Abstract
In this study, we fabricated a novel material composed of magnetic graphene oxide incorporated Fe3O4@polyaniline (Fe3O4@PANI-GO) using a modified Hummers’ method, solvothermal, and two-step polymerisation method. The resulting products were characterised by transmission electron microscopy (TEM), Fourier transform infrared spectroscopy (FT-IR), and X-ray diffraction (XRD). The results indicated that magnetic Fe3O4@PANI particles were successfully loaded onto the surface of the graphene oxide. Further Fe3O4@PANI-GO was investigated to remove bisphenol A(BPA), α-naphthol, and t-octyl-phenol (t-OP) from water samples by magnetic solid phase extraction. Under the optimal conditions, the Fe3O4@PANI-GO composite exhibited good adsorption capacity for t-OP, BPA, and α-naphthol, and the adsorption of these followed a pseudo-second-order kinetic model. Adsorption isotherms fit the Langmuir model, and the adsorption was an endothermic and spontaneous process. This work indicated that Fe3O4@PANI-GO earned great application prospect for removing phenolic contaminants from polluted water.
Introduction
In recent years, magnetic nanoparticles (MNPs) have shown great technological significance in the areas of electronics, catalysis, therapy diagnosis, biosensors, and drug delivery due to their unique superparamagnetic properties1. Iron-based materials (e.g. Fe3O4) have drawn considerable attention as inorganic supports for the synthesis of organic-inorganic hybrid materials because of their potential application in information storage, drug delivery, targeting, and magnetic separation2. Fe3O4 nanoparticles possess many merits such as high surface area, inexpensiveness, easy separation by an external magnetic field, and high reusability, while they also are naturally hydrophilic due to the existence of plentiful hydroxyl groups on the particle surface3. However, since magnetite is highly susceptible to oxidation/dissolution, especially in acidic solutions4, 5, and is easily aggregated, all of these properties cause instability. Thus, magnetic adsorbents are difficult to directly use because of the aggregation and limited adsorption property. An effective strategy is to coat or modify iron oxide nanoparticles with other substrates to enhance the stability of the composite material and improve the special adsorption of target compounds, which is also a successful way to widen the application of the material by coating multifunctional groups on their surfaces.
Polyaniline (PANI) is one of the most technologically important materials based on its environmental stability in a conducting form, unique redox properties, and high conductivity with suitable dopants6, 7. The physicochemical properties of polyaniline and its potential applications in diverse fields such as battery, sensors, and wave-adsorption8-10 have been reviewed. PANI can be easily synthesised by either chemical or electrochemical methods. Recently, bifunctional Fe3O4@PANI nanocomposites have attracted intensive attention for application in nanomaterials due to their novel magnetic and conductive properties. Xuan et al. reported the synthesis of Fe3O4@polyaniline core-shell microspheres with well-defined blackberry-like morphology6. Zhao et al.11 prepared Fe3O4@PANI composite nanoparticles with a core-shell structure and measured their inductive heat property for localised hyperthermia. PANI was also used to easily and efficiently remove pollutants like heavy metal ions and organic contaminant from aqueous solutions12–14. In view of PANI polymers having a wealth of amino and benzene ring groups, the material can adsorb organic compounds and metal ions by π-π interaction and electrostatic interaction. Therefore, PANI is expected to be a promising adsorbent for the removal of aromatic compounds in water. However, the mechanical weakness and poor solubility of PANI greatly hinder further experimental investigation and commercial exploitation of the material15.
Graphene oxide (GO) consists of a hexagonal carbon network bearing hydroxyl and epoxide functional groups on its “basal” plane and is a single sheet of graphite oxide that exhibits good properties for many applications. It can be obtained by exfoliation of graphite oxide16 whereas the edges are mostly decorated by carboxyl and carbonyl groups17. These oxygen-containing functional groups can bind with metal ions and organic contaminants in water. Yang et al. found that the adsorption capacity of Cu(II) on GO was 10 times higher than that of Cu(II) on activated carbon18. Chang et al. prepared Fe3O4/graphene nanocomposites and achieved good adsorption performance for aniline and p-chloroaniline19. Xie et al. developed a facile chemical method to produce a superparamagnetic graphene oxide-Fe3O4 hybrid composite and which was successfully utilised for the removal of dyes from aqueous solution with high removal efficiency20. However, upon the removal of the hydrophilic functional groups on GO, it can lead to aggregated graphene sheets that are a few layers thick21. GO has high hydrophilicity and good dispersibility, making it suitable for direct application as an adsorbent for the separation/preconcentration of organic contaminants. Some graphene materials need to be centrifuged in the last separation steps in order to prepare graphene composite material with high specific surface area and stability22, which is significant for broadening the application of graphene oxide. There exist some reports on the application of PANI in the fabrication of a GO/PANI composite for supercapacitors and high-performance shielding materials23–25, etc., which suggests that PANI can be effectively anchored on a magnetic substrate via strong interactions with GO as the intermediate. This process not only reserves the oxygen-containing functional groups of GO, but also enhances the stability of the magnetic composite.
In present study, Fe3O4@PANI-GO composite was synthesised by decorating GO and PANI, which provided nitrogen-containing functional groups and protected the Fe3O4 nanoparticles. The prepared magnetic composites were investigated as magnetic adsorbents to remove BPA, t-OP, and α-naphthol from aqueous solution. The adsorption parameters of Fe3O4@PANI-GO for the removal of the three phenols from aqueous solution were investigated. The adsorption kinetics, isotherms, and thermodynamic studies were also performed to demonstrate the mechanism of the composite material toward BPA, α-naphthol, and t-OP.
Results
Morphology and structure
Fe3O4@PANI-GO composite was prepared and characterised by Fourier transform infrared spectrometry (FT-IR, Nicolet Magana-IR 750) in the 400 to 4000 cm−1 region. The shape and size distribution of the Fe3O4 and Fe3O4@PANI-GO hybrids were characterised by transmission electron microscopy (TEM, JEM2010F microscope) and scanning electron microscopy (SEM, CAMBRIDGE S-360 microscope). Powder X-ray diffraction (XRD) was performed on a Bruker D8-advance X-ray diffractometer at 40 kV and 40 mA for monochromatised Cu Kα (λ = 1.5406 Å) radiation.
