Summary
The overexpression of miR319 in plants results in delayed senescence, and high levels of miR319‐targeted TCP4 transcription factor cause premature onset of this process. However, the underlying mechanisms of this pathway remain elusive. Here, we found that miR319 overexpression results in a decrease in TCP4 abundance and secondary cell wall formation in the stem. Conversely, constitutive expression of miR319‐resistant TCP4 promotes secondary cell wall formation, indicating that miR319‐mediated TCP4 controls secondary cell wall formation during development. Further analysis revealed that TCP4 might directly bind the promoter of VND7 to activate its expression, which triggers the expression of a VND7 transcriptional network associated with secondary cell wall biosynthesis and programmed cell death and accelerates vessel formation. In addition, the development process gradually increased TCP4 expression. These results suggest that miR319 and its target TCP4 can act as switches that turn on secondary cell wall synthesis and programmed cell death.
Keywords: miR319, TCP4, transcription factor, secondary cell wall biosynthesis, xylem vessel element differentiation
Introduction
Small RNAs, which are typically 20–24 nucleotides long, are important for gene and chromatin regulation in plants (Chen, 2009; Taylor et al., 2014). MicroRNAs (miRNAs), approximately 21 nucleotides in length, negatively regulate target genes by partly pairing to the corresponding mRNA and facilitating its cleavage. Some miRNAs are conserved in different species and are believed to facilitate evolutionarily conserved functions in regulating organogenesis (Chen, 2009). Usually, miRNAs are transcribed by RNA polymerase II into primary‐miRNAs (pri‐miRNAs) in the nucleus. The pri‐miRNAs are processed by microprocessor containing Drosha and dsRNA‐binding protein (DGCR8) to produce precursor‐miRNAs (pre‐miRNAs; Voinnet, 2009). In plants, DICER‐like 1 (DCL1) proteins cleave the pre‐miRNA. Unlike in animals, plant miRNAs are cleaved by DCL1 mainly in the nucleus rather than the cytoplasm, and then the cleaved duplex is translocated into the cytoplasm by HASTY. Once in the cytoplasm, the miRNAs are unwound into single mature miRNAs by a helicase, and the mature miRNAs finally enter the ribonucleoprotein complex known as the RNA‐induced silencing complex, where they regulate targeted gene expression (Chen, 2009; Jones‐Rhoades et al., 2006).
Several plant miRNAs are involved in plant growth, development and the stress response (Chen, 2009; Sunkar, 2010). The first described plant miRNA mutant jaw‐D was identified in the transgenic line overexpressing miR319. The major targets of miR319 are a series of TCP (TEOSINTE BRANCHED1/CYCLOIDEA/PCF) transcription factors, including TCP2/3/4/10/24 (Palatnik et al., 2003). Several studies revealed that miR319 and its targets play multiple roles in plant developmental processes, such as leaf morphogenesis, jasmonic acid biosynthesis, senescence and flower development (Li et al., 2012; Nag et al., 2009; Palatnik et al., 2003; Schommer et al., 2008). In Solanum lycopersicum (tomato), the down‐regulation of several TCPs by ectopic expression of miR319 results in larger leaflets and continuous growth of the leaf margin, whereas reduced levels of miR319 or enhanced levels of TCP decrease leaf sizes (Ori et al., 2007).
Plant cells are enclosed in an extracellular matrix, the cell wall, that imparts structural support and regulate growth and differentiation. All plant cells have a thin primary cell wall and certain cell types such as sclerenchyma cells, also have a secondary wall layer, located between the primary wall and the plasma membrane. Secondary cell walls consist mainly of cellulose, hemicellulose and lignin (Zhong and Ye, 2007). The biosynthetic pathway associated with secondary cell wall formation is highly regulated at the transcriptional level. Several lines of evidence have demonstrated that a network of transcription factors regulates plant secondary wall biosynthesis (Zhong and Ye, 2007). In this network, the NAC (for NAM, ATAF1/2 and CUC2) domain transcription factors, including SND1, NST1, NST2, VND6 and VND7, are master switches that control a set of downstream functional factors, which in turn activate secondary cell wall biosynthesis factors, such as SND2, SND3, MYB20, MYB102 and KNAT7. This activation initiates the expression of secondary cell wall biosynthesis genes, leading to a massive deposition of the secondary wall in cells (Ellis et al., 2014; Zhong et al., 2006).
VND7 belongs to the NAC transcription factor family and is preferentially expressed in differentiating xylem vessel elements (Kubo et al., 2005; Yamaguchi et al., 2010b). VND7 overexpression can induce the ectopic differentiation of protoxylem‐like vessels and result in a pale colour and death (Kubo et al., 2005; Yamaguchi et al., 2010b). Functional suppression of VND7 causes defects in the formation of vessel elements. A broad range of putative direct target genes of VND7 has been identified through transcriptome analysis and encodes transcription factors, irregular xylem proteins and proteolytic enzymes (Yamaguchi et al., 2011). These results strongly suggest that VND7 acts as a key regulator of xylem vessel differentiation.
A previous study showed that miR319 controls jasmonate biosynthesis and senescence through miR319‐targeted TCP4, which can bind to the promoter of the LOX2 gene that is responsible for jasmonic acid biosynthesis (Schommer et al., 2008). However, the pathways that regulate senescence remain unclear. In the present study, we show that TCP4 associates with the promoter region of VND7 to directly activate its expression, which regulates the differentiation of all types of xylem vessels in roots and shoots (Yamaguchi et al., 2008). The disruption of TCP4 resulted in the decreased formation of the secondary cell wall and the decreased differentiation of xylem vessel elements. Furthermore, we found that gradually increasing the level of TCP4 corresponded to a gradual increase in the levels of VND7 transcripts in the development processes. These data suggest that TCP4 may be involved in xylem vessel differentiation via activating VND7 transcription.
Results
Generation of stable transgenic lines overexpressing rTCP4
Plants overexpressing miR319‐resistant TCP4 (rTCP4:GFP) have a shorter life span than wild‐type (WT) plants and do not produce seeds (Schommer et al., 2008). However, three independent transgenic lines with weaker phenotypes, meaning that they produced seeds, were selected in this study. Three lines had long hypocotyls, epinastic cotyledons and smaller rosette leaves; further, their aerial parts were darker green than those of the wild type (Figure 1a), similar to the overexpression of rTCP4:GFP phenotype (Schommer et al., 2008). All three lines exhibited a normal life cycle (Figure 1b). Quantitative real‐time polymerase chain reaction (qRT‐PCR) analysis confirmed the up‐regulation of TCP4 levels (Figure 1c). The rTCP4‐3 transgenic plants showed the highest TCP4 expression level, almost 2.5‐fold higher than WT, while rTCP4‐6 plants showed a twofold increase, and rTCP4‐7 plants showed a 1.7‐fold increase. Notably, even the rTCP4‐7 transgenic plants with slight up‐regulation of TCP4 exhibited the rTCP4‐GFP phenotype (Figure 1a). Jaw‐D, a miR319a overexpressing line, has crinkling leaves with cutting margins (Palatnik et al., 2003). When the rTCP4‐3 line was crossed with jaw‐D plants, the phenotype of the heterozygote F1 plants alleviated the phenotype of the jaw‐D plants (Figure 1d). The fact that slight up‐regulation of TCP4 affects plant growth and development indicates that the TCP4 plays a vital role in these processes.
