Abstract
An appropriate balance between cell survival and cell death is essential for correct pattern formation in the animal tissues and organs. Previous studies have shown that the short-range signalling molecule Hedgehog (Hh) is required for cell proliferation and pattern formation in the Drosophila central wing discs. Signal transduction by one of the Hh targets, the morphogen Decapentaplegic (Dpp), is required for not only cell proliferation, but also cell survival in the pouch cells. However, Hh function in cell survival and cell death has not been revealed. Here, we found that loss of Hh signal activity induces considerable Caspase-dependent cell death in the wing pouch cells, and this process was independent of both Dpp signalling and Jun-N-terminal kinase (JNK) signalling. Loss of Hh induced activation of the pro-apoptotic gene hid and inhibition of diap1. Therefore, we identified an important role of Hh signalling in cell survival during Drosophila wing development.
Introduction
The balance between cell death and cell survival is essential for the development of animal tissues and organs. The disturbance of this balance by massive cell death can result in a great deal of cell loss and can cause developmental defects and diseases1. The lack of survival factors results in ectopic apoptosis and further induces tissue abnormalities.
The cell death pathway is highly conserved across animal species2, 3. Apoptosis, also known as Programmed Cell Death (PCD), is conducted through a strictly regulated progress4. Various types of stimulation, such as X-ray irradiation, mechanical stress and genetic variations, can induce cell death by inducing the expression of pro-apoptotic genes, reaper 5, hid 6, and grim 7 (RHG proteins), and finally by activating Caspases which degrade cellular substrates. There are 7 caspase genes in Drosophila8, divided into two classes: the initiator caspases and effector caspases. The effector caspases Drice9 and Dcp110 are activated by the initiator caspase Dronc11, 12. Caspases are repressed by Inhibitor of Apoptosis Proteins (Diap1) in the absence of cell death stimulation13–15. In the presence of a cell death stimulus, Diap1 is inhibited by RHG proteins. The pan-caspase inhibitor P35 can specifically block the function of the effector caspases Drice and Dcp-1 without affecting the activity of the initiator caspase Dronc16.
The morphogen Decapentaplegic (Dpp) is required for the cell survival to ensure normal tissue morphology by extruding or degrading the damaged cells17, 18. Dpp is expressed in a stripe abutting the A/P compartment boundary and forming a precise concentration gradient along the A/P axis19–22. Dpp binds and activates the receptor complex Thickvein (Tkv)/Punt (Put), which phosphorylates Mad to PMad23. PMad, together wigh Medea (Med), enters the nucleus and regulate the target genes expression, including sal 24 and omb 25–27. One target of PMad, Daughter against dpp (Dad), can regulate Dpp signalling activity via negative feedback28–30. The continuous gradient of Dpp signalling activity is required for the cell survival. Sharp discontinuity of either Dpp signalling or Dpp targets can induce JNK-dependent apoptosis which results in aberrant morphogenesis17, 18, 26. JNK, encoded by basket (bsk) 31, 32 and activated by the MAP kinase kinase Hemipterous (Hep)33, is involved in apoptotic signalling in various tissues.
Dpp is one of the targets of Hedgehog (Hh) which has been considered as a short-range signal34–37. Hh plays a crucial role in proliferation and pattern formation in the central Drosophila wing disc38–41. The components of Hh were initially identified in Drosophila and are conserved in mammals42. In Drosophila wing disc, Hh is expressed in the posterior compartment and secreted into anterior compartment43. The transportation of Hh from posterior to anterior compartment requires Tout-velu (Ttv)44, 45. In anterior compartment, Hh binds to receptor Patched (Ptc) to derepress the activity of a transmembrane protein Smoothened (Smo)44, 46, 47. The activated Smo maintains Cubitus interruptus (Ci) in an active form48. The Ci[act] enters the nucleus and induces target genes expression, including engrailed (en), ptc, Collier (col), and dpp. These target genes are activated in a Hh-concentration dependent manner: The cells close to the AP compartment boundary receive the highest level of Hh and induce the ptc and en; the cells away from the AP compartment boundary will receive the lowest level of Hh and induce the expression of col; the cells between these two type of cells receive the moderate level of Hh and induce the expression of dpp 49–52. ptc acting as the target gene of Hh signaling also inhibits Smo expression in the absence of Hh46.
Previous studies have demonstrated that Hh plays an important role in the proliferation38–40 and patterning41, 53–55. Hh also controls cell survival in germ cells56, 57, neural crest cells58, 59 as well as tumor cells60, 61 in vertebrate. A recent study has shown that in Drosophila eye disc, deregulated Hh signalling promotes cell survival in a non-autonomous manner62. However, it is not clear whether Hh signalling is also involved in the control of cell survival in wing disc. Here, we found that Hh signaling plays an important role in the cell survival in the Drosophila wing pouch. Lacking Hh signaling induced cell death is independent of Dpp and JNK signaling pathways.
Results and Discussion
Down-regulation of Hh signalling results in apoptosis in Drosophila wing disc
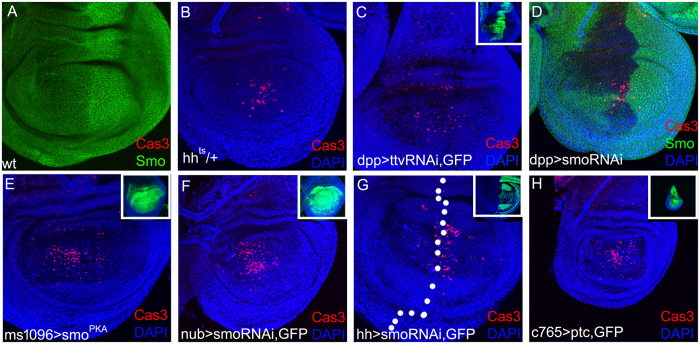
The wild-type wing disc undergoes rapid proliferation with little apoptosis (Fig. 1A). When down-regulating Hh expression using a temperature-sensitive allele, hhts 46, apoptosis, indicated by anti-Caspase-3 staining, occurred in the wing pouch (Fig. 1B). Then, the Hh transportation from the posterior to the anterior was blocked by expressing ttv-RNAi in the dpp-Gal4 domain, obvious apoptosis was consistently observed in the central wing discs (Fig. 1C). Then, we assessed whether Smo mediates the role of Hh in regulating apoptosis. Apparent apoptosis was also induced in the central wing discs when smo was inhibited by the expression of smo-RNAi in the dpp-Gal4 domain (Fig. 1D). To further confirm the above results, Hh signalling activity was suppressed by expressing smo PKA12 (a mutation at the PKA site)35, smo-RNAi, and ptc in all the wing disc cells (driven by c765-Gal4), wing pouch cells (driven by ms1096-Gal4 and nub-Gal4), and posterior cells (driven by hh-Gal4). All these manipulations caused obvious apoptosis in the medial wing discs (Fig. 1E–H). These data suggests that suppression of Hh singling, at the levels of transcription, transportation, or signal transduction, induces cell death in the medial Drosophila wing disc, thereby revealing a new role for Hh signalling in cell survival.