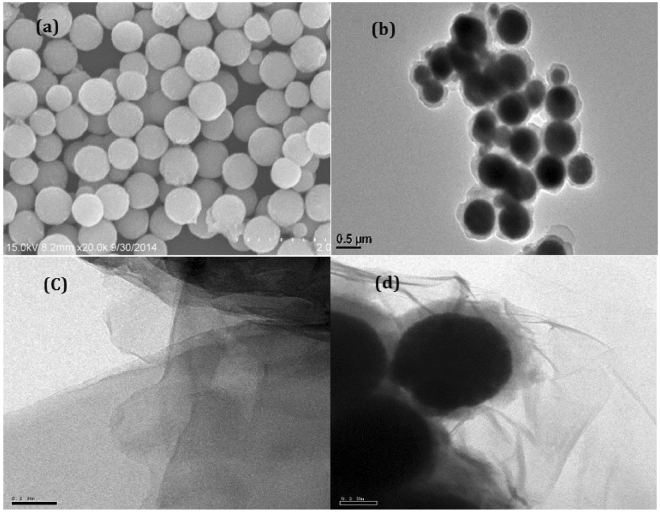
Figure 1(a) shows the SEM of Fe3O4 nanoparticles, which have an average size of about 200–300 nm. Figure 1(b) displays the TEM images of the Fe3O4@PANI core-shell material in which a clear uniform shell of about 50 nm is observed, indicating the successful polymerisation of PANI on the surface of Fe3O4. Figure 1(c,d) shows the TEM images of original GO and Fe3O4@PANI-GO hybrids. It was also clear that Fe3O4@PANI particles highly covered the surface of GO nanosheets (Fig. 1(d)), indicating possible electrostatic attraction between graphene and Fe3O4@PANI microspheres.
Figure 1.
(a) SEM images of the as-prepared Fe3O4; (b) TEM images of Fe3O4@PANI core/shell composite, (c) GO, and (d) Fe3O4@PANI-GO.
The structure of Fe3O4@PANI on graphene oxide was corroborated by XRD measurements. As seen in Fig. 2(a), the XRD pattern of Fe3O4 and Fe3O4@PANI-GO exhibits two peaks; the main peaks of Fe3O4 nanoparticles at 2θ = 18.5°, 30.4°, 35.7°, 43.2°, 54.2°, 57.6°, and 63.1° are assigned to the (111), (220), (311), (400), (422), (511), and (440) reflections, respectively. The diffraction peaks of the graphene oxide composite material are consistent with Fe3O4 nanoparticles, indicating the presence of Fe3O4 nanoparticles in the composites. There are no obvious diffraction peaks for GO (002), suggesting that GO has good interaction with Fe3O4 and PANI, and PANI can be observed in the XRD of Fe3O4@PANI-GO at 2θ = 19.79° 26.
Figure 2.
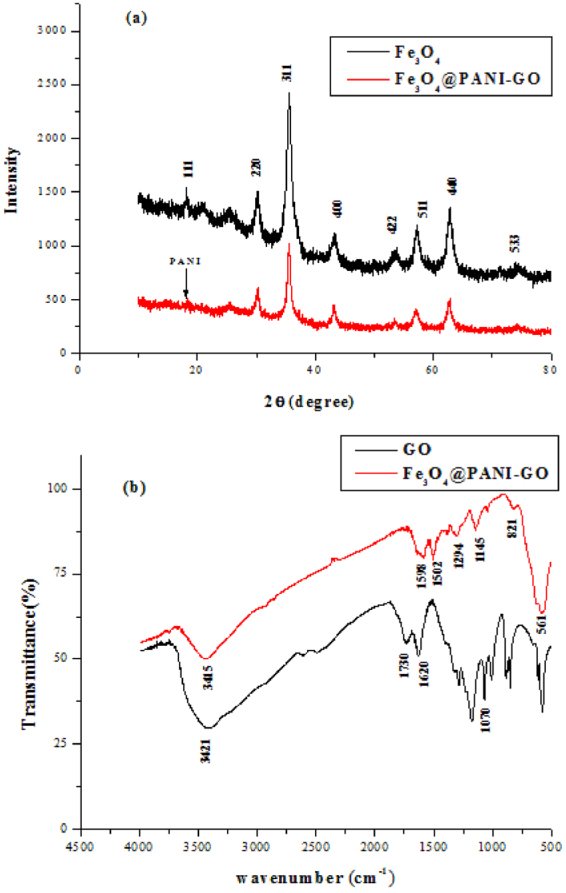
(a) XRD patterns of Fe3O4 and Fe3O4@PANI-GO; (b) FTIR spectra of pure GO and Fe3O4@PANI-GO composites.
Figure 2b shows the FT-IR spectra of GO and Fe3O4@PANI-GO composite material. As GO is concerned, the bands at 1730 and 1070 cm−1 are assigned to the characteristic peaks of C=O and C–O–C, respectively. In the FT-IR spectra of Fe3O4@PANI-GO, the adsorption bands at 1598 and 1502 cm−1 are attributed to the stretching vibration of C=C/C–C of benzenoid ring and quinoid. The band at 1294 cm−1 is the stretching vibration of C–N, which is the characteristic spectral bands of PANI, while the in-plane bending vibration of C=H is at 1145 cm−1. As expected, the characteristic peaks of Fe3O4 microspheres appear around 561 cm−1 and are contributed to the Fe–O bond stretching27–29, and the broad and intense band at 3400 cm−1 is ascribed to the stretching of O–H. In comparison, the same of the peaks of Fe–O and O–H appeared in Fe3O4@PANI-GO composite material, which indicate that Fe3O4@PANI was successfully loaded onto graphene oxide.
