Abstract
Chronic adolescent marijuana use has been linked to the later development of psychiatric diseases such as schizophrenia. GABAergic hypofunction in the prefrontal cortex (PFC) is a cardinal pathological feature of schizophrenia and may be a mechanism by which the PFC loses its ability to regulate sub-cortical dopamine (DA) resulting in schizophrenia-like neuropsychopathology. In the present study, we exposed adolescent rats to Δ-9-tetra-hydrocannabinol (THC), the psychoactive component in marijuana. At adulthood, we characterized the functionality of PFC GABAergic neurotransmission and its regulation of sub-cortical DA function using molecular, behavioral and in-vivo electrophysiological analyses. Our findings revealed a persistent attenuation of PFC GABAergic function combined with a hyperactive neuronal state in PFC neurons and associated disruptions in cortical gamma oscillatory activity. These PFC abnormalities were accompanied by hyperactive DAergic neuronal activity in the ventral tegmental area (VTA) and behavioral and cognitive abnormalities similar to those observed in psychiatric disorders. Remarkably, these neuronal and behavioral effects were reversed by pharmacological activation of GABAA receptors in the PFC. Together, these results identify a mechanistic link between dysregulated frontal cortical GABAergic inhibition and sub-cortical DAergic dysregulation, characteristic of well-established neuropsychiatric endophenotypes.
Introduction
Marijuana is the most widely used illicit drug among adolescents. Although it is still a matter of debate, there is evidence suggesting that chronic exposure to the primary psychoactive phytochemical in cannabis, tetrahydrocannabinol (THC), increases the long-term risk of psychiatric diseases, including schizophrenia1–5. The risk is higher depending upon age of onset of chronic marijuana use (e.g. before 17) and relative THC concentration. Nevertheless, only a minority of cannabis users may be prone to develop psychiatric diseases. It is therefore likely that both environmental factors and genetic predisposition play a role in this causal association. Animal models are useful tools for investigating the long-term behavioral effects of cannabis exposure during adolescence. We have previously demonstrated that chronic THC exposure during adolescent neurodevelopment leads to persistent abnormalities in adulthood resembling schizophrenia, involving neuropathological molecular adaptations in the prefrontal cortex (PFC), concomitant with a hyperactive sub-cortical dopamine (DA) system6. These effects are of particular clinical relevance given the increasing levels of THC in current strains of marijuana7. Nevertheless, the precise neurobiological mechanisms underlying the long-term neuropsychiatric effects of adolescent THC exposure are still unknown.
Adolescence is a highly vulnerable period for brain development, during which the PFC undergoes massive functional remodeling. These cortical remodeling processes include refinement of intrinsic GABAergic function and changes in the excitatory–inhibitory neuronal balance, essential for synchronized cortical activity, regulation of sub-cortical emotional processing centers, and maturation of normal adult behavior and cognition8, 9. Importantly, due to their strategic location on local PFC GABAergic circuits, cannabinoid type 1 receptors (CB1R) play a key role in prefrontal maturational processes, maintaining the balance of excitatory/inhibitory neuronal activity and associated cortical oscillatory states10–12. Consequently, neurodevelopmental impairment of PFC CB1R signaling and associated GABAergic function has the potential to cause long-term pathological prefrontal disinhibition, impaired synchronized cortical activity states and psychopathological deficits in PFC-dependent function8, 13.
The onset of schizophrenia during adolescent neurodevelopment14, 15 is linked to impairments in PFC GABAergic signaling. For example, numerous studies demonstrate cortical reductions in the GABA synthesizing enzyme, glutamic acid decarboxylase-67 (GAD67) corresponding to a loss of GABAergic parvalbumin (PV) interneurons in post-mortem frontal cortical tissues from schizophrenia patients16–24. Altered expression levels of select prefrontal cortical GABAA receptor subunits have also been observed in schizophrenia, characterized by increased expression patterns of GABAA receptor α2 subunits and decreased levels of the γ2 and δ subunits25–27. It has been proposed that this cortical GABAergic dysfunction may underlie disturbances in gamma-band synchronized neuronal activity associated with pathological emotional and cognitive deficits observed in schizophrenia28. For example, cortical gamma oscillation power has been reported to be increased in schizophrenia29, 30, a phenomena that is thought to be associated with the positive symptoms of schizophrenia, including hallucinations31, 32. In addition, decreased activity of inhibitory PFC GABAergic neurons and a resulting dysregulation of PFC pyramidal neuron activity and associated gamma oscillatory states, may be associated with sub-cortical DAergic dysregulation, and related affective and cognitive disturbances in schizophrenia33. For example, knockdown of the α3 GABAA receptor subunit causes a hyper-DAergic, schizophrenia-like phenotype linked to hyperactive DAergic activity states in the ventral tegmental area (VTA)34 consistent with the effects of chronic THC exposure during adolescent neurodevelopment6. Furthermore, GABAA receptor blockade or direct electrical stimulation of the PFC induces schizophrenia-like behaviors and increases sub-cortical DA release35–37.
Using a validated rodent model of adolescent THC exposure, we hypothesized that neurodevelopmental exposure to THC would lead to a molecular, neuronal and behavioral phenotype resembling schizophrenia due to a loss in GABAergic regulation of the PFC and associated sub-cortical DAergic activity patterns. We report that adolescent THC exposure induces a long-term loss of GABAergic inhibition within the PFC that persists into adulthood. This phenotype was characterized by dysregulated γ oscillatory activity, downregulation of GABAergic protein markers, increased medial PFC (mPFC) output neuron activity, hyperactive sub-cortical DAergic activity and a range of cognitive and affective abnormalities resembling to those observed in psychiatric diseases such as schizophrenia. Remarkably, pharmacological activation of GABAA receptors directly in the mPFC, which shares functional-anatomical properties with the human dorsolateral PFC38, 39, reversed these deficits, demonstrating that aberrant PFC-mesolimbic connectivity following adolescent THC exposure is a critical neuropathological mechanism underlying an increased risk for later adulthood schizophrenia-like abnormalities.
Results
THC exposure during adolescence reduces expression levels of GAD67 in the adult mPFC
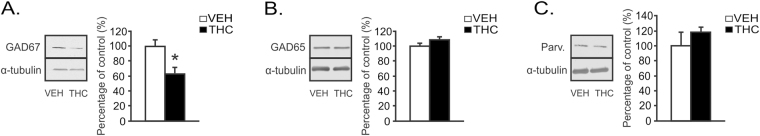
Schizophrenia is associated with lower levels of GAD67 in the dorsolateral PFC17. Using Western Blotting (Fig. 1), we analyzed mPFC protein expression levels of GAD67 comparing adult rats treated with VEH or THC during adolescence. Western blot analysis of GAD 67 revealed a dramatic decrease in adolescent THC vs. VEH-treated rats (t(6) = 2.44; p < 0.05; Fig. 1A). This demonstrated strong reductions in mPFC GABAergic levels following adolescent THC exposure, consistent with schizophrenia neuropsychopathology. Conversely, we did not observe changes in mPFC protein expression levels of either GAD65 or parvalbumin (PV) in adolescent THC vs. VEH-treated rats (t (6) = −0.33; p > 0.05 and t(6) = −0.33; p > 0.05, respectively; Fig. 1B,C).
Figure 1.
Long-term effects of chronic THC exposure during adolescence on mPFC GABAergic markers. (A) Representative western blot for GAD67 expression in the mPFC (left). A significant decrease in GAD67 expression is observed between adolescent VEH and adolescent THC pretreated rats. (B) Representative western blot for GAD65 expression in the mPFC (left). No significant changes in GAD65 were found between groups. (C) Representative western blot for parvalbumin (PV) expression in the mPFC (left). No significant changes in PV were found between groups. n = 8 rats, t-tests; *Indicated p < 0.05. Error bars represent the standard error of the means (SEMs). Western blots for GAD 67, GAD65 and parvalbumin levels are shown in Supplementary Figure 1.
