Abstract
Introduction
Acinetobacter baumannii is an important human nosocomial pathogen; most clinical isolates are multidrug-resistant (MDR). Infections caused by A. baumannii often lead to high morbidity and mortality, with limited treatment options. Owing to the small number of anti-Gram-negative antibiotics in the development pipeline, researchers are looking to other natural compounds. The aim of this study was to determine the in vitro kill kinetics, in vivo efficacy and toxicity of theaflavin–epicatechin combinations against MDR A. baumannii.
Methods
Kill-kinetic assays were performed in Mueller–Hinton 2 broth over 24 h. Toxicity of the compound in the insect model, Galleria mellonella was investigated. The effect of theaflavin–epicatechin combinations on mortality and morbidity were assessed in Acinetobacter baumannii-infected G. mellonella. Larvae were scored for morbidity (melanisation: scale; 0–4) and mortality over 96 h.
Results
Kill-kinetic assays revealed that monotherapy had bacteriostatic activity over 24 h, whereas theaflavin–epicatechin combinations were bactericidal (a >3 log reduction in bacterial numbers at 24 h compared with the starting inoculum). Both polyphenols were non-toxic to G. mellonella at concentrations of up to 1000 mg/kg. In vivo treatment assays showed that the combination significantly increased (t test; p ≤ 0.05) larval survival at 96 h to 86% [±17 standard deviation percentage points (pp)] compared to monotherapy with theaflavin (52% ± 14 pp), epicatechin (44% ± 25 pp) or PBS (31% ± 13 pp). Morbidity was also lower in larvae treated with the combination, compared with monotherapy.
Conclusion
Polyphenol combinations produce effective antibacterial action against A. baumannii and show great potential for the treatment of infections caused by MDR A. baumannii.
Keywords: Acinetobacter, Galleria mellonella, In vivo, Polyphenols, Multidrug-resistant
Introduction
Acinetobacter baumannii is an important human nosocomial pathogen, referred to an ESKAPE pathogen (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species), harbouring multiple genes associated with antimicrobial resistance [1]. Hospital-acquired infections caused by A. baumannii often lead to increased morbidity and mortality [2], due to limited antibiotic therapies. Antibiotics of last resort, such as tigecycline and colistin, are increasingly used, but with the emergence of resistance to these drugs, clinicians may resort to unorthodox antibiotic combination therapies [3].
Potential alternatives to antibiotics includes metal nanoparticles and plant-derived compounds such as polyphenols. Polyphenols play an important role in plant defence systems against microbial pathogens [4]. Several studies have shown that polyphenols have antimicrobial activity against human and animal pathogens including bacteria, fungi and viruses [5–8].
Synergistic activity between polyphenols and some traditional antibiotics has been observed against multidrug-resistant (MDR) bacteria [9–12]. Similarly, combinations of polyphenols have been shown to be effective at inhibiting bacterial growth in vitro, with higher concentrations also leading to bacterial cell death [13–15]. However, no data exist on the in vivo activity of polyphenol combinations. A model in which the in vivo efficacy of polyphenol combinations can rapidly be determined, is the greater wax moth larvae, Galleria mellonella. This Lepidopteran is now a popular model for experimental bacteraemia studies, due to its high throughput, lack of Home Office restrictions and an innate immune system comparable with that of mammals [16]. Until recently, one disadvantage of the G. mellonella model was the lack of standardisation between suppliers, leading to high standard error and poor reproducibility across assays, making statistical analysis difficult. However, a standardised model, with controlled age, size and food supply, is now available. Using the TruLarv G. mellonella model (Biosystems Technology, Exeter, UK), highly reproducible data can be produced.
The aim of this study was to determine the in vitro kill kinetics and the in vivo toxicity and efficacy of theaflavin–epicatechin combinations against MDR strains of A. baumannii in the TruLarv G. mellonella model of infection.