Optimization of adsorption
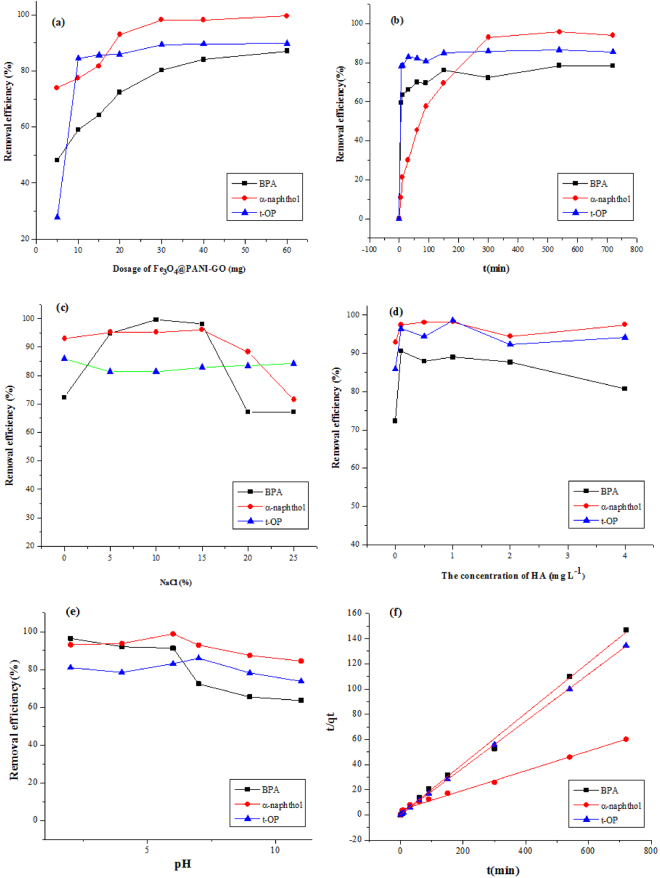
The important parameters that affect the adsorption such as amounts of adsorbents, sample pH, HA, ionic strength were optimized. The results showed that best results were obtained with 60 mg of Fe3O4@PANI-GO dosage at pH6. The salting-out effect and effect of HA were very small (See Fig. 3). The experimental data showed that the adsorption kinetics of BPA, t-OP, and α-naphthol conformed to pseudo-second-order kinetics and the data were exhibited in Fig. 3f and Table 1.
Figure 3.
Optimisation of adsorption parameters and adsorption kinetics. Effects of the (a) amount of adsorbent, (b) contact time, (c) ionic strength, (d) concentration of HA, (e) pH, and (f) pseudo-second-order kinetics.
Table 1.
Comparison results among the kinetic models for BPA, α-naphthol, and t-OP adsorption on Fe3O4@PANI-GO.
| Compound | pseudo-first-order kinetic models | pseudo-second-order kinetic models | intraparticle diffusion model | ||||
|---|---|---|---|---|---|---|---|
| R2 | k1 | R2 | k2 | R2 | Ki | intercept | |
| BPA | 0.9245 | 0.0132 | 0.9991 | 0.0278 | 0.704 | −5.1781 | 4.6182 |
| α-naphthol | 0.9333 | 0.0079 | 0.9958 | 0.0013 | 0.6097 | −47.684 | 9.1623 |
| T-OP | 0.7047 | 0.0146 | 0.9999 | 0.098 | 0.648 | −2.4027 | 5.2762 |
Adsorption isotherm and thermodynamics
The mechanism of adsorption is always in the center of our focusing, and several isotherm models were used to describe the adsorption behavior. The experimental results indicated that Langmuir model fit the adsorption data better than the Freundlich model for the adsorption of BPA, α-naphthol, and t-OP on the Fe3O4@PANI-GO magnetic composites. The thermodynamic data were calculated and demonstated that the adsorption was a spontaneous and endothermic process. These data were presented in Fig. 4, Tables 2 and 3.
Figure 4.
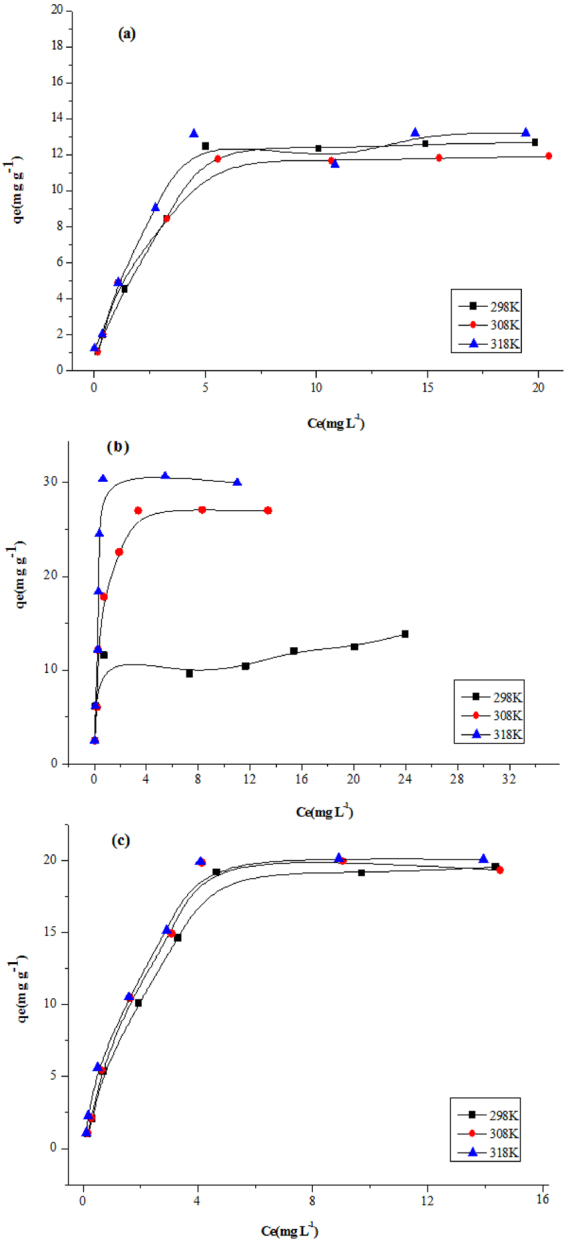
Adsorption isotherms of (a) BPA, (b) α-naphthol,and (c) t-OP.
Table 2.