Adolescent THC exposure increases mPFC putative pyramidal neuron firing and bursting Rates
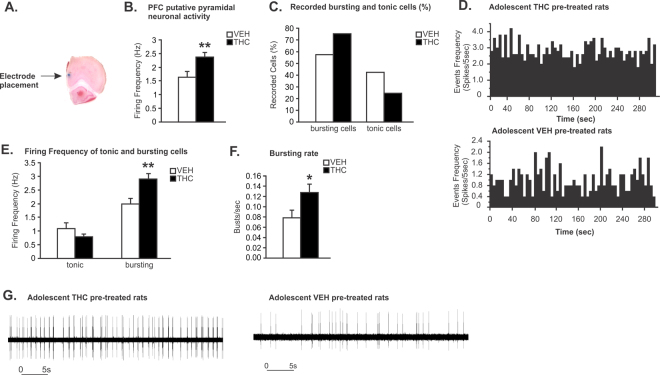
We next evaluated the long-term effects of adolescent THC exposure on adult spontaneous mPFC putative pyramidal neuron activity parameters (See Methods). A microphotograph of a representative mPFC neuronal recording placement is shown in Fig. 2A. In THC treated rats, statistical analysis revealed that THC-exposed rats displayed significantly increased mPFC putative pyramidal neuronal firing frequencies (t (219) = 3.131; p < 0.01; Fig. 2B) relative to VEH controls. In addition, a greater proportion of bursting neurons was observed in THC vs. VEH exposed controls (75.41% vs. 57.58%; Fig. 2C). Representative rastergrams showing spontaneous activity of mPFC putative pyramidal neurons in THC vs. VEH pretreated rats are presented in Fig. 2D. When comparing spontaneous firing frequencies of mPFC putative pyramidal neurons displaying tonic vs. bursting patterns, firing rates of bursting, but not tonic cells, were significantly higher in THC vs. VEH exposed controls (t (147) = −2.862; p < 0.01; Fig. 2E). Finally, in the subpopulation of bursting neurons, spontaneous bursting rates of putative pyramidal neurons were significantly higher in THC vs. VEH exposed controls (t (147) = 2.055; p < 0.05; Fig. 2G). Recording traces comparing bursting patterns in putative PFC pyramidal neurons in THC vs. VEH control rats are presented in Fig. 2G.
Figure 2.
Long-term effects of chronic THC exposure during adolescence on spontaneous mPFC putative pyramidal neuron activity. (A) Microphotograph of a representative mPFC neuronal recording placement. (B) Adolescent THC pretreated rats displayed increasing spontaneous PFC putative pyramidal neuronal firing frequency. (C) Greater proportion of bursting neurons was observed in adolescent THC exposed rats when compared to VEH controls (75.41% vs. 57.58%). (D) Representative rastergrams showing spontaneous activity of putative PFC pyramidal neurons in THC (top) vs. VEH pretreated rats (bottom). (E) Firing frequencies of bursting cells, not tonic cells, were significantly higher in adolescent THC exposed rats when compared to VEH controls. (F) In the bursting cells population, the bursting rate of putative pyramidal neurons of adolescent THC-exposed rats was significantly higher than in VEH controls. (G) Representative examples showing bursting activity of putative PFC pyramidal neurons in THC (left) vs. VEH pretreated rats (right). Two-tailed t-tests; **indicated p < 0.01; *indicated p < 0.05. Error bars represent the standard error of the means (SEMs).
Adolescent THC treatment disrupts spontaneous adult cortical gamma oscillations
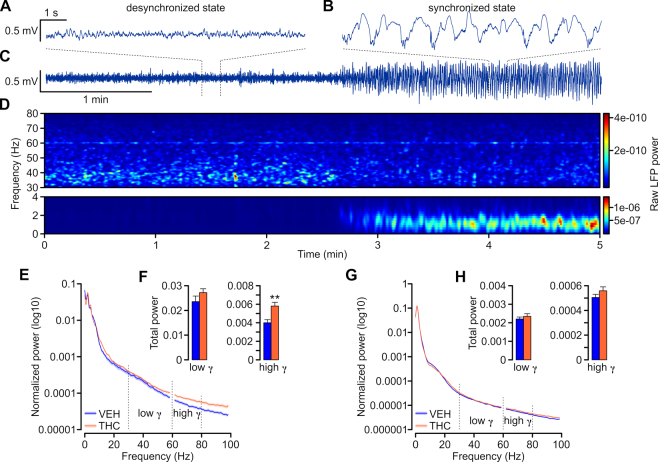
Local field potential (LFP) recordings in the mPFC were performed in urethane anesthetized rats and were typically characterized by occurrence of slow, high-voltage fluctuations (>0.5 mV; Fig. 3B) corresponding to a synchronized cortical state. Occasionally, epochs of desynchronized states occurred, characterized by small-voltage (<0.5 mV) and fast oscillations (Fig. 3A). The strength of the oscillations was assessed by calculating power spectral densities (PSD; window length = 2 s; shift = 0.5 s; Fig. 3D) over a 1-min long recording epoch, averaging PSDs over time and subsequently normalizing the averaged PSD to the total power (normalized total power = 1; values for frequencies between 59 and 61 Hz were excluded from calculations to avoid contamination with 60 Hz noise from power line).
Figure 3.
Adolescent THC-treatment lead to increased high gamma (61–80 Hz) power in the mPFC of adult rat. (A–C) Example recording traces from PFC of urethane anesthetized rat showing different cortical states. Desynchronized state (A) was characterized by small-amplitude fast oscillations while synchronized state (B) by large-amplitude slow oscillations. (C) Five-minute recording showing spontaneous alternation from the desynchronized to synchronized state. (D) Spectrogram calculated for a five-minute recording presented in C showing the temporal changes in the power at different frequencies. Note that upon transition from desynchronized to synchronized state gamma power decreased while the slow delta oscillation gradually emerged. The power values are color-coded as indicated on the right-hand side insets. A peak at around 60 Hz reflect power line frequency and the LFP power values for frequencies between 59–61 Hz were excluded from further analysis. (E,G) Average normalized power spectra corresponding to prefrontal LFP of VEH- (blue) and THC-treated (orange) rats in desynchronized (E) and synchronized (G) states. Note the increased power of high-gamma band (61–80 Hz) in THC-treated rats. (F,H) Bar graphs summarizing the average total power of the low- and high-gamma calculated for desynchronized (F) and synchronized (H) states of VEH (blue) and THC-treated (orange) rats. The high-gamma of THC-treated rats was significantly more powerful than VEH. Two-tailed t-tests; **indicated p < 0.01. Error bars represent the standard error of the means (SEMs).
During the desynchronized states the gamma oscillations were approximately 10 times more powerful than during the synchronized states. The total gamma power, calculated as a sum of power values for frequencies between 30–80 Hz of THC-treated rats did not differ from VEH controls (desynchronized LFPs: VEH = 0.0275 ± 0.0028, THC = 0.0330 ± 0.0018, t (96) = −1.6467; p > 0.05) synchronized LFPs: VEH = 0.0027 ± 0.0001; THC = 0.0029 ± 0.0001; t (172) = −0.9325; p > 0.05). However, the high-gamma oscillations (61–80 Hz) of the desynchronized state were significantly increased in THC-treated rats when compared with VEH-treated rats (VEH = 0.0040 ± 0.0003; THC = 0.0058 ± 0.0004; t (96) = −3.1411; p < 0.01 Fig. 3F). These data suggest impairments of the intracortical inhibitory feedback within the PFC circuit and are consistent with the increased firing of putative pyramidal cells (Fig. 2) and decreased expression of GAD67 in THC-treated rats (Fig. 1A).
Intra-mPFC GABAA receptor activation reverses deficits in short term memory induced by adolescent THC exposure
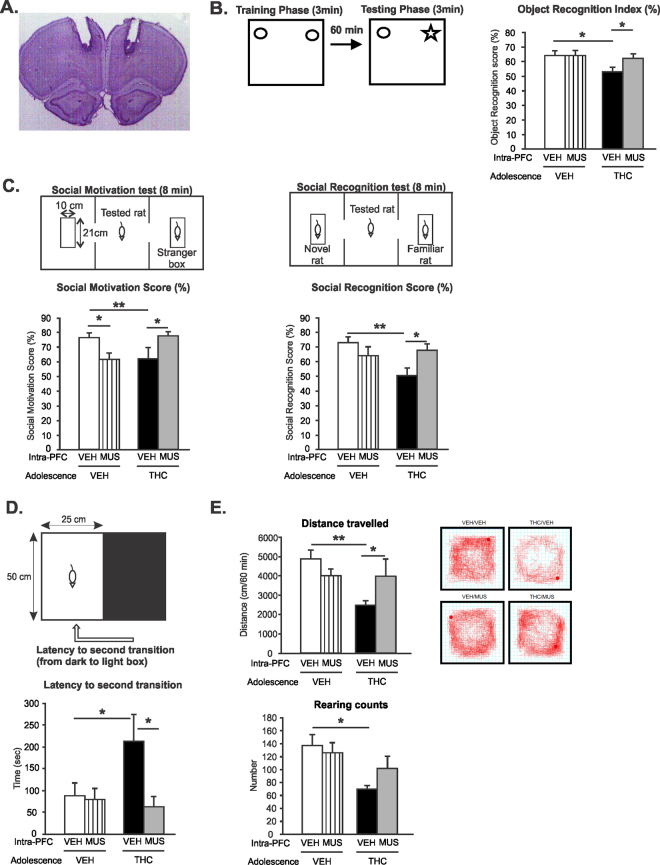
We next mechanistically examined the potential role of PFC GABAA receptor transmission by microinfusing the selective GABAA agonist, muscimol (MUS; 500 ng/0.5 μl) directly into the mPFC to determine if pharmacologically activating GABAA receptors may reverse behavioral abnormalities associated with adolescent THC exposure. A microphotograph of a representative intra-mPFC injector placement is showed in Fig. 4A. Memory deficits are core features of schizophrenia and are thought to be associated with abnormal prefrontal GABA function13. Therefore, we measured short-term memory performance of adult rats pretreated with VEH or THC during adolescence using the object recognition task. Two-way ANOVA revealed a significant main effect of treatment on recognition index (F(1,34) = 4.717; p < 0.05). Post hoc analysis revealed that THC exposure during adolescence induced short-term memory deficits when compared to adolescent VEH pretreated rats (p < 0.05); Fig. 4B). Intra-mPFC MUS treatment reversed adolescent THC-induced short-term memory deficits relative to intra-mPFC VEH controls (p < 0.05; Fig. 4B) while having no effects in adolescent VEH pretreated rats (p > 0.05). Thus, intra-mPFC MUS infusion restored cognitive performances in adolescent THC pretreated rats.