Figure 1.
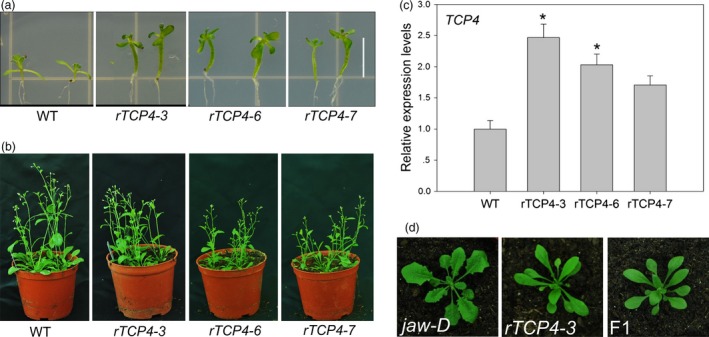
Phenotypes of rTCP4 transgenic plants. (a) Wild‐type and rTCP4 2‐week‐old seedlings. (b) Wild‐type and rTCP4 6‐week‐old seedlings. (c) Relative expression of TCP4 in transgenic plants. Transcript levels were normalized to UBQ10 and then expressed relative to Col‐0. The expression level of TCP4 in the wild type is set to 1. Error bars represent SE of triplicate experiments. (*P < 0.01). (d) Rosettes of 25‐day‐old seedlings. F1, the progeny between rTCP4‐3 and jaw‐D. Bar = 0.5 cm.
MiR319‐targeted TCP4 is involved in xylem vessel element formation
When we transferred the transgenic plants to soil, we found that the hypocotyls of the rTCP4‐3, rTCP4‐6 and rTCP4‐7 transgenic plants were less flexible than WT plants. Thus, we speculated that lignification might be increased in the transformants. To examine this hypothesis, we selected rTCP4‐3 and rTCP4‐6 transgenic plants for further investigation. Histological studies were performed to investigate the differences in secondary wall structure among the WT, jaw‐D plants and rTCP4‐OX lines. Cross‐sections of the basal parts of the stems revealed that xylem vessel elements were more frequent in stems of rTCP4‐OX lines (Figure 2c,d). Transmission electron micrographs showed that the wall thickness of vessel elements in jaw‐D plants was clearly reduced compared with WT plants (Figure 2f,i). The overexpression of rTCP4 resulted in significantly increased wall thickness of the vessels (Figure 2g,h,i), and the magnitude of the effect correlated with the expression level of TCP4 (Figure 1c). These results indicate that TCP4 plays a vital role in vessel formation.
Figure 2.
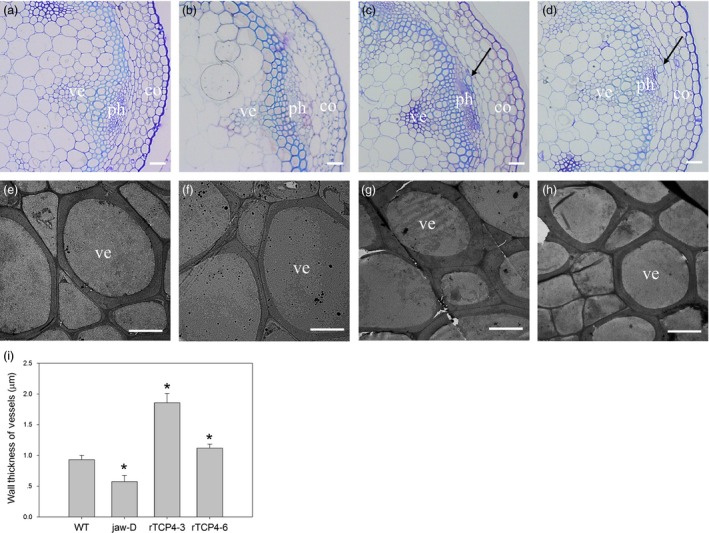
Cross‐sections of the stem in 6‐week‐old plants. (a–d) Cross‐sections of vascular bundles in the 15‐cm‐high stems of 6‐week‐old plants. Note that black arrow indicates increased vessel element formation. (e–h) Transmission electron micrographs of vessel walls in the stems of 6‐week‐old plants. (a, e) Wild type. (b, f) jaw‐D. (c, g) rTCP4‐3. (d, h) rTCP4‐6. co, cortex; ph, phloem; ve, vessel. (i) Wall thickness of the vessel elements in the stems of wild‐type, jaw‐D and transgenic plants. The wall thickness was measured from transmission electron micrographs. The differences in wall thickness of the xylem vessel elements in the stem between the wild type and the other lines are statistically significant (*P < 0.05). The data are the means (μm) of 60 cells, which are from three plants of each genotype. Bars = 20 μm (a–d), 5 μm (e–h).
Overexpression of TCP4 causes increased deposition of lignin and cellulose
Secondary cell walls are mainly composed of lignin, cellulose and hemicellulose. To further investigate the function of TCP4 in the regulation of secondary wall biosynthesis, we examined whether the observed increase in cell wall thickness corresponded to an increased deposition of lignin, cellulose or both. As shown in Figure 3, conventional Wiesner histochemical stains (phloroglucinol‐HCl, in which a violet‐red colour is indicative of lignins) exhibited less intense coloration in the secondary cell walls of jaw‐D plants than in the controls, but higher intensity in the rTCP4‐OX lines (Figure 3a–d). Ectopic lignin staining was also observed in the stem cortex of rTCP4‐OX lines (Figure 3c,d). These data indicate a reduction in lignin content in the secondary cell wall of jaw‐D plants and increased lignin content in the rTCP4‐OX lines.
Figure 3.
