Abstract
Background and Purpose
Alcohol use disorders are a leading cause of preventable deaths worldwide, and stress is a major trigger of relapse. The neuropeptide relaxin‐3 and its cognate receptor, relaxin family peptide receptor 3 (RXFP3), modulate stress‐induced relapse to alcohol seeking in rats, and while the bed nucleus of the stria terminalis has been implicated in this regard, the central nucleus of the amygdala (CeA) also receives a relaxin‐3 innervation and CeA neurons densely express RXFP3 mRNA. Moreover, the CeA is consistently implicated in both stress and addictive disorders. Yohimbine precipitates relapse‐like behaviour in rodents, although exactly how yohimbine induces relapse is unknown, possibly by increasing stress levels and inducing heightened cue reactivity.
Experimental Approach
In the current study, we examined the effects of yohimbine (1 mg·kg−1, i.p.) on anxiety‐like behaviour in alcohol‐experienced rats. Furthermore, we assessed CeA neuronal activation following yohimbine‐induced reinstatement of alcohol seeking and the role of the relaxin‐3/RXFP3 signalling within the CeA in yohimbine‐induced reinstatement to alcohol seeking.
Key Results
Low‐dose yohimbine was anxiogenic in rats with a history of alcohol use. Furthermore, yohimbine‐induced reinstatement of alcohol seeking increased Fos activation in CeA corticotrophin‐releasing factor, dynorphin and GABA neurons compared with naïve and vehicle controls. Bilateral intra‐CeA injections of the selective RXFP3 antagonist, R3(B1‐22)R, attenuated yohimbine‐induced reinstatement of alcohol seeking.
Conclusions
Collectively, these data suggest that the CeA is a node where yohimbine acts to induce reinstatement of alcohol seeking and implicate the relaxin‐3/RXFP3 system within the CeA in this process.
Abbreviations
- AUD
alcohol use disorders
- BNST
bed nucleus of the stria terminalis
- CeA
central nucleus of the amygdala
- CRF
corticotrophin‐releasing factor
- DYN
dynorphin
- EXT
extinction
- iP
Indiana alcohol preferring
- IR
immunoreactivity
- L/D
light/dark
- NDS
normal donkey serum
- NI
nucleus incertus
- RXFP3
relaxin family peptide 3 receptor
- VEH
vehicle
Introduction
Addiction is a chronic relapsing disorder whereby addicts take drugs despite negative effects and consequences. Alcohol use disorders (AUDs) are a leading cause of preventable death worldwide, accounting for 5.5% of the global burden of disease and 4.6% of disability‐adjusted life years (World Health Organisation (WHO), 2014). Self‐medication is a widely recognized hypothesis of drug and alcohol use, with almost 15% of individuals with an anxiety disorder also having a comorbid AUD (Robinson et al., 2009). Stress‐related disorders including anxiety can precipitate alcohol abuse and are a common symptom during withdrawal from alcohol dependence. At a population level, current therapeutics to treat AUDs suffer from low compliance, adverse side effects and high rates of relapse (Jupp and Lawrence, 2010). Up to 90% of addicted individuals relapse within one year of abstinence (Dejong, 1994), most within three months (Sinha, 2008). Therefore, a more detailed understanding of addiction neurobiology may help in the development of new therapeutic targets and treatment strategies to manage both anxiety and substance use disorders.
One potential target for pharmacotherapeutic development to treat AUDs is the cognate GPCR for the conserved neuropeptide, relaxin‐3, known as relaxin family peptide 3 receptor (RXFP3) (Bathgate et al., 2002). Relaxin‐3 is predominantly expressed in GABA neurons in the hindbrain nucleus incertus (NI), which projects widely to forebrain areas, including the amygdala, bed nucleus of the stria terminalis (BNST), hippocampus and lateral hypothalamus, which also express high levels of RXFP3 (Goto et al., 2001; Ma et al., 2007; Olucha‐Bordonau et al., 2012; Santos et al., 2016). The NI relaxin‐3/RXFP3 is implicated in stress and arousal‐related behaviours (Tanaka et al., 2005; Banerjee et al., 2010; Ma and Gundlach, 2015; Walker and Lawrence, 2017). In addition, central administration of the selective RXFP3 antagonist, R3(B1‐22)R, attenuates both operant alcohol self‐administration and stress‐induced relapse to alcohol seeking (Ryan et al., 2013), mediated by corticotrophin‐releasing factor (CRF1) receptor and orexin (OX2) receptor signalling within the NI (Kastman et al., 2016; Walker et al., 2016). At a circuit level, the BNST has been implicated as a node in which relaxin‐3/RXFP3 signalling facilitates alcohol seeking (Ryan et al., 2013). However, since intra‐BNST RXFP3 antagonism only partially attenuated stress‐induced alcohol seeking, other brain regions are probably involved (Ryan et al., 2013). Given that the central nucleus of the amygdala (CeA) is consistently implicated in both addiction and fear/anxiety‐related conditions (see (Gilpin et al., 2015) for review), receives afferent input from relaxin‐3 neurons in the NI (Santos et al., 2016) and contains high levels of RXFP3 mRNA (Ma et al., 2007), we hypothesized that the CeA may constitute another node in which relaxin‐3/RXFP3 signalling mediates alcohol seeking.
The CeA is a major component of the ‘extended amygdala’, a group of interconnected basal forebrain structures that constitute integral parts of the central stress system associated with the negative reinforcement of dependence and with neurotransmitters linked to the positive reinforcing effects of drugs of abuse (Koob and Le Moal, 2005). This nucleus has been identified as a key neural substrate for alcohol drinking and alcohol dependence‐related behaviours (Koob and Volkow, 2010; Gilpin et al., 2015) and is highly stress‐sensitive (Singewald et al., 2003; Funk et al., 2006b, 2016). Studies have reported activation of the CeA following footshock‐induced reinstatement of alcohol seeking (Zhao et al., 2006; Schank et al., 2015); however, this is yet to be examined following yohimbine‐induced reinstatement of alcohol seeking. Furthermore, multiple neuropeptide and neuromodulatory systems are dysregulated by alcohol, including CRF and dynorphin (DYN), which can contribute to excessive alcohol consumption and negative emotional symptoms of alcohol withdrawal by converging on GABA circuitry in the CeA (Roberto et al., 2004; Roberto et al., 2010; Gilpin et al., 2015). For example, acute and chronic alcohol consumption increase GABA transmission in the CeA (Roberto et al., 2004), while GABAA receptor antagonism in the CeA reduces alcohol self‐administration in rats (Hyytia and Koob, 1995).
