Abstract
Natural phytochemicals are attracting increasing interest as anticancer agents. The aim of this study is to evaluate the therapeutic potential of geraniin, a major ellagitannin extracted from Geranium sibiricum L., in human glioma. Human U87 and LN229 glioma cells were treated with different concentrations of geraniin, and cell viability, apoptosis, and gene expression were assessed. The involvement of STAT3 signaling in the action of geraniin was examined. We found that geraniin treatment for 48 h significantly (P < 0.05) impaired the phosphorylation of STAT3 and reduced the expression of downstream target genes Bcl-xL, Mcl-1, Bcl-2, and cyclin D1. Exposure to geraniin led to a concentration-dependent decline in cell viability and increase in apoptosis in glioma cells, but had no significant impact on the viability of normal human astrocytes. Measurement of caspase-3 activity showed that geraniin-treated U87 and LN229 cells showed a 1.8–2.5-fold higher caspase-3 activity than control cells. Overexpression of constitutively active STAT3 significantly (P < 0.05) reversed geraniin-mediated growth suppression and apoptosis, which was accompanied by restoration of Bcl-xL, Mcl-1, Bcl-2, and cyclin D1 expression. In an xenograft tumor mouse model, geraniin treatment significantly retarded tumor growth and induced apoptosis. Western blot analysis confirmed the suppression of STAT3 phosphorylation in glioma xenograft tumors by geraniin. Taken together, these data suggest that geraniin exerts growth-suppressive and pro-apoptotic effects on glioma cells via inhibition of STAT3 signaling and may have therapeutic benefits in malignant gliomas.
Keywords: Apoptosis, Ellagitannin, Glioma, Proliferation, STAT3 signaling
Introduction
Glioblastoma multiforme (GBM) is the most aggressive tumor of the central nervous system (Alexiou et al. 2015). Surgery followed by adjuvant radiation and chemotherapy is usually used to treat GBM (Omuro and DeAngelis 2013; Chinot et al. 2014). Despite improvement in multimodal therapies, the median survival time for malignant gliomas is only 1–2 years (Wen and Kesari 2008; Tanase et al. 2013). Therefore, it is of importance to develop novel therapeutic agents that can be used as a single agent or in combination with existing drugs to treat GBM.
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor that is constitutively activated in many types of cancers (Yeh and Frank 2016). STAT3 activation is implicated in multiple aspects of tumor biology, such as proliferation, survival, invasion, and angiogenesis (Chai et al. 2016). At the molecular level, STAT3 regulates the expression of numerous genes involved in tumor progression, including cyclin D1, c-myc, Bcl-2, Bcl-xL, Mcl-1, and MMP3 (Chai et al. 2016). Aberrant activation of STAT3 has been shown to contribute to glioma tumorigenesis, metastasis, and drug resistance (Gray et al. 2014; Xue et al. 2016). Preclinical studies reported that inhibition of STAT3 signaling suppresses human glioma growth (Yue et al. 2016; Ma et al. 2015). STAT3 is thus suggested as a promising therapeutic target for malignant gliomas.
Geraniin, a major ellagitannin extracted from Geranium sibiricum L., has shown numerous bioactive properties including anti-inflammatory, anti-hyperglycemic, antihypertensive, and antitumor activities (Elendran et al. 2015; Wang et al. 2016; Zhai et al. 2016). This compound was found to trigger apoptotic response in different types of cancer cells, such as breast cancer (Zhai et al. 2016), lung adenocarcinoma (Li et al. 2013), and melanoma (Lee et al. 2008) cells, suggesting its broad anti-cancer potential. Although several other ellagitannins such as punicalagin (Wang et al. 2013) have displayed pro-apoptotic activity in glioma cells, the pharmacological effects of geraniin on malignant gliomas are still unclear.
In this study, we examined the effects of geraniin treatment on the growth and apoptosis of glioma cells both in vitro and in vivo. Since STAT3 signaling plays a pivotal role in glioma development and progression, we checked whether the activity of geraniin in glioma cells was associated with regulation of STAT3-mediated signaling.
