Abstract
Background and Purpose
In cor pulmonale, the increased afterload imposed on the right ventricle (RV) generates a maladaptive response, impairing the contractile cardiac function. Oxidative mechanisms play an important role in the pathophysiology and progression of this disease. The administration of pterostilbene (PTS), a phytophenol with antioxidant potential, may represent a therapeutic option. In the present study, we evaluated the effect of PTS complexed with hydroxypropyl‐β‐cyclodextrin (HPβCD) on hypertrophy, contractile function and oxidative parameters in the RV of rats with pulmonary hypertension, induced by the administration of monocrotaline (MCT).
Experimental Approach
The rats received daily doses of the PTS : HPβCD complex at 25, 50 or 100 mg·kg−1, p.o., for 14 days. The diastolic function, E/A ratio, and systolic function, shortening fraction, fractional area change (FAC) and tricuspid annular plane systolic excursion (TAPSE) of the RV were determined by echocardiography.
Key Results
The PTS : HPβCD complex reduced the production of NADPH oxidase‐dependent superoxide anions and oxidative stress in the RV of MCT‐treated rats in a dose‐dependent manner. At higher doses it prevented the reduction in FAC and TAPSE in MCT‐treated animals.
Conclusions and Implications
The PTS : HPβCD complex prevented the maladaptative remodelling and protected systolic function in the RV of rats with pulmonary hypertension. These cardioprotective mechanisms may be related, in part, to the antioxidant potential of PTS, favoured by the increased p.o. bioavailability promoted by the presence of HPβCD in the complex.
Abbreviations
- CAT
catalase
- DCFH‐DA
2′,7′‐dichloro‐dihydro‐fluorescein diacetate
- FAC
fractional area change
- H2O2
hydrogen peroxide
- HPβCD
hydroxypropyl‐β‐cyclodextrin
- MCT
monocrotaline
- oxLDL
oxidized LDL
- PTS
pterostilbene
- RV
right ventricle
- TAPSE
tricuspid annular plane systolic excursion
Introduction
Pulmonary arterial hypertension is a progressive disease characterized by pulmonary vascular remodelling, which gives rise to increased pulmonary vascular resistance and pressure, with a prevalence of 10–52 cases per million people Peacock et al. (2007). This condition leads to an increase in afterload imposed on the right ventricle (RV) and progressive deterioration of right heart function, leading to cor pulmonale (Weitzenblum and Chaouat, 2009). Initially, the RV adapts to the increased afterload, increasing its wall thickness and contractility (compensatory hypertrophy). However, these compensatory mechanisms are insufficient, eventually generating a maladaptive response characterized by contractile deficiency, hypertrophy and/or dilation and, later, right heart failure (Greyson, 2008; Vonk‐Noordegraaf et al., 2013).
Among the pathological mechanisms involved in maladaptive RV remodelling are increased protein synthesis, activation of neurohormonal signalling (adrenergic and angiotensin pathways) and activation of apoptotic, inflammatory and oxidative pathways (Bogaard et al., 2009; Wrigley et al., 2011; Vonk‐Noordegraaf et al., 2013).
Evidence suggests that ROS and nitrogen species (RNS) and oxidative stress contribute to the transition from hypertrophy to RV dilation and right heart failure (Bogaard et al., 2009). In cardiomyocytes, the reactive species can be produced from mitochondrial oxidative phosphorylation, as well as by the enzymes NADPH oxidases, xanthine oxidase and decoupled NO synthase (Tsutsui et al., 2011). Thus, an increase in oxidants associated with an insufficient response of the primary antioxidant system facilitates the oxidation of lipids and cellular proteins, resulting in cardiomyocyte dysfunction and death (Bello‐Klein et al., 2014). In addition to direct oxidative effects, the ROS generated modify central proteins in the excitation–contraction coupling, impairing the contractile function, as well as activating protein kinases and transcription factors that increase signalling for hypertrophy (Tsutsui et al., 2011).
Cor pulmonale is a very debilitating disease with a poor prognosis and very limited therapeutic options; there are no specific guidelines for its prevention and/or treatment (Greyson, 2008). Few studies have been dedicated to exploring new therapies for the prevention of RV insufficiency due to an overload of pressure and volume (Greyson, 2008), which makes the search for therapeutic alternatives that maintain the function of the right heart quite relevant.
Pterostilbene (PTS), 4‐(3,5 dimethoxystyryl)phenol – a dimethylated analogue of resveratrol naturally found in grapes, blackberries, blueberries, among others – has been found to have many beneficial biological effects: antitumour, as it stimulates apoptosis signalling; anti‐inflammatory, given its inhibitory effect on COX2 and iNOS activities; antidiabetic, due to its negative effect on gluconeogenesis; insulinotropic and antioxidant (Acharya and Ghaskadbi, 2013; McCormack and McFadden, 2013; Lv et al., 2014). This latter effect of PTS may involve the scavenger mechanism and the activation of the nuclear factor erythroid 2‐related factor 2 (Nrf2), a key regulator of the antioxidant response. This mechanism is related to inhibition of the interaction of the Kelch‐like ECH‐associated protein‐1 (Keap1) with Nrf2 (Keap1–Nrf2), with consequent activation of Nrf2, resulting in its translocation to the nucleus, which culminates in transcriptional activation of phase II antioxidants genes and reducers such as glutathione (GSH) (Bhakkiyalakshmi et al., 2016).
Studies have shown the superior beneficial properties of PTS compared with resveratrol, effects that are attributed to increased permeation of cell membranes, due to its superior lipophilicity, as well as its extensive distribution level in cardiac and pulmonary tissues due the favourable pharmacokinetics (Acharya and Ghaskadbi, 2013; Choo et al., 2014). However, this apolar characteristic reduces the water solubility of PTS in the gastrointestinal environment, impairing its dissolution, and consequently, its absorption and biostability, which limits its use as an oral treatment (Helen Chan and Stewart, 1996). In view of this, to improve the p.o. uptake of PTS and potentially enhance its biological effects, the water solubility of PTS can be increased by forming inclusion complexes with hydroxypropyl‐β‐cyclodextrin (HPβCD), a solubility promoting agent that accommodates lipophilic substances in its internal cavity while the hydrophilic outer surface promotes aqueous solubility (López‐Nicolás et al., 2009). Previous studies from our research group have demonstrated the in vitro and in vivo antioxidant capacity of different doses of PTS administered p.o. when complexed with HPβCD (PTS : HPβCD complex), as well as its lack of toxicity to the hepatic and renal function of rats.
Little is known about the effects of PTS on the heart, especially in pathological conditions such as RV failure. Therefore, the aim of the present study was to evaluate the effects of the PTS : HPβCD complex at three different daily doses (25, 50 and 100 mg·kg−1) on RV hypertrophy, as well as haemodynamic, functional and oxidative parameters in rats with pulmonary hypertension induced by monocrotaline (MCT) administration.
Methods
PTS : HPβCD complex
To increase the hydrosolubility of the PTS, it was complexed to HPβCD. The complex was prepared using 0.3 M HPβCD dissolved in water with an excess of PTS (Yeo et al., 2013). The resultant suspension (PTS : HPβCD complex) was stirred for 72 h, at a temperature of 37 ± 0.1°C using a magnetic stirrer (MULTIST Velp®, Usmate Velate, Italy) and a temperature‐controlled bath (IKA). The formulation was filtered, frozen at −18°C and freeze‐dried (Edwards Modulyo EF4) for 48 h. The content of PTS in this lyophilized sample was quantified by HPLC (Shimadzu LC‐20A system; Kyoto, Japan).
