Abstract
Aim
Fixed dose oral tyrosine kinase inhibitors imatinib, sunitinib and pazopanib show a high interpatient variability in plasma exposure. A relationship between plasma exposure and treatment outcome has been established, which supports the rationale for dose optimization of these drugs. The aim of this study was to monitor how many patients reached adequate trough levels after therapeutic drug monitoring‐based dose optimization in daily practice.
Methods
A cohort study was performed in patients treated with imatinib, sunitinib or pazopanib of whom follow‐up drug levels were measured between August 2012 and April 2016. Patients' characteristics were collected by reviewing electronic patient records. Drug levels were measured using high‐performance liquid chromatography coupled with tandem mass spectrometry and trough levels were estimated using a predefined algorithm. Dose interventions were proposed based on trough levels.
Results
In total, 396 trough levels were determined in 109 patients. Median sample frequency per patient was 3. During the first measurement only 38% of patients showed trough levels within the predefined target ranges despite standard dosing; 52% of the patients showed drug levels below and 10% above the target range. In 35 out of 41 patients (85%) dose interventions led to adequate trough levels. Eventually, 64% of the total cohort reached adequate trough levels.
Conclusions
Dose optimization proved an effective tool to reach adequate trough levels in patients treated with imatinib, sunitinib and pazopanib. The percentage of patients with adequate trough levels increased from 38 to 64%. Therapeutic drug monitoring may add to the improvement of efficacy and reduction of toxicity and costs of these treatments.
Keywords: imatinib, individualized dosing, pazopanib, pharmacokinetics, sunitinib, therapeutic drug monitoring
What is Already Known about this Subject
The oral tyrosine kinase inhibitors imatinib, sunitinib and pazopanib show a high interpatient variability in pharmacokinetics but are all used with standard fixed doses.
Due to the correlation between systemic exposure and clinical benefit or toxicity, high variability in systemic exposure may have consequences for the treatment outcome.
What this Study Adds
Therapeutic drug monitoring led to an improvement of reaching the target trough levels from 38 to 64% in the total cohort.
Therapeutic drug monitoring of imatinib, sunitinib and pazopanib seems a feasible tool to individualize the dose and to increase the likelihood of treatment effectiveness and tolerability.
Introduction
The oral tyrosine kinase inhibitors (TKIs) imatinib, sunitinib and pazopanib have been approved at fixed doses for use in different types of cancer, e.g. renal cell cancer and gastrointestinal stromal tumour (GIST). However, these drugs show a high interpatient variability in plasma exposure which might affect treatment outcome. Interpatient variability of approximately 30–60% was found for imatinib, sunitinib and pazopanib plasma exposure 1, 2.
Several factors may influence the systemic exposure, including variability in oral drug absorption and metabolism, drug–drug interactions, food–drug interactions, patient nonadherence and decrease of plasma exposure over time during the first months of treatment 3, 4, 5. Imatinib, sunitinib and pazopanib are metabolized by cytochrome P450 enzyme (CYP) 3A4 in the liver. Therefore, combination with agents that inhibit or induce CYP3A4 may lead to increased or decreased plasma exposure of the TKIs 6, 7, 8. Moreover, imatinib and pazopanib are substrate of the transporter enzyme P‐glycoprotein, which may add to the interpatient variability observed due to drug–drug interactions or polymorphisms 6, 7, 8. In addition, the absorption of pazopanib in the gastrointestinal tract is dependent on a low gastric pH value and for this reason combination with drugs that inhibit gastric acid secretion (proton pump inhibitors, antacids, H2‐antagonists) may lead to decreased pazopanib absorption and plasma exposure 9. Additionally, the plasma exposure of pazopanib is nearly doubled when it is administered after a high‐fat meal instead of after a low‐fat meal or in fasted state 10, 11. Besides these known contributors to variability in plasma exposure also patient characteristics such as age, sex, bodyweight, renal function and liver function may contribute to variability in plasma exposure of TKIs. Despite these factors did not lead to clinically relevant changes in plasma exposure separately, a relevant cumulative effect on plasma exposure cannot be ruled out 2, 5, 12, 13, 14. Since many factors may influence the plasma exposure of imatinib, sunitinib and pazopanib, it is not possible to predict whether an individual patient will reach an adequate plasma exposure using a standard fixed dose of the drug.
For imatinib, sunitinib and pazopanib a relationship between plasma exposure and treatment outcome has been established. In a retrospective analysis of a randomized phase II study by Demetri et al. 15 patients with GIST treated with imatinib showed a better overall survival when the imatinib trough level was >1110 ng ml–1. Additionally, Larson et al. 16 showed retrospectively that the trough level of imatinib was significantly higher in chronic myeloid leukaemia patients who achieved a complete cytogenetic response with a trough level cut off value of 1009 ng ml–1. Since then, several other groups have established the same target exposure for imatinib associated with better treatment outcome 17, 18, 19, 20, 21, 22. Guilhot et al. 23 showed that there was an apparent association between high imatinib trough levels (>3180 ng ml–1) and the occurrence of grade 3/4 adverse effects. For sunitinib the target total trough plasma concentrations of sunitinib plus active metabolite N‐desethyl sunitinib have been deduced from preclinical data and are in the range of 50–100 ng ml–1 24, 25, 26. Subsequently, it was shown that total trough levels > 50 ng ml–1 have been associated with an objective response in a small cohort of patients with advanced solid malignancies treated with sunitinib 50 mg once daily for 4 weeks followed by a 2‐week drug holiday 27. In patients treated with sunitinib 37.5 mg continuously daily dosing minimal total trough levels of 37.5 ng ml–1 have been recommended based on the demonstrated efficacy of sunitinib 37.5 mg continuously daily dosing in GIST patients and dose proportionality of plasma exposure at therapeutic doses 28, 29. However, target trough concentrations for sunitinib plus active metabolite have not been confirmed in retrospective or prospective studies. For sunitinib, Faivre et al. 27 and Mizuno et al. 30 showed that dose limiting toxicities were most often seen in patients with sunitinib doses >75 mg day–1 and sunitinib + N‐desethylsunitinib trough concentrations >90 ng ml–1, respectively. In a retrospective study by Suttle et al., 31 pazopanib trough levels of 20.5 μg ml–1 were associated with improved efficacy in patients with metastatic renal cell carcinoma (mRCC). Additionally, posthoc analyses of the PAZOGIST study showed that trough pazopanib levels ≥20 μg ml–1 were associated with better progression free survival in patients with GIST 32. Lin et al. 33 showed an apparent association between high pazopanib trough levels (>46 μg l–1) and occurrence of adverse effects such as hand–foot syndrome. The standard fixed doses and the identified target trough plasma concentrations, based on retrospective and prospective analyses, are summarized in Table 1.
