Summary
The AP2/ERF family is a plant‐specific transcription factor family whose members have been associated with various developmental processes and stress tolerance. Here, we functionally characterized the drought‐inducible OsERF48, a group Ib member of the rice ERF family with four conserved motifs, CMI‐1, ‐2, ‐3 and ‐4. A transactivation assay in yeast revealed that the C‐terminal CMI‐1 motif was essential for OsERF48 transcriptional activity. When OsERF48 was overexpressed in an either a root‐specific ( ROX O s ERF 48 ) or whole‐body ( OX O s ERF 48 ) manner, transgenic plants showed a longer and denser root phenotype compared to the nontransgenic (NT) controls. When plants were grown on a 40% polyethylene glycol‐infused medium under in vitro drought conditions, ROX O s ERF 48 plants showed a more vigorous root growth than OX O s ERF 48 and NT plants. In addition, the ROX O s ERF 48 plants exhibited higher grain yield than OX O s ERF 48 and NT plants under field‐drought conditions. We constructed a putative OsERF48 regulatory network by cross‐referencing ROX O s ERF 48 root‐specific RNA‐seq data with a co‐expression network database, from which we inferred the involvement of 20 drought‐related genes in OsERF48‐mediated responses. These included genes annotated as being involved in stress signalling, carbohydrate metabolism, cell‐wall proteins and drought responses. They included, OsCML16, a key gene in calcium signalling during abiotic stress, which was shown to be a direct target of OsERF48 by chromatin immunoprecipitation‐qPCR analysis and a transient protoplast expression assay. Our results demonstrated that OsERF48 regulates OsCML16, a calmodulin‐like protein gene that enhances root growth and drought tolerance.
Keywords: OsERF48, drought tolerance, root growth, co‐regulatory network, calmodulin‐like protein
Introduction
Plants dehydrate during limited water conditions, whereupon they close their stomata to avoid further water loss, while experiencing a stress‐induced reduction in growth. Roots are the first organs to sense drought conditions, and their regulation of water uptake is believed to be an important mechanism for managing restricted water availability (Matsuo et al., 2009). A robust root system is therefore both valuable for enhancing plant growth and integral in water stress responses (Uga et al., 2013). Root development is under complex cellular and metabolic control, and many of the associated processes are known to be regulated by specific transcription factors (TFs), including members of the APETALA2/ethylene‐responsive element binding factor (AP2/ERF), MYB, bZIP and NAC families (reviewed by Nakashima et al., 2014).
The AP2/ERF superfamily is one of the largest plant‐specific TF families. Proteins in this family contain a single AP2/ERF domain and are classified into ten subgroups (I–X), each with unique conserved motifs (Nakano et al., 2006). Two motifs have been functionally characterized: one from group VIII (CMVIII‐1), which is identical to an ERF‐associated amphiphilic repression (EAR) motif (Ohta et al., 2001) and the CMIX‐1 domain from Arabidopsis thaliana group IX, which is a transcriptional activation domain with a unique ‘EDLL’ motif (Tiwari et al., 2012). The other conserved motifs have yet to be characterized. AP2/ERF family members have been associated with root developmental processes and stress tolerance. A. thaliana AP2/EREBP PUCH1 and poplar (Populus trichocarpa) PtaERF003 promote lateral root formation (Hirota et al., 2007; Trupiano et al., 2013), while rice (Oryza sativa) ERF3/AP37 is essential for crown root development (Zhao et al., 2015) and, when overexpressed, significantly increases grain yield under field‐drought conditions (Oh et al., 2009). It was proposed that a particularly well‐developed root system in the ERF3/AP37 overexpressing plants was responsible for the increased grain yield (Zhao et al., 2015). In addition, overexpression of the A. thaliana HARDY gene in rice improved water‐use efficiency by increasing root biomass under drought conditions (Karaba et al., 2007). Recently, it was reported that overexpression of OsERF71 (Lee et al., 2016a) and HIGHER YIELD RICE/OsERF137 (Ambavaram et al., 2014) in rice resulted in vigorous root growth that improved grain yield under drought conditions. Finally, overexpression of other AP2/ERF TFs has been found to confer drought tolerance by elevating the levels of osmoprotectants, including proline and soluble sugars (Quan et al., 2010; Wang et al., 2015; Zhang et al., 2010).
In addition to TFs, Ca2+ acts as a core signal transducer and regulator in many plant adaptations to environmental conditions, and elevated levels of cytosolic calcium concentration are central to many stress responses (Zeng et al., 2015). Major transduction routes of Ca2+ signalling involve the calcium‐binding proteins, calmodulin (CaM) and CaM‐like proteins (CML), calcium‐dependent protein kinases (CDPKs) and calcineurin‐B‐like proteins (CBL) (Reddy et al., 2011). CMLs are multifunctional proteins that interact with a broad range of Ca2+ binding downstream targets by directly or indirectly binding to their promoters, in combination with calmodulin‐binding proteins (CBPs). These interactions mediate the regulation of various target proteins, such as protein kinases/phosphatases, transcription factors, metabolic enzymes, ion channels and structural proteins (reviewed by Reddy et al., 2011; Zeng et al., 2015). There is considerable evidence that CMLs play a critical role in Ca2+ signalling during plant adaptations to abiotic stress in tomato (Solanum lycopersicum) (Munir et al., 2016), A. thaliana (Magnan et al., 2008; Park et al., 2010) and rice (Xu et al., 2011). However, the molecular mechanisms by which CMLs regulate stress responses remain unknown.
In the present study, we investigated the roles of OsERF48, a rice gene encoding an ERF TF, by overexpressing it in transgenic rice plants in either a root‐specific or whole‐body expression manner. Root‐specific overexpressors showed improved drought tolerance by a promotion of root growth than whole‐body overexpressors or nontransgenic (NT) control plants. By cross‐referencing RNA‐seq data with a co‐expression database, we constructed a putative regulatory network of OsERF48 in which OsCML16, a calmodulin‐like protein, plays a central role. Our results suggest that OsERF48 regulates OsCML16, which in turn enhances root growth and drought tolerance.
Results
OsERF48 is a drought‐inducible transcriptional activator
OsERF48 (Os08g0408500) transcript levels increased upon exposure of 2‐week‐old rice seedlings to drought and high‐salt conditions, but not upon exposure to the hormone abscisic acid (ABA) and low temperature (Figure 1a). To verify that OsERF48 was localized in the nucleus, which would be expected for a transcription factor, a construct with the full‐length OsERF48 translationally fused to green fluorescent protein (GFP) was transformed into rice protoplasts, together with a gene encoding the nuclear localized reporter OsNF‐YA7‐mCherry as a positive control (Lee et al., 2015). GFP and mCherry fluorescence co‐localized in the nucleus of rice protoplasts (Figure 1b), indicating nuclear localization of OsERF48.
Figure 1.

OsERF48 is a nuclear protein with transcriptional activation. (a) Relative expression of OsERF48 in response to abiotic stresses. Two‐week‐old seedlings were exposed to air‐drying (drought), 400 mm NaCl (salt), 100 μm abscisic acid (ABA) at 28 °C and at 4 °C (low temperature) for the indicated time points. OsUbi1 expression was used as an internal control. Values are the means ± SD (standard deviation) of three independent experiments. (b) Subcellular localization of OsERF48 in rice protoplasts. Protoplasts were transiently co‐transformed with OsERF48‐GFP and the nuclear localization control OsNF‐YA7‐mCherry. Fluorescence was observed using a confocal microscope. (c–d) Transactivation activity of OsERF48 using a yeast system. (c) Schematic structure of OsERF48 full length and deletion mutants. (d) Transformed yeast cells harbouring the indicated constructs on SD/‐Trp and SD/‐Trp/AbA200/X‐α‐gal. NC, negative control ( pGBKT7); PC, positive control ( pGBKT7‐53 + pGADT7‐RecT); AP2/ERF, AP2/ERF domain, CMI, conserved motif of group I, GBD, GAL4 DNA‐binding domain.
To confirm the transcriptional activity of OsERF48, the GAL4 DNA‐binding domain (DBD) was fused to a full‐length or deletion mutants of OsERF48 (Figure S1) and reporter genes expressed in yeast under the control of the GAL4 target‐binding site. Overexpression of the full‐length protein (OsERF48F) or either of two N‐terminal deletion mutants (OsERF48Δc4 and OsERF48Δc3c4) induced expression of the reporter genes, demonstrating their transcriptional activity (Figure 1c, d). However, C‐terminal deletions of CMI‐1 and CMI‐2 (OsERF48Δc1c2), or CMI‐1 only (OsERF48Δc1), resulted in no induction of the reporter genes (Figure 1c, d), suggesting that CMI‐1 is important for the transcriptional activity of OsERF48.
Overexpression of OsERF48 in rice confers drought tolerance at the vegetative stage
To investigate the biological function of OsERF48, we generated three types of transgenic rice plants, in the Ilmi cultivar: one with root‐specific overexpression (ROX OsERF48 ); the second with whole‐body overexpression (OX OsERF48 ); and the third with RNA‐interference (RNAi OsERF48 )‐mediated suppression of the OsERF48 gene (Figure S2a, b). We chose single‐copy T3 homozygous transgenic lines for subsequent analysis (Figure S3) and showed that the OsERF48 transcript levels were elevated in both leaves and roots of OX OsERF48 and only in roots of ROX OsERF48 plants, while they were decreased by 50% compared to nontransgenic control (NT) plants in RNAi OsERF48 roots (Figure 2a).