Animals
Male Wistar adult rats (250–300 g) from the Laboratory Animal Reproduction Centre of the Universidade Federal do Rio Grande do Sul were housed in polypropylene cages (40 × 33 × 17 cm), four per cage, under standard environmental conditions (room temperature, 22 ± 2°C; 12 h light–dark cycle, 07:00–19:00 h.). All rats had free access to food and water. Our experimental protocol was carried out in accordance with the International Guidelines for Use and Care of Laboratory Animals of the National Institutes of Health and with Brazilian Laws for the Scientific Use of Animals. The protocol began after it had been approved by the Ethical Committee for Animal Experimentation at UFRGS (CEUA‐UFRGS # 28218). All efforts were made to minimize animal suffering and to use the minimum number of animals necessary to produce reliable data. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Experimental groups and procedures
Initially, the rats were randomized and divided into equal‐sized groups, called control (CTR) and pulmonary hypertension (MCT) groups. Pulmonary hypertension was induced by administration of monocrotaline (MCT; crotaline – C240 Sigma) 60 mg·kg−1 i.p. (Singal et al., 2000). After 7 days of induction, both CTR and MCT animals received daily the PTS : HPβCD complex in doses corresponding to 25, 50 or 100 mg·kg−1 (CTR25, CTR50 and CTR100; MCT25, MCT50 and MCT100; n = 8 per group) or vehicle (aqueous solution with HPβCD) (CTR0 and MCT0; n = 8 per group) via gavage for a period of 14 days. At the end of the experimental period, the animals were anaesthetized (ketamine 90 mg·kg−1 and xylazine 20 mg·kg−1, i.p.) and submitted to echocardiographic analyses, followed by catheterization of the right jugular vein for evaluation of haemodynamic parameters. The animals were killed by decapitation while still anaesthetized. The anaesthetic methods efficiency was evaluated by verification of decrease of motor reflex, reduction of muscle contraction, and absence of inferior limb movement, according to Laboratory Animal Anaesthesia (Third Edition) (Flecknell, 2009). The liver, heart and lungs were removed and weighed. The ventricles were separated and weighed, and the RV was used for biochemical analysis.
Echocardiographic evaluation
The images were obtained using two‐dimensional mode and M‐mode (Philips HD7 Ultrasound System; Andover, MA, USA) using a S12‐4 transducer (Philips). To determine the right systolic and diastolic function, the following parameters were evaluated: tricuspid flow E/A ratio, RV shortening fraction (FEC), fractional area change (FAC = 100 × end‐diastolic area − end‐systolic area/end‐diastolic area) and tricuspid annular plane systolic excursion (TAPSE) (Rudski et al., 2010).
Evaluation of haemodynamic parameters
Haemodynamic parameters were determined to estimate the effects of the PTS : HPβCD complex on cardiac function. Systolic (right ventricular systolic pressure, mmHg) and final diastolic BP (right ventricular end diastolic pressure, mmHg) was measured by cannulation of the RV, using a catheter connected to a transducer (Strain‐Gauge, Narco Biosystem Miniature Pulse Transducer PR‐155; Houston, TX, USA) and pressure amplifier (Pressure Amplifier HP 8850C). The positive values of the contraction derivative (dP/dt min and dP/dt max, mmHg·s−1) and negative values of the relaxation derivative (dP/dt min, mmHg·s−1) were obtained from the records of the right ventricular pressure wave by determining the maximum and minimum points of each cardiac cycle (Schenkel et al., 2010).
Evaluation of morphometric parameters
Animals were weighed twice a week for dose correction and to evaluate the effect of the treatments on the body weight. The cardiac hypertrophy index infers the increase in heart muscle mass, which is an important characteristic in heart failure, and was calculated as the ratio of the right and left ventricle (LV) weight (mg), corrected by the tibia length (mm) and expressed as a percentage (Hu et al., 2003).
Pulmonary and hepatic congestion
Pulmonary and hepatic congestion are secondary to the development of right heart failure. Lung and liver were conditioned at 65°C and weighed daily until they had a constant mass value. Pulmonary and hepatic congestion was estimated using the wet weight/dry weight ratio (g) (Farahmand et al., 2004).
Tissue preparation
Samples from RV were homogenized (OMNI Tissue Homogenizer, OMNI International, Georgia, USA) for 30 s in 1.15% KCl buffer containing 1% PMSF. The homogenates were centrifuged (1358 × g, 20 min, at 4°C; ALC Multispeed Refrigerated Centrifuge PK 121R, Thermo Electron Corporation, Massachusetts , USA), and the supernatant was collected for subsequent determination of oxidative parameters. The protein concentrations in the samples were determined by the Lowry method using BSA as a standard (Lowry et al., 1951).
Total reactive species
Total reactive species levels were measured using 2′,7′‐dichloro‐dihydro‐fluorescein diacetate (DCFH‐DA) fluorescence emission (Sigma‐Aldrich, Saint Louis, USA). DCFH‐DA is membrane permeable and is rapidly oxidized to the highly fluorescent 2,7‐dichlorofluorescein (DCF) in the presence of intracellular ROS. The samples were excited at 488 nm, and emission was collected with a 525 nm long pass filter. The results are expressed as pmol of DCF mg−1 protein (LeBel et al., 1992).
Activity of NADPH oxidase
NADPH oxidase generates superoxide anions through the transfer of electrons from NADPH to molecular oxygen. The activity of the enzyme was determined in RV homogenate by measuring the consumption of NADPH at 340 nm, its activity being directly proportional to the production of the superoxide anion. The results are expressed as nmol·min−1·mg−1 protein (Wei et al., 2006).
Non‐enzymatic antioxidant defence assay
The sulphydryl content represents a non‐enzymatic antioxidant defence. For the sulphydryl assay, we added 0.1 mM of 5,5‐dithio‐bis‐(2‐nitrobenzoic acid) (DTNB) to 120 μL of RV samples, which were incubated for 30 min at ambient temperature in a dark environment as described by Aksenov and Markesbery (2001). Absorbance was measured at 412 nm (Anthos Zenyth 200rt, Biochrom, Cambridge, UK), and the results are expressed as nmol·mg−1 protein.
Determination of antioxidant enzyme activity
SOD activity was evaluated on the basis of the inhibition of a superoxide radical reaction with pyrogallol, measured at 420 nm. It is expressed as U.mg−1 of protein (Marklund, 1985). Catalase (CAT) activity was measured by following the decrease in hydrogen peroxide (H2O2) absorbance at 240 nm. It was expressed as pmol of H2O2 reduced min−1·mg−1 protein (Aebi, 1984). The balance between SOD and CAT activity was estimated by the SOD/CAT ratio.
Glutathione peroxidase (GPx) activity
GPx is an enzyme that catalyses the reaction of hydroperoxides with reduced glutathione (GSH). In this test, the GPx activity was determined by measuring the consumption of NADPH in the oxidation reaction of GSH. The decrease of absorbance of NADPH at 340 nm was observed. The results were expressed as nmol·min−1·mg−1 protein (Flohé and Günzler, 1984).
Western blot evaluation
Tissue homogenization, electrophoresis and protein transfer were performed as previously described (Laemmli, 1970). Fifty micrograms of protein from RV homogenates were submitted to one‐dimensional SDS‐PAGE in a discontinuous system using an 8–12% (w.v‐1) separating gel and a stacking gel (Laemmli, 1970). The immunodetection was processed using the following primary antibodies: SOD (23 kDa), CAT (64 kDa) (Santa Cruz Biotechnology, Santa Cruz, CA, USA, or Cell Signalling Technology, Beverly, MA, USA). The bound primary antibodies were detected using anti‐rabbit or anti‐mouse HRP‐conjugated secondary antibodies, and the membranes were developed using chemiluminescence detection reagents. The autoradiographs generated were quantitatively measured with an image densitometer (ImageMaster VDS CI, Amersham Biosciences Europe, IT). The MWs of the bands were determined by reference to a standard MW marker (RPN 800 rainbow full range Bio‐Rad, California, USA). The results were normalized using the Ponceau method (Klein et al., 1995).
