Abstract
In human-populated landscapes worldwide, domestic dogs (Canis lupus familiaris) are the most abundant terrestrial carnivore. Although dogs have been used for the protection of livestock from wild carnivores, they have also been implicated as predators of livestock. We used a combination of methods (field surveys, interview surveys, and data from secondary sources) to examine the patterns and factors driving livestock depredation by free-ranging dogs, as well as economic losses to local communities in a Trans-Himalayan agro-pastoralist landscape in India. Our results show that livestock abundance was a better predictor of depredation in the villages than local dog abundance. Dogs mainly killed small-bodied livestock and sheep were the most selected prey. Dogs were responsible for the majority of livestock losses, with losses being comparable to that by snow leopards. This high level of conflict may disrupt community benefits from conservation programs and potentially undermine the conservation efforts in the region through a range of cascading effects.
Electronic supplementary material
The online version of this article (doi:10.1007/s13280-016-0858-6) contains supplementary material, which is available to authorized users.
Keywords: Canis lupus familiaris, Economic loss, High-altitude desert, Human–animal conflict, Human-subsidized carnivore
Introduction
Livestock depredation is an important economic and conservation concern (Clark et al. 1996; Treves and Karanth 2003). The literature on human–animal conflict typically focusses on livestock depredation by large carnivores such as tigers (Harihar et al. 2014; Miller et al. 2015), wolves (Nie 2001; Karlsson and Sjostrom 2007; Kaartinen et al. 2009), and bears (Gunther et al. 2004; Goldstein et al. 2006; Piédallu et al. 2016). However, misidentification of the carnivores responsible for livestock depredation can lead to negative attitudes toward carnivore conservation and reduce the effectiveness of conservation programs. The problem is exacerbated when species such as domestic dogs, which are generally considered benign (or even beneficial) toward livestock, are responsible for depredation (e.g., Echegaray and Vila 2010; Caniglia et al. 2013).
Domestic dogs play diverse and complex roles in human communities (Lescureux and Linnell 2014; Treves and Bonacic 2016), but there is growing evidence that the human–dog relationship is not always one of happy coexistence. Unlike in developed countries, where strict rules and regulations govern the ownership of dogs, in most of the developing world, there is only a loose sense of ownership, and a large proportion of the dog population is free ranging. Due to their adaptability and key biological traits such as early sexual maturity, large litter sizes, and the ability to digest carbohydrates (Moehlman 1989; Axelsson et al. 2013), globally free-ranging dogs are now the most abundant terrestrial carnivore (Gompper 2014). As mid-sized carnivores, dogs can have varied impacts on both wild and domestic species that they interact with. There is now a large body of evidence that dogs are an important threat to native wildlife globally (Young et al. 2011; Hughes and Macdonald 2013; Ritchie et al. 2014; Wierzbowska et al. 2016). However, only a few studies have assessed their specific role as predators of livestock (Blair and Townsend 1983; Bouvier and Arthur 1995; Bergman and Bender 2009) and their role in exacerbating human–wildlife conflict (e.g., Echegaray and Vila 2010).
In this study, we examine the impact of free-ranging dogs as predators of livestock in the Upper Spiti Landscape (USL) of the Trans-Himalayan region of India. India has among the highest population of free-ranging dogs in the world [ca. 59 million (Gompper 2014)] as well as a large livestock population [ca. 512 million (www.dahd.nic.in, accessed on 12–09–2014)]. The study area is a part of the Tibetan plateau and supports traditional pastoralism and agro-pastoralism (Handa 1994; Bishop 1998) along with a unique assemblage of wild herbivores and carnivores (Mishra 1997). The last two decades have seen a rapid increase in tourism and related infrastructure in the Spiti valley. However, in an otherwise resource-poor environment, the absence of a proper garbage disposal system has provided a boost of resources for the free-ranging dog population. A consequential increase in the dog population has resulted in unwanted interactions with both people and wildlife (Hennelly et al. 2015; Kumar and Paliwal 2015; Ghoshal et al. 2016). Due to low resource availability in the lean tourist season, the dogs have started to prey on livestock as well as wildlife. A recent study on human–wildlife conflict in the same landscape found that free-ranging domestic dogs killed more livestock than snow leopard (Panthera uncia) and Tibetan wolf (Canis lupus chanco) (Suryawanshi et al. 2013).
Our study examines the patterns of livestock depredation by free-ranging dogs to understand the drivers of this unique conservation and livelihood challenge. Although prey abundance plays a crucial role in determining predator responses (Korpimaki and Norrdahl 1991; Wellenreuther 2002; Karanth et al. 2004), the effect of anthropogenic influences on predators cannot be neglected (Bino et al. 2010; Rodewald et al. 2011). For domestic dogs, abundance is largely determined by anthropogenic subsidies, which may be in the form of direct feeding by humans, or access to garbage or livestock. Because of the close linkages between the presence of dogs and humans, the factors normally associated with livestock depredation by wild predators may not be as influential for dogs. For example, rapid urbanization affects predator abundance through bottom-up forces in the form of anthropogenic subsidies giving rise to the predation paradox, i.e., predator numbers increase but predation rates decrease (Fischer et al. 2012). On the other hand, a study on dog predation patterns in Poland has shown that predation rates on livestock and wildlife are correlated with local dog abundance (Wierzbowska et al. 2016). Predation rates for predator–naïve prey such as livestock may even increase with increasing predator densities due to the lack of anti-predatory behavior, unlike wild predator–prey systems (Abrams 1993). Thus, for commensal predators like the domestic dog, the question remains as to whether depredation rates are dependent on predator abundance (already determined by bottom-up forces) or on prey abundance (wildlife and/or livestock)?
Our objectives were to test two alternate hypotheses (a) that livestock depredation patterns were governed primarily by the size of the resident dog population (predator abundance hypothesis) or (b) that depredations were a numeric effect of the livestock population, irrespective of the local dog abundance (prey abundance hypothesis). We also determined the village-wise, seasonal, and species-wise patterns of livestock depredation by dogs, and the economic losses suffered by the agro-pastoralist community due to depredation.
Materials and methods
Study area
The USL is a subdivision of Lahaul & Spiti district of Himachal Pradesh (Fig. 1). The area comes under the rain shadow of the Pir Panjal range of the Himalaya and is characterized in the winter by extreme cold (~−40 °C in peak winter) and dry conditions with precipitation mainly in the form of snow. Summer temperatures typically range from 4 to 30 °C, and the predominant vegetation type is ‘alpine scrub’ or ‘dry alpine steppe’ (Champion and Seth 1968). Large mammalian fauna include blue sheep (Pseudois nayaur), ibex (Capra sibirica), and their predators such as the snow leopard (Panthera uncia) and the Tibetan wolf (Canis lupus chanco).
