Abstract
Aims
Methadone is a widely used opioid agonist treatment associated with QT prolongation and torsades de pointes. We investigated the QT interval in patients treated with methadone or buprenorphine using continuous 12‐lead Holter recordings.
Methods
We prospectively made 24‐h Holter recordings in patients prescribed methadone or buprenorphine, compared to controls. After their normal dose a continuous 12‐lead Holter recorder was attached for 24 h. Digital electrocardiograms were extracted hourly from the Holter recordings. The QT interval was measured automatically (H‐scribe software, Mortara Pty Ltd) and checked manually. The QT interval was plotted against heart rate (HR) on the QT nomogram to determine abnormality. Demographics, dosing, medical history and laboratory investigations were recorded.
Results
There were 58 patients (19 methadone, 20 buprenorphine and 19 control); median age 35 years (20–56 years); 33 males. Baseline characteristics were similar. Median dose of methadone was 110 mg day–1 (70–170 mg day–1) and buprenorphine was 16 mg day–1 (12–32 mg day–1). Seven participants had abnormal QT intervals. There was a significant difference in the proportion of prescribed methadone with abnormal QT intervals, 7/19 (37%; 95% confidence interval: 17–61%), compared to controls 0/19 (0%; 95% confidence interval: 0–21%; P = 0.008), but no difference between buprenorphine and controls (0/20). QT vs. HR plots showed patients prescribed methadone had higher QT‐HR pairs over 24 h compared to controls. There was no difference in dose for patients prescribed methadone with abnormal QT intervals and those without.
Conclusions
Methadone is associated with prolonged QT intervals, but there was no association with dose. Buprenorphine did not prolong the QT interval. Twenty four‐hour Holter recordings using the QT nomogram is a feasible method to assess the QT interval in patients prescribed methadone.
Keywords: buprenorphine, Holter recording, methadone, opioid agonist treatment, QT interval
What is Already Known about this Subject
Methadone is an effective opioid agonist treatment associated with QT prolongation and torsades de pointes.
Controversy remains over how to determine the risk of torsades de pointes.
What this Study Adds
Methadone is associated with prolonged QT but not dose dependent QT.
Buprenorphine did not prolong the QT interval.
Twenty four‐hour Holter recordings and the QT nomogram are feasible in assessing the QT in patients prescribed opioid agonist treatment.
Tables of Links
| TARGETS |
|---|
| Voltage‐gated Potassium channels |
| LIGANDS |
|---|
| methadone |
| buprenorphine |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2.
Introduction
Methadone is a first line medication for opioid dependence with demonstrated benefits of reduction of heroin use, improvement in psychosocial functioning including reduction in crime, reduced overdose, reduction in human immunodeficiency virus risk and, more recently, in hepatitis C transmission 3, 4, 5, 6. Buprenorphine, now also a first line treatment for opioid dependence in many countries, has been shown to have benefits similar to methadone 7. Approximately 900 000 patients worldwide were on methadone or buprenorphine in 2009 8.
Methadone is one of a number of medications associated with a prolonged QT interval 9, 10, 11, 12, with an associated increased risk of the rare polymorphic ventricular tachyarrythmia torsades de pointes (TdP). Levo‐acetyl methadol (LAAM), previously also used for treating opioid dependence, was withdrawn from the market due to concerns of QT interval prolongation 13, 14, 15. In contrast, buprenorphine does not appear to be associated with QT interval prolongation 16, 17, 18.
Numerous studies from around the world have reported on the prevalence of QT prolongation in patients on methadone treatment 19, 20, 21, 22, 23, 24, 25. These studies range in sample size from 23 to 436, have prospective and retrospective designs, report on inpatient and outpatient groups, include patients with other conditions (cocaine dependence and HIV) and measure and correct the QT interval differently. QT prolongation is reported in zero up to two‐thirds of patients across different studies, but a range of cut‐off measurements are used to define QTc prolongation, from 430 to 500 ms. Different studies exploring QT interval prolongation have either demonstrated dose dependence 26 or not demonstrated 19, 27 a dose response relationship with methadone doses. Previous studies comparing methadone and buprenorphine have found that methadone prolongs the QT but not buprenorphine 16, 17, 18, 28. Despite this, buprenorphine is on the list of QT drugs at crediblemeds.org as having a possible risk of TdP.
The QT interval measurement in the cardiac cycle is not static and follows a diurnal variation across a 24‐h period. A limitation of using a single QT interval measurement to define whether a patient is above a threshold where there is an elevated risk of TdP is that patients’ QT interval measurements may fluctuate over a 24‐h period.