Figure 2.
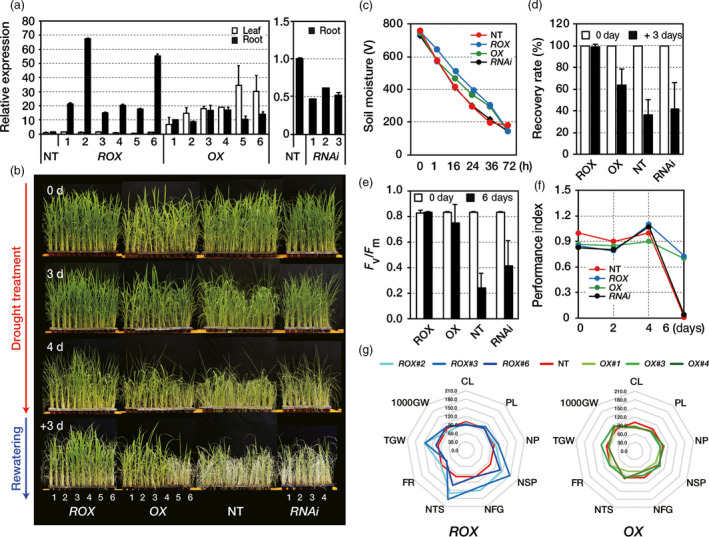
Drought tolerance of OsERF48 overexpressing plants. (a) Relative expression of OsERF48 transcripts in transgenic and nontransgenic (NT) plants. Two‐week‐old roots or leaves from transgenic plants were used for the analysis. OsUbi1 expression was used as an internal control. Values are the means ± SD of three independent experiments. (b–d) All plants were grown in soil for 1 month under well‐watered conditions and were then exposed to drought stress for 4 days, followed by re‐watering for 3 days in the greenhouse. (b) Drought tolerance of transgenic and NT plants. (c) Soil moisture in the pots exposed to drought treatment at the indicated time points. Values are the means ± SD (n = 20). (d) Recovery rate scored 7 days after re‐watering. Values are the means ± SD (n = 30). (e, f) Determination of the photosynthetic viability of transgenic and NT plants under drought conditions. All plants were grown in soil for 2 months under well‐watered conditions and then exposed to drought stress for 6 days. At the indicated time point after exposure to drought stress, the chlorophyll fluorescence (F v /F m ) (e) and performance index (f) of transgenic and NT plants were measured. Each data point represents the mean ± SD (n = 30 points per independent lines of each genotypes). (g) Agronomic traits of transgenic and NT plants under drought conditions. The spider plot represents the agronomic traits by the percentage of the mean values (n = 18), listed in Table 1. Mean measurements from the NT control were assigned a 100% reference value. CL, culm length; PL, panicle length; NP, number of panicle; NSP, number of spikelet per panicle; NFG, number of filled grain; NTS, number of total spikelet; FR, filling rate; TGW, total grain weight; 1000GW, 1000 grain weight. ROX , ROX O s ERF 48 ; OX , OX O s ERF 48 .
To perform a drought tolerance test, 1‐month‐old transgenic and NT plants were exposed to drought by withholding water for several days. Soil moisture showed a constant decrease over this period, indicating that the stress was uniformly applied to the plants (Figure 2c). Visual drought‐associated symptoms, such as leaf rolling and wilting, occurred earlier in NT and RNAi OsERF48 plants than in the OsERF48 overexpressing plants (Figure 2b). Notably, all plants of the ROX OsERF48 lines showed far fewer such symptoms than the other plants after 4 days of drought exposure, and they also had the highest recovery rate (99%) after re‐watering. Two of six OX OsERF48 lines, as well as all the RNAi OsERF48 and NT plants exhibited severe drought symptoms, indicative of a much lower recovery rate than the ROX OsERF48 plants after re‐watering (Figure 2d). To verify the drought tolerance, we selected 3 independent transgenic lines harbouring each construct (#2, 3 and 6 for ROX OsERF48 ; #1, 3 and 4 for OX OsERF48 ; # 1, 2 and 4 for RNAi OsERF48 ) and determined their F v /F m values and performance index, which represent two different indicators of photochemical efficiency, under drought conditions. Leaves of the ROX OsERF48 and the OX OsERF48 plants remained more viable with higher F v /F m values (Figure S4a, b) and performance index values after 6 days of drought treatment than the NT and RNAi OsERF48 plants (Figure 2e, f), indicating a higher drought tolerance in the overexpression plants than in the knock‐down (RNAi) and NT controls.
ROX OsERF48 lines have increased grain yield under drought conditions
Since crop productivity is closely associated with drought stress, we evaluated yield components of the transgenic plants under normal and field‐drought conditions. OsERF48 overexpressing plants exhibited several growth defects, such as reduced plant height, panicle and spikelet number, which collectively contributed to a lower total grain weight under normal growth conditions (Table 1). However, under field‐drought conditions, the number of total spikelets (NTS) and total grain weight (TGW) of the ROX OsERF48 plants were 33%–85% and 6%–47% greater, respectively, while those of the OX OsERF48 plants were similar to the NT plants (Table 1; Figure 2g). These results suggest that the ROX OsERF48 plants showed enhanced drought tolerance during the reproductive stage of growth hence higher grain yield under field‐drought conditions.
Table 1.
Agronomic traits of transgenic rice plants grown under normal and drought conditions
| Genotype | Culm length (cm) | Panicle length (cm) | No. of panicle/hill | No. of spikelet/panicle | No. of total spikelet/hill | Filling rate (%) | Total grain weight (g) | 1000 grain weight (g) |
|---|---|---|---|---|---|---|---|---|
| Normal condition | ||||||||
| NT | 69.4 | 20.3 | 16.6 | 116.3 | 1935.5 | 91.5 | 46.8 | 26.6 |
| ROX‐2 | 58.7** | 21.2 | 10.8** | 120.3 | 1283.1** | 75.5** | 26.2** | 29.2 |
| %Δ | −15.4 | 4.4 | −35.3 | 3.4 | −33.7 | −17.4 | 44.1 | 10.0 |
| ROX‐3 | 63.7** | 21.4 | 13.0** | 123.3 | 1575.9** | 87.2** | 38.0** | 28.0* |
| %Δ | −8.2 | 5.7 | −21.8 | 6.0 | −18.6 | −4.7 | −18.8 | 5.5 |
| ROX‐6 | 63.5** | 19.5 | 11.4** | 126.4** | 1444.4** | 76.6** | 26.9** | 25.3 |
| %Δ | −8.4 | −3.6 | −31.1 | 8.6 | −25.4 | −16.3 | −42.5 | −4.7 |
| OX‐1 | 64.4** | 19.3 | 13.8** | 104.2 | 1387.3** | 85.9** | 33.2** | 28.1 |
| %Δ | −7.1 | −4.8 | −17.2 | −10.4 | −28.3 | 6.1 | −29.0 | 6.0 |
| OX‐3 | 60.6** | 18.8 | 16.8 | 100.1** | 1657.2 | 82.3** | 36.1** | 26.7 |
| %Δ | −12.7 | −7.5 | 0.8 | −13.9 | −14.4 | −10.0 | −22.8 | 0.6 |
| OX‐4 | 60.7** | 18.4 | 15.0 | 87.0** | 1307.4** | 80.6** | 28.3** | 27.0 |
| %Δ | −12.4 | −9.2 | −9.5 | −25.2 | −32.5 | −11.9 | −39.5 | 1.7 |
| Drought condition | ||||||||
| NT | 56.4 | 17.9 | 11.5 | 67.0 | 759.9 | 64.7 | 12.1 | 27.1 |
| ROX‐2 | 54.9 | 17.8 | 12.4 | 98.0** | 1224.8** | 65.5 | 17.7** | 23.9 |
| %Δ | −2.8 | −0.6 | 7.3 | 46.3 | 61.2 | 1.2 | 47.0 | −11.7 |
| ROX‐3 | 49.7** | 19.3 | 13.4 | 120.0** | 1404.0 | 49.5 | 17.6 | 28.6 |
| %Δ | −11.9 | 8.1 | 16.2 | 79.2 | 84.8 | −23.5 | 46.1 | 5.9 |
| ROX‐6 | 51.4** | 17.9 | 10.6 | 92.8** | 1007.1 | 51.9 | 12.8 | 29.3 |
| %Δ | −8.9 | 0.1 | −7.7 | 38.5 | 32.5 | −19.9 | 6.2 | 8.3 |
| OX‐1 | 45.4** | 16.6 | 10.7 | 56.3* | 571.7** | 65.5 | 10.7 | 29.5 |
| %Δ | −19.4 | −7.0 | −7.5 | −15.9 | −24.8 | 1.3 | −11.0 | 9.2 |
| OX‐3 | 48.1** | 15.7 | 10.5 | 70.0 | 740.0 | 65.5 | 14.3 | 30.9 |
| %Δ | −14.6 | −12.0 | −9.3 | 4.4 | −2.6 | 1.2 | 18.8 | 14.2 |
| OX‐4 | 47.9** | 16.3 | 12.1 | 65.5 | 785.3 | 50.7* | 11.3 | 30.1 |
| %Δ | −15.0 | −9.0 | 4.8 | −2.3 | 3.3 | −21.7 | −6.0 | 11.2 |
Each data values represents the mean (n = 30, normal conditions; n = 18, drought conditions) for transgenic and NT control plants. Single (*P < 0.05) and two asterisks (**P < 0.01) represent significant differences by the Student's t‐test between the transgenic and NT plants.