Table 1.
Tyrosine kinase inhibitor target ranges for trough levels
| Drug | Standard dose | Subgroup | Target range (μg l–1) | Level of evidence | References |
|---|---|---|---|---|---|
| Imatinib | 400 mg QD | GIST (metastatic or adjuvant) | 1100–3200 | Based on retrospective and prospective study | Demetri et al. 15, Bouchet et al. 22, Guilhot et al. 23 |
| 800 mg QD | GIST (cKIT exon 9 mutation) | 2200–3200 | Extrapolation from high dose treatment of cKIT exon 9 mutation patients | MetaGIST 35, Guilhot et al. 23 | |
| 400 mg QD | CML | 1000–3200 | Retrospective and prospective study | Larson et al. 16, Picard et al. 19, Guilhot et al. 23 | |
| Sunitinib + N‐desethyl sunitinib | 50 mg QD (intermittent dosing) | mRCC (intermittent dosing) | 50–87.5 | Extrapolation from preclinical data and retrospective analysis by PopPK modelling. | Houk et al. 12, Mendel et al. 25, Faivre et al. 27, Mizuno et al. 30, Yu et al. 28 |
| 37.5 mg QD (CDD) | mRCC (CDD) | 37.5–75 | Extrapolation from 50 mg QD regimen | Mendel et al. 25, Faivre et al. 27, De Wit et al. 46, Yu et al. 28 | |
| 37.5 mg QD | GIST | 37.5–75 | Extrapolation from 50 mg QD regimen | Houk et al. 12, De Wit et al. 46, Yu et al. 28 | |
| Pazopanib | 800 mg QD | mRCC and GIST | 20 500–46 000 | Based on retrospective studies | Suttle et al. 31, Mir et al. 32, Lin et al. 33 |
QD, once daily; GIST: gastrointestinal stromal tumour; CML: chronic myeloid leukaemia; mRCC: metastatic renal cell carcinoma; cKIT: proto‐oncogene cKIT; CDD: continuously daily dosing
Besides the relationship between exposure and clinical efficacy, also relationships between exposure and safety and tolerability have been established for these drugs 12, 16, 31, 34. Due to the correlation between systemic exposure and clinical benefit or toxicity, high variability in systemic exposure may have consequences for treatment outcome. This supports the rationale for therapeutic drug monitoring of imatinib, sunitinib and pazopanib. Dose adjustments based on trough plasma concentrations may contribute to optimization of therapy by reaching plasma concentrations within the therapeutic range in cancer patients. Despite the overwhelming evidence for concentration–effect relationships of these three agents, there has been little attention so far to evaluate the effectiveness of interventions aimed to achieve therapeutic concentrations. Without knowledge of effective interventions, the presence of a therapeutic range is meaningless. Therefore, the aim of this study was to monitor how many patients reached adequate trough levels of the drugs before and after dose optimization based on therapeutic drug monitoring in daily practice. The study was performed in a university medical centre in the Netherlands in which therapeutic drug monitoring of imatinib, sunitinib and pazopanib is nowadays performed as standard of care.
Methods
Patients
This retrospective cohort study was performed in patients treated with imatinib, sunitinib or pazopanib of whom multiple follow‐up drug levels were measured for routine therapeutic drug monitoring between August 2012 and April 2016. An overview of the applied strategy is shown in Figure 1. In two thirds of the patients, time after drug administration (interval between last dose intake and plasma sampling) was documented. Dosing scheme, indication and mutational status of the tumour in case of a GIST were collected by reviewing electronic patient records. Mutational status determines the optimal dosing of imatinib and the preferred treatment. High‐dose imatinib or sunitinib are more likely to give a better outcome in exon 9 metastatic GIST 35.
Figure 1.