A number of formulae have been developed to correct the QT interval for heart rate, as measurements are heart rate dependent. However, limitations exist in all these formulae. A novel approach developed recently has been the use of a QT nomogram that allows plotting of the QT interval against the heart rate (HR) 9, 29. A cut off line on this nomogram is used to ascertain QT interval measurements that would place a patient at elevated risk of TdP. Using a 24‐h Holter recorder in combination with this approach permits a more detailed measurement of QT interval prolongation risk over a 24‐h period. To our knowledge, this method has not been previously used in assessing the risk of QT interval prolongation for patients on opiate agonist treatment.
The aim of the current study was to investigate the use of a 24‐h Holter recording to measure QT intervals and detect abnormal QT intervals based on the QT nomogram in a group of outpatients on methadone or buprenorphine treatment for opioid dependence.
Methods
Study design and setting
This was a prospective study of patients on outpatient opioid agonist treatment (methadone or buprenorphine), which used high resolution 12‐lead Holter recordings to measure the QT interval. Participants were recruited from the Hunter New England Health Drug and Alcohol Clinical Services in Newcastle, NSW, Australia. The service provides opioid agonist treatment, drug counselling, needle syringe services, inpatient withdrawal and hospital outpatient and drug and alcohol consultation liaison services across a wide geographical area in regional NSW. The community‐based opioid agonist treatment programmes treat over 1100 patients each year, with the Newcastle opioid agonist clinic treating approximately 630 patients per year. Patients typically receive supervised dosing of methadone or buprenorphine (as buprenorphine–naloxone sublingual film), with stable patients allowed up to several non‐supervised doses each week. Ethics approval was obtained from the Hunter New England Area Human Research Ethics Committee.
Selection of participants
Participants were patients who accessed Newcastle Drug and Alcohol Clinical Services for either opioid agonist treatment, or who were not on opioid agonist treatment (including patients in cannabis and methamphetamine outpatient counselling programs) and were included as a comparison group. This study aimed to enrol 60 patients: 20 participants on methadone treatment, 20 on buprenorphine treatment, and 20 control participants. Participants on opioid agonist treatment were included if they were on stable opioid agonist treatment with a stable maintenance dose (≥ 60 mg methadone daily or ≥ 12mg buprenorphine) for at least 4 weeks, were adherent to treatment (maximum one missed dose per week), were able to safely comply with and complete the study and were able to wear a portable Holter recorder for a 24‐h period. Patients were excluded if they had a history of violence towards others or unpredictable behaviour, were unable to give an account of their medical, family and drug use history, or were younger than 18 years. Pregnant patients were not included in the study.
Potential participants made themselves known to research staff after viewing advertisements in the clinic waiting area or were approached and informed about the study by study clinicians. If interested, potential participants were assessed to determine if they were eligible for the study using a brief screening form incorporating the inclusion and exclusion criteria. They were then fully informed about the study and asked to provide written consent. Participants were only included on a voluntary basis.
Interventions
Following consent, participants were required to attend Drug and Alcohol Clinical Services on two occasions. Visit one required up to approximately 2 h of the participants' time and involved: a medical assessment to obtain self‐reported drug and alcohol use, opioid agonist treatment history, medical and mental health history, current medications, a physical examination, breath alcohol analysis to estimate the blood alcohol content, an education session on the requirements and limitations of wearing a Holter recorder, and fitting of the Holter recorder (Mortara, Milwaukee, WI, USA) which was attached to the patient with 10 leads to the chest in the manner instructed by the manufacturer 30.
Participants then received their normal opioid agonist treatment dose. Patients taking buprenorphine receiving second daily dosing were required to have their dose administered on the day of Holter recorder fitting. The second visit was 24 h later, took up to approximately 1.5 h and involved removal of the Holter recorder, breath alcohol analysis, collection of blood samples and a urine specimen for drug testing. Blood samples (approximately 10 ml) were collected for blood chemistry analysis (potassium, calcium and magnesium).
Data collection
Clinical histories and patient examination were recorded on case report forms and then subsequently entered into a purpose designed database. Demographics, dosing information and duration of opioid agonist treatment, use of other drugs and alcohol, regular medications, examination (heart rate and blood pressure), investigations (urine drug screen and electrolytes), were then extracted from the database.
From the high‐resolution Holter recordings, 10‐s 12‐lead high‐resolution digital electrocardiogram (ECG) was sampled every hour over the 24‐h period using the manufacturer's software (H‐Scribe; Mortara). ECGs could not be extracted from the Holter recording at exactly the same time each hour due to movement artefact, but an ECG was extracted in each 1‐h period where possible. ECGs were then imported into a second software package (E‐Scribe) which provided on‐screen measurement of the QT interval. The software provided an automated measurement of the QT interval and also the ability to manually adjust this using an overlapped view of the six chest and six limb leads of the ECG. QT interval measurements were excluded if the HR > 150 beats min–1 because the QT interval is difficult or impossible to measure at extreme HR. Two patients had a HR > 150 beats min–1, both on only one occasion, one patient in the buprenorphine group had used amphetamines and the other in the methadone group was prescribed salbutamol.