Materials and methods
Cell culture and treatment
Human glioma cell lines (U87 and LN229) were obtained from the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). They were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS; PAA Laboratories, Pasching, Austria). Normal human astrocytes were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA) and maintained in astrocyte medium (ScienCell Research Laboratories) supplemented with 10% FBS. Geraniin (≥98% in purity; Fig. 1a) was purchased from Wuhan ChemFaces Biochemical Co., Ltd. (Wuhan, China). It was dissolved in 0.1% dimethyl sulfoxide (DMSO) to prepare a stock solution (50 mM). For geraniin treatment, glioma cells were exposed to different concentrations of geraniin (5, 40, and 80 μM) for 48 h and cell viability, apoptosis, and gene expression were analyzed.
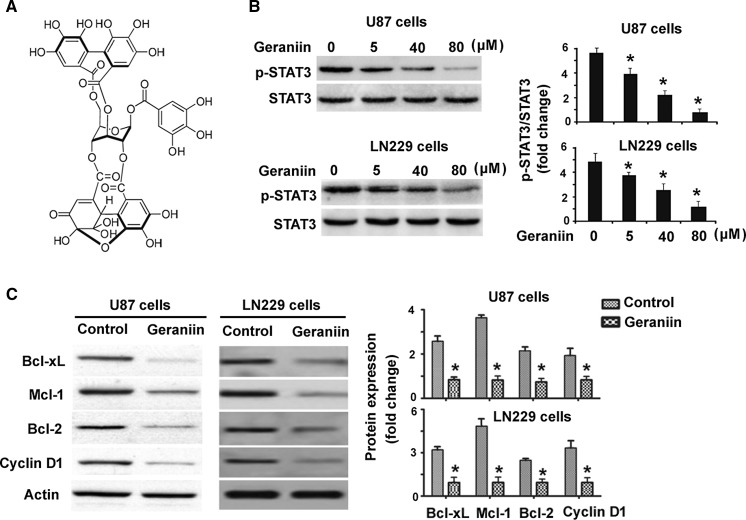
Fig. 1.
Geraniin inhibits constitutive STAT3 activation in glioma cells. a Chemical structure of geraniin. b Western blot analysis of phosphorylated and total STAT3 protein in U87 and LN229 cells treated with different concentrations of geraniin. c Western blot analysis of indicated proteins in U87 and LN229 cells treated with vehicle or 80 μM geraniin. Bar graphs represent densitometric analysis of the Western blots from three independent experiments. *P < 0.05 versus control
Western blot analysis
Protein lysates were prepared using radioimmune precipitation (RIPA) buffer containing protease inhibitors (Sigma-Aldrich, St. Louis, MO, USA), separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred onto nitrocellulose membranes. The membranes were incubated at 4 °C overnight with anti-phospho-STAT3(Y705), anti-STAT3 (Abcam, Cambridge, UK), anti-Bcl-xL, anti-Mcl-1, anti-Bcl-2, anti-cyclin D1, or anti-β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology). Protein bands were visualized using a chemiluminescence detection kit (Pierce, Rockford, IL, USA). Densitometry was performed using the Model GS-800 Calibrated Imaging Densitometer System with Quantity One software (Bio-Rad, Hercules, CA, USA).
Cell viability assay
Cells were plated at a density of 1 × 104 cells/well into 96-well plates and treated with geraniin for 48 h. Cell viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) assay. MTT solution (0.5 mg/mL; Sigma-Aldrich) was added to each well and incubated for 4 h at 37 °C. The formazan crystals were dissolved in dimethyl sulfoxide, and absorbance was recorded at the wavelength of 570 nm.
Detection of apoptotic cells by flow cytometry
Apoptosis was assessed using an Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit, according to the manufacturer’s instructions (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). Cells were stained with Annexin V and propidium iodide (PI) for 15 min in the dark. Apoptotic cells were detected using a FACSCaliber flow cytometer (Becton–Dickinson, Franklin Lakes, NJ, USA).
Caspase-3 activity assay
Cells were lysed in lysis buffer and caspase-3 activity was determined using a colorimetric assay kit (BioVision Inc., Mountain View, CA, USA). Absorbance was read at the wavelength of 405 nm.
Cell transfection
A plasmid expressing constitutively active STAT3 (CA-STAT3) was obtained from Addgene Inc. (Cambridge, MA, USA). Glioma cells were transiently transfected with CA-STAT3 or empty vector (1 μg) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. At 24 h after transfection, cells were exposed to 80 μM geraniin for another 48 h before further analyses.