Methods
Chemicals, Media and Bacterial Isolates
Epicatechin (≥90%) powder, Luria broth base and Mueller–Hinton 2 agar/broth were purchased from Sigma-Aldrich (Dorset, UK). Theaflavin (≥95%) was donated by Unilever (Bedford, UK). Type strain A. baumannii ATCC 19606 was purchased from Pro-Lab Diagnostics (Wirral, UK). Clinical isolates (n = 6) [17] were obtained from the Royal London Hospital (Whitechapel, UK) where they were identified via standard biochemical methods and Maldi-TOF. Isolates represent major clonal strains from the United Kingdom. All isolates were stored long term on cryobeads at −80 °C.
Antimicrobial Susceptibility Testing
Minimum inhibitory concentrations (MICs) of theaflavin and epicatechin were determined alone and in combination against all 6 MDR isolates, performed in 96-well microtitre plates using Mueller–Hinton 2 cation-adjusted broth. Checkerboard assays were used with twofold decreasing concentrations of theaflavin (512–0 mg/L) and epicatechin (1024–0 mg/L) with a bacterial inoculum of 105 colony forming units (CFU) per mL. Plates were incubated at 37 °C for 24 h. Where the MIC was not determined, the dilution above the maximum dose was used in calculating the fractional inhibitory concentration indices (FICIs). Fractional inhibitory concentration indices were calculated using the following equation, as previously described [18]: FICI = FIC of A (MIC of antibiotic A in combination with antibiotic B/MIC of antibiotic A alone) + FIC of B (MIC of antibiotic B in combination with antibiotic A/MIC of antibiotic B alone). FICI values of ≤0.5 suggest a synergistic interaction, >0.5–1.0 as an additive effect, >1.0 to <4 as indifference and a value of ≥4.0 was classed as an antagonistic effect [19]. All experiments were performed in triplicate, on three separate occasions.
Kill-Kinetic Assays
Kill-kinetic assays were performed using strains AB12, AB16, AB210 and the ATCC 19606 type strain. Isolates AB12, AB16 and AB210 were used as they represent the important clonal groups in the UK [17, 20]. In brief, a 1/1000 dilution of an 18-h bacterial culture, equating to approximately 106 CFU/mL, was used as the starting inoculum for each strain. Antimicrobials were added to individual cultures at the following final concentrations: theaflavin (0.5 mg/mL), epicatechin (0.5 mg/mL) and theaflavin and epicatechin combination (0.5 + 0.5 mg/mL). Cultures were incubated at 37 °C under continuous agitation (225 rpm) for 24 h. At set time intervals of 0, 2, 4, 6 and 24 h post-inoculation, 100-μL samples were collected, serially diluted and plated onto Mueller–Hinton 2 agar. Viable counts were performed after incubation at 37 °C for 20 h.
In vivo Toxicity in Galleria mellonella
In vivo testing was conducted using the Galleria mellonella invertebrate model (TruLarv™, Biosystems Technology, Exeter, UK). In brief, ten larvae were injected via the left proleg with 10, 20, 40, 60, 80, 100, 200, 400, 600, 800 or 1000 mg/kg doses of theaflavin or epicatechin, freshly prepared in phosphate-buffered saline (PBS). PBS was used in the control injections. Larvae were incubated at 37 °C for 96 h and were observed every 24 h. The degree of melanisation and mortality was recorded at each observational time point. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Inoculum Testing
To determine the optimum inoculum for staggered larval killing (c. 80% mortality of larvae at 96 h post-inoculation), an inoculum test was performed. In brief, 16 h cultures of A. baumannii strains AB12, AB16, AB210 and the type strain ATCC 19606 in LB broth were washed in PBS before being serially diluted. Colony-forming units were determined after plating the dilutions on nutrient agar and incubating at 37 °C for 24 h. Ten G. mellonella larvae were infected with the 16 h culture dilutions, equating to 103, 104, 105 and 106 CFU/larvae, via a 10 µL injection into the left proleg. Larvae were incubated at 37 °C and scored for survival (live/dead) at 0, 24, 48, 72 and 96 h.