For every ECG, the QT interval measurement and the corresponding HR measurement were plotted on the QT nomogram to determine if the patient had an abnormal QT interval in the 24‐h period and if so the frequency of abnormal QT intervals in the 24‐h period. We used the QT nomogram because it provides a simple QT cut‐off and method to account for HR. 9, 29 The abnormal cut‐off (line) was originally taken from a study by Fossa et al. 31, by digitizing QT‐RR data in their Figure 1 and converting this to a QT‐HR line. The QT nomogram cut‐off was then evaluated systematically and compared to HR correction formulae in a series of 129 cases of drug‐induced TdP vs. a group of 316 patients with overdoses of noncardiotoxic medications (Figure S1) 29. The nomogram was found to be more sensitive and specific than cut‐offs at 440 and 500 ms using Bazett's HR correction 29, and more sensitive than a cut‐off of 500 ms using Fridericia's HR correction 32. Since this original study the QT nomogram has been used in further studies to assess the risk of drug‐induced QT prolongation in patients taking drug overdoses and substance use disorders 32, 33, 34, 35.
Figure 1.
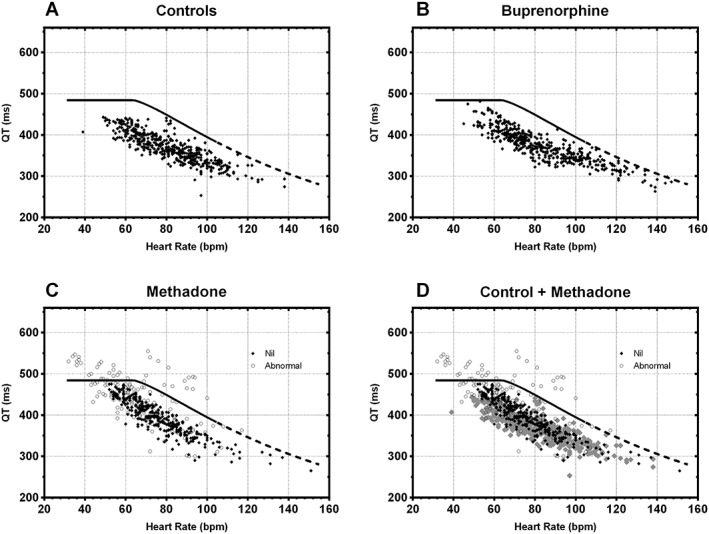
The QT nomogram with plots of multiple QT intervals vs. heart rate (QT‐HR pairs) recorded from each patient including the at‐risk line for (A) the 19 control patients, (B) 20 patients prescribed buprenorphine, (C) 19 patients prescribed methadone and (D) for a comparison of controls and patients prescribed methadone. Points are black diamonds for patients where their QT interval remains under the at‐risk line and open black circles if they are abnormal QT interval. In Panel D, the control patients are in grey
All ECGs obtained from the Holter recorder were promptly reviewed and the QT interval measured within days of the recording. Any cases of QT interval prolongation defined according to the QT nomogram were reviewed by one of the investigators (G.K.I.) for accuracy. If clinically indicated, the participant was referred for further medical review by their treating medical officer, following discussion with the participant.
Outcomes
The primary outcome was the proportion of patients with at least one abnormal QT interval during the 24‐h period in each participant group, defined as abnormal if the plotted QT‐HR pair was above the abnormal line on the QT nomogram 29.
The secondary outcome measures aimed to determine if the magnitude of the QT interval abnormality was associated with the dose ingested; and whether other factors influence the occurrence of an abnormal QT interval in these patient groups – age, sex, other recreational drugs, pre‐existing cardiac disease and electrolyte abnormalities. The proportion (%) of abnormal QT readings occurring in 24 h was also calculated for each patient with an abnormal QT, as the number of hours with an abnormal QT divided by 24.
Analysis
The proportion of patients with an abnormal QT interval occurring in the 24‐h period were compared between the three groups. Proportions are reported with 95% confidence intervals, calculated with Wilson's procedure with a continuity correction. Continuous variables are summarised with medians, interquartile ranges and ranges. The primary outcome comparing the proportion of patients with an abnormal QT interval prescribed methadone or buprenorphine vs. the control group was compared with Fisher's exact test. All graphical and statistical analysis was done using GraphPad Prism software (version 6 for Windows; GraphPad Software, San Diego; CA, USA).
Results
There were 58 participants recruited to the study with Holter data recorded (controls 19, buprenorphine 20, methadone 19). In two patients, the Holter recorder did not record due to technical issues and patient adherence. The median age was 35 years (20–56 years) and 33 were male. The baseline characteristics of the three groups are summarised in Table 1. The use of other psychoactive drugs in the previous 3 months in all three groups was common, including other opioids, alcohol, benzodiazepines, methamphetamine and cannabis (Table 1).