ROX OsERF48 lines have enhanced root growth under drought conditions
Under normal growth conditions, primary roots of ROX OsERF48 and OX OsERF48 plants were 19%, longer than NT roots, resulting in a deeper root growth (Figures 3a, S5a‐c). In addition, the ROX OsERF48 and OX OsERF48 roots had a higher lateral root density by 43.8% and 16.4%, respectively, than the NT plants (Figures 3c, S5d). In contrast, the primary root length of the RNAi OsERF48 plants was similar to that of the NT plants, and the lateral root density was 28% lower (Figure 3d, e). The root‐to‐shoot (R/S) ratio of the ROX OsERF48 and OX OsERF48 plants was 65% and 64% higher, respectively, than the NT plants (Table 2), and this correlated with increased root dry weight values in the ROX OsERF48 and OX OsERF48 plants, which were approximately twice that of the NT plants (Table 2). We concluded that overexpression of OsERF48 caused longer and denser root growth, resulting in a more vigorous root growth phenotype with higher R/S ratio.
Figure 3.

OsERF48 overexpressing lines have vigorous root growth. (a–c) Comparison of 6‐day‐old seedlings roots of OsERF48 overexpression and nontransgenic (NT) plants. (a) Root morphology of plants grown on normal growth media. (b) Root growth of plants grown on a 40% polyethylene glycol (PEG)‐infused media for in vitro drought conditions. (c) Lateral root morphology of plants grown on normal growth media (scale bar, 1 mm). (d) Lateral root morphology of RNAi Os ERF 48 and NT plants (scale bar, 2 mm). (e) Lateral root density of RNAi Os ERF 48 and NT plants. (f) Primary root length of the OsERF48 overexpressing and NT plants grown on the PEG‐infused media. (g) Lateral root density of the OsERF48 overexpressing and NT plants grown on the PEG‐infused media. Lateral root density was measured within a 3‐ to 4.5‐cm region from the root tip. Each data point represents the mean ± SD (n = 15 plants per independent lines of each genotypes). A single (*P < 0.05) and two asterisks (**P < 0.01) represent significant differences, as determined by the student's t‐test between the transgenic and NT plants. PR, primary root; LR, lateral root; ROX , ROX O s ERF 48 ; OX , OX O s ERF 48 ; RNAi, RNAi Os ERF 48 .
Table 2.
Root and shoot dry weights in OsERF48 transgenic and nontransgenic (NT) plants
| Genotype | Shoot | Root | R/S (%) | ||
|---|---|---|---|---|---|
| Dry weight | Δ% | Dry weight | Δ% | ||
| NT | 49.85 ± 1.75 | 10.5 ± 1.63 | 0.21 ± 0.03 | ||
| ROX | |||||
| 2 | 60.13 ± 3.23* | 20.6 | 20.90 ± 1.45** | 98.6 | 0.35 ± 0.03** |
| 3 | 59.13 ± 4.06* | 18.6 | 20.77 ± 1.47** | 97.3 | 0.35 ± 0.04** |
| 6 | 61.47 ± 3.50* | 23.3 | 21.20 ± 2.96* | 101.4 | 0.34 ± 0.07 |
| Average | 60.24 ± 3.50 | 23.3 | 20.96 ± 2.96 | 101.4 | 0.35 ± 0.07 |
| OX | |||||
| 1 | 51.33 ± 4.26 | 3.0 | 21.40 ± 1.47** | 103.3 | 0.42 ± 0.06** |
| 3 | 58.03 ± 4.91 | 16.4 | 19.70 ± 2.88* | 87.2 | 0.34 ± 0.03** |
| 4 | 49.70 ± 2.79 | −0.3 | 16.30 ± 4.19 | 54.9 | 0.33 ± 0.11 |
| Average | 53.87 ± 2.79 | 8.1 | 18.00 ± 4.19 | 71.0 | 0.33 ± 0.11 |
Mean ± SD followed by a single (*P < 0.05) or two asterisks (**P < 0.01) represent significant differences as determined by the Student's t‐test between the transgenic and nontransgenic (NT) plants. ROX, ROX OsERF48 ; OX, OX OsERF48 .
To observe the root phenotype under drought conditions, seedlings were grown in PEG‐infused media (0%, 25% and 40% PEG). Figure 3b shows that under severe drought conditions (40% PEG), roots of the ROX OsERF48 plants were denser and the lateral roots were longer than those of the OX OsERF48 plants. Root growth of the NT and OX OsERF48 plants in 40% PEG‐infused media was severely retarded in terms of primary root length (by 35% and 19%, respectively) and lateral root density (both by 27%). Under the same drought conditions, the ROX OsERF48 plants maintained their root growth with far less of a reduction in primary root length (5.3%) and lateral root density (2.3%) than the other plants. Thus, under drought conditions, the ROX OsERF48 plants had a more vigorous root phenotype than the NT and OX OsERF48 plants, which is consistent with enhanced drought tolerance.
Construction of an OsERF48 transcriptional co‐regulatory network
To identify OsERF48 target genes, we performed a RNA‐seq analysis on roots of two independent ROX OsERF48 lines with high OsERF48 transcript levels (ROX#2 and ROX#6) together with nontransgenic plants grown under normal conditions. A total of 200 differentially expressed genes (DEGs) with significant changes in transcript abundance were found, of which 159 and 41 were up‐ and down‐regulated in ROX OsERF48 roots, respectively, compared to NT roots (Figure S6a, b). To verify the transcriptome profile, we analysed the expression of 18 putatively up‐regulated genes by qRT‐PCR, using ROX#2 and ROX#6 root samples, which gave a similar pattern of gene expression to the RNA‐seq data (Figure S6c).
In order to construct a co‐regulatory network for OsERF48, we selected 56 genes (Table S1) from the 200 DEGs identified in the ROX OsERF48 roots by filtering with the following criteria: (i) genes that were up‐regulated in the ROX OsERF48 roots; (ii) genes that were up‐regulated in drought‐treated rice roots available in a public database (Kawahara et al., 2016); and (iii) genes that were annotated in RAB‐DB (http://rapdb.dna.affrc.go.jp/) and NCBI (http://www.ncbi.nlm.nih.gov) (Figure S6d). We then generated a co‐expression matrix of those 56 genes based on the ‘single‐gene guide’ approach of the RiceFREND (http://ricefrend.dna.affrc.go.jp/) web tool and sorted the genes with high co‐expression frequency (Figure S7). The OsERF48 co‐regulatory network was constructed by applying the 20 selected genes from the co‐expression matrix using the ‘multi‐gene guide’ approach in RiceFREND (Figure 4a; Table S2). The 20 genes, including OsERF48, were classified into three groups based on GO terms: ‘signal transduction’, ‘carbohydrate metabolism’ and ‘stress response’ (Table 3). The expression levels of the nine genes were verified by qRT‐PCR analysis of 2‐week‐old ROX OsERF48 and RNAi OsERF48 root samples (Figure 4b, c). Genes associated with ‘signal transduction’–CALMODULIN‐LIKE PROTEIN 16 (OsCML16), a small calcium‐binding protein, C‐TERMINAL CENTRIN‐LIKE DOMAIN 1 (OsCCD1) and DEHYDRATION RESPONSE ELEMENT‐BINDING PROTEIN 1c (OsDREB1c), ‘drought response’–LATE EMBRYOGENESIS ABUNDANT PROTEIN 23/DEHYDRATION INDUCIBLE PROEIN 1 (OsLEA23/DIP1) and OsLEA24/GALACTINOL SYNTHASE 2 (OsLEA24/OsGolS2), and ‘carbohydrate metabolism’–OsGolS1, RAFFINOSE SYNTHASE 5 (RS5), XYLOGLUCAN ENDOTRANSGLUCOSYLASE‐HYDROLASE 9 (OsXTH9) and ARABINOGALACTAN PROTEIN 3 (OsAGP3) were observed to be up‐regulated in ROX OsERF48 roots and down‐regulated in the RNAi OsERF48 roots, supporting the validity of the constructed network.
Figure 4.

OsERF48 transcriptional co‐regulatory network. (a) OsERF48 and its co‐expressed genes were selected from the differentially expressed genes (DEGs) in ROX O s ERF 48 roots and used as guide input in the RiceFREND web tool to construct this network. The network comprises 67 genes (nodes), of which 20 were present amongst the ROX O s ERF 48 root DEGs. Big nodes represent OsERF48 plus the 19 identified ROX O s ERF 48 root DEGs. All other genes are depicted as small grey circles. Square nodes represent transcription factors. All genes in this network are listed in Table S2. (b–c) qRT‐PCR analysis showing the transcript levels of 9 genes from the network in 2‐week‐old ROX O s ERF 48 (b) and RNAi Os ERF 48 (c) roots. OsUbi1 expression was used as internal control. Values are the means ± SD of three independent experiments. ROX , ROX O s ERF 48 ; OX , OX O s ERF 48 ; RNAi, RNAi Os ERF 48 .
Table 3.
