Abstract
Background and Purpose
Visceral hypersensitivity is responsible for pathogenesis of irritable bowel syndrome (IBS). Therefore, its prevention can help avoid abdominal pain and discomfort in IBS. To find candidate drugs for visceral hypersensitivity, we screened existing medicines for their ability to prevent visceral sensitivity induced by colorectal distension (CRD) in rats and identified chlorpromazine, a typical antipsychotic drug, as a candidate compound. In this study, we investigated the effect of chlorpromazine on visceral hypersensitivity.
Experimental Approach
Visceral sensitivity (visceromotor response) was monitored by measuring the electrical activity of the abdominal external oblique muscle contraction in response to CRD using a barostat apparatus. Visceral hypersensitivity was induced by a colonic instillation of sodium butyrate or acetic acid in neonates.
Key Results
Oral administration of chlorpromazine suppressed butyrate‐induced visceral hypersensitivity to CRD. Interestingly, atypical antipsychotic drugs, quetiapine and risperidone, ameliorated butyrate‐induced visceral hypersensitivity, whereas the typical antipsychotic drugs, haloperidol and sulpiride, did not. Pharmacological analysis using specific inhibitors showed that a selective 5‐HT2A receptor antagonist, ketanserin, suppressed butyrate‐induced visceral hypersensitivity, whereas a selective dopamine D2 receptor antagonist, L‐741626, did not. Furthermore, the 5‐HT2A receptor agonist AL‐34662 stimulated visceral sensitivity to CRD in healthy control rats but not in butyrate‐treated rats. These findings suggest that increased 5‐HT levels in the colon contribute to the induction of visceral hypersensitivity.
Conclusions and Implications
Our results indicate that chlorpromazine ameliorates visceral hypersensitivity and that the 5‐HT2A receptor is a potential therapeutic target for treating abdominal pain and discomfort in IBS.
Abbreviations
- 7OH‐Cpz
7‐hydroxy chlorpromazine
- ASIC
acid‐sensing ion channel
- CRD
colorectal distension
- DRG
dorsal root ganglion
- IBS
irritable bowel syndrome
- p‐CPA
p‐chlorphenylalanine
- TRPV1
transient receptor potential vanilloid 1
- VMR
visceromotor response
Introduction
Irritable bowel syndrome (IBS) is a complex functional gastrointestinal disorder characterized by chronic, recurrent abdominal pain and altered bowel habits (diarrhoea, constipation or alternating diarrhoea/constipation). Its prevalence in the general population is remarkably high (~11% of the world's population) (Chang et al., 2014). Although not life‐threatening, it imposes a large burden on global healthcare and considerably reduces patient quality of life (Canavan et al., 2014). However, no satisfactory pharmacological therapy has been identified for IBS.
Psychiatric comorbidities are highly common in IBS patients (Ladabaum et al., 2012). Centrally acting agents including antidepressants, anxiolytics, antipsychotics and sedatives are two to four times more likely to be prescribed to presumed IBS patients compared to other patients (Grover and Drossman, 2011). Atypical antipsychotic drugs have been effective in patients with severe IBS and abdominal pain (Grover et al., 2009; Martin‐Blanco et al., 2010; Pae et al., 2013). However, the mechanisms underlying the efficacy of these drugs in ameliorating pain are unclear.
Visceral hypersensitivity plays an important role in IBS pathogenesis, and IBS patients have been shown to present visceral hypersensitivity in response to colorectal distension (CRD) (Azpiroz et al., 2007). Therefore, suppression of this pathology could be an effective treatment option for IBS. The mechanisms responsible for visceral hypersensitivity are not completely understood. However, several contributing factors have been suggested, including enhanced visceral perception, altered intestinal microbiota, post‐inflammatory changes in gastrointestinal function and enhanced immunological reactivity (Keszthelyi et al., 2012; Matricon et al., 2012; Simren et al., 2013; Brierley and Linden, 2014). In particular, butyrate, a short‐chain fatty acid produced by bacterial fermentation in the colon, appears to be important in the development of visceral hypersensitivity. Increased faecal butyrate levels have been observed in IBS patients with diarrhoea, and rectal instillation of butyrate increases visceral sensitivity to colonic distension in rats (Treem et al., 1996; Bourdu et al., 2005). 5‐HT (serotonin) is another key molecule regulating visceral perception (Cremon et al., 2011). In fact, a 5‐HT3 receptor antagonist (alosetron) and 5‐HT4 receptor agonist (tegaserod) have been approved for the clinical treatment of diarrhoea‐ and constipation‐predominant IBS respectively. However, adverse effects, such as ischaemic colitis and cardiac toxicity, limit the use of these drugs (Pasricha, 2007). The role of other 5‐HT receptor subtypes, including 5‐HT2Areceptor, in visceral sensitivity, has not been fully investigated.
The 5‐HT2A receptor is mainly located in the brain, spinal cord, sensory neurons and intestines. Recently, the 5‐HT2A receptor antagonist cyproheptadine has been found effective against abdominal pain in children with IBS (Madani et al., 2016). Moreover, the 5‐HT2A receptor antagonist ketanserin showed an antinociceptive effect on abdominal pain in the acetic acid‐induced writhing test (Alhaider, 1991). These findings indicate that the 5‐HT2A receptor is involved in visceral pain sensitivity. In addition to visceral pain, the 5‐HT2A receptor is associated with the potentiation of pain sensation. Further, activation of the 5‐HT2A receptor enhances transient receptor potential vanilloid 1 (TRPV1) function in colon sensory neurons and acid‐sensing ion channels (ASICs) in dorsal root ganglion (DRG) neurons in vitro (Sugiuar et al., 2004; Qiu et al., 2012). These results suggest that the 5‐HT2A receptor participates in peripheral sensitization.
We previously screened a compound library of clinically available drugs for their ability to prevent the visceral pain response to noxious CRD (Asano et al., 2017) and identified aminophylline, a bronchodilator, as a potential candidate. As chlorpromazine strongly suppressed visceral sensitivity to noxious CRD in the same phenotypic screen, it was also considered a candidate drug for abdominal pain. Chlorpromazine is traditionally used for treating schizophrenia, nausea and vomiting and severe hiccups (Adams et al., 2005) and has antagonistic activity at various receptors, including the dopamine D2receptor and 5‐HT2A receptors (Horacek et al., 2006). Chlorpromazine's antagonistic activity against the dopamine D2 and 5‐HT2A receptors is involved in its efficacy in regulating positive or negative symptoms and cognitive symptoms in schizophrenia respectively (Horacek et al., 2006).
In this study, we investigated the effect of chlorpromazine on visceral hypersensitivity to CRD. Our results suggest that 5‐HT2A receptor activation and increased 5‐HT levels in the colon are involved in visceral hypersensitivity.
