutic target for retinal diseases. Oxid. Med. Cell Longev. 2016, 7469326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor RM, Baker DJ, van Deursen JM (2013) Senescent cells: a novel therapeutic target for aging and age‐related diseases. Clin. Pharmacol. Ther. 93, 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SK, Bose SK, Grunwald GK, Myhill P, McCord JM (2006) The induction of human superoxide dismutase and catalase in vivo: a fundamentally new approach to antioxidant therapy. Free Radic. Biol. Med. 40, 341–347. [DOI] [PubMed] [Google Scholar]
- von Otter M, Landgren S, Nilsson S, Zetterberg M, Celojevic D, Bergstrom P, Minthon L, Bogdanovic N, Andreasen N, Gustafson DR, Skoog I, Wallin A, Tasa G, Blennow K, Nilsson M, Hammarsten O, Zetterberg H (2010) Nrf2‐encoding NFE2L2 haplotypes influence disease progression but not risk in Alzheimer's disease and age‐related cataract. Mech. Ageing Dev. 131, 105–110. [DOI] [PubMed] [Google Scholar]
- Palermo V, Falcone C, Calvani M, Mazzoni C (2010) Acetyl‐L‐carnitine protects yeast cells from apoptosis and aging and inhibits mitochondrial fission. Aging Cell 9, 570–579. [DOI] [PubMed] [Google Scholar]
- Palsamy P, Ayaki M, Elanchezhian R, Shinohara T (2012) Promoter demethylation of Keap1 gene in human diabetic cataractous lenses. Biochem. Biophys. Res. Commun. 423, 542–548. [DOI] [PubMed] [Google Scholar]
- Palsamy P, Bidasee KR, Ayaki M, Augusteyn RC, Chan JY, Shinohara T (2014a) Methylglyoxal induces endoplasmic reticulum stress and DNA demethylation in the Keap1 promoter of human lens epithelial cells and age‐related cataracts. Free Radic. Biol. Med. 72, 134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsamy P, Bidasee KR, Shinohara T (2014b) Selenite cataracts: activation of endoplasmic reticulum stress and loss of Nrf2/Keap1‐dependent stress protection. Biochim. Biophys. Acta 1842, 1794–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsamy P, Bidasee KR, Shinohara T (2014c) Valproic acid suppresses Nrf2/Keap1 dependent antioxidant protection through induction of endoplasmic reticulum stress and Keap1 promoter DNA demethylation in human lens epithelial cells. Exp. Eye Res. 121, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Kang KA, Kim KC, Cha JW, Kim EH, Hyun JW (2013) Morin induces heme oxygenase‐1 via ERK‐Nrf2 signaling pathway. J. Cancer Prev. 18, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Han X, Piao MJ, Oh MC, Fernando PM, Kang KA, Ryu YS, Jung U, Kim IG, Hyun JW (2016) Hyperoside induces endogenous antioxidant system to alleviate oxidative stress. J. Cancer Prev. 21, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, Tamashiro KL, Poosala S, Csiszar A, Ungvari Z, Kensler TW, Yamamoto M, Egan JM, Longo DL, Ingram DK, Navas P, de Cabo R (2008) Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc. Natl Acad. Sci. USA 105, 2325–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redza‐Dutordoir M, Averill‐Bates DA (2016) Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 1863, 2977–2992. [DOI] [PubMed] [Google Scholar]
- Robida‐Stubbs S, Glover‐Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann‐Haefelin E, Sabatini DM, Blackwell TK (2012) TOR signaling and rapamycin influence longevity by regulating SKN‐1/Nrf and DAF‐16/FoxO. Cell Metab. 15, 713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529. [DOI] [PubMed] [Google Scholar]
- Rushmore TH, Morton MR, Pickett CB (1991) The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 266, 11632–11639. [PubMed] [Google Scholar]
- Schumann C, Chan S, Khalimonchuk O, Khal S, Moskal V, Shah V, Alani AW, Taratula O, Taratula O (2016) Mechanistic nanotherapeutic approach based on siRNA‐mediated DJ‐1 protein suppression for platinum‐resistant ovarian cancer. Mol. Pharm. 13, 2070–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen SK, Pukazhvanthen P, Abraham R (2008) Plasma homocysteine, folate and vitamin B(12) levels in senile cataract. Indian J. Clin. Biochem. 23, 255–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih PH, Yen GC (2007) Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology 8, 71–80. [DOI] [PubMed] [Google Scholar]
