Abstract
The prognostic significance of gender remains controversial for papillary thyroid carcinoma (PTC). In this study, we investigated the associations between gender and prognosis in a large cohort of patients with PTC or PTMC that was diagnosed in 2010–2013 and recorded in the Surveillance, Epidemiology, and End Results cancer registry. The mean ± standard deviation duration of survival for all patients with PTC during the study period was 21.47 ± 14.04 months. In Kaplan-Meier analyses of the entire cohort of PTC patients, survival curves for all-cause death and cancer-specific death declined more sharply for men than for women. Similar results were observed in analyses of patients with PTCs > 1 cm and PTMC. After adjusting for potential confounders, hazard rates indicated significantly elevated all-cause mortality for men in analyses of all PTCs, PTCs > 1 cm, and PTMCs. However, in a confounder-adjusted analysis of patients with PTMC, the hazard rate did not indicate significantly higher mortality for men than for women. Our study demonstrated that male gender is an independent poor prognostic factor for all PTCs and for PTCs > 1 cm. However, gender is not an independent prognostic factor for cause-specific survival in PTMC.
Introduction
The incidence rate of thyroid cancer rose rapidly in recent decades1. However, the incidence rate of thyroid cancer also appears to have begun to stabilize in recent years, since changes in clinical practice guidelines were initiated in 20092. Papillary thyroid cancer (PTC) accounts for 80–90% of all thyroid malignancies, making it the most common type of thyroid malignancy3–5.
According to World Health Organization guidelines, papillary thyroid microcarcinoma (PTMC) is defined as thyroid carcinoma measuring less than or equal to 1 cm in its greatest dimension6. In recent reports, roughly 18.4% to 50% of the increase in PTC has been attributed to the identification of intrathyroidal papillary thyroid microcarcinomas7–11.
According to previous studies, it is still controversial whether male gender a poor prognostic factor in cases of PTC12, 13. A recent meta-analysis demonstrated that male gender was a strong prognostic factor, and increased the risk of recurrence14. Because of the contradictory results in the prior literature, a large-scale population-based cohort should be investigated to clarify the associations between gender and the prognoses of PTC and PTMC.
The Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (NCI) is the largest publicly available and authoritative source of data on cancer incidence and survival. In the present study, we used this reliable and large-scale research dataset to reevaluate the prognostic significance of male gender for PTC and PTMC. Our analysis was based on the records of 43712 patients with PTCs diagnosed during 2010–2013.
Results
Demographic and clinical features
Baseline clinicopathologic characteristics are summarized and compared between men and women with PTC in Table 1. The mean ± standard deviation (SD) durations of survival during the study period was 21.47 ± 14.04 months for the entire cohort of patients with PTC. Mean age was higher (53.03 ± 15.30 vs 48.68 ± 15.14 years, p < 0.001) and tumor size was larger (22.48 ± 21.79 vs 16.93 ± 17.75 mm, p < 0.001) in men than in women. In patients with PTC, gender was associated with race, T stage, N stage, distant metastasis, extrathyroidal extension, multifocality, radiation, and surgical method (Table 1).
Table 1.
Characteristics of Patients with Papillary Thyroid Cancer from the Surveillance, Epidemiology, and End Results Database (2010–2013).
