Abstract
The traditional soil potassium (K) testing methods fail to accurately predict K requirement by plants. The Diffusive Gradients in Thin-films (DGT) method is promising, but the relationship between the DGT-measured K pool and plant available K is not clear. Wheat (Triticum aestivum L., cv. Frame) was grown in 9 Australian broad acre agricultural soils in a glasshouse trial until the end of tillering growth stage (GS30) with different plant K demands generated by varying plant numbers and pot sizes. Different K concentrations in soils were varied by 4 rates of K fertilizer application. The relative dry matter and K uptake were plotted against the soil K test value (CaCl2, Colwell and NH4OAc and DGT K measurements). To obtain 90% of maximum relative dry matter at low root density (closest to field conditions), the critical value of the NH4OAc K method was 91 (R2 = 0.56) mg kg−1. The DGT K method was not able to accurately predict relative dry matter or K uptake due to a weak extraction force for K from soils with high CEC values. Further endeavor on increasing K extraction force of the DGT method is warranted to obtain accurate plant available K results.
Introduction
Accurate soil testing methods for measuring “plant-available” K would not only maximize crop productivity and quality, but also avoid long term K depletion of fertile farm lands such as what has occurred in grain cropping regions of western and eastern Australia1. To achieve these aims, scientists have long been studying K forms in soils and the mechanisms of K uptake by plant roots. The different (operationally defined) forms of K in soils are solution K, exchangeable K, non-exchangeable K (slowly exchangeable K) and mineral K, with K present in these pools in equilibrium with each other2, 3. Plant available K refers to mainly soluble K and exchangeable K4, although some non-exchangeable K and mineral K can become soluble or exchangeable during a plant growing season. Plants take up K mainly from soil via roots, as K foliar application is not widely employed. Barber et al. proposed that K reached plant roots mainly by diffusion5, and subsequent work by Barber showed that K taken up by corn roots supplied by diffusion accounted for 80% of the overall total K uptake in a fertile Alfisol silt loam2. Other evidence, provided by Mills et al., suggested that approximately 85% of the K moved to root surfaces by diffusion through water films around soil particles6. Further, Baligar reported that diffusion contributed more to K uptake by plants than mass flow, with 99% and 96% of total K uptake occurring by diffusion for corn (20 days growth) and wheat (25 days growth), respectively7. Therefore, it is apparent that plants take up K mainly from the soluble and exchangeable K pools from soils with most of the plant available K moving from the soil solid phase to root surfaces by diffusion. As most K is taken up by roots, the size and shape of roots affect K uptake to a large extent. It was reported that potassium-deficient plants had reduced root to shoot dry weight ratios8, 9. Fusseder and Krauss reported that K uptake by maize in field conditions decreased from 50% to 12% of the possible theoretical uptake based on a mathematic treatment of data when root density varied from >2 cm cm−3 to <2 cm cm−3 10. Consequently, it becomes difficult to predict plant K requirements accurately using soil testing alone as the root growth of plants is unpredictable and dependent on many other factors, such as soil bulk density, soil compaction, soil moisture, temperature and light, other nutrient supply, microorganism activity, etc.
The most common soil tests for predicting available soil K are soil solution extraction and various chemical methods that attempt to either just displace K from the cation exchange complex (exchangeable K), or in addition, measure some of the non-exchangeable K that may contribute to plant uptake during the season (extractable K). The latter tests generally require stronger extracts, and except for extraction with 0.5 mol L−1 NaHCO3 (the Colwell K soil test, reported by Rayment and Lyons to be the most widely used method for measuring available K in Australia11), are only used in specific industries (e.g. sugarcane) or regions (Victoria) where calibrations have been developed12. The solution K method measures K in soil solution and easily exchangeable K from the soil solid phase using extraction in water or 0.01 mol L−1 CaCl2. There are a variety of chemical extraction methods that attempt to measure exchangeable K, but in Australia these typically rely on rapidly replacing K+ on the exchange sites using NH4 +, Ba2+ or Na+ 12. An ion-exchange resin method was used for available K measurement in soils13, and the resin K was linearly correlated with the NH4OAc K results (R = 0.97), illustrating that they measure a similar K pool in soils. A resin disc method allowed direct contact of the resin disc and soil paste, but a poor regression between K uptake and the quantity of K adsorbed by the resin was observed due to luxury K supply associated with the process of direct contact of the disc and soil sample14. Potassium supply rate was measurable using a plant root simulator (PRS, a cation exchange membrane method), expressed as weight per contact area per burial period. However, the sensitivity of the membrane to changes in soil K suppy over time was reported to be the obstacle for simple interpretation of the supply rate measurements15, 16.
It is well established that soil solution K measured using the CaCl2 K method represents an intensity factor while exchangeable K measured using extractants like NH4OAc represents a quantity factor4, 17. Barber reported that K uptake is influenced more by the K quantity factor at high root density situations while K uptake is influenced more by the K intensity factor at low root density situations18. Bell et al. reported that soil solution K values varied by 6–7 fold in different soil types despite similar exchangeable K contents19, so it can be concluded that measures of K intensity and quantity differ greatly across soils with varying soil properties. In particular, soil clay content influence the K levels observed in different extraction methods. For example, Houba et al. acknowledged that CaCl2 did not extract all of the exchangeable K from soils, especially soils with high clay contents20. In a glasshouse trial using Guinea grass, Darunsontaya et al. suggested that the NH4OAc-K method predicted K availability more accurately than other extraction methods (water, HNO3, HNO3-NH4OAc) and the total K method21. However, Krishna reported that response of wheat to K application predicted using the exchangeable K method was soil type dependent, and more specifically related to the K buffering ability associated with clay content22. Similarly, Gourley et al. reported that even commonly used extractable K measures like the Colwell K method were also soil type dependent, with pasture response to K application showing the critical Colwell K values increased with clay content23.
The Diffusive Gradients in Thin-films (DGT) technique has been successfully used to more accurately predict plant available phosphorus (P) compared to traditional extraction methods24–28. The theory of element uptake by the DGT from soil samples has been extensively discussed29–33. Simply, when a DGT device is deployed on water-saturated soil samples, the target element in soil solution diffuses through the diffusive gel and accumulates in the binding gel. When the element concentration at the DGT surface is lowered by uptake by the binding gel, the element from the soil solid phase desorbs to replenish this depletion. Therefore, the fraction of an element measured by the DGT is assumed to incorporate the soluble pool and part of the insoluble pool from the soil solid phase. Tandy et al. proposed that the DGT method could be used for soil K measurement by using Amberlite IRP-69 resin as the binding gel and found a similar accuracy to the NH4OAc K method to predict K concentrations in winter barley grown in pots34. Zhang et al. optimized the resin gel by using a mixed Amberlite and ferrihydrite (MAF) gel (to allow simultaneous measurement of P and K) and the MAF gel showed advantages over the resin gel used by Tandy et al.35. More extensive quantification of the effects of competing cations on K uptake by the DGT will facilitate assessment of the accuracy of the DGT method in predicting plant growth response to K application.