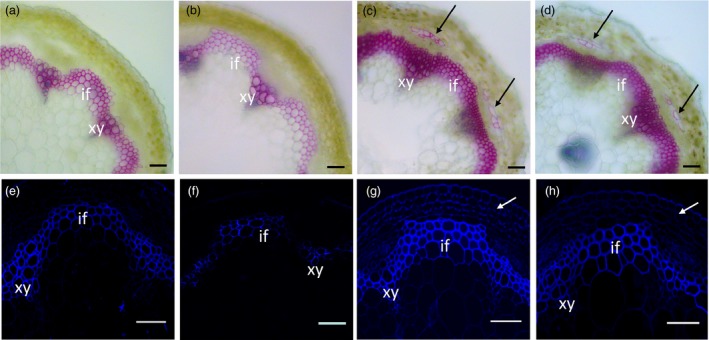
Modulation of lignin and secondary cell wall cellulose by miR319 and TCP4. Stem sections were stained with phloroglucinol‐HCl or Calcofluor White to detect lignin or secondary wall cellulose, respectively. (a‐d) Phloroglucinol‐HCl staining (red colour) revealed weaker lignin signals within the interfascicular fibres and xylem bundles in the jaw‐D plants (b) compared with the control wild‐type plants (a). The strongest signals were observed in the rTCP4‐3 (c) and rTCP4‐6 (d) lines. Note that increased lignin deposition was detected in the stem of rTCP4‐3 (c) and rTCP4‐6 (d). (e–h) Calcofluor White staining (blue colour) of stem sections showing cellulose staining in the walls of interfascicular fibres and xylem cells in seedling stems of the wild type (e), jaw‐D (f), and rTCP4‐3 (g) and rTCP4‐6 (h) lines. Note that strong cellulose signals were detected in the stem cortex of rTCP4‐3 (g) and rTCP4‐6 (h). xy, xylem; if, interfascicular fibre. Bars = 50 μm.
Similarly, histological staining of cellulose in the stems (using Calcofluor White, in which a blue colour is indicative of secondary wall cellulose), under epifluorescence microscopy, revealed weaker fluorescence within the interfascicular fibres and xylem bundles in the jaw‐D plants compared with the control WT plants (Figure 3e,f). However, the fluorescence intensity was stronger in the rTCP4‐OX lines (Figure 3g,h). These results indicate that TCP4 is involved in secondary wall biosynthesis affecting the abundance of both cellulose and lignin.
TCP4 might directly activate the expression of VND7 for xylem vessel element formation
Our data showed that the activation of TCP4 is responsible for secondary cell wall biosynthesis. To characterize this process in more detail, we searched for TCP4‐targeted genes among lignification‐related genes using the TCP‐binding motif ‘GTGGTCCC’ (Schommer et al., 2008) as bait. Intriguingly, we found one ‘GTGGTCCC’ cis element in the promoter region of VND7 (Figure 4a). VND7 is involved in xylem vessel formation (Yamaguchi et al., 2008). We first measured the levels of VND7 transcripts in the different lines. As shown in Figure 4b, the VND7 transcriptional level was lowest in the jaw‐D plants and up‐regulated in the rTCP4‐OX lines. An electrophoretic mobility shift assay (EMSA) revealed that the motif in VND7 was bound by His‐TCP4 but not His alone. The additional unlabeled probes competed for binding in dose‐dependent manner (Figure 4c). The miR319‐resistant rTCP4 cannot be sufficiently cleaved by miR319, due to a mutation at the cleavage site in TCP4 (Schommer et al., 2008). We also found that rTCP4‐HIS bounds to the promoter region of the VND7 (Figure 4c). The qRT‐PCR and EMSA analyses indicated that TCP4 might activate the transcription of VND7 by directly binding to its promoter. Thus, the transient expression system was used next to investigate whether TCP4 activates the expression of VND7. When we co‐transfected the ProVND7:LUC reporter plasmid with the 35S:TCP4 effector plasmid, strong LUC activity was detected (Figure 4d,e). However, in the absence of the effector 35S:TCP4 plasmid, LUC activity was much lower (Figure 4d,e). These results revealed that TCP4 indeed activates transcriptional activity of VND7 in vivo.
Figure 4.
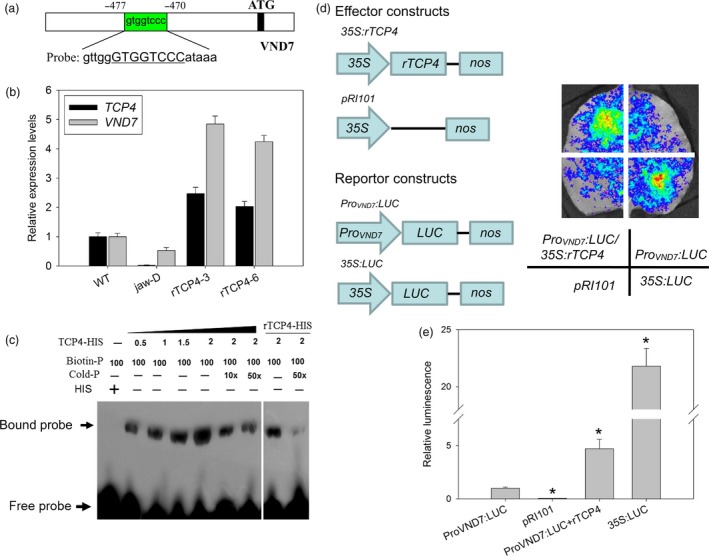
TCP4 binds to the promoter region of VND7. (a) The promoter sequence of the VND7 gene. The green box represents the putative TCP4‐binding element (GTGGTCCC). (b) qRT‐PCR analysis of TCP4 and VND7 expression in Col‐0, jaw‐D plants and rTCP4‐OX lines. The expression levels of TCP4 and VND7 in the wild type are set to 1, respectively. Relative expression of each gene was calculated by normalizing to the value in WT plants. Error bars represent SE of triplicate experiments. (c) In vitro EMSA assay showing that the TCP4‐HIS fusion protein, but not the negative control 6HIS, binds to the VND7 promoter (Probe). rTCP4‐HIS also bounds to the promoter region of the VND7. Biotin‐P indicates the biotin‐labelled probe, and Cold‐P indicates the probe without biotin labelling. Competition for TCP4 binding was performed with 10× and 50× unlabeled probes. Arrow indicates shifted bands. (d) Transient expression of the 35S:TCP4 effector construct with the Pro VND 7 :LUC reporter construct in N. benthamiana leaves. Quantitative analyses of luminescence intensity are shown in (e). The 35S:LUC construct was used as the positive control. The luminescence intensity of Pro VND 7 :LUC is set to 1. Relative luminescence intensity was calculated by normalizing to the value of Pro VND 7 :LUC. The data are the means ± SD of triplicate experiments. (*P < 0.01).
Because VND7 plays a pivotal role in regulating the differentiation of all types of xylem vessels in Arabidopsis (Yamaguchi et al., 2008), we then assessed vessel formation using specific basic fuchsin red staining of xylem and semi‐thin section. As shown in Figure 5, jaw‐D plants showed repressed vessel element differentiation (Figure 5b,f), but more vessel elements were observed in the hypocotyls of the rTCP4‐OX lines (Figure 5c,d,g,h). Similarly, the roots of the jaw‐D plants exhibited weaker signals in vessels (Figure 5j) and repressed vessel element differentiation (Figure 5n). In contrast, the rTCP4‐OX lines showed increased formation of vessel elements (Figure 5k,I,o,p). Overexpression of rTCP4 in jaw‐D plants rescued the vessel element defects of hypocotyls and roots (Figure S1). These results corroborate that TCP4 is involved in the differentiation of xylem vessel elements.