Figure 4.
Effects of Intra-mPFC MUS on adolescent THC-induced behavioral abnormalities. (A) Microphotograph of a representative Intra-mPFC injector placement. (B) THC exposure during adolescence induced short-term memory deficits in the object recognition task. Intra-mPFC MUS treatment reversed adolescent THC-induced short-term memory deficits relative to intra-mPFC VEH controls. (C) THC exposure during adolescence induced lower social motivation (left) and social cognition (right) index in the social interaction task. Intra-mPFC MUS treatment reversed adolescent THC-induced social motivation (left) and social cognition (right) deficits relative to intra-mPFC VEH controls. (D) THC exposure during adolescence increased anxiety levels in the light dark box test. Intra-mPFC MUS reduced adolescent THC-induced anxiety relative to intra-mPFC VEH controls. (E) THC exposure during adolescence decreased locomotion (top) and rearing counts (bottom) in the open field test. Intra-mPFC MUS reduced adolescent THC-induced hypolocomotion relative to intra-mPFC VEH controls (top) but had no effects on rearing counts. Anova 2 factors **indicated p < 0.01; *indicated p < 0.05. Error bars represent the standard error of the means (SEMs).
Intra-mPFC GABAA receptor activation reverses deficits in social cognition induced by adolescent THC exposure
Affective impairment and social cognition represent negative symptoms of schizophrenia40–43. In addition, the PFC plays a critical role in regulating social cognition in both humans and rodents43–46. Therefore, we next compared social motivation/cognition performances in THC vs. VEH treated groups using the social novelty preference test. During the social motivation test, two-way ANOVA revealed a significant interaction between adolescent exposure and PFC treatment factors (F(1,41) = 9.062; p < 0.01). Post hoc analysis revealed that rats exposed to THC during adolescence displayed a lower social motivation index when compared to VEH controls (p < 0.05); Fig. 4C left). Intra-mPFC GABAA receptor activation impaired social motivation performances in VEH treated rats (p < 0.05; Fig. 4C left) while reversing adolescent THC-induced social cognition deficits relative to controls (p < 0.05; Fig. 4C left).
During the social cognition test, two-way ANOVA revealed a significant interaction between adolescent exposure and PFC treatment factors (F(1,41) = 6.67; p < 0.05). Post hoc analysis revealed that rats exposed to THC during adolescence displayed a lower social memory index when compared to VEH controls (p < 0.01); Fig. 4C right). Intra-mPFC GABAA receptor activation reversed adolescent THC-induced social cognition deficits relative to intra-mPFC VEH controls (p < 0.05; Fig. 4C right) while having no effects in adolescent VEH pretreated rats (p > 0.05).
Intra-mPFC GABAAreceptor activation reduces increased anxiety levels following adolescent THC exposure
Next, the effects of intra-mPFC MUS microinfusion on anxiety levels were examined in THC vs. VEH treated rats using the light-dark box anxiety test (Fig. 4D). The latencies to first transition (i.e. moving from the light to dark environment) and second transition (i.e. moving from the dark to light environment) were recorded and compared between the different drug treatment conditions. Two-way ANOVA analysis of the latency to first transition revealed non-significant effects of either adolescent exposure or adult intra-mPFC treatment or interaction between factors, indicating that all groups of rats displayed similar entry latency times (data not shown). However, two-way ANOVA analysis of the latency to second transition indicated a slight yet non-significant effect of adult intra-mPFC treatment (F(1,39) = 3.68; p = 0.062). Post hoc comparisons showed that in adolescent THC pretreated rats the latency to re-emerge from the dark compartment was increased when compared to adolescent VEH pretreated rats (p < 0.05; Fig. 4D, bottom), demonstrating increased anxiety in adolescent THC pretreated rats, consistent with previous findings6. Intra-mPFC MUS reduced the latency to reemerge from the dark compartment in adolescent THC pretreated rats relative to intra-mPFC VEH controls (p < 0.05; Fig. 4D, bottom) while having no effects on adolescent VEH pretreated rats (p > 0.05). Thus, intra-mPFC MUS infusion decreased anxiety levels in adolescent THC pretreated rats.
Intra-mPFC GABAAreceptor activation reduces deficits in exploratory behavior induced by adolescent THC exposure
Adolescent THC exposure has been shown to decrease motivational exploratory behaviors6. Therefore, we next tested if intra-mPFC GABAA receptor activation may reverse this deficit. Two-way ANOVA revealed a significant effect of adolescent treatment on total distance travelled (F(1,40) = 6.145; p < 0.05). Post hoc analysis revealed that adolescent THC pretreated rats showed decreased locomotion when compared to VEH controls (p < 0.01; Fig. 4E, top). However, intra-mPFC MUS restored locomotor activity levels in THC treated rats relative to VEH controls (p < 0.05; Fig. 4E, top) while having no effects on adolescent VEH pretreated rats (p > 0.05). Examples of typical activity plots for adolescent VEH or THC-intra-mPFC or MUS treated rats are presented in Fig. 4E. Analysis of rearing counts revealed a significant main effect of adolescent treatment (F(1,40) = 9.82; p < 0.01). Post hoc analysis demonstrated that THC treated rats exhibited significantly less rearing behavior vs. VEH controls (p < 0.01; Fig. 4E, bottom). However, intra-mPFC MUS had no effects in either of the adolescent VEH or THC pretreated groups (p > 0.05; Fig. 4E, bottom). Thus, intra-mPFC MUS infusion restored exploratory motor behavior in adolescent THC pretreated rats.
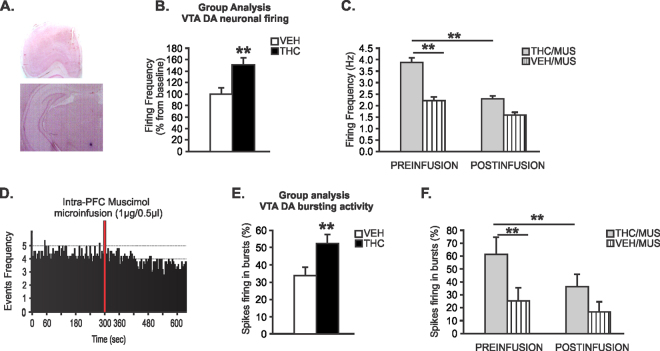
Intra-mPFC GABAA receptor activation reverses sub-cortical DAergic hyperactivity induced by adolescent THC exposure
Given our previous findings of significantly reduced GABAergic PFC GAD67 activity and PFC neuronal hyperactivity in following adolescent THC exposure, we hypothesized that pharmacologically restoring PFC GABAA receptor tone may reverse the hyperactive VTA DA neuron phenotype induced by adolescent THC exposure. Using in vivo, single-unit VTA neuronal recordings in putative DAergic VTA neurons (see Methods) we next examined the effects of intra-mPFC MUS infusions on putative VTA DAergic neuronal frequency and bursting rates. Microphotographs of a representative PFC injector placement and VTA neuronal recording site are shown in Fig. 5A. Collective analysis of baseline firing frequency levels before mPFC micro-infusions of either MUS or VEH showed increased neuronal firing frequency in adolescent THC-pretreated rats (n = 40 neurons) relative to adolescent VEH-pretreated rats (n = 45 neurons) (t(83) = 3.15, p < 0.01, Fig. 5B). For analysis purposes, a neuron was considered to have changed its firing rate if there was a minimum of 10% difference in frequency rate from baseline. Using this previously reported criterion47, qualitative analyses revealed that in VEH pretreated rats, intra-mPFC MUS microinfusions caused mixed effects on firing rates with 10% of putative DA neurons showing increased firing, 40% decreased, and 50% unchanged. In THC pretreated rats that received intra-mPFC MUS, 12% of putative DA neurons showed increased in firing frequency, 46% decreased, and 42% were unchanged. One-way ANOVA comparing pre vs. post-infusion firing rates in sub-populations of putative DA neurons showing decreased firing following mPFC MUS, revealed a significant effect of recording epoch (F(1,22) = 13.002; p < 0.01) on firing rates. Post hoc comparisons revealed that before mPFC MUS infusions, VTA putative DA neuron baseline frequency in THC pretreated rats was significantly increased relative to VEH controls (p < 0.01; Fig. 5C). In addition, in adolescent THC treated, but not VEH controls, the firing frequency of VTA putative DA neurons was significantly reduced by mPFC MUS infusions (p < 0.01; Fig. 5C). Thus, pharmacological activation of GABAA transmission in the mPFC was able to effectively reverse the hyper-DAergic phenotype induced by adolescent THC exposure. A representative rastergram showing the spontaneous activity of a putative DA neuron treated with intra-mPFC muscimol in a THC pretreated rat is presented in Fig. 5D.