Fig. 1.
Map of the study area showing the sampled villages in the Upper Spiti Landscape. The map inset shows the location of the study area in the state of Himachal Pradesh, India.
Map Courtesy: Ecoinformatics Centre, Ashoka Trust for Research in Ecology and Environment and Nature Conservation Foundation
The landscape has the lowest densities of humans in the country (2 person km−2) (www.census2011.co.in) and comprises agro-pastoralists. The livestock assemblage includes sheep, goat, donkey, cattle, yak, cattle–yak hybrids (dzo, dzomo), and horse. Livestock are grazed in pastures except during peak winter, when they are stall-fed. Based on herding practices, we classified livestock as large-bodied free-ranging (yaks and horses) and medium- to small-bodied herded (cattle, donkeys, cow–yak hybrids, goats, and sheep) (Suryawanshi et al. 2013, 2014). The landscape experienced a major change in its agro-economy with the introduction of green pea in the early 1980s, causing a shift from a subsistence/barter-based system to a market-driven one (Mishra et al. 2003a). Tourism infrastructure has expanded over the years and is an important source of income for the local communities. While tourism has benefitted local people, the associated increase in garbage has resulted in large stable populations of dogs in the town of Kaza and in the largest village of Rangrik (Pal 2013).
Field methods
Estimating dog populations
We carried out photographic capture–recapture surveys (Goswami et al. 2007; Sharma and Jhala 2011) to assess dog population size in 25 villages across the landscape from April to June 2013 (Pal 2013). We used a three-day sampling effort for dogs in 23 of 25 villages, and a one-day time-constrained sampling effort for the remaining two smaller hamlets (Gete and Tashigang = 7 households each). For all villages, we walked along all the roads and trails in and around the villages, and photographed every dog that was seen. For the 23 villages, we repeated this exercise over the next 2 days as temporal replicates to determine detection probability in the capture–recapture analysis framework (Pollock et al. 1990). Each dog was identified based on their distinct natural color, sex, and other marks (such as notched ears or scars).
Quantifying predictor variables
To obtain a measure of food availability, we quantified the number and total area of garbage dumps in each village. At each dump, we measured the dimensions (length and breadth) and summed across all dumps in the village to determine the total area of garbage/village. We calculated the distance between each village and the two areas of high dog source populations (Kaza or Rangrik) to determine the impact of distance on livestock depredation rates. We used a least-cost method rather than simple Euclidean distance measures due to the highly rugged terrain. We derived Terrain Ruggedness Index (Riley et al. 1999) from a 30 m × 30 m digital elevation model (Aster Global Digital Elevation Model) using Quantum GIS 2.8.2 (QGIS Development Team 2014). The ruggedness index was used to prepare cost surfaces and a least-cost distance measure between villages was calculated using GRASS GIS (GRASS Development Team 2015). Livestock data for all the villages (except Kaza) were obtained through targeted herder surveys. Livestock data for Kaza were obtained from the Department of Animal Husbandry and data for the number of households in each village were obtained from District Magistrate headquarters in Kaza.
Livestock herding and depredation
Interview surveys were conducted in all 25 villages to collect data on livestock depredation by dogs in the previous year (2012–2013). Due to logistic and time constraints, we performed a convenience sampling (Robinson 2014) where the questionnaire surveys targeted 15–40 % of the total households in the villages. One adult was interviewed in each household with their consent. The questionnaire was administered in Hindi (by RP) and/or Spitian language (by Kesang Chunit, a local field assistant) when required. Data were collected for each head of livestock that was reportedly killed by dogs, with specific information on kill location (whether corral/agricultural field/pasture/inside village), month, time (morning/evening), sex and age of livestock, and herder attendance (presence/absence). We also obtained a detailed account of herding practices in each village (collected with the help of experienced herders). At the end of the year (Jan–Feb 2014), key and prominent herders were interviewed (CH and Kesang Chunit) to collect information on village-level livestock mortality for the year 2013. Herders confirmed predator identities through direct sightings or signs around or on the kill. Since they needed to report livestock losses and causes of death to the owner for compensation payments (if livestock were lost to wild carnivores), we expected that their records would be fairly accurate. The study area has also been the focus of intense research and conservation efforts for the last two decades and the collection of village-level livestock mortality data has been an annual exercise since 2009. The research field staff also verified herder accounts based on occasional field visits.
Analytical methods
Dog population estimates
Individual capture history for the identified dogs was constructed using a standard “X-matrix format” (Otis et al. 1978) and the program CAPTURE was used to analyze the capture history data. Population closure was tested using the “Close Test” program (Stanley and Burnham 1999). CAPTURE produces abundance estimates from seven different models that differ in their assumptions about capture probability, where models assume no variability (M o), or assume differences in capture probabilities due to heterogeneity/individual variation (M h), behavior (M b), and time (M t). Pairwise combinations of model assumptions (M bh, M th, M tb) were also generated. Population estimates of dogs obtained from this analysis were used as a predictive variable in determining factors of livestock depredation.
Correlates of livestock depredation
We summarized the information on livestock depredation by dogs generated from interviews using standard descriptive statistics. We used compositional analysis to determine selectivity (use vs available) of livestock prey by dogs (Aebischer et al. 1993) where
To identify the influence of potential determinants on the number of livestock lost to dogs, we fitted generalized linear models using a Poisson distribution. Our response variable was the number of livestock lost to dogs in every village, while the explanatory variables were the number of households and livestock abundance (for the prey abundance hypothesis), and garbage area, dog population, and distance to high-dog population centers (for predator abundance hypothesis). We considered the total number of livestock, except for adults of large-bodied livestock, as potential available prey for dogs.
We tested for multicollinearity in the dataset and found that dog population estimates, garbage area, and the number of households were correlated. Among the three, we opted for dog population estimate and garbage area as important variables but made sure that these two variables were not used in combination within the GLM models. As the overdispersion parameter was greater than one, we fitted negative binomial models to the data. For each model, we used additive combinations of the explanatory variables in a nested manner. To prevent over-parameterization of the model, only meaningful interactions between explanatory variables were considered. We included an interaction term between dog population estimate and distance to high-dog population centers. All the variables were scaled to mean for GLM analysis. Akaike information criterion corrected for a small sample size (AICc) was used to assess model weights and the models were ranked using ∆AICc. We used Akaike weights (AICc w i) to determine the relative support for a model and accounted for model selection uncertainty by averaging the estimates of the coefficients of main effect variables across all models (Burnham and Anderson 2002).