The aims of this research were to 1) investigate the accuracy of DGT K relative to existing soil K tests for predicting wheat response (growth and plant K uptake) to fertilizer K application in different soils types with a range of plant available K concentrations; and 2) compare the predictive performances of these soil tests under differing plant K demands and root densities.
Methods
Soil characterization
Nine soils from typical grain producing areas across Australia with known low available K concentrations were dried and sieved to ≤2 mm for use in a glasshouse trial. The soils varied in texture and other inherent soil properties in an attempt to capture soil types prone to K deficiency in Australia (Table 1). Soil pH was measured using 0.01 mol L−1 CaCl2 solution with a soil to solution ratio of 1:536; organic carbon was measured using the Walkley and Black method36; exchangeable cations (Ca, Mg, Na and K) and cation exchange capacity (CEC) were extracted using 1 mol L−1 NH4OAc36; soil particle size was determined using the method described by Bowman and Hutka37; and water holding capacity was measured using the same method as Jenkinson and Powlson38.
Table 1.
Basic physical and chemical properties of the soils, and soil K testing values on control soils using different soil testing methods.
| Site | Abbreviation | State | EC (µS cm−1) | pH | Organic Carbon (%) | CaCl2 K (mg kg−1) | Colwell K (mg kg−1) | NH4OAc K (mg kg−1) | DGT K (mg L−1) | Exchangeable Ca (cmol kg−1) | Exchangeable Mg (cmol kg−1) | CEC (cmol kg−1) | Clay (%) | Sand (%) | Silt (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Karoonda | KD | SA | 93 | 6.43 | 0.28 | 73 | 71 | 77 | 7.2 | 0.7 | 0.2 | 1.0 | 3 | 95 | 2 |
| Lake Bolac | LB | VIC | 90 | 5.78 | 1.33 | 71 | 69 | 76 | 10.0 | 1.9 | 0.3 | 2.4 | 3 | 92 | 4 |
| Ngarkat | NK | SA | 17 | 6.55 | 0.62 | 20 | 21 | 22 | 2.6 | 0.9 | 0.1 | 1.0 | 4 | 94 | 2 |
| Gindie B | QB | QLD | 47 | 6.95 | 0.47 | 11 | 28 | 34 | 0.6 | 9.6 | 7.1 | 17.1 | 67 | 18 | 15 |
| Capella B | QC | QLD | 48 | 7.04 | 0.60 | 21 | 81 | 94 | 1.0 | 18.0 | 8.9 | 17.3 | 68 | 20 | 12 |
| Kingaroy | QL | QLD | 59 | 5.30 | 1.11 | 34 | 65 | 56 | 1.9 | 2.3 | 1.3 | 27.5 | 41 | 43 | 16 |
| Jandowae | QS | QLD | 67 | 6.01 | 0.51 | 44 | 100 | 135 | 1.2 | 7.2 | 6.2 | 14.9 | 66 | 12 | 22 |
| Regans Ford | RF | WA | 141 | 6.14 | 2.17 | 48 | 52 | 56 | 6.4 | 3.1 | 0.3 | 3.6 | 4 | 94 | 2 |
| Wickepin | WN | WA | 69 | 5.32 | 0.81 | 32 | 47 | 37 | 3.9 | 0.7 | 0.1 | 1.0 | 6 | 88 | 6 |
Glasshouse trial
A glasshouse trial was designed to investigate the effects of differing plant demand and root density on K uptake by wheat, established by growing plants in two contrasting soil volumes with differing plant densities, and to assess whether these parameters affected the wheat response to K fertilizer and to compare the soil analytical methods to estimate wheat K fertiliser requirements. The soils were amended with nutrient solutions containing 100 mg kg−1 of nitrogen (as NH4NO3), 3 mg kg−1 of copper (as CuSO4•5H2O), 5 mg kg−1 of magnesium (as MgSO4•7H2O), 3 mg kg−1 of manganese (as MnSO4•H2O) and 10 mg kg−1 of zinc (as ZnSO4•7H2O), which altogether resulted in a total of 15 mg kg−1 sulfur. Phosphorus (as H3PO4) was applied at different rates for each soil to provide sufficient available P for wheat plants based on phosphorus buffering index (PBI) values and initial P status as assessed by DGT P25. In order to obtain a plant dry matter response curve for wheat, K (as KCl) was applied at 4 different rates (Table 2) in each soil type. After addition of nutrient solutions, the soils were thoroughly mixed and incubated for 2 days. The pots were sealed with plastic bags to prevent moisture loss and nutrient leaching. Initially 500 g of each soil (3 replicates) was placed into a small pot (12 cm diameter at the top, 10 cm diameter at the bottom, 11 cm high), another 1250 g of each soil (3 replicates) was placed into a large pot (14 cm diameter at the top, 11.5 cm diameter at the bottom, 14.5 cm high) and 100 g was left as a subsample for soil analysis.
Table 2.
Root densities and wheat dry matter in response to K application; SD means the standard deviation; DM means dry matter; RDM means relative dry matter and R2 is the coefficient obtained by fitting the Mitscherlich curve, where “—” means no R2 obtained due to: a) the response in the fertilized treatment was unexpectedly below the controls, dry mass in the control soil is taken as the DMmax and b) a linear response was observed, dry mass of 120% of the highest K rate is taken as the DMmax; different letters mean significant difference observed at P ≤ 0.05.