Figure 5.
Effects of Intra-mPFC MUS on adolescent THC-induced sub-cortical hyperdopaminergia. (A) microphotograph of a representative mPFC microsyringe and VTA neuronal recording placements. (B) Increased VTA putative DA neuronal firing frequency in adolescent THC-pretreated rats relative to adolescent VEH-pretreated rats. (C) Before intra-mPFC MUS microinfusion, sub-population of VTA putative DA neurons firing frequency in adolescent THC pretreated rats were significantly increased compared to adolescent VEH pretreated rats. Intra-mPFC MUS reduced firing frequency of VTA putative DA in adolescent THC-pretreated rats relative to adolescent VEH-pretreated rats. (D) Representative rastergram showing the spontaneous activity of 1 putative DA neuron treated with intra-mPFC muscimol in adolescent THC pretreated rat. (E) Increased VTA putative DA neurons bursting levels in adolescent THC vs. VEH pretreated rats. (F) Before intra-mPFC MUS microinfusion, sub-population of VTA putative DA neurons spikes firing in bursts in adolescent THC pretreated rats were significantly increased compared to adolescent VEH pretreated rats. Intra-mPFC MUS reduced spikes firing in bursts of VTA putative DA in adolescent THC-pretreated rats relative to adolescent VEH-pretreated rats. Two-way repeated measures ANOVA or Two-tailed t-tests; **indicated p < 0.01. Error bars represent the standard error of the means (SEMs).
Similar to VTA putative DA neuron firing frequency rates, collective analysis of spontaneous baseline levels of putative DA neuron bursting activity comparing THC vs. VEH control neurons demonstrated increased bursting rates specifically in THC-treated rats (t(83) = −2.86; p < 0.01; Fig. 5E). One-way ANOVA comparing the sub-populations of neurons from THC and VEH rats showing intra-mPFC MUS-induced decreased bursting levels showed a significant effect of recording epoch (F(1,11) = 13.12; p < 0.01) on bursting rate levels. Similar to firing frequency, post hoc comparisons revealed that baseline bursting levels of VTA putative DA neurons recorded in THC treated rats were significantly increased relative to VEH controls (p < 0.05; Fig. 5F). In addition, in adolescent THC, but not VEH treated rats, intra-mPFC MUS significantly reduced bursting rates relative to pre-infusion levels (p < 0.01; Fig. 5F). Thus, intra-mPFC activation of GABAA receptor transmission was able to effectively reverse the hyper-bursting VTA putative DA neuron phenotype induced by adolescent THC exposure. mPFC VEH microinfusions had no effects on either DA neuron firing frequency or bursting levels in either VEH or THC treated rats (data not shown). These results suggest that hyperexcitability of VTA DA neurons induced by adolescent exposure to THC can be attenuated by pharmacologically restoring the inhibitory balance within the mPFC.
Discussion
In the present study, we demonstrate that adolescent THC exposure induces persistent prefrontal GABA hypofunction in adulthood characterized by reduced expression levels of the GABAergic marker GAD67, increased spontaneous PFC pyramidal neuron bursting and firing rates and potentiated high gamma power oscillatory activity. Furthermore, these cortical neuronal abnormalities occurred in concert with a sub-cortical hyperDAergic neuronal phenotype in the VTA. Finally, adolescent THC exposure induced cognitive and affective disturbances characterized by impairments in short-term memory, social motivation and cognition, higher anxiety levels and decreased motivation. Remarkably, these behavioral impairments were reversed by pharmacological activation of GABAA receptors in the adult mPFC which concomitantly, reversed sub-cortical hyperDAergic activity in the VTA. Together, these results identify a mechanistic link between dysregulated frontal cortical GABAergic inhibition and sub-cortical DAergic dysregulation, characteristic of well-established schizophrenia endophenotypes.
First, the observed decrease of GAD67 in the adult mPFC following adolescent THC exposure is consistent with schizophrenia-related cortical neuropathology. Decreased cortical GABA function is one of the most reliable neuropathological markers observed in post-mortem analyses of the schizophrenic brain48. Both mRNA and protein expression of GAD67 are reduced in the dorsolateral PFC of individuals with schizophrenia16, 19, 21–23 suggesting a reduction in cortical GABA synthesis and release. Furthermore, consistent with previous findings in schizophrenia, we observed no PFC GAD65 alterations49. Our results are consistent with previous studies showing GAD67 reductions in selective parvalbumin (PV)- and CCK-containing PFC interneuron populations following adolescent THC exposure50. Similar to previous reports, we did not find any THC-induced changes in total PFC PV levels, suggesting that, despite a deficit in cortical GABAergic synthesis, overall population levels of PV interneurons are likely not attenuated50.
Consistent with a loss of cortical GABAergic inhibition, we observed a significant potentiation in both firing and bursting rates of putative mPFC pyramidal neurons, demonstrating a persistent dysregulation of cortical excitatory/inhibitory balance following adolescent THC exposure. This increase in firing frequency was concomitant with a general increase in the proportion of mPFC neurons displaying bursting patterns of activity, suggesting a general shift from tonic to bursting neuronal activity states. In the PFC, pyramidal burst-firing generally indicates greater signal to noise ratio, which in turn can pathologically amplify the strength of selective signals entering the cortex. Moreover, altered burst-firing patterns of pyramidal PFC neurons can be indicators of dysregulated pyramidal cell function due to changes in the integration of multiple neuronal inputs51, which may underlie cognitive, motivational and affective deficits observed in psychiatric disorders. Importantly, cannabinoid transmission strongly modulates PFC pyramidal neuron encoding of associative emotional memories. For example, acute overstimulation of CB1R transmission in the PFC causes profound misattribution of emotional salience cues measured in associative fear memory paradigms, both by increasing the firing frequency and associative bursting activity of PFC neurons52, 53. Thus, adolescent THC exposure induces a PFC neuronal phenotype consistent with deficits in the ability to appropriately integrate competing cortical inputs in terms of their affective and/or attentional relevance, consistent with the profound behavioral abnormalities observed following adolescent THC exposure.
Consistent with our observation of mPFC neuronal hyperactivity and loss of GABAergic inhibitory tone, we observed a significant and persistent potentiation in gamma-band oscillatory activity states in the adult mPFC, following adolescent THC exposure. In the healthy brain, the interplay between GABA interneurons and excitatory pyramidal cells is critical for the generation of synchronous gamma oscillations54. This cortical oscillatory activity represents a fundamental mechanism to enable the normal functioning of distributed cortical networks and supports a variety of higher order information processing necessary for normal cognition, learning and memory. Considerable evidence demonstrates that dysregulated PFC gamma oscillations are a critical neuropathological feature of schizophrenia and may serve as a neurodevelopmental signature for the onset of first-episode schizophrenia55. For example, affective and social cognition disturbances are associated with abnormal patterns of PFC gamma activity in schizophrenia56, 57. In addition, hallucinations and reality distortion have been correlated with increased cortical gamma power in schizophrenia58, 59. High (>60 Hz) gamma-band oscillatory dysregulation may contribute to schizophrenia-related perceptual and cognitive deficits60–62. While numerous studies have reported reduced evoked levels of cortical gamma band activity, other studies have found that schizophrenia patients demonstrate potentiated spontaneous gamma band oscillatory patterns in the PFC and during working memory tasks63, 64, believed to be related to a loss of cortical GABAergic inhibitory mechanisms.
At the cellular level, CB1R transmission plays a role in the control of neuronal network gamma oscillations. For example, acute CB1R stimulation alters neuronal network oscillations in the gamma-frequency ranges65–67 in both hippocampus and cortex. Similarly, 5 days of exposure to a synthetic CB1R agonist during early adolescence in rats impaired the modulation of cortical oscillatory activity evoked by hippocampal stimulation8. Thus, acute or chronic CB1R activation can alter network oscillations in the mammalian PFC. Given the critical role of CB1R signaling in frontal cortical maturation8 and maintenance of normal oscillatory activity, the present findings provide further evidence for the critical role of neurodevelopmental CB1R signaling in the maturation of inhibitory cortical networks.
