Abstract
Seed germination behavior is an important factor in the distribution of species. Many studies have shown that germination is controlled by phylogenetic constraints, however, it is not clear whether phylogenetic constraints or environmental cues explain seed germination of a genus from a common ancestor. In this study, seed germination under different temperature- and water-regimes [induced by different osmotic potentials of polyethylene glycol (PEG)] was investigated in the phylogenetically-related Caragana species that thrive in arid, semiarid, semihumid and humid environments. The results showed that the final percentage germination (FPG) decreased from 95% in species from arid habitats to 0% in species from humid habitats, but with no significant phylogenetic signal. Rather, the response of seed germination to temperature and PEG varied greatly with species from arid to humid habitats and was tightly linked to the ecological niche of the species, their seed coat structure and abscisic acid concentration. The findings are not consistent with the hypothesis that within a family or a genus, seed germination strategies can be a stable evolutionary trait, thus constraining interspecific variation, but the results clearly show that seed germination of Caragana species distributed across a range of habitats has adapted to the environment of that habitat.
Introduction
The ‘how and why’ of plant species’ distribution has been a subject of fundamental importance throughout the history of plant ecology1. Among several traits that account for species’ distribution, the germination of the seed is a critical step in the life cycle of a plant2–4 that is particularly vulnerable to environmental stress3, 5, 6. The most favorable period for seed germination of a species varies according to it’s geographic distribution and life cycle7, 8. Therefore, investigation of seed behaviour of species in response to a combination of biotic and abiotic factors may help to understand factors related to the distribution of the species5.
Among biotic factors, it is considered that seed germination is genetically determined and the phylogenetic signal is a significant constraint, termed a phylogenetic constraint6, 9, to the evolution and expression of seed traits. As a result of phylogenetic constraints, closely-related species do not move too far from their optimum niche and share similar seed-germination traits or niche preferences6, 9, 10. For example, when phylogenetic analysis is used to infer the evolution, there is strong evidence of phylogenetic constraints on seed size11. As seed size is positively correlated with mean time to germination, this suggests that seed germination is likely to be a phylogenetically-conserved trait3. Zhang et al.6 found that closely-related species shared similar germination times (temporal niche preferences). Romulea species that grow in different Mediterranean habitats exhibit strong phylogenetic constraints on the phenology of seed germination regardless of their habitat of origin12, and species in North American forests have germination strategies that match those of co-genetic species presently occurring in East Asia13, 14. These findings lend support to the hypothesis that within a family or a genus, germination strategies can be a stable evolutionary trait, thus constraining interspecific variation in germination6, 9, 10, 12. However, other studies have reported that seed germination differs within a family or a genus. For example, Stellaria species that grow in dry grasslands and in shady deciduous forests have some germinating seeds, while those that grow in open forest have completely dormant seeds at maturity in early summer15. Similar to Stellaria species, Nothofagus species along altitudinal gradient produce seeds that vary considerably in their germination1. These results, at least to some degree, indicate a smaller phylogenetic constraint on seed germination within a family or a genus, but how widespread this is still needs to be confirmed.
Among abiotic factors, temperature and water status are two of the most important factors that regulate seed germination1, 16. Studies have shown that the seed germination of species from distinct environments vary significantly in response to various temperature and water regimes1, 8, 17–20, that is, species have different optimal temperatures for seed germination and different abilities to germinate at low water potential8, 16, 19, the two main influences likely to account for species’ distribution1. For species from the same genus, seed germination traits adapt to local ecological factors, and species-specific germination preferences are different15, 21, 22. However, it is not clear whether seed germination, and consequently species’ distribution, of species that have evolved from the same ancestor, but diversified to distinct environments, have been strongly shaped by environmental cues.
The seed coat acts as a barrier to water permeability23–25, while abscisic acid (ABA) plays a important role in the induction and maintenance of seed dormancy24, 26–28 that can be reversed by gibberellic acid (GA3)28, so that both permeability and dormancy play an important role in seed germination. Therefore, if the phylogenetic signal constrains germination, seed coat structure and seed ABA content within a family or a genus are likely to be similar, while if environmental cues shape germination, they are likely to vary.