Expression level of the 20 genes in the OsERF48 co−regulatory network
| Description | ID | ROX/WT.fc | ROX/WT.pval | Dro/con* | Co−exp. freq.† | References | Calmodulin−binding motif (in 2−kb promoter)‡ |
|---|---|---|---|---|---|---|---|
| Signal transduction | |||||||
| OsCML16 | Os01g0135700 | 4.9 | 0.003 | 6.00 | 18 | Yu et al. (2013)4 | −1181, −1061, −901 |
| OsCCD1 | Os06g0683400 | 2.8 | 0.044 | 3.74 | 13 | Jing et al. (2016)1 | −445, −399 |
| OsERF048 | Os08g0408500 | 6.0 | 0.000 | 2.53 | 7 | In this study Oh et al. (2009)1 | −808, −640 |
| OsDREB1c | Os06g0127100 | 5.2 | 0.006 | 5.21 | 20 | Ito et al. (2006)2 | −1889, −1811, −1803, −1791,− 284, −175 |
| OsDERF5 | Os02g0764700 | 4.2 | 0.006 | 4.61 | 13 | Oh et al. (2009)1 | −1992, −1923, −1918, −1865,−1859, −1758, −56 |
| OsAP2−39 | Os04g0610400 | 3.1 | 0.028 | 4.02 | 13 | Oh et al. (2009)1 | −1350, −1308, −1268, −1155, −1117, −1098, −1057, −894, −779 |
| OsAP37/OsERF3 | Os01g0797600 | 2.2 | 0.025 | 2.86 | 8 | Oh et al. (2009)1; Zhao et al. (2015)1 | −1474, −1431, −1418, −1381, −1362, −1309, −1193, −1085, −31 |
| Carbohydrate metabolic processes | |||||||
| OsXTH9 | Os04g0604300 | 3.1 | 0.000 | 3.26 | 7 | Yang et al. (2006)2; Osato et al. (2006)3 | −1263, −1253, −1234, −821, −791, −648, −622 |
| OsAGP24 | Os06g0318800 | 2.6 | 0.010 | 3.35 | 7 | Gong et al. (2012)3 | −492, −303 |
| OsAGP3 | Os03g0188500 | 2.4 | 0.011 | 2.01 | 3 | Gong et al. (2012)3 | −835, −750, −333 |
| RS5 | Os01g0170000 | 2.8 | 0.000 | 2.69 | 4 | Wu et al. (2009)4 | −1479 |
| OsGolS1 | Os03g0316200 | 3.6 | 0.000 | 4.83 | 7 | Wu et al. (2009)4; Taji et al. (2002)3; Shimosaka and Ozawa (2015)3; Zhuo et al. (2013)3 | |
| OsGolS2/ OsLEA24 | Os07g0687900 | 3.3 | 0.002 | 5.30 | 9 | He et al. (2017)4; Wu et al. (2009)4 | −1703, −1700 |
| Response to stimulus | |||||||
| DIP1/ OsLEA23 | Os02g0669100 | 2.2 | 0.004 | 2.39 | 13 | Jung et al. (2015)4; He et al. (2017)4 | −1859, −1795, −1460, −791, −785, −431, −295, −198 |
| OsPSY3 | Os09g0555500 | 2.0 | 0.000 | 6.89 | 13 | Welsch et al. (2008)2 | −1943, −1906, −333, −273, −213, −177, −155, −94, −83, −4 |
| OsSAP12 | Os03g0241900 | 2.6 | 0.011 | 2.94 | 11 | Merewitz et al. (2011)1 | −1812, −1480 |
| OsJAZ3 | Os03g0180800 | 3.9 | 0.006 | 5.28 | 7 | Ye et al. (2009)1; Seo et al. (2011)4 | −958 |
| OsVQ1 | Os01g0278000 | 2.2 | 0.021 | 3.34 | 12 | Li et al. (2014)2 | −1398, −1368, −1316, −1302, −1264, −1252, −1250 |
| OsABCG1 | Os01g0121600 | 2.2 | 0.000 | 3.55 | 9 | Matsuda et al. (2012)2 | |
| Mss4 | Os05g0126800 | 2.5 | 0.001 | 2.40 | 7 | −667, −581, −489, −256, −63 | |
*Fold‐change (log2) between roots of drought‐treated/not‐treated controls in wild‐type rice plant (Kawahara et al., 2016).
†Represents a co‐expression frequency between the indicated gene and the 56 candidate genes identified in ROX OsERF48 roots based on co‐expression matrix shown in Figure S7.
‡Represents presence of calmodulin‐binding motifs from the translation start site (+1) determined using PlantPAN (similar score = 1). Superscripts 1–4 on references reports observations of the following: (1) Its overexpression has drought tolerance or enhanced root growth. (2) Induced by abiotic stresses. (3) Orthologous gene related with abiotic stress. (4) Up‐regulated in transgenic plants having abiotic stress tolerance.
OsERF48 directly binds to the promoter of OsCML16, a key gene in calcium signalling in response to abiotic stress
We found that OsCML16 was co‐expressed with 18 of the 56 candidate genes (Table 3), and that it was located in the centre of the co‐regulatory network (Figure 4a). We also identified many calmodulin‐binding motifs in the promoter regions of the genes in the OsERF48 co‐regulatory network (Table 3). We therefore hypothesized that the drought‐induced OsERF48 binds directly to the OsCML16 promoter, which then transduces a drought response to other downstream target genes. To examine the interaction between OsERF48 and the OsCML16 promoter, we generated myc‐tagged OsERF48 over‐expressing transgenic plants (ROX‐Myc OsERF48 ) in order to conduct a chromatin immunoprecipitation (ChIP) assay (Figures S2c and S4). Three candidate genes, OsCML16, OsLEA24/OsGolS2 and OsDREB1c, were selected for their high transcript levels (Figure 4b) and the presence of AP2/ERF cis‐regulatory elements in their promoter regions (Figure 5a–c). Os02g0771600 was also chosen as a negative control, since it contains AP2/ERF cis‐regulatory elements in its promoter region (Figure 5d), yet it is absent from the network. The ChIP assay of the ROX‐Myc OsERF48 roots revealed highly enriched genomic DNA fragments in all the OsCML16 promoter positions (P1 to P4) containing AP2/ERF cis‐regulatory elements (Figure 5e). OsERF48 also bound to the P2 position of the OsDREB1c promoter with low affinity (Figure 5f). Genomic DNA fragments from the promoter regions of both OsLEA24/OsGolS2 and Os02g0771600 (negative control) were not enriched (Figure 5g, h).
Figure 5.

Chromatin immunoprecipitation (ChIP)‐qPCR and transient protoplast expression assays showing that OsERF48 interacts with the OsCML16 promoter. (a–h) Two‐week‐old ROX‐Myc Os ERF 48 and nontransgenic (NT) roots were used in the ChIP‐qPCR experiments with an anti‐myc antibody. (a–d) Promoter region showing ChIP‐qPCR target positions (P1 to P4 or P1 to P3) with AP2/ERF cis‐regulatory elements. (e–h) ChIP‐qPCR data show an enrichment of chromatin DNA fragments at the indicated promoter region compared to NT plants. P1 to P4 in the OsCML16 promoter (a, e), P1 to P3 in the OsDREB1c promoter (b, f) and OsLEA24 promoter (c, g), and P1 to P3 in the Os02g0771600 gene promoter as a negative control (d, h). The relative enrichment was normalized with total input. Values are the means ± SD of three independent experiments. (i, j) Transient protoplast expression assay using a dual‐luciferase reporter system. (i) Schematic diagram of the reporter, internal control and two effector constructs. (j) Relative fLUC (fLUC/rLUC) activity in rice protoplasts. Values are the means ± SD of three independent experiments.
To test whether the interaction of OsERF48 with the OsCML16 promoter activates transcription of the latter, a transient protoplast expression assay using a dual‐luciferase reporter system was performed. OsCML16p::fLUC (firefly luciferase) and CaMV35Sp::rLUC (renilla luciferase) constructs were used as a reporter and an internal control, respectively. Two effector plasmids, CaMV35Sp::OsERF48F and CaMV35Sp::OsERF48Δc1, were transiently co‐expressed in rice protoplasts together with the reporter and the internal control, as indicated (Figure 5i, j). The OsERF48F (full‐length ORF) construct effectively activated the reporter gene expression, while the OsERF48Δc1 construct did not (Figure 5j), supporting our hypothesis that drought‐induced OsERF48 directly binds to the promoter of OsCML16 via AP2/ERF cis‐acting regulatory elements, thereby activating its transcription.
Discussion
OsERF48 belongs to AP2/ERF group Ib, members of which contain four distinct conserved motifs (CMI‐1‐4). Here, we showed that the CMI‐1 motif is important for transcriptional activity using a yeast transactivation assay and a transient protoplast expression assay. The highly conserved acidic domain, EIDWD, found in the CMI‐1 region of OsERF48, appears to be essential for transactivation (Remacle et al., 1997) and is conserved in legume (Medicago truncatula), A. thaliana and maize (Zea mays) orthologs (Figure S8).
We showed that root‐specific overexpression of OsERF48 is more effective than whole‐body overexpression in promoting root growth and enhancing drought tolerance. ROX OsERF48 transgenic plants maintained their root growth, in terms of primary root length and lateral root density, under in vitro drought conditions (40% PEG‐infused media). Additionally, under normal growth conditions, the root dry weight of ROX OsERF48 plants was twofold higher than that of NT plants. We propose that OsERF48‐mediated root modification enhances water uptake by increasing the total root surface area. Such robust root system‐mediated drought tolerance was previously observed in rice when AtEDT1/HDG11 and HYR were overexpressed (Ambavaram et al., 2014; Yu et al., 2013). Deep rooting architecture caused by overexpressing DEEP ROOTING 1 (DRO1), a QTL for deep rooting, in rice results in increased grain yield under drought conditions, by enhancing the capacity for water extraction from deep soil layers (Uga et al., 2013).