The parameters for isotherms at three different temperatures.
| T | phenols | Langmuir | Freundlich | Temkin | Dubinin–Radushkevich | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R 2 | q m (mg/g) | b(L/mg) | R L | R 2 | n | K f (L/g) | R 2 | b T | K T | Kd | R 2 | E(KJ/mol) | ||
| 298 K | BPA | 0.991 | 14.430 | 0.453 | 0.306 | 0.934 | 1.815 | 3.396 | 0.931 | 2.841 | 1.070 | 7.564 | 0.852 | 0.257 |
| α-naphthol | 0.977 | 13.193 | 1.037 | 0.088 | 0.765 | 5.297 | 7.466 | 0.800 | 1.272 | 1.095 | 50.431 | 0.959 | 0.100 | |
| T-OP | 0.975 | 24.155 | 0.367 | 0.353 | 0.921 | 1.479 | 5.002 | 0.951 | 4.780 | 1.025 | 7.098 | 0.915 | 0.265 | |
| 308 K | BPA | 0.996 | 13.158 | 0.571 | 0.260 | 0.919 | 1.936 | 3.460 | 0.952 | 2.583 | 1.077 | 8.586 | 0.888 | 0.241 |
| α-naphthol | 0.999 | 28.169 | 2.351 | 0.041 | 0.860 | 2.460 | 13.846 | 0.939 | 4.807 | 1.012 | 26.067 | 0.914 | 0.139 | |
| T-OP | 0.973 | 23.419 | 0.454 | 0.306 | 0.908 | 1.507 | 5.443 | 0.936 | 4.785 | 1.024 | 8.250 | 0.924 | 0.246 | |
| 318 K | BPA | 0.986 | 14.065 | 0.731 | 0.215 | 0.908 | 2.674 | 4.981 | 0.825 | 1.945 | 1.074 | 5.739 | 0.920 | 0.295 |
| α-naphthol | 0.999 | 30.769 | 5.417 | 0.018 | 0.755 | 2.649 | 20.186 | 0.795 | 4.811 | 1.009 | 41.205 | 0.927 | 0.110 | |
| T-OP | 0.990 | 23.041 | 0.617 | 0.245 | 0.923 | 1.662 | 6.340 | 0.953 | 4.463 | 1.023 | 12.289 | 0.947 | 0.202 | |
Table 3.
Thermodynamic parameters for the absorption of phenols onto Fe3O4@PANI-GO.
| Compound | T | −∆G0(kJ/mol) | ∆S0(J/mol.K) | ∆H0(kJ/mol) |
|---|---|---|---|---|
| BPA | 298 K | 2.367 | 101.41 | 27.69 |
| 308 K | 3.901 | |||
| 318 K | 4.372 | |||
| α-naphthol | 298 K | 1.856 | 36.88 | 9.189 |
| 308 K | 1.985 | |||
| 318 K | 2.519 | |||
| T-OP | 298 K | 1.599 | 76.17 | 21.25 |
| 308 K | 1.889 | |||
| 318 K | 3.143 |
Reusability
As a new adsorbent was concerned, the reusability was often an important parameter. In this study, it was investigated with ten recycles. The results were shown in Fig. 5, and the results indicated that the Fe3O4@PANI-GO magnetic composite was a good adsorbent with almost no loss of the recovery of BPA, α-naphthol, and t-OP after ten cycles.
Figure 5.
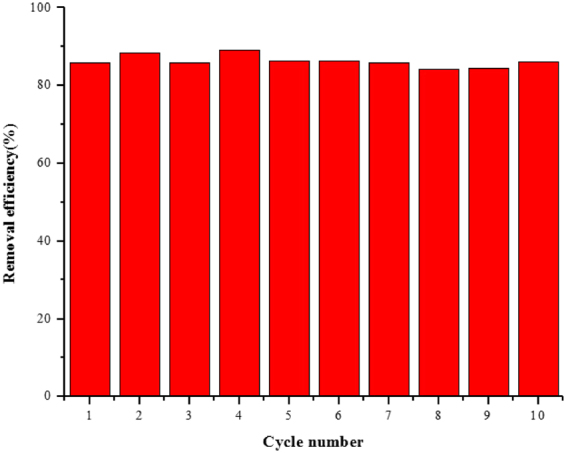
Recycling of Fe3O4@PANI-GO in the removal of t-OP at T = 298 K.
Discussion
Adsorption
The adsorption of BPA, t-OP, and α-naphthol was performed by designing a series of experiments. The effect of the amount of adsorbent was investigated with an initial concentration of 5, 5 and 10 mg L−1 for BPA, t-OP and α-naphthol, respectively. (Fig. 3(a)). It was observed that the removal efficiency of the target compound adsorbed increased when the adsorbent dosage increased from 5 to 60 mg. The removal efficiency reached 91.32, 95.93, and 98.86% for BPA, t-OP, and α-naphthol, respectively. The removal rates of three phenols reached a steady state, and increased very small, and only the removal rate of BPA still had a slight increase, however the increase was very small. Therefore, 60 mg of Fe3O4@PANI-GO was used.
The effect of salinity was an important parameter and was often optimized parameter, herein it was checked with the concentration of NaCl in the range of 0–25% (w/v). Figure 3(c) shows the results of the effect of ionic strength on the adsorption of BPA, α-naphthol, and t-OP onto Fe3O4@PANI-GO. It was observed that the removal efficiency of BPA increased within the NaCl concentration of 0–10% (w/v) and then decreased to the initial level when the NaCl concentration up to 25%. As α-naphthol was concerned, its removal rate kept constant within NaCl concentration range of 0−15% and then decreased with increase of NaCl concentration up to 25%. For t-OP, no significant influence was observed in the concentration range of 5–25% (w/v).
The presence of humic acid (HA) may have affected the adsorption capacity of phenols due to its competition for surface adsorption sites on the composites. As shown in Fig. 3(d), an interesting phenomenon occurred in which a small amount of humic acid promoted the adsorption of three phenols. The best results were achieved when the concentration of humic acid was 0.1 mgL−1 for these three phenols. The adsorption efficiency of BPA increased to the maximum value of 90.7% with 0.1 mgL−1 humic acid, yet the continuous increase of humic acid concentration resulted in the decrease of BPA adsorption. For α-naphthol and t-OP, no significant influence of HA was observed in the HA concentration range of 0.1–4 mg L−1. The independence of humic acid concentration on phenols adsorption is important for the application of Fe3O4@PANI-GO in the removal of some organic pollutants from wastewaters since HA concentration may vary in different samples. Therefore, the effect of HA on the extraction efficiencies of target compounds in real water samples was negligible.
To optimise the pH for maximum adsorption capacity of BPA, α-naphthol, and t-OP on Fe3O4@PANI-GO magnetic composites, a series of adsorption experiments were carried at various pH values. The effects of pH on adsorption percentages of phenols were investigated from pH2 to pH11. As shown in Fig. 3(e), the Fe3O4@PANI-GO composite material adsorbed the phenols effectively in the range of pH 2–7. However, the adsorption rate declined sharply and even decreased to about 4.4% for α-naphthol at pH 9.0 and 11.0 and had no obvious effect for t-OP over the whole pH range. These phenomena could be explained by the net charge of graphene, BPA, and other organic matter at different pH values30. The strong adsorption of phenolic organic pollutants on the magnetic nanocomposites might be attributed to physical adsorption: the donor-acceptor interactions between the electrons of the aromatic ring and the graphene sheets31. Therefore, the kinetic and isotherm experiments were operated at pH 6.0.