Given our evidence for a profound hypo-GABAergic PFC phenotype following adolescent THC exposure and studies linking downregulation of cortical GABAA receptor expression levels with schizophrenia-related deficits68, we examined the potential mechanistic role of mPFC GABAA receptor transmission by pharmacologically activating mPFC GABAA receptors. Remarkably, we found that direct mPFC GABAA receptor activation reversed adolescent THC-induced impairments in short-term memory as well as motivational and anxiety-related abnormalities in adult rats, while having no effects on adolescent VEH pretreated rats. Thus, it appears that under normal conditions, such as in adolescent VEH pretreated rats, enhancing mPFC GABAergic transmission has no effects on cognitive performance. In contrast, under pathological conditions involving hypo-GABAergic function, such as in adolescent THC pretreated rats, activation of mPFC GABAA transmission can reverse these deficits. Therefore, similar to observations in schizophrenia35, 69, 70 decreasing prefrontal GABAA transmission and disinhibition of the mPFC can disrupt cognitive and emotional function. However, these cognitive and emotional impairments can be restored by increasing prefrontal GABAA transmission. Similarly, it has been shown that mPFC inactivation via infusion of the GABAA receptor agonist muscimol, did not affect working and reference memories in rats tested in the radial-arm maze task. In contrast, mPFC disinhibition via infusions of the GABAA receptor antagonist bicuculline impairs both working and reference memory71 and impairs performance of PFC-independent cognitive tasks, suggesting that hyperactivity of the frontal lobes may interfere with cognitive/mnemonic processes mediated by downstream structures71. Thus, it is possible that in adolescent THC pretreated rats, memory processes may be disrupted by disinhibitory increases in mPFC activity, consistent with the mPFC glutamatergic hyperactivity that we found in these animals. Since the mPFC sends excitatory projections to the VTA72, it is possible that mPFC disinhibition-induced cognitive impairments may be driven by aberrant patterns of excitatory outflow to sub-cortical, mesolimbic structures. Therefore, although decreasing prefrontal GABAA transmission/disinhibition of the mPFC impaired cognitive performance, inhibition of mPFC neural activity with GABA agonists had no effect on cognitive performance in adolescent VEH pretreated rats but might selectively inhibit mPFC hyperactivity in adolescent THC pretreated rats.
Interestingly, we found that intra-mPFC GABAA receptor activation reversed social behavior deficits in adolescent THC pretreated rats, but caused impairments in VEH control rats. Similar results have been observed in previous studies. For example, intra-mPFC muscimol was shown to block social familiarity-induced reductions in anxiety in healthy rats73. Thus, under normal conditions, enhancing mPFC GABAergic transmission appears to suppress social motivation. However, under pathological conditions involving hypo-GABAergic function, such as those induced by adolescent THC exposure, increasing mPFC GABAergic tone may reverse social deficits and cognition, as demonstrated by the present findings, suggesting that the normal balance between excitation and inhibition within the prefrontal circuitry is crucial for the integration of socially relevant cues and social memory formation and that adolescent THC exposure may compromise normal social cognition by disturbing an intra-mPFC inhibitory GABAergic network.
Interestingly, clinical studies have demonstrated therapeutic effects of GABA-modulating drugs on schizophrenia-related symptoms, including cognitive deficits, hallucinations, agitation and anxiety74, 75. Moreover, increasing GABAA receptor function in schizophrenia patients with chronic administration of a GABAA α2/3-selective agonist was shown to reverse working memory deficits and cognitive control impairments, as well as normalizing gamma band power abnormalities associated with these symptoms68. To our knowledge, the present findings represent the first demonstration that a THC-induced neurodevelopmental cortical GABAergic deficit can be functionally reversed by direct pharmacological intervention in adulthood.
Increasing mPFC GABAergic tone not only rescued adolescent THC-induced affective/cognitive deficits, but also normalized VTA DAergic activity levels. Dysregulation of the mesocorticolimbic DA pathway is a cardinal neuropathological feature associated with schizophrenia-related symptoms. In terms of PFC-VTA interactions, the PFC sends dense glutamatergic, regulatory projections to VTA DA neurons76. Thus, we hypothesized that our observed sub-cortical DAergic hyperactive phenotype may be directly linked to hyperactive excitatory drive from the PFC. Consistent with this model, previous studies have reported that PFC GABAA receptor antagonist infusions induced schizophrenia-like cognitive and affective disturbances in rodents and primates35, 77. Furthermore, the partial GABAA receptor agonist imidazenil reverses methionine-induced schizophrenia-like deficits in rodents, including social interaction and sensorimotor gating impairments78. In addition, knockout of the GABAA α3-subunit was shown to induce a hyper-DAergic state and sensorimotor gating deficits which could be reversed with antipsychotics34. Since tonic DA release is thought to be controlled by PFC GLUTergic afferents79, one possible mechanistic explanation is that direct activation of PFC GABAA transmission within the PFC, may reverse PFC overdrive to sub-cortical DA targets and associated cognitive and affective deficits, as suggested by the present findings.
In summary, our results demonstrate that hypofunction of inhibitory PFC GABAergic neurotransmission plays a crucial role in the behavioral and neuronal effects observed following adolescent THC exposure. These findings improve our knowledge of the precise neurobiological mechanisms leading to the long-term deleterious effects of THC exposure during adolescence and the later risk of schizophrenia onset. More importantly, these findings demonstrate that THC-induced neurodevelopmental insults to the PFC-mesolimbic circuitry may be reversible by restoration of GABAergic cortical tone, even into adulthood.
Methods
Animals and housing
Adolescent Sprague-Dawley rats were obtained at postnatal day (PND) 28 from Charles River Laboratories (Quebec, Canada). At arrival, rats were pair housed under controlled conditions (12 h light/dark cycle, constant temperature, and humidity) with free access to food and water. All procedures were performed in accordance with Governmental and Institutional guidelines for appropriate rat care and experimentation. The experimental protocols were approved by the Canadian Council on Animal Care and the Animal Care Committee at Western University, Ontario.
Adolescent THC administration
Rats treated with THC (Cayman Chemical) received twice daily injections of THC (2.5 mg/kg; Days 1–3; 5 mg/kg; Days 4–7; 10 mg/kg, Days 8–11). Control groups received the same injection schedule (volume adjusted per body weight) with vehicle (VEH). Increasing doses of THC were administered to counter the development of drug tolerance80. These doses of THC were chosen based on our and others previously published protocols6, 50, 81–83 and are known to produce long-term behavioral and molecular and neuronal effects in rats. Importantly, this escalating THC exposure protocol mimics a heavy use regiment of marijuana exposure, with the 2.5 mg/kg dose corresponding to approximately one cannabis cigarette, the 5 mg/kg dose corresponding to approximately two cannabis cigarettes, and the 10 mg/kg dose corresponding to approximately four cannabis cigarettes50. THC in ethanol was dissolved in cremophor and saline (1:1:18). Ethanol was then evaporated using nitrogen gas to remove it from the final THC solution. All injections were administered intraperitoneally (i.p). The adolescent exposure experiments began at PND 35. Experimental procedures were initiated following a 30 days drug-free period (at PND 75).
Protein Expression Analyses
At adulthood (PND75), rats received an overdose of sodium pentobarbital (240 mg/kg, i.p., EuthanylTM). Under deep anesthesia rats were decapitated and brains removed and frozen. Coronal sections (60 μm) containing the PFC were cut on a cryostat and slide mounted. Bilateral micro-punches of the mPFC, were obtained for protein isolation. The western blotting procedure was performed as described previously84. Primary antibody dilutions were as follows: α-tubulin (1:120 000; Sigma-Aldrich), GAD67 (1:1000; Cell Signaling Technology), GAD65 (1:200; Santa Cruz Biotechnology) and Parvalbumin (1:2000; Sigma-Aldrich). Species appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (Thermo Scientific) were all used at a dilution of 1:20 000.
Neuronal Activity Recordings and Analysis
Extracellular single-unit electrophysiological recordings were performed in vivo in adult (>PND 75) VEH and THC pretreated rats during adolescence. The recordings were taken from either putative glutamatergic (GLUT) PFC neurons (cells/animals: VEH = 99/6, THC = 122/10) or dopaminergic (DA) VTA neurons (cells/animals: VEH = 45/24, THC = 40/20). Rats were anesthetized with urethane (1.4 g/kg, i.p., Sigma-Aldrich) and placed in a stereotaxic frame with body temperature maintained at 37 °C. A scalp incision was made and a hole was drilled in the skull overlaying the targeted structure at the following coordinates: mPFC: AP: +2.7 to +3.5 mm from bregma, L: ±0.8 to ±1 mm, DV: −2.5 to −4 mm from the dural surface; VTA: AP: −5.2 mm from bregma, L: ±0.8 to ±1 mm, DV: −6.5 to −9 mm from the dural surface. Recordings were taken with glass microelectrodes (average impedance of 6–10 MΩ) filled with 2 M sodium acetate solution containing 2% pontamine sky blue (Sigma-Aldrich). A bone screw was placed over the cerebellum and was connected with the return of the headstage and served as a reference electrode. Extracellular signals were amplified (×5000) using a MultiClamp 700B amplifier (Molecular Devices), digitized at 25 kHz and recorded on the computer using a Digidata 1440 A and pClamp software (Molecular Devices). The wideband signal of PFC recordings was fed to two channels of the digitizer and filtered to obtain single unit recordings (band pass between 0.3 and 3 kHz) and local field potentials (LFPs; low pass at 0.3 kHz). For the VTA electrophysiology only unit data were recorded.