| Characteristic | Variable | Female (n = 33614) | Male (n = 10098) | P |
|---|---|---|---|---|
| Age | Mean ± SD | 48.68 ± 15.14 | 53.03 ± 15.30 | <0.001 |
| <45 year | 13597(40.5%) | 2962(29.3%) | <0.001 | |
| ≥ 45 year | 20017(59.5%) | 7136(70.7%) | ||
| Race | white | 26843(81.1%) | 8484(85.1%) | <0.001 |
| black | 2381(7.2%) | 502(5.0%) | ||
| other | 3875(11.7%) | 989(9.9%) | ||
| Tumor size | Mean ± SD | 16.93 ± 17.75 | 22.48 ± 21.79 | <0.001 |
| ≤ 1.0 cm | 14425(42.9%) | 3347(33.1%) | <0.001 | |
| > 1.0 cm | 19189(57.1%) | 6751(66.9%) | ||
| T stage | T1 | 21435(64.0%) | 5203(51.7%) | <0.001 |
| T2 | 5293(15.8%) | 1794(17.8%) | ||
| T3 | 5956(17.7%) | 2595(25.8%) | ||
| T4 | 826(2.5%) | 473(4.7%) | ||
| N stage | N0 | 26685(80.7%) | 7047(70.9%) | <0.001 |
| N1 | 6391(19.3%) | 2889(29.1%) | ||
| Distant metastasis | No | 33347(99.2%) | 9861(97.7%) | <0.001 |
| Yes | 267(0.8%) | 236(2.3%) | ||
| Extrathyroidal extension | No | 26225(84.9%) | 7263(79.1%) | <0.001 |
| Yes | 4664(15.1%) | 1915(20.9%) | ||
| Multifocal tumor | No | 19884(59.8%) | 5525(55.5%) | <0.001 |
| Yes | 13355(40.2%) | 4436(44.5%) | ||
| Surgery | Noa | 427(1.3%) | 201(2.0%) | <0.001 |
| Yesb | 33107(98.7%) | 9827(98.0%) | ||
| Radiation | None or refused | 17793(54.3%) | 4689(47.8%) | <0.001 |
| performed | 14958(45.7%) | 5111(52.2%) | ||
| All cause death | No | 33128(98.6%) | 9706(96.1%) | <0.001 |
| Yes | 486(1.4%) | 392(3.9%) | ||
| Cancer-specific death | No | 33497(99.7%) | 9991(98.9%) | <0.001 |
| Yes | 117(0.3%) | 107(1.1%) |
aRecommended but not Performed OR Not recommended; bPerformed.
Baseline clinicopathologic characteristics are summarized and compared between men and women with PTC > 1 cm in Table 2. The mean ± SD duration of survival for patients with PTC > 1 cm was 21.52 ± 14.00 months. Mean age was higher (52.26 ± 15.98 vs 47.31 ± 15.83 years, p < 0.001) and tumor size was larger (25.53 ± 19.32 vs 30.90 ± 22.18 mm, p < 0.001) in men than in women. PTC > 1 cm in male patients was more frequently associated with race, T stage, N stage, distant metastasis, extrathyroidal extension, multifocality, radiation, and surgical method (Table 2).
Table 2.
Characteristics of Patients with Papillary Thyroid Cancer ≥ 1 cm from the Surveillance, Epidemiology, and End Results Database (2010–2013).
| Characteristic | Variable | Female (n = 19189) | Male (n = 6751) | P |
|---|---|---|---|---|
| Age | Mean ± SD | 47.31 ± 15.83 | 52.26 ± 15.98 | <0.001 |
| <45 year | 8653(45.1%) | 2178(32.3%) | <0.001 | |
| ≥45 year | 10536(54.9%) | 4573(67.7%) | ||
| Race | white | 15098(79.9%) | 5648(84.7%) | <0.001 |
| black | 1347(7.1%) | 327(4.9%) | ||
| other | 2452(13.0%) | 694(10.4%) | ||
| Tumor size | Mean ± SD | 25.53 ± 19.32 | 30.90 ± 22.18 | <0.001 |
| T stage | T1 | 7891(41.3%) | 2105(31.3%) | <0.001 |
| T2 | 5293(27.7%) | 1794(26.7%) | ||
| T3 | 5155(27.0%) | 2369(35.3%) | ||
| T4 | 759(4.0%) | 452(6.7%) | ||
| N stage | N0 | 13727(73.2%) | 4300(65.1%) | <0.001 |
| N1 | 5031(26.8%) | 2309(34.9%) | ||
| Distant metastasis | No | 18945(98.7%) | 6539(96.9%) | <0.001 |
| Yes | 244(1.3%) | 211(3.1%) | ||
| Extrathyroidal extension | No | 13400(77.6%) | 4323(71.9%) | <0.001 |
| Yes | 3877(22.4%) | 1690(28.1%) | ||
| Multifocal tumor | No | 10560(55.9%) | 3503(52.7%) | <0.001 |
| Yes | 8355(44.1%) | 3139(47.3%) | ||
| Surgery | Noa | 335(1.8%) | 173(2.6%) | <0.001 |
| Yesb | 18799(98.2%) | 6548(97.4%) | ||
| Radiation | None or refused | 7045(37.8%) | 2309(35.3%) | <0.001 |
| performed | 11570(62.2%) | 4228(64.7%) | ||
| All cause death | No | 18851(98.2%) | 6479(96.0%) | <0.001 |
| Yes | 338(1.8%) | 272(4.0%) | ||
| Cancer-specific death | No | 19080(99.4%) | 6651(98.5%) | <0.001 |
| Yes | 109(0.6%) | 100(1.5%) |
aRecommended but not Performed OR Not recommended; bPerformed.