Quantifying economic losses
Finally, we summarized the livestock losses incurred due to dogs, wild carnivores, and disease for the year 2013–2014 and calculated their associated financial losses. We used the average annual sale price for the different livestock types (based on age and sex) obtained from market sources. Financial loss was quantified in Indian rupees, which was then converted to USD (@ $1 = 68 INR in 2015) to enable comparison with other studies. All statistical analyses were performed using the R programming environment (version 3.1.1) (R Core Team 2015).
Results
Dog population estimates
For two hamlets, the time-constrained sampling resulted in a count of one dog from one village and no dogs for the other. We obtained sufficient data to conduct a mark–recapture analysis for 12 out of the 23 villages sampled. For the remaining 11 villages, due to the low number of dogs seen, we used the naïve estimate (not accounting for detection probability) of the total number of uniquely identifiable dogs as the minimum population (Table S1). We estimated 541 (±59.03 SE) dogs across 12 villages with the township of Kaza and the largest village (Rangrik) accounting for 74 % of the total dog population (Table S2). We recorded an additional 29 dogs in the remaining villages resulting in a minimum population size of ~570 dogs.
Patterns of dog depredation and herding practices
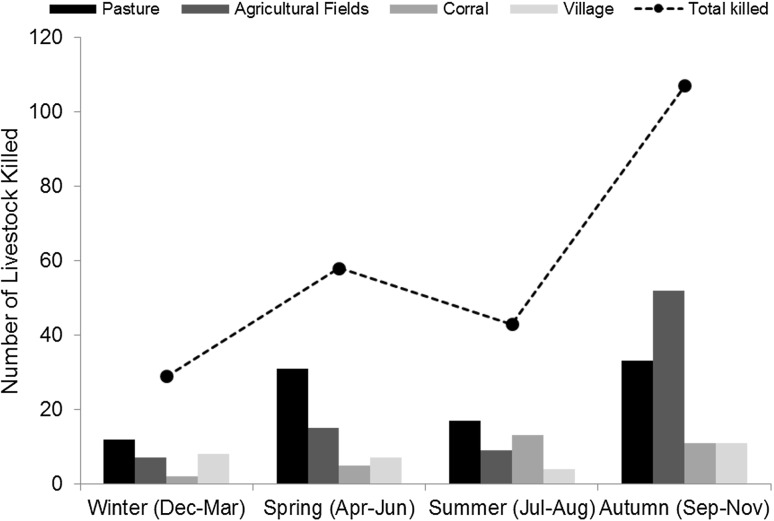
We recorded 238 livestock mortalities by dogs between Jan 2012 and May 2013 across 287 respondents interviewed in 25 villages. Sheep and goats comprised 80 % of the kills. Most predation events occurred during the day (62 % compared to evening) and in pastures (40 %) or agricultural fields (35 %) compared to corrals or inside villages. Fifty-seven percent of predation events occurred in the absence of the herders. About 46 % of the livestock losses took place during autumn followed by spring (24 %), summer (18 %), and winter (12 %). During spring and summer, most of the depredation by dogs occurred in the pastures, while during autumn most of the depredation took place in the agricultural fields (Fig. 2).
Fig. 2.
The seasonal patterns and location of livestock loss show that more livestock were killed in autumn compared to the other seasons, and more livestock were killed in pastures, except in autumn where they were more targeted in the agricultural fields
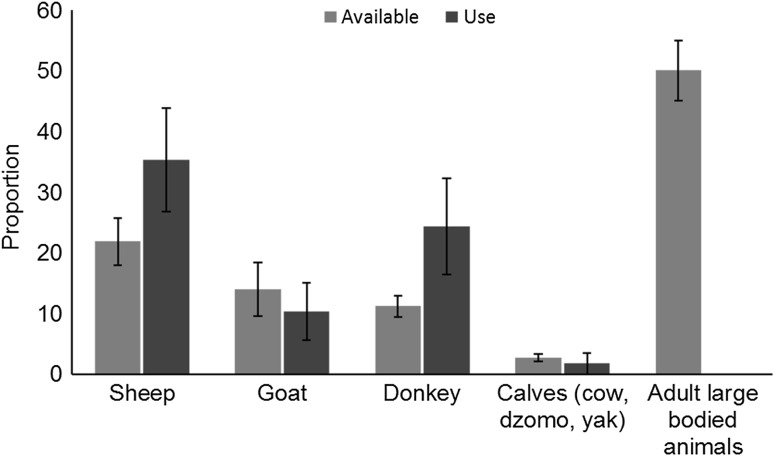
Dogs did not use the available livestock species at random but selected certain kinds of livestock as prey (Compositional analysis, λ = 0.102, P = 0.002). Sheep were the most selected prey, followed by donkeys and goats based on availability (Fig. 3). Calves and adults of larger-sized livestock (yak, horses, dzomo, and cow) were not preferred (losses of large livestock comprised 5 % of the total losses due to dogs).
Fig. 3.
Proportion of livestock available and proportion being used by dogs (in terms of depredation) in the Upper Spiti Landscape
Herding patterns in the landscape varied according to season and agricultural activities (Table 1). Medium- (cow, cow–yak hybrids, and donkeys) and small-bodied livestock (sheep, goat) were usually accompanied by herders to the pastures. They were however left unattended in the fields to forage on crop residue in autumn. Large-bodied livestock (yak and horses) were free ranging and were only brought back to villages during peak winters. In other times of the year, these animals were brought to villages for short durations for specific purposes (i.e., yaks for ploughing the fields and horses for cultural events).
Table 1.
Livestock herding patterns across seasons in the Upper Spiti Landscape by the resident agro-pastoral community
| Jan–Feb–Mar | Apr–May–Jun | Jul–Aug–Sep | Oct–Nov–Dec | |
|---|---|---|---|---|
| Agricultural activity | No agriculture Peak winter |
Cropping season | Cropping + harvest (Aug–Sep) | Post-harvest grain processing |
| Livestock types | ||||
| Small (sheep and goat) | Inside corral within house (Feb–Mar) | In pastures during day. Inside corral at night outside the house. One herder “riyok” particularly for sheep and goat along with people accompanying from the village | In pastures till about mid-August. After harvest in September, feed on stubble in agricultural fields for manure input. Herded in corrals at night outside the house | Herded in pastures from Nov till peak winter but within ca. 2 km radius of the village |
| Medium (donkey, cow, cow–yak hybrids) | Inside corral within house (Feb–Mar) | In pastures during day. Inside corral at night outside the house. | In pastures and sometimes around agricultural fields. Herded in corrals at night outside the house | In pastures within ca. 2 km range of village. Herded in corral at night outside the house |
| Large (yak, horse) | Inside corral (Feb–Mar) | In pastures. Not herded | In pastures. Not herded | In pastures. Not herded |
Determinants of livestock depredation
Our initial analysis (n vill = 25) indicated that livestock abundance had a positive influence on the magnitude of livestock depredation (β livestock = 0.53, SE = 0.28), while garbage and dog population had a negative influence (β garbage = −1.06, SE = 0.54; β dogpop = −0.42, SE = 0.37). Distance to high-dog population centers had an indeterminate influence (β dist = 0.03, SE = 0.3). Garbage area was an important variable explaining depredation followed by livestock abundance (Table 2). However, we decided to exclude the township of Kaza and the largest village (Rangrik) from the GLM analysis since they had the largest areas under garbage and, importantly, no small-bodied livestock for the last 10 years.