- Shui YB, Beebe DC (2008) Age‐dependent control of lens growth by hypoxia. Invest. Ophthalmol. Vis. Sci. 49, 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shui YB, Fu JJ, Garcia C, Dattilo LK, Rajagopal R, McMillan S, Mak G, Holekamp NM, Lewis A, Beebe DC (2006) Oxygen distribution in the rabbit eye and oxygen consumption by the lens. Invest. Ophthalmol. Vis. Sci. 47, 1571–1580. [DOI] [PubMed] [Google Scholar]
- Sikdar S, Papadopoulou M, Dubois J (2016) What do we know about sulforaphane protection against photoaging? J. Cosmet. Dermatol. 15, 72–77. [DOI] [PubMed] [Google Scholar]
- Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, de Magalhaes JP, Martinez PA, McCord JM, Miller BF, Muller M, Nelson JF, Ndukum J, Rainger GE, Richardson A, Sabatini DM, Salmon AB, Simpkins JW, Steegenga WT, Nadon NL, Harrison DE (2016) Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha‐glucosidase inhibitor or a Nrf2‐inducer. Aging Cell 15, 872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM (2004) Decline in transcriptional activity of Nrf2 causes age‐related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl Acad. Sci. USA 101, 3381–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd DJ, Lee AH, Glimcher LH (2008) The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol. 8, 663–674. [DOI] [PubMed] [Google Scholar]
- Townsend BE, Johnson RW (2016) Sulforaphane induces Nrf2 target genes and attenuates inflammatory gene expression in microglia from brain of young adult and aged mice. Exp. Gerontol. 73, 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma SD, Chandrasekaran K, Kovtun S (2013) Sulforaphane‐induced transcription of thioredoxin reductase in lens: possible significance against cataract formation. Clin. Ophthalmol. 7, 2091–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T, Takacs‐Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F (2003) Genetics: influence of TOR kinase on lifespan in C. elegans . Nature 426, 620. [DOI] [PubMed] [Google Scholar]
- Velmurugan K, Alam J, McCord JM, Pugazhenthi S (2009) Synergistic induction of heme oxygenase‐1 by the components of the antioxidant supplement Protandim. Free Radic. Biol. Med. 46, 430–440. [DOI] [PubMed] [Google Scholar]
- Vinson JA (2006) Oxidative stress in cataracts. Pathophysiology 13, 151–162. [DOI] [PubMed] [Google Scholar]
- Wang F, Ma J, Han F, Guo X, Meng L, Sun Y, Jin C, Duan H, Li H, Peng Y (2016) DL‐3‐n‐butylphthalide delays the onset and progression of diabetic cataract by inhibiting oxidative stress in rat diabetic model. Sci. Rep. 6, 19396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Yu Z, Sunchu B, Shoaf J, Dang I, Zhao S, Caples K, Bradley L, Beaver LM, Ho E, Lohr CV, Perez VI (2017) Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2‐independent mechanism. Aging Cell 16, 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SP, Yang XZ, Cao GP (2015) Acetyl‐l‐carnitine prevents homocysteine‐induced suppression of Nrf2/Keap1 mediated antioxidation in human lens epithelial cells. Mol. Med. Rep. 12, 1145–1150. [DOI] [PubMed] [Google Scholar]
- Yu R, Chen C, Mo YY, Hebbar V, Owuor ED, Tan TH, Kong AN (2000) Activation of mitogen‐activated protein kinase pathways induces antioxidant response element‐mediated gene expression via a Nrf2‐dependent mechanism. J. Biol. Chem. 275, 39907–39913. [DOI] [PubMed] [Google Scholar]
- Yu S, Khor TO, Cheung KL, Li W, Wu TY, Huang Y, Foster BA, Kan YW, Kong AN (2010) Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice. PLoS ONE 5, e8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Kang KA, Piao MJ, Maeng YH, Lee KH, Chang WY, You HJ, Kim JS, Kang SS, Hyun JW (2009) Cellular protection of morin against the oxidative stress induced by hydrogen peroxide. Chem. Biol. Interact. 177, 21–27. [DOI] [PubMed] [Google Scholar]
- Zhang R, Kang KA, Kang SS, Park JW, Hyun JW (2011) Morin (2′,3,4′,5,7‐pentahydroxyflavone) protected cells against gamma‐radiation‐induced oxidative stress. Basic Clin. Pharmacol. Toxicol. 108, 63–72. [DOI] [PubMed] [Google Scholar]
- Zheng XY, Xu J, Chen XI, Li W, Wang TY (2015) Attenuation of oxygen fluctuation‐induced endoplasmic reticulum stress in human lens epithelial cells. Exp. Ther. Med. 10, 1883–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]