Next, we focused on the baseline clinicopathologic characteristics of patients with PTMC, which are summarized in Table 3. The mean ± SD survival months for PTMC patients was 21.38 ± 14.09 months. Mean age was higher (50.50 ± 13.96 vs 54.61 ± 13.71 years, p < 0.001) and tumor size was larger (5.49 ± 2.94 vs 5.49 ± 2.98 mm, p = 0.0909) in men than in women. PTMC in male patients was more frequently associated with race, T stage, N stage, distant metastasis, extrathyroidal extension, multifocality, and radiation (all p < 0.05), but not with surgical method (p = 0.189) (Table 3).
Table 3.
Characteristics of Patients with Papillary Thyroid Microcarcinoma from the Surveillance, Epidemiology, and End Results Database (2010–2013).
| Characteristic | Variable | Female (n = 14425) | Male (n = 3347) | P |
|---|---|---|---|---|
| Age | Mean ± SD | 50.50 ± 13.96 | 54.61 ± 13.71 | <0.001 |
| <45 year | 4944(34.3%) | 784(23.4%) | <0.001 | |
| ≥ 45 year | 9481(65.7%) | 2563(76.6%) | ||
| Race | white | 11745(82.7%) | 2836(85.8%) | <0.001 |
| black | 1034(7.3%) | 175(5.3%) | ||
| other | 1423(10.0%) | 295(8.9%) | ||
| Tumor size | Mean ± SD | 5.49 ± 2.94 | 5.49 ± 2.98 | 0.909 |
| T stage | T1 | 13544(94.0%) | 3098(92.6%) | 0.013 |
| T3 | 801(5.6%) | 226(6.8%) | ||
| T4 | 67(0.4%) | 21(0.6%) | ||
| N stage | N0 | 12958(90.5%) | 2747(82.6%) | <0.001 |
| N1 | 1360(9.5%) | 580(17.4%) | ||
| Distant metastasis | No | 14402(99.8%) | 3322(99.3%) | <0.001 |
| Yes | 23(0.2%) | 25(0.7%) | ||
| Extrathyroidal extension | No | 12825(94.2%) | 2940(92.9%) | 0.005 |
| Yes | 787(5.8%) | 225(7.1%) | ||
| Multifocal tumor | No | 9324(65.1%) | 2022(60.9%) | <0.001 |
| Yes | 5000(34.9%) | 1297(39.1%) | ||
| Surgery | Noa | 92(0.6%) | 28(0.8%) | 0.189 |
| Yesb | 14308(99.4%) | 3279(99.2%) | ||
| Radiation | None or refused | 10748(76.0%) | 2380(72.9%) | <0.001 |
| performed | 3388(24.0%) | 883(27.1%) | ||
| All cause death | No | 14277(%) | 3227(%) | <0.001 |
| Yes | 148(%) | 120(%) | ||
| Cancer-specific death | No | 14417(99.9%) | 3340(99.8%) | 0.006 |
| Yes | 8(0.1%) | 7(0.2%) |
aRecommended but not Performed OR Not recommended; bPerformed.