Animal experiments
U87 cells (2 × 106 cells/mouse) were subcutaneously injected to the right flank of male BALB/c nude mice (4 week old; Shanghai Laboratory Animal Center, Chinese Academy of Sciences, Shanghai, China). When xenograft tumors grew to ~100 mm3, mice were randomly assigned into the following 2 groups (n = 4): control group (0.1% DMSO) and geraniin (60 mg/kg) group. Geraniin was administered once daily by oral gavage. Tumor volume was measured every 4 days. At 20 days after the initial treatment, mice were sacrificed and tumors were weighed. Tumor samples were processed for apoptosis analysis by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining using an apoptotic cell detection kit (Nanjing KeyGen Biotech Co., Ltd.). This study was approved by the Ethics Committee for the Use and Care of Laboratory Animals at Kunming Medical University (Kunming, China).
Statistical analysis
Data are expressed as mean ± standard deviation. Statistical significance was determined by Student’s t test or one-way analysis of variance (ANOVA) followed by the Tukey’s test. P < 0.05 was considered to be statistically significant.
Results
Geraniin inhibits constitutive STAT3 activation in glioma cells
We first examined the effect of geraniin on STAT3 signaling activation. As shown in Fig. 1b, geraniin at 5–80 μM significantly suppressed the phosphorylation of STAT3 in U87 cells, compared to control cells (P < 0.05). Such suppression was in a concentration-dependent fashion. Similarly, geraniin-treated LN229 cells displayed significantly lower levels of phosphorylated STAT3 than control cells. Analysis of the expression of several downstream targets of STAT3 further demonstrated that geraniin at 80 μM caused a significant reduction of Bcl-xL, Mcl-1, Bcl-2, and cyclin D1 protein in both U87 and LN229 cells (Fig. 1c).
Geraniin suppresses cell viability and induces apoptosis in glioma cells
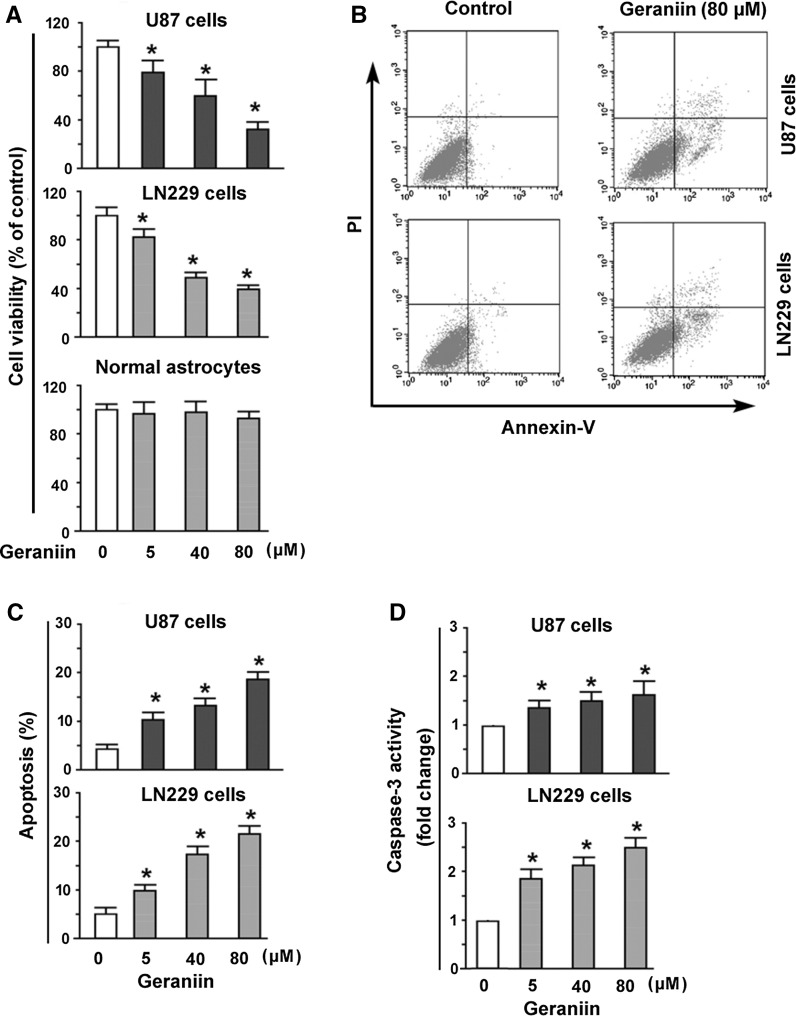
Given the regulation of STAT3 signaling by geraniin, we investigated the effect of geraniin on glioma growth. MTT assay showed that exposure to geraniin for 48 h led to a concentration-dependent inhibition of the viability of U87 and LN229 cells (Fig. 2a). However, geraniin treatment did not affect the viability of normal human astrocytes up to the maximal concentration used. Apoptosis analysis after Annexin-V/PI staining showed that geraniin promoted apoptotic death in U87 and LN229 cells in a concentration-dependent manner (Fig. 2b, c). Measurement of caspase-3 activity, a well-established marker of apoptosis, further confirmed that geraniin-treated U87 and LN229 cells showed a 1.8–2.5-fold increase in caspase-3 activity, compared to corresponding controls (P < 0.05; Fig. 2d).