Treatment Assays
Sixteen larvae were infected with 105 CFU/larvae of ATCC 19606, AB12, AB16 or AB210. Thirty minutes post-inoculation, a second injection was administered of theaflavin (20 mg/kg in PBS), epicatechin (40 mg/kg in PBS), a combination of theaflavin and epicatechin (20 + 40 mg/kg) or PBS. Larvae were incubated at 37 °C in Petri dishes lined with Whatman™ filter paper and scored for survival (live/dead) at 0, 24, 48, 72 and 96 h. All insects were also scored for melanisation over 96 h. Melanisation was quantified based on a reversed scoring method [16], whereby a score of 4 indicated total melanisation of the larvae, 2 equalled melanin spots over the larvae, 1 equalled discolouration of the tail and a score of 0 equalled no melanisation. All in vivo experiments were carried out in triplicate on 3 separate occasions.
Statistical Analyses
Time–kill curves (CFU/mL vs. time) and survival curves were plotted using GraphPad Prism 7.0 software. Synergy was defined as bactericidal activity (≥2 log10 difference in CFU/mL) of the combination compared to the single agent after 24 h incubation. Unpaired Student’s t tests were performed to check for significant variance.
Survival curves were analysed using the log rank test with a p value of ≤0.05 indicating statistical significance. At the 96-h time-point, percentage survival of all antibiotic-treated larvae was calculated using combined data from replicate experiments. One-tailed, unpaired t tests were used to compare mean values, as described previously [21].
Results
Broth microtitre testing revealed high MICs for both theaflavin and epicatechin (256–512 and 1024–2048 mg/L, respectively) for all A. baumannii isolates tested (Table 1). However, when used in combination, MICs were significantly (p ≤ 0.05) reduced for both theaflavin and epicatechin (16–64 and 16–512 mg/L, respectively). The FICs indicate synergistic activity of the theaflavin and epicatechin combination (≤0.5).
Table 1.
Mean minimum inhibitory concentrations and fractional inhibitory concentrations for theaflavin (TF) and epicatechin (EC) alone and in combination versus Acinetobacter baumannii isolates used
| Isolates | Description | MIC (mg/L) | MIC in combination (mg/L) | FICI | ||
|---|---|---|---|---|---|---|
| TF | EC | TF | EC | |||
| A. baumannii NCTC 19606 | Type strain | 512 | 2048 | 64 | 32 | 0.14 |
| A. baumannii AB12 | Clinical isolate of PFGE-defined UK lineage: ‘South East clone’ | 512 | 2048 | 16 | 16 | 0.04 |