We found that, under field‐drought conditions, the ROX OsERF48 plants produced a higher grain yield than the OX OsERF48 and the NT control plants. Similar observations were made in our previous studies with whole‐body and root‐specific overexpression of OsNACs (Jeong et al., 2010; Redillas et al., 2012) and OsERF71 (Lee et al., 2016a) in rice. Root‐specific overexpressors always presented a higher grain yield than the whole‐body overexpressors and NT plants under field‐drought conditions. Moreover, those root‐specific overexpressors had similar levels of grain yield with NT plants under normal growth conditions. This was likely due to less of a trade‐off in the root‐specific overexpressors of the TFs than in the whole‐body overexpressors. In the current study, however, root‐specific overexpression of OsERF48 showed defects in shoot growth and reduced grain yield under normal growth conditions. We believed that both whole‐body and root‐specific overexpression of OsERF48 might have caused some negative effects on the normal growth of corresponding transgenic plants. Ito et al. (2006) also observed that the overexpression of AP2/ERF TFs showed shoot growth retardation of transgenic rice plants under normal growth conditions. A use of stress‐inducible promoter such as the RD29A promoter (Datta et al., 2012; Liu et al., 2014) could be an alternative way to avoid the growth defects.
We constructed a putative OsERF48 regulatory network by cross‐referencing ROX OsERF48 root RNA‐seq data with a co‐expression network database, from which we inferred the involvement of 20 drought‐related genes. These genes were associated with stress signalling, carbohydrate metabolism, cell‐wall proteins and drought response and included OsCML16, encoding a calmodulin‐like protein, which was located at the centre of our OsERF48 co‐regulatory network, indicating an important regulatory role (Figure 4a). Many studies have suggested that CMLs are major calcium ion sensors and function in mediating plant stress tolerance (Magnan et al., 2008; Munir et al., 2016; Park et al., 2010; Xu et al., 2011). Other lines of evidence also indicate that downstream target genes regulated by CMLs include kinases, metabolic proteins, cytoskeletal proteins, ion channels and pumps, and TFs (Zeng et al., 2015). Indeed, similar categories of genes, such as TFs (OsDREB1c, OsDERF5, OsAP2‐39 and AP37), cell‐wall proteins (OsXTH9, OsAGP24 and OsAGP3) and carbohydrate metabolic enzymes (OsGolS1, OsGolS2/OsLEA24 and RS5), were included in the OsERF48 co‐regulatory network. Most of these genes appear to be connected to OsERF48 via OsCML16. These observations led us to propose that drought‐induced OsERF48 binds to the OsCML16 promoter, which then interacts with other downstream target genes. This hypothesis was supported by the results of ChIP and transient protoplast expression assays, which indicated that OsERF48 binds to the promoter of, and activates, OsCML16 (Figure 5e, j). Although OsLEA24/OsGolS2 was up‐regulated in the ROX OsERF48 plants, we found no evidence that it is a direct target of OsERF48, since we did not observe OsERF48 binding to its promoters (Figure 5g). We identified numerous calmodulin‐binding motifs in the promoter regions of the 20 genes in the co‐regulatory network (Table 3), suggesting possible interaction of OsCML16 with those promoters, and consequently involvement in the drought stress response.
We found evidence in the literature (Table 3) that other genes in the OsERF48 co‐regulatory network are also closely associated with drought tolerance and root growth. For example, OsDERF5, OsAP2‐39 and AP37 were reported to be stress‐inducible AP2/ERF genes in rice (Oh et al., 2009), and AP37 promotes crown root development, as well as its overexpression increases drought tolerance and grain yield under drought conditions (Oh et al., 2009; Zhao et al., 2015). Plant XTH proteins play a role in cell‐wall restructuring and in cell expansion during root growth (Vilches‐Barro and Maizel, 2015), and rice XTH and XYLOSE ISOMERASE genes were up‐regulated under water‐deficit conditions, where both were associated with maintenance of root growth in rice plants (Yang et al., 2006). RNA interference‐mediated suppression of AtXTH18 in A. thaliana caused a reduction in root length and cell size (Osato et al., 2006), indicating its importance in root phenotype determination. Arabinogalactan proteins are plant cell‐wall glycoproteins that have been reported to be associated with various plant growth and developmental processes, such as cell expansion and proliferation, cell‐wall plasticization, salt tolerance and root growth (Seifert and Roberts, 2007). During early root development of cotton (Gossypium hirsutum), GhAGP31 has been shown to be involved in the response to cold stress (Gong et al., 2012), and OsXTH9 and OsAGP3, which we observed were both up‐regulated in ROX OsERF48 roots and down‐regulated in RNAi OsERF48 roots, have been shown to be involved in root growth and abiotic stress tolerance. Similarly, OsGolS2/OsLEA24, OsGolS1 and RS5 were also up‐ and down‐regulated in the ROX OsERF48 and the RNAi OsERF48 roots, respectively (Figure 4a, b). GolS and RS are key enzymes in the biosynthesis of raffinose family oligosaccharides (RFO), such as raffinose, stachyose and galactinol. Overexpression plants of the genes encoding the enzymes involved in RFO biosynthesis, such as GolS and RS were shown to result in intracellular accumulation of RFO, which serve as osmoprotectants and may aid in drought tolerance (Shimosaka and Ozawa, 2015; Taji et al., 2002; Zhuo et al., 2013). Overexpression of OsWRKY11 was shown to cause an up‐regulation of OsGolS1, OsLEA24/OsGolS2 and RS5, resulting in elevated raffinose levels and increased drought tolerance (Wu et al., 2009). These reports suggest that up‐regulation of the OsGolS1, OsLEA24/OsGolS2 and RS5 in ROX OsERF48 plants may trigger the accumulation of RFO in roots, which is consistent with the ROX OsERF48 plants having a drought tolerance.
Endogenous levels of ABA and Ca2+ increase upon exposure to various abiotic stresses. Many genes regulated by ABA are therefore involved in the Ca2+ signal transduction (Tuteja, 2007). Conversely, genes that are not responsive to ABA are also involved in Ca2+ signalling. For example, overexpression of OsCML4, a calcium‐signalling gene that is not responsive to ABA, conferred drought tolerance via ROS‐scavenging (Yin et al., 2015). Similarly, our ABA‐independent OsERF48 regulated the OsCML16 which resulted in drought tolerance through enhanced root growth. Together, these observations support that the ABA‐dependent and ABA‐independent pathways cross‐talk and Ca2+ is a common second messenger of the crosstalk during abiotic stresses (Roychoudhury et al., 2013).
We have demonstrated that OsERF48 overexpression enhances root growth and drought tolerance under drought conditions. We constructed a putative co‐regulatory network of OsERF48 by cross‐referencing ROX OsERF48 root RNA‐seq data with a co‐expression network database. A number of genes that are known to confer drought tolerance were present in the network. The calmodulin‐like protein gene, OsCML16, a direct target of OsERF48, appears to transduce OsERF48 actions to downstream target genes that together confer the acquired root phenotype and drought tolerance of the ROX OsERF48 plants. In addition, we present evidence of a role for OsERF48 as an upstream regulator of OsCML16.
Experimental procedures
Plasmid construction for rice transformation
To generate OsERF48 overexpressing transgenic rice, the OsERF48 (Os08g0804500) coding sequence was amplified from rice (O. sativa cv Ilmi) cDNA using a high‐fidelity DNA polymerase PrimeSTAR (TaKaRa, Kyoto, Japan). The amplified OsERF48 fragment was ligated into the pPZP‐PGD1 vector for whole‐body expression (OX OsERF48 ) (Park et al., 2012) and pPZP‐RCc3 for root‐specific expression (ROX OsERF48 ) (Jeong et al., 2010). For the RNAi (RNAi OsERF48 ) construct, a 289 bp OsERF48 fragment (−137 to +152 from the translational start ATG) was sub‐cloned into the pENTRd‐TOPO vector (Invitrogen, Carlsbad, CA) and transferred to the pGOS2‐RNAi vector (Lee et al., 2016a) using the Gateway cloning system (Invitrogen). For the myc‐tagged OsERF48 (ROX‐Myc OsERF48 ) construct, the OsERF48 coding sequence was fused to 3′ end of the sequence encoding a 6xMyc tag in the pE3n vector (Dubin et al., 2008), and the fused fragment was transferred to the p700RCc3 vector (Jeong et al., 2010). Transgenic rice (O. sativa cv Ilmi) was obtained by Agrobacterium tumefaciens (EHA101 for ROX OsERF48 and OX OsERF48 ; LBA4404 for RNAi OsERF48 and ROX‐Myc OsERF48 )‐mediated transformation. To verify the copy number, a Southern blot was performed as previously described (Lee et al., 2016b). Vector maps and primer sequences used in this study are listed in Figure S1 and Table S3, respectively.