Fig. 2.
Geraniin suppresses cell viability and induces apoptosis in glioma cells. a U87 and LN229 NSCLC cells and normal human astrocytes were treated with geraniin at 5–80 μM for 48 h and tested for cell viability by the MTT assay. b, c Flow cytometric analysis of apoptosis after Annexin-V/PI staining. b Representative dot plots of Annexin V-FITC/PI staining of U87 and LN229 50 cells treated with or without geraniin (80 μM). c Geraniin at 5–80 μM caused a concentration-dependent apoptosis in U87 and LN229 cells. d Measurement of caspase-3 activity in U87 and LN229 cells treated with indicated concentrations of geraniin. *P < 0.05 versus control
Overexpression of STAT3 reverses geraniin-mediated growth suppression and apoptosis in glioma cells
Next, we checked whether geraniin-mediated growth suppression and apoptosis in glioma cells are associated with inactivation of STAT3 signaling. To this end, we performed rescue experiments with constitutively active STAT3. Delivery of constitutively active STAT3 was found to almost completely reverse geraniin-mediated growth suppression in U87 and LN229 cells (Fig. 3a). Consistently, geraniin-induced apoptosis was significantly (P < 0.05) blocked by overexpression of constitutively active STAT3 (Fig. 3b). At the molecular level, overexpression of STAT3 restored the expression of Bcl-xL, Mcl-1, Bcl-2, and cyclin D1 protein in geraniin-treated U87 cells (Fig. 3c).
Fig. 3.
Overexpression of STAT3 reverses geraniin-mediated growth suppression and apoptosis in glioma cells. Glioma cells were transfected with constitutively active STAT3-expressing plasmid or empty vector 24 h before exposure to 80 μM geraniin. Measurement of a cell viability and b apoptosis in U87 and LN229 cells after indicated treatments. *P < 0.05. c Western blot analysis of indicated proteins in U87 cells. Representative Western blots from three independent experiments are shown
Geraniin retards the growth of human glioma cells in vivo
Finally, we explored the in vivo effect of geraniin on glioma growth in a mouse model. Nude mice with U87 xenograft tumors were administered with a single dose of geraniin or an equal volume of vehicle and tumor growth was measured every 4 days for 20 days. It was found that geraniin treatment significantly (P < 0.05) suppressed tumor growth, compared to the control group (Fig. 4a). Final tumor weight in the geraniin-treated group was about 44% of that in the control group (0.31 ± 0.08 vs. 0.69 ± 0.11 g, P < 0.05; Fig. 4b). TUNEL analysis revealed that geraniin-treated tumors had significantly higher TUNEL-positive cells than the control group, 9.4 ± 1.5 versus 2.9 ± 0.8% (Fig. 4c). Western blot analysis confirmed that geraniin treatment significantly decreased the phosphorylation of STAT3 in U87 xenograft tumors (Fig. 4d). Collectively, geraniin shows the ability to suppress glioma growth in vivo.
Fig. 4.