The precise mechanisms by which yohimbine precipitates reinstatement of operant alcohol seeking are not fully understood. Yohimbine is an α2‐adrenoceptor antagonist that increases noradrenaline release in several brain areas (Tanaka et al., 2000), elevates glucocorticoid levels in both rats (Marinelli et al., 2007) and humans (Vythilingam et al., 2000) and elicits anxiety in both rodents (Pellow et al., 1987) and humans (McDougle et al., 1995). A recent study reported that yohimbine reinstates operant responding in rats previously trained with lever pressing resulting in cue presentation with or without reward delivery, suggesting yohimbine may not necessarily act as a stressor (Chen et al., 2015). Indeed, low doses of yohimbine that reinstate operant responding are not anxiogenic in alcohol‐naïve rats (Arrant et al., 2013). However, alcohol (and other drugs of abuse) induces adaptations in brain stress systems (Koob, 2009a; Zhou and Kreek, 2014; Walker et al., 2016), which may influence the impact of yohimbine in alcohol‐experienced rats. In healthy human subjects, alcohol and yohimbine have additive effects on subjective reports of intoxication and anxiety (McDougle et al., 1995), while yohimbine increases anxiety and cortisol levels in recently detoxified alcoholic subjects (Krystal et al., 1994) and increases subjective craving in human alcoholics (Umhau et al., 2011). Furthermore, yohimbine has been used extensively in animal models of stress‐alcohol interaction, including stress‐induced reinstatement of alcohol seeking paradigms (Marinelli et al., 2007; Richards et al., 2008; Le et al., 2013; Ryan et al., 2013; Funk et al., 2016; Kastman et al., 2016; Walker et al., 2016). To our knowledge, previous investigations of the effects of yohimbine on anxiety‐like behaviour and neuronal activation have examined alcohol‐naïve rats and employed yohimbine doses typically higher than required to induce reinstatement of drug seeking (Singewald et al., 2003; Funk et al., 2006b).
Here, we demonstrate for the first time that low doses of yohimbine are anxiogenic in male iP rats with previous alcohol/abstinence experience. Furthermore, we demonstrate that CRF, dynorphin and GABA neuronal activation increases in the CeA following yohimbine‐induced reinstatement of alcohol seeking and identify the CeA as another node within the ‘stress‐relapse’ circuitry in which the relaxin‐3/RXFP3 system acts to mediate stress (yohimbine)‐induced reinstatement of alcohol seeking.
Methods
Animals
Adult male alcohol‐preferring (iP) rats were obtained from the breeding colony of The Florey Institute of Neuroscience and Mental Health. Parental stock was obtained from Professor T.K. Li (while at Indiana University, IN, USA). This strain of rat was chosen as they voluntarily consume high levels of ethanol resulting in relevant blood ethanol concentrations (Ryan et al., 2013; Walker et al., 2016). Rats were housed two per cage with a littermate in a 12 h light/dark (L/D) cycle (0700–1900 h), with access to food (laboratory chow) and water ad libitum. After surgery, rats were single housed. All behavioural studies were performed in accordance with the Prevention of Cruelty to Animals Act (2004), under the guidelines of the National Health and Medical Research Council Code of Practice for the Care and Use of Animals for Experimental Purposes in Australia (2013) and approved by The Florey Animal Ethics Committee. All efforts were made to minimize animal suffering and reduce the number of animals used. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Alcohol self‐administration
Male iP rats were trained to self‐administer alcohol [10% (v.v‐1)] under operant conditions using a fixed ratio of 3 (FR3, three lever presses for one reward delivery) during 20 min sessions (Ryan et al., 2013). Two levers were available during the session, one that delivered a 100 μL alcohol reward (active lever), and the other that delivered the same volume of water (inactive lever). Operant conditioning chambers supplied by Med Associates (St Albans, VT, USA) were employed. Each chamber was housed individually in sound‐attenuation cubicles, and chambers were connected to a computer running Med‐PC IV software (Med Associates) to record data. Within the chambers, a house light provided soft illumination during operant conditioning sessions. Retractable levers (exerted during operant conditioning sessions) were positioned below a stimulus light and adjacent to a fluid receptacle. Availability of alcohol was conditioned by the presence of an olfactory cue [S+; one drop of vanilla essence (Queen Foods, Alderley, QLD, Australia)] placed directly underneath the alcohol‐paired lever of the operant conditioning chamber. A 1 s light stimulus (CS+) occurred when the FR3 requirement was obtained with both the alcohol‐paired and water‐paired lever. Rats underwent an extended period of alcohol self‐administration (>7 weeks, five consecutive days·week−1).
Light–dark box paradigm
Following alcohol self‐administration, male iP rats (n = 24) underwent 10 days of home cage abstinence and were subsequently tested for anxiety‐like behaviour in a 10 min L/D box test. Experiments were conducted between 10:00 and 13:00 h. Rats were placed in the 42 cm (length) × 42 cm (width) × 40 cm (height) automated locomotor cell (Med Associates). Half of the locomotor cell was covered by the ‘dark’ box made of plastic opaque to visible light, but transparent to photo beams. A small opening (7 × 7 cm) enabled rats to enter or leave the ‘dark’ side. The light side was lit by an array of light‐emitting diodes (475 LUX in the centre), creating an aversive stimulus. Movements of rats were tracked using activity monitor software (Med Associates). Rats were administered either the α2‐adrenoceptor antagonist yohimbine, dissolved in distilled water (1 mg·kg−1, i.p., Tocris Bioscience, Bristol, UK) or vehicle (VEH; 1 mL·kg−1, i.p.) 30 min prior to being placed in the dark side of the box to commence the 10 min session. Boxes were cleaned and dried between each test.
Extinction and yohimbine‐induced reinstatement of alcohol seeking
In order to model relapse of alcohol seeking, rats underwent extinction (EXT)‐reinstatement testing (Ryan et al., 2013). During extinction training, no cues were present and there was no programmed response following instrumental responses. Extinction sessions continued until mean responding on the alcohol‐paired lever were <15 lever presses for three consecutive days (~10 days in total). Rats were habituated to i.p. injections for the final 4–5 days of extinction training. To examine reinstatement, lever responding was measured in rats during a 20 min operant session, 30 min following administration of yohimbine (1 mg·kg−1, i.p.) under the same conditions used during self‐administration with the exception that there were no deliveries of ethanol or water.