| A. baumannii AB16 | Clinical isolate of PFGE-defined UK lineage: ‘OXA-23 clone 2’ | 256 | 1024 | 64 | 128 | 0.38 |
| A. baumannii AB184 | Clinical isolate of PFGE-defined UK lineage: ‘T’ | 256 | 1024 | 64 | 256 | 0.50 |
| A. baumannii AB186 | Clinical isolate of PFGE-defined UK lineage: ‘Burn’ | 512 | 2048 | 16 | 512 | 0.28 |
| A. baumannii AB210 | Clinical isolate of PFGE-defined UK lineage: ‘OXA-23 clone 1’ | 512 | 2048 | 32 | 16 | 0.07 |
| A. baumannii AB211 | Clinical isolate of PFGE-defined UK lineage: ‘OXA-23 clone 1’ | 256 | 1024 | 64 | 256 | 0.50 |
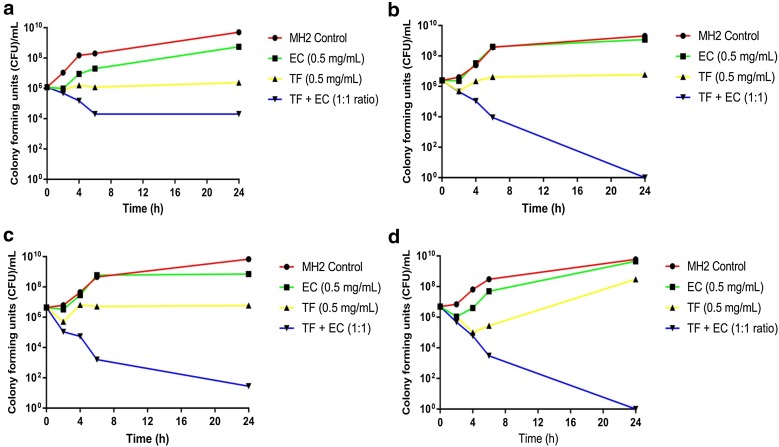
Data from the kill-kinetic assays showed that A. baumannii exposed to epicatechin alone regrew (2–3 log10 CFU/mL) after 24 h (Fig. 1). No significant difference between EC and the no-drug control was observed. In 2/3 isolates tested, theaflavin produced bacteriostatic activity, with CFU/mL maintained over 24 h. However, with AB210, regrowth was observed after 4 h. The combination of theaflavin and epicatechin had a greater impact than any agent alone on the kill kinetics of A. baumannii. For ATCC 19606, a >2 log10 reduction in CFU/mL at 24 h was observed. However, significant (p ≤ 0.05) differences were observed between the combination and monotherapy alone against AB12, AB16 and AB210, with a >3 log10 reduction in CFU/mL at 24 h, indicating synergy and bactericidal activity.
Fig. 1.
Kill kinetics of epicatechin (EC), theaflavin (TF), EC + TF combinations and a Mueller–Hinton control versus multidrug-resistant Acinetobacter baumannii strains. a ATCC 19696, b AB12, c AB16 and d AB210
Results from the in vivo toxicity testing showed no insect deaths after injection with any of the polyphenols at any concentration over 96 h. No melanisation was observed in any insect, indicating the absence of observable, phenotypic toxicological effects of theaflavin and epicatechin in Galleria mellonella.
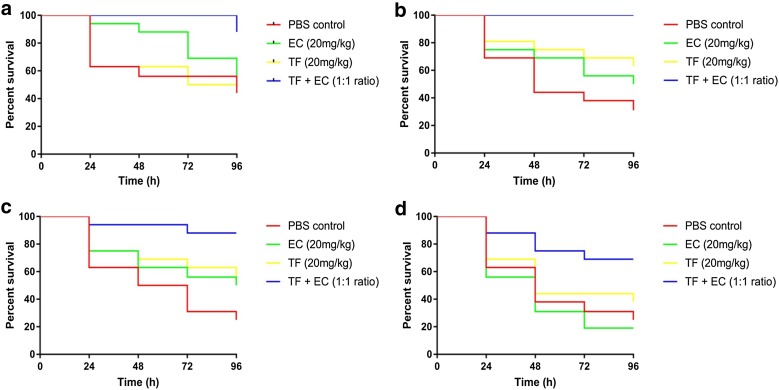
Data from the in vivo treatment assays suggest that monotherapy with theaflavin or epicatechin affords greater larval survival than PBS over 96 h, with overall survival rates of 52% [±14 standard deviation percentage points (pp)] and 44 (±22 pp), respectively (Fig. 2). However, when treated with the theaflavin–epicatechin combination, larval survival rates significantly increased to 86% (±17 pp) (p ≤ 0.05).
Fig. 2.