Geraniin inhibits the growth of human glioma cells in vivo. a Tumor growth curves were determined for U87 xenograft tumors treated with geraniin (60 mg/kg) or vehicle every 4 days for 20 days. b Tumors were removed and weighed at 20 days after geraniin treatment. c TUNEL analysis was performed to detect apoptosis in U87 xenograft tumors after geraniin treatment. Top representative sections with TUNEL staining. Bottom quantification of the percentage of TUNEL-positive cells in tumors. Scale bar 100 μm. d Western blot analysis of STAT3 phosphorylation in U87 xenograft tumors. Bottom quantification of the Western blots. *P < 0.05 versus control
Discussion
A wide variety of natural ellagitannins have exhibited antiproliferative activity in cancer cells and represent an important source of anticancer phytochemicals (Ismail et al. 2016). For instance, davidiin extracted from a medicinal herb Polygonum capitatum was reported to suppress tumor growth in hepatocellular carcinoma (Wang et al. 2014). The ellagitannin punicalagin can exert cytoprotective effects on lung cancer cells exposed to benzo[a]pyrene, a potent mutagen (Zahin et al. 2014). Ellagitannins are effective in inhibiting multiple cancer-related signaling pathways including Akt and nuclear factor-kappaB (NF-κB) signaling (Heber 2008; Adams et al. 2006). In this study, we showed that the ellagitannin geraniin had the ability to suppress STAT3 signaling in glioma cells. Treatment with geraniin caused a concentration-dependent inhibition of STAT3 phosphorylation, which was accompanied by a significant decline in the expression of downstream target genes including Bcl-xL, Mcl-1, Bcl-2, and cyclin D1. Constitutive activation of STAT3 signaling is known to be required for glioma growth (Xue et al. 2016). Our results suggest that geraniin may have anticancer potential in glioma. Another ellagitannin punicalagin can also exert cytotoxic effects against glioma cells (Wang et al. 2013). These findings suggest that natural ellagitannins may represent an important source of anticancer biomolecules.
Previous studies have demonstrated that geraniin can induce significantly apoptosis in breast cancer, lung adenocarcinoma, and melanoma cells (Lee et al. 2008; Li et al. 2013; Zhai et al. 2016). Consistently, we found that geraniin exposure led to a significant decrease in cell viability and increase in apoptosis in glioma cells. In vivo studies confirmed that geraniin treatment significantly retarded tumor growth and induced apoptosis in a glioma xenograft mouse model. These results highlight the anticancer potential of geraniin in glioma. Besides apoptotic death, induction of cell cycle arrest may also contribute to the growth-suppressive activity of geraniin. This hypothesis is supported by the finding that geraniin treatment led to a marked downregulation of cyclin D1, a key regulator of cell cycle progression in glioma cells (Xu et al. 2014).
In contrast to cancer cells, normal human astrocytes were less sensitive to geraniin. It has been reported that geraniin confers protection against γ-radiation-induced apoptosis in Chinese hamster lung fibroblast (V79-4) cells (Kang et al. 2011) and splenocytes isolated from C57BL/6 mice (Bing et al. 2013). These observations suggest that geraniin seems to be selectively cytotoxic to cancer cells. The molecular basis for this tumor selectivity is still unclear. In this study, we showed that overexpression of constitutively active STAT3 significantly rescued the viability of glioma cells exposed to geraniin. Moreover, geraniin treatment significantly suppressed the phosphorylation of STAT3 in glioma xenograft tumors. Previous studies have demonstrated that pharmacological inhibition of STAT3 activation causes cytotoxic effects against glioma cells (Ma et al. 2015; Mukthavaram et al. 2015), which is consistent with our findings. Taken together, inhibition of constitutive STAT3 activation accounts, at least partially, for geraniin-mediated cytotoxic effects against glioma cells.
STAT3 as a transcription factor is implicated in the regulation of many cancer-related genes (Chai et al. 2016). Our data further demonstrated that the expression of Bcl-xL, Mcl-1, Bcl-2, and cyclin D1 in geraniin-treated cells was restored by overexpression of STAT3. Bcl-xL, Mcl-1, and Bcl-2 are well-known anti-apoptotic proteins and contribute to the survival of glioma cells in response to cytotoxic agents (Harmalkar et al. 2015; Karpel-Massler et al. 2015). Cyclin D1 is a key gene involved in cell cycle progression. Knockdown of cyclin D1 modulates several aspects of glioma biology, leading to suppression of growth and invasion and induction of apoptosis (Wang et al. 2012). The downregulation of Bcl-xL, Mcl-1, Bcl-2, and cyclin D1 expression by geraniin provides a molecular explanation for its pro-apoptotic activity in glioma cells.