Abiotic stress treatment and qRT‐PCR analysis
Rice (O. sativa cv. Ilmi) seeds were germinated on Murashige‐Skoog (MS) medium (Duchefa Biochemie, Haarlem, Netherlands), transferred to soil and grown for 14 days in a green house at 28 °C. For abiotic stress treatments, soil was removed from the roots of the seedlings, and drought stress was induced by air‐drying the seedlings, while salinity stress and ABA treatment were imposed by incubating the seedlings in water containing 400 mm NaCl and 100 μm ABA, respectively at 28 °C. Low temperature stress was induced by incubating seedlings in water and placing them inside a cooler at 4 °C. Leaves and roots from the stress‐treated plants were sampled at the indicated time points. Total RNA was extracted from rice leaves or roots using the TRIzol reagent (Invitrogen) in accordance with the manufacturer's instructions.
cDNA was synthesized using Revertaid™ reverse transcriptase (ThermoFisher Scientific, Waltham, MA), and real‐time PCR analysis was performed using the Solg™ 2× real‐time PCR smart mix with evagreen (Solgent, Seoul, Korea) with a Mx3000P real‐time PCR system (Agilent Technologies, Palo Alto, CA). The OsUbi1 gene (Os06g0681400) was used as an internal standard, and three biological replicates were analysed. Values are the means ± SD (standard deviation) of three independent experiments. All primer sequences are listed in Table S3.
Drought tolerance evaluation at the vegetative stage
OsERF48 transgenic and NT control plants (O. sativa cv. Ilmi) were germinated on MS media at 28 °C for 3 days. Thirty plants from each line were transplanted into ten soil pots (4 × 4 × 6 cm) within a container (59 × 38.5 × 15 cm; three plants per pot) and grown for 4 weeks in a greenhouse at 28–30 °C. Pots were moved from the container for a 4‐day drought treatment and returned into the container for re‐watering until the plants recovered.
To measure chlorophyll fluorescence and the performance index, 2‐week‐old plants were transplanted into a 15‐cm‐diameter × 14‐cm‐tall pot within another larger container (66 × 45.3 × 22.5 cm) and grown for 2 months. By removing the pots from the container, drought stress was performed for 6 days. After a 1‐h dark adaptation, the longest leaves from each plant were selected and measured at their apex, middle and base regions using the Handy‐pea fluorimeter (Hansatech Instrument, Norfolk, UK). Thirty readings per line were averaged using the Handy‐pea software (version 1.31). Chlorophyll a fluorescence (F v/F m) and the performance index were measured and analysed according to the equations of the JIP test (Redillas et al., 2011). Drought‐induced symptoms were visualized using a NEX‐5N camera (Sony, Kyoto, Japan), and soil moisture was measured using a SM150 soil moisture sensor (Delta T Devices, Cambridge, UK) at the indicated time points.
Field‐drought tolerance evaluation at the reproductive stage
Evaluation of yield components of transgenic and nontransgenic (NT) plants was performed in the rice paddy field at Kyungpook National University, Gunwi (128:34E/36:15N), Korea. A randomized design was introduced for three replicates using three different 10‐m2 plots. Thirty seedlings were planted in the paddy field for normal growth, and 18 seedlings were planted in a pot (15 cm diameter × 14 cm tall) for field‐drought conditions. All plants were applied with fertilizer at 70N/40P/70K kg/ha after the last paddling. To make the field‐drought conditions, plants were grown in a tank with a rain‐off shelter to cover rice plants from rain. During 10 days before and after heading, intermittent drought stress was applied by draining water from the tank. When complete leaf‐rolling was observed in the plants after the first drought treatment, they were irrigated overnight and allowed to recover. Plants were then subjected to the second round of drought treatment until another complete leaf‐rolling occurred. After two times of drought stress treatments, all plants were irrigated until harvest. Yield parameters were scored with the 30 and 18 plants per each line from three different plots for normal and drought field conditions, respectively. The results were compared with those of NT controls, using analysis by SPSS software.
Characterization of root phenotypes
Seeds were immersed in water at 37 °C for 1 day, sowed on 1/2 MS medium containing 1% agar without sucrose and grown vertically for 1 day in the dark and 5 days under a 16 : 8 light : dark photoperiod at 28 °C. For in vitro drought conditions, polyethylene glycol (PEG)‐infused plates were prepared by dissolving solid PEG8000 in a sterilized solution of 1/2 MS medium with 6 mm 4‐morpholineethanesulfonic acid hydrate (MES) buffer (pH 5.7), followed by overlaying of the PEG solution onto 1.5% agar‐solidified half‐strength MS medium with 6 mm MES buffer (pH 5.7). The agar medium and PEG solution were equilibrated for at least 12 h, and the excess PEG solution was removed before use. Drought stress strength was considered to be proportional to the concentration of the overlaid PEG solution: 0% (control); 25% and 40% (drought stress; Verslues et al., 2006). To measure primary root length and lateral root number, images were taken using a NEX‐5N camera (Sony, Kyoto, Japan) and analysed with ImageJ software (https://imagej.nih.gov). The number of lateral roots was counted within a 3‐ to 4.5‐cm region from the root tip using a stereomicroscope (Leica, Bensheim, Germany).
Protein subcellular localization using rice protoplasts
The OsERF48, OsNF‐YA7, GFP and mCherry coding regions without stop codons were amplified using the high‐fidelity DNA polymerase PrimeSTAR (TaKaRa) and specific primers (Table S3). Using the In‐fusion cloning system (TaKaRa), multiple PCR products (OsERF48 and GFP for the OsERF48‐GFP construct and OsNF‐YA7 and mCherry for the OsNF‐YA7‐mCherry construct) were cloned into the pHBT vector (GenBank accession number EF090408), which harboured the constitutive 35S promoter. These vectors were transiently expressed in rice protoplasts, as previously described (Jung et al., 2015). Eighteen hours after transformation, GFP and mCherry fluorescence were observed using a SP8 STED laser scanning confocal microscope (Leica, Bensheim, Germany).
Transactivation assay in yeast
Five OsERF48 mutants with conserved motifs (CMI‐1‐4) separately deleted (Figure S2) were produced using the high‐fidelity DNA polymerase PrimeSTAR (TaKaRa). Primer combinations and sequences are listed in Table S3. Each deletion fragment was cloned into the pGBKT7 vector (Clontech, Palo Alto, CA) using the In‐fusion cloning system (TaKaRa). All of the mutant vectors were transformed into the Y2HGold yeast strain through LiAc‐mediated transformation according to the manufacturer's instructions (Clontech). Transformants were cultured at 30 °C on Synthetic Dropout (SD)/‐Trp and SD/Trp with 200 ng/mL of aureobasidin A (AbA) and 40 μg/mL of X‐α‐gal.
RNA‐seq analysis
Total RNA was extracted from 2‐week‐old transgenic and nontransgenic (NT) roots using a RNeasy plant kit and treated with DNase I according to the manufacturer's instructions (Qiagen, Hilden, German). RNA samples from two biological replicates with two technical replicates each were prepared, from the two different transgenic lines (ROX#2 and ROX#6). Processing of library construction, next‐generation sequencing (NGS) sequencing and DEG analysis was performed by Macrogen Inc., Seoul, Korea. DEGs were defined by an expression change ≥2‐fold with a P value <0.05. For validation of candidate genes from the RNA‐seq analysis, RT‐PCR was performed of root material from 2‐week old transgenic and WT plants, prepared separately from the RNA‐seq sample. All primer sequences are listed in Table S3.
ChIP‐qPCR assay
Two‐week‐old ROX‐Myc OsERF48 and NT plants grown on soil were hydroponically adapted in water for 3 days and then fixed by cross‐linking with 1% formaldehyde under vacuum for 15 min. Cross‐linking was stopped by the addition of glycine to a final concentration of 125 mm and application of vacuum for 10 min. After washing the plants in cold water, roots were collected and frozen in liquid nitrogen, and stored at −80 °C. The ChIP assay was performed as described by Chung et al. (2009), except that an anti‐myc antibody (SC‐789; Santa Cruz Biotech, Santa Cruz, CA) used. The ChIP product was analysed via quantitative PCR on a Mx3000P real‐time PCR system (Agilent Technologies). The enrichment values were normalized to the input sample. Values are the means ± SD of three independent experiments. All primer sequences are listed in Table S3.
Protoplast isolation and transactivation assay
The OsERF48F, OsERF48Δc1 and a promoter region of OsCML16 were amplified by PCR using a high‐fidelity DNA polymerase PrimeSTAR (TaKaRa). For effector constructs, OsERF48F and OsERF48Δc1 were cloned into the pHBT vector (GenBank accession number EF090408) containing the 35S promoter, and for the reporter construct, the OsCML16 promoter region was cloned into the pGST6‐LUC‐NOS vector (GenBank accession number EF090412.1) using the In‐fusion cloning system (TaKaRa). Protoplast isolation from shoots of 10‐day‐old rice seedlings (O. sativa cv. Ilmi) and PEG‐mediated transformation were performed as previously described (Jung et al., 2015). Fifteen microlitres of vector solution, including 3 μg of effector, 1 μg of reporter and 1 μg of internal control were transfected into the isolated protoplast solution harbouring up to 3.5 × 106 cells. Dual‐luciferase activity was analysed using the dual‐luciferase reporter assay system (Promega, Madison, WI) and measured with an Infinite M200 system (Tecan Systems, San Jose, CA). Three independent transfections for each sample were performed, and the relative luciferase activity was calculated as the ratio between fLUC and rLUC. The 35S::rLUC construct was used as an internal control. Primer sequences are listed in Table S3.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Figure S1 Predicted domain and motifs of OsERF48 and their nucleotide and amino acid sequences.
Figure S2 Vectors used for rice transformation.
Figure S3 Southern blot analysis of OsERF48 overexpression lines.
Figure S4 Drought tolerance of transgenic and nontransgenic (NT).
Figure S5 Vigorous root growth in OsERF48 overexpressors.