Table 1.
Demographics, details of opioid agonist treatment, drug use, cardiac history, urine drug screen and electrolytes
| Controls | % | Methadone | % | Buprenorphine | % | |
|---|---|---|---|---|---|---|
| Number | 19 | 19 | 20 | |||
| Male | 13 | 68 | 8 | 42 | 12 | 60 |
| Age (years; range) | 35 (21–54) | 36 (20–56) | 36 (24–46) | |||
| Opioid agonist treatment | ||||||
| Dose (mg) | 110 (70–170) | 16 (12–32) | ||||
| Total duration b (years) | 8.75 (0.33–30) | 3.6 (0.17–23) | ||||
| Self‐reported drug use in past 3 months | ||||||
| Heroin/other opioids (nonprescribed) | 8 | 42 | 2 | 11 | 5 | 25 |
| Cannabis | 8 | 42 | 11 | 58 | 9 | 45 |
| Methamphetamine | 6 | 32 | 7 | 37 | 11 | 55 |
| Benzodiazepines | 5 | 26 | 5 | 26 | 8 | 40 |
| Alcohol | 13 | 68 | 6 | 32 | 9 | 45 |
| Prescribed QT medication a | 2 | 11 | 0 | 0 | 1 | 5 |
| Urine drug screen | ||||||
| Cocaine | 0 | 1 | 5 | 0 | ||
| Methamphetamines | 1 | 5 | 2 | 11 | 5 | 25 |
| Benzodiazepines | 2 | 11 | 7 | 37 | 4 | 20 |
| THC | 4 | 21 | 10 | 53 | 8 | 40 |
| Cardiac history | ||||||
| Syncope | 4 | 21 | 5 | 26 | 3 | 15 |
| Family history of sudden death | 0 | 0 | 0 | 0 | 2 | 10 |
| Vital observations | ||||||
| Heart rate | 78 (60–102) | 70 (49–86) | 76 (55–118) | |||
| Systolic blood pressure | 128 (102–153) | 119 (92–149) | 119 (96–163) | |||
| Electrolytes | ||||||
| Magnesium (mmol l –1 ) | 0.81 (0.67–0.89) | 0.80 (0.66–0.92) | 0.85 (0.70–0.94) | |||
| Calcium (mmol l –1 ) | 2.41 (2.11–2.54) | 2.35 (2.23–2.56) | 2.36 (2.23–2.51) | |||
| Potassium (mmol l –1 ) | 4.6 (3.8–5.0) | 4.3 (3.8–5.0) | 4.5 (3.8–5.3) | |||
Escitalopram (1), valproate (2)
Includes both buprenorphine and methadone for both groups
In total, there were seven patients who developed an abnormal QT interval; all were prescribed methadone. There was a significant difference in the proportion of patients on methadone with an abnormal QT interval 7 of 19 (37%, 95% confidence interval: 17–61%), compared to controls (0/19, 0%, 95% confidence interval: 0–21%; P = 0.008). There was no difference between patients prescribed buprenorphine and controls, with neither having any abnormal QT intervals. Plots of the Holter recordings for each patient group are shown in Figure 1, including a comparison of patients prescribed methadone and controls. The individual plots for the seven patients with abnormal QT intervals is shown in Figure S2. Figure 1D shows that in addition to the seven patients with abnormal QT intervals, the remainder of patients prescribed methadone also had longer QT intervals compared to controls, seen by the upwards shift of the QT‐HR cloud. One patient prescribed buprenorphine had one QT‐HR pair above the line for a HR of 145 beats min–1 where measurement of the QT interval is inaccurate due to the rapid HR. 34 This patient was taking methamphetamine confirmed on urine drug screening.
None of the patients prescribed methadone were on other medications known to cause QT interval prolongation and none had a history or family history of sudden collapse or significant cardiac disease. There was no difference between patients prescribed methadone who had an abnormal QT interval and those that did not (Table 2), except there were more men among the patients with an abnormal QT interval. The median proportion of abnormal QT readings occurring over the 24 h period in the seven patients was 25% (4–46%).
Table 2.