In conclusion, this work demonstrates the anticancer effects of geraniin on glioma cells both in vitro and in vivo, which are causally linked to inhibition of STAT3 activation and downregulation of Bcl-xL, Mcl-1, Bcl-2, and cyclin D1. Geraniin may represent a promising therapeutic agent for malignant gliomas.
References
- Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54:980–985. doi: 10.1021/jf052005r. [DOI] [PubMed] [Google Scholar]
- Alexiou GA, Tsamis KI, Kyritsis AP. Targeting tumor necrosis factor-related apoptosis-inducing ligand (TRAIL): a promising therapeutic strategy in gliomas. Semin Pediatr Neurol. 2015;22:35–39. doi: 10.1016/j.spen.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Bing SJ, Ha D, Kim MJ, Park E, Ahn G, Kim DS, Ko RK, Park JW, Lee NH, Jee Y. Geraniin down regulates gamma radiation-induced apoptosis by suppressing DNA damage. Food Chem Toxicol. 2013;57:147–153. doi: 10.1016/j.fct.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Chai EZ, Shanmugam MK, Arfuso F, Dharmarajan A, Wang C, Kumar AP, Samy RP, Lim LH, Wang L, Goh BC, Ahn KS, Hui KM, Sethi G. Targeting transcription factor STAT3 for cancer prevention and therapy. Pharmacol Ther. 2016;162:86–97. doi: 10.1016/j.pharmthera.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- Elendran S, Wang LW, Prankerd R, Palanisamy UD. The physicochemical properties of geraniin, a potential antihyperglycemic agent. Pharm Biol. 2015;53:1719–1726. doi: 10.3109/13880209.2014.1003356. [DOI] [PubMed] [Google Scholar]
- Gray GK, McFarland BC, Nozell SE, Benveniste EN. NF-κB and STAT3 in glioblastoma: therapeutic targets coming of age. Expert Rev Neurother. 2014;14:1293–1306. doi: 10.1586/14737175.2014.964211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmalkar M, Upraity S, Kazi S, Shirsat NV. Tamoxifen-induced cell death of malignant glioma cells is brought about by oxidative-stress-mediated alterations in the expression of BCL2 family members and is enhanced on miR-21 Inhibition. J Mol Neurosci. 2015;57:197–202. doi: 10.1007/s12031-015-0602-x. [DOI] [PubMed] [Google Scholar]
- Heber D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett. 2008;269:262–268. doi: 10.1016/j.canlet.2008.03.043. [DOI] [PubMed] [Google Scholar]
- Ismail T, Calcabrini C, Diaz AR, Fimognari C, Turrini E, Catanzaro E, Akhtar S, Sestili P. Ellagitannins in cancer chemoprevention and therapy. Toxins (Basel) 2016;8(5):E151. doi: 10.3390/toxins8050151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KA, Lee IK, Zhang R, Piao MJ, Kim KC, Kim SY, Shin T, Kim BJ, Lee NH, Hyun JW. Radioprotective effect of geraniin via the inhibition of apoptosis triggered by γ-radiation-induced oxidative stress. Cell Biol Toxicol. 2011;27:83–94. doi: 10.1007/s10565-010-9172-4. [DOI] [PubMed] [Google Scholar]
- Karpel-Massler G, Ba M, Shu C, Halatsch ME, Westhoff MA, Bruce JN, Canoll P, Siegelin MD. TIC10/ONC201 synergizes with Bcl-2/Bcl-xL inhibition in glioblastoma by suppression of Mcl-1 and its binding partners in vitro and in vivo. Oncotarget. 2015;6:36456–36471. doi: 10.18632/oncotarget.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Tsai CY, Kao JY, Kao MC, Tsai SC, Chang CS, Huang LJ, Kuo SC, Lin JK, Way TD. Geraniin-mediated apoptosis by cleavage of focal adhesion kinase through up-regulation of Fas ligand expression in human melanoma cells. Mol Nutr Food Res. 2008;52:655–663. doi: 10.1002/mnfr.200700381. [DOI] [PubMed] [Google Scholar]
- Li J, Wang S, Yin J, Pan L. Geraniin induces apoptotic cell death in human lung adenocarcinoma A549 cells in vitro and in vivo. Can J Physiol Pharmacol. 2013;91:1016–1024. doi: 10.1139/cjpp-2013-0140. [DOI] [PubMed] [Google Scholar]