Data and statistical analysis
The data and statistical analysis in this study comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). The normal distribution of results was evaluated using the Shapiro–Wilk test. Parametric results were analysed using two‐way ANOVA and treatment with different doses of PTS : HPβCD complex (25, 50 or 100 mg·kg−1) as independent variables. Tukey's test was performed to detect differences between groups. Differences were considered significant when P < 0.05. The results are expressed as the mean ± SD. The data were analysed using the Sigma Stat Programme (Jandel Scientific Co., v. 11.0, San Jose, CA, USA).
Materials
PTS was purchased from Changsha Organic Herb (Changsha, China). HPβCD was supplied by Roquette Frères (Lestrem, France). MCT (Crotaline – C240 from Sigma‐Aldrich, Saint Louis, USA). All reagents used were of analytical or HPLC grade.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b).
Results
Echocardiographic evaluation
When evaluating the functional echocardiographic measurements, a reduction in tricuspid flow (E/A ratio) and FEC in MCT animals was observed, and these parameters were not altered by the administration of the PTS : HPβCD complex at any of the doses tested (Figure 1A, B). However, we found a reduction in FAC (Figure 1C) and TAPSE (Figure 1D) in MCT0 animals in relation to CTR0. Administration of the PTS : HPβCD complex increased the FAC after the administration of the 100 mg·kg−1 dose (Figures 1C and 2A), whereas the TAPSE was increased by both the 100 and 50 mg·kg−1 doses (Figures 1D and 2B).
Figure 1.
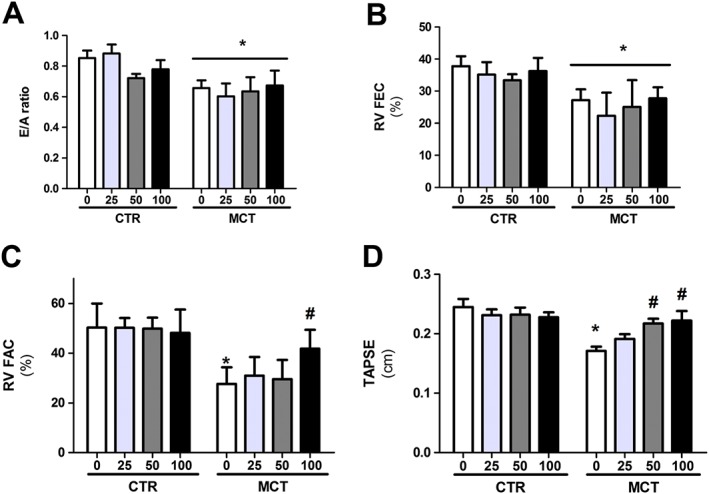
Effect of p.o. administration of PTS : HPβCD complex at different doses (25, 50 and 100 mg·kg−1) on functional echocardiographic measurements. Tricuspid flow (E/A ratio) (A), FEC (B), FAC (C) and TAPSE (D) of control (CTR0, CTR25, CTR50 and CTR100) and MCT (MCT0, MCT25, MCT50 and MCT100) rats treated via gavage for 14 days. Values are expressed as mean ± SD, n = 8 per group; two‐way ANOVA with Tukey's post hoc test was performed. *Significantly different compared with CTR0; #significantly different compared with MCT0.
Figure 2.
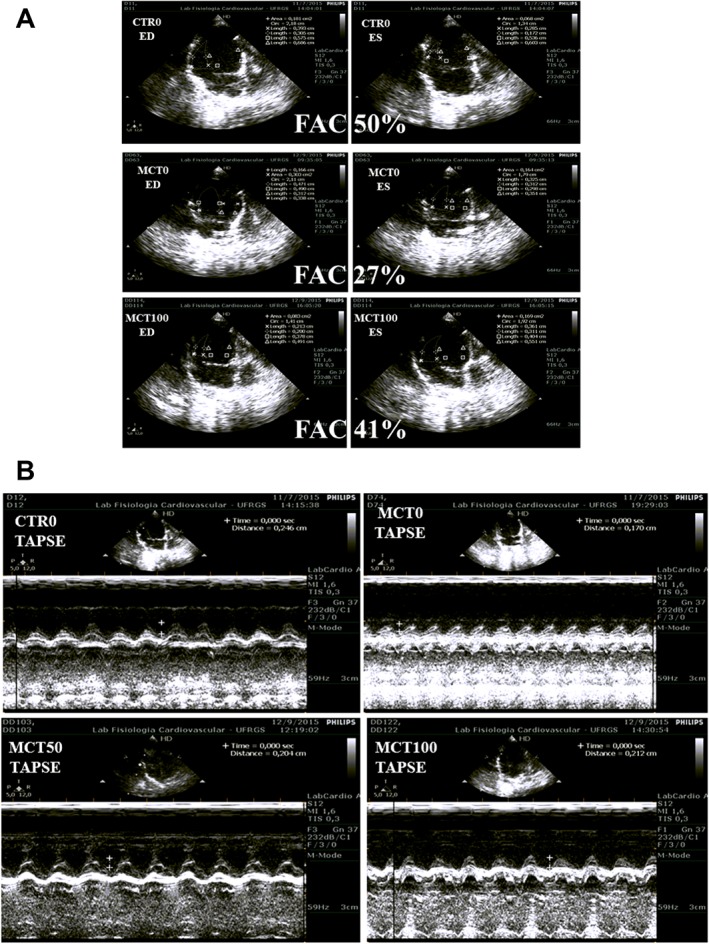
Representative image of echocardiographic data in the RV of control and MCT‐treated rats. (A) FAC is used to demonstrate changes in the FAC of the control, MCT0 and MCT100 groups. (B) The TAPSE is marked for the groups CTR0, MCT0, MCT50 and MCT100. The endocardial border is traced in apical four‐chamber views from the tricuspid annulus along the free wall to the apex, then back to the annulus, along the interventricular septum at end diastole (ED) and end systole (ES). RV FAC 50%: normal subject; (middle) moderately dilated RV, RV FAC 40%: (middle) moderately dilated; and RV FAC 21%: markedly dilated.
Evaluation of haemodynamic parameters
Pressure recordings in the RV during systole (SPRV) and diastole (DPRV) showed that MCT animals presented an elevation in SPRV and DPRV in relation to CTR (Table 1). Interestingly, the PTS : HPβCD complex at the dose of 100 mg·kg−1 increased the DPRV in the MCT100 group compared with the MCT0 group. Also, the dP/dt max (contractility) and dP/dt min (relaxation) derivatives were higher in the MCT groups compared with the CTR groups; however, these parameters were not altered by the PTS : HPβCD complex at any of the doses tested (Table 1).
Table 1.
Haemodynamic parameters of control (CTR) rats and rats with heart failure (MCT) after 14 days of p.o. administration with the PTS : HPβCD complex at different doses (25, 50 and 100 mg·kg−1)
| Groups | RVSP (mmHg) | RVEDP (mmHg) | dP/dt max (mmHg) | dP/dt min (mmHg) | HR (beat.min‐1) |
|---|---|---|---|---|---|
| CTR0 | 30.36 ± 5.25 | 2.12 ± 1.13 | 1607.42 ± 318.13 | −893.25 ± 194.34 | 230.89 ± 17.13 |
| CTR25 | 30.79 ± 4.30 | 1.81 ± 1.67 | 1969.42 ± 503.01 | −1101.29 ± 307.29 | 235.00 ± 27.09 |
| CTR50 | 34.33 ± 5.85 | 3.15 ± 1.38 | 1552.84 ± 355.68 | −997.97 ± 217.28 | 236.89 ± 19.15 |
| CTR100 | 32.91 ± 4.44 | 3.25 ± 1.37 | 1347.95 ± 268.73 | −893.56 ± 164.25 | 287.57 ± 14.48 |
| MCT0 | 71.06 ± 5.8a | 5.31 ± 1.16a | 3022.37 ± 355.68a | −1771.91 ± 217.28a | 256.25 ± 19.15 |
| MCT25 | 62.20 ± 5.65a | 4.10 ± 1.18a | 2382.91 ± 355.52a | −1403.51 ± 217.28a | 252.33 ± 15.64 |
| MCT50 | 63.04 ± 3.9a | 3.45 ± 1.03a | 2749.65 ± 290.41a | −1475.51 ± 117.41a | 237.33 ± 18.55 |
| MCT100 | 67.56 ± 6.49a | 8.77 ± 1.26b , c | 3557.38 ± 355.68a | −2361.22 ± 217.28a | 258.25 ± 19.15 |
Values are expressed as mean ± SD, n = 8 animals per group. Two‐way ANOVA and Tukey's post hoc tests were performed. dP/dt max, contractility index; dP/dt min, relaxation index; HR, heart rate; RVSP, right ventricular systolic pressure; RVEDP, right ventricular end diastolic pressure.