Immunohistochemistry
Another cohort of alcohol‐experienced rats were randomly divided into three groups; group 1 (n = 5) were administered yohimbine (1 mg·kg−1, i.p.) and, 30 min later, underwent reinstatement testing. Group 2 (n = 5) were administered yohimbine (1 mg·kg−1, i.p.) but returned to their home cage and not subjected to reinstatement. Group 3 (n = 5) were administered vehicle (1 mL·kg−1, i.p.), while another group of age‐matched alcohol‐naïve rats (n = 5) did not receive any intervention before perfusion. Rats were anaesthetized (pentobarbitone 100 mg·kg−1, i.p., Virbac, Milperra, NSW, Australia) 60 min after the end of reinstatement testing (or equivalent time) and transcardially perfused. Rats were perfused with 400 mL PBS (0.1 M, pH 7.4) followed by 400 mL 1% paraformaldehyde (PFA, Sigma‐Aldrich, Sydney, NSW, Australia) in PBS with 15% (v.v‐1) picric acid (Sigma‐Aldrich), decapitated, brains removed and post‐fixed (1 h) in 50 mL perfusion solution. Brains were incubated in 20% sucrose in PBS (50 mL) at 4°C overnight, before being frozen over liquid nitrogen and stored at −80°C. Coronal sections (50 μm) were cut on a cryostat at −18°C (Cryocut, 1800; Leica Microsystems, Heerbrugg, Switzerland) and collected into PBS in a 1/3 series. Sections were pre‐blocked in 10% normal donkey serum (NDS, Millipore, Billerica, MA, USA) and 0.5% Triton‐X (BDH Chemicals, QLD, Australia) in PBS, for 1 h at room temperature. Sections were then incubated in a primary antibody mix of either rabbit anti‐CRF (1:1000, PBL#C70) (Sawchenko et al., 1984), guinea pig anti‐prodynorphin (pDYN, 1:1000; Neuromics, Edina, MN, USA, GP10109) or rabbit anti‐GABA (1:1000; Sigma Aldrich, A2052), with 1:500 goat anti‐c‐Fos (Santa Cruz Biotechnology, Santa Cruz, CA, USA, #SC‐52‐G), 2% NDS in PBS containing 0.1% TritonX‐100 (48–72 h at 4°C). Sections were washed 3 × 5 min in PBS and incubated for 2 h with donkey anti‐goat‐Alexa 647 (1:400; Life Technologies, Carlsbad, CA, USA, A21447) and donkey anti‐rabbit‐Alexa 488 (1:400; Life Technologies, A21206). For pDYN, sections were incubated for 2 h in Biotin‐SP affinity pure donkey anti‐guinea pig (1:500; Jackson ImmunoResearch, West Grove, PA, USA, JI706065148), washed 3 × 5 min in PBS and then incubated for 2 h at room temperature with donkey anti‐goat‐Alexa 647 (1:400; Life Technologies, A21447) and streptavidin‐Alexa 488 (1:400; Life Technologies, S11223). Sections were washed 3 × 5 min, mounted on microscope slides and coverslipped with fluorescence mounting medium (DAKO, Carpentaria, CA, USA). Images were acquired on a confocal microscope (Leica DM LB2, Leica Microsystems, Wetzlar, Germany) and quantified using Image J (NIH). The number of CRF, pDYN, GABA, Fos‐positive neurons and % CRF, %pDYN or %GABA neurons that expressed Fos were determined unilaterally in a 1‐in‐3 series throughout the CeA from bregma 2.1 mm (Paxinos and Watson, 1986) by an investigator blinded to treatment allocation.
Stereotaxic implantation of central nucleus of the amygdala cannulae
After stabilization of alcohol self‐administration, a separate cohort of rats was anaesthetized and underwent bilateral stereotaxic implantation of cannulae into the CeA. Rats were deeply anaesthetized with isoflurane [5% (v.v‐1) induction, 2% maintenance], positioned in a stereotaxic frame (Stoelting Co. Wood Dale, IL, USA), and the scalp shaved and cleaned with proviodine‐iodine [10% (w.v‐1); Orion Laboratories, Arkles Bay, New Zealand]. Depth of anesthesia was monitored using the paw‐pinch test. A small incision was made to expose the skull. Four pits were drilled into the skull and screws (1.4 mm diameter and 2 mm length; Mr Specs, Parkdale, Australia) inserted. A hole was drilled through the skull, and a stainless steel 26 gauge bilateral cannula cut 8 mm below the pedestal (PlasticsOne, Roanoke, VA, USA) was implanted relative to bregma: anteroposterior, −2.2 mm; mediolateral, ±4.4 mm and dorsoventral, −9.5 mm (Paxinos and Watson, 1986). Cannulae were fixed in place using dental cement (Vertex‐Dental, Zeist, The Netherlands). Patency was maintained by inserting a dummy, which projected 1.5 mm beyond the tip (PlasticsOne) and analgesia (Meloxicam; 3 mg·kg−1, i.p., Troy Laboratories, Glendenning, NSW, Australia) plus antibiotic (Baytril, enrofloxacin, 3 mg·kg−1, i.p, Bayer Health Care, Pymble, NSW, Australia) were administered.
Central nucleus of the amygdala infusions
After recovery from surgery, rats re‐acquired alcohol responding to pre‐surgical levels before extinction training as described. Once the extinction criteria were met, rats underwent a yohimbine‐induced reinstatement session, whereby yohimbine (1 mg·kg−1, i.p.) was administered 30 min prior to test (Ryan et al., 2013). During this session, rats (n = 19) received bilateral intra‐CeA infusions of either the selective RXFP3 receptor antagonist R3(B1‐22)R (1 μg in 0.5 μL aCSF) (Ryan et al., 2013) or vehicle, directly prior to reinstatement testing in a randomized manner. This dose was chosen as it reduced yohimbine‐induced reinstatement of alcohol seeking within the BNST (Ryan et al., 2013). Subsequently, rats received 2 days of alcohol reacquisition to re‐stabilize alcohol consumption and were then re‐extinguished. A second reinstatement test was performed in which rats received the alternate intra‐CeA treatment in a counterbalanced manner. Rats in which the injections were outside the CeA were used as anatomical controls (n = 7). Bilateral CeA infusions were made using 40 cm polyethylene connectors (PlasticsOne) attached to 1 μL microsyringes (SGE Analytical Science, Ringwood, VIC, Australia). R3(B1‐22)R or vehicle was infused bilaterally (0.25 μL·min−1) by an automated syringe pump (Harvard Apparatus, Holliston, MA, USA), and injectors were left in place for 2 min after infusion. After completion of behavioural testing, rats were anaesthetized with pentobarbitone (100 mg·kg−1, i.p.). Correct cannula positioning was verified by infusing methylene blue (0.25 μL per side) under anaesthesia. Rats were subsequently decapitated, brains collected, frozen over liquid nitrogen and sectioned for injection site validation, which was performed by an investigator blinded to the identity of the treatment.
Data and statistical analysis
All data analysis and generation of histograms were performed using GraphPad Prism Version 6 for Windows (GraphPad Software Inc., San Diego, CA, USA; www.graphpad.com). L/D box data were examined with Student's unpaired t‐test. Differences in the number of CRF‐immunoreactive (IR), pDYN‐IR, GABA‐IR, Fos‐IR, co‐localization of CRF, pDYn or GABA with Fos‐IR and % of activated CRF, pDYN or GABA cells were analysed using one‐way ANOVA with post hoc Tukey's multiple comparison tests. Reinstatement and time course data were analysed using repeated measures (RM) two‐way ANOVA with post hoc Tukey's multiple comparison tests. Latency to reward was analysed using Student's t‐test. Differences were considered significant at P < 0.05. Data are presented as mean ± SEM. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b).
Results
Low‐dose yohimbine provokes anxiety in alcohol‐experienced rats
We assessed the effect of yohimbine administration on anxiety‐like behaviour in adult male iP rats with prior alcohol self‐administration experience. During alcohol self‐administration, rats averaged 92 ± 9 active lever responses (0.59 ± 0.05 g·kg−1 per session alcohol intake). Following 10 days of home cage abstinence, rats were tested for anxiety‐like behaviour in the L/D box 30 min following yohimbine (1 mg·kg−1, i.p.) or vehicle (1 mL·kg−1, i.p.) administration. Yohimbine‐treated rats spent significantly less time in the light side of the locomotor cell (Student's unpaired t‐test, P < 0.05, Figure 1).