Figure 5.

Modulation of vessel elements formation by miR319 and TCP4. Xylem vessels were stained with basic fuchsin red and visualized under a confocal microscope in (a–d) and (i–l). (a–d) Confocal laser scanning microscopy images of hypocotyls from Col‐0 (a), jaw‐D plants (b) and rTCP4‐OX lines (c and d). (e–h) Cross‐section of hypocotyls in the 1‐week‐old seedlings. (i–l) Confocal laser scanning microscopy images of roots from Col‐0 (i), jaw‐D plants (j) and rTCP4‐OX lines (k and l). (m–p) Cross‐section of roots in the 1‐week‐old seedlings. White and black arrows indicate protoxylem vessels. White arrows in (b) indicate the absence of vessels on one side. The phenotype was also found in (f). White arrows in (c, d) and (k, l) indicate the additional formation of vessels in the roots and hypocotyls, respectively. The phenotype was also found in (g, h) and (o, p). Bars = 20 μm.
Quantitative RT‐PCR analysis of genes involved in secondary wall formation
Our data showed that TCP4 can bind the VND7 promoter to modulate its transcriptional level. Previous studies showed that VND7 also modulates a series of transcription factor genes including MYB46 and MYB83, which up‐regulate the expression of many genes related to secondary cell wall formation both in fibres and vessels (McCarthy et al., 2009; Zhong et al., 2007). LBD30 forms a positive feedback loop with VND7 during xylem vessel formation (Soyano et al., 2008; Zhou et al., 2009). We found lower transcriptional levels of MYBs and LBD30 in jaw‐D plants and higher transcriptional levels in the rTCP4‐OX lines compared with WT (Figure 6). CESA4/IRX5, CESA7/IRX3 and CESA8/IRX1 are cellulose synthases that function in the synthesis of the secondary cell wall (Taylor et al., 2003). IRX8 and IRX10 are required for normal amounts of hemicellulose and cellulose in secondary cell wall formation (Brown et al., 2005; Zeng et al., 2016). Here, we also found lower transcriptional levels of these genes in jaw‐D plants and up‐regulation in the rTCP4‐OX lines (Figure 6). Two plant cysteine proteases, XCP1 and XCP2, that are also the direct target genes of VND7 (Yamaguchi et al., 2011), may be involved in autolysis of programmed cell death (PCD) during the xylem differentiation of tracheary elements (TEs) (Avci et al., 2008). The expression levels of these proteases strikingly corresponded to the TCP4 transcript level.
Figure 6.
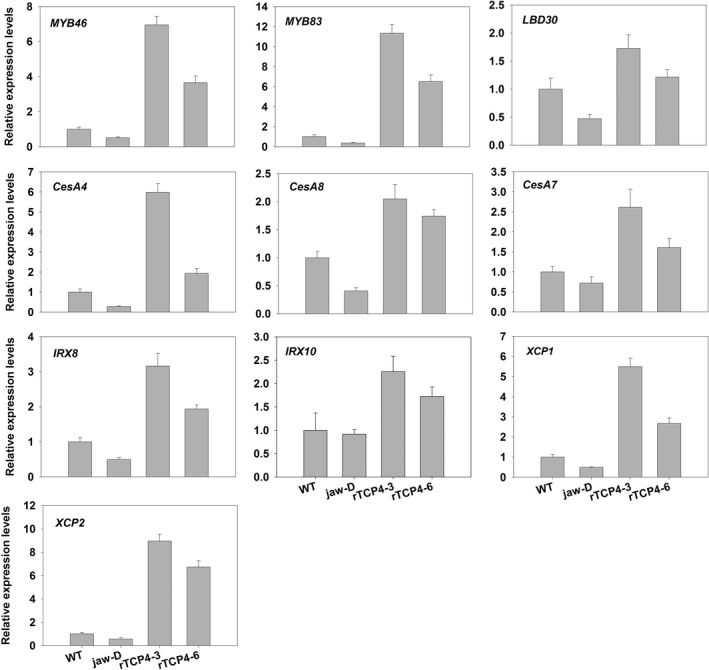
qRT‐PCR analysis of secondary wall‐associated formation genes in 2‐week‐old seedlings. Bars show the relative expression levels of each gene in the wild type, jaw‐D plants and rTCP4‐OX lines. The expression level of each gene in the wild type is set to 1. Relative gene expression was calculated by normalizing to the value in WT plants. Error bars represent SE of triplicate experiments.
Consistent with the histological data showing that TCP4 overexpression caused ectopic deposition of lignin, we found that TCP4 overexpression substantially induced the expression of genes involved in lignin biosynthetic pathways (Figure 7). Both laccase genes LAC4 and LAC17, which are involved in secondary wall formation (Berthet et al., 2011; Zhou et al., 2009), were also activated by TCP4 (Figure 7).
Figure 7.

qRT‐PCR analysis of lignin biosynthesis genes in 2‐week‐old seedlings. Bars show the relative expression levels of each gene in the wild type, jaw‐D plants and rTCP4‐OX lines. The expression level of each gene in the wild type is set to 1. Relative gene expression was calculated by normalizing to the value in WT plants. Error bars represent SE of triplicate experiments.
To further confirm that TCP4 could activate the expression of cellulose and lignin biosynthesis genes, the rTCP4 was subcloned into pER8 (Zuo et al., 2000), downstream of an estrogen‐inducible promoter, and the resulting plasmids were introduced into wild‐type Arabidopsis via the floral‐dip method (Clough and Bent, 1998). Two‐week‐old transgenic plants were treated with 2 μm estradiol, and estradiol activation of TCP4 substantially induced the expression of cellulose and lignin biosynthesis genes (Figure 8). Together, these results demonstrate that TCP4 is a transcriptional activator of cellulose and lignin biosynthesis during secondary wall formation.
Figure 8.