Survival analysis of patients with PTC
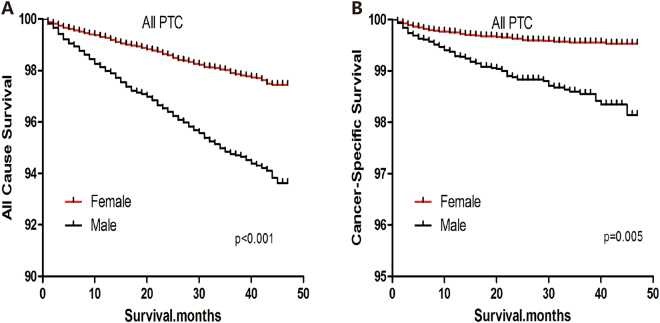
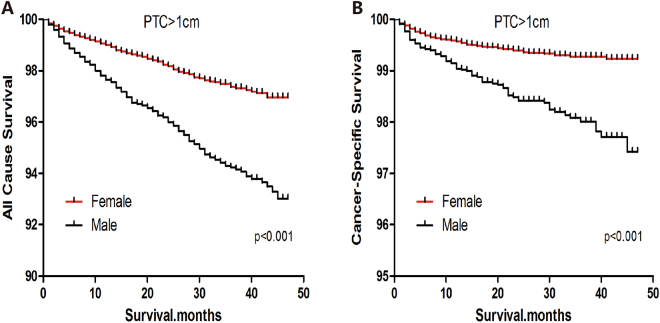
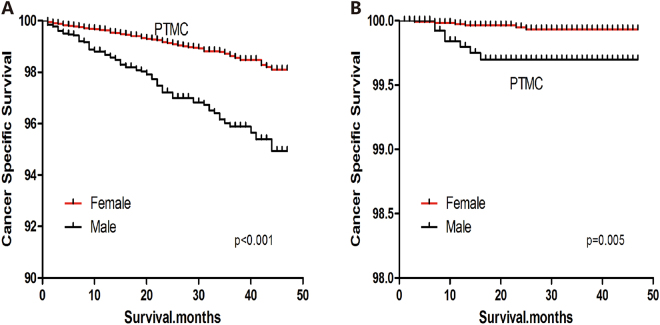
In a Kaplan-Meier analysis of the entire cohort of PTC patients, all-cause survival declined sharply for men, but declined more modestly for women (Log-rank test, p < 0.001) (Fig. 1A). Similar results were observed for cause-specific survival (Log-rank test, p = 0.005) (Fig. 1B). Among patients with PTC > 1 cm, all-cause and cause-specific survival curves were poorer for men than women (Log-rank test, both p < 0.001) (Fig. 2A,B). Among patients with PTMC, all-cause and cause-specific survival curves were also poorer for men than women (Log-rank test; all-cause death, p < 0.001; cancer-specific death, p = 0.005) (Fig. 3A,B).
Figure 1.
All-cause survival (A) and cancer-specific survival (B) for men and women with papillary thyroid carcinoma.
Figure 2.
All-cause survival (A) and cancer-specific survival (B) for men and women with papillary thyroid carcinomas >1 cm.
Figure 3.
All-cause survival (A) and cancer-specific survival (B) for men and women with papillary thyroid microcarcinoma.
In the entire cohort of patients with PTC, the rate of all-cause death per 1000 person-years was 7.16 (95% confidence interval [CI], 6.52-7.87) for men and 18.28 (95% CI, 16.40-20.37) for women (Table 4). Comparing male vs. female patients, the hazard ratio (HR) for all-cause mortality was 2.53 (95% CI, 2.20-2.90). After adjustment for patient age at diagnosis, race, multifocality, tumor size, extrathyroidal invasion, lymph node metastasis, distant metastasis, radiation, and surgical treatment, these HRs for all-cause and cause-specific mortality became 1.63 (95% CI, 1.39-1.91) and 1.67 (95% CI, 1.42-1.97), respectively. Therefore, male gender had a robust effect on all-cause death in the overall cohort of PTC patients; this effect remained even after additional adjustment for conventional clinicopathological risk factors and treatment methods (Table 4). We subsequently investigated all of the abovementioned associations in subgroup analyses of patients with PTC > 1 cm and PTMC. Similar results were observed (Table 4).
Table 4.