Methods
Animals
Male Wistar rats (4 to 5 weeks old, 150–200 g) or primiparaous late pregnant Wistar female rats were obtained from Charles River Laboratories Japan (Yokohama, Japan). Animals were housed under conditions of controlled temperature (22–24°C) and light (12 h light: 12 h dark cycle) for 1–2 weeks before experiments. The standard pellet diets (Rodent Diet CE‐2, CLEA Japan, Inc.) were fed to the experimental animals ad libitum. The experiments and procedures described here were performed in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health (NIH, USA) and were approved by the Animal Care Committees of Keio University and St. Marianna University. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Assessment of visceromotor response (VMR) to CRD
VMR to CRD was monitored as described previously (Asano et al., 2017). Briefly, rats were deeply anaesthetized with an intraperitoneal injection of mixture of medetomidine chloride (0.5 mg·kg−1), midazolam (2.5 mg·kg−1) and butorphanol tartrate (2.5 mg·kg−1). We ensured adequate depth of anesthesia by testing pedal withdrawal and palpebral reflexes. The skin was incised (1 cm‐long incision), and the external oblique muscle of the abdomen was exposed. Electromyography electrodes (Starmedical, Tokyo, Japan) were sutured into the external oblique muscle to record the EMG. Electrode leads were tunnelled s.c. and exteriorized at the nape of the neck for future access. Incised skin was closed with nylon sutures, and the rats were given butorphanol tartrate (2.5 mg·kg−1) and viccillin (30 mg·kg−1) s.c. After surgery, rats were housed individually and allowed to recuperate for 5 to 7 days before being used in the CRD study. During the recovery period, several rats broke the EMG electrodes and were excluded from VMR assessment. On the day of the CRD test, rats were restrained in a plastic conical tube cage (diameter, 6 cm; height, 15 cm), 15 min before electromyography. Rats were not habituated to the tube prior to experiments. A polyethylene barostat balloon (length, 2 cm; maximum diameter, 1.5 cm; volume, 2–3 mL) was inserted in the colon, positioned 1 cm proximal to the rectum and connected to a balloon catheter, which was anchored with tape to the base of the tail. Pressure and volume of the balloon were controlled and monitored by a barostat device (Distender Series II; G & J Electronics, Toronto, Canada) connected to it. Conscious rats were subjected to graded phasic CRD (10, 20, 40, 60 and 80 mmHg; each distension in triplicate; duration, 20 s; interstimulus interval, 150 s) to estimate drug activity.
EMG data were collected and analysed using the 8 STAR software package (Star Medical, Tokyo, Japan). EMG amplitude (mV·s) resulting from contraction of the external oblique muscle was quantified by calculating the AUC of the voltage alteration graph during CRD. EMG amplitude data were corrected by subtracting the baseline EMG data, which were collected 20 s before each CRD. The AUC (mmHg·mV·s) of EMG amplitude (mV·s) – balloon pressure (mmHg) curve – was also determined.
Colonic compliance (pressure–volume curves) was monitored during phasic CRD in control rats and presented as change in cylinder volume and balloon pressure.
Chlorpromazine, sulpiride, haloperidol, risperidone or quetiapine were administered p.o. 2 h before the CRD test. Chlorpromazine was dissolved in saline, whereas other drugs were suspended in 1% methylcellulose. Ketanserin, L‐741,626, AL‐34662 and 7OH‐Cpz were also suspended in 1% methylcellulose but were administered i.p. 15 min before the CRD study. In the 5‐HT depletion experiment, rats were administered p‐chlorphenylalanine (p‐CPA; 300 mg·kg−1, p.o.) with 0.1% Tween‐80 or vehicle 30 min before butyrate application, once daily for 3 days.
Sodium butyrate‐ or acetic acid‐induced visceral hypersensitivity to CRD
A butyrate enema was performed using a previously described method with some modifications (Asano et al., 2012). Briefly, rats were instilled with 1 mL sodium butyrate solution (110 mg·mL−1, pH 6.9) or saline into the colon twice daily for 3 days (day 1 to 3). On day 4, rats were subjected to CRD experiments.
Acetic acid‐induced colonic hypersensitivity was induced as described previously (Winston et al., 2007). Primiparous late pregnant Wistar female rats were individually housed for about a week prior to giving birth (10–15 pups per rat). The 10‐day‐old rat pups were subjected to intracolonic injection of 0.2 mL acetic acid (0.5% in saline) 2 cm from the anus; control rats received an equal volume of saline. At 5–6 weeks of age, VMR to CRD was measured in both groups of male rats.
Western blot analysis
Rats were killed by decapitation, and tissue samples from the sigmoid colon and L5‐S1 dorsal root ganglia were collected, frozen with liquid nitrogen and stored at −80°C until Western blot analysis. Tissue samples were homogenized in RIPA buffer [50 mM Tris–HCl (pH 7.2), 150 mM NaCl, 1% sodium deoxycholate, 1% SDS, 1% NP‐40] containing protease inhibitor cocktail (Sigma, St. Louis, MO, USA) and phosphatase inhibitor cocktail (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Samples were centrifuged at 15 000 × g at 4°C, and the supernatants were collected. Total protein concentration of the sample was determined using the Bradford method. Samples were subjected to electrophoresis on 10% polyacrylamide SDS gel (Thermo Fisher Scientific, Rockford, IL, USA), after which the separated proteins were transferred to PVDF membranes. Membranes were blocked for 30 min with Tris phosphate buffer containing 0.1% Tween‐80 (T‐TBS) and 5% skimmed milk and incubated overnight at 4°C with T‐TBS containing the appropriate primary antibodies. Antibodies, including mouse monoclonal anti‐5‐HT2A receptor (5‐HT2A R) antibody (MABN1595, Merck Millipore, Darmstadt, Germany), rabbit polyclonal anti‐phosphorylated ERK1/2 antibody (#9101, Cell Signalling Technology), rabbit polyclonal anti‐ERK1/2 antibody (#9102, Cell Signalling Technology) and mouse monoclonal anti‐β‐actin antibody (Sigma, St. Louis, MO, USA) were used at 1:2000, 1:2000, 1:2000 and 1:1000 dilutions respectively. The membranes were washed three times with T‐TBS, incubated for 60 min at room temperature with horseradish peroxidase‐conjugated secondary antibodies in T‐TBS containing 2% skimmed milk and washed three times with T‐TBS. Signal was developed in ECL™ Prime solution (GE Healthcare) and imaged using ImageQuant™ LAS4000 image analyser (GE Healthcare). Intensity of immunoreactive bands was quantified using Image J software (NIH) and normalized to β‐actin levels. Data are expressed as the fold change over saline groups.
Measurement of 5‐HT levels in colonic tissues
After the 3 day butyrate treatment, the rats were killed and colon tissues were collected to determine tissue 5‐HT levels using an enzyme immunoassay kit (Beckman Coulter, Inc., Fullerton, CA, USA), according to the manufacturer's instructions.
Statistical analyses
Data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). In all animal studies, a co‐worker blinded to the experimental protocol randomized animals into groups. The values presented were derived from at least two independent experiments. All data are expressed as mean ± SEM values. Normalization was performed for the quantitative analysis of Western blots. Statistical analyses were performed using SPSS 24.0 software (IBM, Armonk, NY, USA). One or two‐way ANOVA followed by Dunnett's test or Student's t‐test for unpaired results was used to evaluate differences between more than two groups or between two groups respectively. Two‐way ANOVA with repeated measures followed by Bonferroni's method was used for the phasic CRD test. Differences were considered significant at P < 0.05.