| Soil | Pot size | K rate (mg kg−1) | P (mg kg−1) | Root density (g m−3) | SD of root density | DMcontrol (g pot−1) | RDM of control soils (%) | DMmax (g pot−1) | DMmax predicted (g pot−1) | R2 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | K 1 | K 2 | K 3 | ||||||||||
| KD | Small | 0 | 50 | 150 | 300 | 300 | 169bcd | 74 | 0.39bcd | 75 | 0.59 | 0.53 | 0.64 |
| LB | 0 | 50 | 150 | 300 | 300 | 102abc | 20 | 0.34abc | 83 | 0.41 | 0.41 | 0.73 | |
| NK | 0 | 50 | 150 | 300 | 300 | 49a | 29 | 0.15a | 39 | 0.39 | 0.39 | 1.00 | |
| QB | 0 | 50 | 250 | 500 | 500 | 144bcd | 73 | 0.28abc | 38 | 0.77 | 0.73 | 0.92 | |
| QC | 0 | 50 | 250 | 500 | 500 | 148cd | 38 | 0.59d | 68 | 0.86 | 0.86 | 1.00 | |
| QL | 0 | 50 | 150 | 300 | 300 | 187d | 57 | 0.32abc | 49 | 0.75 | 0.65 | 0.79 | |
| QS | 0 | 50 | 250 | 500 | 500 | 131bcd | 51 | 0.48bcd | 63 | 0.82 | 0.76 | 0.88 | |
| RF | 0 | 50 | 150 | 300 | 300 | 179cd | 84 | 0.46bcd | 80 | 0.58 | 0.58 | 1.00 | |
| WN | 0 | 50 | 150 | 300 | 300 | 86ab | 19 | 0.22ab | 67 | 0.33 | 0.32 | 0.96 | |
| KD | Large | 0 | 50 | 150 | 300 | 300 | 18a | 4 | 0.30 cd | 100 | 0.30 | 0.30a | — |
| LB | 0 | 50 | 150 | 300 | 300 | 26ab | 3 | 0.25abc | 100 | 0.25 | 0.25a | — | |
| NK | 0 | 50 | 150 | 300 | 300 | 15a | 10 | 0.11a | 41 | 0.28 | 0.27 | 0.93 | |
| QB | 0 | 50 | 250 | 500 | 500 | 27ab | 13 | 0.26c | 55 | 0.49 | 0.48 | 0.99 | |
| QC | 0 | 50 | 250 | 500 | 500 | 30c | 8 | 0.42d | 87 | 0.50 | 0.48 | 0.74 | |
| QL | 0 | 50 | 150 | 300 | 300 | 15a | 4 | 0.27c | 86 | 0.34 | 0.32 | 0.61 | |
| QS | 0 | 50 | 250 | 500 | 500 | 23ab | 5 | 0.43d | 70 | 0.51 | 0.61b | — | |
| RF | 0 | 50 | 150 | 300 | 300 | 18a | 7 | 0.25bc | 100 | 0.25 | 0.25a | — | |
| WN | 0 | 50 | 150 | 300 | 300 | 19a | 5 | 0.13ab | 59 | 0.24 | 0.22 | 0.92 | |
Five pre-germinated seeds of wheat (Triticum aestivum L., cv. Frame) were sown in each pot and thinned to two plants in the small pots and one in the large pots after one week, at the two-leaf growth stage. Soil moisture was maintained at approximately 65% of the water holding capacity throughout the experiment. A randomized block design was employed and pot positions were changed randomly twice a week within the block to minimize any effects due to spatial variations in growing conditions (e.g. light, temperature and humidity) within the glasshouse. During the course of the experiment, the temperature ranged from 22 to 24 °C and relative humidity ranged from 25 to 88%. Wheat plants were harvested 28 days after sowing, at the end of tillering (GS30) stage39. Since the root density obtained between treatments in each soil was similar (assessed visually), wheat roots were removed by washing from only one replicate of each K rate from both small pots and large pots. Roots were dried to constant weight, resulting in 4 replicate samples for each soil from which root density was calculated.
Soil analyses
Extraction method details
Subsamples separated before planting were dried to constant weight at 40 °C and sieved to 2 mm before soil K testing. CaCl2-K was extracted using 0.01 mol L−1 CaCl2 at a soil to solution ratio of 1:10 for 2 h40; Colwell K was extracted using 0.5 mol L−1 NaHCO3 (pH 8.5) at a soil to solution ratio of 1:100 for 16 h36, 41; and exchangeable K (extracted using NH4OAc) was measured using 1 mol L−1 NH4OAc at a soil to solution ratio of 1:10 for 30 min36. Potassium in the eluents from the above tests was analyzed by an Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES, PerkinElmer, Optima 7000DV) at λ of 766.490 nm.
DGT methods
A mixed Amberlite and ferrihydrite (MAF) gel was used as the binding gel to measure K and other cations. The MAF gel and diffusive gel were prepared as described previously35, 42. Standard DGT devices (DGT Research Ltd, Lancaster, UK) with an effective sampling area of 2.54 cm2 were used to load the gel assemblies (MAF gel, diffusive gel and filter paper). The absorbed cations on the resin gel were eluted and measured according to the methods described by Zhang et al.43. The concentration of K measured by the DGT method (CDGT) was calculated as described in the previous publications35, 42, 43.
Plant relative dry matter and plant K analyses
The dry weight of aboveground plant parts was recorded after drying in an oven at 60 °C to constant weight. Maximum plant dry matter obtained for each soil was calculated by fitting a Mitscherlich response function in SigmaPlot 12.0 to describe the response in plant dry matter to applied K rates using Eq. (1):
| 1 |
where DM 0 is the wheat dry mass obtained in soils; a equals dry mass increase due to K application, b is the curvature coefficient resulting in (DM 0 + a) equating to the maximum dry matter (DM max); relative dry matter (RDM) at each treatment was calculated as the ratio of dry matter yield to the DM max obtained expressed as a percentage.
Plant tissue K concentration was analyzed using an acid digestion method44, 45. Plant samples (0.5 g, above ground part) were digested in 5 mL of nitric acid until the volume reduced to 1 mL, and then the solution was diluted to 20 mL for filtration. The filtered solution was then analyzed by an ICP-OES as outlined above. Overall plant K uptake was calculated by multiplying the dry mass by the tissue K concentration.
Comparison of different soil testing methods
The accuracy of the soil testing methods for predicting wheat relative dry matter and K uptake across the different soils was compared using the coefficient of determination derived from fitting a Mitscherlich curve (Eq. 1) to the data. The critical value of each soil test method for indicating K deficiency was assessed as the value that delivered 90% of the maximum dry matter46, 47. When fitting the Mitscherlich curve, all curves were scaled to a relative basis by setting (DM 0+a) to 100. The coefficient of determination was used to identify the most accurate soil testing method for predicting wheat dry matter response. For the DGT K method, any treatments where competing cations could have affected the measured value for K43 were excluded.
Statistical analysis
Correlations between the values obtained from the different soil K test methods on control soils were assessed using the Spearman correlation method in the SigmaPlot 12.0 software. Analysis of variance (ANOVA) was performed using IBM SPASS Statistics 20 to assess whether the measured root density and dry mass were significantly different between soil types within each pot size.
Results
Correlations between soil tests
A significant correlation was obtained between the Colwell K and the NH4OAc K methods (R2 = 0.93, P < 0.01). However, no significant correlation (p ≤ 0.05) was found between the Colwell K or NH4OAc K methods and the DGT K method. The CaCl2 K method had moderate correlation with the Colwell K (R2 = 0.49, P < 0.01) and NH4OAc K methods (R2 = 0.51, P < 0.01), and correlated well with the DGT K method (R2 = 0.77, P < 0.01).
Wheat responses to K
Contrasting root densities were obtained in small and large pots, as measured after wheat plants were harvested (Table 2). Significant differences in root densities were found between wheat roots in different soils in both small and large pots (P ≤ 0.05). Wheat grown in some control soils showed symptoms of K deficiency, i.e. yellowing tips and edges on leaves and stunted growth, reflecting low available K contents in those soils. Good wheat dry matter responses to K application were observed in most of the small pots (Table 2). Generally, wheat growth responses to K applications were larger in the small pots compared to that in the large pots, except for soils NK and WN. In the large pots containing soils KD, LB and RF, the response to K fertilizer application was negative, so DM max was set at the dry mass in the control soil. In the large pots of soil QS, linear responses to K were observed, so DM max was set as 120% of the dry mass for the highest K rate48. The predicted maximum dry matter was close to what obtained on each soil from the experiment (Table 2).