Figure 1.
Hh signalling activity is required for cell survival in Drosophila wing disc. In this and subsequent figures, wing discs are oriented with dorsal up and anterior left. (A) In the wild-type wing disc, there is no obvious apoptosis indicated by anti-Caspase-3 staining (red). The smo expression pattern is revealed by anti-Smo staining (green). (B) Heterozygote of a hh temperature-sensitive mutant allele showing the induction of apparent cell death (red). (C) Suppression of Hh transportation from posterior to anterior by expressing ttv-RNAi in the dpp-Gal4 domain (inset panel, green) results in apparent cell death (red). (D) Smo (green) is suppressed by expressing smo-RNAi in the dpp-Gal4 domain, and that induces massive cell death (red). (E) Suppressing smo by expressing a mutant smo PKA in all the wing pouch cells induces massive cell death (red). ms1096-Gal4 is expressed in all the wing pouch cells with a higher activity within the dorsal compartment (See inset panel, green). (F and G) Suppressing smo by expressing smo-RNAi in large regions induces massive cell death (red). The nub-Gal4 domain covers the pouch region (See inset panel in F, green). hh-Gal4 is expressed only within the posterior compartment (See inset panel in G, green). (H) Suppressing Smo activity by expressing the inhibitor gene ptc in all the wing cells under the c765-Gal4 driver results in small wing discs with massive cell death (red). Note that panel H is also from a 3rd instar larvae and is shown at the same magnification with other pannels. When ptc is expressed in the whole wing disc, the wing disc size is reduced apparently due to a proliferation defect.
Apoptosis induced by the lack of Hh signalling is Dpp-independent
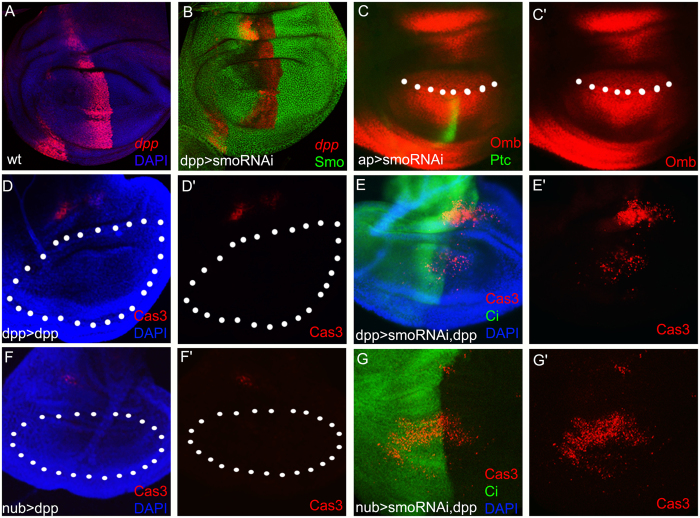
dpp, one target gene of Hh signalling, has been demonstrated to be an important survival factor24–26. To test whether the apoptosis caused by suppression of Hh signalling is due to the reduction of Dpp signalling, we examined the dpp expression using a dpp-lacZ reporter. In the wild-type background, dpp is expressed in a stripe of cells along the AP boundary (Fig. 2A). When Hh signalling was suppressed by smo-RNAi, the dpp transcription level was mildly reduced compared with that in wild type (Fig. 2A and B). Ptc, which is only expressed in a narrow stripe of cells just anterior to the AP compartment boundary by sensing the highest level of Hh, is a direct readout of Hh signalling. To obtain an internal control, we used a dorsal-specific driver, ap-Gal4, to express smo-RNAi (Fig. 2C). Ptc was abolished completely in the ap-Gal4 region (Fig. 2C), while Omb, one of the targets of Dpp signalling, was still detectable. The apoptosis was consistently observed in the ap > smoRNAi wing disc (Fig. S1). These data implied that the cell death might be a direct consequence of the suppression of Hh signalling and not a side effect of the reduction in Dpp signalling. To test this possibility, we co-expressed dpp with smo-RNAi to see whether the apoptosis can be rescued. In the control, dpp was solely expressed in either the dpp-Gal4 or the nub-Gal4 region, and there was no cell death in the pouch region except in the notum region (Fig. 2D and F). When dpp was co-expressed with smo-RNAi in the dpp-Gal4 domain, the apoptosis was still present in the wing pouch (Fig. 2E). The failure of dpp in the rescue experiment was confirmed in the nub-Gal4 domain (Fig. 2G). Taken together, the cell death caused by the suppression of Hh signalling is a direct consequence of the Hh pathway and not a side effect of disturbance in Dpp signalling.
Figure 2.
Cell death caused by Hh signalling reduction is dpp-independent. (A) The dpp expression pattern in wild-type wing discs is revealed by the dpp-lacZ enhancer trap line (red). (B) dpp-lacZ is still present (red) when Smo (green) is suppressed within the dpp-Gal4 domain. (C) Omb (red) is still detectable when smo is inhibited in the dorsal compartment by the dorsal-specific driver ap-Gal4. The Hh target Ptc is apparently inhibited within the dorsal compartment (green). The dotted line indicates the boundary between the dorsal and ventral compartments. (D and F) In control experiments, dpp is expressed within the dpp-Gal4 domain (D) and the nub-Gal4 domain (F). No cell death occurs within the wing pouch regions (dotted regions) except for a patch of dead cells (red) in the presumptive hinge domains, which might be a side effect of overgrowth induced by excess Dpp. (E and G) The apoptosis (red) is still induced even when dpp is co-expressed with smo-RNAi in the dpp-Gal4 (E) and nub-Gal4 (G) domains. A specific marker for the anterior compartment, Ci, is revealed by anti-Ci staining (green), to show the midline of overgrown wing discs.