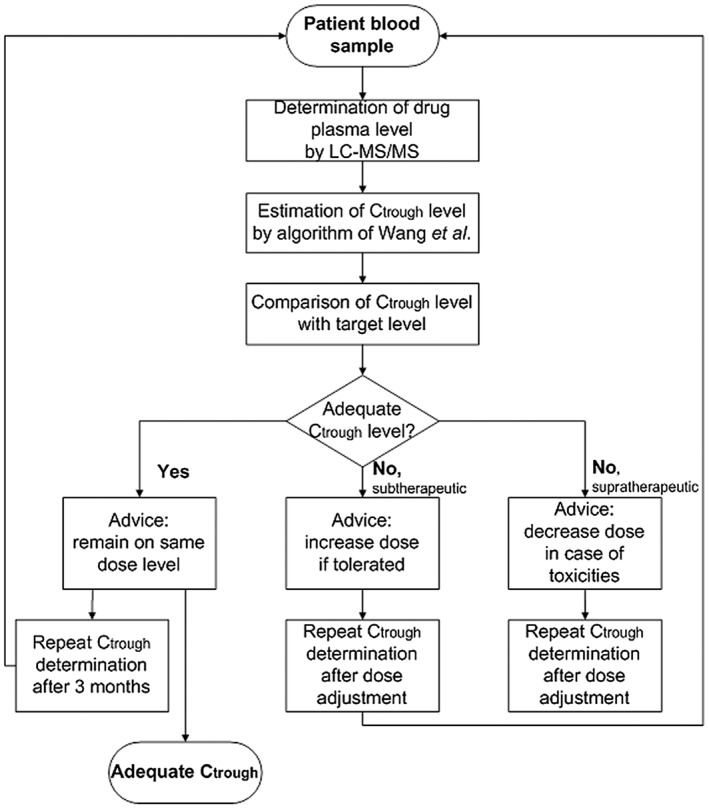
Flowchart of the applied strategy for therapeutic drug monitoring. (Ctrough: plasma trough level; LC–MS/MS: liquid chromatography coupled to tandem mass spectrometry)
Bioanalysis
Whole‐blood samples were collected in EDTA plasma tubes or clot activator serum tubes. Samples were centrifuged within 24 h of collection. EDTA plasma or serum was stored cold (4–8°C) until the day of analysis for a maximum of 14 days. Plasma or serum concentrations of imatinib, sunitinib, N‐desethyl sunitinib and pazopanib were determined by a previously validated high‐performance liquid chromatography coupled with tandem mass spectrometry detection method 36. The lower limits of quantification for imatinib, sunitinib, N‐desethyl sunitinib and pazopanib were 100 ng ml–1, 2 ng ml–1, 2 ng ml–1 and 1 μg ml–1, respectively.
Trough plasma concentrations
Blood samples were drawn during follow‐up visits at the outpatient clinic at least 7 to 14 days after start of therapy and after a dose intervention to make sure steady‐state pharmacokinetics was reached. Most patients took their medication early in the morning or late in the evening. Therefore, the plasma samples were drawn 12–24 h after the last dosage. Real trough plasma concentrations were not always available. Trough plasma concentrations were estimated, as previously proposed by Wang et al. 37, using the interval between last dose intake and blood sampling and the mean elimination half‐life of the drugs. The formulae used for this purpose were:
Conc 24h, Conc 12h = Ctrough = calculated plasma trough levels for drugs with once daily or twice daily administration, respectively.
Conc measured = measured drug plasma concentration in ng ml–1 or μg ml–1
Interval = interval between last dose intake and blood sampling in hours
t½ = mean terminal half‐life of the drug in hours
Formula 1 was used to calculate plasma trough levels for drugs with once daily (QD) administration, and formula 2 was used for drugs with twice‐daily administration. Mean elimination half‐lives used in the calculation were 18, 50 and 31 h for imatinib, sunitinib plus N‐desethyl sunitinib and pazopanib, respectively 37, 38, 39, 40. For each drug, target ranges for plasma concentrations were defined based on published data and are shown in Table 1.
Therapeutic drug monitoring
For each patient sample, trough plasma concentrations were compared with the predefined target trough concentrations. A hospital pharmacist formulated a dosing advice based on the plasma concentration outcome aiming to reach the target dose after implementation of this single advise, without fixed dose steps. The individual dosing advice was reported in the electronic patient record. For patients with trough plasma concentration within the target range the advice was to maintain the same dose level if toxicity was manageable. For patients with trough plasma concentrations below the target range, the advice was to increase the dose to a level that would be sufficient to reach target plasma concentrations based on linear pharmacokinetics. For patients with trough plasma concentration above the target range and signs of toxicity, the advice was to decrease the dose to a level that would be sufficient to reach plasma concentrations within the target range based on linear pharmacokinetics. The availability of dose units was taken into account by formulation of the dose advice. Sunitinib was used in doses from 12.5 mg QD per day to 75 mg QD. Because of the long elimination half time of sunitinib and its metabolite dose advices included also different doses every other day (e.g. 25 mg and 37.5 mg every other day). Imatinib was used in doses from 200 mg QD to 400 mg twice‐daily. Pazopanib was used in doses between 400 mg QD and 1000 mg QD. Additional advices for pazopanib dosing in order to increase the bioavailability included splitting the dose in a twice‐daily regimen (as suggested by Yu et al. 5) or administration in combination with breakfast instead of in fasted state (as suggested by Lubberman et al. 11).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 41, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 42.
Results
Patients
In total, 396 trough levels of imatinib, sunitinib and pazopanib were determined in 109 patients between August 2012 and April 2016. Median sample frequency per patient was 3 (range 2–13). Patient characteristics are presented in Table 2. Seventy patients were taking imatinib of whom 290 plasma concentrations were measured. Twenty‐seven patients using sunitinib were included, of whom 75 plasma concentrations were measured. Pazopanib was used by 12 patients of whom 31 plasma concentrations were measured.
Table 2.
Sample and treatment characteristics
| Imatinib (n = 70) | Sunitinib (n = 27) | Pazopanib (n = 12) | |
|---|---|---|---|
| Male sex, n (%) | 36 (51) | 23 (85) | 8 (75) |
| Age, median (range) | 63 (8–94) | 62 (26–77) | 62 (29–74) |
| Drug levels samples, n | 290 | 75 | 31 |
| Number of drug levels per patient, median (range) | 3 (2–13) | 2 (2–8) | 3 (2–3) |
| Indication, n | GIST: 68 | mRCC: 23 | mRCC: 8 |
| ALL: 1 | GIST: 4 | STS: 4 | |
| CML: 1 | |||
| Treatment goal, n (%) | Palliative: 47 (67) | NR | NR |
| Adjuvant: 19 (27) | |||
| Neoadjuvant: 4 (6) | |||
| Mutation in GIST | cKIT exon 11: 41 | ||
| cKIT exon 9: 4 | |||
| cKIT exon 17: 1 | |||
| BCR‐ABL: 1 | |||
| PDGFRA exon 18: 6 | |||
| PDGFRA exon 12: 1 | |||
| unknown: 16 |
GIST: gastrointestinal stromal tumour; ALL: acute lymphoblastic leukaemia; CML: chronic myeloid leukaemia; mRCC: metastatic renal cell carcinoma; STS: soft tissue sarcoma; cKIT: proto‐oncogene cKIT; BCR‐Abl: fusion gene of breakpoint cluster region gene and Abelson murine leukaemia viral oncogene; PDGFRA: platelet‐derived growth factor receptor‐α; NR: not relevant for therapeutic drug monitoring.