Survival curves for Galleria mellonella infected with 104 CFU/larvae of multidrug-resistant A. baumannii strains. a ATCC 19696, b AB12, c AB16 and d AB210, treated with phosphate-buffered saline (PBS), epicatechin (EC), theaflavin (TF) or EC + TF combinations
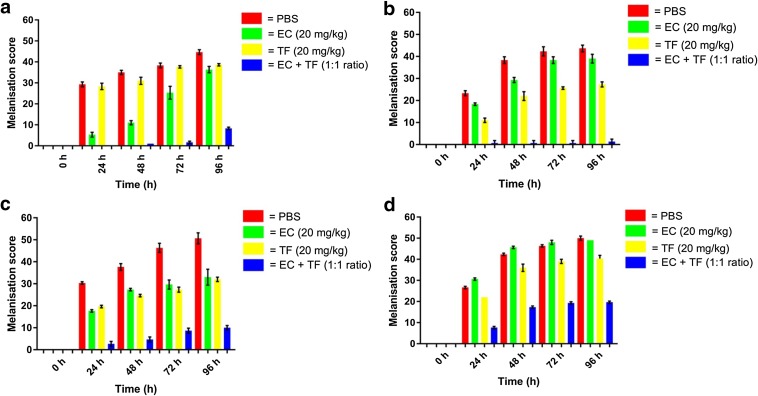
High levels of morbidity were observed over 96 h in A. baumannii-infected larvae, ‘treated’ with PBS alone (Fig. 3). Medium to high melanisation scores were recorded at 96 h for infected larvae treated with theaflavin or epicatechin monotherapy. Low melanisation scores were observed for the theaflavin–epicatechin combination.
Fig. 3.
Effect of treatments of phosphate buffered saline (PBS), epicatechin (EC), theaflavin (TF) or EC + TF combination on morbidity (melanisation) over 96 h in Galleria mellonella infected with 104 CFU/larva Acinetobacter baumannii. a ATCC 19696, b AB12, c AB16 and d AB210, with higher melanisation scores indicating greater morbidity
Discussion
The high MICs for theaflavin and epicatechin were not unexpected and are likely due to non-specific binding and/or degradation/dimerization of the polyphenols, which have been previously highlighted as factors reducing activity [22, 23].
Data from the time–kill assays clearly demonstrated that the theaflavin–epicatechin combination was not only synergistic but also bactericidal. This confirms previous kill-kinetic studies using polyphenolics, which also showed that combinations of polyphenols are synergistic and significantly reduce CFU/mL [24].
The lack of toxicity, at high administrated concentrations, may provide greater support for the use of polyphenols as treatments for bacterial infections. However, these observations might be due, in part, to the natural diet of G. mellonella larvae, which includes bee honeycomb that is naturally abundant in phenolic and polyphenolic compounds [25]. Therefore, G. mellonella larvae may have evolved to tolerate such compounds. Potential toxicity should be further assessed in mammalian models.
Data from the in vivo treatment assays demonstrated that monotherapy with either theaflavin or epicatechin was more efficacious than ‘treatment’ with PBS alone. Previous studies using Caenorhabditis elegans [26] found that monotherapy with epigallocatechin gallate (EGCG) increased survival rates of Escherichia coli infected nematodes by 28.9–76% compared with uninfected controls. However, the C. elegans model is incubated at 25 °C and not 37 °C (as with G. mellonella) and infection/treatment relies on the animal’s ability to consume the inoculum/antimicrobial. This might have had an impact on the growth of the bacteria and therefore the susceptibility to EGCG. Treatment with the polyphenol combination was significantly more effective at preventing larval death than monotherapy. These data support the use of combination therapies and clearly demonstrate that the theaflavin–epicatechin combination is an effective treatment in an invertebrate in vivo model of infection.
High melanisation scores are indicative of a substantial immune response. The medium to high melanisation scores of larvae treated with theaflavin–epicatechin indicate that monotherapy is not adequate for reducing morbidity. A significantly reduced phenotypically detected immune response was observed in larvae treated with the theaflavin–epicatechin combination. This indicates that the combination reduced overall morbidity and mortality, compared with monotherapy. Further studies using mammalian models are now warranted.
The number of clinical isolates used, although representative of epidemic clonal groups in the UK, was relatively small and is thus a limitation of this work. Another limitation of note is the use of an invertebrate model of infection, which lacks an adaptive immune response and the detoxification systems found in mammals.