The characteristics of the patients prescribed methadone comparing those with a normal QT vs. those with an abnormal QT
| Characteristics | Normal | % | Abnormal QT | % |
|---|---|---|---|---|
| Number | 12 | 7 | ||
| Male | 4 | 33% | 4 | 57% |
| Age (years; range) | 36 (20–52) | 35 (25–56) | ||
| Opioid agonist treatment | ||||
| Dose (mg) | 115 (70–160) | 100 (75–170) | ||
| Urine Drug Screen | ||||
| Cocaine | 1 | 8% | 0 | 0% |
| Methamphetamines | 1 | 8% | 1 | 14% |
| Benzodiazepines | 2 | 17% | 5 | 71% |
| THC | 7 | 58% | 3 | 43% |
| Electrolytes | ||||
| Magnesium (mmol l –1 ) | 0.81 (0.76–0.92) | 0.83 (0.66–0.89) | ||
| Calcium (mmol l –1 ) | 2.33 (2.25–2.56) | 2.44 (2.23–2.50) | ||
| Potassium (mmol l –1 ) | 4.3 (3.8–5.0) | 4.5 (4.1–4.8) | ||
Discussion
The study found that the QT interval is prolonged in a group of patients taking methadone for opioid agonist treatment, with over a third having an abnormal QT interval based on the QT nomogram. We could not find an association between methadone dose and QT interval prolongation. Buprenorphine was not associated with QT interval prolongation. QT interval assessment using Holter recordings was possible in patients prescribed opioid agonist treatment.
Previous studies assessing the QT interval for patients on opioid agonist treatment have used other methods of QT interval measurement and HR correction. The advantage of the QT nomogram is that it separates the HR correction from the measurement of the QT interval and provides a cloud of QT‐HR pairs for each patient. Visually, this provides a semiquantitative way of comparing patients prescribed methadone and those not. Figure 1D demonstrates this with the upward shifting of patients prescribed methadone.
Measurement of the QT interval also varies significantly between previous studies, with some only measuring one lead 28, and others measuring multiple leads but taking the longest, rather than the median measurement of the QT interval 18. Decades of research into the measurement of the QT interval in the assessment of drug induced QT intervals, recommends using multiple leads and taking the median 36, 37. Most problematic is the reliance on the automated measurement using standard ECG machines, which is known to be inaccurate, particularly for abnormal QT interval measurements 37.
The lack of relationship between methadone dose and QT interval prolongation is unusual and differs to other drugs, which have demonstrated associations between dose and QT interval prolongation 38, 39. Previous studies have reported this for methadone before 19, 27. The reason for this is unclear, but may be simply due to the small sample size in our study. It may also be due to large interindividual differences in oral bioavailability and a poor relationship between dose and methadone concentrations 40. Measurement of methadone concentrations should be undertaken in future studies to further explore the relationship between methadone exposure (dose), methadone concentrations and the pharmacodynamics of QT interval changes using Holter recordings.
Our study supports previous studies of buprenorphine that did not report QT prolongation. A small number of studies directly compared QT interval prolongation in patients prescribed methadone and buprenorphine 16, 17, 18, 28. A Norwegian study found that 26/173 (15%) patients on methadone had a QTc > 470 ms and eight (4.6%) at QTc > 500 ms, while none in the buprenorphine group had an abnormal QTc 28. A randomised controlled trial inducting and maintaining 165 opioid dependent patients onto methadone, buprenorphine or LAAM found QT interval prolongation in the methadone (23%) and LAAM (28%) groups, but not in the buprenorphine group 17. A 5‐year follow‐up study of methadone and buprenorphine users found methadone but not buprenorphine was associated with a prolonged QT interval 16. A study of spontaneous adverse drug events found that methadone is associated with cardiac arrhythmias, while buprenorphine is not 41. A recent study in healthy volunteers found that transdermal buprenorphine did not cause QTc prolongation at therapeutic doses, but caused mild prolongation at supratherapeutic doses 42.
The current study demonstrates the potential feasibility of using Holter recordings in clinical practice to measure the QT interval in patients on opioid agonist treatment. A high proportion of all patients were able to wear a 24‐h Holter recorder, and return with measureable readings. The method of using 24‐h Holter recording combined with using a QT nomogram may provide more sensitive assessment of QT interval abnormalities for patients on opioid agonist treatment. A single 12‐lead ECG done at a single time point after a methadone dose may easily miss an abnormal QT. The seven patients with abnormal QT intervals, only had an abnormal QT for part of the 24‐h period, demonstrating the importance of sampling every hour for a 24‐h period. Given the significant implications of abnormal readings for patients on methadone maintenance, i.e. risks with continuing methadone treatment and the need to transfer to buprenorphine maintenance or reduce off methadone, more accurate assessment of the QT interval is essential. Transfer from methadone to buprenorphine maintenance can be difficult given the risk of precipitated withdrawal when transferring from high dose methadone 43, 44, and withdrawal off methadone is associated with increased opioid overdose risk 45.