Cell death induced by the lack of Hh signalling is JNK independent
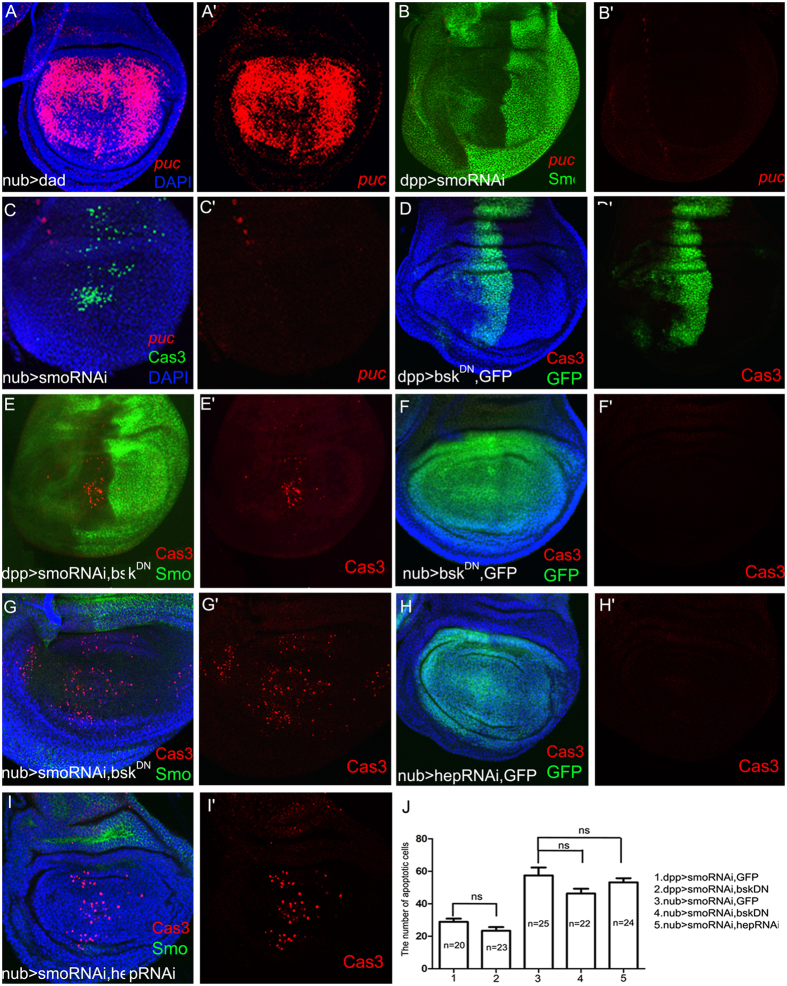
Previous studies have shown that JNK signalling plays a vital role in cell morphology, cell invasion, and apoptosis26, 31, 63, 64. JNK, monitored by puc-lacZ 65, was activated when Dpp signalling was inhibited by expressing its inhibitor dad (Fig. 3A). However, there was no ectopic puc-lacZ expression when Hh signalling was inhibited (Fig. 3B and C). Furthermore, the apoptosis was not reduced when JNK signalling was inhibited by co-expressing a dominant negative form of bsk (bsk DN) (Fig. 3D,E,F,G and J) or by co-expressing hep-RNAi (Fig. 3H,I and J). These data suggested that the apoptosis caused by the suppression of Hh signalling is independent of JNK signalling.
Figure 3.
The cell death induced by suppressing Hh signalling is JNK-independent. (A) Ectopic JNK signalling activity can be revealed by using a puc-lacZ. In a positive control experiment, puc-lacZ reporter (red) is activated when Dpp signalling is suppressed by expressing dad within the nub-Gal4 domain. (B,C) Suppression of Hh signalling by expressing smo-RNAi does not induce ectopic puc-lacZ (red). (D–I) Inhibition of JNK signalling by expressing a dominant negative form of bsk DN (E and G) or by suppressing an upstream effector (by expressing hep-RNAi) (I) can not rescue the apoptosis induced by expressing smo-RNAi. (D,F and H) The control experiments show no apoptosis when bsk DN or hep-RNAi is expressed alone. (J) Statistics for the apoptotic cell number per wing disc of each genotype mentioned above. ns stands for no significant difference.
Cell death induced by the lack of Hh signalling is mediated by hid and diap1
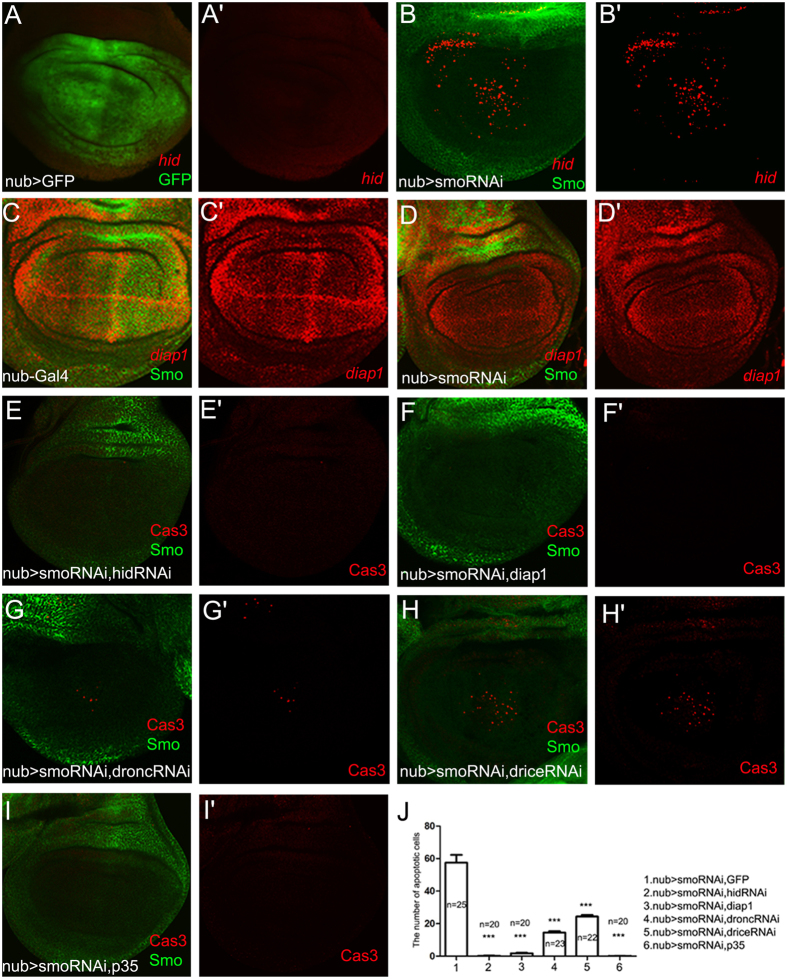
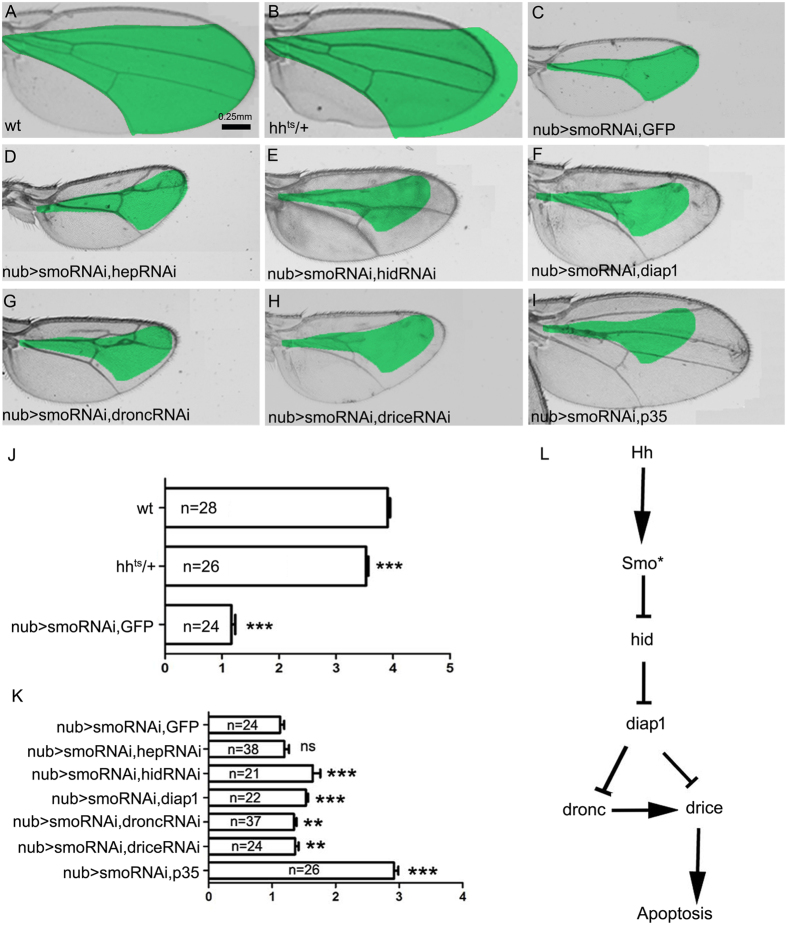
Apoptosis is a highly conserved pathway in both invertebrate and vertebrate systems. The key mediators, including Hid, Drice, Dronc, and Diap1, were mentioned in the introduction section. Subsequently, we tested whether there was a link between the cell death pathway and the Hh pathway. When smo was suppressed in the wing pouch, the transcription of hid-lacZ was markedly increased (Fig. 4A and B), while the transcription of diap1-lacZ was reduced (Fig. 4C and D). Co-express hid-RNAi and smo-RNAi in the nub-Gal4 domain supppressed the cell death (Fig. 4E and J). The cell death was also suppressed completely when diap1 was co-expressed with smo-RNAi in the nub-Gal4 domain (Fig. 4F and J). Next, we examined the roles of an initiator caspase (Dronc) and an effector caspase (Drice). Use of either dronc-RNAi or drice-RNAi partially rescued the apoptosis (Fig. 4G,H and J) compared with the control (Fig. 1F). This cell death was suppressed completely when P35 was co-expressed with smo-RNAi in the nub-Gal4 domain (Fig. 4I and J). Taken together, we demonstrated that the cell death caused by the suppression of Hh signalling is at least partially mediated by the activation of the proapoptotic gene hid and by inhibition of diap1.