Figure 1.

Yohimbine (YOH) administration in alcohol‐experienced iP rats decreased the % time spent in the light compartment of the L/D box test (*P < 0.05). Data shown as mean ± SEM (n = 12 per group).
GABA, CRF and pDYN neurons in the CeA are activated by yohimbine‐induced reinstatement of alcohol seeking
Next, we examined neuronal activation in the CeA of alcohol‐naïve rats or rats that underwent extended alcohol self‐administration/extinction followed by vehicle administration, yohimbine administration or yohimbine‐induced reinstatement of alcohol seeking. During the last 5 days of alcohol self‐administration, rats averaged 90 ± 7 active lever responses (0.59 ± 0.04 g·kg−1 per session alcohol intake). No differences were observed in ethanol self‐administration (Figure 2A) or extinction (Figure 2B) across groups. For rats that underwent reinstatement, two‐way ANOVA revealed a main effect of treatment (F (2, 10) = 67.72, P < 0.05), lever (F (1, 5) = 146.3, P < 0.05) and a treatment × lever interaction (F (2, 10) = 72.05, P < 0.05). Post hoc analysis revealed that active lever responding significantly decreased during extinction training (P < 0.05) and yohimbine precipitated reinstatement of active lever (P < 0.05), but not inactive lever (P > 0.05), responding Figure 2C.
Figure 2.
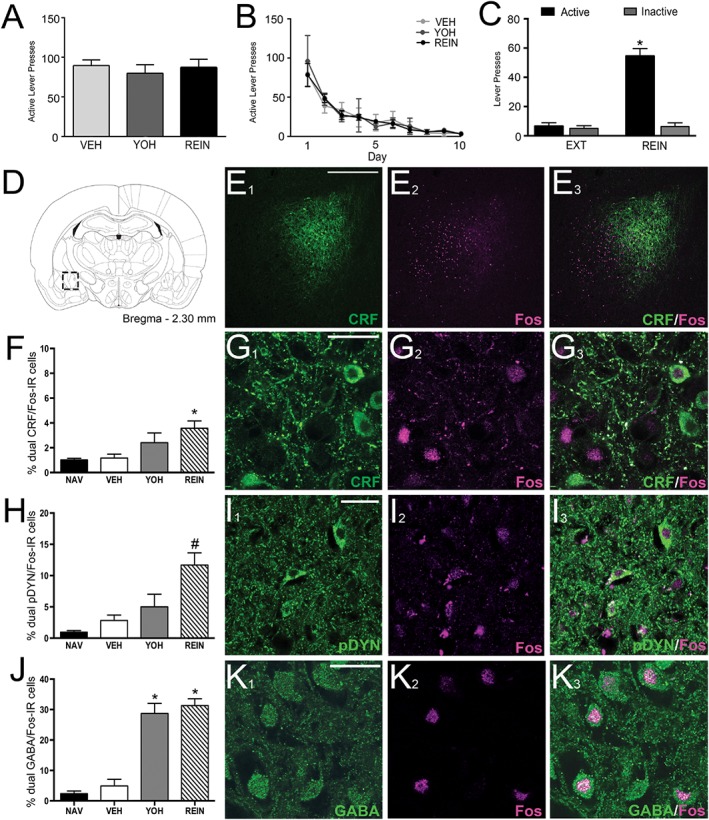
Yohimbine‐induced reinstatement of alcohol seeking activates CRF, pDYN and GABA neurons in the CeA of iP rats. Active lever responding during (A) the last 5 days of self‐administration and (B) extinction (EXT) training across treatment groups. (C) Yohimbine administration induced reinstatement of active lever responding (*P < 0.05), but not inactive lever responding. (D) Rat brain schematic outlining CeA region in which CRF, pDYN, GABA and Fos‐IR was quantified. (E1–3) Representative low magnification micrographs of CRF‐, Fos‐ and merged‐IR in the CeA. Histogram illustrating (F) % CRF cells, (H) % DYN cells, (J) % GABA cells activated in naïve (NAV), VEH‐treated, yohimbine (YOH)‐treated and rats that underwent yohimbine‐induced reinstatement of alcohol seeking [reinstatement (REIN)]. (G 1–3) Representative high magnification micrograph of CRF (green), Fos (magenta) and co‐localization in a REIN rat. (I 1–3) Representative image of pDYN (green), Fos (magenta) and co‐localization in a REIN rat. (K 1–3) Representative high magnification images of GABA (green), Fos (magenta) and co‐localization in a REIN rat. Rats that underwent reinstatement displayed significantly increased %CRF neurons expressing Fos, compared with VEH (*P < 0.05) and a significant increase in % pDYN neurons expressing Fos, compared with VEH‐ and YOH‐treated animals (#P < 0.05). YOH‐treated and REIN rats displayed significantly increased Fos‐IR and %GABA neurons expressing Fos, compared with VEH (*P < 0.05). Scale bar: low magnification, 150 μm; high magnification, 20 μm. All data expressed as mean ± SEM, n = 5 per group.
Subsequently, we examined neuronal activation in the CeA as reflected by Fos‐IR following yohimbine‐induced reinstatement of alcohol seeking. One‐way ANOVA revealed an overall main effect of treatment on the number of Fos‐positive neurons in three separate series of adjacent sections (lowest F = 10.38, P < 0.05). Post hoc analysis showed a significant increase in the number of Fos‐positive neurons in the yohimbine‐treated and yohimbine‐induced reinstatement rats, compared with vehicle or naïve (P values <0.05, Table 1). No differences were observed between naïve and vehicle‐treated rats (P > 0.05). Furthermore, no significant differences were observed in the number of GABA‐IR (F (3, 16) = 0.946, P > 0.05), CRF‐IR (F (3, 16) = 1.61, P > 0.05) or pDYN‐IR (F (3, 16) = 0.75, P > 0.05) across treatment groups (Table 1). One‐way ANOVA revealed an overall main effect of treatment on the number of GABA/Fos‐IR cells (F (3, 16) = 28.61, P < 0.05) and % GABA neurons co‐expressing Fos‐IR (F (3, 16) = 54.75, P < 0.05). Post hoc analysis revealed a significant increase in the number GABA/Fos‐ IR and %GABA/Fos‐positive neurons in the yohimbine‐treated and yohimbine‐induced reinstatement rats, compared with all other groups (P values <0.05, Figure 2J/K). No differences were observed between naïve and vehicle‐treated rats (P > 0.05; Table 1).
Table 1.