Hazard Ratios of sex for the all cause deaths of PTC.
| Versions | Sex | All Cause Deaths, No. | % | All Cause Deaths per 1,000 Person-Years | 95% CI | Unadjusted | Adjustment 1* | Adjustment 2† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratios | 95% CI | Hazard Ratios | 95% CI | Hazard Ratios | 95% CI | |||||||||
| All PTC | Female | 486 | 1.45 | 7.16 | 6.52–7.87 | 1 [Reference] | 1 [Reference] | 1 [Reference] | ||||||
| Male | 392 | 3.89 | 18.28 | 16.40–20.37 | 2.53 | 2.20–2.90 | <0.001 | 1.63 | 1.39–1.91 | <0.001 | 1.67 | 1.42–1.97 | <0.001 | |
| PTC > 1 cm | Female | 338 | 1.76 | 9.13 | 8.17–10.20 | 1 [Reference] | 1 [Reference] | 1 [Reference] | ||||||
| Male | 272 | 4.03 | 20.56 | 18.16–23.28 | 2.27 | 1.93–2.66 | <0.001 | 1.46 | 1.21–1.77 | <0.001 | 1.52 | 1.25–1.84 | <0.001 | |
| PTMC | Female | 148 | 1.03 | 4.54 | 3.78–5.44 | 1 [Reference] | 1 [Reference] | 1 [Reference] | ||||||
| Male | 120 | 3.59 | 13.50 | 10.81–16.85 | 2.84 | 2.16–3.73 | <0.001 | 2.07 | 1.54–2.78 | <0.001 | 2.06 | 1.52–2.77 | <0.001 | |
PTC: Papillary thyroid cancer; PTMC: Papillary Thyroid Microcarcinoma.
*Adjustment 1 was made for patient age at diagnosis, race, multifocality, tumor size, extrathyroidal invasion, lymph node metastasis and distant metastasis.
†Adjustment 2 was made for patient age at diagnosis, race, multifocality, tumor size, extrathyroidal invasion, lymph node metastasis, distant metastasis, radiation and surgery treatment.
Furthermore, we analyzed cancer-specific deaths. In the entire cohort of patients with PTC, the rate of cancer-specific deaths per 1000 person-years was 1.76 (95% CI, 1.46-2.13) for men and 5.42 (95% CI, 4.44-6.62) for women (Table 5). Comparing male vs. female patients, the HR for cancer-specific deaths was 3.09 (95% CI, 2.38-4.02). After adjustment for conventional clinicopathological risk factors and treatment methods, the HRs for cancer-specific deaths were 1.50 (95% CI, 1.10-2.06) and 1.53 (95% CI, 1.11-2.10), respectively. Similar results were observed for patients with PTC > 1 cm. However, among patients with PTMC, the HR for cancer-specific deaths was 3.89 (95% CI, 1.41-10.71) without adjustment and 1.23 (95% CI, 0.36-4.16; p = 0.743) after adjustment for patient age at diagnosis, race, multifocality, tumor size, extrathyroidal invasion, lymph node metastasis, and distant metastasis. Moreover, after the HR became 0.77 (95% CI, 0.19-3.16; p = 0.769) after adjustment for patient age at diagnosis, race, multifocality, tumor size, extrathyroidal invasion, lymph node metastasis, distant metastasis, radiation, and surgical treatment (Table 5).
Table 5.
Hazard Ratios of sex for the cancer specific deaths of PTC.