Performance of soil tests to predict dry matter response to K fertilizer
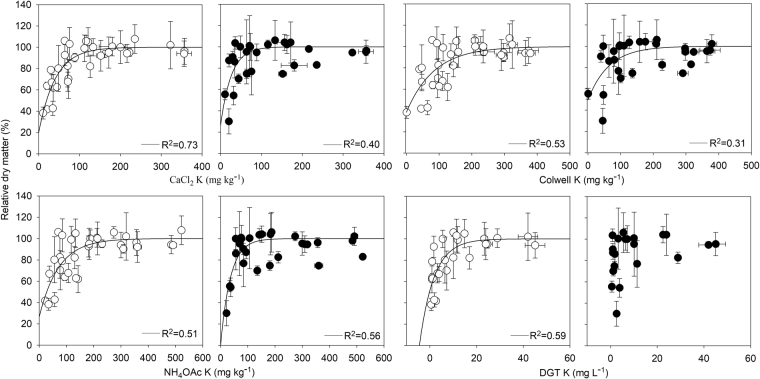
The relationships between soil extractable K derived using the different methods and the relative dry matter response of wheat to K fertilizer in all soils are shown in Fig. 1. In the small pots, good relationships of wheat relative dry matter to extractable K values were obtained for the CaCl2 K method (R2 = 0.73), while all other tests relatively showed poorer relationships (R2 0.51–0.59, Table 3). However in the larger pots, good relationships of wheat relative dry matter to measured soil K were obtained for the NH4OAc K method (R2 = 0.56, Fig. 1). CaCl2 K (R2 = 0.40) and Colwell K (R2 = 0.31) methods showed poor correlation relationships. The DGT K method performed poorly and no significant relationship (P ≤ 0.05) could be derived. The critical concentrations for different soil K test methods in all the soils at two root densities were shown in Table 3. Compared to the results obtained in all soils, the relationships between soil extractable K derived and the relative dry matter response of wheat to K fertilizer using different methods in control soils showed similar relationships. In the small pots, good relationships of wheat relative dry matter to extractable K values were obtained for the CaCl2 K method (R2 = 0.64), followed by the DGT K method (R2 = 0.57), while Colwell K and NH4OAc K methods showed no correlation relationships. In the larger pots, good relationships of wheat relative dry matter to measured soil K were obtained for the NH4OAc K method (R2 = 0.75), followed by the Colwell K (R2 = 0.70) method. CaCl2 K (R2 = 0.55) methods showed a relative poor correlation relationship and the DGT K method performed poorly and no significant relationship (P ≤ 0.05) could be derived.
Figure 1.
Relationship between extractable K with and the relative dry matter response of wheat to K fertilizer (open symbols represents the relative dry matter obtained from small pots and closed symbols represents the relative dry matter obtained from large pots).
Table 3.
Critical values and coefficient of determinations for each test method for obtaining 90% of maximum dry matter at two root densities; “—” denotes no significant relationship was observed.
| Soil test | CaCl2 K | Colwell K | NH4OAc K | DGT K | |
|---|---|---|---|---|---|
| mg kg−1 | |||||
| High demand and root density | Critical value | 83 | 151 | 142 | 10 |
| R2 | 0.73 | 0.53 | 0.51 | 0.59 | |
| Low demand and root density | Critical value | 57 | 114 | 91 | — |
| R2 | 0.40 | 0.31 | 0.56 | — | |
Performance of soil tests to predict K uptake by plants
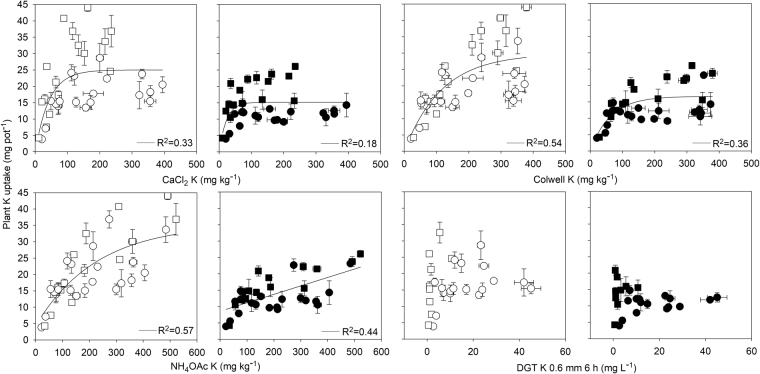
The amounts of K uptake (mg K pot−1) in the small pots were higher than that in the large pots due to more plants were grown in the small pots (Fig. 2). The coefficients of determination (R2) between extractable K and plant K uptake in wheat shoots were <0.44 for the large pots and <0.57 for the small pots (Fig. 2). NH4OAc K method was best able to predict plant K uptake (R2 = 0.57 in small pots; R2 = 0.44 in large pots), with a fairly similar level of accuracy found for the Colwell K method (R2 = 0.54 in small pots; R2 = 0.36 in large pots). The CaCl2 K method was less effective at predicting plant K uptake (R2 = 0.33 in small pots; R2 = 0.18 in large pots), while there was no correlation between the plant K uptake and the amounts of soil K extracted by the DGT method in each pot size. While using the control soils only, the best predictor was also the NH4OAc K method (R2 = 0.60 in small pots; R2 = 0.83 in large pots). A fairly similar level of accuracy was also found for the Colwell K method (R2 = 0.55 in small pots; R2 = 0.82 in large pots). The CaCl2 K method was less effective at predicting plant K uptake (R2 = 0.44 in small pots; R2 = 0.54 in large pots), while there was no correlation between the plant K uptake and the amounts of soil K extracted by the DGT method in each pot size.
Figure 2.
Relationship between extractable K and plant K uptake in wheat shoots grown in small (open symbols) or large (closed symbols) pots and receiving 4 rates of K fertilizer. Soils with CEC < 10 cmol kg−1 are represented by circles while square symbols represent soils with CEC > 10 cmol kg−1.