Table 2.
Generalized linear models to identify variables influencing livestock depredation by dogs with associated number of parameters (K), Akaike information criterion values corrected for sample sizes AICc, ∆AICc, AICc weights (AICc w i), cumulative weights, and log likelihood (LL). Models < 4∆AICc are shown. In the analyses for 23 villages, the villages of Kaza and Rangrik (having large garbage dumps, but no small-bodied livestock) have been dropped
| Models | K | AICc | ∆AICc | AICc w i | Cum.wt | LL |
|---|---|---|---|---|---|---|
| 25 villages | ||||||
| Garbage + livestock | 4 | 153.43 | 0.00 | 0.29 | 0.29 | −71.71 |
| Garbage | 3 | 154.48 | 1.05 | 0.17 | 0.46 | −73.67 |
| Dogpop + livestock + dist + dogpop × dist | 6 | 154.96 | 1.53 | 0.13 | 0.60 | −69.15 |
| Livestock | 3 | 155.19 | 1.76 | 0.12 | 0.72 | −74.02 |
| Garbage + livestock + dist | 5 | 156.58 | 3.15 | 0.06 | 0.78 | −71.71 |
| Dogpop | 3 | 157.04 | 3.61 | 0.05 | 0.82 | −74.95 |
| Garbage + dist | 4 | 157.31 | 3.88 | 0.04 | 0.86 | −73.65 |
| 23 villages | ||||||
| Livestock | 3 | 144.72 | 0.00 | 0.22 | 0.22 | −68.73 |
| Livestock + garbage | 4 | 144.92 | 0.19 | 0.20 | 0.41 | −67.35 |
| Garbage | 3 | 146.58 | 1.85 | 0.09 | 0.50 | −69.66 |
| Dogpop + livestock | 4 | 146.58 | 1.86 | 0.09 | 0.58 | −68.18 |
| Dogpop + livestock + garbage | 5 | 147.28 | 2.56 | 0.06 | 0.64 | −66.88 |
| Dogpop | 3 | 147.33 | 2.61 | 0.06 | 0.70 | −70.04 |
| Livestock + dist | 4 | 147.39 | 2.67 | 0.06 | 0.76 | −68.59 |
| Dist | 3 | 147.67 | 2.94 | 0.05 | 0.81 | −70.20 |
| Livestock + garbage + dist | 5 | 148.03 | 3.30 | 0.04 | 0.85 | −67.25 |
| Dogpop + livestock + dist + dogpop × dist | 6 | 148.49 | 3.77 | 0.03 | 0.88 | −65.62 |
garbage area under garbage in each village, livestock abundance of small- and medium-bodied livestock for each village, dogpop dog population estimate in each village, dist distance to high dog source
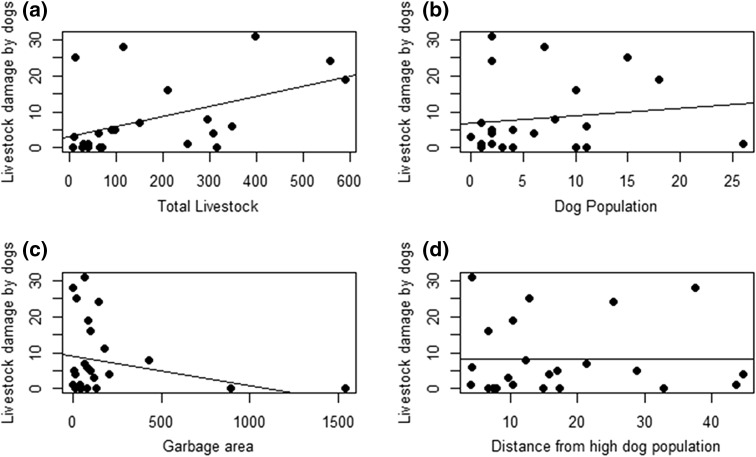
After excluding Kaza and Rangrik from the analysis, the multicollinearity between dog population and garbage was weak (VIF < 2) for the remaining 23 villages, and hence we used these variables in combination with other variables to predict livestock depredation levels. The best model explaining livestock depredation by dogs included only livestock abundance followed by the model including livestock and garbage as additive terms. Two closely related models (within 2 ∆AICc) included one with only garbage, followed by a model with local dog population in combination with livestock and garbage (Table 2). The model-averaged β estimates indicated that livestock abundance, local dog population, and distance to high-dog population centers had a positive influence on patterns of livestock depredation (β livestock = 0.58, SE = 0.3; β dogpop = 0.31, SE = 0.29; β dist = 0.11, SE = 0.32) (Fig. 4a, b, d), while garbage had a negative influence (β garbage = −0.61, SE = 0.39) (Fig. 4c). However, both local dog populations and distance to high-dog population center had high variability around the estimates and were therefore poor predictors.
Fig. 4.
a The number of livestock lost per village has a stronger relationship with the total population of livestock in that village. b There is, however, a weak positive relationship with the size of the local dog populations across villages. c The number of livestock lost per village is weakly negatively correlated to garbage area. d However, there is no relationship between livestock loss per village and distance from high-dog population area (Kaza or Rangrik)
Financial losses
We recorded a total of 441 cases of livestock losses for the year 2013 across 29 villages within the landscape. Depredation removed 4.5 % of the total livestock population, followed by disease (1 %). Depredation by both dogs and native carnivores accounted for 340 livestock losses (77 %) followed by disease (18 %) and unknown factors (5 %). Dogs contributed to the majority of livestock losses (63.5 %) followed by snow leopards (28.5 %) and wolves (8 %). The total value of livestock losses reported for one year due to depredation and diseases was USD 46 662. Dogs were the main cause of the economic losses (USD 17 522), followed by snow leopards (USD 15 029), disease (USD 11 846), and wolves (USD 2265). The average economic loss/household/year was USD 54 and 40 % this loss could be attributed to dogs alone.