Putative mPFC pyramidal cells were identified based on established criteria85: (1) firing frequency < 10 Hz, (2) waveform shape, and (3) action potential duration > 2.5 ms. Cells exhibiting 3 consecutive spikes with inter-spike intervals < 45 ms were classified as burst-firing cells85. The electrophysiological properties of spontaneously active pyramidal neurons were sampled in the mPFC by making vertical passes of the electrode through the pyramidal cell body region. These tracks were made in a predefined pattern, with each track separated by 200 µm. After an individual putative pyramidal neuron was isolated, its spontaneous activity was recorded for 5 min. Two parameters of activity were sampled, the basal firing rate and the bursting rate.
VTA DA neurons were identified according to well established electrophysiological features86: (1) action potential width > 2.5 ms, (2) spontaneous firing rate between 2–5 Hz, (3) a triphasic waveform consisting of a notch on the rising phase followed by a delayed after potential, and (4) a single irregular or burst firing pattern. For intra-mPFC microinfusions of VEH or Muscimol (MUS, 1 μg/0.5 μl) a 10 μl Hamilton syringe was slowly lowered into the mPFC using the same stereotaxic coordinates as described above. The firing frequencies of collective VTA putative DA neurons from adolescent THC pretreated rats before microinfusions of VEH or muscimol into the mPFC were averaged and normalized to the average firing frequency of collective VTA neurons from adolescent VEH pretreated rats. The response patterns of isolated VTA neurons to microinfusion of VEH or MUS into the mPFC were determined by comparing the neuronal frequency rates between the 5 min preinfusion vs. postinfusion recording epochs. We also analyzed the proportion of DA neuronal spikes fired in burst mode. The onset of a burst was defined as the occurrence of two consecutive spikes with an interspike interval of 80 ms86. The percentage of burst spikes was calculated by dividing the number of spikes occurring in bursts by the total number of spikes occurring in the same period of time. We sampled a total of n = 85 VTA DA neurons (Adolescent VEH pretreated-Intra mPFC VEH group; n = 15 cells in 7 rats; Adolescent VEH pretreated- Intra-mPFC MUS group; n = 30 cells in 17 rats; Adolescent THC pretreated- Intra mPFC VEH group; n = 14 cells in 7 rats; Adolescent THC pretreated- Intra mPFC MUS group; n = 26 cells in 13 rats).
LFP signals were analyzed using NeuroExplorer (Nex Technologies). First, the signals were decimated to 1 kHz, and lowpass filtered (IIR Butterworth filter at 170 Hz; filter order set to 3). Subsequently a spectrogram function was used to calculate the power of oscillations at frequencies between 0–100 Hz (window length 2 s; shift 0.5 s). One minute long recording epochs were used for estimating the average power spectrum distributions. Epochs were selected such as either the desynchronized (relatively small signal amplitude devoid of slow oscillations) or synchronized (relatively large signal amplitude with slow oscillations present) cortical state could be easily distinguished. Power values for a given frequency were averaged over time of the recording epoch and normalized so that the sum of all power spectrum values equals 1. The total power was calculated by adding all the power values at frequencies between 0–59 and 61–100 Hz. Power values at 60 ± 1 Hz were excluded from all the calculations. Gamma band was defined as frequency between 30–80 Hz and subcategorized into low gamma (30–59 Hz) and high gamma (61–80 Hz). Statistics are based on 98 recordings of desynchronized state (VEH = 37, THC = 61) and 174 recordings of synchronized state (VEH = 107, THC = 67). Every recording was taken at different electrode location throughout mPFC.
For histological analyses the recording electrodes positions were marked with an iontophoretic deposit of pontamine sky blue dye (−20 mA, continuous current for 15 min). Histological analysis was performed as described previously47. No mPFC cells were recorded outside the anatomical boundaries of the mPFC as defined by Paxinos and Watson (2007). One recorded rat was excluded from electrophysiological data analysis because its cells were recorded outside the anatomical boundaries of the VTA as defined by Paxinos and Watson (2007).
Surgical Procedures
At adulthood (PND) 75, rats were anesthetized with an intraperitoneal injection of ketamine (80 mg/mL, Vetoquinol)-xylazine (6 mg/kg, Bayer) mixture. To minimize pain and inflammation, meloxicam (1 mg/kg; s.c., Boehringer Ingelheim) was administered before and after surgeries. Rats were positioned in a Kopf stereotaxic device for cannulae implantation. Stainless steel guide cannulae (22-gauge) were implanted bilaterally into the mPFC with the coordinates (15° angle, in mm from bregma): AP: +2.9 L: ±1.9 mm: DV: −3 mm from the dural surface. The guide cannulae were secured into position by jeweler’s screws and dental acrylic cement. Rats were singly housed post-surgery and behavioral tests were initiated after one week of recovery.
Intra-mPFC Microinfusions
Intra-mPFC microinfusions of either vehicle (VEH, NaCl 0.9%) or the selective GABAA receptor agonist, muscimol (500 ng/0.5 µl, Sigma-Aldrich, diluted in NaCl 0.9%) were performed immediately before behavioral experiments. A total volume of 0.5 μl per hemisphere was delivered via a 28 gauge injector over a period of 1 min. Microinjectors were left in place for an additional 1 min following infusions to ensure adequate diffusion from the tip.
Behavioral Testing
Object recognition
Rats were tested using the object recognition task as described previously87. This task evaluates the ability of the rat to discriminate between the familiarity of previously encountered objects; normal rats typically spend more time exploring a novel object than a familiar object. The test sessions consisted of two 3-min trials. During the first trial (T1 acquisition trial), each rat was placed in the center of an arena containing two identical objects placed in the far corners 15 cm from the side wall. After a delay of 60 min during which the rat was returned to its cage, and both objects were replaced (one by an identical copy, the other by a novel object in the same location), the rat was returned to the arena for the second trial (T2 test trial). Between rats, both the role (familiar or novel object) and the relative position of the two objects were randomly counterbalanced. Object exploration was considered when the head of the rat was facing the object or the rat was touching or sniffing the object. Times spent in exploration were videotaped with a video-tracking system (ANY-maze; Stoelting) and analyzed by an experimenter blind to the treatment conditions. Exploration times were recorded and used to calculate object recognition index [time spent with novel object/total time exploring both objects] *100. The final number of rats in each group was as follows: Adolescent VEH-Intra-mPFC VEH (VEH/VEH) group, n = 10; Adolescent VEH-Intra-mPFC MUS (VEH/MUS) group, n = 9; Adolescent THC-Intra-mPFC VEH (THC/VEH) group, n = 9; Adolescent THC-Intra-mPFC MUS (THC/MUS) group, n = 10.
Social motivation and social cognition
Rats were tested using a social interaction procedure as described previously6. Briefly, this task evaluates 2 distinguishable aspects of social behavior: (1) social affiliation/motivation and (2) social recognition memory. Rats were habituated to the test arena for 13 min, 24 h before testing. Testing consisted of 2 successive 8-min phases. During the first phase, we analyzed social motivation, that is, the propensity to spend time with an unfamiliar male rat (stranger rat) enclosed in a small wire cage compared with time spent with an identical but empty cage. During the second phase, occurring just after the first one, we analyzed social recognition, that is, the propensity to spend time with a novel unfamiliar rat (novel stranger) rather than with the familiar stranger rat (encountered during the first phase). The locations of stranger vs. novel rats in the left vs. right side chambers were counterbalanced between trials. Times spent in exploration were videotaped with a video-tracking system (ANY-maze; Stoelting) and analyzed by an experimenter blind to the treatment conditions. After each test, chambers and cages were cleaned with 50% ethanol to avoid olfactory cue bias. Exploration times were recorded and used to calculate a social motivation or cognition index [time spent with stranger (or novel stranger)/total time exploring both rats] *100. The final number of rats in each group was as follows: Adolescent VEH-Intra-mPFC VEH (VEH/VEH) group, n = 12; Adolescent VEH-Intra-mPFC MUS (VEH/MUS) group, n = 11; Adolescent THC-Intra-mPFC VEH (THC/VEH) group, n = 12; Adolescent THC-Intra-mPFC MUS (THC/MUS) group, n = 10.