Relationship between K concentration in shoot and relative dry matter
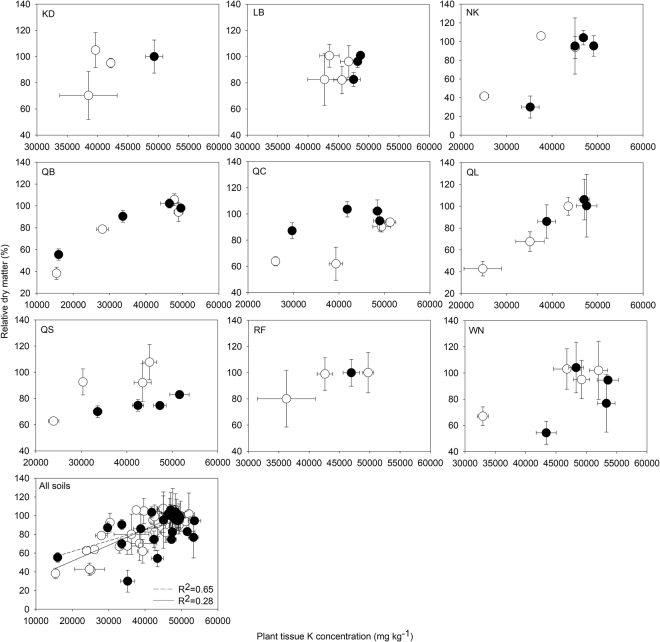
Generally, higher relative dry matter was observed in plants with higher K concentrations in each soil (Fig. 3). A moderate correlation between wheat relative dry matter and plant tissue K concentrations was observed in the small pots (R2 = 0.65), with 42600 mg K kg−1 required to obtain 90% relative dry matter. Soils NK and WN seemed to behave differently under the different root densities and were the only two soils where responses to K were larger in the large pot-low root density conditions. The relationship between dry matter and tissue K concentration in large pots was improved when soils NK and WN were excluded from the results, rising from an R2 = 0.28 to R2 = 0.63. The overall critical tissue concentration to obtain 90% relative dry matter was 42800 mg kg−1 for the plants in the large pots, which was very similar to the critical value obtained in the small pots.
Figure 3.
Relationships between concentrations of K in shoots and relative dry matter for wheat grown in 9 contrasting soil types (open symbol represent the values obtained from small pots and closed symbols represent the values obtained from large pots). The final figure is a combined analysis across all 9 soil types, with the dashed and continuous lines representing the relationship curve for small pots and large pots, respectively. R2 in the large pots rises from 0.28 to R2 = 0.63 when soils NK and WN were excluded from the results.
Discussion
Correlations between soil tests
Based on the correlations between soil K testing methods, they can be categorized into 3 groups: 1) CaCl2 K method, 2) exchangeable K methods (Colwell K and NH4OAc K) and 3) DGT K method. A high correlation between the Colwell K (exchangeable K) and NH4OAc K (exchangeable K) methods (slope of NH4OAc K/Colwell K = 1.11, R2 = 0.93, P < 0.01) suggests that these two methods measure a similar K pool, commonly referred to as exchangeable K. While both extractants in effect measure both soil solution K and readily desorbable K on exchange sites, the former is usually an extremely small fraction of the quantity measured– except where recent fertilizer or manure applications have been made49. The bulk of K measured in these tests therefore refers to the pool of K that is in a form immediately available for movement into the soil solution in response to depletion by plant root uptake50. In soils that have limited mineral K sources, exchangeable K is therefore effectively an index of the capacity of the soil to supply bioavailable K over an extended period (a quantity factor). The close correlation between the Colwell K and NH4OAc K methods extraction methods is consistent with findings from the review by McLean and Watson4. Although the soil to solution ratio higher and extracting period is shorter than that for the Colwell K method, the slight increase in extraction efficiency with the NH4OAc K method is mainly due to the similarity in hydrated radius of the NH4 + and K+ and a higher extraction concentration, allowing more complete displacement of K from the jagged edges and interlayer positions in tetrahedral sheets of clay minerals.
The lower concentration and larger size of Ca2+ relative to Na+ and NH4 + mean that the CaCl2 method is much less effective at displacing K from tightly held exchange positions on the solid phase, meaning the measurement is more closely related to soil solution K (a measure of the intensity of K supply) than to exchangeable K. The lesser but still significant (R2 = 0.49–0.51) correlation between the CaCl2 K method and the Colwell K and NH4OAc K methods may be at least partly due to the effect of recent fertilizer applications disproportionately increasing the fraction of Colwell K and NH4OAc K extracts that consists of soil solution K. Soils with high buffer power (typically high cation exchange capacity) hold increasing proportions of K tightly bound on soil solid phase, even with fresh fertilizer additions. While this K can be measured by the NH4OAc or Colwell K methods, it cannot be measured by solution K methods (e.g. CaCl2 K method). So the differences between the quantity and intensity extraction methods are likely to increase as CEC and soil buffer power increase.
The moderate correlations between the DGT K method and the CaCl2 K method (R2 = 0.77, P < 0.05) are consistent with the fact that both are defacto measures of soil solution K (a surrogate K intensity factor), in agreement with Zhang et al.’s result43. However the DGT K method adds a diffusion factor purported to better relate to the rate of diffusive supply to a DGT surface upon dilution of the solution K phase and K resupply from the soil solid phase. Furthermore, the extracting force of K from soils with high CEC by DGT K method is weak, as lower CDGT values were obtained on soils with higher CEC values which were amended with more K before wheat growing (last component in Fig. 2). This may explain the lack of correlation between the DGT K results and those of Colwell K and NH4OAc K methods (in contrast to that for CaCl2 K method).
Performance of soil tests to predict relative dry matter and K uptake
The results obtained by using all soils and control soils were comparable because the relative dry matter and K uptake response to soil testing values using different extracting methods at two root densities showed similar accuracy. The wheat roots were constricted in the pots in the glasshouse trial and a net-like root mat was observed at the bottom of all pots, but particularly for the small pots. Due to the limited soil volume and increased plant number in the small pots, there is the likelihood that the available K in the small pots was exhausted, and that plant growth was restricted before the biomass was measured compared to the plants in the large pots. It was reported that root competition could cause a reduction in the availability of a soil resource51. Root volume confinement could also result in an increased abscisic acid concentration within root system and decreased dry weight in biomass52, 53. The root density of winter wheat in a field experiment (soil depth ≤ 20 cm) was reported to be around 0.15 g cm−3 by Yao et al.54, which was close to the root density obtained from the large pots in this study. Therefore, we assumed that the root density in the large pot was more representative of the field conditions than that in the small pots.
The present study showed good correlations between wheat relative dry matter and K extracted by the NH4OAc K method at lower root densities. The critical value (90% relative dry matter) for the NH4OAc K method was 91 mg kg−1 at low root density (large pots) in all soils. While there are few reported studies developing critical soil test K values using the NH4OAc K method in the literature, Brennan and Bell reported a critical Colwell K critical value of 40–64 mg kg−1 for grain yield of wheat in field condition, with the range related to contrasting soil types55. This value was much lower than the 114 mg K kg−1 value we derived in this study, with the difference attributed to differences in plant growth stage, root density, water content and access to greater soil volumes (e.g. subsoil K) by roots in field conditions. Regardless, our data suggest the NH4OAc K methods were more accurate than other methods for predicting wheat response to K applications. The difference in critical values of soil K test is influenced by the ratio of other cations and their plant uptake. Also, the rate of K supply from less available soil K pools may not be sufficient to meet crop demand where rooting patterns or slow diffusion limits the supply to the crop especially in vertisols. This suggest the estimation of reliable critical soil K test values still needs further investigation for grain cropping soils of Australia.