Chemicals
7‐Hydroxy chlorpromazine (7OH‐Cpz) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Methylcellulose, sodium butyrate, haloperidol and quetiapine were obtained from Wako Pure Chemical Industries (Osaka, Japan). Medetomidine chloride (Domitor®) and butorphanol tartrate (Vetorphale®) were purchased from Meiji Seika Pharma Co., Ltd. (Tokyo, Japan). Midazolam was purchased from SANDOZ (Tokyo, Japan). 5‐HT, p‐chlorphenylalanine, sulpiride and chlorpromazine hydrochloride (chlorpromazine) were purchased from Sigma (St. Louis, MO, USA). Risperidone (Ris) was obtained from LKT laboratories, Inc. (St Paul, MN, USA). AL‐34662 was obtained from Cayman Chemical Company (Ann Arbor, MI, USA). L‐741,626 and ketanserin tartrate (ketanserin) were obtained from Abcam (Cambridge, UK).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org/, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b).
Results
Effect of chlorpromazine on butyrate‐induced visceral hypersensitivity in rats
Effect of oral administration of chlorpromazine on butyrate‐induced visceral hypersensitivity, which is a model of IBS, was investigated in rats. Rats subjected to butyrate treatment showed increase in VMR to CRD compared with that in saline‐treated control rats (Figure 1A, B). Oral pre‐administration of chlorpromazine significantly decreased this response in a dose–response manner (Figure 1A, B). Interestingly, chlorpromazine did not affect visceral sensitivity in healthy control rats except at a high dose of 40 mg·kg−1 (Figure 1C) nor was colonic compliance affected (Figure 1D). In a preliminary experiment, we observed that 12 mg·kg−1 chlorpromazine significantly induced a cataleptic response in healthy rats (an adverse effect). However, there was no significant difference between inhibitory actions of 4 and 12 mg·kg−1 chlorpromazine in Figure 1A, B. Thus, we decided to use 4 mg·kg−1 chlorpromazine for subsequent experiments. We also assessed the effect of chlorpromazine on visceral hypersensitivity induced by colonic instillation of dilute acetic acid to neonatal rats, another model of IBS. As shown in Figure 1E, chlorpromazine restored visceral sensitivity stimulated by acetic acid to the levels observed in control rats.
Figure 1.
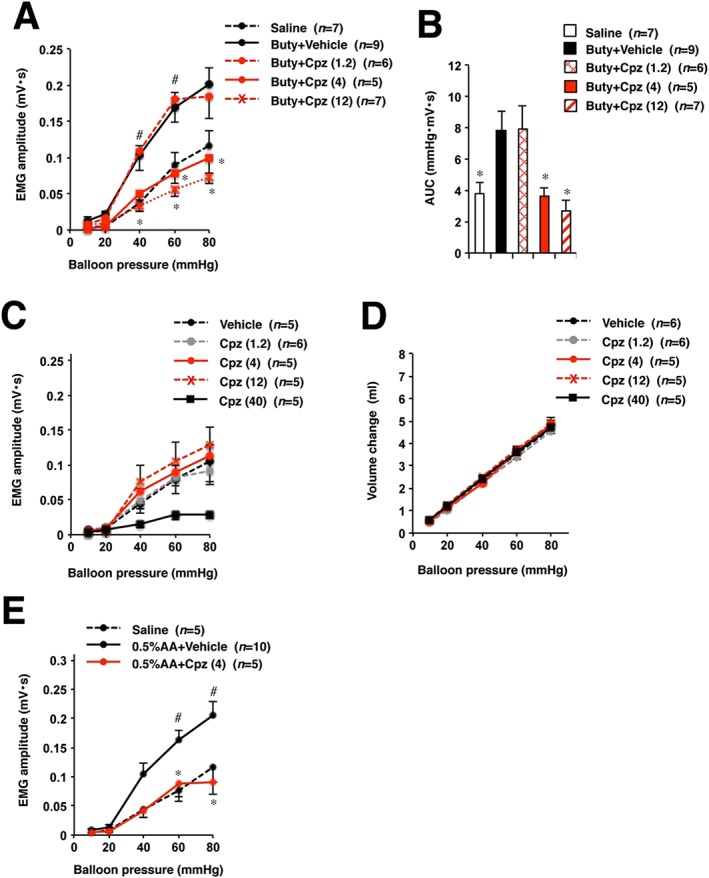
Effects of chlorpromazine on visceral sensitivity to CRD in rats without or with butyrate or acetic acid treatment. Rats were administered saline, sodium butyrate (Buty) or 0.5% acetic acid (0.5% AA) in neonates (A, B, E). Rats were orally administered the indicated doses of chlorpromazine (Cpz) (mg·kg−1) or vehicle (saline), then two hours later, the CRD study was performed (A–E). (A) Visceral sensitivity to CRD (EMG amplitude) was determined. (B) Collective AUC data from (A). (C, D) Visceral sensitivity to CRD and colonic compliance in healthy control rats were examined. Values are mean ± SEM; * or # P < 0.05 (*, vs. vehicle; #, vs. saline).
The effect of antipsychotics other than chlorpromazine on visceral hypersensitivity to CRD was also evaluated. Typical antipsychotic drugs sulpiride and haloperidol and atypical antipsychotics quetiapine and risperidone were analysed. In addition to inhibiting the dopamine D2 receptor, quetiapine and risperidone are 5‐HT2A receptor antagonists. According to a previous report, sulpiride, haloperidol, quetiapine and risperidone show <500, 20, 0.13 and 0.33 5‐HT2A/D2 K i ratios respectively (Horacek et al., 2006). We examined whether the inhibition of 5‐HT2A receptor by antipsychotic drugs is associated with the suppression of visceral hypersensitivity. As shown in Figure 2A, B, quetiapine and risperidone suppressed butyrate‐induced visceral hypersensitivity to CRD, whereas sulpiride and haloperidol did not.
Figure 2.
