Tables of Links
| LIGANDS |
|---|
| Fluoxetine |
| Nifedipine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2, 3, 4.
Whether fluoxetine, a selective serotonin reuptake inhibitor, is an effective treatment for Raynaud's phenomenon (RP) has been debated for about 20 years. Based on one positive efficacy trial 5 and some preliminary observations 6, fluoxetine is recommended in RP secondary to systemic sclerosis (SSc), after failure of calcium channel blockers 7. However, when one looks closely at the available evidence, the lack of a homogeneous effect of fluoxetine in RP patients is obvious.
The crossover study comparing the efficacy of nifedipine and fluoxetine in 56 patients with primary or secondary RP showed a significant improvement in the Raynaud's condition score (RCS) [4.35 (0.39) vs. 2.3 (0.35); P = 0.0002] and daily frequency of attacks [2.98 (0.31) vs. 1.7 (0.25); P = 0.003] 4. However, when looked at more carefully, subgroup analysis showed a significant benefit for RCS and the frequency of attacks in primary RP, while only RCS was significantly improved in patients with secondary RP. Likewise, the secondary criterion of percentage of rewarming after cold challenge was positive in primary RP [33.4% (±7.5%) vs. 58.8% (±8.7%); P = 0.03] but negative in secondary RP [31.6% (±6.4%) vs. 31.2% (±8.2%); P = 0.97]. Furthermore, we could hypothesize that the antidepressant activity of fluoxetine may have a significant impact on a subjective measurement of self‐reported outcomes such as RCS. Owing to the discovery of thrombocyte dysfunction correlated with an increase in intraplatelet serotonin in RP, the antiaggregant effect of fluoxetine was hypothesized to be the main mechanism 8. However, later studies using antithrombotic drugs were disappointing and evidence of their benefit in RP is now limited 9.
The involvement of the serotoninergic pathway in vascular tone is complex; serotonin causes direct vasoconstriction through 5HT2A, 5HT2B and 5HT1B receptors 10. Experimental data also suggest that serotonin released from adrenergic nerves inhibits calcitonin gene‐related peptide‐containing nerve‐dependent vasodilation 11. By contrast, vasodilation is mediated through 5HT7 and 5HT2B receptors, located on smooth muscle cells and on the endothelium, respectively 10. Endothelium‐dependent vasodilation would be secondary to increased nitric oxide (NO) bioavailability, through enhanced endothelial NO synthase activity 11, 12. Mechanisms underlying direct activity on smooth muscle cells may involve activation of calcium‐sensitive potassium channels 13 and inhibition of voltage‐gated calcium channels 12 (Figure 1).
Figure 1.
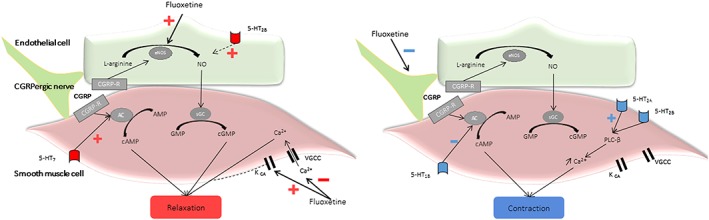
Co‐existing vasodilator–vasoconstrictor pharmacodynamic effects of fluoxetine. AC, adenylyl cyclase; AMP, adenosine monophosphate; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; CGRP, calcitonin gene‐related peptide; eNOS, endothelial NO synthase; GMP, guanosine monophosphate; Kca, calcium‐sensitive potassium channel; NO, nitric oxide; PLCβ, phospholipase C beta; R‐CGRP, calcitonin gene‐related peptide receptor; sGC, soluble guanylate cyclase; VGCC, voltage‐gated calcium channels
Whether fluoxetine increases, through the reduction in serotonin reuptake into platelets, or decreases, through the sequestration of serotonin at the intestinal level, the plasma serotonin concentration is still controversial 14. However, this probably has a limited impact, considering that vasomodulation mediated by fluoxetine is not dependent on plasma serotonin concentration 15.