For prediction of K uptake, Colwell K and NH4OAc K methods seemed to be more accurate than other methods in control soils at low root densities, with this difference maintained when freshly fertilized soils were included in the assessment (R2 = 0.36 for the Colwell K and R2 = 0.44 for the NH4OAc K method) (Fig. 2). In the small pots, two plants with larger biomass generated a high K demand, and the high density plant roots were able to take up nearly all the available K from soils. Consequently, diffusion and/or soil supply rate contributes very little to K uptake by plants in the small pots, but the total available K amounts in soils represent plant acquisition at high demand and root density. Therefore, the NH4OAc K method should best predict K uptake by plants at high root density, as the NH4OAc K method is considered the quantity factor for soil available K measuring. The accuracy of predicting K uptake also decreased as K demand decreased in the large pots. On the contrary, plants took up K easily in a large pot compared to that in a small pot. Because there was no high K demand produced by aggressive plant biomass, plants took up K in a limited space around the roots, representing the dynamics associated with nutrient supply and plant acquisition. In the large pots, plant roots continued to be able to explore “new” space in the pots with relative sufficient K+ presented in the soil solution, where diffusive supply factors also become more notable as K+ diffused from “new” soil to exploited soil. The performance of DGT for predicting K uptake was poor at high root density, but the performance was also unexpectedly poor at low root density as well. Compared to other methods, high K uptakes by plants from soils with high CEC values (soils with CEC >10 cmol kg−1 as shown in square symbols in Fig. 2) was observed, but the measured DGT K values were low. That is to say, there was a proportion of available K in soils that can’t be measured by the DGT K method, but had been used by plants. Therefore, we can concluded that the extraction force of the DGT device for K is too weak on the soils with high CEC values and DGT K method may not be a suitable soil test method to assess the plant available K in certain soil types.
Effects of root density on critical values for soil tests
The performance of the soil tests to predict response to K fertilizer was affected by the different root densities induced by varying pot size. The critical value increased with increased root density (Table 3). The reason is presumably that higher root density creates a stronger demand on soil K pools thereby requiring a greater amount of available K to satisfy this demand. This indicates that calibrating soil tests with crop fertilizer requirements for K is difficult under glasshouse conditions as root densities are often higher than in the field, so critical values determined under glasshouse conditions are unlikely to accurately predict responses in the field. These inaccuracies will increase as pot size decreases.
We hypothesized that at high root density (small pots) the capacity measures (Colwell K and NH4OAc K methods) would correlate better with plant response due to significant K depletion, while at low rooting density it was more likely that intensity measures of soil K (CaCl2 and DGT) might correlate well with plant response to K fertilizer. However, the intensity measures of soil K unexpectedly showed worse correlations with plant response (both dry matter and K uptake) to K fertilizer compared to the capacity measures at low root density. There was also the likelihood that K demands by plants in the small pots were larger than that soils could supply due to limited soil volume/mass employed, thereby being the reason that more response of wheat relative dry matter to K application was observed.
Plant K concentration relationship with relative dry matter
Although plant K concentration in tissue can vary with time and in different parts of the same plant56, 57, K concentration of the whole plant (dry mass basis) stays at a similar level at the same stage of plant growth58. Moderate correlations between wheat relative dry matter and tissue K concentration (R2 = 0.65 at high root density; R2 = 0.63 at low root density when soils NK and WN were excluded) were observed across the soils (Fig. 3). A consistent correlation relationship of wheat relative dry matter and tissue K concentration at different root densities suggests that plant tissue K concentration could be used to infer wheat growth constraints in terms of K limitations for diagnose purposes. The correlation relationship deteriorated when values from soils NK and WN were included in the analysis, resulting in a lowered coefficient of determination in the low root density situation. The failure to achieve a strong growth response to K in soils NK and WN was probably due to some unpredicted factors during the growth period in the large pots (e.g. poor aeration, or water accumulation at the bottom of the pots).
Conclusions
This study investigated four soil testing methods to predict wheat response to K application in 9 agricultural soils in a glasshouse trial under two root densities. The predictive accuracy of soil K testing methods varied with root density. Comparing the plant growing conditions to that in the field, the low root density of plant roots in large pots was a more realistic approximation of field conditions. In these larger pots the NH4OAc K method had the highest accuracy for predicting wheat relative dry matter and plant K uptake in response to K application across all soils. The critical value for 90% wheat relative dry matter at the end of tillering (GS30) stage for the NH4OAc K method was 91 mg kg−1. Although the DGT technique measures elements in soil solution and parts from the soil solid phase, it failed to accurately predict wheat relative dry matter or plant K uptake responses to K applications, presumably because the extraction force of K from soils by the DGT was too weak compared to that by plant roots. Further study is needed to validate the performance of these soil K test methods in field conditions.
Acknowledgements
The first author receives funding from the China Scholarship Council (CSC). This research was supported by the University of Adelaide and Grain Research and Development Corporation (GRDC). The authors also thank CSIRO for the ICP-OES analyses. The authors also would like to acknowledge the assistance in supplying soil to the trials by Dr. Mike Bell (The University of Queensland), Dr. Craig Scanlan (Development of Agriculture and Food WA) and Qiu Ma (Curtin University). We would also like to express great appreciation to the anonymous reviewers for thoughtful and professional comments and suggestions.
Author Contributions
Zhang, Y. wrote the manuscript; Nachimuthu, G. and Zhang, Y. performed the experiment; Mason, S., McLaughlin, M. and McNeill, A. joined the discussion of experiment design, revised and edited the manuscript; Bell, M. revised the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bell, M., Moody, P., Klepper, K. & Lawrence, D. in Proceedings of the 19th World Congress of Soil Science: Soil solutions for a changing world, Brisbane, Australia, 1–6 August 2010. Symposium 3.3.1 Integrated nutrient management. 302–305.
- 2.Barber, S. A. Soil Nutrient Bioavailability. 91 (John Wiley & Sons Inc, 1995).
- 3.Huang, P. M., Li, Y. & Sumner, M. E. Handbook of soil sciences. 2nd ed. edn, (CRC; Taylor & Francis, 2012).
- 4.McLean, E. O. & Watson, M. E. In Potassium in Agriculture (ed. Robert D. Munson) 277–308 (Madison, Wisconsin; USA, 1985).