Significantly different compared with CTR (P < 0.05).
Significantly different compared with MCT0 (P < 0.05).
Significantly different compared with MCT25 and MCT50 (P < 0.05).
Weight measurements and morphometry
The results show that there was no difference in the initial body weight of the animals. However, there was a reduction in weight gain in the MCT group in relation to the CTR group (P < 0.05), and this parameter was not affected by the PTS : HPβCD complex at any of the doses tested (Table 2). However, we observed that MCT animals had an increase in RV weight, RV/tibia index and RV/LV weight in relation to CTR, parameters that were reduced by the PTS : HPβCD complex at a dose of 100 mg·kg−1 (Table 2).
Table 2.
Body weight and morphometric measurements of control rats (CTR) and rats with heart failure (MCT) after 14 days of p.o. administration with the PTS : HPβCD complex at 25, 50 or 100 mg·kg−1 dose
| Groups | Initial BW (g) | BW variation (%) | RV gross weight (mg) | RV weight/tíbia (mg·mm−1) | RV weight/LV weight (mg·mg−1) |
|---|---|---|---|---|---|
| CTR0 | 238 ± 9.2 | 53.3 ± 3.0 | 200 ± 13.3 | 5.9 ± 0.4 | 0.31 ± 0.02 |
| CTR25 | 243 ± 9.2 | 49.6 ± 3.0 | 197 ± 13.0 | 5.4 ± 0.3 | 0.28 ± 0.02 |
| CTR50 | 231 ± 10.6 | 55.9 ± 3.5 | 188 ± 15.2 | 5.0 ± 0.5 | 0.27 ± 0.02 |
| CTR100 | 250 ± 9.21 | 54.8 ± 3.0 | 192 ± 13.2 | 5.3 ± 0.4 | 0.29 ± 0.02 |
| MCT0 | 238 ± 9.5 | 35.6 ± 2.3a | 291 ± 9.9a | 8.0 ± 0.3a | 0.48 ± 0.01a |
| MCT25 | 248 ± 6.9 | 37.0 ± 2.1a | 259 ± 10.0a | 7.2 ± 0.4a | 0.42 ± 0.02a |
| MCT50 | 236 ± 6.5 | 33.0 ± 2.3a | 262 ± 9.9a | 7.3 ± 0.4a | 0.43 ± 0.01a |
| MCT100 | 254 ± 6.9 | 30.1 ± 3.0a | 222 ± 10.0a , b | 6.2 ± 0.30a , b | 0.34 ± 0.01b , c |
Values are expressed as mean ± SD, n = 8 animals per group. Two‐way ANOVA and Tukey's post hoc tests were performed. BW, body weight.
Significantly different compared with CTR (P < 0.05).
Significantly different compared with MCT0 (P < 0.05).
Significantly different compared with MCT25 and MCT50 (P < 0.05).
Pulmonary and hepatic congestion
According to the results, there was an increase in the pulmonary congestion index in MCT animals, as evidenced by increased wet/dry weight (g) and wet lung/body weight (mg·g−1), parameters that were not altered by administration of the PTS : HPβCD complex (Table 3). However, in relation to pulmonary congestion, no significant differences were found between groups (Table 3).
Table 3.
Lungs and liver congestion indexes of control rats (CTR) and rats with heart failure (MCT) after 14 days of p.o. administration with PTS : HPβCD complex at 25, 50 or 100 mg·kg−1 dose
| Groups | Lungs wet weight/dry weight (g) | Lungs wet weight/BW (mg·g−1) | Liver wet weight/dry weight (g) | Liver wet weight/BW (mg·g−1) |
|---|---|---|---|---|
| CTR0 | 2.4 ± 0.12 | 2.97 ± 0.35 | 2.85 ± 0.04 | 31.64 ± 2.43 |
| CTR25 | 2.2 ± 0.13 | 2.69 ± 0.14 | 2.86 ± 0.04 | 31.86 ± 2.43 |
| CTR50 | 2.43 ± 0.14 | 3.11 ± 0.41 | 2.84 ± 0.05 | 32.89 ± 2.80 |
| CTR100 | 2.31 ± 0.12 | 2.31 ± 0.35 | 2.86 ± 0.04 | 32.87 ± 2.43 |
| MCT0 | 2.7 ± 0.10a | 4.31 ± 0.27a | 2.95 ± 0.03 | 35.56 ± 1.43 |
| MCT25 | 2.57 ± 0.10a | 4.37 ± 0.26a | 2.86 ± 0.03 | 34.37 ± 1.83 |
| MCT50 | 2.36 ± 0.11a | 3.85 ± 0.27a | 2.85 ± 0.03 | 34.10 ± 1.85 |
| MCT100 | 2.68 ± 0.10a | 3.62 ± 0.29a | 2.88 ± 0.04 | 36.56 ± 1.98 |
Values are expressed as mean ± SD, n = 8 animals per group. Two‐way ANOVA and Tukey's post hoc tests were performed. BW, body weight.
Significantly different compared with CTR group.
Oxidative parameters
The results show that there was no reduction in the concentration of total ROS in the RV of rats in this experimental protocol (Figure 3A). We observed an increase in NADPH oxidase activity in the MCT0 group in relation to CTR0 (Figure 3B). Administration of the PTS : HPβCD complex at the dose of 100 mg·kg−1 reduced NADPH oxidase activity in the animals with heart failure induced by pulmonary hypertension; however, this same dose increased NADPH oxidase activity in the CTR animals (Figure 3B). There was also a negative correlation of NADPH oxidase activity with TAPSE (correlation coefficient: −0.967; P = 0.0331) and FAC (correlation coefficient: −0.592; P = 0.00229) of the RV, indicating an association between these parameters. In contrast, the levels of non‐enzymatic antioxidants were not altered in our experimental protocol (Figure 3C).
Figure 3.