Mean counts of Fos‐IR, CRF‐IR, pDYN‐IR, GABA‐IR and number and percentages of dual peptide/Fos‐IR cells in the CeA of iP rats
| pDYN‐IR | Fos‐IR | pDYN/Fos‐IR | % pDYN/Fos‐IR | |
|---|---|---|---|---|
| NAV | 157.2 (9) | 9.7 (2) | 1.5 (1) | 1.0 (1) |
| VEH | 162.8 (6) | 25.8 (6) | 4.6 (1) | 2.8 (1) |
| YOH | 149.8 (3) | 133.4 (13)* | 7.6 (1) | 5.1 (1) |
| REIN | 151.8 (6) | 213 (21)*, # | 18 (4)*, # | 11.9 (2)*, # |
| CRF‐IR | Fos‐IR | CRF/Fos‐IR | % CRF/Fos‐IR | |
|---|---|---|---|---|
| NAV | 157.6 (5) | 6.1 (1) | 1.6 (1) | 1.0 (1) |
| VEH | 163.9 (14) | 20.4 (6) | 2.0 (1) | 1.2 (1) |
| YOH | 152.2 (6) | 137.8 (4)* | 3.6 (1) | 2.4 (1) |
| REIN | 135.9 (10) | 217.2 (63)* | 4.9 (1)* | 3.6 (1)* |
| GABA‐IR | Fos‐IR | GABA/Fos‐ IR | % GABA/Fos‐IR | |
|---|---|---|---|---|
| NAV | 560.2 (17) | 28 (3) | 12.8 (3) | 2.3 (1) |
| VEH | 508.2 (28) | 60.2 (14) | 25.6 (6) | 5.0 (3) |
| YOH | 405.2 (45) | 230.3 (28)* | 119.5 (24)* | 29.5 (10)* |
| REIN | 507.4 (30) | 274.4 (11)* | 158.2 (13)* | 31.2 (7)* |
The number of CRF‐IR, pDYN‐IR, GABA‐IR, Fos‐IR and co‐localization of cells was counted in adjacent sections in a 1/3 series throughout the CeA from bregma 2.1–2.4 mm of alcohol naïve (NAV); VEH‐treated; yohimbine (YOH)‐treated and yohimbine‐induced reinstatement (REIN)‐treated iP rats. One‐way ANOVA showed no significant difference in number of CRF‐IR, pDYN‐IR and GABA‐IR cells across treatment groups (P > 0.05), while one‐way ANOVA with Tukey's multiple comparisons revealed a significant difference between NAV/VEH animals and YOH/REIN animals for number of Fos‐IR cells, number of GABA/Fos‐IR cells and % GABA/Fos‐IR cells. Number of CRF/Fos cells and % CRF/Fos‐IR cells were also different in REIN rats compared to NAV/VEH groups, while the number of pDYN/Fos cells and %pDYN/Fos cells were significantly different from NAV/VEH/YOH‐treated rats.
P < 0.05 compared with VEH‐treated animals,
P < 0.05 compared with YOH‐treated. n = 5 per group. Data are presented as group means with SEM in parentheses.
The % CRF neurons co‐expressing Fos‐IR (F (3, 16) = 5.32, P < 0.05) also displayed a significant difference. Post hoc analysis revealed a significant increase in %CRF/Fos‐positive neurons in the yohimbine‐induced reinstatement rats, compared with naïve and vehicle‐treated groups (P values <0.05, Figure 2F/G). No differences were observed between naïve and vehicle‐treated rats (P > 0.05; Table 1). One‐way ANOVA also revealed a significant increase in % pDYN neurons co‐expressing Fos‐IR (F (3, 16) = 16.15, P < 0.05), with post hoc analysis showing a significant increase in the % pDYN/Fos‐positive neurons in the yohimbine‐induced reinstatement rats, compared with naïve, vehicle‐ and yohimbine‐treated groups (P values <0.05, Figure 2H/I). Once again, no differences were observed between naïve and vehicle‐treated rats (P > 0.05; Table 1).
Bilateral administration of R3(B1‐22)R into CeA attenuates yohimbine‐induced reinstatement of alcohol seeking in iP rats
Lastly, we investigated the effect of bilateral intra‐CeA injections of R3(B1‐22)R on yohimbine‐induced reinstatement of alcohol seeking. Rats made 122 ± 9 active lever responses, consuming 0.76 ± 0.04 g·kg−1 per session of alcohol during self‐administration training prior to and following surgery. RM two‐way ANOVA revealed a main effect of lever (F (1, 18) = 203.1, P < 0.05), treatment (F (2, 36) = 39.16, P < 0.05) and a treatment × lever interaction (F (2, 36) = 53.31, P < 0.05). Tukey's post hoc analysis indicated that yohimbine precipitated alcohol seeking in vehicle‐treated rats (EXT vs. VEH, P < 0.05), while R3(B1‐22)R injected bilaterally into the CeA significantly attenuated yohimbine‐induced reinstatement (VEH vs. R3(B1‐22)R, P < 0.05; Figure 3A), with ~42% reduction in active lever responding compared with vehicle‐treated rats. No differences were observed on the inactive lever (P values >0.05). Analysis of time course data indicated an effect of R3(B1‐22)R on active lever responding over time with a main effect of treatment (F (1, 34) = 11.26, P < 0.05) and time (F (3, 102) = 29.27, P < 0.05). Post hoc analysis revealed a significant reduction at the early stage of the reinstatement session when responding is at its highest (P < 0.05; Figure 3D), although no difference in latency to active lever response was observed (Student's t‐test P < 0.05; Figure 3C). Injection sites were validated histologically for all R3(B1‐22)R‐treated rats (Figure 3E). Rats in which the injection sites were adjacent to the CeA were analysed separately as anatomical controls. RM two‐way ANOVA revealed a main effect of lever (F (1,6) = 36.19, P < 0.05), treatment (F (2, 12) = 5.61, P < 0.05) and a treatment × lever interaction (F (2, 12) = 5.61, P < 0.05). Tukey's post hoc analysis indicated that yohimbine precipitated alcohol seeking in vehicle‐treated rats (EXT vs. VEH, P < 0.05); however, R3(B1‐22)R injected immediately adjacent to the CeA failed to significantly attenuate yohimbine‐induced reinstatement (VEH vs. R3(B1‐22)R, P = 0.467, Figure 3B), with a non‐significant ~22% reduction in active lever responding compared with vehicle‐treated rats. No differences were observed on the inactive lever (P values >0.05).
Figure 3.

R3(B1‐22)R injected bilaterally into the CeA attenuates yohimbine‐induced reinstatement for alcohol seeking in iP rats. Active lever and inactive lever responding following within subject counterbalanced (A) bilateral intra‐CeA (n = 19) or (B) anatomical control (n = 7) injections of either VEH or R3(B1‐22)R. (C) Latency to active lever responding for VEH‐ and R3(B1‐22)R‐treated rats and (D) time course of lever responding throughout reinstatement session. (E) Neuroanatomical representation of injection sites for R3(B1‐22)R‐treated rats. Green circles represent CeA injections, and red circles represent anatomical controls. Data were analysed by RM two‐way ANOVA with post hoc Tukey's multiple comparison test and expressed as mean ± SEM. Extinguished rats (EXT), VEH‐ and R3(B1‐22)R‐treated rats. Yohimbine induced reinstatement of alcohol seeking in VEH‐treated rats (EXT vs. VEH #P < 0.05), which was attenuated by R3(B1‐22)R microinjection within the CeA (VEH vs. R3(B1‐22)R *P < 0.05). Furthermore, time course data revealed a reduction in responding by R3(B1‐22)R treated rats during the first 5 min (VEH vs. R3(B1‐22)R, *P < 0.05), but did not significantly increase latency to active lever responding.