There are a number of limitations of the study, including the potential for other prescription or illicit drugs to be taken during the 24 h period. Urine drug screening confirmed that many of the patients were taking cannabis and benzodiazepines, and a smaller group were using methamphetamines. Cannabis and benzodiazepine use have not been associated with QT interval prolongation. Methamphetamine is believed to prolong QT interval based on a single retrospective study of dependent methamphetamine users where only a single ECG was taken; there was no information on how the QT interval was measured and Bazett's correction was used in a population likely to have increased heart rates 46. Other studies of methamphetamine toxicity do not report QT interval prolongation 47, 48, and TdP has not be reported. There was one patient prescribed buprenorphine and confirmed methamphetamine use in this study, with a very rapid HR. This would certainly have an incorrectly assigned long QT interval if Bazett's formula was used for correction. However, plotting this on the QT nomogram demonstrates that it is in a region where the HR is so fast that accurate measurement of the QT interval is not possible and it is known that tachycardia is protective against TdP 29.
Conclusion
Methadone was associated with a prolonged QT interval in over one third of patients, but there was no association with dose. Buprenorphine did not prolong the QT interval. The use of 24‐h Holter recordings combined with the QT nomogram appears to be feasible in patients prescribed methadone and should be considered when assessing these patients for an abnormal QT interval.
Competing Interests
The are no competing interests to declare.
We thank the patients who participated in the study, Hunter New England Health clinical staff and Rohan Holland for recruiting patients.
G.K.I. is supported by an NHMRC Senior Research Fellowship 1061041. The study was funded by NSW Health Drug and Alcohol Research Grants Program.
Supporting information
Figure S1 Plots of QT vs. heart rate showing a visual representation of the line of abnormality for the QT nomogram (black line) comparing 129 positive cases of drug induced torsade de pointes (red circles) and 316 control cases (blue circles); modified from Chan et al., 2007 and Berling and Isbister, 2015
Figure S2 Individual patient plots of QT vs. heart rate showing the line of abnormality for the QT nomogram (black line), including the proportion (%) of abnormal QT intervals over the 24‐h period for each patient
Isbister, G. K. , Brown, A. L. , Gill, A. , Scott, A. J. , Calver, L. , and Dunlop, A. J. (2017) QT interval prolongation in opioid agonist treatment: analysis of continuous 12‐lead electrocardiogram recordings. Br J Clin Pharmacol, 83: 2274–2282. doi: 10.1111/bcp.13326.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE, et al The Concise Guide to PHARMACOLOGY 2015/16: Voltage-gated ion channels. Br J Pharmacol 2015; 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev 2009; 8: CD002209. [DOI] [PubMed] [Google Scholar]
- 4. Garcia‐Portilla MP, Bobes‐Bascaran MT, Bascaran MT, Saiz PA, Bobes J. Long term outcomes of pharmacological treatments for opioid dependence: Does methadone still lead the pack? Br J Clin Pharmacol 2014; 77: 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gowing LR, Hickman M, Degenhardt L. Mitigating the risk of HIV infection with opioid substitution treatment. Bull World Health Organ 2013; 91: 148–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turner KM, Hutchinson S, Vickerman P, Hope V, Craine N, Palmateer N, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction 2011; 106: 1978–1988. [DOI] [PubMed] [Google Scholar]
- 7. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014; 6: CD002207. [DOI] [PubMed] [Google Scholar]
- 8. Mathers BM, Degenhardt L, Ali H, Wiessing L, Hickman M, Mattick RP, et al. HIV prevention, treatment, and care services for people who inject drugs: A systematic review of global, regional, and national coverage. Lancet 2010; 375: 1014–1028. [DOI] [PubMed] [Google Scholar]
- 9. Isbister GK, Page CB. Drug induced QT prolongation: The measurement and assessment of the QT interval in clinical practice. Br J Clin Pharmacol 2013; 76: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MC. QTc interval screening in methadone treatment. Ann Intern Med 2009; 150: 387–395. [DOI] [PubMed] [Google Scholar]
- 11. Pearson EC, Woosley RL. QT prolongation and torsades de pointes among methadone users: reports to the FDA spontaneous reporting system. Pharmacoepidemiol Drug Saf 2005; 14: 747–753. [DOI] [PubMed] [Google Scholar]