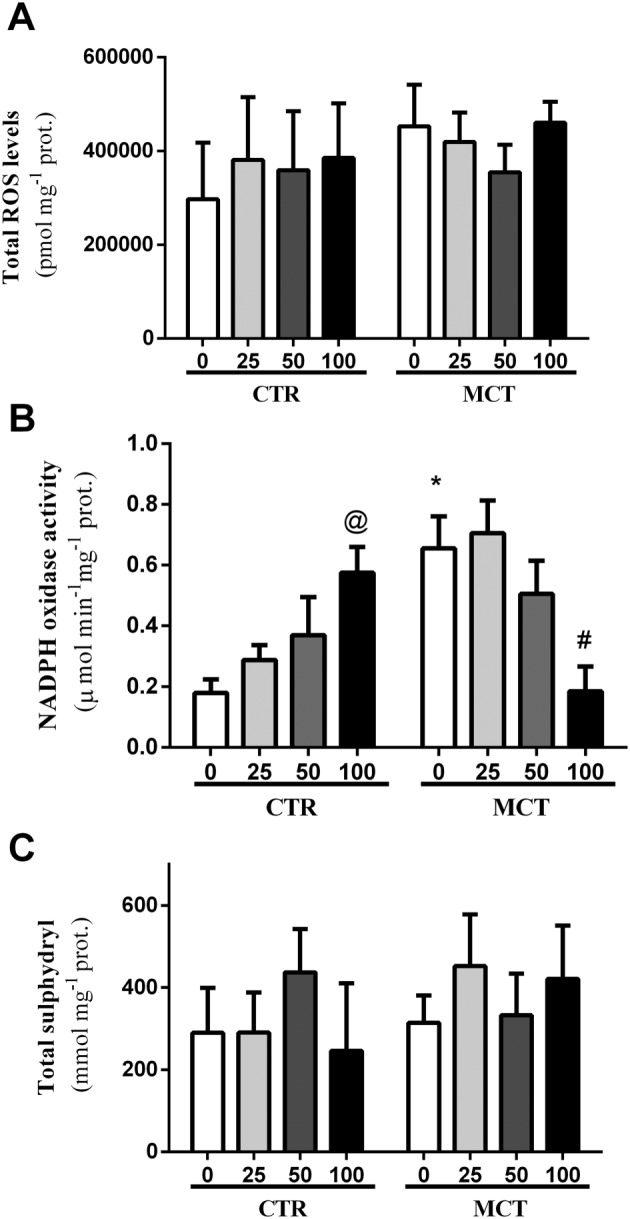
Effect of p.o. administration of PTS : HPβCD complex at different doses (25, 50 and 100 mg·kg−1) on oxidative parameters. Total ROS levels (A), NADPH oxidase activity (B) and total sulphydryl content (C) in the RV of control (CTR0 and CTR25: CTR50 and CTR100) and MCT (MCT0, MCT25, MCT50 and MCT100) rats treated via gavage for 14 days. Values are expressed as mean ± SD, n = 8 per group; two‐way ANOVA with Tukey's post hoc test was performed. * Significantly different compared with CTR0; # significantly different compared with MCT0; and @ different compared with CTR.
When evaluating the activity of antioxidant enzymes, we detected that heart failure induced by pulmonary hypertension reduced SOD activity (Figure 4A) and increased CAT activity (Figure 4C). The PTS : HPβCD complex restored the SOD activity at the dose of 100 mg·kg−1 (Figure 4A), while the CAT activity was reduced at the three doses tested (Figure 4C). We observed a reduction in the SOD/CAT ratio in the RV of the MCT group in relation to CTR (Figure 4E). Administration of the PTS : HPβCD complex did not change this parameter.
Figure 4.
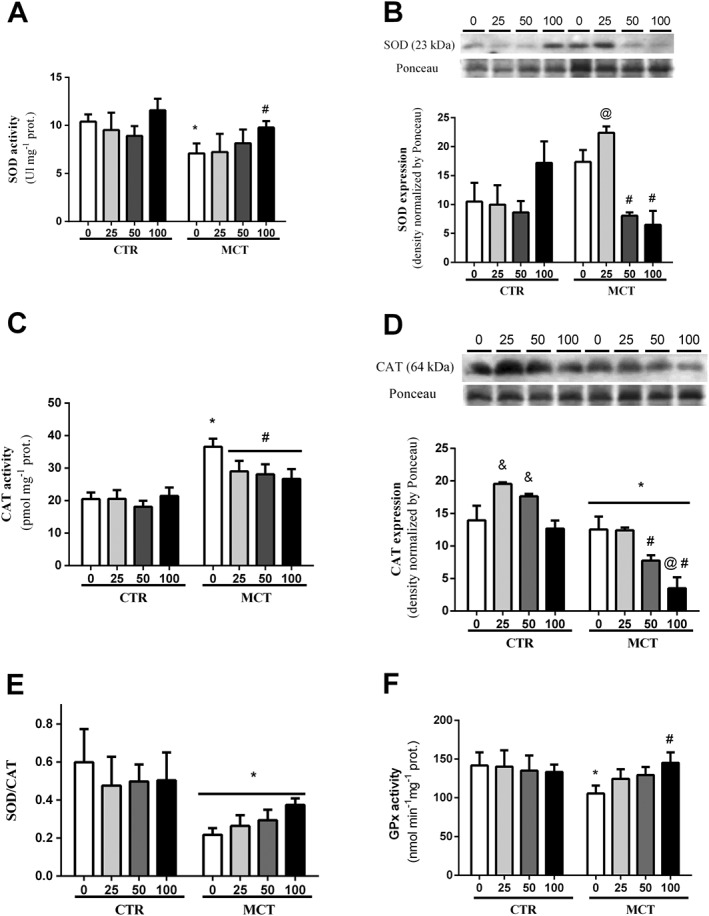
Activity and expression of antioxidant enzymes. Effect of p.o. administration of PTS : HPβCD complex at different doses (25, 50 and 100 mg·kg−1) on SOD activity (A), SOD expression (B), CAT activity (C), CAT expression (D), SOD/CAT ratio (E) and GPx activity (F) in RV of control (CTR0, CTR25, CTR50 and CTR100) and MCT (MCT0, MCT25, MCT50 and MCT100) rats treated via gavage for 14 days. Values are expressed as mean ± SD, n = 8 per group; two‐way ANOVA with Tukey's post hoc test was performed. * Significantly different compared with CTR0; # significantly different to MCT0; @ significantly different to CTR; and & significantly different to CTR0 and CTR100.
In relation to GPx activity, we observed a decrease in its activity in the MCT0 group in relation to the CTR0 group (Figure 4F). Administration of the PTS : HPβCD complex at the dose of 100 mg·kg−1 restored the activity in the animals with heart failure induced by pulmonary hypertension (Figure 4F).
Western blot evaluation
The expression of antioxidant enzymes was also measured in the heart tissue. We observed an increase in the immunocontent of the SOD enzyme in the RV of the MCT25 animals compared with the other MCT groups (Figure 4B), as well as a reduction in the immunocontent of the SOD enzyme in the MCT50 and MCT100 groups in relation to MCT0 (Figure 4B). CAT expression was decreased in the MCT groups in relation to CTR (Figure 4D). Administration of the PTS : HPβCD complex at doses of 50 and 100 mg·kg−1 reduced CAT expression compared with the MCT0 and MCT25 groups, and this effect was even more pronounced at the dose of 100 mg·kg−1 (Figure 3D). Additionally, the PTS : HPβCD complex increased CAT expression in the control group (CTR25 and CTR50) (Figure 4D).
Pterostilbene modulates NADPH oxidase activity in right ventricle
NADPH oxidases are transmembrane proteins, composed of two catalytic subunits (gp91phox and p22phox) and four regulatory subunits (p47phox, p40phox, p67phox and Rac1). NADPH oxidases transfer electrons, reducing oxygen to superoxide anion (Bedard and Krause, 2007). In the heart, the activation of NADPH oxidase is due to hypertrophic stimuli, through angiotensin II, adrenaline and mechanical overload, and its overactivation is related to cardiac injury and failure (Bedard and Krause, 2007; Borchi et al., 2010). Our findings suggest that administration of PTS is able to reduce NADPH oxidase activity, diminishing anion superoxide production (Figure 2B), associated to the inductor effect of antioxidant response in RV, which can be observed by its effect on GPx and SOD activities (Figure 4A, F). This results in the prevention of maladaptative remodelling and protection of systolic function.
Discussion
In cor pulmonale, the increase in ROS associated with a defective antioxidant system is considered a determinant for the progression and severity of the disease (Bogaard et al., 2009). Thus, the use of natural antioxidant products, such as the phytophenol PTS, may represent a therapeutic strategy against oxidative/nitrosative imbalance in cardiovascular diseases (Kosuru et al., 2016). Studies that focus on exploring the effects of PTS on the heart are scarce. This is the first study to evaluate the effects of PTS complexed to HPβCD on cardiac function. In the present study, p.o. administration of a PTS : HPβCD complex reduced oxidative stress, prevented RV maladaptive remodelling and improved right systolic function in rats with cor pulmonale.