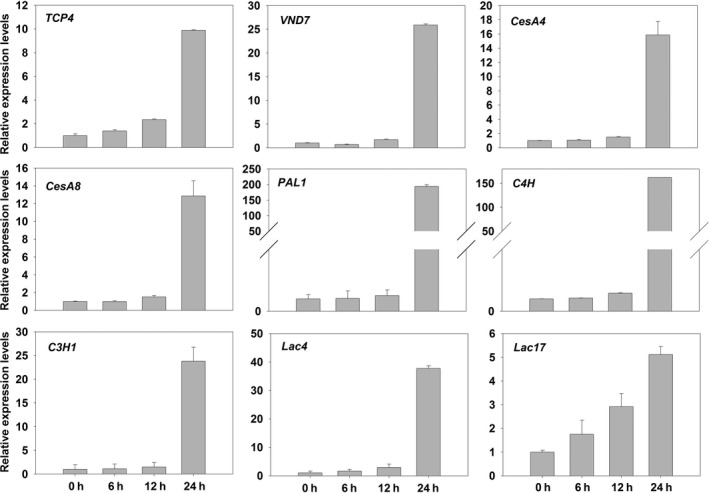
Induction of the secondary cell wall biosynthetic genes by estradiol activation of rTCP4. Bars show the relative expression levels of TCP4,VND7 and secondary cell wall biosynthetic genes expression in 2‐week‐old pER8‐rTCP4‐4 seedlings treated with 2 μm estradiol for various periods of time. The expression levels of TCP4 and VND7 in the 2‐week‐old pER8‐rTCP4‐4 seedlings without treatment are set to 1, respectively. Relative gene expression was calculated by normalizing to the value in the 2‐week‐old pER8‐rTCP4‐4 seedlings without treatment. Error bars represent SE of triplicate experiments.
Inducible overexpression of rTCP4 is sufficient to induce ectopic xylem vessel formation
To further confirm that TCP4 is involved in xylem vessel formation, pER8‐rTCP4‐4 transgenic seeds were grown in MS medium with or without 2 μm estradiol. Under estradiol‐free growth conditions, pER8‐rTCP4‐4 seedlings showed a phenotype similar to the wild type (Figure 9a). In the presence of estradiol treatment, pER8‐rTCP4‐4 seedlings exhibit long hypocotyls, epinastic cotyledons and smaller rosette leaves (Figure 9b). Cross‐sections of hypocotyls and roots of pER8‐rTCP4‐4 seedlings without estradiol treatment did not show obvious differences from the wild type (Figure 9c,e). In contrast, estradiol treatment increased the production of xylem vessel elements in pER8‐rTCP4‐4 seedlings (Figure 9d,f).
Figure 9.
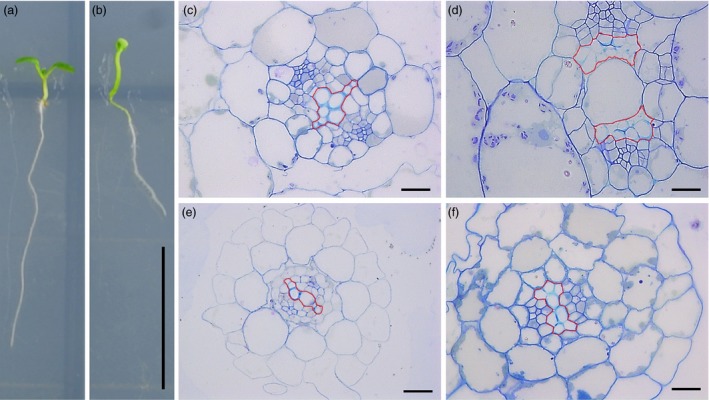
Inducible overexpression of rTCP4 increased xylem vessel element formation in transgenic Arabidopsis. (a) Phenotype of 1‐week‐old pER8‐rTCP4‐4 seedlings growing in MS medium without 2 μm estradiol. (b) Phenotype of 1‐week‐old pER8‐rTCP4‐4 seedlings growing in MS medium with 2 μm estradiol. (c) Cross‐sections of hypocotyls in (a) showing similar phenotype with wild type. (d) Cross‐sections of hypocotyls in (b) showing increased xylem vessel formation. (e) Cross‐sections of roots in (a) showing similar phenotype with wild type. (f) Cross‐sections of roots in (b) showing increased xylem vessel formation. Bars = 1 cm (a, b), 20 μm (c–f).
Transcriptional levels of TCP4 and VND7 are regulated by developmental processes
The expression of miR319 varied depending on the developmental state (Nag et al., 2009). As TCP4 is the target gene of miR319, we measured the expression levels of TCP4 and VND7 during different developmental periods. As shown in Figure 10, we found that the transcriptional levels of TCP4 and VND7 gradually increased during plant development and reached a maximum level after 5 weeks of growth. This result indicates that a positive correlation exists between TCP4 and VND7.
Figure 10.
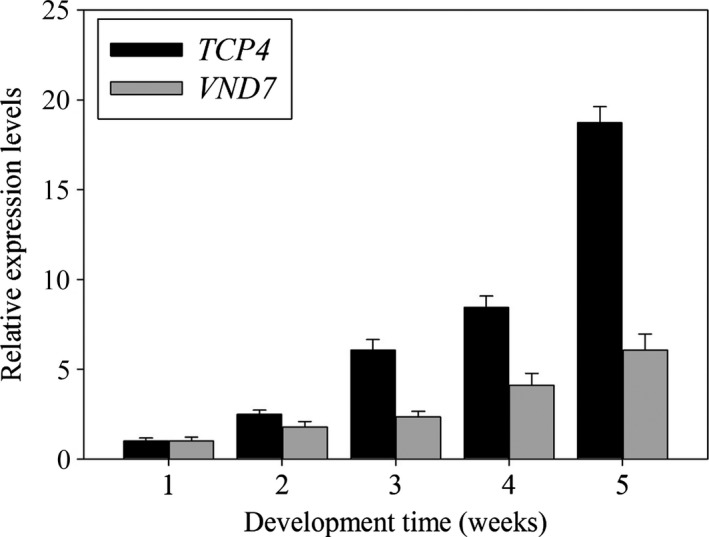
Dynamic expression of Arabidopsis TCP4 and VND7 mRNA. qRT‐PCR analysis of TCP4 and VND7 expression changes during development. The expression levels of TCP4 and VND7 in the 1‐week‐old wild‐type plants are set to 1, respectively. Relative gene expression was calculated by normalizing to the value in the 1‐week‐old wild‐type plants. Error bars represent SE of triplicate experiments.
Discussion
Previous studies demonstrated that miR319 and its targets, which comprise a set of TCP transcription factor genes, regulate various developmental physiological processes, such as leaf growth (Palatnik et al., 2003; Schommer et al., 2014), leaf senescence (Sarvepalli and Nath, 2011; Schommer et al., 2008) and petal development (Nag et al., 2009). Here, we discovered that miR319 and its target gene TCP4 simultaneously control plant developmental processes and senescence by modulating VND7‐dependent xylem vessel formation and PCD. These findings suggest that plants strictly adjust TCP4 levels to coordinate two key processes in plant biology: development and senescence.