- 5.Barber SA, Walker JM, Vasey EH. Mechanisms for movement of plant nutrients from soil and fertilizer to plant root. J. Agric. Food Chem. 1963;11:204–207. doi: 10.1021/jf60127a017. [DOI] [Google Scholar]
- 6.Mills, H. A., Jones, J. B. & Mills, H. A. Plant analysis handbook II: a practical sampling, preparation, analysis, and interpretation guide. iii, 422 (Micromacro Publishing, 1996).
- 7.Baligar VC. Potassium uptake by plants, as characterized by root density, species and K/Rb ratio. Plant Soil. 1985;85:43–53. doi: 10.1007/BF02197799. [DOI] [Google Scholar]
- 8.Cakmak I, Hengeler C, Marschner H. Partitioning of shoot and root dry-matter and carbohydrates in bean-plants suffering from phoshphorus, potassium and magnesium-deficiency. J. Exp. Bot. 1994;45:1245–1250. doi: 10.1093/jxb/45.9.1245. [DOI] [Google Scholar]
- 9.Marschner H, Kirkby EA, Cakmak I. Effect of mineral nutritional status on shoot-root partitioning of photoassimilates and cycling of mineral nutrients. J. Exp. Bot. 1996;47:1255–1263. doi: 10.1093/jxb/47.Special_Issue.1255. [DOI] [PubMed] [Google Scholar]
- 10.Fusseder A, Kraus M. Individual root competition and exploitation of the immobile macronutrients in the root space of maize. Flora. 1986;178:11–18. doi: 10.1016/S0367-2530(17)30199-8. [DOI] [Google Scholar]
- 11.Rayment, G. E. & Lyons, D. J. Soil Chemical Methods- Australia. (CSIRO Publishing, 2010).
- 12.Gourley, K. In Soil Analysis– an Interpretation Manual (eds K. I. Peverill, L. A. Sparrow, & D. J. Reuter) Ch. 233, 229–245 (CSIRO, 1999).
- 13.Raij BV, Quaggio JA, Silva NMd. Extraction of phosphorus, potassium, calcium, and magnesium from soils by an ion‐exchange resin procedure. Commun. Soil Sci. Plant Anal. 1986;17:547–566. doi: 10.1080/00103628609367733. [DOI] [Google Scholar]
- 14.Datta SC, Singhal SK, Mandal D. Development of resin disc soil testing in rice crop in relation to kinetics of nutrient adsorption on resin. Commun. Soil Sci. Plant Anal. 2012;43:1109–1120. doi: 10.1080/00103624.2012.662560. [DOI] [Google Scholar]
- 15.Woods, M. S., Rossi, F. S. & Ketterings, Q. M. Potassium supply rate as measured by exchange membranes in a calcareous sand. Applied Turfgrass Science3, doi:10.1094/ats-2006-0323-01-rs (2006).
- 16.Bremer, E., Andronak, L. & Greer, K. In Crops and Soils Vol. 47, 22–25 (American Society of Agronomy, 2014).
- 17.Beckett PHT. Studies on soil potassium II: The ‘immediate’ Q/I relations of labile potassium in the soil. J. Soil Sci. 1964;15:9–23. doi: 10.1111/j.1365-2389.1964.tb00240.x. [DOI] [Google Scholar]
- 18.Barber, S. A. In Chemistry in the Soil Environment Vol. 40 (eds R. H. Dowdy, J Ryan, V. V. Volk, & D. E. Baker) 1–12 (American Society of Agronomy; Soil Science Society of America, 1981).
- 19.Bell MJ, Moody PW, Harch GR, Compton B, Want PS. Fate of potassium fertilisers applied to clay soils under rainfed grain cropping in south-east Queensland, Australia. Aust. J. Soil Res. 2009;47:60–73. doi: 10.1071/SR08088. [DOI] [Google Scholar]
- 20.Houba VJG, Novozamsky I, Lexmond TM, van der Lee JJ. Applicability of 0.01 M CaCl2 as a single extraction solution for the assessment of the nutrient status of soils and other diagnostic purposes. Commun. Soil Sci. Plant Anal. 1990;21:2281–2290. doi: 10.1080/00103629009368380. [DOI] [Google Scholar]
- 21.Darunsontaya T, Suddhiprakarn A, Kheoruenromne I, Prakongkep N, Gilkes RJ. The forms and availability to plants of soil potassium as related to mineralogy for upland Oxisols and Ultisols from Thailand. Geoderma. 2012;170:11–24. doi: 10.1016/j.geoderma.2011.10.002. [DOI] [Google Scholar]
- 22.Krishna, K. R. In Soil fertility and crop production (ed. K. R. Krishna) Ch. 6, 141–153 (Science Publishers Inc., 2002).
- 23.Gourley, C. J. P. et al. Making better fertiliser decisions for grazed pastures in Australia (Department of Primary Industries, 2007).
- 24.Mason S, McLaughlin M, Johnston C, McNeill A. Soil test measures of available P (Colwell, resin and DGT) compared with plant P uptake using isotope dilution. Plant Soil. 2013;373:711–722. doi: 10.1007/s11104-013-1833-7. [DOI] [Google Scholar]
- 25.Mason S, McNeill A, McLaughlin MJ. Prediction of wheat response to an application of phosphorus under field conditions using diffusive gradients in thin-films (DGT) and extraction methods. Plant Soil. 2010;337:243–258. doi: 10.1007/s11104-010-0521-0. [DOI] [Google Scholar]
- 26.Six L, Pypers P, Degryse F, Smolders E, Merckx R. The performance of DGT versus conventional soil phosphorus tests in tropical soils - An isotope dilution study. Plant Soil. 2012;359:267–279. doi: 10.1007/s11104-012-1192-9. [DOI] [Google Scholar]
- 27.Speirs S, Scott B, Moody P, Mason S. Soil phosphorus tests II: A comparison of soil test-crop response relationships for different soil tests and wheat. Crop & Pasture Science. 2013;64:469–479. doi: 10.1071/CP13111. [DOI] [Google Scholar]
- 28.Zhang H, Davison W, Gadi R, Kobayashi T. In situ measurement of dissolved phosphorus in natural waters using DGT. Anal. Chim. Acta. 1998;370:29–38. doi: 10.1016/S0003-2670(98)00250-5. [DOI] [Google Scholar]
- 29.Nolan AL, Lombi E, McLaughlin MJ. Metal bioaccumulation and toxicity in soils-Why bother with speciation? Aust. J. Chem. 2003;56:77–91. doi: 10.1071/CH02226. [DOI] [Google Scholar]
- 30.Mason S, Hamon R, Nolan A, Zhang H, Davison W. Performance of a mixed binding layer for measuring anions and cations in a single assay using the diffusive gradients in thin films technique. Anal. Chem. 2005;77:6339–6346. doi: 10.1021/ac0507183. [DOI] [PubMed] [Google Scholar]
- 31.Ernstberger H, Zhang H, Tye A, Young S, Davison W. Desorption kinetics of Cd, Zn, and Ni measured in soils by DGT. Environ. Sci. Technol. 2005;39:1591–1597. doi: 10.1021/es048534d. [DOI] [PubMed] [Google Scholar]
- 32.Harper MP, Davison W, Zhang H, Tych W. Kinetics of metal exchange between solids and solutions in sediments and soils interpreted from DGT measured fluxes. Geochim. Cosmochim. Acta. 1998;62:2757–2770. doi: 10.1016/S0016-7037(98)00186-0. [DOI] [Google Scholar]
- 33.Huang J, Bennett WW, Teasdale PR, Gardiner S, Welsh DT. Development and evaluation of the diffusive gradients in thin films technique for measuring nitrate in freshwaters. Anal. Chim. Acta. 2016;923:74–81. doi: 10.1016/j.aca.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Tandy S, et al. A new method for determination of potassium in soils using diffusive gradients in thin films (DGT) Environ. Chem. 2012;9:14–23. doi: 10.1071/EN11070. [DOI] [Google Scholar]
- 35.Zhang Y, Mason S, McNeill A, McLaughlin MJ. Optimization of the diffusive gradients in thin films (DGT) method for simultaneous assay of potassium and plant-available phosphorus in soils. Talanta. 2013;113:123–129. doi: 10.1016/j.talanta.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 36.Rayment, G. E. & Higginson, F. R. Australian laboratory handbook of soil and water chemical methods. (Inkata Press, 1992).