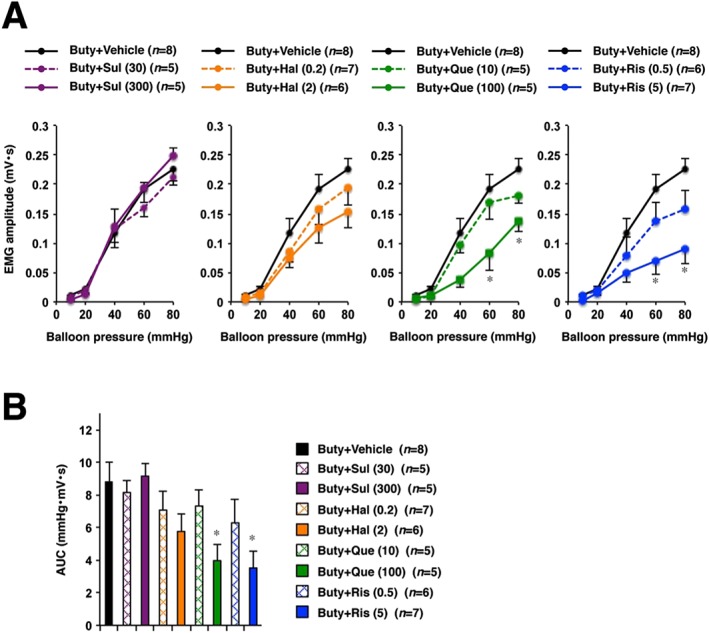
Effects of antipsychotic drugs on butyrate‐induced visceral hypersensitivity to CRD in rats. The indicated doses (mg·kg−1) of sulpiride (Sul), haloperidol (Hal), quetiapine (Que), risperidone (Ris) or vehicle (1% methylcellulose) were administered p.o. to butyrate (Buty)‐treated rats. Two hours later, the CRD study was performed (A, B). (B) shows the collective AUC data from (A). Values are mean ± SEM; * P < 0.05 vs. vehicle. Each graph of Buty + Vehicle group in four panels represents the same data as in (A).
Role of 5‐HT2A receptor in visceral hypersensitivity
Next, it was investigated whether 5‐HT2A or dopamine D2 receptor antagonists could inhibit sodium butyrate‐induced increase in visceral sensitivity. Results showed that a selective 5‐HT2A receptor antagonist, ketanserin, ameliorated butyrate‐induced visceral hypersensitivity to CRD, whereas a selective dopamine D2 receptor antagonist, L‐741,626, did not. To confirm these findings, the effect of 7OH‐Cpz, which does not bind to 5‐HT2A receptors, was also assessed. 7OH‐Cpz (4.2 mg·kg−1) was administered p.o. to rats at a dose equivalent to that of chlorpromazine. 7OH‐Cpz did not suppress butyrate‐induced visceral hypersensitivity to CRD (Figure 3A, B).
Figure 3.
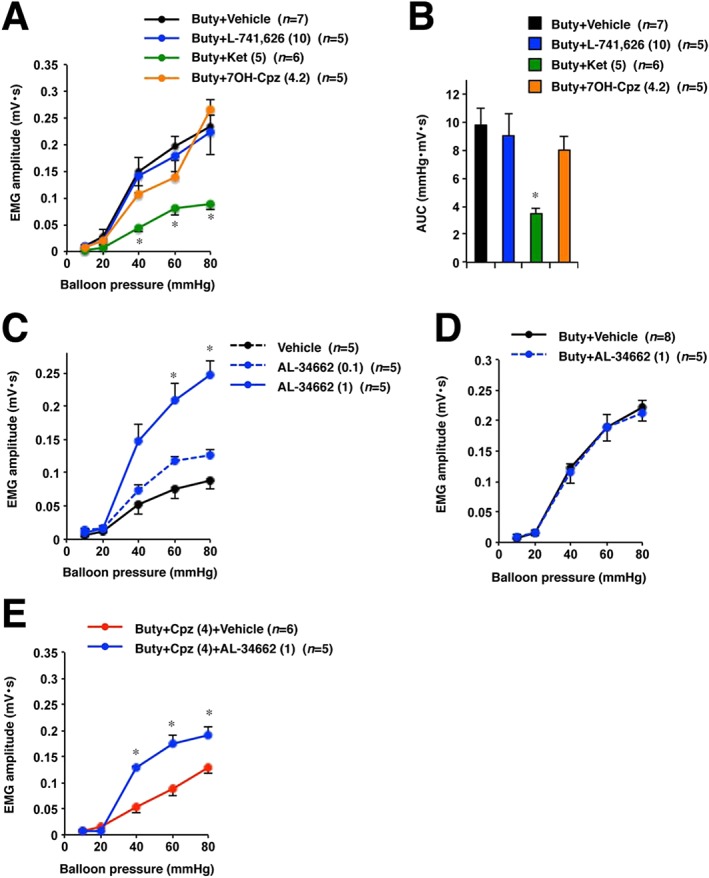
Pharmacological study of role of the 5‐HT2A receptor in visceral sensitivity to CRD. Rats treated with sodium butyrate (Buty) for 3 days were administered, i.p., the indicated doses (mg·kg−1) of L‐741626 (a selective dopamine D2 receptor antagonist) (A, B), ketanserin (Ket) (a selective 5‐HT2A receptor antagonist) (A, B), AL‐34662 (a selective 5‐HT2A receptor agonist) (D, E) or vehicle (1% methylcellulose), 15 min before the CRD study. The indicated doses (mg·kg−1) of chlorpromazine (Cpz) (E) or 7OH‐Cpz (A, B) were administered, p.o., to butyrate‐treated rats, 2 h before the CRD test. (C) The indicated doses of AL‐34662 (mg·kg−1) or vehicle (1% methylcellulose) were administered, i.p., to healthy control rats. Fifteen minutes later, the CRD study was performed. (B) Collective AUC data from (A). Values are mean ± SEM; * P < 0.05, vs. vehicle.
Moreover, we investigated whether a 5‐HT2A receptor agonist stimulated visceral sensitivity to CRD in healthy rats to study the involvement of 5‐HT2A receptor activation in the stimulation of visceral sensitivity. AL‐34662, a selective 5‐HT2A receptor agonist, stimulated visceral sensitivity to CRD in control rats (Figure 3C). Additionally, we investigated the effect of AL‐34662 on visceral sensitivity in rats treated with butyrate to determine the sensitivity of 5‐HT2A receptor in butyrate‐treated rats. AL‐34662 did not affect the VMR to CRD in butyrate‐treated rats (Figure 3D) and significantly restored butyrate‐induced visceral hypersensitivity to CRD in the presence of chlorpromazine (Figure 3E).
Mechanisms underlying the inhibitory effect of chlorpromazine on visceral hypersensitivity
Activation of ERK1/2 in DRG neurons plays an important role in butyrate‐induced colonic hypersensitivity to CRD (Xu et al., 2013). We examined the effect of chlorpromazine on the expression of phospho‐ERK1/2 in DRG neurons after butyrate treatment. As shown in Figure 4A, B, administration of sodium butyrate increased the expression of phospho‐ERK1/2, whereas oral pre‐administration of chlorpromazine inhibited this effect. To determine whether 5‐HT2A receptor antagonism is involved in the attenuation of ERK1/2 activation, we examined the effects of ketanserin and L‐741626 on the expression of phospho‐ERK1/2 in butyrate‐treated rats. Application of ketanserin but not L‐741626 to butyrate‐treated rats decreased phospho‐ERK1/2 expression in DRG neurons (Figure 4C, D).
Figure 4.