Adsorption kinetics
The effect of contact time on the amount of organics adsorbed was investigated, and results are presented in Fig. 3(b). It only took 5 min for BPA and t-OP to attain 59.41% and 78.04%, respectively, and for α-naphthol, the adsorption equilibrium was reached after 300 min. These results demonstrated that a fast adsorption process and the adsorbed amount of these phenols reached equilibrium values very quickly. The time-dependent adsorption capacity was obtained to study the kinetics for the adsorption of these phenols on Fe3O4@PANI-GO. The kinetics of adsorption is important considering that it controls the process efficiency. The adsorption model that describes the sorption of a solute onto a solid surface can be expressed by pseudo-first-order, pseudo-second-order, or intraparticle diffusion model27–29. The best-fit model was selected based on the linear regression correlation coefficient values (R2).
The pseudo-first-order kinetic model is expressed as:
| 1 |
The pseudo-second-order kinetic model is present in the following equation as:
| 2 |
The intraparticle diffusion model is defined as follows:
| 3 |
where qe and qt were the amounts of Fe3O4@PANI-GO adsorbed (mg g−1) at equilibrium and at time t(min) respectively; and k1, k2, and ki are the rate constants.
A good linear relationship between t/qt and t was obtained. The slopes and intercepts of each linear plot in Fig. 3(f) are used to calculate the kinetic parameters for BPA, t-OP, and α-naphthol adsorption, and the results are listed in Table 1. The correlation coefficients, R2, of the pseudo-second-order kinetic model for the adsorption of BPA, α-naphthol, and t-OP onto Fe3O4@PANI-GO were determined to be 0.9991, 0.9958, and 0.9999, respectively, which are much higher than that of the pseudo-first-order and intraparticle diffusion models. Clearly, the pseudo-second-order kinetic curves gave a good fit to the experimental kinetic data with a much higher correlation coefficient (R2). Furthermore, the experimental adsorption capacity (qe, exp) was also in accordance with the calculated adsorption capacity (qe, cal) obtained from the pseudo-second-order model. These results suggest that the pseudo-second-order kinetic model offers a more appropriate description of the adsorption process.
Adsorption isotherms
Adsorption isotherms describe the distribution of adsorbed molecules between the liquid phase and solid phase. The adsorption isotherms for the removal of BPA, t-OP, and α-naphthol were studied using an adsorbent dosage of 20–50 mg. Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich isotherm models were used to describe the adsorption process32–35.
The adsorption isotherm of the Langmuir model assumes monolayer adsorption on a perfectly smooth and homogeneous surface. It has been successfully applied to many pollutant adsorption processes from aqueous solution. The equation is expressed as:
| 4 |
where qe is the adsorption capacity (mg g−1) at the equilibrium point; Ce is the equilibrium concentration of BPA, α-naphthol, and t-OP (mg L−1); qm represents the maximum adsorption capacity of the adsorbent (mg g−1); and b is the Langmuir adsorption constant (L mg−1).The value of RL indicates the shape of the isotherm to be unfavourable (RL > 1), linear (RL = 1), favourable (0 < RL < 1), or irreversible (RL = 0) in which RL values between 0 and 1 indicate favourable adsorption. Figure 4 exhibits the Langmuir adsorption isotherms of BPA, α-naphthol, and t-OP on as-prepared Fe3O4@PANI-GO at three different temperatures. The adsorption capacity, qm, and adsorption constant, b, can be determined from the slope and intercept of a linearized plot of Ce/qe vs Ce, as presented in Table 2. The maximum adsorption capacities (qm) calculated according to the Langmuir model were 14.43, 13.19, and 24.15 mg g−1 for BPA, α-naphthol, and t-OP on Fe3O4@PANI-GO composites, respectively. The RL values were obtained in the range of 0.0350–0.5638, thereby confirming that the adsorption is a favourable process. Comparing of the qm values for phenols, the graphene oxide composite has a higher absorbability for t-OP than does BPA and α-naphthol.
The Freundlich isotherm is an empirical equation employed for heterogeneous systems and adsorption at multilayers. The equation is expressed as:
| 5 |
where kf and n are Freundlich constants that indicate the relative sorption capacity and sorption intensity, respectively. If 1 < n < 10, the adsorption is favourable31. Hence, it can be seen (Table 2) that the adsorption of BPA, α-naphthol, and t-OP on Fe3O4@PANI-GO composites were favourable in this research.
The Temkin isotherm considers the effects of indirect adsorbent/adsorbate interactions on adsorption isotherms. The isotherm assumes that the heat of adsorption of all the molecules in the layer decreases linearly with coverage due to adsorbent-adsorbate interactions. The equation is expressed as:
| 6 |
Which can be linearized to:
| 7 |
where KT is the constant of Temkin isotherm (g−1); and bT is the Temkin isotherm constant related to the heat of adsorption (kJ mol−1).
The Dubinin-Radushkevich isotherm is used to estimate the characteristic porosity and the apparent free energy of adsorption. The Dubinin-Radushkevich equation is expressed as:
| 8 |
where KD (mol2 kJ−2) is the mean free energy E (kJ mol−1) of adsorption per molecule of the sorbate when it is transferred to the surface of the solid from infinity in the solution, which was calculated by E = −(2KD)−0.5; and ε is Polanyi potential constant given as RT ln(1 + 1/Ce). The E value can be used to estimate the type of adsorption. If 8 < E < 16 kJ mol−1, the adsorption can be explained by ion exchange. If E < 8 kJ mol−1, it is physical absorption, and if E > 16 kJ mol−1, it is chemical adsorption.
In Table 2, the higher correlation coefficients(R2) indicate that the Langmuir model fit the adsorption data better than the Freundlich model for the adsorption of BPA, α-naphthol, and t-OP on the Fe3O4@PANI-GO magnetic composites. The mechanism of Fe3O4@PANI-GO adsorption toward the phenols might be based on van der Waals interactions occurring between the hexagonally arrayed carbon atoms in the graphene oxide sheet and the aromatic backbones of the organics. The second reason might be due to the strong π-stacking interaction between the benzene ring of the organics and the large delocalized π-electron system of GO32. Thus, it is suggested that the graphene oxide magnetic adsorbent has a higher adsorption capacity for BPA, α-naphthol, and t-OP removal. Further, it is clear that the sorption energy values (E) of the Dubinin-Radushkevich model for BPA, α-naphthol, and t-OP are similar to each other, which are 0.257, 0.010, and 0.265 kJ mol−1, respectively, at 298k.These indicated that the adsorption was physical adsorption, and the positive values indicated that the adsorption process was endothermic.