Light dark box
This test is based upon a rat’s natural aversion to bright environments and attributes greater time spent in an illuminated environment as reflecting lower anxiety levels. The test was performed as previously described6. At the start of the experiment, a rat was placed in the center of the lighted box with its head facing the wall opposite the door and was allowed to freely explore both compartments for a period of 8 min. A zone entry was considered to have begun when the rat placed all 4 paws in that zone. Experiments were videotaped with a video-tracking system (ANY-maze;Stoelting) and analyzed by an experimenter blind to treatment conditions. Behaviors analyzed included latency time to leave the dark box and enter the light box (latency to second transition), which is thought to be the most reliable indicator of anxiety-like behavior and is sensitive to both anxiogenic and anxiolytic treatments88. The final number of rats in each group was as follows: Adolescent VEH-Intra-mPFC VEH (VEH/VEH) group, n = 9; Adolescent VEH-Intra-mPFC MUS (VEH/MUS) group, n = 12; Adolescent THC-Intra-mPFC VEH (THC/VEH) group, n = 12; Adolescent THC-Intra-mPFC MUS (THC/MUS) group, n = 10.
Open field test
Rats were placed in an automated open field activity chamber (San Diego Instruments, San Diego, CA, USA) for 60 min. Total distance travelled and vertical counts were recorded and analyzed. The final number of rats in each group was as follows: Adolescent VEH-Intra-mPFC VEH (VEH/VEH) group, n = 11; Adolescent VEH-Intra-mPFC MUS (VEH/MUS) group, n = 12; Adolescent THC-Intra-mPFC VEH (THC/VEH) group, n = 12; Adolescent THC-Intra-mPFC MUS (THC/MUS) group, n = 9.
Statistical analysis
The data were analyzed using t-tests, one or two-way ANOVA. Post hoc analyses were calculated using Fisher’s LSD. Densitometry values for Western blots were acquired with Kodak digital analysis software and analyzed with t-tests.
Data availability
The authors declare that the data supporting the findings of this study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
This work was supported by the Canadian Institutes of Health Research (CIHR; MOP 246144) and the National Science and Engineering Research Council of Canada (NSERC).
Author Contributions
In vivo Electrophysiological recordings were performed by J.R., C.P.K., K.M. and H.J.S. Behavioural experiments were performed by J.R. Tissue extractions were performed by J.R., C.E.L.J., W.J.R. and S.R.L. Western blots were performed by C.E.L.J. and W.J.R. Experimental design and conception were done by J.R., W.J.R. and S.R.L. Analyses were performed by J.R. and H.J.S. The paper has been written by J.R. and S.R.L. The project was supervised by S.R.L.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-11645-8
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Andréasson S, Allebeck P, Engström A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2:1483–1486. doi: 10.1016/S0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- 2.Arseneault L, et al. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: Examination of the evidence. British Journal of Psychiatry. 2004;184:110–117. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- 4.Stefanis NC, et al. Early adolescent cannabis exposure and positive and negative dimensions of psychosis. Addiction. 2004;99:1333–1341. doi: 10.1111/j.1360-0443.2004.00806.x. [DOI] [PubMed] [Google Scholar]
- 5.Renard J, Krebs MO, Le Pen G, Jay TM. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front Neurosci. 2014;8:1–14. doi: 10.3389/fnins.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renard, J. et al. Adolescent Cannabinoid Exposure Induces a Persistent Sub-Cortical Hyper-Dopaminergic State and Associated Molecular Adaptations in the Prefrontal Cortex. Cereb. Cortex doi:10.1093/cercor/bhv335 (2016). [DOI] [PubMed]
- 7.Smith N. High potency cannabis: the forgotten variable. Addiction (Abingdon, England) 2005;100:1551–1558. doi: 10.1111/j.1360-0443.2005.01295.x. [DOI] [PubMed] [Google Scholar]
- 8.Cass DK, et al. CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex. Mol. Psychiatry. 2014;19:536–43. doi: 10.1038/mp.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caballero A, Flores-Barrera E, Cass DK, Tseng KY. Differential regulation of parvalbumin and calretinin interneurons in the prefrontal cortex during adolescence. Brain Struct. Funct. 2014;219:395–406. doi: 10.1007/s00429-013-0508-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long LE, Lind J, Webster M, Weickert C. Developmental trajectory of the endocannabinoid system in human dorsolateral prefrontal cortex. BMC Neuroscience. 2012;13:87. doi: 10.1186/1471-2202-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heng L, Beverley JA, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 2011;65:278–286. doi: 10.1002/syn.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellgren M, et al. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur. Neuropsychopharmacol. 2008;18:826–834. doi: 10.1016/j.euroneuro.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volk DW, Lewis DA. GABA Targets for the Treatment of Cognitive Dysfunction in Schizophrenia. Curr. Neuropharmacol. 2005;3:45–62. doi: 10.2174/1570159052773396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? In. Neuroscience and Biobehavioral Reviews. 2003;27:3–18. doi: 10.1016/S0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 15.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Straub RE, et al. Allelic variation in GAD1 (GAD67) is associated with\rschizophrenia and influences cortical function and gene\rexpression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- 17.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto T, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guidotti A, et al. Decrease in Reelin and Glutamic Acid Decarboxylase 67 (GAD 67) Expression in Schizophrenia and Bipolar Disorder. Arch. Gen. Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 20.Akbarian S, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 21.Volk, D. W., Austin, M. C., Pierri, J. N., Sampson, A. R. & Lewis, D. A. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch. Gen. Psychiatry57, 237–245 (2000). [DOI] [PubMed]
- 22.Hashimoto T, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J. Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curley AA, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am. J. Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo T-U, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc. Natl. Acad. Sci. USA. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vawter MP, et al. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: A preliminary study. Schizophr. Res. 2002;58:11–20. doi: 10.1016/S0920-9964(01)00377-2. [DOI] [PubMed] [Google Scholar]
- 26.Maldonado-Avilés JG, et al. Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. Am. J. Psychiatry. 2009;166:450–459. doi: 10.1176/appi.ajp.2008.08101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huntsman, M. M., Tran, B. V., Potkin, S. G., Bunney, Jr., W. E. & Jones, E. G. Altered ratios of alternatively spliced long and short g2 subunit mRNAs of the g-amino butyrate type A receptor in prefrontal cortex of schizophrenics. Proc Natl Acad Sci USA95, 15066–71. (1998). [DOI] [PMC free article] [PubMed]
- 28.Aoki F, Fetz EE, Shupe L, Lettich E, Ojemann GA. Increased gamma-range activity in human sensorimotor cortex during performance of visuomotor tasks. Clin. Neurophysiol. 1999;110:524–537. doi: 10.1016/S1388-2457(98)00064-9. [DOI] [PubMed] [Google Scholar]
- 29.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardin JA, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–7. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon E, Williams L, Haig AR, Wright J, Meares RA. Symptom profile and ‘gamma’ processing in schizophrenia. Cogn. Neuropsychiatry. 2001;6:7–19. doi: 10.1080/13546800042000016. [DOI] [Google Scholar]
- 32.Lee KH, Williams LM, Breakspear M, Gordon E. Synchronous Gamma activity: A review and contribution to an integrative neuroscience model of schizophrenia. Brain Research Reviews. 2003;41:57–78. doi: 10.1016/S0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- 33.Nakazawa K, et al. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62:1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yee BK, et al. A schizophrenia-related sensorimotor deficit links alpha 3-containing GABAA receptors to a dopamine hyperfunction. Proc Natl Acad Sci USA. 2005;102:17154–17159. doi: 10.1073/pnas.0508752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enomoto T, Tse MT, Floresco SB. Reducing prefrontal gamma-aminobutyric acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol. Psychiatry. 2011;69:432–441. doi: 10.1016/j.biopsych.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 36.Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci. Lett. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-W. [DOI] [PubMed] [Google Scholar]
- 37.Taber MT, Das S, Fibiger HC. Cortical Regulation of Subcortical Dopamine Release: Mediation via the Ventral Tegmental Area. J. Neurochem. 1995;65:1407–1410. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- 38.Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 39.Uylings HBM, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behavioural Brain Research. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 40.Seillier A, Martinez AA, Giuffrida A. Phencyclidine-induced social withdrawal results from deficient stimulation of cannabinoid CB1 receptors: implications for schizophrenia. Neuropsychopharmacology. 2013;38:1816–24. doi: 10.1038/npp.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ochsner KN. The Social-Emotional Processing Stream: Five Core Constructs and Their Translational Potential for Schizophrenia and Beyond. Biological Psychiatry. 2008;64:48–61. doi: 10.1016/j.biopsych.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Millan MJ, et al. Selective blockade of dopamine D3 versus D2 receptors enhances frontocortical cholinergic transmission and social memory in rats: A parallel neurochemical and behavioural analysis. J. Neurochem. 2007;100:1047–1061. doi: 10.1111/j.1471-4159.2006.04262.x. [DOI] [PubMed] [Google Scholar]