Figure S6 Transcriptome profile of ROX OsERF48 roots compared with nontransgenic (NT) roots.
Figure S7 Co‐expression matrix of 56 candidate genes identified amongst the differentially expressed genes (DEGs) in ROX OsERF48 roots compared to wild type using the RiceFREND web tool (http://ricefrend.dna.affrc.go.jp/). Red boxes indicate pairings of each gene.
Figure S8 Alignment of amino acid sequences from the CMI‐1 region of OsERF48 and orthologs.
Table S1 Fifty‐six candidate genes identified from the differentially expressed genes (DEGs) from the RNA‐seq analysis of ROX OsERF48 roots.
Table S2 Nodes (genes) constituting the OsERF48 co‐regulatory network.
Table S3 Primers used in this study.
Acknowledgements
We are grateful to Dr. Dong‐Keun Lee for comments in writing the manuscript. This research was supported by the Rural Development Administration under the Next Generation BioGreen 21 Program (Project No. PJ011829012016 to J‐KK) and by the Basic Science Research Program through the National Research Foundation of Korea, Ministry of Education (NRF‐2013R1A6A3A04060627 to PJC).
Accession numbers: GSE93081 (RNA‐seq).
Contributor Information
Joo‐Won Suh, Email: jwsuh@mju.ac.kr.
Ju‐Kon Kim, Email: jukon@snu.ac.kr.
References
- Ambavaram, M.M. , Basu, S. , Krishnan, A. , Ramegowda, V. , Batlang, U. , Rahman, L. , Baisakh, N. et al. (2014) Coordinated regulation of photosynthesis in rice increases yield and tolerance to environmental stress. Nat. Commun. 5, 5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, P.J. , Kim, Y.S. , Jeong, J.S. , Park, S.‐H. , Nahm, B.H. and Kim, J.‐K. (2009) The histone deacetylase OsHDAC1 epigenetically regulates the OsNAC6 gene that controls seedling root growth in rice. Plant J. 59, 764–776. [DOI] [PubMed] [Google Scholar]
- Datta, K. , Baisakh, N. , Ganguly, M. , Krishnan, S. , Yamaguchi Shinozaki, K. and Datta, S.K. (2012) Overexpression of Arabidopsis and rice stress genes’ inducible transcription factor confers drought and salinity tolerance to rice. Plant Biotechnol. J. 10, 579–586. [DOI] [PubMed] [Google Scholar]
- Dubin, M.J. , Bowler, C. and Benvenuto, G. (2008) A modified gateway cloning strategy for overexpressing tagged proteins in plants. Plant Methods, 4, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, S.‐Y. , Huang, G.‐Q. , Sun, X. , Li, P. , Zhao, L.‐L. , Zhang, D.‐J. and Li, X.‐B. (2012) GhAGP31, a cotton non‐classical arabinogalactan protein, is involved in response to cold stress during early seedling development. Plant Biol. 14, 447–457. [DOI] [PubMed] [Google Scholar]
- He, X. , Li, L. , Xu, H. , Xi, J. , Cao, X. , Xu, H. , Rong, S. et al. (2017) A rice jacalin‐related mannose‐binding lectin gene, OsJRL, enhances Escherichia coli viability under high salinity stress and improves salinity tolerance of rice. Plant Biol. J. 19, 257–267. [DOI] [PubMed] [Google Scholar]
- Hirota, A. , Kato, T. , Fukaki, H. , Aida, M. and Tasaka, M. (2007) The auxin‐regulated AP2/EREBP gene PUCHI is required for morphogenesis in the early lateral root primordium of Arabidopsis . Plant Cell, 19, 2156–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, Y. , Katsura, K. , Maruyama, K. , Taji, T. , Kobayashi, M. , Seki, M. , Shinozaki, K. et al. (2006) Functional analysis of rice DREB1/CBF‐type transcription factors. Plant Cell Physiol. 47, 141–153. [DOI] [PubMed] [Google Scholar]
- Jeong, J.S. , Kim, Y.S. , Baek, K.H. , Jung, H. , Ha, S.‐H. , Choi, Y.D. , Kim, M. et al. (2010) Root‐specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol. 153, 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing, P. , Zou, J. , Kong, L. , Hu, S. , Wang, B. , Yang, J. and Xie, G. (2016) OsCCD1, a novel small calcium‐binding protein with one EF‐hand motif, positively regulates osmotic and salt tolerance in rice. Plant Sci. 247, 104–114. [DOI] [PubMed] [Google Scholar]
- Jung, H. , Lee, D.K. , Choi, Y.D. and Kim, J.‐K. (2015) OsIAA6, a member of the rice Aux/IAA gene family, is involved in drought tolerance and tiller outgrowth. Plant Sci. 236, 304–312. [DOI] [PubMed] [Google Scholar]
- Karaba, A. , Dixit, S. , Greco, R. , Aharoni, A. , Trijatmiko, K.R. , Marsch‐Martinez, N. , Krishnan, A. et al. (2007) Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proc. Natl Acad. Sci. USA, 104, 15270–15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara, Y. , Oono, Y. , Wakimoto, H. , Ogata, J. , Kanamori, H. , Sasaki, H. , Mori, S. et al. (2016) TENOR: database for comprehensive mRNA‐Seq experiments in rice. Plant Cell Physiol. 57, e7. [DOI] [PubMed] [Google Scholar]
- Lee, D.K. , Kim, H.I. , Jang, G. , Chung, P.J. , Jeong, J.S. , Kim, Y.S. , Bang, S.W. et al. (2015) The NF‐YA transcription factor OsNF‐YA7 confers drought stress tolerance of rice in an abscisic acid independent manner. Plant Sci. 241, 199–210. [DOI] [PubMed] [Google Scholar]
- Lee, D.K. , Jung, H. , Jang, G. , Jeong, J.S. , Kim, Y.S. , Ha, S.H. , Choi, Y.D. et al. (2016a) Overexpression of the OsERF71 transcription factor alters rice root structure and drought resistance. Plant Physiol. 172, 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D.K. , Park, S.‐H. , Seong, S.Y. , Kim, Y.S. , Jung, H. , Choi, Y.D. and Kim, J.‐K. (2016b) Production of insect‐resistant transgenic rice plants for use in practical agriculture. Plant Biotechnol. Rep. 10, 391–401. [Google Scholar]
- Li, N. , Li, X. , Xiao, J. and Wang, S. (2014) Comprehensive analysis of VQ motif‐containing gene expression in rice defense responses to three pathogens. Plant Cell Rep. 33, 1493–1505. [DOI] [PubMed] [Google Scholar]
- Liu, C. , Mao, B. , Ou, S. , Wang, W. , Liu, L. , Wu, Y. , Chu, C. et al. (2014) OsbZIP71, a bZIP transcription factor, confers salinity, and drought tolerance in rice. Plant Mol. Biol. 84, 19–36. [DOI] [PubMed] [Google Scholar]
- Magnan, F. , Ranty, B. , Charpenteau, M. , Sotta, B. , Galaud, J.P. and Aldon, D. (2008) Mutations in AtCML9, a calmodulin‐like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J. 56, 575–589. [DOI] [PubMed] [Google Scholar]
- Matsuda, S. , Funabiki, A. , Furukawa, K. , Komori, N. , Koike, M. , Tokuji, Y. , Takamure, I. et al. (2012) Genome‐wide analysis and expression profiling of half‐size ABC protein subgroup G in rice in response to abiotic stress and phytohormone treatments. Mol. Genet. Genomics, 287, 819–835. [DOI] [PubMed] [Google Scholar]
- Matsuo, N. , Ozawa, K. and Mochizuki, T. (2009) Genotypic differences in root hydraulic conductance of rice (Oryza sativa L.) in response to water regimes. Plant Soil, 316, 25–34. [Google Scholar]
- Merewitz, E.B. , Gianfagna, T. and Huang, B. (2011) Protein accumulation in leaves and roots associated with improved drought tolerance in creeping bentgrass expressing an ipt gene for cytokinin synthesis. J. Exp. Bot. 62, 5311–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir, S. , Liu, H. , Xing, Y. , Hussain, S. , Ouyang, B. , Zhang, Y. , Li, H. et al. (2016) Overexpression of calmodulin‐like (ShCML44) stress‐responsive gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses. Sci. Rep. 6, 31772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano, T. , Suzuki, K. , Fujimura, T. and Shinshi, H. (2006) Genome‐wide analysis of the ERF gene family in arabidopsis and rice. Plant Physiol. 140, 411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima, K. , Yamaguchi‐Shinozaki, K. and Shinozaki, K. (2014) The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front Plant Sci. 5, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, S.‐J. , Kim, Y.S. , Kwon, C.‐W. , Park, H.K. , Jeong, J.S. and Kim, J.‐K. (2009) Overexpression of the transcription factor AP37 in rice improves grain yield under drought conditions. Plant Physiol. 150, 1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta, M. , Matsui, K. , Hiratsu, K. , Shinshi, H. and Ohme‐Takagi, M. (2001) Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell, 13, 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osato, Y. , Yokoyama, R. and Nishitani, K. (2006) A principal role for AtXTH18 in Arabidopsis thaliana root growth: a functional analysis using RNAi plants. J. Plant. Res. 119, 153–162. [DOI] [PubMed] [Google Scholar]