Discussion
These experiments provide evidence that low‐dose yohimbine (1 mg·kg−1) produces anxiety‐like behaviour in male iP rats with the same history of alcohol self‐administration followed by abstinence as used in our model of relapse to alcohol seeking. Furthermore, in these alcohol‐experienced rats, Fos immunohistochemistry revealed that CRF, pDYN and GABA neurons within the CeA are activated following yohimbine‐induced reinstatement of alcohol seeking. We also provide evidence for the involvement of relaxin‐3/RXFP3 signalling within the CeA in yohimbine‐induced reinstatement of alcohol seeking in iP rats.
Effects of yohimbine on anxiety‐like behaviour have been previously observed in alcohol‐naïve rats at doses ranging from 2.5 to 10 mg·kg−1 (Pellow et al., 1985; Baldwin et al., 1989), but these effects were not observed at a lower 1 mg·kg−1 dose (Pellow et al., 1985; Arrant et al., 2013), except in rats that have been housed in isolation (Cai et al., 2012). Given these data and recent evidence suggesting yohimbine reinstates lever pressing in rats that were previously trained with lever pressing resulting in cue presentation with or without reward delivery (Chen et al., 2015), yohimbine may, theoretically at least, act in a similar manner to reinstate alcohol seeking. In this study, we tested male iP rats after 10 days of abstinence from alcohol, to align with the duration of extinction training when rats do not have access to alcohol. Under these conditions, yohimbine (1 mg·kg−1) was anxiogenic. Notably, as withdrawal from alcohol alters stress systems (Zorrilla et al., 2001), it is possible that long‐term alcohol experience plus subsequent abstinence contribute to the anxiogenic properties of low‐dose yohimbine. Accordingly, we suggest that although yohimbine may increase cue reactivity, in our model of alcohol seeking, yohimbine is also an anxiogenic acute stressor that apparently contributes to reinstatement of alcohol seeking.
Low‐dose yohimbine also increased neuronal activation in the CeA of alcohol experienced/abstinent rats. These data are in line with studies in alcohol‐naïve rats, whereby higher doses of yohimbine also result in activation of the CeA (Singewald et al., 2003; Funk et al., 2006b, 2016). We also observed an increase in CeA Fos‐IR following yohimbine‐induced reinstatement, similar to that observed following footshock‐induced reinstatement of alcohol seeking (Zhao et al., 2006; Schank et al., 2015). Both footshock stress and yohimbine activate similar brain regions, increase CRF mRNA and induce relapse to drug (including alcohol) seeking driven via a CRF‐dependent mechanism (Le et al., 2000; Funk et al., 2006a,b; Marinelli et al., 2007). Therefore, findings that both yohimbine and yohimbine‐induced reinstatement of alcohol seeking increase Fos‐IR within the CeA implicate the CeA as a key structure in the precipitation of ‘relapse’ after exposure to multiple stressors, including yohimbine.
Notably, the majority of Fos‐IR was localized within the lateral and capsular divisions of the CeA, while sparse within the medial division of the CeA. The lateral and capsular divisions of the CeA receive concentrated dopaminergic innervation (Freedman and Cassell, 1994) and express a variety of neuropeptides implicated in addictive disorders. These include CRF, DYN, cocaine and amphetamine‐regulated transcript, neuropeptide Y and nociceptin (Koylu et al., 1997; Roberto et al., 2012). Neuropeptides have been attributed a prominent role in negative affective aspects of drug and alcohol abuse (Koob, 2009b). The relationship between stress‐related disorders and addiction‐related behaviours has been a focus of research in recent years, driven in part by high comorbidity in the clinical setting (Volkow, 2004). The CRF/CRF1 system has been strongly implicated in these behaviours (see Koob, 2013, for review). CRF neurons in the CeA are a combination of GABAergic and glutamatergic, projection and interneurons (Pomrenze et al., 2015), and alcohol and CRF signalling in the CeA increases GABAergic and glutamatergic transmission (Roberto et al., 2004).
More than half of the neurons activated following yohimbine‐induced reinstatement of alcohol seeking were GABA positive, representing activation of ~30% of all GABAergic neurons in the CeA. Due to the topography of Fos‐IR, we further assessed the involvement of two neuropeptides strongly implicated in stress‐induced relapse, CRF and DYN. Significant increases in % dual CRF/Fos‐IR and DYN/Fos‐IR were observed following yohimbine‐induced reinstatement of alcohol seeking. These data are potentially an under estimate caused by limitations in the detection of cytoplasmic CRF peptide without colchicine pretreatment, which we deliberately avoided due to confounds with behavioural and Fos expression studies. Nevertheless, our findings clearly establish that subpopulations of both CRF and DYN neurons within the CeA are activated during yohimbine‐induced reinstatement of alcohol seeking.
An endogenous ligand for the κ opioid receptor, DYN, is implicated in alcohol abuse and dependence, as well as the reinforcing and rewarding properties of other drugs of abuse. Further, the DYN/κ receptor system induces dysphoric/anhedonic properties, which may contribute to alcohol seeking and consumption (Walker et al., 2012). In line with this, we observed an increase in activation of DYN‐positive neurons within the CeA following yohimbine‐induced reinstatement of alcohol seeking. Furthermore, the CRF and DYN systems within the CeA are also closely linked, with 1/3 of CRF neurons also expressing DYN (Marchant et al., 2007).
Emerging evidence suggests that CRF signalling interacts with the relaxin‐3/RXFP3 system to modulate both stress responses and alcohol seeking (Tanaka et al., 2005; Banerjee et al., 2010; Ma et al., 2013; Ma and Gundlach, 2015; Walker et al., 2016; Walker and Lawrence, 2017). Given that the CeA is a stress‐sensitive node in reward circuitry (Funk et al., 2006a; Koob, 2009a; Koob, 2009b; Gilpin et al., 2015), receives relaxin‐3 innervation from the NI (Goto et al., 2001; Olucha‐Bordonau et al., 2012; Santos et al., 2016) and expresses dense RXFP3 mRNA (Ma et al., 2007), we tested the effects of the selective RXFP3 receptor antagonist, R3(B1‐22)R, on yohimbine‐induced reinstatement of alcohol seeking. Intra‐CeA microinjection of R3(B1‐22)R (1 μg) attenuated yohimbine‐induced reinstatement of alcohol seeking in iP rats. In contrast, R3(B1‐22)R injected immediately adjacent to the CeA had no effect, suggesting the action is specifically associated within the CeA. Antagonism of CeA RXFP3 decreased responding at the beginning of the test session, when responding is at its highest; however, it did not alter latency, suggesting the effect of treatment is not due to sedation or a deficit in procedural memory. Furthermore, central administration of a 10‐fold higher dose of R3(B1‐22)R has no effect on locomotor activity in iP rats, suggesting the antagonist did not impact general activity (Ryan et al., 2013).