Thermodynamic studies
Batch adsorption was performed at different temperatures (298, 308, and 318 K), and the results are summarised in Table 3. The thermodynamic parameters were estimated in order to evaluate the feasibility and exothermic nature of the adsorption process. Free energy of adsorption (∆G°), enthalpy (∆H°), and entropy (∆S°) changes were calculated to predict the nature of adsorption.
The free energy of adsorption (∆G°) can be related to the equilibrium constant, K0 (L mol−1), where K0 can be obtained from the intercept of the plot ln(qe/Ce) vs. qe. The adsorption standard free energy changes (∆G°) can be calculated according to following equation:
| 9 |
where R is the gas universal constant (8.314 J/mol K); K0 is the equilibrium constant; and T is the absolute temperature.
The van’t Hoff equation was used to determine K0:
| 10 |
Which can be converted to
| 11 |
| 12 |
where ∆H0 and ∆S0 values can be obtained from the slope and intercept by plotting lnK0 vs. 1/T. The thermodynamic parameters are listed in Table 3. The negative values of free energy of adsorption (∆G0) increased when the temperature increased, which indicates that the adsorption process is spontaneous. The positive standard enthalpy change (∆H0) also suggests that the interaction of BPA, α-naphthol, and t-OP adsorbed by Fe3O4@PANI-GO is endothermic, which is supported by the increased adsorption of BPA, α-naphthol, and t-OP with the increase in temperature.
Regeneration
To investigate the possibility of regeneration of the Fe3O4@PANI-GO adsorbent, desorption experiments were performed in which organic pollutants were easily dissolved in organic solvents. Because of the theory of “similarity and intermiscibility”, we selected methanol as the desorption solvent. After adsorption, desorption was carried out by shaking the Fe3O4@PANI-GO with 3 mL methanol. 5 min was allotted as the extraction and desorption time, and the removal efficiencies of phenols are shown in Fig. 5.
From Fig. 5, it is observed that desorption of t-OP was achieved from the solution by using 3 mL methanol, and desorption ratio of 86.03% was obtained over ten adsorption/desorption cycles, which indicated that Fe3O4@PANI-GO sorbents were stable during the MSPE procedure and shows good reusability.
Conclusions
In summary, we developed a facile method for the preparation of the magnetic composite material, Fe3O4@polyaniline with incorporated GO, and TEM, SEM, FT-IR, and XRD investigation revealed the characteristics of the composites. The Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich adsorption models were applied to describe the equilibrium isotherms. The calculated maximum adsorption capacities were 14.43, 13.19, and 24.15 mg g−1 for BPA, α-naphthol, and t-OP on the Fe3O4@PANI-GO composite, respectively. In addition, the adsorption capacity of Fe3O4@PANI-GO for t-OP was higher. The negative adsorption standard free energy changes and positive standard enthalpy change indicate that the adsorption was spontaneous and endothermic. Furthermore, owing to the excellent dispersion in water and hydrophilicity of GO, the stability of the hybrid material in water is attributed to GO. Furthermore, the magnetic separation technology provided a rapid and effective method for separating magnetic materials from aqueous phase and displayed good regeneration capacity. This research indicates that Fe3O4@PANI-GO can be used as an effective sorbent for the simple and rapid removal of organic pollutants from water samples.
Materials and Methods
Chemicals and materials
Graphite powder (500 meshes) was purchased from J&K Chemical Ltd (Beijing, China). Concentrated sulfuric acid (H2SO4), K2S2O8, H2O2, KMnO4, HCl, Ferric chloride hexahydrate (FeCl3·6H2O), sodium acetate (CH3COONa), ethylene glycol (C2H6O2), ethanol, and ammonium peroxodisulfate ((NH4)2S2O8; APS) were all of analytical grade and used without further purification. Ultrapure water and double deionized water were used throughout the experiment.
Preparation of graphene oxide (GO) and Fe3O4 nanoparticles
Graphene oxide (GO) was synthesised from natural graphite powder using a modified Hummers’ method36, 37. Fe3O4 microspheres were synthesised following a solvothermal method reported previously38, 39. FeCl3 . 6H2O (4.32 g) and sodium acetate (12.0 g) were dispersed in 80 mL ethylene glycol and stirred vigorously for 30 min at room temperature, then transferred to a Teflon-lined stainless steel autoclave (100 mL, capacity) and heated to reflux for 8 h at 200 °C. In this case, FeCl3·6H2O was used as the iron source, and NaAc was used as the reductant. NaAc was also used for electrostatic stabilisation to prevent the agglomeration of the particles and to assist in the reduction of Fe3+ to Fe3O4 40. The obtained Fe3O4 was washed with deionized water and ethanol several times and then dried at 60 °C for 6 h.
Preparation of Fe3O4@PANI-GO composite material
0.1 g Fe3O4 was dissolved in 50 mL deionized water containing 1 mL 0.02 M HCl aqueous solution in which HCl was used as a dopant. Then, FeCl3.6H2O and aniline monomer were added and sonicated for 10 min. After that, the mixture was mechanically stirred for 10 h in an ice bath for short-chain polymerization. APS and GO were then slowly added to the suspension under constant stirring. The long-chain oxidative polymerization was further continued for 12 h. After the reaction, the prepared particles were collected using a magnet and washed with deionized water and ethanol, then the obtained Fe3O4@PANI-GO composite was dried at 60 °C in a vacuum.
Adsorption experiment
Adsorptions of BPA, t-OP, and α-naphthol by Fe3O4@PANI-GO magnetic composites were performed by batch adsorption techniques in glass vials at T = 25 ± 1 °C, except for the thermodynamic experiments in which additional temperatures of 35 ± 1 °C and 45 ± 1 °C were used. 25 mL solution spiked with a known initial concentration was shaken with 20 mg magnetic Fe3O4@PANI-GO on a shaker at 270 rpm.