Discussion
Our study demonstrates that dogs can take a heavy toll on domestic herbivores, potentially having a major impact on the livelihoods of marginal communities. The majority of kills by dogs were of small-bodied livestock (sheep and goat), and sheep was the most selected prey. Dogs also killed medium-bodied livestock such as donkeys in greater proportion than their availability. Our analysis provides support for the prey abundance hypothesis, because of the stronger predictive power of livestock abundance rather than predator abundance in explaining predation rates. These results contrast with Wierzbowska et al. (2016), who showed that livestock depredation was better predicted by local dog abundances rather than prey abundance. These differences in patterns could in part be explained by prey naivety as well as predator familiarity. Where livestock depredation by dogs is a recent phenomenon (such as in our study area), livestock may not display anti-predator behaviors to dogs as they would to wild predators such as wolves (Laporte et al. 2010). Even for wolves, the factors explaining depredation patterns of wild prey versus livestock are different. For example, wolf density was a better predictor of kill rates of wild herbivores (Vucetich et al. 2002), whereas overall livestock losses were correlated with the size of the flock per farm (Iliopoulos et al. 2009). Indeed, in some cases other ecological factors such as ruggedness of terrain used by herded livestock contributed more to depredation by wolves than herd size of small-bodied livestock (Suryawanshi et al. 2013). Thus, for naïve prey such as livestock, novel predators such as dogs may not be perceived as a danger, even though the numerical impact of depredation is similar to wild predators.
Our expectation that distance from high-dog population centers would influence livestock depredation was not supported in the analysis. In Kaza and Rangrik, the human-generated organic material is disposed as waste, whereas in most of the other villages the daily organic waste generated is utilized for fodder or composted for agriculture. The high resources in these large villages have resulted in the highest local dog abundances. The spillover populations from Kaza and Rangrik have moved to villages where there are more small-bodied livestock (Anonymous 2011). The small populations of dogs in these villages seem to be maintained by livestock carcasses during peak winters. Winter is the parturition season for many livestock species and as per local reports there is very high mortality of young animals which provides an easy resource for dogs in this season. Additionally, our mark–recapture surveys showed that dogs are moving between villages and it is therefore highly likely that only a subset of the dog population may be responsible for most of the livestock depredation. If so, this would explain the weak effect of local dog population size in explaining depredation and the lack of support for the predator abundance hypothesis.
Small-bodied livestock are most susceptible to depredation by dogs and are killed both in the presence and in the absence of herders. In the post-harvest (Sept–Oct) season, small-bodied livestock were killed when left unattended in agricultural fields, and in spring and summer (Apr–Aug) most of the depredations occurred in the pastures where they were herded. Although speculative, we feel that the herding practices of the villages may also contribute to the patterns of depredation seen here. Our observations in recent years suggests that the use of migrant labor, especially children, for herding livestock has increased in the Trans-Himalaya, which has possibly caused a decline in the vigilance levels in herding. Local villagers, as well as key herders, reported observing the presence of large numbers of dogs in packs (ranged from 8 to 15 dogs) in the pastures intermittently across the seasons. Dogs were also reported to be in the pastures continuously for 3–5 days, which is when they are likely to have preyed on livestock. The herders also expressed their concern for protecting and managing herds in the presence of large packs of dogs during such events.
Depredation by free-ranging dogs was not only responsible for a majority of the livestock losses but also contributed substantially to the average economic loss/household/year. Economically, losses due to dogs and snow leopards were almost comparable. This is because dogs killed more smaller-bodied livestock species, while snow leopards killed fewer but more expensive large-bodied livestock (yak and horses). We also believe that some livestock deaths that were attributed to wolves may instead be due to dogs as wolves are rare, and there is a strong negative perception of wolves in the landscape (Suryawanshi et al. 2013, 2014). As a result, it is likely that actual levels of dog depredation are somewhat higher than the levels that we estimated from village surveys. These high rates of depredation by free-ranging dogs can disrupt community benefits from conservation programs such as livestock insurance programs. In such programs, a premium is collected from owners, based on livestock size and risks associated with loss to wild carnivores, and contributes to a communal fund to offset depredation costs (Mishra et al. 2003b). Compensation is generally paid only for insured livestock killed by wild carnivores and not by dogs. For medium- and lower-income households who are already suffering a substantial economic burden from dog attacks, any further loss from wild carnivores is unlikely to be tolerated, potentially resulting in poor conservation outcomes.
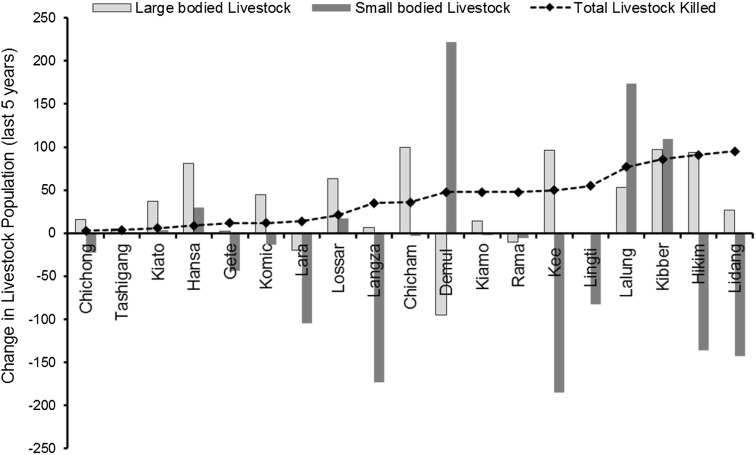
Patterns of livestock composition have also been changing across the landscape over time. Many villages along the Spiti river valley have reduced their small livestock holding due to increased access to development as well as dog depredation over the years (Fig. 5). However, some of the villages higher up in the mountains (Lalung, Kibber, Langza, Chicham, Demul, Hikkim) that still maintain small-bodied livestock are facing continued predation pressures by dogs. Except for three villages (Demul, Lalung, and Kibber) where there has been an increase in small stock in the last 5 years, most villages have witnessed reduction in their small stock and in one of the villages (Gete), people have stopped keeping sheep and goats since 2013. Although dogs did not select larger-bodied livestock as prey in our study, there is more recent evidence that they are killing calves of larger-bodied livestock (CH personal observations and communication with herders during Dec 2014–Jan 2015). This trend may increase in the future due to the reduction in small-bodied livestock population across the landscape (Anonymous 2011).
Fig. 5.