The CeA therefore represents an additional locus where RXFP3 signalling is involved in yohimbine‐induced reinstatement to alcohol seeking, with previous reports implicating the BNST (Ryan et al., 2013). The degree of attenuation of reinstatement observed after bilateral intra‐CeA R3(B1‐22)R administration is similar to that observed when the peptide antagonist was delivered within the BNST. The CeA and BNST have extensive interconnectivity, and both regions are involved in controlling fear and anxiety responses to environmental stimuli (see Tovote et al., 2015, for review). Relaxin‐3/RXFP3 signalling may act jointly in these regions to facilitate yohimbine‐induced reinstatement of alcohol seeking; however, the involvement of other brain regions cannot be ruled out. Our immunohistochemical data suggest that GABA, CRF and DYN signalling in the CeA is activated following yohimbine‐induced reinstatement of alcohol seeking, which may be influenced by intra‐CeA relaxin‐3/RXFP3 signalling to drive relapse to alcohol seeking. Given that RXFP3 is a Gi/o coupled G protein receptor and that the CeA has a complex network of intrinsic connectivity, relaxin‐3/RXFP3 signalling in the CeA may facilitate relapse to alcohol seeking via disinhibition of downstream CeA signalling. This and other anatomical and molecular questions require further investigation.
Collectively, our results emphasize the role of the CeA as an interface of stress and relapse to alcohol seeking. We provide evidence that low doses of yohimbine increase anxiety‐like behaviour in iP rats with previous alcohol experience. Furthermore, in alcohol‐experienced rats, low‐dose yohimbine causes robust activation of CeA GABAergic neurons (plus CRF and DYN) and reinstatement of alcohol seeking. Relaxin‐3/RXFP3 signalling within the CeA contributes to yohimbine‐induced reinstatement of alcohol seeking. We therefore suggest that the relaxin‐3/RXFP3 system represents a legitimate potential target for the treatment of both anxiety and substance use disorders.
Author contributions
A.J.L., L.C.W. and H.E.K. contributed to the design and analysis of the study; A.J.L. and A.L.G. jointly supervised the project. L.C.W., H.E.K. and E.V.K. conducted experiments and performed related analysis; L.C.W. and A.J.L. wrote the manuscript. All authors reviewed the content and approved the final version of the manuscript.
Conflict of interest
L.C.W., H.E.K., E.V.K., A.L.G. and A.J.L. declare no competing conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
We thank Dr Johan Rosengren, School of Biomedical Sciences, University of Queensland, for the supply of R3(B1‐22)R and Dr Wylie W. Vale (in memoriam), Dr Paul E. Sawchenko and Dr Joan Vaughan from the Salk Institute for Biological Studies for the generous gift of the antisera against CRF. These studies were supported by a National Health and Medical Research Council (NHMRC) of Australia project grant (1079893) to A.J.L. and A.L.G., both of whom are NHMRC (Australia) Research Fellows (A.J.L. 1116930 and A.L.G. 1106330). L.C.W. is supported by an Australian Postgraduate Scholarship, and H.E.K. was supported by a Melbourne International Research Scholarship. We acknowledge the Victorian State Government Infrastructure Program.
Walker, L. C. , Kastman, H. E. , Krstew, E. V. , Gundlach, A. L. , and Lawrence, A. J. (2017) Central amygdala relaxin‐3/relaxin family peptide receptor 3 signalling modulates alcohol seeking in rats. British Journal of Pharmacology, 174: 3359–3369. doi: 10.1111/bph.13955.
References
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al (2015b). The Concise Guide to PHARMACOLOGY 2015/2016: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E et al (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrant AE, Schramm‐Sapyta NL, Kuhn CM (2013). Use of the light/dark test for anxiety in adult and adolescent male rats. Behav Brain Res 256: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin HA, Johnston AL, File SE (1989). Antagonistic effects of caffeine and yohimbine in animal tests of anxiety. Eur J Pharmacol 159: 211–215. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Shen PJ, Ma S, Bathgate RA, Gundlach AL (2010). Swim stress excitation of nucleus incertus and rapid induction of relaxin‐3 expression via CRF1 activation. Neuropharmacology 58: 145–155. [DOI] [PubMed] [Google Scholar]
- Bathgate RA, Samuel CS, Burazin TC, Layfield S, Claasz AA, Reytomas IG et al (2002). Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene. Novel members of the relaxin peptide family. J Biol Chem 277: 1148–1157. [DOI] [PubMed] [Google Scholar]
- Cai L, Bakalli H, Rinaman L (2012). Yohimbine anxiogenesis in the elevated plus maze is disrupted by bilaterally disconnecting the bed nucleus of the stria terminalis from the central nucleus of the amygdala. Neuroscience 223: 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Fiscella KA, Bacharach SZ, Tanda G, Shaham Y, Calu DJ (2015). Effect of yohimbine on reinstatement of operant responding in rats is dependent on cue contingency but not food reward history. Addict Biol 20: 690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander S, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejong W (1994). Relapse prevention: an emerging technology for promoting long‐term drug abstinence. Int J Addict 29: 681–705. [DOI] [PubMed] [Google Scholar]
- Freedman L, Cassell M (1994). Distribution of dopaminergic fibers in the central division of the extended amygdala of the rat. Brain Res 633: 243–252. [DOI] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF (2006a). Corticotropin‐releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self‐administration in withdrawn, ethanol‐dependent rats. J Neurosci 26: 11324–11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Li Z, Le AD (2006b). Effects of environmental and pharmacological stressors on c‐fos and corticotropin‐releasing factor mRNA in rat brain: relationship to the reinstatement of alcohol seeking. Neuroscience 138: 235–243. [DOI] [PubMed] [Google Scholar]
- Funk D, Coen K, Tamadon S, Li Z, Loughlin A, Le AD (2016). Effects of prazosin and doxazosin on yohimbine‐induced reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 233: 2197–2207. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M (2015). The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry 77: 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Swanson LW, Canteras NS (2001). Connections of the nucleus incertus. J Comp Neurol 438: 86–122. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Koob GF (1995). GABAA receptor antagonism in the extended amygdala decreases ethanol self‐administration in rats. Eur J Pharmacol 283: 151–159. [DOI] [PubMed] [Google Scholar]
- Jupp B, Lawrence AJ (2010). New horizons for therapeutics in drug and alcohol abuse. Pharmacol Ther 125: 138–168. [DOI] [PubMed] [Google Scholar]
- Kastman HE, Blasiak A, Walker L, Siwiec M, Krstew EV, Gundlach AL et al (2016). Nucleus incertus Orexin2 receptors mediate alcohol seeking in rats. Neuropharmacology 110: 82–91. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2009a). Brain stress systems in the amygdala and addiction. Brain Res 1293: 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2009b). Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 56 (Suppl 1): 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2013). Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry 4: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2005). Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci 8: 1442–1444. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Ling NC, DeSouza EB, Kuhar MJ (1997). Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J Neuroendocrinol 9: 823–833. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Cooney N, Kranzler HR, Charney DS (1994). Specificity of ethanol like effects elicited by serotonergic and noradrenergic mechanisms. Arch Gen Psychiatry 51: 898–911. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y (2000). The role of corticotrophin‐releasing factor in stress‐induced relapse to alcohol‐seeking behavior in rats. Psychopharmacology (Berl) 150: 317–324. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Coen K, Li Z, Shaham Y (2013). Role of corticotropin‐releasing factor in the median raphe nucleus in yohimbine‐induced reinstatement of alcohol seeking in rats. Addict Biol 18: 448–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Gundlach AL (2015). Ascending control of arousal and motivation: role of nucleus incertus and its peptide neuromodulators in behavioural responses to stress. J Neuroendocrinol 27: 457–467. [DOI] [PubMed] [Google Scholar]