- Park, H.C. , Park, C.Y. , Koo, S.C. , Cheong, M.S. , Kim, K.E. , Kim, M.C. , Lim, C.O. et al. (2010) AtCML8, a calmodulin‐like protein, differentially activating CaM‐dependent enzymes in Arabidopsis thaliana . Plant Cell Rep. 29, 1297–1304. [DOI] [PubMed] [Google Scholar]
- Park, S.‐H. , Bang, S.W. , Jeong, J.S. , Jung, H. , Redillas, M.C. , Kim, H.I. , Lee, K.H. et al. (2012) Analysis of the APX, PGD1 and R1G1B constitutive gene promoters in various organs over three homozygous generations of transgenic rice plants. Planta, 235, 1397–1408. [DOI] [PubMed] [Google Scholar]
- Quan, R. , Hu, S. , Zhang, Z. , Zhang, H. , Zhang, Z. and Huang, R. (2010) Overexpression of an ERF transcription factor TSRF1 improves rice drought tolerance. Plant Biotechnol. J. 8, 476–488. [DOI] [PubMed] [Google Scholar]
- Reddy, A.S.N. , Ali, G.S. , Celesnik, H. and Day, I.S. (2011) Coping with stresses: roles of calcium‐ and calcium/calmodulin‐regulated gene expression. Plant Cell, 23, 2010–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redillas, M.C.F.R. , Strasser, R.T. , Jeong, J.S. , Kim, Y.S. and Kim, J.‐K. (2011) JIP test to evaluate drought‐tolerance of transgenic rice overexpressing OsNAC10. Plant Biotechnol. Rep. 5, 169–175. [Google Scholar]
- Redillas, M.C.F.R. , Jeong, J.S. , Kim, Y.S. , Jung, H. , Bang, S.W. , Choi, Y.D. , Ha, S.H. et al. (2012) The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnol. J. 10, 792–805. [DOI] [PubMed] [Google Scholar]
- Remacle, J.E. , Albrecht, G. , Brys, R. , Braus, G.H. and Huylebroeck, D. (1997) Three classes of mammalian transcription activation domain stimulate transcription in Schizosaccharomyces pombe . EMBO J. 16, 5722–5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury, A. , Paul, S. and Basu, S. (2013) Cross‐talk between abscisic acid‐dependent and abscisic acid‐independent pathways during abiotic stress. Plant Cell Rep. 32, 985–1006. [DOI] [PubMed] [Google Scholar]
- Seifert, G.J. and Roberts, K. (2007) The biology of arabinogalactan proteins. Annu. Rev. Plant Biol. 58, 137–161. [DOI] [PubMed] [Google Scholar]
- Seo, J.‐S. , Joo, J. , Kim, M.‐J. , Kim, Y.‐K. , Nahm, B.H. , Song, S.I. , Cheong, J.J. et al. (2011) OsbHLH148, a basic helix‐loop‐helix protein, interacts with OsJAZ proteins in a jasmonate signaling pathway leading to drought tolerance in rice. Plant J. 65, 907–921. [DOI] [PubMed] [Google Scholar]
- Shimosaka, E. and Ozawa, K. (2015) Overexpression of cold‐inducible wheat galactinol synthase confers tolerance to chilling stress in transgenic rice. Breed Sci. 65, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taji, T. , Ohsumi, C. , Iuchi, S. , Seki, M. , Kasuga, M. , Kobayashi, M. , Yamaguchi‐Shinozaki, K. et al. (2002) Important roles of drought‐ and cold‐inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana . Plant J. 29, 417–426. [DOI] [PubMed] [Google Scholar]
- Tiwari, S.B. , Belachew, A. , Ma, S.F. , Young, M. , Ade, J. , Shen, Y. , Marion, C.M. et al. (2012) The EDLL motif: a potent plant transcriptional activation domain from AP2/ERF transcription factors. Plant J. 70, 855–865. [DOI] [PubMed] [Google Scholar]
- Trupiano, D. , Yordanov, Y. , Regan, S. , Meilan, R. , Tschaplinski, T. , Scippa, G.S. and Busov, V. (2013) Identification, characterization of an AP2/ERF transcription factor that promotes adventitious, lateral root formation in Populus. Planta, 238, 271–282. [DOI] [PubMed] [Google Scholar]
- Tuteja, N. (2007) Abscisic acid and abiotic stress signaling. Plant Signal Behav. 2, 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uga, Y. , Sugimoto, K. , Ogawa, S. , Rane, J. , Ishitani, M. , Hara, N. , Kitomi, Y. et al. (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 45, 1097–1102. [DOI] [PubMed] [Google Scholar]
- Verslues, P.E. , Agarwal, M. , Katiyar‐Agarwal, S. , Zhu, J. and Zhu, J.‐K. (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 45, 523–539. [DOI] [PubMed] [Google Scholar]
- Vilches‐Barro, A. and Maizel, A. (2015) Talking through walls: mechanisms of lateral root emergence in Arabidopsis thaliana . Curr. Opin. Plant Biol. 23, 31–38. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Han, H. , Yan, J. , Chen, F. and Wei, W. (2015) A new AP2/ERF transcription factor from the oil plant Jatropha curcas confers salt and drought tolerance to transgenic tobacco. Appl. Biochem. Biotechnol. 176, 582–597. [DOI] [PubMed] [Google Scholar]
- Welsch, R. , Wüst, F. , Bär, C. , Al‐Babili, S. and Beyer, P. (2008) A third phytoene synthase is devoted to abiotic stress‐induced abscisic acid formation in rice and defines functional diversification of phytoene synthase genes. Plant Physiol. 147, 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X. , Kishitani, S. , Ito, Y. and Toriyama, K. (2009) Accumulation of raffinose in rice seedlings overexpressing OsWRKY11 in relation to desiccation tolerance. Plant Biotechnol. 26, 431–434. [Google Scholar]
- Xu, G.‐Y. , Rocha, P.S.C.F. , Wang, M.‐L. , Xu, M.‐L. , Cui, Y.‐C. and Li, L.‐Y. (2011) A novel rice calmodulin‐like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta, 234, 47–59. [DOI] [PubMed] [Google Scholar]
- Yang, L. , Wang, C.C. , Guo, W.D. , Li, X.B. , Lu, M. and Yu, C.L. (2006) Differential expression of cell wall related genes in the elongation zone of rice roots under water deficit. Russ. J. Plant Physiol. 53, 390–395. [Google Scholar]
- Ye, H. , Du, H. , Tang, N. , Li, X. and Xiong, L. (2009) Identification and expression profiling analysis of TIFY family genes involved in stress and phytohormone responses in rice. Plant Mol. Biol. 71, 291–305. [DOI] [PubMed] [Google Scholar]
- Yin, X.M. , Huang, L.F. , Zhang, X. , Wang, M.L. , Xu, G.Y. and Xia, X.J. (2015) OsCML4 improves drought tolerance through scavenging of reactive oxygen species in rice. J. Plant Biol. 58, 68–73. [Google Scholar]
- Yu, L. , Chen, X. , Wang, Z. , Wang, S. , Wang, Y. , Zhu, Q. , Li, S. et al. (2013) Arabidopsis enhanced drought tolerance1/HOMEODOMAIN GLABROUS11 confers drought tolerance in transgenic rice without yield penalty. Plant Physiol. 162, 1378–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, H. , Xu, L. , Singh, A. , Wang, H. , Du, L. and Poovaiah, B.W. (2015) Involvement of calmodulin and calmodulin‐like proteins in plant responses to abiotic stresses. Front Plant Sci. 6, 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Liu, W. , Wan, L. , Li, F. , Dai, L. , Li, D. , Zhang, Z. et al. (2010) Functional analyses of ethylene response factor JERF3 with the aim of improving tolerance to drought and osmotic stress in transgenic rice. Transgenic Res. 19, 809–818. [DOI] [PubMed] [Google Scholar]
- Zhao, Y. , Cheng, S. , Song, Y. , Huang, Y. , Zhou, S. , Liu, X. and Zhou, D.‐X. (2015) The interaction between rice ERF3 and WOX11 promotes crown root development by regulating gene expression involved in cytokinin signaling. Plant Cell, 27, 2469–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo, C. , Wang, T. , Lu, S. , Zhao, Y. , Li, X. and Guo, Z. (2013) A cold responsive galactinol synthase gene from Medicago falcata (MfGolS1) is induced by myo‐inositol and confers multiple tolerances to abiotic stresses. Physiol. Plant. 149, 67–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Predicted domain and motifs of OsERF48 and their nucleotide and amino acid sequences.
Figure S2 Vectors used for rice transformation.
Figure S3 Southern blot analysis of OsERF48 overexpression lines.
Figure S4 Drought tolerance of transgenic and nontransgenic (NT).
Figure S5 Vigorous root growth in OsERF48 overexpressors.
Figure S6 Transcriptome profile of ROX OsERF48 roots compared with nontransgenic (NT) roots.
Figure S7 Co‐expression matrix of 56 candidate genes identified amongst the differentially expressed genes (DEGs) in ROX OsERF48 roots compared to wild type using the RiceFREND web tool (http://ricefrend.dna.affrc.go.jp/). Red boxes indicate pairings of each gene.
Figure S8 Alignment of amino acid sequences from the CMI‐1 region of OsERF48 and orthologs.
Table S1 Fifty‐six candidate genes identified from the differentially expressed genes (DEGs) from the RNA‐seq analysis of ROX OsERF48 roots.
Table S2 Nodes (genes) constituting the OsERF48 co‐regulatory network.
Table S3 Primers used in this study.