Change in small-bodied livestock and large-bodied livestock for 19 villages from 2010 to 2015 and the cumulative damage caused by dogs
Dogs not only targeted livestock but also preyed on wildlife, as reported widely (Young et al. 2011; Ritchie et al. 2014). During the winter of 2013 (Jan-March), we received nine independent eye-witness reports of blue sheep death by dogs (Pal 2013). Locals also observed dogs chasing ibex herds and preying on woolly hare (Lepus oiostolus). The shepherd dogs maintained by the migratory Gaddi pastoralists were also observed to hunt Himalayan marmots (Marmota himalayana) in the nearby Chandra Tal area (high-altitude lake and a Ramsar site). This suggests that there could be more widespread impacts of domestic dogs on the alpine ecosystems.
Conclusion
The emergence of domestic dogs as one of the main threats to livestock is an outcome of improved financial opportunities in the landscape through tourism and unplanned infrastructure development. With dogs being responsible for much of the livestock losses in the landscape, this could have a disruptive effect on existing conservation efforts, primarily the livestock insurance program, especially if predation on calves and foals increases (Mishra et al. 2003b). Current programs to manage garbage and control dog populations by the Capture–Neuter–Release method are likely to take several years of sustained effort before any significant reduction is seen in the dog population (Totton et al. 2010). It is therefore imperative that dog populations be reduced and controlled through a combination of sustained management regimes such as reducing food subsidies through garbage management, removing un-owned dogs, and focusing on responsible dog ownership. In the interim, considering that it is likely that only a subset of the dog population engages in livestock depredation, a targeted and consistent effort should be made to capture and remove dogs that are known to predate on livestock, thus providing an immediate relief to livestock owners. In the long term, improving herding practices for the resident agro-pastoralist communities could help in mitigating losses not only from dogs, but also from wild predators.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Financial support for this study was provided through the International Foundation for Science grant to CH. We would like to thank the Himachal Pradesh Forest Department, particularly the Divisional Forest Officer, Kaza, Shri Rajesh Sharma and Range Officer, Kaza, Shri Devender Singh Chauhan for their logistic support. We would like to thank the Animal Husbandry Department, Kaza, for facilitating secondary data collection. CH would like to thank Charudutt Mishra, NCF for helping in conceptualizing the paper and Ajay Bijoor, NCF for overall logistic support in field. We thank Maria Thaker for providing useful comments on the manuscript. We are thankful to the entire NCF field crew in Kibber: Chunit Kesang, Tanzin Thinley, Tanzin Thuktan, Rinchen Tobgye, Lobzang Gyalson, Kalzang Gurmet, Chudim Dorje, Sherup, Lama Tenzing, Tashi Gonpo, Takpa Tanzin, field crew from Lalung village, and the reserve guards of Chicham and Lossar village for immense support during fieldwork. We would like to thank the respondents and herders for their support and participation in data collection. Finally, we thank the three anonymous reviewers whose suggestions have improved the quality of the manuscript.
Biographies
Chandrima Home
is a Doctoral Candidate at the Ashoka Trust for Research in Ecology and the Environment and Manipal University. She is interested in the ecology of mesocarnivores in human-altered landscapes and human dimensions of ecology.
Ranjana Pal
is a Researcher in the National Mission for Sustaining Himalayan Ecosystem (NMSHE) Program at the Wildlife Institute of India. She is interested in population ecology, landscape ecology, and human dimensions in wildlife management.
Rishi Kumar Sharma
is a Conservation Biologist at World Wildlife Fund (WWF). His research interests include applied ecology and human dimensions of conservation.
Kulbhushansingh R. Suryawanshi
is a Scientist at the Nature Conservation Foundation, Mysore, and the Snow Leopard Trust, Seattle. His primary research interests include population ecology and human–wildlife interactions.
Yash Veer Bhatnagar
is a Senior Scientist at the Nature Conservation Foundation, Mysore, and the Snow Leopard Trust, Seattle. He is interested in integrated conservation of mountain ecosystems and is involved with participatory landscape-level management planning for conservation of such areas.
Abi Tamim Vanak
is an Associate Professor (Fellow) at the Ashoka Trust for Research in Ecology and the Environment, Bangalore, a Wellcome Trust/DBT India Alliance Intermediate Fellow, and an Honorary Research Associate at the School of Life Sciences, University of KwaZulu-Natal, South Africa. His research interests include animal movement ecology, disease ecology, and ecology of human-subsidized carnivores.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13280-016-0858-6) contains supplementary material, which is available to authorized users.
Contributor Information
Chandrima Home, Phone: +91(80)-23635555, Email: chandrima.home@atree.org.
Ranjana Pal, Phone: +91(135) 264112, Email: ranjana.biocon@gmail.com.
Rishi Kumar Sharma, Phone: +91 (11) 4150 4782, Email: rishi.eco@gmail.com.
Kulbhushansingh R. Suryawanshi, Phone: +91(80) 23648778, Email: kulbhushan@ncf-india.org
Yash Veer Bhatnagar, Phone: +91(821) 2515601, Email: yash@ncf-india.org.
Abi Tamim Vanak, Phone: +91(80)-23635555, Email: avanak@atree.org.
References
- Abrams PA. Why predation rate should not be proportional to predator density. Ecology. 1993;74:726–733. doi: 10.2307/1940800. [DOI] [Google Scholar]
- Aebischer NJ, Robertson PA, Kenward RE. Compositional analysis of habitat use from animal radio tracking data. Ecology. 1993;74:1313–1325. doi: 10.2307/1940062. [DOI] [Google Scholar]
- Anonymous. 2011. Management plan for the Upper Spiti Landscape including the Kibber Wildlife Sanctuary. Nature Conservation Foundation, Wildlife Wing—Himachal Pradesh Forest Department and Youth Groups in Spiti.