- Ma S, Bonaventure P, Ferraro T, Shen PJ, Burazin TC, Bathgate RA et al (2007). Relaxin‐3 in GABA projection neurons of nucleus incertus suggests widespread influence on forebrain circuits via G‐protein‐coupled receptor‐135 in the rat. Neuroscience 144: 165–190. [DOI] [PubMed] [Google Scholar]
- Ma S, Blasiak A, Olucha‐Bordonau FE, Verberne AJ, Gundlach AL (2013). Heterogeneous responses of nucleus incertus neurons to corticotrophin‐releasing factor and coherent activity with hippocampal theta rhythm in the rat. J Physiol 591: 3981–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Densmore VS, Osborne PB (2007). Coexpression of prodynorphin and corticotrophin‐releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division. J Comp Neurol 504: 702–715. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y et al (2007). The CRF1 receptor antagonist antalarmin attenuates yohimbine‐induced increases in operant alcohol self‐administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 195: 345–355. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Krystal JH, Price LH, Heninger GR, Charney DS (1995). Noradrenergic response to acute ethanol administration in healthy subjects: comparison with intravenous yohimbine. Psychopharmacology (Berl) 118: 127–135. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olucha‐Bordonau FE, Otero‐Garcia M, Sanchez‐Perez AM, Nunez A, Ma S, Gundlach AL (2012). Distribution and targets of the relaxin‐3 innervation of the septal area in the rat. J Comp Neurol 520: 1903–1939. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1986). The rat brain in stereotaxic coordinates. Academic: London. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M (1985). Validation of open:closed arm entries in an elevated plus‐maze as a measure of anxiety in the rat. J Neurosci Methods 14: 149–167. [DOI] [PubMed] [Google Scholar]
- Pellow S, Johnston AL, File SE (1987). Selective agonists and antagonists for 5‐hydroxytryptamine receptor subtypes, and interactions with yohimbine and FG 7142 using the elevated plus‐maze test in the rat. J Pharm Pharmacol 39: 917–928. [DOI] [PubMed] [Google Scholar]
- Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A et al (2015). A transgenic rat for investigating the anatomy and function of corticotrophin releasing factor circuits. Front Neurosci 9: 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A et al (2008). Inhibition of orexin‐1/hypocretin‐1 receptors inhibits yohimbine‐induced reinstatement of ethanol and sucrose seeking in Long‐Evans rats. Psychopharmacology (Berl) 199: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR (2004). Increased GABA release in the central amygdala of ethanol‐dependent rats. J Neurosci 24: 10159–10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M et al (2010). Corticotropin releasing factor‐induced amygdala gamma‐aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry 67: 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, Siggins GR (2012). The central amygdala and alcohol: role of gamma‐aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb Perspect Med 2: a012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J, Sareen J, Cox BJ, Bolton J (2009). Self‐medication of anxiety disorders with alcohol and drugs: results from a nationally representative sample. J Anxiety Disord 23: 38–45. [DOI] [PubMed] [Google Scholar]
- Ryan PJ, Kastman HE, Krstew EV, Rosengren KJ, Hossain MA, Churilov L et al (2013). Relaxin‐3/RXFP3 system regulates alcohol‐seeking. Proc Natl Acad Sci U S A 110: 20789–20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos FN, Pereira CW, Sanchez‐Perez AM, Otero‐Garcia M, Ma S, Gundlach AL et al (2016). Comparative distribution of relaxin‐3 inputs and calcium‐binding protein‐positive neurons in rat amygdala. Front Neuroanat 10: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Vale WW (1984). Coticotrophin releasing factor: Co‐expression within distinct subsets of oxytocin‐, vassopressin‐ and neurotensin‐ immunoreactive neurons in the hypothalamus of the male rat. J Neurosci 4: 1118–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Nelson BS, Damadzic R, Tapocik JD, Yao M, King CE et al (2015). Neurokinin‐1 receptor antagonism attenuates neuronal activity triggered by stress‐induced reinstatement of alcohol seeking. Neuropharmacology 99: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T (2003). Induction of c‐Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry 53: 275–283. [DOI] [PubMed] [Google Scholar]
- Sinha R (2008). Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141: 105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Yoshida M, Emoto H, Ishii H (2000). Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol 405: 397–406. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Iijima N, Miyamoto Y, Fukusumi S, Itoh Y, Ozawa H et al (2005). Neurons expressing relaxin 3/INSL 7 in the nucleus incertus respond to stress. Eur J Neurosci 21: 1659–1670. [DOI] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Luthi A (2015). Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16: 317–331. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT et al (2011). Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology 36: 1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND (2004). The reality of comorbidity: depression and drug abuse. Biol Psychiatry 56: 714–717. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Anderson GM, Owens MJ, Halaszynski TM, Bremner JD, Carpenter LL et al (2000). Cerebrospinal fluid corticotropin‐releasing hormone in healthy humans: effects of yohimbine and naloxone. J Clin Endocrinol Metab 85: 4138–4145. [DOI] [PubMed] [Google Scholar]
- Walker BM, Valdez GR, McLaughlin JP, Bakalkin G (2012). Targeting dynorphin/kappa opioid receptor systems to treat alcohol abuse and dependence. Alcohol 46: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Lawrence AJ (2017). CRF and the nucleus incertus: a node for integration of stress signals. Nat Rev Neurosci 18: 158. [DOI] [PubMed] [Google Scholar]
- Walker LC, Kastman HE, Koeleman JA, Smith CM, Perry CJ, Krstew EV et al (2016). Nucleus incertus corticotrophin‐releasing factor 1 receptor signalling regulates alcohol seeking in rats. Addict Biol . https://doi.org/10.1111/adb.12426. [DOI] [PubMed] [Google Scholar]
- World Health Organisation (WHO) (2014). Global status report on alcohol and health.
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin‐Fardon R, Weiss F (2006). Activation of group II metabotropic glutamate receptors attenuates both stress and cue‐induced ethanol‐seeking and modulates c‐fos expression in the hippocampus and amygdala. J Neurosci 26: 9967–9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Kreek MJ (2014). Alcohol: a stimulant activating brain stress responsive systems with persistent neuroadaptation. Neuropharmacology 87: 51–58. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F (2001). Changes in levels of regional CRF‐like‐immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 158: 374–381. [DOI] [PubMed] [Google Scholar]