The indication for use of imatinib in this cohort was mainly GIST (68 patients; 97%). Additionally, one patient was treated with imatinib for Philadelphia‐chromosome positive acute lymphoblastic leukaemia, and one patient for chronic myeloid leukaemia. Two thirds of the patients (47 patients, 67%) were treated with imatinib as a palliative therapy in metastatic GIST. Twenty‐three patients (33%) were treated in a (neo‐) adjuvant setting. More than half of the patients with GIST (41 patients; 59%) had a tumour harbouring an activating mutation in cKIT exon 11 and four patients had a tumour harbouring a mutation in cKIT exon 9. In this cohort, sunitinib was primarily used in treatment of mRCC (23 patients; 85%). Four patients used sunitinib as a second line treatment for GIST. The main indication for pazopanib therapy was mRCC. In addition, four patients were treated with pazopanib for soft tissue sarcoma.
Therapeutic drug monitoring: trough levels and dose interventions
After start of treatment on the standard drug dose, the first trough level measurement showed that only 37.6% of patients had trough levels within the predefined target ranges (see Table 3 and Figure 2). From the patients treated with imatinib 29 patients (41.4%) showed an adequate trough level during the first trough level measurement. More than half of the patients (39 patients; 55.7%) showed subtherapeutic imatinib trough levels and two patients (2.9%) showed supratherapeutic trough levels. For sunitinib only a quarter of patients (seven patients; 25.9%) reached adequate trough levels during the first measurement, 14 patients (51.9%) showed subtherapeutic trough levels and six patients (22.2%) supratherapeutic trough levels. From the patients treated with pazopanib five patients (41.7%) had an adequate trough level during the first trough level measurement. Additionally, four patients (33.3%) showed subtherapeutic trough levels and three patients (25.0%) supratherapeutic trough levels. Overall, 52.3% of patients showed subtherapeutic and 10.1% of patients showed supratherapeutic trough levels during the first trough level measurement.
Table 3.
Pharmacokinetic measurements
| Imatinib (n = 70) | Sunitinib (n = 27) | Pazopanib (n = 12) | Total (n = 109) | |||||
|---|---|---|---|---|---|---|---|---|
| First | Last | First | Last | First | Last | First | Last | |
| C trough (μg l –1 or mg l –1 ), geometric mean (CV%) [95%CI] | 862 (70) [721–1031] | 1205 (52) [1064–1365] | 35.8 (92) [23.9–53.5] | 50.4 (35) [44.2–57.6] | 25.9 (64) [16.9–39.7] | 23.1 (67) [14.4–37.1] | NA | NA |
| Subtherapeutic C trough, n (%) | 39 (55.7) | 28 (40) | 14 (51.9) | 5 (18.5) | 4 (33.3) | 3 (25) | 57 (52.3) | 36 (33.0) |
| Supratherapeutic C trough, n (%) | 2 (2.9) | 0 (0) | 6 (22.2) | 2 (7.4) | 3 (25) | 1 (8.3) | 11 (10.1) | 3 (2.8) |
| Adequate C trough, n (%) | 29 (41.4) | 42 (60) | 7 (25.9) | 20 (74.1) | 5 (41.7) | 8 (66.7) | 41 (37.6) | 70 (64.2) |
Ctrough: estimated trough level; CI: confidence interval; CV: coefficient of variation; start: first pharmacokinetic sample per patient; end: last measured pharmacokinetic sample per patient.
Figure 2.
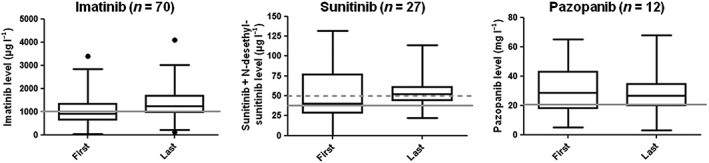
Boxplots representing median, lower and upper quartile plasma concentrations of imatinib, sunitinib plus desethylsunitinib and pazopanib measured during the first and last measurement. De grey lines represent the minimal trough level. The boxes represent the 25–75% percentiles and the whiskers represent the 2.5–97.5% percentiles
Dose interventions based on estimated trough levels were proposed in 72 (66.1%) patients and implemented in 41 (37.6%) patients. In total, 56.9% of the proposed dose interventions were implemented in clinical practice (see Table 4). From the patients with dose interventions 85.4% reached adequate trough levels within the study period. The effectiveness of dose interventions was highest in patients treated with imatinib. In this patient group 95% of implemented interventions led to adequate trough levels. In patients treated with sunitinib and pazopanib implemented dose interventions led to adequate trough levels in 76.4 and 75% of cases, respectively. For imatinib the percentage of patients in which the proposed interventions were implemented was the lowest, namely 46.5%. On the contrary, for sunitinib this percentage was the highest (77.3%).
Table 4.