Conclusion
Polyphenol combinations show great potential for the treatment of infections caused by MDR A. baumannii. Significant increases in survival were observed when A. baumannii-infected larvae were treated with a theaflavin–epicatechin combination, compared with monotherapy. To our knowledge, this is the first study to unequivocally demonstrate antibacterial activity of polyphenols in G. mellonella and the first in vivo report where combinations of polyphenols have showed antibacterial activity against bacterial pathogens.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors and no funding was received for the publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Jonathan W. Betts, Michael Hornsey, David W. Wareham and Roberto M. La Ragione have nothing to disclose.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/CB98F0607627E6CD.
References
- 1.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197(8):1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 2.Santajit S, Indrawattana N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int. 2016 doi: 10.1155/2016/2475067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phee LM, Betts JW, Bharathan B, Wareham DW. Colistin and fusidic acid, a novel potent synergistic combination for treatment of multidrug-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother. 2015;59(8):4544–4550. doi: 10.1128/AAC.00753-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daglia M. Polyphenols as antimicrobial agents. Curr Opin Biotechnol. 2012;23(2):174–181. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Steinmann J, Buer J, Pietschmann T, Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol. 2013;168(5):1059–1073. doi: 10.1111/bph.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YS, Schiller NL, Oh KH. Antibacterial effects of green tea polyphenols on clinical isolates of methicillin-resistant Staphylococcus aureus. Curr Microbiol. 2008;57:542–546. doi: 10.1007/s00284-008-9239-0. [DOI] [PubMed] [Google Scholar]
- 7.Friedmann M, Henika PR, Levin CE, Mandrill RE, Kozukue N. Antimicrobial activities of tea catechins and theaflavins and tea extracts against Bacillus cereus. J Food Prot. 2006;69(2):354–361. doi: 10.4315/0362-028X-69.2.354. [DOI] [PubMed] [Google Scholar]
- 8.Park BJ, Park JC, Taguchi H, Fukushima K, Hyon SH. Antifungal susceptibility of epigallocatechin-3-O-gallate (EGCg) on clinical isolates of pathogenic yeasts. Biochem Biophys Res Comm. 2006;347(2):401–405. doi: 10.1016/j.bbrc.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Al Razqan GS, Kwon DH. Antibacterial activity of epicatechin-3-gallate (EGCG) and its synergism with β-lactam antibiotics sensitize carbapenem-associated multidrug-resistant clinical isolates of Acinetobacter baumannii. Phytomedicine. 2017;24:49–55. doi: 10.1016/j.phymed.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Cui Y, Kim SH, Kin H, Yeom J, Ko K, Park W, Park S. AFM probing the mechanism of synergistic effects of the green tea polyphenol (−)epigallocatechin-3-gallate (EGCG) with cefotaxime against extended-spectrum β-lactamase (ESBL)-producing Escherichia coli. PLoS ONE. 2012 doi: 10.1371/journal.pone.0048880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stapleton PD, Shah S, Hamilton-Miller JM, Hara Y, Nagakoa Y, Kumagai A, Uesato S, Taylor PW. Anti-Staphylococcus aureus activity and oxacillin resistance modulating capacity of 3-O-acyl-catechins. Int J Antimicrob Agents. 2004;24:374–380. doi: 10.1016/j.ijantimicag.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Zhao WH, Hu ZQ, Okubo S, Hara Y, Shimamura T. Mechanism of synergy between epigallocatechin gallate and β-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45(6):1737–1742. doi: 10.1128/AAC.45.6.1737-1742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su Y, Ma L, Wen Y, Wang H, Zhang S. Studies of the in vitro antibacterial activities of several polyphenols against clinical isolates of methicillin-resistant Staphylococcus aureus. Molecules. 