Schommer et al. (2008) found that jaw‐D plants with high miR319 expression levels could delay senescence by reducing TCP4 expression and jasmonic acid (JA) biosynthesis. Given that JA also mediates cell wall lignification (Denness et al., 2011), it is possible that the lower level of miR319 during maturity results in a high level of TCP4 expression, which promotes JA synthesis, and that the produced JA synergistically contributes to lignin biosynthesis. Although exogenous application of JA caused premature senescence in attached and detached leaves in WT Arabidopsis, TCP4‐targeted LOX2 expression was sharply reduced during leaf senescence (He et al., 2002). LOX2‐RNAi plants and WT plants did not show any differences in senescence initiation and progression based on the assessment of chlorophyll loss during natural senescence (Seltmann et al., 2010). These results indicate that the TCP‐targeted LOX2 in the JA biosynthesis pathway is not necessary for promoting senescence in Arabidopsis, and a parallel pathway exists during the control of senescence. The differentiation of vessel cells results in an orchestrated construction of the secondary cell wall structure involving cellulosic thickening and lignification, PCD and cellular autolysis (Fukuda, 1997). Systematic analysis of gene expression revealed that many genes involved in both secondary wall formation and modification and PCD are simultaneously expressed just before morphological changes occur in TEs (Kubo et al., 2005; Ohashi‐Ito et al., 2010; Yamaguchi et al., 2010a, 2011). In this study, we showed that TCP4 binds to the promoter of VND7 and activates its expression. VND7, an essential NAC transcription factor, activates downstream networks to promote PCD of TEs and xylem vessel differentiation (Yamaguchi et al., 2011). Overexpression of VND7 causes seedlings to become pale in colour and results in their premature death (Yamaguchi et al., 2010a). TCP4 and VND7 showed a similar expression pattern in vascular tissue as shown by a promoter GUS assay (Koyama et al., 2007; Yamaguchi et al., 2008). During the promotion of maturation, the transcriptional levels of TCP4 and VND7 gradually increased during plant development. These findings suggest that TCP4 initiates PCD, at least partly, by activating the VND7 gene through the VND7 transcriptional network for secondary wall formation.
Development and senescence are widely studied processes that are fundamental for sessile plant survival in nature. Leaf senescence represents the final stage of leaf development and is critical for plant relocation of nutrients from the leaves to the reproducing seeds (Lim et al., 2007). Here, we uncovered a novel mechanism of miR319 in coordinating development and senescence in Arabidopsis. Development modulates miR319‐mediated TCP4 expression to activate the downstream VND7 network and thereby promotes secondary cell wall formation and PCD. Thus, low TCP4 levels during the juvenile‐to‐adult/senescence transition retard xylem vessel differentiation. In contrast, high TCP4 levels in adult/senescence plants promote rapid xylem vessel differentiation, which contributes to transport of water and minerals and provides mechanic strength to the entire plants, suggesting that proper levels of active TCP4 are critical for plant development. Thus, this study reveals a novel role for miR319 in coordinating plant developmental processes with senescence responses.
Experimental procedures
Plant materials and growth conditions
Arabidopsis thaliana ecotype Columbia (Col‐0) was used as the WT control. Jaw‐D seeds were obtained from Prof. Detlef Weigel (Max Planck Institute for Developmental Biology). Surface‐sterilized seeds were germinated on Murashige and Skoog (MS) medium. The seeds were kept at 4 °C for 3 days and then transferred to a greenhouse (22 °C) under long‐day conditions (16 h light/day). Seedlings were transplanted to the soil 10 days after planting.
Transgenic plants
The ORF of rTCP4 was amplified with the primer pairs 5′‐ATGTCTGACGACCAATTCCATCACC‐3′ and 5′‐TCAATGGCGAGAAATAGAGGAAGCA‐3′ using rTCP4‐GFP plasmid (Schommer et al., 2008) and then used EZ cloning into pBI121 and pER8, respectively. The resulting plasmids were introduced into Agrobacterium GV3101. Transgenic Arabidopsis lines expressing rTCP4 were obtained by Agrobacterium‐mediated flower dipping transformation. The transformants were selected on MS medium containing 50 mg/L kanamycin for pBI121‐rTCP4 and 20 mg/L hygromycin for pER8‐rTCP4.
Histology
Inflorescence stems were collected at developmental stage 6.2 (Boyes et al., 2001), corresponding to a height of 15 cm. The basal parts of the stem were fixed at 4 °C overnight with 2% glutaraldehyde in PBS (33 mm Na2HPO4 and 1.8 mm NaH2PO4, pH 7.2). After fixation, tissues embedded in low viscosity (Spurr's) resin (Electron Microscopy Sciences, PA) were sectioned (1 μm thick) and stained with toluidine blue for light microscopy. For observation of subcellular structures, 85‐nm‐thick sections were poststained with uranyl acetate and lead citrate and observed using a JEM‐1230 transmission electron microscope (JEOL, Tokyo, Japan). The wall thickness of xylem vessel elements in the stem was measured.
For examination of lignified cell walls in stems, 50‐μm‐thick sections were stained for 5 min with 1% phloroglucinol in 6 N HCl to identify lignin, which was shown as a bright red colour. Semithin sections were processed according to Sun et al. (2013). One‐micrometer‐thick sections were stained for cellulose with 0.01% Calcofluor White and observed with an ultraviolet fluorescence microscope as described previously (Zhou et al., 2009). Under the conditions used, only secondary walls exhibited brilliant fluorescence. For vessel visualizations, the roots and hypocotyls of 7‐day‐old seedlings were cleared and stained with 0.01% basic fuchsin as described previously (Dharmawardhana et al., 1992).
Protein expression and purification
The TCP4 and rTCP4 expression constructs pET32a‐TCP4 and pET32a‐rTCP4 were transformed into the Escherichia coli strain Rosseta™, respectively. LB (100 mL) containing 100 mg/mL ampicillin was inoculated with 1 mL of overnight culture and grown at 37 °C to mid‐log phase. Recombinant protein expression was induced with 0.5 mm isopropyl b‐L‐thiogalactoside. Cells were harvested after 3 h of induction. Cells were lysed according to the instructions of the MagneHis™ Protein Purification System (Promega, WI). The lysate was centrifuged, and the supernatant was loaded onto a Ni‐NTA spin column (Promega). Recombinant protein was eluted in a 200 μL volume containing 500 mm imidazole. The eluted protein was dialysed against 50 mm NaH2PO4, 300 mm NaCl and 10% glycerol for 6 h. The purification was monitored by Western blot using anti‐His HRP‐conjugated antibodies (Qiagen, Beijing, China).
Emsa
A DNA probe was generated by end‐labelling a double‐stranded oligo (5′‐ATTGTTGGGTGGTCCCATAAAAAT‐3′) containing one TCP4‐binding site with a biotin label at the 3′ end. The binding reaction was conducted in a total volume of 20 μL containing 100 fmol of probe, 1× binding buffer (20 mm HEPES‐KOH, pH 7.8, 100 mm KCl, 1 mm EDTA, 0.1% BSA, 10 ng herring sperm DNA and 10% glycerol) and 1 μg of purified protein. The mixture was incubated for 30 min at room temperature and loaded on a 6% native polyacrylamide gel. Electrophoresis was conducted at 6 V/cm for 45 min with 0.25× Tris‐borate buffer at room temperature.