Figure 4.
Cell death induced by the lack of Hh signalling activity requires the activation of the pro-apoptotic gene hid and the reduction of diap1. (A) In the control nub > GFP wing disc, there is no apparent hid-lacZ expression (red). (B) The pro-apoptotic gene hid (red) is activated in the wing pouch when smo-RNAi is expressed within the nub-Gal4 domain. (C,D) diap1-lacZ (red) is apparently reduced in the medial region of the nub > smo-RNAi wing discs (D) compared with the control (C). (E) Suppressing pro-apoptotic gene hid by expressing hid-RNAi efficiently suppresses the cell death compared with the control (Fig. 1F). (F) Co-expression of diap1 with smo-RNAi efficiently suppresses the cell death. (G) Suppressing initiator caspase activity by expressing dronc-RNAi largely suppresses the cell death induced by smo-RNAi expression. (H) Suppressing effector caspase activity by expressing drice-RNAi reduces the cell death induced by smo-RNAi expression. (I) Co-expression of P35 with smo-RNAi suppresses the cell death completely. (J) Statistical analysis for the apoptotic cell number per wing disc of each genotype mentioned above. Means ± SEM indicated *** are significantly different (pairwise comparisons performed using t-tests, p < 0.0001).
Cell death induced by the lack of Hh signalling led to small adult wings
To assay the apoptosis effect on adult wing, we measured the size of the medial wing where apoptosis always occur in the manipulations of Hh signalling. Compared with the wild-type adult wing (Fig. 5A), reduction of Hh signalling by hhts (Fig. 5B and J) and smo-RNAi (Fig. 5C and J) in the whole wing blade resulted in an obvious reduction in wing size. There was no significant difference in adult wing size between nub > smo-RNAi, GFP and nub > smo-RNAi, hep-RNAi (Fig. 5C, D and K). Suppression of the pro-apoptotic genes hid by hid-RNAi showed rescue effect in adult wing size (Fig. 5C,E and K). Co-expressing diap1 with smo-RNAi in the nub-Gal4 domain had an obvious rescue effect of adult wing size compared with smo-RNAi alone (Fig. 5C,F and K). However, the adult wing size of nub > smo-RNAi, hid-RNAi and nub > smo-RNAi, diap1 did not restore to the wild type size, which may be due to the proliferation effect of Hh signalling. Suppression of the initiator caspase and the effector caspase showed a slight rescue effect in adult wing size (Fig. 5C,G,H and K). Co-expressing the pan-caspase inhibitor P35 could largely rescue the adult wing size (Fig. 5I and K). These data suggest that the wing size is regulated not only by proliferation control, but also by cell survival control of Hh signalling.