- Axelsson E, Ratnakumar A, Arendt M-L, Maqbool K, Webster MT, Perloski M, Liberg O, Arnemo JM, et al. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature. 2013;495:360–364. doi: 10.1038/nature11837. [DOI] [PubMed] [Google Scholar]
- Bergman D, Bender S. Dogs gone wild : Feral dog damage in the United States. In: Boulanger J, editor. Proceedings of the 13th WDM conference. New York: Saratoga Springs; 2009. pp. 177–183. [Google Scholar]
- Bino G, Dolev A, Yosha D, Guter A, King R, Saltz D, Kark S. Abrupt spatial and numerical responses of overabundant foxes to a reduction in anthropogenic resources. Journal of Applied Ecology. 2010;47:1262–1271. doi: 10.1111/j.1365-2664.2010.01882.x. [DOI] [Google Scholar]
- Bishop N. Himalayan herders. In: Spindler G, Spindler L, editors. Case studies in cultural anthropology. Fort Worth, TX: Hartcourt Brace; 1998. [Google Scholar]
- Blair BJ, Townsend TW. Dog predation of domestic sheep in Ohio. Journal of Range Management. 1983;36:527–528. doi: 10.2307/3897961. [DOI] [Google Scholar]
- Bouvier M, Arthur C. Protection et indemnisation des degats d’ours aux troupeaux domestiques dans les Pyrenees occidentales: Fonctionnement, importance economique et role dans la protection de l’ours. In: Bourliere F, Barre V, Camerra J, Herrenschmidt V, Moutou F, Servheen C, Stuart S, Saint Girons M, editors. Proceedings on the management and restoration of small and relictual bear populations. Paris: Museum of Natural History; 1995. pp. 510–521. [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multi model inference: A practical information-theoretic approach. 2. New York: Springer; 2002. [Google Scholar]
- Caniglia R, Fabbri E, Mastrogiuseppe L, Randi E. Who is who? Identification of livestock predators using forensic genetic approaches. Forensic Science International: Genetics. 2013;7:397–404. doi: 10.1016/j.fsigen.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Champion HG, Seth SK. A revised survey of the forest types of India. Delhi: Manager of Publications, GOI; 1968. [Google Scholar]
- Clark TW, Curlee AP, Reading RP. Crafting effective solutions to the large carnivore conservation problem. Conservation Biology. 1996;10:940–948. doi: 10.1046/j.1523-1739.1996.10040940.x. [DOI] [Google Scholar]
- Echegaray J, Vila C. Noninvasive monitoring of wolves at the edge of their distribution and the cost of their conservation. Animal Conservation. 2010;13:157–161. doi: 10.1111/j.1469-1795.2009.00315.x. [DOI] [Google Scholar]
- Fischer JD, Cleeton SH, Lyons TP, Miller JR, Fischer JD, Cleeton SH, Timothy P. Urbanization and the predation paradox: The role of trophic dynamics in structuring vertebrate communities. BioScience. 2012;62:809–818. doi: 10.1525/bio.2012.62.9.6. [DOI] [Google Scholar]
- Ghoshal A, Bhatnagar YV, Mishra C, Suryawanshi K. Response of the red fox to expansion of human habitation in the Trans-Himalayan mountains. European Journal of Wildlife Research. 2016;62:131–136. doi: 10.1007/s10344-015-0967-8. [DOI] [Google Scholar]
- Goldstein I, Paisley S, Wallace R, Jorgenson JP, Cuesta F, Castellanos A. Andean bear-livestock conflicts: A review. Ursus. 2006;17:8–15. doi: 10.2192/1537-6176(2006)17[8:ABCAR]2.0.CO;2. [DOI] [Google Scholar]
- Gompper ME. The dog-human-wildlife interface: Assessing the scope of the problem. In: Gompper ME, editor. Free-ranging dogs and wildlife conservation. Oxford: Oxford University Press; 2014. pp. 9–54. [Google Scholar]
- Goswami VR, Madhusudan MD, Karanth KU. Application of photographic capture–recapture modelling to estimate demographic parameters for male Asian elephants. Animal Conservation. 2007;10:391–399. doi: 10.1111/j.1469-1795.2007.00124.x. [DOI] [Google Scholar]
- GRASS Development Team. 2015. Geographic Resources Analysis Support System (GRASS) Software, Version 7.0. Open Source Geospatial Foundation. http://grass.osgeo.org.
- Gunther KA, Haroldson MA, Frey K, Cain SL, Copeland J, Schwartz CC. Grizzly bear–human conflicts in the Greater Yellowstone ecosystem, 1992–2000. Ursus. 2004;15:10–22. doi: 10.2192/1537-6176(2004)015<0010:GBCITG>2.0.CO;2. [DOI] [Google Scholar]
- Handa O. Tabo Monastery and Buddhism in the Trans-Himalaya: Thousand years of existence of the Tabo Chos-Khor. New Delhi: Indus Publishing; 1994. [Google Scholar]
- Harihar A, Ghosh-Harihar M, MacMillan DC. Human resettlement and tiger conservation—Socio-economic assessment of pastoralists reveals a rare conservation opportunity in a human-dominated landscape. Biological Conservation. 2014;169:167–175. doi: 10.1016/j.biocon.2013.11.012. [DOI] [Google Scholar]
- Hennelly L, Habib B, Lyngdoh S. Himalayan wolf and feral dog displaying mating behaviour in Spiti Valley, India, and potential conservation threats from sympatric feral dogs. Canid Biology and Conservation. 2015;18:27–30. [Google Scholar]
- Hughes J, Macdonald DW. A review of the interactions between free-roaming domestic dogs and wildlife. Biological Conservation. 2013;157:341–351. doi: 10.1016/j.biocon.2012.07.005. [DOI] [Google Scholar]
- Iliopoulos Y, Sgardelis S, Koutis V, Savaris D. Wolf depredation on livestock in central Greece. Acta Theriologica. 2009;54:11–22. doi: 10.1007/BF03193133. [DOI] [Google Scholar]
- Kaartinen S, Luoto M, Kojola I. Carnivore-livestock conflicts: Determinants of wolf (Canis lupus) depredation on sheep farms in Finland. Biodiversity and Conservation. 2009;18:3503–3517. doi: 10.1007/s10531-009-9657-8. [DOI] [Google Scholar]
- Karanth KU, Nichols JD, Kumar NS, Link WA, Hines JE. Tigers and their prey: Predicting carnivore densities from prey abundance. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4854–4858. doi: 10.1073/pnas.0306210101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J, Sjostrom M. Human attitudes towards wolves, a matter of distance. Biological Conservation. 2007;137:610–616. doi: 10.1016/j.biocon.2007.03.023. [DOI] [Google Scholar]
- Korpimaki E, Norrdahl K. Numerical and functional responses of kestrels, short-eared owls, and long-eared owls to vole densities. Ecology. 1991;72:814–826. doi: 10.2307/1940584. [DOI] [Google Scholar]
- Kumar A, Paliwal R. Feral dogs of Spiti Valley, Himachal Pradesh: An emerging threat for wildlife and human life. Current Science. 2015;108:1799–1800. [Google Scholar]