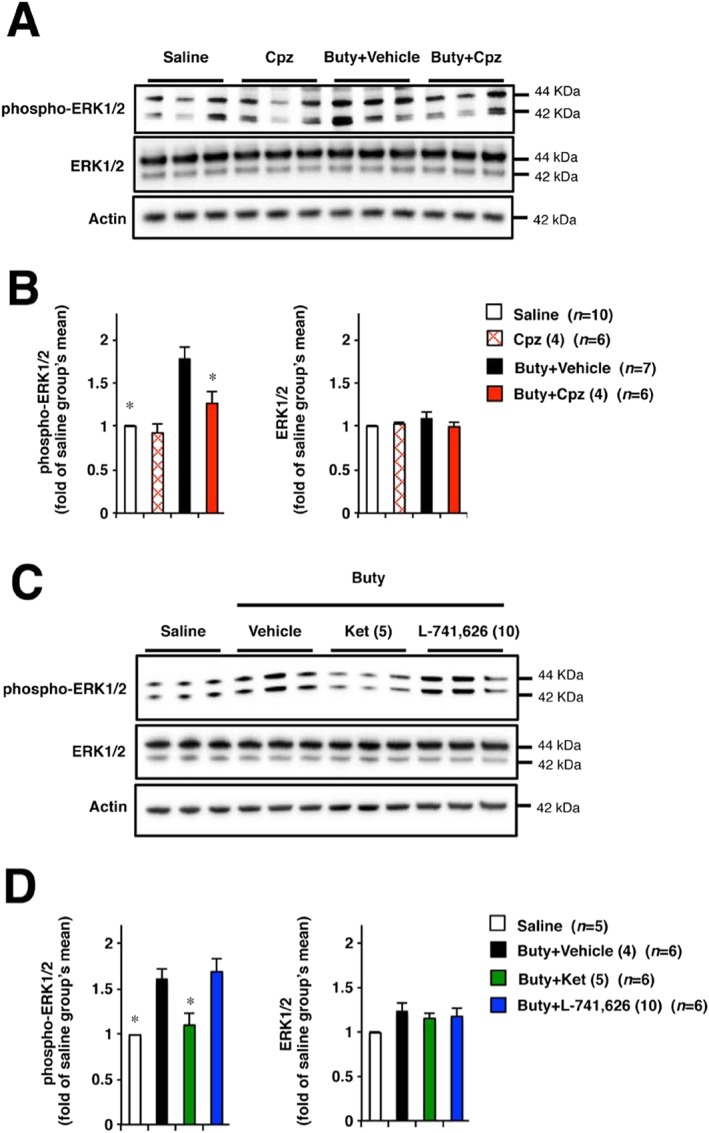
Effect of chlorpromazine, ketanserin or L‐741626 on the butyrate‐induced activation of ERK1/2 in DRG neurons. Rats were intracolonically administered sodium butyrate or saline twice daily for 3 days (A–D). On the next day after the last administration, chlorpromazine (4 mg·kg−1) or vehicle (saline) was administered p.o. to these rats (A, B). Rats treated with butyrate were given ketanserin (5 mg·kg−1), L‐741626 (10 mg·kg−1) or vehicle (1% methylcellulose) (C, D), i.p. Two hours (A, B) or 15 min (C, D) later, the L5‐S1 DRG were removed from rats and tissues were subjected to Western blot analysis. (A) and (C) show representative data of immunoblotting. (B) and (D) show the relative data of band intensity at (A) and (C) respectively. Values are mean ± SEM; * P < 0.05, vs. vehicle.
Moreover, expression of 5‐HT2A receptors in DRG neurons and colon was evaluated and shown not to change after butyrate treatment (Figure 5A–C). Next, we determined colonic 5‐HT levels. Butyrate treatment significantly increased 5‐HT levels in colonic tissues (Figure 5D). Subsequently, we investigated the effect of a 5‐HT synthesis inhibitor p‐CPA on butyrate‐induced hypersensitivity (Qin et al., 2010). After oral administration of p‐CPA for 3 days, colonic 5‐HT levels significantly decreased in butyrate‐treated rats (Figure 6A), whereas stimulated visceral sensitivity to CRD improved (Figure 6B).
Figure 5.
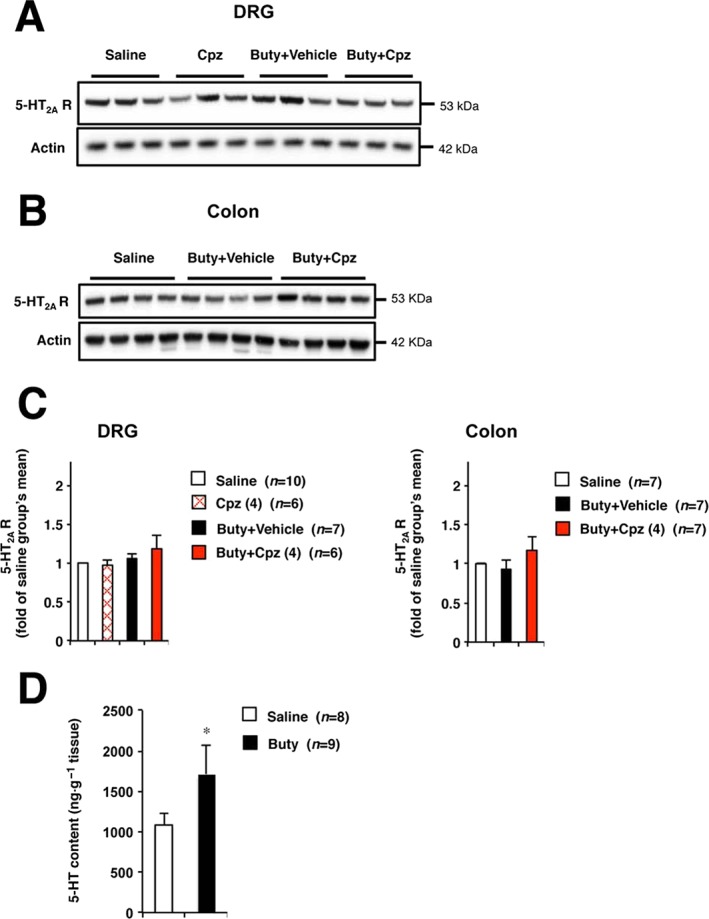
The expression of 5‐HT2A receptors in DRG neurons and colon, and colonic 5‐HT contents. Rats were administered sodium butyrate or saline via colonic instillation twice daily for 3 days (A–D). After the last administration, chlorpromazine (4 mg·kg−1) or vehicle (saline) was administered p.o. Two hours later, the L5‐S1 DRG or colon was removed from the rats and tissues were subjected to Western blot analysis (A–C) or 5‐HT elisa assay (D). (A) and (B) show representative data of immunoblotting. (C) The relative data of band intensity at (A) and (B). Values are mean ± SEM; * P < 0.05 vs. saline.
Figure 6.