| Versions | Sex | Cancer Specific Deaths, No. | % | Cancer Specific Deaths per 1,000 Person–Years | 95% CI | Unadjusted | Adjustment 1* | Adjustment 2† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratios | 95% CI | p | Hazard Ratios | 95% CI | p | Hazard Ratios | 95% CI | p | ||||||
| All PTC | Female | 117 | 0.35 | 1.76 | 1.46–2. 13 | 1 [Reference] | 1 [Reference] | 1 [Reference] | ||||||
| Male | 107 | 1.06 | 5.42 | 4.44–6.62 | 3.09 | 2.38–4.02 | <0.001 | 1.50 | 1.10–2.06 | 0.011 | 1.53 | 1.11–2.10 | 0.010 | |
| PTC > 1 cm | Female | 109 | 0.57 | 2.91 | 2.39–3.54 | 1 [Reference] | 1 [Reference] | 1 [Reference] | ||||||
| Male | 100 | 1.48 | 7.43 | 6.04–9.14 | 2.62 | 2.00–3.45 | <0.001 | 1.46 | 1.05–2.02 | 0.024 | 1.52 | 1.09–2.11 | 0.014 | |
| PTMC | Female | 8 | 0.06 | 0.23 | 0.10–0.52 | 1 [Reference] | 1 [Reference] | 1 [Reference] | ||||||
| Male | 7 | 0.21 | 1.21 | 0.58–2.54 | 3.89 | 1.41–10.71 | 0.009 | 1.23 | 0.36–4.16 | 0.743 | 0.77 | 0.19–3.16 | 0.769 | |
PTC: Papillary thyroid cancer; PTMC: Papillary Thyroid Microcarcinoma.
*Adjustment 1 was made for patient age at diagnosis, race, multifocality, tumor size, extrathyroidal invasion, lymph node metastasis and distant metastasis.
†Adjustment 2 was made for patient age at diagnosis, race, multifocality, tumor size, extrathyroidal invasion, lymph node metastasis, distant metastasis, radiation and surgery treatment.
Discussion
Several studies have reported conflicting results regarding the effects of male gender on the prognosis of PTC. Kruijff and colleagues demonstrated the risk of structural recurrence in men was 2.44 times that in women, with a median follow-up period of 31 months15. A meta-analysis also showed that male gender was an independent poor prognostic factor, and that the risk of recurrence in men was 1.53 times that in women14. However, only 4 of the 13 articles included in this meta-analysis reported that male gender was a poor prognostic factor for PTC.
Oyer et al. concluded that there was no difference in disease-specific survival between male and female patients with the same disease stage, according to an analysis of the SEER database from 1988–200316. Nilubol and Grogan et al. also reported that male gender was not an independent prognostic factor of cancer specific survival in PTC patients12, 17. Similar result was also reported by Matsuzu et al.’s study which recorded 1088 PTC patients with a median follow-up period of 17.6 years18.
These inconsistent results introduce a dilemma into the treatment of PTC. More aggressive treatment options (such as total thyroidectomy, or lymph node dissection followed by radioactive iodine ablation) should be considered for men if male gender is a negative independent prognostic factor in PTC. The purpose of this study was to investigate whether male gender was a poor prognostic factor in PTC. Throughout our analyses of the SEER database, male gender was associated with elevated risks of all-cause death and cancer-specific death, both for PTC as a whole and for PTCs > 1 cm. Therefore, based on our results, relatively more aggressive treatment should be considered for these subgroups of patients.
Among patients with PTMC, male gender was associated with significantly elevated risks of all-cause death. However, we found that this association did not remain statistically significant after adjusting for patient age at diagnosis, race, multifocality, and other risk-associated clinicopathological characteristics and treatment methods. This may be attributable to the extremely good prognosis of PTMC, which as 15-year disease-specific survival rates as high as 99% and overall survival of 97.5%19, 20.
Recent studies have reported that estrogen can regulate thyroid cell proliferation by binding to both ERα, and ERβ. These studies attempted to investigate the associations between estrogen receptor (ER) expression and tumor aggressiveness and prognosis in PTC, but obtained inconsistent conclusions21–23. Yi et al. investigated the clinical implications of ER coding genes in PTC and found that ESR1 expression and the ESR ratio were associated with aggressive clinicopathological factors and poor overall survival in women21. Huang et al. further observed that ERα stimulates PTC growth and progression, whereas ERb1 inhibits PTC growth and progression23. Since ER expression levels differ between men and women, we speculate that ER level may be one of the underlying reasons for the gender differences in PTC tumor aggressiveness and prognosis. However, further research (including both genomic and epidemiologic investigations) should be performed to reveal the source of the gender disparity.