Transactivation of VND7 promoter activity by TCP4 in Nicotiana benthamiana leaves
The transient expression assays were performed in N. benthamiana leaves according to previously described methods (Chen et al., 2011). The VND7 promoter was amplified with the primer pairs 5′‐TTTCATCAGTACCTGATCCAGC‐3′ and 5′‐GTGTCTTTTTGGAAGCTATTGC‐3′, cloned into the pMD18T vector (Takara, Dalian, China) and verified by sequencing. The VND7 promoter was then fused with the luciferase reporter gene LUC through EZ cloning into the plant binary vector pRI101 to generate the reporter construct Pro VND7 :LUC. The TCP4 effector construct was 35S:TCP4, and the TCP4 coding region was amplified by PCR with the primer pairs. Five independent determinations were assessed. The experiment was repeated three times with similar results.
Quantitative RT‐PCR
The aforementioned Arabidopsis seedlings were used for quantitative real‐time PCR (qRT‐PCR) analysis of gene expression. Total RNAs were extracted using Trizol reagent (Invitrogen, CA) and reverse transcribed using M‐MLV (Promega). Real‐time PCR was performed with the ABI7500 real‐time PCR system using TransStart ® Top Green qPCR SuperMix (TransGen, Beijing, China). The relative gene expression level was calculated by normalizing against the internal control Ubiquitin10. Three technical replicates were carried out for each sample. Primers are listed in Table S1.
Statistical analysis
Statistical analysis and exponential curve fitting were performed using SigmaPlot 10.0 (Systat Software Inc., San Jose, CA) software. Results are expressed as mean ± SD. Student's t‐tests were performed, and P‐values provided in results.
Conflict of interest
The authors declare no competing interests.
Supporting information
Figure S1 Cross‐sections of hypocotyls and roots of F1 seedlings.
Table S1 Primers used for qRT‐PCR analysis in this study.
Acknowledgments
We thank Prof. Detlef Weigel (Max Planck Institute Developmental Biology, Germany) for the jaw‐D seeds, rTCP4‐GFP plasmid and critical reading of the manuscript. We are grateful to Dr. Hongtao Li for practical assistance with operation of the ABI7500. We gratefully thank Prof. Anja Geitmann (McGill University, Canada) and Prof. Jinling Huang (East Carolina University, USA) for revising the manuscript about writing style, English language and syntax. This work was financially supported by the Major State Basic Research Development Program of China (2010CB951704) and the National Natural Science Foundation of China to Y. P. Y. (41271058) and X. D. S. (31400244).
Accession numbers: UBQ10 (At4 g05320); TCP4 (AT3 g15030); VND7 (AT1 g71930); LBD30 (At4 g00220); MYB46 (At5 g12870); MYB83 (AT3 g08500); CesA4 (At5 g44030); CesA7 (AT5 g17420); CesA8 (At4 g18780); IRX8 (AT5 g54690); IRX10 (AT1 g27440); XCP1 (At4 g35350); XCP2 (At1 g20850); LAC4 (At2 g38080); LAC17 (At5 g60020); PAL1 (At2 g37040); C4H (At2 g30490); HCT (At5 g48930), C3H1 (At2 g40890).
Contributor Information
Xudong Sun, Email: sunxudong@mail.kib.ac.cn.
Yunqiang Yang, Email: yangyunqiang@mail.kib.ac.cn.
References
- Avci, U. , Petzold, H.E. , Ismail, I.O. , Beers, E.P. and Haigler, C.H. (2008) Cysteine proteases XCP1 and XCP2 aid micro‐autolysis within the intact central vacuole during xylogenesis in Arabidopsis roots. Plant J. 56, 303–315. [DOI] [PubMed] [Google Scholar]
- Berthet, S. , Demont‐Caulet, N. , Pollet, B. , Bidzinski, P. , Cezard, L. , Le Bris, P. , Borrega, N. et al (2011) Disruption of LACCASE4 and 17 results in tissue‐specific alterations to lignification of Arabidopsis thaliana stems. Plant Cell, 23, 1124–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes, D.C. , Zayed, A.M. , Ascenzi, R. , McCaskill, A.J. , Hoffman, N.E. , Davis, K.R. and Görlach, J. (2001) Growth stage–based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell, 13, 1499–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D.M. , Zeef, L.A.H. , Ellis, J. , Goodacre, R. and Turner, S.R. (2005) Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. Plant Cell, 17, 2281–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. (2009) Small RNAs and their roles in plant development. Ann. Rev. Cell Dev. 25, 21–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Sun, J. , Zhai, Q. , Zhou, W. , Qi, L. , Xu, L. , Wang, B. et al (2011) The basic helix‐loop‐helix transcription factor MYC2 directly represses PLETHORA expression during jasmonate‐mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell, 23, 3335–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough, S.J. and Bent, A.F. (1998) Floral dip: a simplified method for Agrobacterium‐mediated transformation of Arabidopsis thaliana . Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Denness, L. , McKenna, J.F. , Segonzac, C. , Wormit, A. , Madhou, P. , Bennett, M. , Mansfield, J. et al (2011) Cell wall damage‐induced lignin biosynthesis is regulated by a reactive oxygen species‐and jasmonic acid‐dependent process in Arabidopsis. Plant Physiol. 156, 1364–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmawardhana, D. , Ellis, B. and Carlson, J. (1992) Characterization of vascular lignification in Arabidopsis thaliana . Can. J. Bot. 70, 2238–2244. [Google Scholar]
- Ellis, B. , Schuetz, M. , Douglas, C. and Samuels, L. (2014) Manipulating lignin deposition. Can. J. Plant Sci. 94, 1043–1049. [Google Scholar]
- Fukuda, H. (1997) Tracheary element differentiation. Plant Cell, 9, 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y. , Fukushige, H. , Hildebrand, D.F. and Gan, S. (2002) Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 128, 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones‐Rhoades, M.W. , Bartel, D.P. and Bartel, B. (2006) MicroRNAs and their regulatory roles in plants. Annu. Rev. Plant Biol. 57, 19–53. [DOI] [PubMed] [Google Scholar]
- Koyama, T. , Furutani, M. , Tasaka, M. and Ohme‐Takagi, M. (2007) TCP transcription factors control the morphology of shoot lateral organs via negative regulation of the expression of boundary‐specific genes in Arabidopsis. Plant Cell, 19, 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo, M. , Udagawa, M. , Nishikubo, N. , Horiguchi, G. , Yamaguchi, M. , Ito, J. , Mimura, T. et al (2005) Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev. 19, 1855–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Li, B. , Shen, W.H. , Huang, H. and Dong, A. (2012) TCP transcription factors interact with AS2 in the repression of class‐I KNOX genes in Arabidopsis thaliana . Plant J. 71, 99–107. [DOI] [PubMed] [Google Scholar]