Figure 5.
The phenotypes of adult wings. (A) Wild-type adult wing. The area between L2 and L5 veins is measured. The region between L2 and L5 veins of each control wing (A and C) is marked in green and compared in each manipulation (B,D–H) because most of the cell death induced by the suppression of Hh signalling occurs in the presumptive region between the L2 and L5 veins. (B) The hh ts adult wing is smaller than the wild-type wing (A). (D) Co-expressing hep-RNAi does not rescue the small size between L2 and L5 compared with the expression of smo-RNAi (C). (E) Co-expressing hid-RNAi increases the indicated area compared with the control wing in C. (F) Co-expressing diap1 increases the indicated area compared with the control wing in C. (G) Co-expressing dronc-RNAi increases the indicated area compared with the control wing in C. (H) Co-expressing drice-RNAi increases the indicated area compared with the control wing in C. (I) The wing area is rescued by co-overexpressing P35. (J and K) Statistics for the green-marked wing regions in each genotype mentioned above. Means ± SEM indicated by ** or *** are significantly different (pairwise comparisons performed using t-tests, p < 0.001 or p < 0.0001). (L) A model of the genetic pathway regulating cell survival by Hh signalling.
The wing veins’ pattern was also altered. Consistent with previous reports, lacking Hh signalling lead to loss of L3 and L4 veins (Fig. 5C)41, 55, 66. When the cell death was suppressed by hid-RNAi or d ronc-RNAi, the L4 vein was rescued (Fig. 5E and G). When the cell death was suppressed by diap1 or drice-RNAi, the L3 and L4 veins were only partially rescued up to the proximal region with fusion effect (Fig. 5F and H). The L3 and L4 veins could be completely rescued only when the cell death is suppressed by P35 (Fig. 5I). Therefore, Hh signalling regulates the medial wing pattern formation, at least in part, by control of cell survival.
Various signalling pathways are involved in cell survival. The Hippo/Warts/Yorkie (Hpo/Wts/Yki) pathway is known to control apoptosis. Hpo negatively regulates the transcription factor Yki by phosphorylating it. The dephosphorylation of Yki activates the target gene diap1 to inhibit apoptosis67–69. Notch and Wingless (Wg) promote cell survival by inhibiting Caspase70–72. Epidermal Growth Factor Receptor (EGFR) is required for cell survival in the Drosophila eye disc, where it inhibits the pro-apoptotic gene hid 73–75. Dpp is involved in cell survival by activating the downstream target genes omb 24 and sal 25–27. Here, we found that Hh is also involved in cell survival in the Drosophila wing disc through hid and diap1, and we present a model to explain the possible genetic regulation (Fig. 5L). Although hid-RNAi and diap1 can efficiently suppress the cell death induced by smo-RNAi expression (Fig. 4E,F and J), the adult wings are not restored to wild type size (Fig. 5E,F and K). We can not rule out a possibility of compensational mechanism between the Hh-regulated cell survival and proliferation. However, the disruption of any of the above signalling pathways can induce apoptosis. There must be a mechanism by which a cell integrates all of these signals to determine its survival status. Our results suggest that Smo is the most downstream component of Hh signalling that is related to cell survival. To better understand how Hh-Smo signalling promotes cell survival, the potential mediators between Smo and apoptosis pathway need to be identified. In the Drosophila eye, diap1 is up-regulated by deregulated Hh signalling62. In the Drosophila wing, diap1 is apparently suppressed under the condition of Hh loss-of-function (Fig. 4D and D’). Hh has been reported essential for the cell survival in vertebrate56–61. A recent study has shown that the requirement of Hh in cell survival in pancreatic cancer cells is dependent on the up-regulation of baculoviral IAP repeat-containing 3 (BIRC3) gene which belongs to IAP family76. Therefore, Hh is functionally conserved in cell survival control in both vertebrate and invertebrate.
Materials and methods
Drosophila stocks
The following transgenes were used: dpp-Gal4 77, ms1096-Gal4 78, nub-Gal4 79, hh-Gal4 (BL#45169), c765-Gal4 80, UAS-ttvRNAi (VDRC#4871), UAS-smoRNAi (VDRC#9542), UAS-smo PKA35, UAS-ptc (BL#5817), UAS-dpp (BL#1486), UAS-diap1 81, UAS-droncRNAi 82, UAS-driceRNAi (VDRC#28065), UAS-P35 (BL#5073), UAS-dad 28, UAS-bsk DN (BL#6409), UAS-hepRNAi (VDRC#47507), UAS-hidRNAi (a gift from Lei Xue). Mutant alleles used were: hh ts (BL#1684). Enhancer trap lines used were: dpp-lacZ 83, puc-lacZ (BL#11173), hid-lacZ 84, diap1-lacZ 85. Larvae were raised at 25 °C unless stated otherwise. For efficient expression of RNAi transgenes, larvae were raised at 29 °C.
Dissection of larvae
Wing imaginal discs were dissected from 3rd instar Drosophila larvae according to a standard protocol and were fixed for 30 min in 4% paraformaldehyde in PBT (PBS with 0.3% Triton X-100).
Immunohistochemistry
Fixed wing imaginal discs were stained with antibodies according to standard procedures. The primary antibodies used were: rabbit anti-Caspase3, 1:200 (Cell Signaling Technology); mouse anti-β-galactosidase, 1:2000 (Promega); rabbit anti-β-galactosidase 1:2000 (Promega); rat anti-Ci, 1:200 (DSHB); mouse anti-En, 1:200 (DSHB); mouse anti-Smo, 1:200 (DSHB); mouse anti-Ptc, 1:200 (DSHB). Secondary antibodies used were goat anti-mouse DyLight 549, goat anti-rat DyLight 549, and goat anti-rabbit DyLight 488, 1:200 (Agrisera). Images were collected using a Leica TCS-SP2-AOBS confocal microscope.