Dose interventions per patient
| Imatinib (n = 70) | Sunitinib (n = 27) | Pazopanib (n = 12) | Total (n = 109) | |
|---|---|---|---|---|
| Dosage advices by hospital pharmacist (n = 109) | ||||
| Patients with the advice to maintain current dose, n (%) | 27 (38.6) | 5 (18.5) | 5 (41.7) | 37 (33.9) |
| Patients with dose adjustment advice, n (%) | 43 (61.4) | 22 (81.5) | 7 (58.3) | 72 (66.1) |
| Implementation of proposed dose interventions (n = 72) | ||||
| Patients for whom proposed interventions were implemented, n (% of proposed interventions) | 20 (46.5) | 17 (77.3) | 4 (57.1) | 41 (56.9) |
| Patients for whom proposed interventions were not implemented, n (% of proposed interventions) | 23 (43.5) | 5 (22.7) | 3 (42.9) | 31 (43.1) |
| Effect of dose interventions (n = 41) | ||||
| Patients with adequate C trough after intervention, n (% of implemented interventions) | 19 (95.0) | 13 (76.5) | 3 (75.0) | 35 (85.4) |
| Patients with inadequate C trough despite intervention, n (% of implemented interventions) | 1 (5%) | 4 (23.5) | 1 (25.0) | 6 (14.6) |
GIST: gastrointestinal stromal tumour; ALL: acute lymphoblastic leukaemia; CML: chronic myeloid leukaemia; mRCC: metastatic renal cell carcinoma; STS: soft tissue sarcoma; cKIT: proto‐oncogene cKIT; BCR‐Abl: fusion gene of breakpoint cluster region gene and Abelson murine leukaemia viral oncogene; PDGFRA: platelet‐derived growth factor receptor‐α; Ctrough: trough level.
Eventually, 64.2% of patients reached trough levels within the predefined target ranges after dose optimization (imatinib: 60%; sunitinib: 74.1%; pazopanib: 66.7%). For imatinib, 28 patients (40.0%) showed subtherapeutic trough levels during the last measurement and none of the patients had supratherapeutic imatinib trough levels. Within the group of sunitinib patients, the number of patients with subtherapeutic and supratherapeutic trough levels decreased to five patients (18.5%) and two patients (7.4%), respectively. For pazopanib, one patient (8.3%) remained with a supratherapeutic trough level and three patients (25.0%) with a subtherapeutic trough level.
The geometric mean imatinib trough level increased from 862 μg l–1 during the first measurement to 1205 μg l–1 during the last measurement. The interpatient variability [coefficient of variation (CV%)] of the first and last imatinib trough levels decreased from 70 to 52%. The geometric mean sunitinib trough levels increased from 35.8 to 50.4 μg l–1. Additionally, the interpatient variability of trough levels decreased markedly from 92 to 35%. The mean pazopanib trough level decreased with approximately 10% from 25.9 to 23.1 mg l–1. Additionally, the interpatient variabilities between the first and last measurements were comparable with 64 and 67%, respectively.
Discussion
The objective of this study was to evaluate the effect of therapeutic drug monitoring in daily practice by monitoring how many patients reached adequate trough levels of imatinib, sunitinib and pazopanib before and after dose recommendations. Multiple follow‐up trough levels were measured in 109 cancer patients. Initially, only 38% of patients had an adequate trough level. Of the patients undergoing dose intervention based on trough level, 85% of patients reached an adequate trough level within the study period. In the total cohort (patients with and without implemented dose interventions), the percentage of patients with adequate trough levels increased from 38 to 64%. This implies that measurement of plasma drug levels might add to the improvement of efficacy and reduced toxicity of patients treated with imatinib, sunitinib and pazopanib.
The rationale for this study was based on the demonstrated plasma exposure–response relationship for imatinib, sunitinib and pazopanib, and the large interpatient variability observed for these drugs in clinical practice. Therefore, it was reasoned that fixed dosing may lead to underdosing or overdosing due to low or high drug exposure. However, a limitation is that the predefined target trough levels have not been established in large prospective randomized clinical trials thus far but are derived from retrospective analyses. Therefore, the extent to which dose individualization has an impact on treatment outcome/toxicity it is not known yet. Likewise, in our study we did not correlate plasma exposure to treatment outcome, since we studied a small cohort of patients covering a large variety of solid tumours and no control group. However, target levels for these drugs have been demonstrated in various studies and, therefore, the predefined target levels could be regarded appropriate for use in this study.
The algorithm used to estimate plasma trough levels in this study was previously described by Wang et al. 37 . With this method imatinib trough levels of plasma samples obtained at different time points in the elimination phase can be estimated with acceptable deviations from real measured trough levels 37. Since the time to peak plasma level for imatinib, suntinib and pazopanib are comparable and the elimination half‐lives of sunitinib and pazopanib are even longer than the elimination half‐life of imatinib, it was justified to use this method of Wang et al. 37 to calculate imatinib, sunitinib and pazopanib trough levels in our study.
During the first measurement only 25.9% of patients treated with sunitinib had an adequate trough level. This percentage is much lower than the percentages of 48 and 51% that were described in previous cohort and dose optimization studies 2, 43. This discrepancy is probably due to the fact that the previous studies only defined minimal effective target levels (>50 ng ml–1) and the current study also included supratherapeutic trough levels, which were observed in 22.2% of the patients. Combining all patients with sunitinib trough levels above the minimal effective target level results in 48.1% of patients which is comparable to previous studies. Moreover, in the first part of the study period, some selection bias could have been introduced for imatinib and sunitinib since TDM was not performed as standard of care in those days, and TDM was mainly performed in case of suspected toxicity or suboptimal drug response. Thus, there could have been included a disproportional amount of more extreme trough levels. This selection bias could possibly explain the low percentage of adequate trough levels in patients treated with sunitinib and could possibly overestimate the effect of therapeutic drug monitoring in this patient population. However, the effect of selection bias seemed less apparent for imatinib and pazopanib. Adequate imatinib trough levels were reached in 41% during the first trough level measurement and this percentage is comparable to the most recent cohort studies (44%) 44. Lastly, the percentage of patients on pazopanib with adequate trough levels was also comparable to a previous study (41.7 vs. 43.3%) 45.