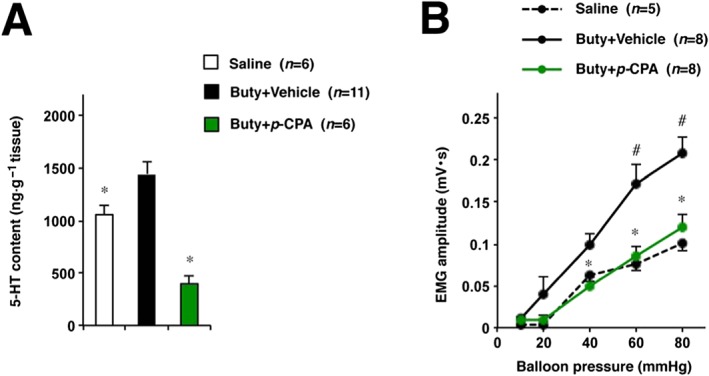
Effects of p‐CPA on butyrate‐induced visceral hypersensitivity to CRD in rats. Rats were administered 300 mg·kg−1 p‐CPA (a 5‐HT synthesis inhibitor) or vehicle (0.1% Tween‐80), p.o., once daily for 3 days. Thirty minutes after each treatment, sodium butyrate or saline was administered via colonic administration. The next day, after the last administration of butyrate, the CRD test was performed (A). Colonic tissues removed from rats were subjected to 5‐HT elisa assay (B). Values are mean ± SEM; * or # P < 0.05 (*, vs. vehicle; #, vs. saline).
Discussion
Various types of drugs including psychoactive agents are prescribed to IBS patients, and many target molecules for IBS drugs have been proposed. However, an appropriate pharmacological therapy has not yet been established. Furthermore, although some antipsychotic drugs are prescribed to treat pain symptoms in IBS, their pain amelioration mechanisms are unclear. Previously, in a phenotypic screening, we identified aminophylline as a drug that suppressed visceral sensitivity response to repeated CRD. Aminophylline suppressed stress‐induced visceral hypersensitivity by inhibiting adenosine A2 receptors (Asano et al., 2017). In the same screen, chlorpromazine showed similar effects. Chlorpromazine has been used clinically as an antipsychotic drug for a long time. However, the effects of chlorpromazine on IBS have not been investigated in any non‐clinical or clinical study. Herein, we demonstrated that chlorpromazine suppressed visceral hypersensitivity in an IBS animal model, by inhibiting 5‐HT2A receptor signalling.
Oral administration of chlorpromazine prevented butyrate‐ or acetic acid‐induced visceral hypersensitivity to CRD without affecting visceral sensitivity under normal conditions or affecting colonic compliance. This activity profile suggests that chlorpromazine could be beneficial for IBS patients. Furthermore, atypical antipsychotic drugs risperidone and quetiapine improved colonic hypersensitivity in rats. Whereas sulpiride and haloperidol, which did not show this effect in our analysis, are preferential dopamine D2 receptor antagonists, quetiapine and risperidone inhibit both 5‐HT2A receptor and dopamine D2 receptor. Therefore, 5‐HT2A receptor inhibition may be involved in the suppression of visceral hypersensitivity. Although previous studies have suggested the possibility of atypical antipsychotics as a treatment option for IBS (Pae et al., 2013), this is the first report in an animal model.
Pre‐administration of a subtype‐specific 5‐HT2A receptor antagonist significantly suppressed visceral sensitivity to CRD in rats subjected to butyrate treatment. In contrast, preferential dopamine D2 receptor inhibitor did not affect visceral hypersensitivity. This result does not conflict with previous reports suggesting that central activation of dopamine D2 receptor produces antinociceptive action against CRD (Nozu et al., 2016; Okumura et al., 2016). Our results indicate that chlorpromazine‐induced amelioration of visceral hypersensitivity could have been mediated by 5‐HT2A receptor antagonism. To confirm this, we examined 7OH‐Cpz, which does not bind to 5‐HT2A receptor (Suzuki et al., 2013), and AL‐34662, a subtype‐specific 5‐HT2A receptor agonist, in the presence of chlorpromazine. As 7OH‐Cpz does not inhibit 5‐HT2A receptor but does bind to D2 receptor (Suzuki et al., 2013), we anticipated that 7OH‐Cpz treatment would have no inhibitory effect on visceral hypersensitivity. As expected, 7OH‐Cpz did not suppress butyrate‐induced visceral hypersensitivity to CRD, indicating that 5‐HT2A receptor antagonism was involved in chlorpromazine‐induced inhibition of visceral hypersensitivity. Moreover, AL‐34662 induced visceral hypersensitivity in response to CRD in normal rats, but not in butyrate‐treated rats, suggesting that the activation of 5‐HT2A receptor might be the mechanism responsible for sodium butyrate‐induced visceral hypersensitivity to CRD. Lack of visceral sensitivity to CRD stimulation by AL‐34662 in butyrate‐treated rats may imply that the 5‐HT2A receptor was already fully occupied by 5‐HT produced due to butyrate treatment. Additionally, as AL‐34662 is unable to cross the blood–brain barrier (Sharif et al., 2007), 5‐HT2A receptors expressed in peripheral tissues (such as the colon) rather than those in the central nervous system appear to be involved in the inhibitory effects of chlorpromazine. However, our data do not exclude the possibility of a central mechanism of chlorpromazine, as 5‐HT2A receptors are expressed in the brain and spinal cord and chlorpromazine is a centrally acting agent. Furthermore, inhibition of spinal 5‐HT2A receptors was reported to decrease nociceptive responses to noxious stimuli (Rahman et al., 2011), and chlorpromazine was shown to elicit a spinal blockade of nociception in rats (Chen et al., 2012). Thus, further studies are required to determine whether the amelioration of colonic hypersensitivity by chlorpromazine originates from peripheral or central actions of this drug.
As demonstrated earlier, 5‐HT2A receptor antagonists have an analgesic effect on inflammatory pain (Nitanda et al., 2005; Cervantes‐Duran et al., 2016). In the present study, we demonstrated the inhibitory effect of a 5‐HT2A antagonist on the visceral pain response in non‐inflammatory models (Bourdu et al., 2005). Inflammatory pain models, such as neuropathic pain and formalin stimulation, were shown to increase the expression of 5‐HT2A receptors in DRG neurons (Okamoto et al., 2002; Cervantes‐Duran et al., 2016); however, colonic butyrate application did not alter the expression of 5‐HT2A receptors in this study. This may be because the pathophysiology of pain sensation differs between our models and previous inflammatory pain models.