- Laporte I, Muhly TB, Pitt JA, Alexander M, Musiani M. Effects of wolves on elk and cattle behaviors: Implications for livestock production and wolf conservation. PLoS ONE. 2010;5:e11954. doi: 10.1371/journal.pone.0011954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescureux N, Linnell JDC. Warring brothers: The complex interactions between wolves (Canis lupus) and dogs (Canis familiaris) in a conservation context. Biological Conservation. 2014;171:232–245. doi: 10.1016/j.biocon.2014.01.032. [DOI] [Google Scholar]
- Miller JRB, Jhala YV, Jena J, Schmitz OJ. Landscape-scale accessibility of livestock to tigers: Implications of spatial grain for modeling predation risk to mitigate human-carnivore conflict. Ecology and Evolution. 2015;5:1354–1367. doi: 10.1002/ece3.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra C. Livestock depredation by large carnivores in the Indian trans-Himalaya: Conflict perceptions and conservation prospects. Environmental Conservation. 1997;24:338–343. doi: 10.1017/S0376892997000441. [DOI] [Google Scholar]
- Mishra C, Prins HHT, Van Wieren SE. Diversity, risk mediation, and change in a Trans-Himalayan agropastoral system. Human Ecology. 2003;31:595–609. doi: 10.1023/B:HUEC.0000005515.91576.8f. [DOI] [Google Scholar]
- Mishra C, Allen P, McCarthy T, Madhusudan MD, Bayarjargal A, Prins HHT. The role of incentive programs in conserving the snow leopard. Conservation Biology. 2003;17:1512–1520. doi: 10.1111/j.1523-1739.2003.00092.x. [DOI] [Google Scholar]
- Moehlman PD. Intraspecific variation in canid social systems. In: Gittleman JL, editor. carnivore Behavior, ecology and evolution. Berlin: Springer; 1989. pp. 143–163. [Google Scholar]
- Nie MA. The sociopolitical dimensions of wolf management and restoration in the United States. Human Ecology Review. 2001;8:1–12. [Google Scholar]
- Otis D, Burnham K, White G, Anderson D. Statistical inference from capture data on closed animal populations. Wildlife Monographs. 1978;62:3–135. [Google Scholar]
- Pal, R. 2013. Estimates of dog abundance and livestock predation along a gradient of village sizes in the Spiti Valley, Himachal Pradesh. MSc Thesis. Guru Gobind Singh Indraprastha University, New Delhi.
- Piédallu B, Quenette P-Y, Mounet C, Lescureux N, Borelli-Massines M, Dubarry E, Camarra J-J, Gimenez O. Spatial variation in public attitudes towards brown bears in the French Pyrénées. Biological Conservation. 2016;197:90–97. doi: 10.1016/j.biocon.2016.02.027. [DOI] [Google Scholar]
- Pollock KH, Nichols JD, Brownie C, Hines JE. Statistical inference for capture-recapture experiments. Wildlife Monographs. 1990;107:1–97. [Google Scholar]
- QGIS Development Team. 2014. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org.
- R Core Team. 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/.
- Riley S, DeGloria S, Elliot R. A terrain ruggedness index that quantifies topographic heterogeneity. Intermountain Journal of Science. 1999;5:23–27. [Google Scholar]
- Ritchie EG, Dickman CR, Letnic M, Vanak AT. Dogs as predators and trophic regulators. In: Gompper ME, editor. Free-ranging dogs and wildlife conservation. Oxford: Oxford University Press; 2014. pp. 55–68. [Google Scholar]
- Robinson OC. Sampling in interview-based qualitative research: A theoretical and practical guide. Qualitative Research in Psychology. 2014;11:25–41. doi: 10.1080/14780887.2013.801543. [DOI] [Google Scholar]
- Rodewald AD, Kearns LJ, Shustack DP. Anthropogenic resource subsidies decouple predator-prey relationships. Ecological Applications. 2011;21:936–943. doi: 10.1890/10-0863.1. [DOI] [PubMed] [Google Scholar]
- Sharma RK, Jhala YV. Monitoring tiger populations using intensive search in a capture–recapture framework. Population Ecology. 2011;53:373–381. doi: 10.1007/s10144-010-0230-9. [DOI] [Google Scholar]
- Stanley T, Burnham K. A closure test for time-specific capture-recapture data. Environmental and Ecological Statistics. 1999;6:197–209. doi: 10.1023/A:1009674322348. [DOI] [Google Scholar]
- Suryawanshi KR, Bhatnagar YV, Redpath S, Mishra C. People, predators and perceptions: Patterns of livestock depredation by snow leopards and wolves. Journal of Applied Ecology. 2013;50:550–560. doi: 10.1111/1365-2664.12061. [DOI] [Google Scholar]
- Suryawanshi KR, Bhatia S, Bhatnagar YV, Redpath S, Mishra C. Multiscale factors affecting human attitudes toward snow leopards and wolves. Conservation Biology. 2014;28:1657–1666. doi: 10.1111/cobi.12320. [DOI] [PubMed] [Google Scholar]
- Totton SC, Wandeler AI, Zinsstag J, Bauch CT, Ribble CS, Rosatte RC, McEwen SA. Stray dog population demographics in Jodhpur, India following a population control/rabies vaccination program. Preventive Veterinary Medicine. 2010;97:51–57. doi: 10.1016/j.prevetmed.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Treves A, Bonacic C. Humanity’s dual response to dogs and wolves. Trends in Ecology & Evolution. 2016;31:489–491. doi: 10.1016/j.tree.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Treves A, Karanth KU. Human-carnivore conflict and perspectives on carnivore management worldwide. Conservation Biology. 2003;17:1491–1499. doi: 10.1111/j.1523-1739.2003.00059.x. [DOI] [Google Scholar]
- Vucetich JA, Peterson RO, Schaefer CL. The effect of prey and predator densities on wolf predation. Ecology. 2002;83:3003–3013. doi: 10.1890/0012-9658(2002)083[3003:TEOPAP]2.0.CO;2. [DOI] [Google Scholar]
- Wellenreuther M. Response of predators to prey abundance: Separating the effects of prey density and patch size. Journal of Experimental Marine Biology and Ecology. 2002;273:61–71. doi: 10.1016/S0022-0981(02)00145-4. [DOI] [Google Scholar]
- Wierzbowska IA, Hędrzak M, Popczyk B, Okarma H, Crooks KR. Predation of wildlife by free-ranging domestic dogs in Polish hunting grounds and potential competition with the grey wolf. Biological Conservation. 2016;201:1–9. doi: 10.1016/j.biocon.2016.06.016. [DOI] [Google Scholar]
- Young JK, Olson KA, Reading RP, Amgalanbaatar S, Berger J. Is wildlife going to the dogs? Impacts of feral and free-roaming dogs on wildlife populations. BioScience. 2011;61:125–132. doi: 10.1525/bio.2011.61.2.7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.