Some inherent limitations must be taken into consideration in the interpretation of our results. The major limitations of this study are that data regarding recurrence are not captured in SEER, and that designation of cancer-specific death is susceptible to overestimation bias, particularly for diseases such as PTMC. Furthermore, vascular invasion, family history, and other histologic findings were not evaluated or included in our study. In addition, the molecular markers such as BRAF point mutation and TERT promotor point mutation, were not obtained in our study or adjusted for in our analyses. Furthermore, given the generally favorable prognosis of PTC, the relatively short study period and follow-up (2010–2013) is a limitation to our analysis.
Conclusion
Our study demonstrated that male gender is an independent poor prognostic factor for all PTCs and for PTCs > 1 cm. However, gender could actually have substantial prognostic relevance for PTMC, but that you do not have enough data or a long enough follow-up period to determine this reliably. These results may be instructive for clinicians. In general, more aggressive treatments should be provided to men with all PTCs and PTCs > 1 cm.
Methods and Materials
Database
We investigated PTC and PTMC in a large cohort of patients from SEER. The SEER project is a United States population-based cancer registry that began in 1973 and is supported by the National Cancer Institute and the Centers for Disease Control and Prevention. It covers approximately 30% of the population of the United States and contains data across multiple geographic regions on incidence, prevalence, mortality, population-based variables, primary characteristics of the tumor, and other attributes.
Data collection and analysis
We examined SEER data for 2010–2013 and selected patients with a diagnosis of PTC, as defined by a combination of ICD-O site code of C73.9 (i.e., thyroid) and papillary histology. The following diagnostic codes were included in the study: “papillary carcinoma,” “papillary adenocarcinoma,” “oxyphilic adenocarcinoma”, “papillary adenocarcinoma,” and “papillary cyst-adenocarcinoma.” Demographic information, age, sex, tumor size, extrathyroidal extension, multifocality, nodal, metastasis, surgical treatment and radiation treatment were compiled from the SEER dataset, and a survival analysis was performed to evaluate the associations between gender and prognosis.
Statistical analysis
Patients were followed up until December 2013. The outcomes measures were thyroid carcinoma-specific mortality and all-cause mortality. Patient survival curves were investigated using Kaplan-Meier analyses, log-rank tests, and Cox proportional hazards regression analyses. HRs were used to show the magnitude of the effect of stages on cancer-specific mortality. Ninety-five percent CIs were used to indicate the significance of the risks. All P values were 2-sided and P less than .05 was regarded as indicating statistical significance. Analyses were performed using SPSS version 19.0 (IBM Corp, Armonk, NY, USA), Stata/SE version 12 (Stata Corp, College Station, TX, USA), and GraphPad Prism version 6 (GraphPad Software Inc, La Jolla, CA, USA).
Author Contributions
All authors contributed to the design of the study and writing of the manuscript. C.P.L. and T.W.C. undertook the research, W.Z., Y.Q.X. and S.T.W. performed the analyses, Z.M.L. and T.H. wrote the main manuscript text and prepared figures. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Chunping Liu and Tianwen Chen contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zeming Liu, Email: 529716391@qq.com.
Tao Huang, Email: huangtaowh@163.com.