- 37.Bowman, G. & Hutka, J. In Soil physical measurement and interpretation for land evaluation (eds Neil. McKenzie, Keppel. Coughlan, & Hamish. Cresswell) Ch. 17, 224–239 (CSIRO Publishing, 2002).
- 38.Jenkinson DS, Powlson DS. The effects of biocidal treatments on metabolism in soil- V: A method for measuring soil biomass. Soil Biol. Biochem. 1976;8:209–213. doi: 10.1016/0038-0717(76)90005-5. [DOI] [Google Scholar]
- 39.Zadoks JC, Chang TT, Konzak CF. Decimal code for growth stages of cereals. Weed Research. 1974;14:415–421. doi: 10.1111/j.1365-3180.1974.tb01084.x. [DOI] [Google Scholar]
- 40.Salomon E. Extraction of soil potassium with 0.01 M calcium chloride compared to official Swedish methods. Commun. Soil Sci. Plant Anal. 1998;29:2841–2854. doi: 10.1080/00103629809370159. [DOI] [Google Scholar]
- 41.Colwell JD. An automated procedure for the determination of phosphorus in sodium hydrogen carbonate extracts of soils. Chem. Ind. 1965;22:893–895. [Google Scholar]
- 42.Zhang H, Davison W. Performance characteristics of diffusion gradients in thin films for the in situ measurement of trace metals in aqueous solution. Anal. Chem. 1995;67:3391–3400. doi: 10.1021/ac00115a005. [DOI] [Google Scholar]
- 43.Zhang Y, Mason S, McNeill A, McLaughlin MJ. Application of the diffusive gradients in thin films technique for potassium measurement in agricultural soils: Effects of competing cations on potassium uptake by the resin gel. Anal. Chim. Acta. 2014;842:27–34. doi: 10.1016/j.aca.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 44.Zarcinas BA, Cartwright B, Spouncer LR. Nitric acid digestion and multielement analysis of plant-material by inductively coupled plasma spectrometry. Commun. Soil Sci. Plant Anal. 1987;18:131–146. doi: 10.1080/00103628709367806. [DOI] [Google Scholar]
- 45.McBeath TM, McLaughlin MJ, Noack SR. Wheat grain yield response to and translocation of foliar-applied phosphorus. Crop & Pasture Science. 2011;62:58–65. doi: 10.1071/CP10237. [DOI] [Google Scholar]
- 46.Holford I, Morgan J, Bradley J, Cullis B. Yield responsiveness and response curvature as essential criteria for the evaluation and calibration of soil phosphate tests for wheat. Aust. J. Soil Res. 1985;23:167–180. doi: 10.1071/SR9850167. [DOI] [Google Scholar]
- 47.Menzies N, Kusumo B, Moody P. Assessment of P availability in heavily fertilized soils using the diffusive gradient in thin films (DGT) technique. Plant Soil. 2005;269:1–9. doi: 10.1007/s11104-004-1725-y. [DOI] [Google Scholar]
- 48.Dyson CB, Conyers MK. Methodology for online biometric analysis of soil test-crop response datasets. Crop & Pasture Science. 2013;64:435–441. doi: 10.1071/CP13009. [DOI] [Google Scholar]
- 49.Hinsinger, P. In Encyclopedia of Soil Science Vol. 2 (ed Rattan Lal) 1354–1358 (Taylor and Francis, 2006).
- 50.Barber, S. A. Soil nutrient bioavailability: A mechanistic approach. 2nd Edition edn, (John Wiley & Sons, 1995).
- 51.Schenk HJ. Root competition: beyond resource depletion. J. Ecol. 2006;94:725–739. doi: 10.1111/j.1365-2745.2006.01124.x. [DOI] [Google Scholar]
- 52.Hurley MB, Rowarth JS. Resistance to root growth and changes in the concentrations of ABA within the root and xylem sap during root-restriction stress. J. Exp. Bot. 1999;50:799–804. doi: 10.1093/jxb/50.335.799. [DOI] [Google Scholar]
- 53.Cooper AJ. The influence of container volume, solution concentration, pH and aeration on dry matter partition by tomato plants in water culture. Journal of Horticultural Science. 1972;47:341–347. doi: 10.1080/00221589.1972.11514476. [DOI] [Google Scholar]
- 54.Yao S, Kang Y, Liu H. Research on root growth and distribution of winter wheat under sprinkler and surface irrigation. Journal of Water Resources & Water Engineering. 2010;21:1–5. [Google Scholar]
- 55.Brennan RF, Bell MJ. Soil potassium-crop response calibration relationships and criteria for field crops grown in Australia. Crop & Pasture Science. 2013;64:514–522. doi: 10.1071/CP13006. [DOI] [Google Scholar]
- 56.Barraclough PB, Leigh RA. Grass yield in relation to potassium supply and the concentration of cations in tissue water. J. Agric. Sci. 1993;121:157–168. doi: 10.1017/S0021859600077017. [DOI] [Google Scholar]
- 57.Greenwood DJ, et al. Comparison of the effects of potassium fertilizer on the yield, potassium content and quality of 22 different vegetable and agricultural crops. J. Agric. Sci. 1980;95:441–456. doi: 10.1017/S0021859600039496. [DOI] [Google Scholar]
- 58.Greenwood DJ, Stone DA. Prediction and measurement of the decline in the critical-K, the maximum-K and total cation plant concentrations during the growth of field vegetable crops. Ann. Bot. 1998;82:871–881. doi: 10.1006/anbo.1998.0775. [DOI] [Google Scholar]