- Lim, P.O. , Kim, H.J. and Nam, H.G. (2007) Leaf senescence. Annu. Rev. Plant Biol. 58, 115–136. [DOI] [PubMed] [Google Scholar]
- McCarthy, R.L. , Zhong, R. and Ye, Z.‐H. (2009) MYB83 is a direct target of SND1 and acts redundantly with MYB46 in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell Physiol. 50, 1950–1964. [DOI] [PubMed] [Google Scholar]
- Nag, A. , King, S. and Jack, T. (2009) miR319a targeting of TCP4 is critical for petal growth and development in Arabidopsis. Proc. Natl Acad. Sci. USA, 106, 22534–22539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi‐Ito, K. , Oda, Y. and Fukuda, H. (2010) Arabidopsis VASCULAR‐RELATED NAC‐DOMAIN6 directly regulates the genes that govern programmed cell death and secondary wall formation during xylem differentiation. Plant Cell, 22, 3461–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori, N. , Cohen, A.R. , Etzioni, A. , Brand, A. , Yanai, O. , Shleizer, S. , Menda, N. et al (2007) Regulation of LANCEOLATE by miR319 is required for compound‐leaf development in tomato. Nat. Genet. 39, 787–791. [DOI] [PubMed] [Google Scholar]
- Palatnik, J.F. , Allen, E. , Wu, X. , Schommer, C. , Schwab, R. , Carrington, J.C. and Weigel, D. (2003) Control of leaf morphogenesis by microRNAs. Nature, 425, 257–263. [DOI] [PubMed] [Google Scholar]
- Sarvepalli, K. and Nath, U. (2011) Hyper‐activation of the TCP4 transcription factor in Arabidopsis thaliana accelerates multiple aspects of plant maturation. Plant J. 67, 595–607. [DOI] [PubMed] [Google Scholar]
- Schommer, C. , Palatnik, J.F. , Aggarwal, P. , Chetelat, A. , Cubas, P. , Farmer, E.E. , Nath, U. et al (2008) Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol. 6, 1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schommer, C. , Debernardi, J.M. , Bresso, E.G. , Rodriguez, R.E. and Palatnik, J.F. (2014) Repression of cell proliferation by miR319‐regulated TCP4. Mol. Plant, 7, 1533–1544. [DOI] [PubMed] [Google Scholar]
- Seltmann, M.A. , Stingl, N.E. , Lautenschlaeger, J.K. , Krischke, M. , Mueller, M.J. and Berger, S. (2010) Differential impact of lipoxygenase 2 and jasmonates on natural and stress‐induced senescence in Arabidopsis. Plant Physiol. 152, 1940–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano, T. , Thitamadee, S. , Machida, Y. and Chua, N.H. (2008) ASYMMETRIC LEAVES2‐LIKE19/LATERAL ORGAN BOUNDARIES DOMAIN30 and ASL20/LBD18 regulate tracheary element differentiation in Arabidopsis . Plant Cell, 20, 3359–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X.D. , Feng, Z.H. , Meng, L.S. , Zhu, J. and Geitmann, A. (2013) Arabidopsis ASL11/LBD15 is involved in shoot apical meristem development and regulates WUS expression. Planta, 237, 1367–1378. [DOI] [PubMed] [Google Scholar]
- Sunkar, R. (2010) MicroRNAs with macro‐effects on plant stress responses Seminars in Cell & Developmental Biology, 21, 805–811. [DOI] [PubMed] [Google Scholar]
- Taylor, N.G. , Howells, R.M. , Huttly, A.K. , Vickers, K. and Turner, S.R. (2003) Interactions among three distinct CesA proteins essential for cellulose synthesis. Proc. Natl Acad. Sci. USA, 100, 1450–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, R.S. , Tarver, J.E. , Hiscock, S.J. and Donoghue, P.C. (2014) Evolutionary history of plant microRNAs. Trends Plant Sci. 19, 175–182. [DOI] [PubMed] [Google Scholar]
- Voinnet, O. (2009) Origin, biogenesis, and activity of plant microRNAs. Cell, 136, 669–687. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, M. , Kubo, M. , Fukuda, H. and Demura, T. (2008) Vascular‐related NAC‐DOMAIN7 is involved in the differentiation of all types of xylem vessels in Arabidopsis roots and shoots. Plant J. 55, 652–664. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, M. , Goué, N. , Igarashi, H. , Ohtani, M. , Nakano, Y. , Mortimer, J.C. , Nishikubo, N. et al (2010a) VASCULAR‐RELATED NAC‐DOMAIN6 and VASCULAR‐RELATED NAC‐DOMAIN7 effectively induce transdifferentiation into xylem vessel elements under control of an induction system. Plant Physiol. 153, 906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, M. , Ohtani, M. , Mitsuda, N. , Kubo, M. , Ohme‐Takagi, M. , Fukuda, H. and Demura, T. (2010b) VND‐INTERACTING2, a NAC domain transcription factor, negatively regulates xylem vessel formation in Arabidopsis. Plant Cell, 22, 1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, M. , Mitsuda, N. , Ohtani, M. , Ohme‐Takagi, M. , Kato, K. and Demura, T. (2011) VASCULAR‐RELATED NAC‐DOMAIN7 directly regulates the expression of a broad range of genes for xylem vessel formation. Plant J. 66, 579–590. [DOI] [PubMed] [Google Scholar]
- Zeng, W. , Lampugnani, E.R. , Picard, K.L. , Song, L. , Wu, A.‐M. , Farion, I.M. , Zhao, J. et al (2016) Asparagus IRX9, IRX10, and IRX14A are components of an active xylan backbone synthase complex that forms in the Golgi apparatus. Plant Physiol. 171, 93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, R. and Ye, Z.‐H. (2007) Regulation of cell wall biosynthesis. Curr. Opin. Plant Biol. 10, 564–572. [DOI] [PubMed] [Google Scholar]
- Zhong, R. , Demura, T. and Ye, Z.‐H. (2006) SND1, a NAC domain transcription factor, is a key regulator of secondary wall synthesis in fibers of Arabidopsis. Plant Cell, 18, 3158–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, R. , Richardson, E.A. and Ye, Z.‐H. (2007) The MYB46 transcription factor is a direct target of SND1 and regulates secondary wall biosynthesis in Arabidopsis. Plant Cell, 19, 2776–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. , Lee, C. , Zhong, R. and Ye, Z.H. (2009) MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell, 21, 248–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, J. , Niu, Q.W. and Chua, N.H. (2000) Technical advance: an estrogen receptor‐based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24, 265–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Cross‐sections of hypocotyls and roots of F1 seedlings.
Table S1 Primers used for qRT‐PCR analysis in this study.