References
- 1.Mao Y, Xing M. Recent incidences and differential trends of thyroid cancer in the USA. Endocrine-related cancer. 2016;23(4):313–322. doi: 10.1530/ERC-15-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, et al. Cancer statistics in China, 2015. CA: a cancer journal for clinicians. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, et al. A comparison of the clinicopathological features and prognoses of the classical and the tall cell variant of papillary thyroid cancer: a meta-analysis. Oncotarget. 2017;8(4):6222–6232. doi: 10.18632/oncotarget.14055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haugen BR, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid: official journal of the American Thyroid Association. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Q, et al. Multifocality and total tumor diameter predict central neck lymph node metastases in papillary thyroid microcarcinoma. Annals of surgical oncology. 2013;20(3):746–752. doi: 10.1245/s10434-012-2654-2. [DOI] [PubMed] [Google Scholar]
- 7.Leenhardt L, Grosclaude P, Cherie-Challine L. Increased incidence of thyroid carcinoma in france: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid: official journal of the American Thyroid Association. 2004;14(12):1056–1060. doi: 10.1089/thy.2004.14.1056. [DOI] [PubMed] [Google Scholar]
- 8.Leboulleux S, Tuttle RM, Pacini F, Schlumberger M. Papillary thyroid microcarcinoma: time to shift from surgery to active surveillance? The lancet Diabetes & endocrinology. 2016;4(11):933–942. doi: 10.1016/S2213-8587(16)30180-2. [DOI] [PubMed] [Google Scholar]
- 9.Xiang J, et al. New clinical features of thyroid cancer in eastern China. Journal of visceral surgery. 2010;147(1):e53–56. doi: 10.1016/j.jviscsurg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Garrel R, et al. Cervical lymphadenopathies signaling thyroid microcarcinoma. Case study and review of the literature. European annals of otorhinolaryngology, head and neck diseases. 2011;128(3):115–119. doi: 10.1016/j.anorl.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Huang T. Papillary thyroid microcarcinoma and active surveillance. The lancet Diabetes & endocrinology. 2016;4(12):974–975. doi: 10.1016/S2213-8587(16)30269-8. [DOI] [PubMed] [Google Scholar]
- 12.Nilubol N, Zhang L, Kebebew E. Multivariate analysis of the relationship between male sex, disease-specific survival, and features of tumor aggressiveness in thyroid cancer of follicular cell origin. Thyroid: official journal of the American Thyroid Association. 2013;23(6):695–702. doi: 10.1089/thy.2012.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YH et al. Is Male Gender a Prognostic Factor for Papillary Thyroid Microcarcinoma? Annals of surgical oncology. (2017). [DOI] [PubMed]
- 14.Guo K, Wang Z. Risk factors influencing the recurrence of papillary thyroid carcinoma: a systematic review and meta-analysis. International journal of clinical and experimental pathology. 2014;7(9):5393–5403. [PMC free article] [PubMed] [Google Scholar]
- 15.Kruijff S, et al. Patterns of structural recurrence in papillary thyroid cancer. World journal of surgery. 2014;38(3):653–659. doi: 10.1007/s00268-013-2286-0. [DOI] [PubMed] [Google Scholar]
- 16.Oyer SL, Smith VA, Lentsch EJ. Reevaluating the prognostic significance of age in differentiated thyroid cancer. Otolaryngology–head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2012;147(2):221–226. doi: 10.1177/0194599812441587. [DOI] [PubMed] [Google Scholar]
- 17.Grogan RH, et al. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery. 2013;154(6):1436–1446. doi: 10.1016/j.surg.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Matsuzu K, et al. Thyroid lobectomy for papillary thyroid cancer: long-term follow-up study of 1,088 cases. World journal of surgery. 2014;38(1):68–79. doi: 10.1007/s00268-013-2224-1. [DOI] [PubMed] [Google Scholar]
- 19.Yu XM, Wan Y, Sippel RS, Chen H. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Annals of surgery. 2011;254(4):653–660. doi: 10.1097/SLA.0b013e318230036d. [DOI] [PubMed] [Google Scholar]
- 20.Shi X, et al. Differential Clinicopathological Risk and Prognosis of Major Papillary Thyroid Cancer Variants. The Journal of clinical endocrinology and metabolism. 2016;101(1):264–274. doi: 10.1210/jc.2015-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi JW et al. Upregulation of the ESR1 Gene and ESR Ratio (ESR1/ESR2) is Associated with a Worse Prognosis in Papillary Thyroid Carcinoma: The Impact of the Estrogen Receptor alpha/beta Expression on Clinical Outcomes in Papillary Thyroid Carcinoma Patients. Annals of surgical oncology. 2017. [DOI] [PubMed]
- 22.Vannucchi G, et al. Impact of estrogen and progesterone receptor expression on the clinical and molecular features of papillary thyroid cancer. European journal of endocrinology / European Federation of Endocrine Societies. 2015;173(1):29–36. doi: 10.1530/EJE-15-0054. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, et al. Differential expression patterns and clinical significance of estrogen receptor-alpha and beta in papillary thyroid carcinoma. BMC cancer. 2014;14:383. doi: 10.1186/1471-2407-14-383. [DOI] [PMC free article] [PubMed] [Google Scholar]