Caragana species have the same ancestor, but radiated from arid areas of northern China to humid and forested areas of East Asia and differentiated into different species depending on whether the habitats were arid, semiarid, semihumid or humid29, 30. These provide a valuable resource for a comparative study of the influence of phylogenetic constraints and environmental cues on seed germination. In this study, we hypothesized that over the wide range of environments, from arid to humid, in which the Caragana genus has evolved, the different species would exhibit great variation and small phylogenetic constraint in seed germination, thereby reflecting the distinct habitats and bioclimatic areas in which they occur. Specific questions were (1) whether phylogenetic constraints limited seed germination of Caragana species; if not, then: (2) whether the responses of seed germination to temperature and water status (using polyethylene glycol 6000) differed greatly, therefore reflecting the distinct habitats and bioclimatic environments in which they are distributed; if yes, (3) whether the variation in seed germination was related to differentiation in seed coat structure and ABA content; and (4) whether GA3 can reverse the effects of ABA.
Results
Site germination and phylogenetic signal
Using the tetrazolium test, the ratio of viable seeds to total seeds in all Caragana species was greater than 90%. While the mean daytime temperature during germination varied from only 17 °C to 20 °C, the final germination percentage (FGP) of well-watered seeds at the sites of collection varied greatly among species, decreasing from 95% in species from arid habitats to 0% in species from humid habitats (Fig. 1). There was no significant phylogenetic signal in FPG (Table 1) based on observed Page’s λ = 0 (P = 0.99 against likelihood estimate with λ set to 0).
Figure 1.
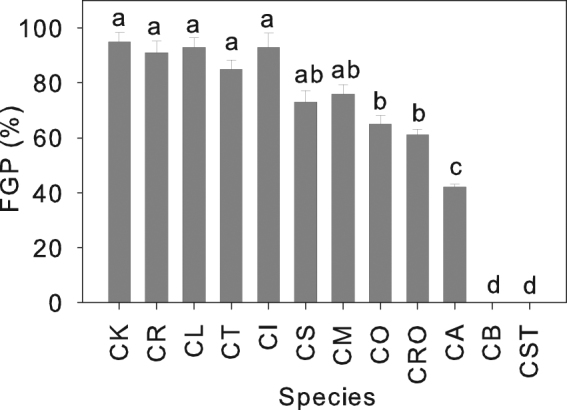
Final germination percentage (FGP) in seeds of 12 Caranaga species. Seeds of four species [C. korshinskii (CK), C. roborovskyi (CR), C. leucophloea (CL) and C. tibetica (CT)] from arid habitats, three species [C. intermedia (CI), C. stenophylla (CS) and C. microphylla (CM)] from semiarid habitats, four species [C. opulens (CO), C. rosea (CRO), C. arborescens (CA) and C. boisi (CB)] from semihumid habitats and one species [C. stipitata (CST)] from a humid habitat were incubated under well-watered conditions at the site of collection in Petri dishes covered with soil to keep the seeds in the dark. The mean daytime temperatures during germination varied from 17 to 20 °C. Data are means + one SE of the mean (n = 5). Species with different letters are significantly different from each other (P < 0.05).
Table 1.
Phylogenetic signal of seed germination traits based on the phylogenetic tree (Supplementary Fig. S1) of 12 Caragana species.
| Trait | n | observed λ | P 0 Value (against λ = 0) | P 1 Value (against λ = 1) |
|---|---|---|---|---|
| FGP | 12 | 0 | 0.99 | <0.0001 |
n, number of species; observed λ, likelihood estimate of observed λ; P 0, likelihood estimate with λ set to 0 (no phylogenetic signal); P 1, likelihood estimate with λ set to 1 (maximum phylogenetic signal); FGP, final germination percentage. The phylogenetic signal was estimated as Pagel’s λ using a maximum likelihood framework (see Statistical analysis in Methods).