Complexation with cyclodextrin promotes the hydrosolubility, protects bioactive molecules from degradation (against changes in temperature, pH and exposure to light) and greatly increases the p.o. bioavailability of stilbenes, as observed for PTS (>59%) and resveratrol (>45%), increasing its potential pharmacological effects (Lin and Ho, 2011; Yeo et al., 2013).
In this study, the morphometric and haemodynamic changes in the RV caused by pulmonary hypertension agree with the findings of other authors (Mosele et al., 2012; Colombo et al., 2015). Increased pressure in systole and diastole as well as increased RV contractility and relaxation may be considered as adaptive mechanisms for pulmonary arterial hypertension and sustained afterload imposed on the RV (Vonk‐Noordegraaf et al., 2013). In this context, the change in geometry (more rounded shape) and increase in right ventricular mass (hypertrophy) are due to an increase in protein synthesis induced by stretching and by the presence of additional sarcomeres in cardiomyocytes (Bogaard et al., 2009). In our study, the RV hypertrophy index results showed that administration of the PTS : HPβCD complex at a dose of 100 mg·kg−1 inhibited this increase, demonstrating the beneficial effect of this treatment on ventricular remodelling. Evidence shows that PTS inhibits the proliferation and growth of muscle cells (Park et al., 2010; McCormack and McFadden, 2013), events that are also related to cardiac hypertrophy (Han et al., 2009). Similarly, resveratrol produces anti‐hypertrophic effects in rat cardiomyocytes, both in vitro and in vivo, by activating AMP‐activated protein kinase and inhibiting PKB (Akt), reducing the content and rate of protein synthesis (Chan et al., 2008). The flavonoid quercetin administered p.o. also prevents cardiac hypertrophy induced by pressure overload in rats by inhibiting the MAPK signalling pathway, which plays an important role in cardiac hypertrophy (Han et al., 2009).
The size of the chamber as well as the thickness of the ventricular free wall are determinants of the demand and consumption of oxygen by the myocardium, contributing to the production of mitochondrial ROS (Giordano, 2005). Another important source of ROS in the RV is via the activation of the enzyme NADPH oxidase, increasing the concentration of the superoxide anion, which can stimulate the production of other reactive species that are capable of damaging cellular macromolecules and can result in the dysfunction and death of cardiac cells (Bedard and Krause, 2007; Redout et al., 2007). In parallel, the superoxide anion increase participates in the regulation of cell signalling in cardiomyocytes under afterload stress conditions, favouring maladaptive RV remodelling (Seddon et al., 2007). In the present study, rats that received MCT showed increased NADPH oxidase activity, similar to that described by Redout et al. (2007). Increased NADPH oxidase activation and expression in cardiomyocytes may occur in response to pressure overload, generating oxidative stress and cardiac failure (Bedard and Krause, 2007). We observed that the PTS: HPβCD complex at a dose of 100 mg·kg−1 reduced the production of NADPH oxidase‐dependent superoxide anions in the RV by modulating the activity of this enzyme. In contrast, the PTS: HPβCD complex stimulated the activity of NADPH oxidase in the heart of control animals. Chakraborty et al. (2010) reported that the production of ROS increased in cells in culture after incubation with PTS. In parallel, this ROS may lead to the rupture of the Keap1‐Nrf2 complex, activating Nrf2 and inducing an adaptation of the antioxidant system, preparing the cell to respond more efficiently to challenges, similar to the induction of a hormesis effect. Phytochemicals have been described as modulators of NADPH oxidase (Maraldi, 2013). In this context, extracts enriched with anthocyanins or catechins reduced the expression of NADPH oxidase and prevented cardiac hypertrophy in rats (Al‐Awwadi et al., 2005).
In addition, the intracellular increase of superoxide anion may lead to the generation of other reactive species such as H2O2, hydroxyl and peroxy nitrite radicals, which may make the redox environment more oxidized. This could impair the functioning of antioxidant enzymes such as SOD and CAT, compromising the primary antioxidant defence system (Halliwell and Gutteridge, 2007). It has been reported that H2O2, the product of SOD, oxidizes the histidine residue of cytosolic SOD, inactivating it (Uchida and Kawakishi, 1994). These mechanisms could be determinants of the impairment in RV SOD activity of the MCT rats in this study, compromising the intracellular elimination of superoxide anions. In contrast, the improvement in SOD activity induced by the administration of the PTS : HPβCD complex (100 mg·kg−1 dose) may be a result of the scavenging potential of PTS phenolic hydroxyl groups, which stabilize free radicals through their reducing ability (Perron and Brumaghim, 2009). This results in lower superoxide anion concentrations and, consequently, reduces the need for the expression of SOD. Other authors also noted that PTS modulates the activity of antioxidant enzymes (SOD and CAT) in vascular endothelial cells exposed to high levels of oxidized LDLs (oxLDLs), as well as in acetaminophen‐injured hepatocytes (Zhang et al., 2012; El‐Sayed et al., 2015).
Measurements of CAT activity in the RV presented a different activity pattern compared with SOD. The increase in CAT activity in MCT‐treated rats may reflect a compensatory mechanism against the high availability of H2O2 in cardiomyocytes that protects the RV against oxidative damage (Dieterich et al., 2000). The PTS : HPβCD complex reduced CAT activity at the three doses administered, preserving the antioxidant potential of the enzyme. Still, there was a reduction in the immunocontent of CAT at the higher doses. Similar effects have also been described by other authors. A reduction in the activity and expression of CAT induced by polyphenols in cardiac and muscular cells, respectively, occurs through the induction of non‐enzymatic antioxidants and the neutralization of reactive species (Babu et al., 2006; Goutzourelas et al., 2015). In contrast, the PTS : HPβCD complex increased CAT protein expression in the control groups. This could be the result of a PTS‐induced pro‐oxidant action that provides adaptation of antioxidant system, in order to activate mechanisms of cellular protection (Chakraborty et al., 2010). The SOD/CAT ratio reflects the balance between the activity of the two enzymes, since the SOD product is a CAT substrate (Halliwell and Gutteridge, 2007). In contrast, it was observed that the PTS : HPβCD complex did not change the SOD/CAT ratio reduced by MCT. In addition, the PTS : HPβCD complex (100 mg·kg−1 dose) treatment also elevated GPx activity in the RV of rats with pulmonary arterial hypertension. This enzyme plays an important role in the control of redox homeostasis, and an increase in its levels will reduce oxidative stress, compensating for the reduced CAT levels. However, the cardio‐protective effects of PTS are not limited to its antioxidant action. Consistently, studies have shown that PTS has anti‐inflammatory properties (in addition to its antioxidant effects) in the heart mediated through various mechanisms, such as inhibition of COX2 and inducible NOS activities of NF‐κB signalling, decreasing myeloperoxidase activity, inhibition of angiotensin‐converting enzyme, elevation of endothelial NOS and a reduction in the levels of TNF‐α and IL‐1 (Lv et al., 2014; Yu et al., 2017). Therefore, the PTS‐induced cardio‐protective action may depend on several of these effects.