2014;19:12630–12639. doi: 10.3390/molecules190812630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betts JW, Murphy C, Kelly SM, Haswell SJ. Minimum inhibitory and bactericidal concentrations of theaflavin and synergistic combinations with epicatechin and quercetin against clinical isolates of Stenotrophomonas maltophilia. J Microbiol Biotechnol Food Sci. 2012;1(5):1250–1258. [Google Scholar]
- 15.Betts JW, Kelly SM, Haswell SJ. Antibacterial effects of theaflavin and synergy with epicatechin against clinical isolates of Acinetobacter baumannii and Stenotrophomonas maltophilia. Int J Antimicrob Agents. 2011;38:421–425. doi: 10.1016/j.ijantimicag.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Tsai CJ, Loh JM, Proft T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence. 2017;7(3):214–229. doi: 10.1080/21505594.2015.1135289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornsey M, Ellington MJ, Doumith M, Thomas CP, Gordon NC, Wareham DW, Quinn J, Lolans K, Livermore DM, Woodford N. AdeABC-mediated efflux and tigecycline MICs for epidemic clones of Acinetobacter baumannii. J Antimicrob Chemother. 2010;65(8):1589–1593. doi: 10.1093/jac/dkq218. [DOI] [PubMed] [Google Scholar]
- 18.Hall MJ, Middleton RF, Westmacott D. The fractional inhibitory concentration (FIC) index as a measure of synergy. J Antimicrob Chemother. 1983;11:427–433. doi: 10.1093/jac/11.5.427. [DOI] [PubMed] [Google Scholar]
- 19.Karlowsky JA, Hoban DJ, Zhanel GG, Goldstein BP. In vitro interactions of anidulafungin with azole antifungals, amphotericin B and 5-fluorocytosine against Candida species. Int J Antimicrob Agents. 2006;27(2):174–177. doi: 10.1016/j.ijantimicag.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Hornsey M, Loman N, Wareham DW, Ellington MJ, Pallen MJ, Turton JF, Underwood A, Gaulton T, Thomas CP, Doumith M, Livermore DM, Woodford N. Whole-genome sequencing of two Acinetobacter baumannii isolates from a single patient, where resistance developed during tigecycline therapy. J Antimicrob Chemother. 2011;66(7):1499–1503. doi: 10.1093/jac/dkr168. [DOI] [PubMed] [Google Scholar]
- 21.Hornsey MA, Wareham DW. In vivo efficacy of glycopeptide-colistin combination therapies in a Galleria mellonella model of Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2011;55(7):3534–3537. doi: 10.1128/AAC.00230-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozdal T, Capanoglu E, Altay FL. A review on protein–phenolic interactions and associated changes. Food Res Int. 2013;51(2):954–970. doi: 10.1016/j.foodres.2013.02.009. [DOI] [Google Scholar]
- 23.Spencer JE, Chaudry F, Pannala AS, Srai SK, Debnam E, Rice EC. Decomposition of cocoa procyanidians in the gastric milieu. Biochem Biophys Res Comm. 2000;272(1):236–241. doi: 10.1006/bbrc.2000.2749. [DOI] [PubMed] [Google Scholar]
- 24.Betts JW, Wareham DW. In vitro activity of curcumin in combination with epigallocatechin gallate (EGCG) versus multidrug-resistant Acinetobacter baumannii. BMC Microbiol. 2014;14:172. doi: 10.1186/1471-2180-14-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Israili ZH. Antimicrobial properties of honey. Am J Ther. 2014;21(4):304–323. doi: 10.1097/MJT.0b013e318293b09b. [DOI] [PubMed] [Google Scholar]
- 26.Lee KM, Kim WS, Lim J, Nam S, Youn M, Nam SW, Kim Y, Kim SH, Park W, Park S. Antipathogenic properties of green tea polyphenol epigallocatechin gallate at concentrations below the MIC against Enterohemorrhagic Escherichia coli O157:H7. J Food Protect. 2009;72(2):325–333. doi: 10.4315/0362-028X-72.2.325. [DOI] [PubMed] [Google Scholar]