In rat DRG neurons, butyrate‐induced colonic hypersensitivity is mediated by ERK1/2 activation (induction of ERK1/2 phosphorylation) (Xu et al., 2013). Prevention of ERK1/2 phosphorylation in DRG neurons by an MEK inhibitor attenuated the butyrate‐induced enhancement of DRG neuronal excitability and colonic hypersensitivity (Xu et al., 2013). However, mechanisms based on an overexpression of acid‐sensing ion channels (ASICs), the T‐type calcium channel (Cav3.2) or phosphorylated voltage‐gated potassium channel subunit 4.2 (pKv4.2) in colonic sensory neurons have also been proposed (Marger et al., 2011; Xu et al., 2013). These changes would result in the stimulation of neuronal firing, leading to colonic hypersensitivity. In this study, butyrate treatment increased the phosphorylation of ERK1/2, whereas chlorpromazine prevented this activation without altering 5‐HT2A receptor expression in DRG neurons. Moreover, ketanserin but not L‐741626 suppressed butyrate‐induced ERK1/2 activation, suggesting that 5‐HT2A receptors may be involved in ERK1/2 activation in DRG neurons. Additionally, chlorpromazine improved visceral hypersensitivity induced by intracolonic injection of dilute acetic acid in neonatal rats. Recent study using the acetic acid model has shown that increased expression of 5‐HT and 5‐HT2A receptors in colon and DRG neurons is involved in the induction of visceral hypersensitivity (Chen et al., 2016). Present study focused on the role of 5‐HT2A receptor and 5‐HT in butyrate‐induced visceral hypersensitivity, as these mechanisms have not been investigated previously in this model.
Expression of 5‐HT2A receptors in DRG neurons and colon did not change after butyrate instillation, in agreement with AL‐34662 not stimulating visceral pain response in rats treated with butyrate. Therefore, we focused on colonic 5‐HT levels. Butyrate treatment increased 5‐HT levels in the colon, in agreement with the findings of a previous study (Grider and Piland, 2007), which showed that butyrate causes 5‐HT release in the rat colon. These findings indicate that the change in 5‐HT2A receptor expression does not contribute to the inhibitory effect of chlorpromazine on visceral hypersensitivity, whereas increased levels of 5‐HT may be responsible for inducing visceral hypersensitivity. To confirm this hypothesis, we examined the effect of p‐CPA, an inhibitor of 5‐HT synthesis, on visceral hypersensitivity. 5‐HT depletion via p‐CPA treatment attenuated butyrate‐induced colonic hypersensitivity. As elevated 5‐HT levels or 5‐HT2A receptor expression in the colon and DRG neurons are associated with acetic acid‐induced visceral hypersensitivity (Chen et al., 2016), increased colon 5‐HT levels may be a visceral hypersensitivity mechanism shared by butyrate and acetic acid models. Moreover, inhibition of 5‐HT2A receptor signalling by chlorpromazine may account for antinociceptive effects in these models. As ASIC3 and TRPV1 are required for visceral sensitivity to CRD, and 5‐HT2A receptor signalling enhances TRPV1 and ASICs activity in sensory neurons (Sugiuar et al., 2004; Jones et al., 2005; Qiu et al., 2012), activation of 5‐HT2A receptors in our study may also be associated with modulation of these channels. However, we could not exclude the possibility that other effects of chlorpromazine, including histamine H1 receptor antagonism and anti‐calmodulin activity, may also be involved in the inhibitory effect observed in this study (Horacek et al., 2006; Olah et al., 2007). Taken together, our results indicate that butyrate‐induced increase in colonic 5‐HT levels potentiates visceral sensitivity via the 5‐HT2A receptor, with chlorpromazine potentially preventing the binding of released 5‐HT to 5‐HT2A receptors.
In conclusion, we propose that the ameliorating effects of chlorpromazine on visceral hypersensitivity may be beneficial for IBS patients with abdominal pain and discomfort. The inhibitory effect of chlorpromazine on visceral hypersensitivity is likely to be mediated by inhibition of 5‐HT2A receptor signalling, and we suggest the 5‐HT2A receptor as a potential therapeutic target protein for abdominal pain and discomfort in IBS.
Author contributions
T.A., K.T., M.T. and T.M. conceived and designed the experiments; T.A., A.T., K.T., R.T., H.S. and H.M. carried out the analysis and interpretation; and T.A performed the drafting and critical revising of the manuscript.
Conflict of interest
T.M. is the chairman and director of LTT Bio‐Pharma Co., Ltd. T.A. and M.T. belong to an endowed research division of LTT Bio‐Pharma Co., Ltd. The other authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
We gratefully acknowledge Ayumi Kanada for technical assistance with animal experiments. We would like to thank Editage for English language editing. This work was supported by Grants‐in‐Aid for Scientific Research from the Ministry of Health, Labour, and Welfare of Japan, as well as the Center of Innovation Program from Japan Science and Technology Agency, Scientific Technique Research Promotion Program for Agriculture, Forestry and Food Industry, and Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Asano, T. , Tanaka, K. , Tada, A. , Shimamura, H. , Tanaka, R. , Maruoka, H. , Mizushima, T. , and Takenaga, M. (2017) Ameliorative effect of chlorpromazine hydrochloride on visceral hypersensitivity in rats: possible involvement of 5‐HT2A receptor. British Journal of Pharmacology, 174: 3370–3381. doi: 10.1111/bph.13960.
References
- Adams CE, Rathbone J, Thornley B, Clarke M, Borrill J, Wahlbeck K et al (2005). Chlorpromazine for schizophrenia: a Cochrane systematic review of 50 years of randomised controlled trials. BMC Med 3: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaider AA (1991). Antinociceptive effect of ketanserin in mice: involvement of supraspinal 5‐HT2 receptors in nociceptive transmission. Brain Res 543: 335–340. [DOI] [PubMed] [Google Scholar]
- Asano T, Tanaka K, Suemasu S, Ishihara T, Tahara K, Suzuki T et al (2012). Effects of beta‐(1,3‐1,6)‐D‐glucan on irritable bowel syndrome‐related colonic hypersensitivity. Biochem Biophys Res Commun 420: 444–449. [DOI] [PubMed] [Google Scholar]
- Asano T, Tanaka KI, Tada A, Shimamura H, Tanaka R, Maruoka H et al (2017). Aminophylline suppresses stress‐induced visceral hypersensitivity and defecation in irritable bowel syndrome. Sci Rep 7: 40214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J et al (2007). Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil 19: 62–88. [DOI] [PubMed] [Google Scholar]
- Bourdu S, Dapoigny M, Chapuy E, Artigue F, Vasson MP, Dechelotte P et al (2005). Rectal instillation of butyrate provides a novel clinically relevant model of noninflammatory colonic hypersensitivity in rats. Gastroenterology 128: 1996–2008. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Linden DR (2014). Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat Rev Gastroenterol Hepatol 11: 611–627. [DOI] [PubMed] [Google Scholar]
- Canavan C, West J, Card T (2014). Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther 40: 1023–1034. [DOI] [PubMed] [Google Scholar]
- Cervantes‐Duran C, Vidal‐Cantu GC, Godinez‐Chaparro B, Granados‐Soto V (2016). Role of spinal 5‐HT2 receptors subtypes in formalin‐induced long‐lasting hypersensitivity. Pharmacol Rep 68: 434–442. [DOI] [PubMed] [Google Scholar]