Adult wing imaging
Adult wing images were collected using an inverted microscope (AMG EVOS, America).
Adult wing measurement
The area of the adult wing was measured using Image-J software, and the calculation and measurement were carried out using GraphPad Prism 5 Project.
Electronic supplementary material
Acknowledgements
We thank the Bloomington stock center, TsingHua Fly Center, Jin Jiang, Andreas Bergmann, Lei Xue, Wenzhe Li for fly stocks. This research was financially supported by the National Key R&D Program [2017YFD0201200], the National Natural Science Foundation of China [NSFC31372255], and the 973 Program [2013CB127603].
Author Contributions
J.S. developed the concept and designed the experiments. J.L. performed the experiments. J.L., D.W. and J.S. analyzed the data and wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-10550-4
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 2.Cashio P, Lee TV, Bergmann A. Genetic control of programmed cell death in Drosophila melanogaster. Seminars in Cell & Developmental Biology. 2005;16:225–235. doi: 10.1016/j.semcdb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Hay BA, Guo M. Caspase-Dependent Cell Death in Drosophila. Cell and Developmental Biology. 2006;22:623–650. doi: 10.1146/annurev.cellbio.21.012804.093845. [DOI] [PubMed] [Google Scholar]
- 4.Conradt, B. In Annual Review of Genetics Vol. 43 Annual Review of Genetics 493-523 (2009). [DOI] [PMC free article] [PubMed]
- 5.White K, et al. Genetic Control of Programmed Cell Death in Drosophila. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- 6.Grether ME, Abrams JM, Agapite J, White K, Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- 7.Morimura S, Maves L, Chen Y, Hoffmann FM. Decapentaplegic overexpression affects Drosophila wing and leg imaginal disc development and wingless expression. Developmental Biology. 1996;177:136–151. doi: 10.1006/dbio.1996.0151. [DOI] [PubMed] [Google Scholar]
- 8.Dorstyn L, Kumar S. A biochemical analysis of the activation of the Drosophila caspase DRONC. Cell Death Differ. 2008;15:461–470. doi: 10.1038/sj.cdd.4402288. [DOI] [PubMed] [Google Scholar]
- 9.Fraser AG, Evan GI. Identification of a Drosophila melanogaster ICE/CED-3-related protease, drICE. Embo Journal. 1997;16:2805–2813. doi: 10.1093/emboj/16.10.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song Z, Mccall K, Steller H. DCP-1, a Drosophila cell death protease essential for development. Science. 1997;275:536–540. doi: 10.1126/science.275.5299.536. [DOI] [PubMed] [Google Scholar]
- 11.Dorstyn L, Kumar S. A biochemical analysis of the activation of the Drosophila caspase DRONC. Cell Death & Differentiation. 2008;15:461–470. doi: 10.1038/sj.cdd.4402288. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins CJ, et al. The Drosophila caspase DRONC cleaves following glutamate or aspartate and is regulated by DIAP1, HID, and GRIM. Journal of Biological Chemistry. 2000;275:27084–27093. doi: 10.1074/jbc.M000869200. [DOI] [PubMed] [Google Scholar]
- 13.Matakatsu H, Tadokoro R, Gamo S, Hayashi S. Repression of the wing vein development in Drosophila by the nuclear matrix protein plexus. Development. 1999;126:5207–5216. doi: 10.1242/dev.126.23.5207. [DOI] [PubMed] [Google Scholar]
- 14.Meier P, Silke J, Leevers SJ, Evan GI. The Drosophila caspase DRONC is regulated by DIAP1. Embo J. 2000;19:598–611. doi: 10.1093/emboj/19.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zachariou A, et al. IAP-antagonists exhibit non-redundant modes of action through differential DIAP1 binding. Embo J. 2003;22:6642–6652. doi: 10.1093/emboj/cdg617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clem RJ, Fechheimer M, Miller LK. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science. 1991;254:1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- 17.Gibson MC, Perrimon N. Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science. 2005;307:1785–1789. doi: 10.1126/science.1104751. [DOI] [PubMed] [Google Scholar]
- 18.Shen J, Dahmann C. Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science. 2005;307:1789–1790. doi: 10.1126/science.1104784. [DOI] [PubMed] [Google Scholar]
- 19.Akiyama T, et al. Dally Regulates Dpp Morphogen Gradient Formation by Stabilizing Dpp on the Cell Surface. Developmental Biology. 2008;313:408–419. doi: 10.1016/j.ydbio.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Entchev EV, Schwabedissen A, Gonzálezgaitán M. Gradient formation of the TGF-beta homolog Dpp. Cell. 2000;103:981–991. doi: 10.1016/S0092-8674(00)00200-2. [DOI] [PubMed] [Google Scholar]
- 21.Fujise M, et al. Dally regulates Dpp morphogen gradient formation in the Drosophila wing. Development. 2003;130:1515–1522. doi: 10.1242/dev.00379. [DOI] [PubMed] [Google Scholar]
- 22.Teleman AA, Cohen SM. Dpp gradient formation in the Drosophila wing imaginal disc. Current Biology. 2000;10:293–300. doi: 10.1016/S0960-9822(00)00378-X. [DOI] [PubMed] [Google Scholar]
- 23.Becker K. The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nature Reviews Genetics. 2007;8:663–674. doi: 10.1038/nrg2166. [DOI] [PubMed] [Google Scholar]
- 24.Organista MF, De Celis JF. The Spalt transcription factors regulate cell proliferation, survival and epithelial integrity downstream of the Decapentaplegic signalling pathway. Biology Open. 2013;2:37–48. doi: 10.1242/bio.20123038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon, E. & Guerrero, I. The transcription factor optomotor-blind antagonizes Drosophila haltere growth by repressing decapentaplegic and hedgehog targets. Plos One10 (2015). [DOI] [PMC free article] [PubMed]
- 26.Adachi Yamada T, Fujimurakamada K, Nishida Y, Matsumoto K. Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature. 1999;400:166–169. doi: 10.1038/22112. [DOI] [PubMed] [Google Scholar]
- 27.Del ARD, Terriente FJ, Díazbenjumea FJ. The role of the T-box gene optomotor-blind in patterning the Drosophila wing. Developmental Biology. 2004;268:481–492. doi: 10.1016/j.ydbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Tsuneizumi K, et al. Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature. 1997;389:627–631. doi: 10.1038/39362. [DOI] [PubMed] [Google Scholar]