The interpatient variability (CV%) for sunitinib (70%) and imatinib (90%) trough levels at starting point of our cohort was much higher than described in previous studies (approximately 35%). This can be explained by the fixed dose regimen on which the interpatient variation was based in these studies: sunitinib was dosed at 37.5 mg QD and imatinib at 400 mg QD. In our cohort, however, different dosing schedules of sunitinib and imatinib were used. The starting dose for sunitinib was 37.5 mg QD (continuously daily dosing) or 50 mg QD (intermittent dosing) and starting dose for imatinib was 400 mg QD (sensitive mutations) or 800 mg QD (less sensitive cKit mutation). In addition, selection bias as mentioned above can be an explanation for the high interpatient variability. The interpatient variability for pazopanib in our study was comparable to the study of Verheijen et al. (64 vs. 72%) 45. The intended effect of individualized dosing based on trough levels is to minimize the interpatient variability in trough levels, and thereby to reduce toxicity and to increase efficacy. The largest decrease in interpatient variability (CV%) was seen for sunitinib (from 92 to 35%). This large effect is possibly related to the large percentage of implemented dose advices for sunitinib. The interpatient variability for imatinib decreased from 70 to 52%. As mentioned above, the large variability for this drug can be explained by the two dosing schemes with large difference in daily dose. Remarkably, the interpatient variability for pazopanib did not alter after therapeutic drug monitoring, which possibly can be explained by the low percentage of follow up of dose advices or the difficulty to overcome the limited gastrointestinal absorption of pazopanib 5, 46. Moreover, as shown in previously published data the intrapatient variability of pazopanib compared to the interpatient is relatively large (24.7 vs. 27.3%) and it is known that the utility of therapeutic drug monitoring is limited for drugs with this pharmacokinetic characteristic 46. The intrapatient variability compared to the interpatient variability for the other drugs is relatively modest (26.5 vs. 44.7% for imatinib and 16.2 vs. 42.3% for sunitinib, respectively) and therefore utility of therapeutic drug monitoring is expected to be larger 47, 48.
The percentage of patients that reached adequate trough levels increased from 38% to 64% during this study. The reason that the proportion of patients with adequate trough levels at the end of the study is not larger is mainly explained by the duration of the study period. At the end of the study period, the dose optimization was not yet completed for all patients. Additionally, it can be due to patient nonadherence, no follow‐up of advice given by the hospital pharmacist to the treating oncologist, or the tendency of plasma exposure to decrease after long‐term treatment as was previously shown for imatinib, sorafenib and pazopanib 3, 5, 49, 50. Reasons for no follow‐up of advice, as in 31 of 72 advices in this study, were, e.g. expected intolerability of higher doses, activity of the treatment based on radiological evaluation and lack of evidence of higher dose imatinib in GIST in the adjuvant setting.
The current study retrospectively assessed the effect of therapeutic drug monitoring on the number of patients that reached adequate trough levels of imatinib, sunitinib and pazopanib. It was shown that adequate trough levels can be reached using TKI dosing based on trough levels. Therefore, therapeutic drug monitoring may already be used to support clinical decision making in daily practice, when physicians suspect inadequate trough levels based on toxicity or lack of response. However, since the effect of reaching adequate trough levels was not correlated to treatment outcome in this exploratory study, effect of therapeutic drug monitoring on treatment efficacy and safety cannot be established yet. Future research is necessary to find an answer to this research question. At present, there are some initiatives to study treatment effectiveness and tolerability using therapeutic drug monitoring, prospectively. One of the initiatives is a national study assessing the clinical outcomes in GIST patients treated with TKIs and therapeutic drug monitoring compared with matched‐controls that receive standard of care (NCT02331914).
In conclusion, the number of patients that reached adequate trough levels of imatinib, sunitinib or pazopanib roughly increased from one third to two thirds of the patients after dose optimization based on trough levels in daily practice. Therefore, therapeutic drug monitoring of these drugs seems a feasible tool to individualize the dose and to increase the likelihood of treatment effectiveness and tolerability in daily practice. Further research is needed to establish the therapeutic efficacy and safety of imatinib, sunitinib and pazopanib dosing based on trough levels instead of standard fixed doses.
Competing Interests
The authors declare no potential conflicts of interest.
Lankheet, N. A. G. , Desar, I. M. E. , Mulder, S. F. , Burger, D. M. , Kweekel, D. M. , van Herpen, C. M. L. , van der Graaf, W. T. A. , and van Erp, N. P. (2017) Optimizing the dose in cancer patients treated with imatinib, sunitinib and pazopanib. Br J Clin Pharmacol, 83: 2195–2204. doi: 10.1111/bcp.13327.