In cor pulmonale, increased oxidative stress is correlated with a decrease in right ventricular function, which is considered to be the main determinant of functional capacity and survival of patients (van de Veerdonk et al., 2011). In this study, RV dysfunction was confirmed using classical echocardiographic parameters of diastolic function (E/A ratio) and systolic function (FEC, FAC and TAPSE) (Rudski et al., 2010; Kimura et al., 2015). FAC expresses the percentage variation in the RV area between diastole and final systole, with values below 35% indicating systolic dysfunction (Rudski et al., 2010). In addition, TAPSE is a parameter of the overall RV function that describes longitudinal apex shortening to the base (Rudski et al., 2010). We observed that pulmonary hypertension (MCT animals) impaired both diastolic and systolic function. Administration of the PTS : HPβCD complex at higher doses (50 and 100 mg·kg−1) prevented the reduction in FAC and TAPSE, indicating protection of systolic function. These results were correlated with the decrease in NADPH oxidase activity observed in our study, since there is a negative correlation of this enzyme activity with the functional parameters of the RV. Resveratrol has also been shown to improve systolic function and reduce hypertrophy in hypertensive rats and these effects were attributed to a reduction in oxidative stress (Thandapilly et al., 2010). In another study, improvement of the cardiac dysfunction induced by pressure overload in rodents was also observed after treatment with flavonoids extracted from propolis and also after treatment with the isoflavonoid pueranine (Yuan et al., 2014; Sun et al., 2016).
In summary, our findings suggest that administration of the PTS : HPβCD complex reduces NADPH oxidase‐dependent superoxide anion concentration and oxidative stress in RV in a dose‐dependent manner, resulting in the prevention of maladaptative remodelling and protection of systolic function. These cardioprotective mechanisms may be related, in part, to the antioxidant effects of PTS, favoured by the increased p.o. bioavailability promoted by the presence of HPβCD in the complex. These cardioprotective effects of PTS may represent an adjuvant therapy for the pathological changes that occur in cor pulmonale.
Author contributions
D.S.L. participated in all phases (experimental and writing) of this manuscript. P.T. did the biochemical analysis and statistics; B.G.D.L.S. the haemodynamic analysis; R.C. the echocardiography analysis and V.D.O. the Western Blot analysis. J.H.P.B. took care of the animals. C.C.‐C. was involved in the biochemical analysis and S.E.B. in the pterostisbene–cyclodextrin complexation. A.B.‐K. provided intellectual contributions and V.L.B. and A.S.D.R.A. created the project and wrote the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This work was supported by CNPq, CAPES and FAPERGS, Brazilian Research Agencies. This work was funded by the Brazilian Research Agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS).
dos Santos Lacerda, D. , Türck, P. , Gazzi de Lima‐Seolin, B. , Colombo, R. , Duarte Ortiz, V. , Poletto Bonetto, J. H. , Campos‐Carraro, C. , Bianchi, S. E. , Belló‐Klein, A. , Linck Bassani, V. , and Sander da Rosa Araujo, A. (2017) Pterostilbene reduces oxidative stress, prevents hypertrophy and preserves systolic function of right ventricle in cor pulmonale model. British Journal of Pharmacology, 174: 3302–3314. doi: 10.1111/bph.13948.
References
- Acharya JD, Ghaskadbi SS (2013). Protective effect of pterostilbene against free radical mediated oxidative damage. BMC Complement Altern Med 13: 238–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi H (1984). [13] Catalase in vitro In: Methods in Enzymology. Academic Press: New York, USA, pp. 121–126. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Markesbery WR (2001). Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer's disease. Neurosci Lett 302: 141–145. [DOI] [PubMed] [Google Scholar]
- Al‐Awwadi NA, Araiz C, Bornet A, Delbosc S, Cristol J‐P, Linck N et al (2005). Extracts enriched in different polyphenolic families normalize increased cardiac NADPH oxidase expression while having differential effects on insulin resistance, hypertension, and cardiac hypertrophy in high‐fructose‐fed rats. J Agric Food Chem 53: 151–157. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu PVA, Sabitha KE, Shyamaladevi CS (2006). Therapeutic effect of green tea extract on oxidative stress in aorta and heart of streptozotocin diabetic rats. Chem Biol Interact 162: 114–120. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause K‐H (2007). The NOX family of ROS‐generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245. [DOI] [PubMed] [Google Scholar]
- Bello‐Klein A, Khaper N, Llesuy S, Vassallo DV, Pantos C (2014). Oxidative stress and antioxidant strategies in cardiovascular disease. Oxid Med Cell Longev 2014: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakkiyalakshmi E, Dineshkumar K, Karthik S, Sireesh D, Hopper W, Paulmurugan R et al (2016). Pterostilbene‐mediated Nrf2 activation: mechanistic insights on Keap1:Nrf2 interface. Bioorg Med Chem 24: 3378–3386. [DOI] [PubMed] [Google Scholar]
- Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF (2009). The right ventricle under pressure: cellular and molecular mechanisms of right‐heart failure in pulmonary hypertension. Chest 135: 794–804. [DOI] [PubMed] [Google Scholar]
- Borchi E, Bargelli V, Stillitano F, Giordano C, Sebastiani M, Nassi PA et al (2010). Enhanced ROS production by NADPH oxidase is correlated to changes in antioxidant enzyme activity in human heart failure. Biochim Biophys Acta (BBA) Mol Basis Dis 1802: 331–338. [DOI] [PubMed] [Google Scholar]
- Chakraborty A, Gupta N, Ghosh K, Roy P (2010). In vitro evaluation of the cytotoxic, anti‐proliferative and anti‐oxidant properties of pterostilbene isolated from Pterocarpus marsupium. Toxicol In Vitro 24: 1215–1228. [DOI] [PubMed] [Google Scholar]
- Chan AYM, Dolinsky VW, Soltys C‐LM, Viollet B, Baksh S, Light PE et al (2008). Resveratrol inhibits cardiac hypertrophy via AMP‐activated protein kinase and Akt. J Biol Chem 283: 24194–24201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q‐Y, Yeo SCM, Ho PC, Tanaka Y, Lin H‐S (2014). Pterostilbene surpassed resveratrol for anti‐inflammatory application: potency consideration and pharmacokinetics perspective. J Funct Foods 11: 352–362. [Google Scholar]
- Colombo R, Siqueira R, Conzatti A, Fernandes TRG, Tavares AMV, Araújo ASdR, et al. (2015). Aerobic exercise promotes a decrease in right ventricle apoptotic proteins in experimental Cor pulmonale. J Cardiovasc Pharmacol 66: 246–253. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich S, Bieligk U, Beulich K, Hasenfuss G, Prestle J (2000). Gene expression of antioxidative enzymes in the human heart. Circulation 101: 33. [DOI] [PubMed] [Google Scholar]
- El‐Sayed E‐SM, Mansour AM, Nady ME (2015). Protective effects of pterostilbene against acetaminophen‐induced hepatotoxicity in rats. J Biochem Mol Toxicol 29: 35–42. [DOI] [PubMed] [Google Scholar]
- Farahmand F, Hill MF, Singal PK (2004). Antioxidant and oxidative stress changes in experimental cor pulmonale. Mol Cell Biochem 260: 21–29. [DOI] [PubMed] [Google Scholar]
- Flecknell P (2009). Laboratory Animal Anaesthesia (3rd ed.) Chapter 2 ‐ Anaesthesia. Academic Press. pp. 19–78. https://doi.org/10.1016/B978‐0‐12‐369376‐1.00002‐2
- Flohé L, Günzler WA (1984). Assays of glutathione peroxidase. Methods Enzymol 105: 114–121. [DOI] [PubMed] [Google Scholar]