- 43.Bicks LK, Koike H, Akbarian S, Morishita H. Prefrontal cortex and social cognition in mouse and man. Front. Psychol. 2015;6:1–15. doi: 10.3389/fpsyg.2015.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell JP, Macrae CN, Banaji MR. Dissociable Medial Prefrontal Contributions to Judgments of Similar and Dissimilar Others. Neuron. 2006;50:655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 45.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 46.Wood JN, Romero SG, Makale M, Grafman J. Category-specific representations of social and nonsocial knowledge in the human prefrontal cortex. J Cogn Neurosci. 2003;15:236–248. doi: 10.1162/089892903321208178. [DOI] [PubMed] [Google Scholar]
- 47.Loureiro M, Renard J, Zunder J, Laviolette SR. Hippocampal Cannabinoid Transmission Modulates Dopamine Neuron Activity: Impact on Rewarding Memory Formation and Social Interaction. Neuropsychopharmacology. 2014;40:1436–1447. doi: 10.1038/npp.2014.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends in Neurosciences. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fatemi SH, et al. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol. Psychiatry. 2002;52:805–810. doi: 10.1016/S0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- 50.Zamberletti E, et al. Alterations of prefrontal cortex GABAergic transmission in the complex psychotic-like phenotype induced by adolescent delta-9-tetrahydrocannabinol exposure in rats. Neurobiol. Dis. 2014;63:35–47. doi: 10.1016/j.nbd.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 51.Homayoun H, Moghaddam B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J. Neurosci. 2006;26:8025–8039. doi: 10.1523/JNEUROSCI.0842-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laviolette SR, Grace AA. Cannabinoids Potentiate Emotional Learning Plasticity in Neurons of the Medial Prefrontal Cortex through Basolateral Amygdala Inputs. J. Neurosci. 2006;26:6458–6468. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan H, et al. Cannabinoid transmission in the basolateral amygdala modulates fear memory formation via functional inputs to the prelimbic cortex. J Neurosci. 2011;31:5300–5312. doi: 10.1523/JNEUROSCI.4718-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buzsáki, G. & Wang, X.-J. Mechanisms of Gamma Oscillations. Annu. Rev. Neurosci. 203–225, doi:10.1146/annurev-neuro-062111-150444 (2012). [DOI] [PMC free article] [PubMed]
- 55.Symond MB, Harris AWF, Gordon E, Williams LM. ‘Gamma synchrony’ in first-episode schizophrenia: A disorder of temporal connectivity? Am. J. Psychiatry. 2005;162:459–465. doi: 10.1176/appi.ajp.162.3.459. [DOI] [PubMed] [Google Scholar]
- 56.Gandal MJ, et al. GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl. Psychiatry. 2012;2:e142. doi: 10.1038/tp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams LM, et al. Emotion-elicited gamma synchrony in patients with first-episode schizophrenia: A neural correlate of social cognition outcomes. J. Psychiatry Neurosci. 2009;34:303–313. [PMC free article] [PubMed] [Google Scholar]
- 58.Lee KH, Williams LM, Haig A, Gordon E. ‘Gamma (40 Hz) phase synchronicity’ and symptom dimensions in schizophrenia. Cogn Neuropsychiatry. 2003;8:57–71. doi: 10.1080/713752240. [DOI] [PubMed] [Google Scholar]
- 59.Baldeweg T, Spence S, Hirsch SR, Gruzelier J. Gamma-band electroencephalographic oscillations in a patient with somatic hallucinations. Lancet (London, England) 1998;352:620–621. doi: 10.1016/S0140-6736(05)79575-1. [DOI] [PubMed] [Google Scholar]
- 60.Hamm JP, Gilmore CS, Picchetti NAM, Sponheim SR, Clementz BA. Abnormalities of neuronal oscillations and temporal integration to low- and high-frequency auditory stimulation in schizophrenia. Biol. Psychiatry. 2011;69:989–996. doi: 10.1016/j.biopsych.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsuchimoto R, et al. Reduced high and low frequency gamma synchronization in patients with chronic schizophrenia. Schizophr. Res. 2011;133:99–105. doi: 10.1016/j.schres.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 62.Grützner C, et al. Deficits in high- (>60 Hz) gamma-band oscillations during visual processing in schizophrenia. Front. Hum. Neurosci. 2013;7:88. doi: 10.3389/fnhum.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barr MS, et al. Evidence for excessive frontal evoked gamma oscillatory activity in schizophrenia during working memory. Schizophr. Res. 2010;121:146–152. doi: 10.1016/j.schres.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 64.Farzan F, et al. Evidence for gamma inhibition deficits in the dorsolateral prefrontal cortex of patients with schizophrenia. Brain. 2010;133:1505–1514. doi: 10.1093/brain/awq046. [DOI] [PubMed] [Google Scholar]
- 65.Morrison PD, et al. Disruption of frontal theta coherence by Delta9-tetrahydrocannabinol is associated with positive psychotic symptoms. Neuropsychopharmacology. 2011;36:827–836. doi: 10.1038/npp.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holderith N, et al. Cannabinoids attenuate hippocampal gamma oscillations by suppressing excitatory synaptic input onto CA3 pyramidal neurons and fast spiking basket cells. J. Physiol. 2011;589:4921–4934. doi: 10.1113/jphysiol.2011.216259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hajós M, Hoffmann WE, Kocsis B. Activation of Cannabinoid-1 Receptors Disrupts Sensory Gating and Neuronal Oscillation: Relevance to Schizophrenia. Biol. Psychiatry. 2008;63:1075–1083. doi: 10.1016/j.biopsych.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 68.Lewis DA, et al. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am. J. Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piantadosi PT, Floresco SB. Prefrontal Cortical GABA Transmission Modulates Discrimination and Latent Inhibition of Conditioned Fear: Relevance for Schizophrenia. Neuropsychopharmacology. 2014;39:2473–2484. doi: 10.1038/npp.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paine TA, Slipp LE, Carlezon WA. Schizophrenia-Like Attentional Deficits Following Blockade of Prefrontal Cortex GABAA Receptors. Neuropsychopharmacology. 2011;36:1703–1713. doi: 10.1038/npp.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Auger, M. L. & Floresco, S. B. Prefrontal cortical GABA modulation of spatial reference and working memory. Int. J. Neuropsychopharmacol. 18 (2015). [DOI] [PMC free article] [PubMed]
- 72.French ED, Dillon K, Wu X. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport. 1997;8:649–652. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- 73.Lungwitz EA, et al. The role of the medial prefrontal cortex in regulating social familiarity-induced anxiolysis. Neuropsychopharmacology. 2014;39:1009–1019. doi: 10.1038/npp.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Delini-Stula A, Berdah-Tordjman D, Neumann N. Partial benzodiazepine agonists in schizophrenia: expectations and present clinical findings. Clin. Neuropharmacol. 1992;15(Suppl 1):405A–406A. doi: 10.1097/00002826-199201001-00211. [DOI] [PubMed] [Google Scholar]
- 75.Delini-Stula A, Berdah-Tordjman D. Antipsychotic effects of bretazenil, a partial benzodiazepine agonist in acute schizophrenia - A study group report. J. Psychiatr. Res. 1996;30:239–250. doi: 10.1016/0022-3956(96)00003-9. [DOI] [PubMed] [Google Scholar]
- 76.Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J. Comp. Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- 77.Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J. Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tremolizzo L, et al. Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol. Psychiatry. 2005;57:500–509. doi: 10.1016/j.biopsych.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 79.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-U. [DOI] [PubMed] [Google Scholar]
- 80.González S, Cebeira M, Fernández-Ruiz J. Cannabinoid tolerance and dependence: A review of studies in laboratory animals. in. Pharmacology Biochemistry and Behavior. 2005;81:300–318. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 81.Rubino T, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- 82.Rubino T, et al. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33:2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- 83.Realini N, et al. Chronic URB597 treatment at adulthood reverted most depressive-like symptoms induced by adolescent exposure to THC in female rats. Neuropharmacology. 2011;60:235–43. doi: 10.1016/j.neuropharm.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 84.Lyons D, et al. Opiate exposure and withdrawal induces a molecular memory switch in the basolateral amygdala between ERK1/2 and CaMKIIα-dependent signaling substrates. J. Neurosci. 2013;33:14693–704. doi: 10.1523/JNEUROSCI.1226-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parsegian A, Glen WB, Lavin A, See RE. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol. Psychiatry. 2011;69:253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons–1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- 87.Ennaceur A, Delacour J. A new one-trial test for neurobio- logical studies of memory in rats. Behav. Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-X. [DOI] [PubMed] [Google Scholar]
- 88.Ardayfio P, Kim K-S. Anxiogenic-like effect of chronic corticosterone in the light-dark emergence task in mice. Behav. Neurosci. 2006;120:249–256. doi: 10.1037/0735-7044.120.2.249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available from the corresponding author on reasonable request.