References
- 1. de Wit D, Guchelaar HJ, den Hartigh J, Gelderblom H, van Erp NP. Individualized dosing of tyrosine kinase inhibitors: are we there yet? Drug Discov Today 2015; 20: 18–36. [DOI] [PubMed] [Google Scholar]
- 2. Lankheet NA, Knapen LM, Schellens JH, Beijnen JH, Steeghs N, Huitema AD. Plasma concentrations of tyrosine kinase inhibitors imatinib, erlotinib, and sunitinib in routine clinical outpatient cancer care. Ther Drug Monit 2014; 36: 326–334. [DOI] [PubMed] [Google Scholar]
- 3. Eechoute K, Fransson MN, Reyners AK, de Jong FA, Sparreboom A, van der Graaf WT, et al. A long‐term prospective population pharmacokinetic study on imatinib plasma concentrations in GIST patients. Clin Cancer Res 2012; 18: 5780–5787. [DOI] [PubMed] [Google Scholar]
- 4. Klumpen HJ, Samer CF, Mathijssen RH, Schellens JH, Gurney H. Moving towards dose individualization of tyrosine kinase inhibitors. Cancer Treat Rev 2011; 37: 251–260. [DOI] [PubMed] [Google Scholar]
- 5. Yu H, van Erp N, Bins S, Mathijssen RH, Schellens JH, Beijnen JH, et al. Development of a pharmacokinetic model to describe the complex pharmacokinetics of Pazopanib in cancer patients. Clin Pharmacokinet 2017; 56: 293–303. [DOI] [PubMed] [Google Scholar]
- 6. Herbrink M, Nuijen B, Schellens JH, Beijnen JH. Variability in bioavailability of small molecular tyrosine kinase inhibitors. Cancer Treat Rev 2015; 41: 412–422. [DOI] [PubMed] [Google Scholar]
- 7. van Erp NP, Gelderblom H, Guchelaar HJ. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev 2009; 35: 692–706. [DOI] [PubMed] [Google Scholar]
- 8. van Leeuwen RW, van Gelder T, Mathijssen RH, Jansman FG. Drug‐drug interactions with tyrosine‐kinase inhibitors: a clinical perspective. Lancet Oncol 2014; 15: e315–e326. [DOI] [PubMed] [Google Scholar]
- 9. Tan AR, Gibbon DG, Stein MN, Lindquist D, Edenfield JW, Martin JC, et al. Effects of ketoconazole and esomeprazole on the pharmacokinetics of pazopanib in patients with solid tumors. Cancer Chemother Pharmacol 2013; 71: 1635–1643. [DOI] [PubMed] [Google Scholar]
- 10. Heath EI, Chiorean EG, Sweeney CJ, Hodge JP, Lager JJ, Forman K, et al. A phase I study of the pharmacokinetic and safety profiles of oral pazopanib with a high‐fat or low‐fat meal in patients with advanced solid tumors. Clin Pharmacol Ther 2010; 88: 818–823. [DOI] [PubMed] [Google Scholar]
- 11. Lubberman F, Gelderblom H, Jansman F, Colbers A, Van der Graaf WTA, Mulders P, et al. Food intervention to make therapy with pazopanib more patient‐friendly and affordable. J Clin Oncol 2016; 34 (suppl): abstr 11040. [Google Scholar]
- 12. Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta‐analysis. Cancer Chemother Pharmacol 2010; 66: 357–371. [DOI] [PubMed] [Google Scholar]
- 13. Widmer N, Decosterd LA, Csajka C, Leyvraz S, Duchosal MA, Rosselet A, et al. Population pharmacokinetics of imatinib and the role of alpha‐acid glycoprotein. Br J Clin Pharmacol 2006; 62: 97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu H, Steeghs N, Kloth JS, de Wit D, van Hasselt JG, van Erp NP, et al. Integrated semi‐physiological pharmacokinetic model for both sunitinib and its active metabolite SU12662. Br J Clin Pharmacol 2015; 79: 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demetri GD, Wang Y, Wehrle E, Racine A, Nikolova Z, Blanke CD, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol 2009; 27: 3141–3147. [DOI] [PubMed] [Google Scholar]
- 16. Larson RA, Druker BJ, Guilhot F, O'Brien SG, Riviere GJ, Krahnke T, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic‐phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood 2008; 111: 4022–4028. [DOI] [PubMed] [Google Scholar]
- 17. Awidi A, Ayed AO, Bsoul N, Magablah A, Mefleh R, Dweiri M, et al. Relationship of serum imatinib trough level and response in CML patients: long term follow‐up. Leuk Res 2010; 34: 1573–1575. [DOI] [PubMed] [Google Scholar]
- 18. Koren‐Michowitz M, Volchek Y, Naparstek E, Gavish I, Levi I, Rowe JM, et al. Imatinib plasma trough levels in chronic myeloid leukaemia: results of a multicentre study CSTI571AIL11TGLIVEC. Hematol Oncol 2012; 30: 200–205. [DOI] [PubMed] [Google Scholar]
- 19. Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard MA, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard‐dose imatinib in chronic myeloid leukemia. Blood 2007; 109: 3496–3499. [DOI] [PubMed] [Google Scholar]
- 20. Singh N, Kumar L, Meena R, Velpandian T. Drug monitoring of imatinib levels in patients undergoing therapy for chronic myeloid leukaemia: comparing plasma levels of responders and non‐responders. Eur J Clin Pharmacol 2009; 65: 545–549. [DOI] [PubMed] [Google Scholar]
- 21. Takahashi N, Wakita H, Miura M, Scott SA, Nishii K, Masuko M, et al. Correlation between imatinib pharmacokinetics and clinical response in Japanese patients with chronic‐phase chronic myeloid leukemia. Clin Pharmacol Ther 2010; 88: 809–813. [DOI] [PubMed] [Google Scholar]
- 22. Bouchet S, Poulette S, Titier K, Moore N, Lassalle R, Abouelfath A, et al. Relationship between imatinib trough concentration and outcomes in the treatment of advanced gastrointestinal stromal tumours in a real‐life setting. Eur J Cancer 2016; 57: 31–38. [DOI] [PubMed] [Google Scholar]
- 23. Guilhot F, Hughes TP, Cortes J, Druker BJ, Baccarani M, Gathmann I, et al. Plasma exposure of imatinib and its correlation with clinical response in the tyrosine kinase inhibitor optimization and selectivity trial. Haematologica 2012; 97: 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet‐derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther 2003; 2: 471–478. [PubMed] [Google Scholar]
- 25. Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet‐derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 2003; 9: 327–337. [PubMed] [Google Scholar]
- 26. Murray LJ, Abrams TJ, Long KR, Ngai TJ, Olson LM, Hong W, et al. SU11248 inhibits tumor growth and CSF‐1R‐dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis 2003; 20: 757–766. [DOI] [PubMed] [Google Scholar]
- 27. Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 2006; 24: 25–35. [DOI] [PubMed] [Google Scholar]
- 28. Yu H, Steeghs N, Nijenhuis CM, Schellens JH, Beijnen JH, Huitema AD. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. Clin Pharmacokinet 2014; 53: 305–325. [DOI] [PubMed] [Google Scholar]
- 29. George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, Deprimo SE, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer 2009; 45: 1959–1968. [DOI] [PubMed] [Google Scholar]
- 30. Mizuno T, Fukudo M, Terada T, Kamba T, Nakamura E, Ogawa O, et al. Impact of genetic variation in breast cancer resistance protein (BCRP/ABCG2) on sunitinib pharmacokinetics. Drug Metab Pharmacokinet 2012; 27: 631–639. [DOI] [PubMed] [Google Scholar]
- 31. Suttle AB, Ball HA, Molimard M, Hutson TE, Carpenter C, Rajagopalan D, et al. Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Br J Cancer 2014; 111: 1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mir O, Cropet C, Toulmonde M, Cesne AL, Molimard M, Bompas E, et al. Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): a randomised, multicentre, open‐label phase 2 trial. Lancet Oncol 2016; 17: 632–641. [DOI] [PubMed] [Google Scholar]
- 33. Lin Y, Ball HA, Suttle B, Mehmud F, Amado RG, Hutson TE, et al Relationship between plasma pazopanib concentration and incidence of adverse events in renal cell carcinoma. Genitourinary Cancer Symposium 2011, 2011.