- 12. Butler B, Rubin G, Lawrance A, Batey R, Bell J. Estimating the risk of fatal arrhythmia in patients in methadone maintenance treatment for heroin addiction. Drug Alcohol Rev 2011; 30: 173–180. [DOI] [PubMed] [Google Scholar]
- 13. Wieneke H, Conrads H, Wolstein J, Breuckmann F, Gastpar M, Erbel R, et al. Levo‐alpha‐acetylmethadol (LAAM) induced QTc‐prolongation ‐ results from a controlled clinical trial. Eur J Med Res 2009; 14: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deamer RL, Wilson DR, Clark DS, Prichard JG. Torsades de pointes associated with high dose levomethadyl acetate (ORLAAM). J Addict Dis 2001; 20: 7–14. [DOI] [PubMed] [Google Scholar]
- 15. McCance‐Katz EF. Opioids and cardiogram abnormalities: providing treatment based on understanding the risks and benefits. Addiction 2008; 103: 1994–1995. [DOI] [PubMed] [Google Scholar]
- 16. Fareed A, Patil D, Scheinberg K, Blackinton Gale R, Vayalapalli S, Casarella J, et al. Comparison of QTc interval prolongation for patients in methadone versus buprenorphine maintenance treatment: A 5‐year follow‐up. J Addict Dis 2013; 32: 244–251. [DOI] [PubMed] [Google Scholar]
- 17. Wedam EF, Bigelow GE, Johnson RE, Nuzzo PA, Haigney MC. QT‐interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch Intern Med 2007; 167: 2469–2475. [DOI] [PubMed] [Google Scholar]
- 18. Stallvik M, Nordstrand B, Kristensen O, Bathen J, Skogvoll E, Spigset O. Corrected QT interval during treatment with methadone and buprenorphine – relation to doses and serum concentrations. Drug Alcohol Depend 2013; 129: 88–93. [DOI] [PubMed] [Google Scholar]
- 19. Peles E, Linzy S, Kreek MJ, Adelson M. Prospective study of QTc changes among former opiate addicts since admission to methadone maintenance treatment: benzodiazepine risk. J Addict Med 2013; 7: 428–434. [DOI] [PubMed] [Google Scholar]
- 20. van den Beuken‐van Everdingen MH, Geurts JW, Patijn J. Prolonged QT interval by methadone: relevance for daily practice? A prospective study in patients with cancer and noncancer pain. J Opioid Manag 2013; 9: 263–267. [DOI] [PubMed] [Google Scholar]
- 21. Katz DF, Albright K, Krantz MJ. An ECG‐based cardiac safety initiative is well received by opioid treatment program staff: Results from a qualitative assessment. J Addict Dis 2013; 32: 387–392. [DOI] [PubMed] [Google Scholar]
- 22. Vallecillo G, Mojal S, Roquer A, Martinez D, Rossi P, Fonseca F, et al. Risk of QTc prolongation in a cohort of opioid‐dependent HIV‐infected patients on methadone maintenance therapy. Clin Infect Dis 2013; 57: 1189–1194. [DOI] [PubMed] [Google Scholar]
- 23. Ben Bassat OK, Peles E, Schreiber S, Adelson M, Zeltser D, Viskin S, et al. Response of QT interval in methadone maintenance treated patients to the rapid changes in heart rate provoked by brisk standing: comparison to healthy controls and patients with long QT syndrome. J Electrocardiol 2013; 46: 519–523. [DOI] [PubMed] [Google Scholar]
- 24. Chang KC, Huang CL, Liang HY, Chang SS, Wang YC, Liang WM, et al. Gender‐specific differences in susceptibility to low‐dose methadone‐associated QTc prolongation in patients with heroin dependence. J Cardiovasc Electrophysiol 2012; 23: 527–533. [DOI] [PubMed] [Google Scholar]
- 25. Mayet S, Gossop M, Lintzeris N, Markides V, Strang J. Methadone maintenance, QTc and torsade de pointes: Who needs an electrocardiogram and what is the prevalence of QTc prolongation? Drug Alcohol Rev 2011; 30: 388–396. [DOI] [PubMed] [Google Scholar]
- 26. Florian J, Garnett CE, Nallani SC, Rappaport BA, Throckmorton DC. A modeling and simulation approach to characterize methadone QT prolongation using pooled data from five clinical trials in MMT patients. Clin Pharmacol Ther 2012; 91: 666–672. [DOI] [PubMed] [Google Scholar]
- 27. Roy AK, McCarthy C, Kiernan G, McGorrian C, Keenan E, Mahon NG, et al. Increased incidence of QT interval prolongation in a population receiving lower doses of methadone maintenance therapy. Addiction 2012; 107: 1132–1139. [DOI] [PubMed] [Google Scholar]
- 28. Anchersen K, Clausen T, Gossop M, Hansteen V, Waal H. Prevalence and clinical relevance of corrected QT interval prolongation during methadone and buprenorphine treatment: a mortality assessment study. Addiction 2009; 104: 993–999. [DOI] [PubMed] [Google Scholar]
- 29. Chan A, Isbister GK, Kirkpatrick CM, Dufful SB. Drug‐induced QT prolongation and torsades de pointes: evaluation of a QT nomogram. QJM 2007; 100: 609–615. [DOI] [PubMed] [Google Scholar]