- Chang L, Lembo A, Sultan S (2014). American Gastroenterological Association Institute Technical Review on the pharmacological management of irritable bowel syndrome. Gastroenterology 147: 1149–1172.e2. [DOI] [PubMed] [Google Scholar]
- Chen MX, Chen Y, Fu R, Liu SY, Yang QQ, Shen TB (2016). Activation of 5‐HT and NR2B contributes to visceral hypersensitivity in irritable bowel syndrome in rats. Am J Transl Res 8: 5580–5590. [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Chu CC, Chen YC, Kan CD, Wang JJ (2012). Promazine and chlorpromazine for prolonged spinal anesthesia in rats. Neurosci Lett 521: 115–118. [DOI] [PubMed] [Google Scholar]
- Cremon C, Carini G, Wang B, Vasina V, Cogliandro RF, De Giorgio R et al (2011). Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol 106: 1290–1298. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grider JR, Piland BE (2007). The peristaltic reflex induced by short‐chain fatty acids is mediated by sequential release of 5‐HT and neuronal CGRP but not BDNF. Am J Physiol Gastrointest Liver Physiol 292: G429–G437. [DOI] [PubMed] [Google Scholar]
- Grover M, Dorn SD, Weinland SR, Dalton CB, Gaynes BN, Drossman DA (2009). Atypical antipsychotic quetiapine in the management of severe refractory functional gastrointestinal disorders. Dig Dis Sci 54: 1284–1291. [DOI] [PubMed] [Google Scholar]
- Grover M, Drossman DA (2011). Centrally acting therapies for irritable bowel syndrome. Gastroenterol Clin North Am 40: 183–206. [DOI] [PubMed] [Google Scholar]
- Horacek J, Bubenikova‐Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P et al (2006). Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs 20: 389–409. [DOI] [PubMed] [Google Scholar]
- Jones RC 3rd, Xu L, Gebhart GF (2005). The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid‐sensing ion channel 3. J Neurosci 25: 10981–10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keszthelyi D, Troost FJ, Masclee AA (2012). Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Methods to assess visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 303: G141–G154. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladabaum U, Boyd E, Zhao WK, Mannalithara A, Sharabidze A, Singh G et al (2012). Diagnosis, comorbidities, and management of irritable bowel syndrome in patients in a large health maintenance organization. Clin Gastroenterol Hepatol 10: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani S, Cortes O, Thomas R (2016). Cyproheptadine use in children with functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr 62: 409–413. [DOI] [PubMed] [Google Scholar]
- Marger F, Gelot A, Alloui A, Matricon J, Ferrer JF, Barrere C et al (2011). T‐type calcium channels contribute to colonic hypersensitivity in a rat model of irritable bowel syndrome. Proc Natl Acad Sci U S A 108: 11268–11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Blanco A, Pascual JC, Soler J, Valdeperez A, Perez V (2010). Quetiapine in the treatment of refractory irritable bowel syndrome: a case report. Prog Neuropsychopharmacol Biol Psychiatry 34: 715–716. [DOI] [PubMed] [Google Scholar]
- Matricon J, Meleine M, Gelot A, Piche T, Dapoigny M, Muller E et al (2012). Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment Pharmacol Ther 36: 1009–1031. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitanda A, Yasunami N, Tokumo K, Fujii H, Hirai T, Nishio H (2005). Contribution of the peripheral 5‐HT2A receptor to mechanical hyperalgesia in a rat model of neuropathic pain. Neurochem Int 47: 394–400. [DOI] [PubMed] [Google Scholar]
- Nozu T, Miyagishi S, Nozu R, Takakusaki K, Okumura T (2016). Water avoidance stress induces visceral hyposensitivity through peripheral corticotropin releasing factor receptor type 2 and central dopamine D2 receptor in rats. Neurogastroenterol Motil 28: 522–531. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Imbe H, Morikawa Y, Itoh M, Sekimoto M, Nemoto K et al (2002). 5‐HT2A receptor subtype in the peripheral branch of sensory fibers is involved in the potentiation of inflammatory pain in rats. Pain 99: 133–143. [DOI] [PubMed] [Google Scholar]
- Okumura T, Nozu T, Kumei S, Takakusaki K, Miyagishi S, Ohhira M (2016). Levodopa acts centrally to induce an antinociceptive action against colonic distension through activation of D2 dopamine receptors and the orexinergic system in the brain in conscious rats. J Pharmacol Sci 130: 123–127. [DOI] [PubMed] [Google Scholar]
- Olah Z, Josvay K, Pecze L, Letoha T, Babai N, Budai D et al (2007). Anti‐calmodulins and tricyclic adjuvants in pain therapy block the TRPV1 channel. PLoS One 2: e545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae CU, Lee SJ, Han C, Patkar AA, Masand PS (2013). Atypical antipsychotics as a possible treatment option for irritable bowel syndrome. Expert Opin Investig Drugs 22: 565–572. [DOI] [PubMed] [Google Scholar]
- Pasricha PJ (2007). Desperately seeking serotonin …. A commentary on the withdrawal of tegaserod and the state of drug development for functional and motility disorders. Gastroenterology 132: 2287–2290. [DOI] [PubMed] [Google Scholar]
- Qin HY, Luo JL, Qi SD, Xu HX, Sung JJ, Bian ZX (2010). Visceral hypersensitivity induced by activation of transient receptor potential vanilloid type 1 is mediated through the serotonin pathway in rat colon. Eur J Pharmacol 647: 75–83. [DOI] [PubMed] [Google Scholar]
- Qiu F, Qiu CY, Liu YQ, Wu D, Li JD, Hu WP (2012). Potentiation of acid‐sensing ion channel activity by the activation of 5‐HT(2) receptors in rat dorsal root ganglion neurons. Neuropharmacology 63: 494–500. [DOI] [PubMed] [Google Scholar]
- Rahman W, Bannister K, Bee LA, Dickenson AH (2011). A pronociceptive role for the 5‐HT2 receptor on spinal nociceptive transmission: an in vivo electrophysiological study in the rat. Brain Res 1382: 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif NA, McLaughlin MA, Kelly CR (2007). AL‐34662: a potent, selective, and efficacious ocular hypotensive serotonin‐2 receptor agonist. J Ocul Pharmacol Ther 23: 1–13. [DOI] [PubMed] [Google Scholar]
- Simren M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S et al (2013). Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 62: 159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiuar T, Bielefeldt K, Gebhart GF (2004). TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5‐hydroxytryptamine receptor activation. J Neurosci 24: 9521–9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Gen K, Inoue Y (2013). Comparison of the anti‐dopamine D(2) and anti‐serotonin 5‐HT(2A) activities of chlorpromazine, bromperidol, haloperidol and second‐generation antipsychotics parent compounds and metabolites thereof. J Psychopharmacol 27: 396–400. [DOI] [PubMed] [Google Scholar]
- Treem WR, Ahsan N, Kastoff G, Hyams JS (1996). Fecal short‐chain fatty acids in patients with diarrhea‐predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Pediatr Gastroenterol Nutr 23: 280–286. [DOI] [PubMed] [Google Scholar]
- Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ (2007). The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology 132: 615–627. [DOI] [PubMed] [Google Scholar]
- Xu D, Wu X, Grabauskas G, Owyang C (2013). Butyrate‐induced colonic hypersensitivity is mediated by mitogen‐activated protein kinase activation in rat dorsal root ganglia. Gut 62: 1466–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]