The effects of pH, ionic strength, and humic acid and the adsorption kinetics and adsorption isotherms were investigated by batch experiments. The initial concentrations of the solutions were 5 mg L−1 for BPA and t-OP, and 10 mg L−1 for α-naphthol in the pH and kinetics experiments. The maximum adsorption capacity and adsorption isotherms were calculated according to different concentrations of organics in the range of 2–35 for α-naphthol and 1–30 mg L−1 for BPA and t-OP, respectively.
After adsorption, the solution was separated by a magnet and analysed using a high-performance liquid chromatography (HPLC, Shimadzu SPD-10A, Wondasil-C18, superb 5 µm) system equipped with a UV-Vis detector. The detection wavelength was set at 225, 225, and 280 nm for BPA, t-OP, and α-naphthol, respectively. The mobile phase was composed of methanol and water (80/20, v/v), and the flow rate was set at 1 mL min−1. The absorption capacity (q, mg g−1) was calculated using Equation (1):
| 13 |
where C0 and Ce are the initial and equilibrium concentrations of phenols in the solution (mg L−1), respectively; m is the mass of the adsorbent (g); and V (L) is the solution volume.
Data Availability
All data generated or analysed during this study are included in this published article.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21377167).
Author Contributions
Prof. Q.X. Zhou designed the experiment and revised the paper. Miss Y.Q. Wang carried out the experiments and wrote the text. Dr. J.P. Xiao prepared the material and characterized them. Prof. Fan had done the kinetic experiments and gave some good suggestions on the revision of the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jiang K, et al. Facile synthesis of Ag@Pd satellites-Fe3O4 core nanocomposites as efficient and reusable hydrogenation catalysts. Chem. Commun. 2011;47:11924–11926. doi: 10.1039/c1cc14675k. [DOI] [PubMed] [Google Scholar]
- 2.Yao YJ, et al. Fabrication of Fe3O4/SiO2 core/shell nanoparticles attached to graphene oxide and its use as an adsorbent. J. Colloid. Interf. Sci. 2012;379:20–26. doi: 10.1016/j.jcis.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Zamani F, Izadi E. PolyvinylaminecoatedFe3O4@SiO2 magnetic microspheres forKnoevenagelcondensation. Chinese. J. Catal. 2014;35:21–27. doi: 10.1016/S1872-2067(12)60685-8. [DOI] [Google Scholar]
- 4.Chandra V, et al. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano. 2010;4:3979–3986. doi: 10.1021/nn1008897. [DOI] [PubMed] [Google Scholar]
- 5.Zhang WJ, et al. Synthesis of water-soluble magnetic graphene nanocomposites for recyclable removal of heavy metal ions. J. Mater. Chem. A. 2013;1:1745–1753. doi: 10.1039/C2TA00294A. [DOI] [Google Scholar]
- 6.Xuan SH, Wang YXJ, Leung KCF, Shu KY. Synthesis of Fe3O4@polyaniline core/shell microspheres with well-defined blackberry-like morphology. J. Phys. Chem. C. 2008;112:18804–18809. doi: 10.1021/jp807124z. [DOI] [Google Scholar]
- 7.Wang J, Deng B, Chen H, Wang X, Zheng J. Removal of aqueous Hg(II) by polyaniline: sorption characteristics and mechanisms. Environ. Sci. Technol. 2009;43:5223–5228. doi: 10.1021/es803710k. [DOI] [PubMed] [Google Scholar]
- 8.Mirmohseni A, Solhjo R. Preparation and characterization of aqueous polyaniline battery using a modified polyaniline electrode. Eur. Polym. J. 2003;39:219–223. doi: 10.1016/S0014-3057(02)00202-1. [DOI] [Google Scholar]
- 9.Lin MH, et al. Electrochemical immunoassay of benzo[a]pyrene based on dual amplification strategy of electron-accelerated Fe3O4/polyaniline platform and multi-enzyme-functionalized carbon sphere label. Anal. Chim. Acta. 2012;722:100–106. doi: 10.1016/j.aca.2012.01.059. [DOI] [PubMed] [Google Scholar]
- 10.Li QL, Zhang CR, Li JQ. Photocatalysis and wave-absorbing properties of polyaniline/TiO2 microbelts composite by in situ polymerization method. Appl. Surf. Sci. 2010;257:944–948. doi: 10.1016/j.apsusc.2010.07.098. [DOI] [Google Scholar]
- 11.Zhao DL, Zhang HL, Zeng XW, Xia QS, Tang JT. Inductive heat property of Fe3O4/polymer composite nanoparticles in an ac magnetic field for localized hyperthermia. Biomed. Mater. 2006;1:198–201. doi: 10.1088/1748-6041/1/4/004. [DOI] [PubMed] [Google Scholar]
- 12.Gao Q, Zheng HB, Luo D, Ding, Feng YQ. Facile synthesis of magnetic one-dimensional polyaniline and its application in magnetic solid phase extraction for fluoroquinolones in honey samples. Anal. Chim. Acta. 2012;720:57–62. doi: 10.1016/j.aca.2011.12.067. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Wu SN, Chen JP. Modification of activated carbon by polyaniline for enhanced adsorption of aqueous arsenate. Ind. Eng. Chem. Res. 2007;46:2133–2140. doi: 10.1021/ie0611352. [DOI] [Google Scholar]
- 14.Yang J, Wu JX, Lu QF, Lin TT. Facile preparation of lignosulfonate–graphene oxide–polyaniline ternary nanocomposite as an effective adsorbent for Pb(II) ions. ACS Sustain. Chem. Eng. 2014;2:1203–1211. doi: 10.1021/sc500030v. [DOI] [Google Scholar]
- 15.Li J, et al. Hybrid composites of conductive polyaniline and nanocrystalline titanium oxide prepared via self-assembling and graft polymerization. Polymer. 2006;47:7361–7367. doi: 10.1016/j.polymer.2006.08.059. [DOI] [Google Scholar]
- 16.Dikin DA, et al. Preparation and characterization of graphene oxide paper. Nature. 2007;448:457–460. doi: 10.1038/nature06016. [DOI] [PubMed] [Google Scholar]