- 30. Calver L, Isbister GK. High dose droperidol and QT prolongation: analysis of continuous 12‐lead recordings. Br J Clin Pharmacol 2014; 77: 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fossa AA, Wisialowski T, Magnano A, Wolfgang E, Winslow R, Gorczyca W, et al. Dynamic beat‐to‐beat modeling of the QT‐RR interval relationship: analysis of QT prolongation during alterations of autonomic state versus human ether a‐go‐go‐related gene inhibition. J Pharmacol Exp Ther 2005; 312: 1–11. [DOI] [PubMed] [Google Scholar]
- 32. Berling I, Isbister GK. The half RR rule: a poor rule of thumb and not a risk assessment tool for QT interval prolongation. Acad Emerg Med 2015; 22: 1139–1144. [DOI] [PubMed] [Google Scholar]
- 33. Berling I, Isbister GK. Prolonged QT risk assessment in antipsychotic overdose using the QT nomogram. Ann Emerg Med 2015; 66: 154–164. [DOI] [PubMed] [Google Scholar]
- 34. Calver L, Page CB, Downes MA, Chan B, Kinnear F, Wheatley L, et al. The safety and effectiveness of droperidol for sedation of acute behavioral disturbance in the emergency department. Ann Emerg Med 2015; 66: 230–8 e1. [DOI] [PubMed] [Google Scholar]
- 35. Scott AJ, Dunlop AJ, Brown A, Sadler C, Isbister GK. The prevalence of QT prolongation in a population of patients with substance use disorders. Drug Alcohol Rev 2017; 36: 239–44. [DOI] [PubMed] [Google Scholar]
- 36. Malik M, Camm AJ. Evaluation of drug‐induced QT interval prolongation: implications for drug approval and labelling. Drug Saf 2001; 24: 323–351. [DOI] [PubMed] [Google Scholar]
- 37. Isbister GK, Calver L, van Gorp F, Stokes B, Page CB. Inter‐rater reliability of manual QT measurement and prediction of abnormal QT,HR pairs. Clin Toxicol (Phila) 2009; 47: 884–888. [DOI] [PubMed] [Google Scholar]
- 38. Friberg LE, Isbister GK, Duffull SB. Pharmacokinetic‐pharmacodynamic modelling of QT interval prolongation following citalopram overdoses. Br J Clin Pharmacol 2006; 61: 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Isbister GK, Balit CR, Macleod D, Duffull SB. Amisulpride overdose is frequently associated with QT prolongation and torsades de pointes . J Clin Psychopharmacol 2010; 30: 391–395. [DOI] [PubMed] [Google Scholar]
- 40. Ferrari A, Coccia CP, Bertolini A, Sternieri E. Methadone – metabolism, pharmacokinetics and interactions. Pharmacol Res 2004; 50: 551–559. [DOI] [PubMed] [Google Scholar]
- 41. Kao DP, Haigney MC, Mehler PS, Krantz MJ. Arrhythmia associated with buprenorphine and methadone reported to the Food and Drug Administration. Addiction 2015; 110: 1468–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harris SC, Morganroth J, Ripa SR, Thorn MD, Colucci S. Effects of buprenorphine on QT intervals in healthy subjects: results of 2 randomized positive‐ and placebo‐controlled trials. Postgrad Med 2017; 129: 69–80. [DOI] [PubMed] [Google Scholar]
- 43. Oretti R. A retrospective evaluation of inpatient transfer from high‐dose methadone to Buprenorphine substitution therapy. J Subst Abuse Treat 2015; 57: 102–105. [DOI] [PubMed] [Google Scholar]
- 44. Rosado J, Walsh SL, Bigelow GE, Strain EC. Sublingual buprenorphine/naloxone precipitated withdrawal in subjects maintained on 100 mg of daily methadone. Drug Alcohol Depend 2007; 90: 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kimber J, Larney S, Hickman M, Randall D, Degenhardt L. Mortality risk of opioid substitution therapy with methadone versus buprenorphine: a retrospective cohort study. Lancet Psychiatry 2015; 2: 901–908. [DOI] [PubMed] [Google Scholar]
- 46. Haning W, Goebert D. Electrocardiographic abnormalities in methamphetamine abusers. Addiction 2007; 102 (Suppl 1): 70–75. [DOI] [PubMed] [Google Scholar]
- 47. Richards JR, Bretz SW, Johnson EB, Turnipseed SD, Brofeldt BT, Derlet RW. Methamphetamine abuse and emergency department utilization. West J Med 1999; 170: 198–202. [PMC free article] [PubMed] [Google Scholar]
- 48. Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med 1998; 16: 567–573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Plots of QT vs. heart rate showing a visual representation of the line of abnormality for the QT nomogram (black line) comparing 129 positive cases of drug induced torsade de pointes (red circles) and 316 control cases (blue circles); modified from Chan et al., 2007 and Berling and Isbister, 2015
Figure S2 Individual patient plots of QT vs. heart rate showing the line of abnormality for the QT nomogram (black line), including the proportion (%) of abnormal QT intervals over the 24‐h period for each patient