- 29.Bökel C, Schwabedissen A, Entchev E, Renaud O, González-Gaitán M. Sara endosomes and the maintenance of Dpp signaling levels across mitosis. Science. 2006;314:1135–1139. doi: 10.1126/science.1132524. [DOI] [PubMed] [Google Scholar]
- 30.Inoue H, et al. Interplay of signal mediators of decapentaplegic (Dpp): molecular characterization of mothers against dpp, Medea, and daughters against dpp. Molecular Biology of the Cell. 1998;9:2145–2156. doi: 10.1091/mbc.9.8.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/S0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 32.Weston CR, Davis RJ. The JNK signal transduction pathway. Current Opinion in Cell Biology. 2002;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Glise B, Bourbon H. Noselli & Stéphane. hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell. 1995;83:451–461. doi: 10.1016/0092-8674(95)90123-X. [DOI] [PubMed] [Google Scholar]
- 34.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 35.Jia J, Tong C, Wang B, Luo L, Jiang J. Hedgehog signalling activity of Smoothened requires phosphorylation by protein kinase A and casein kinase I. Nature. 2004;432:1045–1050. doi: 10.1038/nature03179. [DOI] [PubMed] [Google Scholar]
- 36.Roy, S., Hsiung, F. & Kornberg, T. B. Specificity of Drosophila cytonemes for distinct signaling pathways. Science332, 354–358 (2011). [DOI] [PMC free article] [PubMed]
- 37.Chen Y, Jiang J. Decoding the phosphorylation code in Hedgehog signal transduction. Cell Res. 2013;23:186–200. doi: 10.1038/cr.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duman-Scheel M, Weng L, Xin S, Du W. Hedgehog regulates cell growth and proliferation by inducing Cyclin D and Cyclin E. Nature. 2002;417:299–304. doi: 10.1038/417299a. [DOI] [PubMed] [Google Scholar]
- 39.Casso DJ, Biehs B, Kornberg TB. A novel interaction between hedgehog and Notch promotes proliferation at the anterior-posterior organizer of the Drosophila wing. Genetics. 2011;187:485–499. doi: 10.1534/genetics.110.125138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christiansen AE, Tian D, Bergmann A. Ligand-independent activation of the Hedgehog pathway displays non-cell autonomous proliferation during eye development in Drosophila. Mechanisms of Development. 2012;129:98–108. doi: 10.1016/j.mod.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mullor JL, Calleja M, Capdevila J, Guerrero I. Hedgehog activity, independent of decapentaplegic, participates in wing disc patterning. Development. 1997;124:1227–1237. doi: 10.1242/dev.124.6.1227. [DOI] [PubMed] [Google Scholar]
- 42.Mohler J. Requirements for hedgehog, a segmental polarity gene, in patterning larval and adult cuticle of Drosophila. Genetics. 1988;120:1061–1072. doi: 10.1093/genetics/120.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strigini M, Cohen SM. Wingless gradient formation in the Drosophila wing. Curr Biol. 2000;10:293–300. doi: 10.1016/S0960-9822(00)00378-X. [DOI] [PubMed] [Google Scholar]
- 44.Ingham PW, Taylor AM, Nakano Y. Role of the Drosophila patched gene in positional signalling. Nature. 1991;353:184–187. doi: 10.1038/353184a0. [DOI] [PubMed] [Google Scholar]
- 45.The I, Bellaiche Y, Perrimon N. Hedgehog movement is regulated through tout velu-dependent synthesis of a heparan sulfate proteoglycan. Molecular Cell. 1999;4:633–639. doi: 10.1016/S1097-2765(00)80214-2. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Struhl G. Dual roles for patched in sequestering and transducing Hedgehog. Cell. 1996;87:553–563. doi: 10.1016/S0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- 47.Denef N, Neubuser D, Perez L, Cohen SM. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell. 2000;102:521–531. doi: 10.1016/S0092-8674(00)00056-8. [DOI] [PubMed] [Google Scholar]
- 48.Li W, Ohlmeyer JT, Lane ME, Kalderon D. Function of protein kinase A in hedgehog signal transduction and Drosophila imaginal disc development. Cell. 1995;80:553–562. doi: 10.1016/0092-8674(95)90509-X. [DOI] [PubMed] [Google Scholar]
- 49.Briscoe J, Chen Y, Jessell TM, Struhl G. A hedgehog-insensitive form of patched provides evidence for direct long-range morphogen activity of sonic hedgehog in the neural tube. Mol Cell. 2001;7:1279–1291. doi: 10.1016/S1097-2765(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 50.Dessaud E, et al. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450:717–720. doi: 10.1038/nature06347. [DOI] [PubMed] [Google Scholar]
- 51.Nahmad M, Stathopoulos A. Dynamic interpretation of hedgehog signaling in the Drosophila wing disc. PLoS Biol. 2009;7:e1000202. doi: 10.1371/journal.pbio.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Irons, D. J., Wojcinski, A., Glise, B. & Monk, N. A. Robustness of positional specification by the Hedgehog morphogen gradient. Dev Biol342, 180–193 (2010). [DOI] [PubMed]
- 53.Strigini M, Cohen SM. A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development. 1997;124:4697–4705. doi: 10.1242/dev.124.22.4697. [DOI] [PubMed] [Google Scholar]
- 54.Vervoort M, Crozatier M, Valle D, Vincent A. The COE transcription factor Collier is a mediator of short-range Hedgehog-induced patterning of the Drosophila wing. Current Biology. 1999;9:632–639. doi: 10.1016/S0960-9822(99)80285-1. [DOI] [PubMed] [Google Scholar]
- 55.Crozatier M, Glise B, Vincent A. Connecting Hh, Dpp and EGF signalling in patterning of the Drosophila wing; the pivotal role of collier/knot in the AP organiser. Development. 2002;129:4261–4269. doi: 10.1242/dev.129.18.4261. [DOI] [PubMed] [Google Scholar]
- 56.Juho-Antti M, et al. Hedgehog signalling promotes germ cell survival in the rat testis. Reproduction. 2011;142:711. doi: 10.1530/REP-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cobourne MT, Hardcastle Z, Sharpe PT. Sonic hedgehog regulates epithelial proliferation and cell survival in the developing tooth germ. Journal of Dental Research. 2001;80:1974. doi: 10.1177/00220345010800110501. [DOI] [PubMed] [Google Scholar]