Germination under controlled conditions
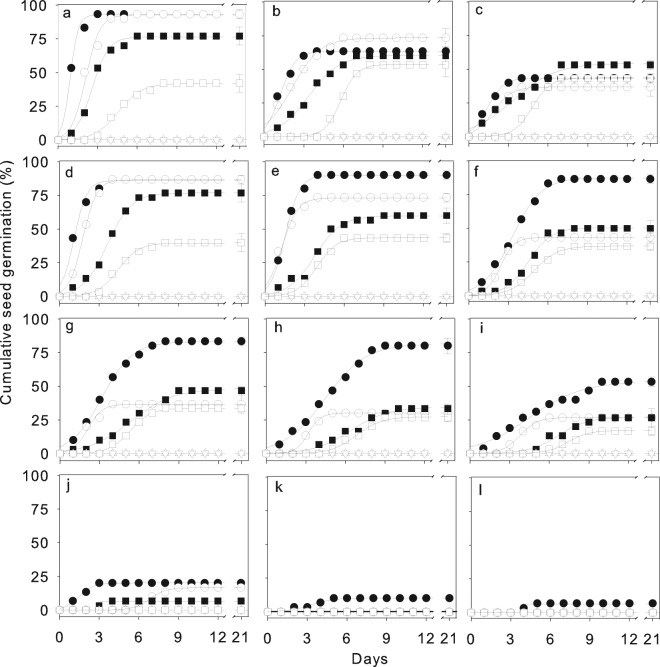
The FGP of the six Caragana species varied significantly with the temperature regime and the water/osmotic potential of the PEG solution and there was significant interaction between species and temperature regime and species and water/osmotic potential (Table 2). At 20/10 °C, FGP gradually decreased from 95% to 42%, and the time required for seeds to reach 50% of final FGP (T50) increased gradually from 0.9 d to 4.6 d in the species from an arid habitat, C. korshinkii, to the species from a semihumid habitat, C. arborescens. With increasing temperature, FGP decreased dramatically in species from arid and semiarid habitats, but not in C. arborescens, a species from a semihumid habitat, in which T50 also did not change significantly (Figs 2 and S2, Table S1). As the water/osmotic potential decreased, FGP of C. korshinskii, the species from an arid habitat, did not decrease significantly until −0.8 MPa, then decreased to 20% at −1.2 MPa and to 6% at −1.8 MPa, while FGP in the species from semiarid and semihumid habitats decreased significantly as the water/osmotic potential decreased below −0.2 MPa, so that no seeds germinated at −1.6 MPa in the species from a semiarid habitat and at −1.2 MPa in the species from a semihumid habitat (Figs 2 and S3). T50 tended to increase with the decreasing water/osmotic potential of the PEG solution (Table S1). Seeds of C. boisi and C. stipitata, did not absorb water and germinate at any temperature or PEG treatment (Figs 2, S2 and S3, Table S1).
Table 2.
Analysis of the final germination percentage of the six Caragana species in relation to temperatures and their interaction, in relation to water/osmotic potential treatments and their interaction, and in relation to gibberellic acid treatments and their interaction.
| Source of variance | df | MS | F-value | P-value |
|---|---|---|---|---|
| Temperature | ||||
| Species (S) | 5 | 5462 | 71.6 | <0.001 |
| Temperature (T) | 2 | 1820 | 23.9 | <0.001 |
| S × T | 10 | 618 | 8.2 | <0.001 |
| Water/osmotic potential | ||||
| Species (S) | 5 | 8982 | 232 | <0.001 |
| Water/osmotic potential (W) | 9 | 9741 | 252 | <0.001 |
| S × W | 45 | 526 | 13.6 | <0.001 |
| Gibberellic acid (GA 3 ) | ||||
| Species (S) | 5 | 148.6 | 147.5 | <0.001 |
| GA3 concentration (C) | 6 | 29.6 | 29.3 | <0.001 |
| S × C | 30 | 4.6 | 4.5 | <0.001 |
df, degrees of freedom; MS, mean squares; F-value, value of the F-statistic; P-value, probability.
Figure 2.