- Ma JW, Zhang Y, Li R, Ye JC, Li HY, Zhang YK, Ma ZL, Li JY, Zhong XY, Yang X. Tetrandrine suppresses human glioma growth by inhibiting cell survival, proliferation and tumour angiogenesis through attenuating STAT3 phosphorylation. Eur J Pharmacol. 2015;764:228–239. doi: 10.1016/j.ejphar.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Mukthavaram R, Ouyang X, Saklecha R, Jiang P, Nomura N, Pingle SC, Guo F, Makale M, Kesari S. Effect of the JAK2/STAT3 inhibitor SAR317461 on human glioblastoma tumorspheres. J Transl Med. 2015;13:269. doi: 10.1186/s12967-015-0627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- Tanase CP, Enciu AM, Mihai S, Neagu AI, Calenic B, Cruceru ML. Anti-cancer therapies in high grade gliomas. Curr Proteom. 2013;10:246–260. doi: 10.2174/1570164611310030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang Q, Cui Y, Liu ZY, Zhao W, Wang CL, Dong Y, Hou L, Hu G, Luo C, Chen J, Lu Y. Knockdown of cyclin D1 inhibits proliferation, induces apoptosis, and attenuates the invasive capacity of human glioblastoma cells. J Neurooncol. 2012;106:473–484. doi: 10.1007/s11060-011-0692-4. [DOI] [PubMed] [Google Scholar]
- Wang SG, Huang MH, Li JH, Lai FI, Lee HM, Hsu YN. Punicalagin induces apoptotic and autophagic cell death in human U87MG glioma cells. Acta Pharmacol Sin. 2013;34:1411–1419. doi: 10.1038/aps.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ma J, Chow SC, Li CH, Xiao Z, Feng R, Fu J, Chen Y. A potential antitumor ellagitannin, davidiin, inhibited hepatocellular tumor growth by targeting EZH2. Tumour Biol. 2014;35:205–212. doi: 10.1007/s13277-013-1025-3. [DOI] [PubMed] [Google Scholar]
- Wang P, Qiao Q, Li J, Wang W, Yao LP, Fu YJ. Inhibitory effects of geraniin on LPS-induced inflammation via regulating NF-κB and Nrf2 pathways in RAW 264.7 cells. Chem Biol Interact. 2016;253:134–142. doi: 10.1016/j.cbi.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- Xu Z, Zeng X, Tian D, Xu H, Cai Q, Wang J, Chen Q. MicroRNA-383 inhibits anchorage-independent growth and induces cell cycle arrest of glioma cells by targeting CCND1. Biochem Biophys Res Commun. 2014;453:833–838. doi: 10.1016/j.bbrc.2014.10.047. [DOI] [PubMed] [Google Scholar]
- Xue J, Zhou A, Wu Y, Morris SA, Lin K, Amin S, Verhaak R, Fuller G, Xie K, Heimberger AB, Huang S. miR-182-5p induced by STAT3 activation promotes glioma tumorigenesis. Cancer Res. 2016;76:4293–4304. doi: 10.1158/0008-5472.CAN-15-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JE, Frank DA. STAT3-interacting proteins as modulators of transcription factor function: implications to targeted cancer therapy. Chem Med Chem. 2016;11:795–801. doi: 10.1002/cmdc.201500482. [DOI] [PubMed] [Google Scholar]
- Yue P, Lopez-Tapia F, Paladino D, Li Y, Chen CH, Namanja AT, Hilliard T, Chen Y, Tius MA, Turkson J. Hydroxamic acid and benzoic acid-based STAT3 inhibitors suppress human glioma and breast cancer phenotypes in vitro and in vivo. Cancer Res. 2016;76:652–663. doi: 10.1158/0008-5472.CAN-14-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahin M, Ahmad I, Gupta RC, Aqil F. Punicalagin and ellagic acid demonstrate antimutagenic activity and inhibition of benzo[a]pyrene induced DNA adducts. Biomed Res Int. 2014;2014:467465. doi: 10.1155/2014/467465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai JW, Gao C, Ma WD, Wang W, Yao LP, Xia XX, Luo M, Zu YG, Fu YJ. Geraniin induces apoptosis of human breast cancer cells MCF-7 via ROS-mediated stimulation of p38 MAPK. Toxicol Mech Methods. 2016;26:311–318. doi: 10.3109/15376516.2016.1139025. [DOI] [PubMed] [Google Scholar]