- Giordano FJ (2005). Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115: 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutzourelas N, Stagos D, Housmekeridou A, Karapouliou C, Kerasioti E, Aligiannis N et al (2015). Grape pomace extract exerts antioxidant effects through an increase in GCS levels and GST activity in muscle and endothelial cells. Int J Mol Med 36: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greyson CR (2008). Pathophysiology of right ventricular failure. Crit Care Med 36: S57–S65. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J (2007). Free Radicals in Biology and Medicine, Fourth edn: New York, USA. [Google Scholar]
- Han J‐J, Hao J, Kim C‐H, Hong J‐S, Ahn H‐Y, Lee Y‐S (2009). Quercetin prevents cardiac hypertrophy induced by pressure overload in rats. J Vet Med Sci 71: 737–743. [DOI] [PubMed] [Google Scholar]
- Helen Chan O, Stewart BH (1996). Physicochemical and drug‐delivery considerations for oral drug bioavailability. Drug Discov Today 1: 461–473. [Google Scholar]
- Hu LW, Benvenuti LA, Liberti EA, Carneiro‐Ramos MS, Barreto‐Chaves MLM (2003). Thyroxine‐induced cardiac hypertrophy: influence of adrenergic nervous system versus renin‐angiotensin system on myocyte remodeling. Am J Physiol Regul Integr Comp Physiol 285: R1473–R1480. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Daimon M, Morita H, Kawata T, Nakao T, Okano T et al (2015). Evaluation of right ventricle by speckle tracking and conventional echocardiography in rats with right ventricular heart failure. Int Heart J 56: 349–353. [DOI] [PubMed] [Google Scholar]
- Klein D, Kern RM, Sokol RZ (1995). A method for quantification and correction of proteins after transfer to immobilization membranes. Biochem Mol Biol Int 36: 59–66. [PubMed] [Google Scholar]
- Kosuru R, Rai U, Prakash S, Singh A, Singh S (2016). Promising therapeutic potential of pterostilbene and its mechanistic insight based on preclinical evidence. Eur J Pharmacol 789: 229–243. [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- LeBel CP, Ischiropoulos H, Bondy SC (1992). Evaluation of the probe 2′,7′‐dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress Chem Res Toxicol 5: 227–231. [DOI] [PubMed] [Google Scholar]
- Lin H‐s, Ho PC (2011). Preclinical pharmacokinetic evaluation of resveratrol trimethyl ether in sprague‐dawley rats: the impacts of aqueous solubility, dose escalation, food and repeated dosing on oral bioavailability. J Pharm Sci 100: 4491–4500. [DOI] [PubMed] [Google Scholar]
- López‐Nicolás JM, Rodríguez‐Bonilla P, Méndez‐Cazorla L, García‐Carmona F (2009). Physicochemical study of the complexation of pterostilbene by natural and modified cyclodextrins. J Agric Food Chem 57: 5294–5300. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275. [PubMed] [Google Scholar]
- Lv M, Liu K, Fu S, Li Z, Yu X (2014). Pterostilbene attenuates the inflammatory reaction induced by ischemia/reperfusion in rat heart. Mol Med Rep 11: 724–728. [DOI] [PubMed] [Google Scholar]
- Maraldi T (2013). Natural compounds as modulators of NADPH oxidases. Oxid Med Cell Longev 2013: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund SL (1985). CRC Handbook of Methods for Oxygen Radical Research. CRC Press: Boca Raton, Florida. [Google Scholar]
- McCormack D, McFadden D (2013). A review of pterostilbene antioxidant activity and disease modification. Oxid Med Cell Longev 2013: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosele F, Tavares AMV, Colombo R, Caron‐Lienert R, Araujo ASR, Ribeiro MF et al (2012). Effects of purple grape juice in the redox‐sensitive modulation of right ventricular remodeling in a pulmonary arterial hypertension model. J Cardiovasc Pharmacol 60: 15–22. [DOI] [PubMed] [Google Scholar]
- Park E‐S, Lim Y, Hong J‐T, Yoo H‐S, Lee C‐K, Pyo M‐Y et al (2010). Pterostilbene, a natural dimethylated analog of resveratrol, inhibits rat aortic vascular smooth muscle cell proliferation by blocking Akt‐dependent pathway. Vascul Pharmacol 53: 61–67. [DOI] [PubMed] [Google Scholar]
- Peacock AJ, Murphy NF, McMurray JJV, Caballero L, Stewart S (2007). An epidemiological study of pulmonary arterial hypertension. Eur Respir J 30: 104–109. [DOI] [PubMed] [Google Scholar]
- Perron NR, Brumaghim JL (2009). A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys 53: 75–100. [DOI] [PubMed] [Google Scholar]
- Redout EM, Wagner MJ, Zuidwijk MJ, Boer C, Musters RJP, van Hardeveld C et al (2007). Right‐ventricular failure is associated with increased mitochondrial complex II activity and production of reactive oxygen species. Cardiovasc Res 75: 770–781. [DOI] [PubMed] [Google Scholar]
- Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K et al (2010). Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr 23: 685–713. [DOI] [PubMed] [Google Scholar]
- Schenkel PC, Tavares AMV, Fernandes RO, Diniz GP, Bertagnolli M, da Rosa Araujo AS et al (2010). Redox‐sensitive prosurvival and proapoptotic protein expression in the myocardial remodeling post‐infarction in rats. Mol Cell Biochem 341: 1–8. [DOI] [PubMed] [Google Scholar]
- Seddon M, Looi YH, Shah AM (2007). Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart 93: 903–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal PK, Khaper N, Farahmand F, Belló‐Klein A (2000). Oxidative stress in congestive heart failure. Curr Cardiol Rep 2: 206–211. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G‐w, Qiu Z‐d, Wang W‐n, Sui X, Sui D‐j (2016). Flavonoids extraction from propolis attenuates pathological cardiac hypertrophy through PI3K/AKT signaling pathway. Evid Based Complement Alternat Med 2016: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thandapilly SJ, Wojciechowski P, Behbahani J, Louis XL, Yu L, Juric D et al (2010). Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. Am J Hypertens 23: 192–196. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, Kinugawa S, Matsushima S (2011). Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 301: H2181–H2190. [DOI] [PubMed] [Google Scholar]
- Uchida K, Kawakishi S (1994). Identification of oxidized histidine generated at the active site of Cu,Zn‐superoxide dismutase exposed to H2O2. Selective generation of 2‐oxo‐histidine at the histidine 118. J Biol Chem 269: 2405–2410. [PubMed] [Google Scholar]
- van de Veerdonk MC, Kind T, Marcus JT, Mauritz G‐J, Heymans MW, Bogaard H‐J et al (2011). Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 58: 2511–2519. [DOI] [PubMed] [Google Scholar]
- Vonk‐Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J et al (2013). Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 62: D22–D33. [DOI] [PubMed] [Google Scholar]
- Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM‐E, Clark SE et al (2006). Angiotensin II‐induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem 281: 35137–35146. [DOI] [PubMed] [Google Scholar]
- Weitzenblum E, Chaouat A (2009). Cor pulmonale. Chron Respir Dis 6: 177–185. [DOI] [PubMed] [Google Scholar]
- Wrigley BJ, Lip GYH, Shantsila E (2011). The role of monocytes and inflammation in the pathophysiology of heart failure. Eur J Heart Fail 13: 1161–1171. [DOI] [PubMed] [Google Scholar]
- Yeo SCM, Ho PC, Lin H‐S (2013). Pharmacokinetics of pterostilbene in Sprague‐Dawley rats: the impacts of aqueous solubility, fasting, dose escalation, and dosing route on bioavailability. Mol Nutr Food Res 57: 1015–1025. [DOI] [PubMed] [Google Scholar]
- Yu Z, Wang S, Zhang X, Li Y, Zhao Q, Liu T (2017). Pterostilbene protects against myocardial ischemia/reperfusion injury via suppressing oxidative/nitrative stress and inflammatory response. Int Immunopharmacol 43: 7–15. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zong J, Zhou H, Bian Z‐Y, Deng W, Dai J et al (2014). Puerarin attenuates pressure overload‐induced cardiac hypertrophy. J Cardiol 63: 73–81. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou G, Song W, Tan X, Guo Y, Zhou B et al (2012). Pterostilbene protects vascular endothelial cells against oxidized low‐density lipoprotein‐induced apoptosis in vitro and in vivo. Apoptosis 17: 25–36. [DOI] [PubMed] [Google Scholar]