- 17.Su SW, Chen BB, He M, Hu B, Xiao ZW. Determination of trace/ultratrace rare earth elements in environmental samples by ICP-MS after magnetic solid phase extraction with Fe3O4@SiO2@polyaniline–graphene oxide composite. Talanta. 2014;119:458–466. doi: 10.1016/j.talanta.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Yang ST, et al. Folding/aggregation of graphene oxide and its application in Cu2+ removal. J. Colloid Interface Sci. 2010;351:122–127. doi: 10.1016/j.jcis.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 19.Chang YP, Ren CL, Qu JC, Chen XG. Preparation and characterization of Fe3O4/graphene nanocomposite and investigation of its adsorption performance for aniline and p-chloroaniline. Appl. Surf. Sci. 2012;261:504–509. doi: 10.1016/j.apsusc.2012.08.045. [DOI] [Google Scholar]
- 20.Xie GX, et al. A facile chemical method to produce superparamagnetic graphene oxide–Fe3O4 hybrid composite and its application in the removal of dyes from aqueous solution. J. Mater. Chem. 2012;22:1033–1039. doi: 10.1039/C1JM13433G. [DOI] [Google Scholar]
- 21.Zhu Y, et al. Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater. 2010;22:3906–3924. doi: 10.1002/adma.201001068. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Chen ZM, Chen BL. Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol. 2014;48:4817–4825. doi: 10.1021/es405227u. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Hao Q, Yang X, Lu L, Wang X. ACS Appl. Mater. Interf. 2010. Effect of graphene oxide on the properties of its composite with polyaniline; pp. 821–828. [DOI] [PubMed] [Google Scholar]
- 24.Li ZF, et al. ACS Appl. Mater. Interf. 2013. Fabrication of high-surface-area graphene/polyaniline nanocomposites and their application in supercapacitors; pp. 2685–2691. [DOI] [PubMed] [Google Scholar]
- 25.Singh K, et al. Nanostructured graphene/Fe3O4 incorporated polyaniline as a high performance shield against electromagnetic pollution. Nanoscale. 2013;5:2411–2420. doi: 10.1039/c3nr33962a. [DOI] [PubMed] [Google Scholar]
- 26.He HK, Gao C. ACS Appl. Mater. Interf. 2010. Supraparamagnetic, conductive, and processable multifunctional graphene nanosheets coated with high-density Fe3O4 nanoparticles; pp. 3201–3210. [DOI] [PubMed] [Google Scholar]
- 27.Yan XB, Chen JT, Yang J, Xue QJ, Miele P. Fabrication of free-standing, electrochemically active, and biocompatible graphene oxide-polyaniline and graphene−polyaniline hybrid papers . ACS Appl. Mater. Interf. 2010;2:2521–2529. doi: 10.1021/am100293r. [DOI] [PubMed] [Google Scholar]
- 28.Baykal A, et al. Acid functionalized multiwall carbon nanotube/magnetite (MWCNT)-COOH/Fe3O4 hybrid: Synthesis, characterization and conductivity evaluation J. Inorg. Organomet. P. 2013;23:726–735. doi: 10.1007/s10904-013-9839-4. [DOI] [Google Scholar]
- 29.Xuan SH, Wang YXJ, Yu JC, Leung KCF. Preparation, characterization, and catalytic activity of core/shell Fe3O4@polyaniline@Au nanocomposites. Langmuir. 2009;25:11835–11843. doi: 10.1021/la901462t. [DOI] [PubMed] [Google Scholar]
- 30.Li XH, et al. Fe3O4–graphene hybrids: nanoscale characterization and their enhanced electromagnetic wave absorption in gigahertz range. J. Nanopart Res. 2013;15:1472–1483. doi: 10.1007/s11051-013-1472-1. [DOI] [Google Scholar]
- 31.Liu PB, Huang Y, Zhang X. Superparamagnetic Fe3O4nanoparticles on graphene–polyaniline: Synthesis, characterization and their excellent electromagnetic absorption properties. J. Alloy. Compd. 2014;596:25–31. doi: 10.1016/j.jallcom.2014.01.188. [DOI] [Google Scholar]
- 32.Zhu HY, Jiang R, Xiao L, Zeng GM. Preparation, characterization, adsorption kinetics and thermodynamics of novel magnetic chitosan enwrapping nanosized γ-Fe2O3 and multi-walled carbon nanotubes with enhanced adsorption properties for methyl orange. Bioresour. Technol. 2010;101:5063–5069. doi: 10.1016/j.biortech.2010.01.107. [DOI] [PubMed] [Google Scholar]
- 33.Karthik R, Meenakshi S. Removal of hexavalent chromium ions using polyaniline/silica gel composite. J. Water Pro. Eng. 2014;1:37–45. doi: 10.1016/j.jwpe.2014.03.001. [DOI] [Google Scholar]
- 34.Yao W, Shen C, Lu Y. Fe3O4@C@polyaniline trilaminar core–shell composite microspheres as separable adsorbent for organic dye. Compos. Sci. Technol. 2013;87:8–13. doi: 10.1016/j.compscitech.2013.07.023. [DOI] [Google Scholar]
- 35.Xu J, Wang L, Zhu YF. Decontamination of bisphenol A from aqueous solution by graphene adsorption. Langmuir. 2012;28:8418–8425. doi: 10.1021/la301476p. [DOI] [PubMed] [Google Scholar]
- 36.Ji LQ, et al. Facile synthesis of multiwall carbon nanotubes/iron oxides for removal of tetrabromobisphenol A and Pb(II) J. Mater. Chem. 2012;22:15853–15862. doi: 10.1039/c2jm32896h. [DOI] [Google Scholar]
- 37.Wang C, Feng C, Gao XX, Wu QH, Wang Z. Preparation of a graphene-based magnetic nanocomposite for the removal of an organic dye from aqueous solution. Chem. Eng. J. 2011;173:92–97. doi: 10.1016/j.cej.2011.07.041. [DOI] [Google Scholar]
- 38.Deng XJ, Lu LL, Li HW, Luo F. The adsorption properties of Pb(II) and Cd(II) on functionalized graphene prepared by electrolysis method. J. Hazard. Mater. 2010;183:923–930. doi: 10.1016/j.jhazmat.2010.07.117. [DOI] [PubMed] [Google Scholar]
- 39.Saeung S, Boonamnuayvitaya V. Adsorption of formaldehyde vapor by amine-functionalized mesoporous silica materials. J. Environ. Sci. 2008;20:379–384. doi: 10.1016/S1001-0742(08)60059-5. [DOI] [PubMed] [Google Scholar]
- 40.Sheha RR, Metwally E. Equilibrium isotherm modeling of cesium adsorption onto magnetic materials. J Hazard. Mater. 2007;143:354–61. doi: 10.1016/j.jhazmat.2006.09.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.