In light of the clinical discrepancies described above, we raise the hypothesis that in SSc, endothelial dysfunction could explain the reduced vasodilator effect of fluoxetine, and could even switch the balance between vasoconstriction and vasodilation.
We therefore believe that there is insufficient scientific evidence to recommend fluoxetine as a treatment in SSc‐related RP. A well‐designed, double‐blinded clinical trial that properly stratifies patients according to RP aetiology would address this question.
Competing Interests
All authors have completed the Unified Competing Interest form and declare no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Khouri, C. , Gailland, T. , Lepelley, M. , Roustit, M. , and Cracowski, J.‐L. (2017) Fluoxetine and Raynaud's phenomenon: friend or foe?. Br J Clin Pharmacol, 83: 2307–2309. doi: 10.1111/bcp.13314.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 2015; 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 2015; 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coleiro B, Marshall SE, Denton CP, Howell K, Blann A, Welsh KI, et al. Treatment of Raynaud's phenomenon with the selective serotonin reuptake inhibitor fluoxetine. Rheumatology 2001; 40: 1038–1043. [DOI] [PubMed] [Google Scholar]
- 6. Jaffe IA. Serotonin reuptake inhibitors in Raynaud's phenomenon. Lancet 1995; 345: 1378. [DOI] [PubMed] [Google Scholar]
- 7. Kowal‐Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, et al Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 2017;https://doi.org/10.1136/annrheumdis‐2016‐209909. [DOI] [PubMed] [Google Scholar]
- 8. Biondi ML, Marasini B, Bianchi E, Agostoni A. Plasma free and intraplatelet serotonin in patients with Raynaud's phenomenon. Int J Cardiol 1988; 19: 335–339. [DOI] [PubMed] [Google Scholar]
- 9. Pauling JD, O'Donnell VB, Mchugh NJ. The contribution of platelets to the pathogenesis of Raynaud's phenomenon and systemic sclerosis. Platelets 2012; 24: 503–515. [DOI] [PubMed] [Google Scholar]
- 10. Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation. Pharmacol Rev 2012; 64: 359–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta M, Neavin D, Liu D, Biernacka J, Hall‐Flavin D, Bobo WV, et al. TSPAN5, ERICH3 and selective serotonin reuptake inhibitors in major depressive disorder: pharmacometabolomics‐informed pharmacogenomics. Mol Psychiatry 2016; 21: 1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ungvari Z, Pacher P, Kecskeméti V, Koller A. Fluoxetine dilates isolated small cerebral arteries of rats and attenuates constrictions to serotonin, norepinephrine, and a voltage‐dependent Ca2+ channel opener. Stroke 1999; 30: 1949–1954. [DOI] [PubMed] [Google Scholar]
- 13. Pereira CA, Ferreira NS, Mestriner FL, Antunes‐Rodrigues J, Evora PRB, Resstel LBM, et al. Chronic fluoxetine treatment increases NO bioavailability and calcium‐sensitive potassium channels activation in rat mesenteric resistance arteries. Eur J Pharmacol 2015; 765: 375–383. [DOI] [PubMed] [Google Scholar]
- 14. Fujii H, Takatori S, Zamami Y, Hashikawa‐Hobara N, Miyake N, Tangsucharit P, et al. Adrenergic stimulation‐released 5‐HT stored in adrenergic nerves inhibits CGRPergic nerve‐mediated vasodilatation in rat mesenteric resistance arteries. Br J Pharmacol 2012; 166: 2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ofek K, Schoknecht K, Melamed‐Book N, Heinemann U, Friedman A, Soreq H. Fluoxetine induces vasodilatation of cerebral arterioles by co‐modulating NO/muscarinic signalling. J Cell Mol Med 2012; 16: 2736–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]