- 58.Brito JM, Teillet MA, Douarin NML. An Early Role for Sonic Hedgehog from Foregut Endoderm in Jaw Development: Ensuring Neural Crest Cell Survival. Proceedings of the National Academy of Sciences. 2006;103:11607–11612. doi: 10.1073/pnas.0604751103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahlgren SC, Bronnerfraser M. Inhibition of Sonic hedgehog signaling in vivo results in craniofacial neural crest cell death. Current Biology. 1999;9:1304–1314. doi: 10.1016/S0960-9822(00)80052-4. [DOI] [PubMed] [Google Scholar]
- 60.Lin EH, et al. Hedgehog pathway maintains cell survival under stress conditions, and drives drug resistance in lung adenocarcinoma. Oncotarget. 2016;7:24179–24193. doi: 10.18632/oncotarget.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Céline DB, et al. Sonic Hedgehog Promotes Tumor Cell Survival by Inhibiting CDON Pro-Apoptotic Activity. PLoS Biology,11,8(2013-8-6) 2013;11:e1001623. doi: 10.1371/journal.pbio.1001623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Christiansen AE, et al. Non-cell autonomous control of apoptosis by ligand-independent Hedgehog signaling in Drosophila. Cell Death & Differentiation. 2013;20:302–311. doi: 10.1038/cdd.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agnès F, Suzanne M, Noselli S. The Drosophila JNK pathway controls the morphogenesis of imaginal discs during metamorphosis. Development. 1999;126:5453–5462. doi: 10.1242/dev.126.23.5453. [DOI] [PubMed] [Google Scholar]
- 64.Tripura C, Chandrika NP, Susmitha VN, Noselli S, Shashidhara LS. Regulation and activity of JNK signaling in the wing disc peripodial membrane during adult morphogenesis in Drosophila. International Journal of Developmental Biology. 2011;55:583–590. doi: 10.1387/ijdb.103275ct. [DOI] [PubMed] [Google Scholar]
- 65.Martín-Blanco E, et al. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in. Drosophila. Genes & Development. 1998;12:557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crozatier M, Glise B, Khemici V, Vincent A. Vein-positioning in the Drosophila wing in response to Hh; new roles of Notch signaling. Mechanisms of Development. 2003;120:529–535. doi: 10.1016/S0925-4773(03)00041-8. [DOI] [PubMed] [Google Scholar]
- 67.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pan D. The hippo signaling pathway in development and cancer. Developmental Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oh H, Irvine KD. Yorkie: the final destination of Hippo signaling. Trends in Cell Biology. 2010;20:410–417. doi: 10.1016/j.tcb.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giraldez AJ, Cohen SM. Wingless and Notch signaling provide cell survival cues and control cell proliferation during wing development. Development. 2003;130:6533–6543. doi: 10.1242/dev.00904. [DOI] [PubMed] [Google Scholar]
- 71.Johnston LA, Sanders AL. Wingless promotes cell survival but constrains growth during Drosophila wing development. Nature Cell Biology. 2003;5:827–833. doi: 10.1038/ncb1041. [DOI] [PubMed] [Google Scholar]
- 72.Graves HK, Woodfield SE, Yang CC, Halder G, Bergmann A. Notch signaling activates Yorkie non-cell autonomously in Drosophila. Plos One. 2012;7:e37615. doi: 10.1371/journal.pone.0037615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bergmann A, Agapite J, Mccall K, Steller H. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell. 1998;95:331–341. doi: 10.1016/S0092-8674(00)81765-1. [DOI] [PubMed] [Google Scholar]
- 74.Kurada P, White K. Ras Promotes Cell Survival in Drosophila by Downregulating hid Expression. Cell. 1998;95:319–329. doi: 10.1016/S0092-8674(00)81764-X. [DOI] [PubMed] [Google Scholar]
- 75.Yu SY, et al. A pathway of signals regulating effector and initiator caspases in the developing Drosophila eye. Development. 2002;129:3269–3278. doi: 10.1242/dev.129.13.3269. [DOI] [PubMed] [Google Scholar]
- 76.Gan H, et al. SHh-Gli1 signaling pathway promotes cell survival by mediating baculoviral IAP repeat-containing 3 (BIRC3) gene in pancreatic cancer cells. Tumor Biology. 2016;37:1–8. doi: 10.1007/s13277-016-4898-0. [DOI] [PubMed] [Google Scholar]
- 77.Shen W, Mardon G. Ectopic eye development in Drosophila induced by directed dachshund expression. Development. 1997;124:45–52. doi: 10.1242/dev.124.1.45. [DOI] [PubMed] [Google Scholar]
- 78.Capdevila J, Guerrero I. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. Embo Journal. 1994;13:4459–4468. doi: 10.1002/j.1460-2075.1994.tb06768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calleja M, Moreno E, Pelaz S, Morata G. Visualization of gene expression in living adult Drosophila. Science. 1996;274:252–255. doi: 10.1126/science.274.5285.252. [DOI] [PubMed] [Google Scholar]
- 80.Nellen D, Burke R, Struhl G, Basler K. Direct and long-range action of a DPP morphogen gradient. Cell. 1996;85:357–368. doi: 10.1016/S0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 81.Bruce A, Wassarman, David A, Rubin, Gerald M. Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell. 1996;83:1253–1262. doi: 10.1016/0092-8674(95)90150-7. [DOI] [PubMed] [Google Scholar]
- 82.Leulier F, et al. Systematic in vivo RNAi analysis of putative components of the Drosophila cell death machinery. Cell Death & Differentiation. 2006;13:1663–1674. doi: 10.1038/sj.cdd.4401868. [DOI] [PubMed] [Google Scholar]
- 83.Su Y, et al. Sequential phosphorylation of smoothened transduces graded hedgehog signaling. Science Signaling. 2011;4:2750–2750. doi: 10.1126/scisignal.2001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grether ME, Abrams JM, Agapite J, White K, Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes & Development. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- 85.Ryoo HD, Bergmann A, Gonen H, Ciechanover A, Steller H. Regulation of Drosophila IAP1 degradation and apoptosis by reaper and ubcD1. Nature Cell Biology. 2002;4:432–438. doi: 10.1038/ncb795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.