- 34. Widmer N, Decosterd LA, Leyvraz S, Duchosal MA, Rosselet A, Debiec‐Rychter M, et al. Relationship of imatinib‐free plasma levels and target genotype with efficacy and tolerability. Br J Cancer 2008; 98: 1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. MetaGIST . Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta‐analysis of 1,640 patients. J Clin Oncol 2010; 28: 1247–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Erp NP, de Wit D, Guchelaar HJ, Gelderblom H, Hessing TJ, Hartigh J. A validated assay for the simultaneous quantification of six tyrosine kinase inhibitors and two active metabolites in human serum using liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2013; 937: 33–43. [DOI] [PubMed] [Google Scholar]
- 37. Wang Y, Chia YL, Nedelman J, Schran H, Mahon FX, Molimard M. A therapeutic drug monitoring algorithm for refining the imatinib trough level obtained at different sampling times. Ther Drug Monit 2009; 31: 579–584. [DOI] [PubMed] [Google Scholar]
- 38. EMA . Sutent; EPAR‐Product Information 2008. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/000687/WC500057737.pdf (last accessed 2013 August).
- 39. EMA . Glivec; EPAR‐Product Information 2009. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/000406/WC500022207.pdf (last accessed 2013 July 2nd 2013).
- 40. EMA . Votrient, EPAR‐Product Information 2010. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/001141/WC500094272.pdf (last accessed 2013 August).
- 41. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 2016; 44 (D1): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alexander SP, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 2015; 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lankheet NA, Kloth JS, Gadellaa‐van Hooijdonk CG, Cirkel GA, Mathijssen RH, Lolkema MP, et al. Pharmacokinetically guided sunitinib dosing: a feasibility study in patients with advanced solid tumours. Br J Cancer 2014; 110: 2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Farag S, Verheijen RB, Martijn Kerst J, Cats A, Huitema AD, Steeghs N. Imatinib pharmacokinetics in a large observational cohort of gastrointestinal stromal tumour patients. Clin Pharmacokinet 2017; 287: 287–292. [DOI] [PubMed] [Google Scholar]
- 45. Verheijen RB, Bins S, Mathijssen RH, Lolkema MP, van Doorn L, Schellens JH, et al. Individualized Pazopanib dosing: a prospective feasibility study in cancer patients. Clin Cancer Res 2016; 22: 5738–5746. [DOI] [PubMed] [Google Scholar]
- 46. de Wit D, van Erp NP, den Hartigh J, Wolterbeek R, den Hollander‐van Deursen M, Labots M, et al. Therapeutic drug monitoring to individualize the dosing of pazopanib: a pharmacokinetic feasibility study. Ther Drug Monit 2015; 37: 331–338. [DOI] [PubMed] [Google Scholar]
- 47. Yoo C, Ryu MH, Kang BW, Yoon SK, Ryoo BY, Chang HM, et al. Cross‐sectional study of imatinib plasma trough levels in patients with advanced gastrointestinal stromal tumors: impact of gastrointestinal resection on exposure to imatinib. J Clin Oncol 2010; 28: 1554–1559. [DOI] [PubMed] [Google Scholar]
- 48. Zhang AYF, Fox P, Coulter S, Balakrishnar B, Liddle C, Gurney H. Effect of toxicity‐adjusted dose (TAD) of sunitinib on intra‐patient variation of trough levels: a longitudinal study in metastatic renal cell cancer (mRCC). J Clin Oncol 2014; 32 (15s): 2579. [Google Scholar]
- 49. Arrondeau J, Mir O, Boudou‐Rouquette P, Coriat R, Ropert S, Dumas G, et al. Sorafenib exposure decreases over time in patients with hepatocellular carcinoma. Invest New Drugs 2012; 30: 2046–2049. [DOI] [PubMed] [Google Scholar]
- 50. Boudou‐Rouquette P, Ropert S, Mir O, Coriat R, Billemont B, Tod M, et al. Variability of sorafenib toxicity and exposure over time: a pharmacokinetic/pharmacodynamic analysis. Oncologist 2012; 17: 1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]