Cumulative seed germination of six Caragana species under different temperature- and water treatments. Seeds of species from arid (C. korshinskii, ●), semiarid (C. intermedia, ○, and C. microphylla, ■), semihumid (C. arborescens, □, and C. boisi, △), and humid (C. stipitata, ▽) habitats incubated at alternating (12/12 h) temperatures of 20/10 °C (a), 25/15 °C (b), 30/20 °C (c), and at water/osmotic potentials of PEG-6000 solution of 0.0 MPa (d), −0.2 MPa (e), −0.4 MPa (f), −0.6 MPa (g), −0.8 MPa (h), −1.0 MPa (i), −1.2 MPa (j), −1.6 MPa (k) and −1.8 MPa (l) at 20/10 °C. No seeds of C. boisi and C. stipitata absorbed water and germinated at any temperature and water/osmotic potential, and no seeds germinated in any species at a water/osmotic potential of −2.0 MPa (data not shown). Data are means ± one SE of the mean of the final germination percentage when larger than the symbol (n = 5); the lines are the fitted logistic curve (see Methods).
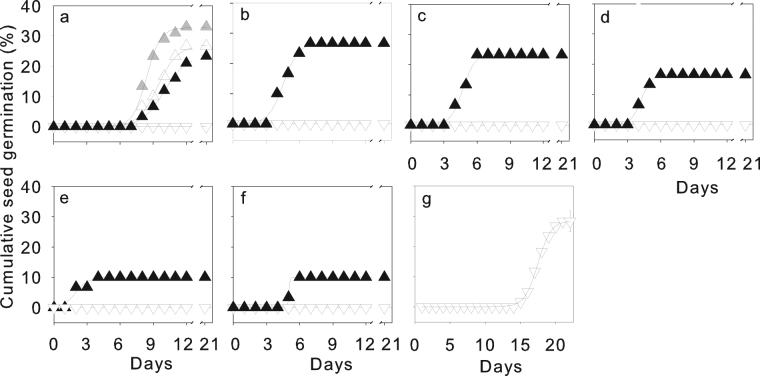
Cracked seeds of C. boisi germinated, and FGP reached about 23% at 20/10 °C, increased slightly with warmer temperatures (Fig. 3a), and decreased dramatically with a decrease in the water/osmotic potential of the PEG solution (Fig. 3b–f, Table S2). However, cracked seeds of C. stipatata did not geminate and heat shock, cold stratification, smoking and ethanol treatment did not induce C. stipatata seeds to germinate, but the 3-h daily washing treatment induced about 30% of the seeds to germinate (Fig. 3g).
Figure 3.
Cumulative germination of cracked seeds of C. boisi and C. stipitata. Cracked seeds of C. boisi (▲, △,  ) and C. stipitata (▽) from a semihumid and humid habitat, respectively, incubated at alternating (12/12 h) temperatures of 20/10 °C (▲), 25/15 °C (△), 30/20 °C (
) and C. stipitata (▽) from a semihumid and humid habitat, respectively, incubated at alternating (12/12 h) temperatures of 20/10 °C (▲), 25/15 °C (△), 30/20 °C ( ) at a water/osmotic potential of 0.0 MPa (a), and water/osmotic potentials of PEG-6000 solution of 0.0 MPa (b), −0.2 MPa (c), −0.4 MPa (d), −0.6 MPa (e), −0.8 MPa (f) at 20/10 °C. Cracked seeds of C. stipitata did not germinate at any temperature and water/osmotic potential, and no seeds germinated in C. boisi at water/osmotic potentials below −0.8 MPa (data not shown). Cumulative germination of cracked seeds of C. stipitata washed with water for 3 h each day during the incubation period (g). Data are means ± one SE of the mean of the final germination percentage when larger than the symbol (n = 5); the lines are the fitted logistic curve (see Methods).
) at a water/osmotic potential of 0.0 MPa (a), and water/osmotic potentials of PEG-6000 solution of 0.0 MPa (b), −0.2 MPa (c), −0.4 MPa (d), −0.6 MPa (e), −0.8 MPa (f) at 20/10 °C. Cracked seeds of C. stipitata did not germinate at any temperature and water/osmotic potential, and no seeds germinated in C. boisi at water/osmotic potentials below −0.8 MPa (data not shown). Cumulative germination of cracked seeds of C. stipitata washed with water for 3 h each day during the incubation period (g). Data are means ± one SE of the mean of the final germination percentage when larger than the symbol (n = 5); the lines are the fitted logistic curve (see Methods).
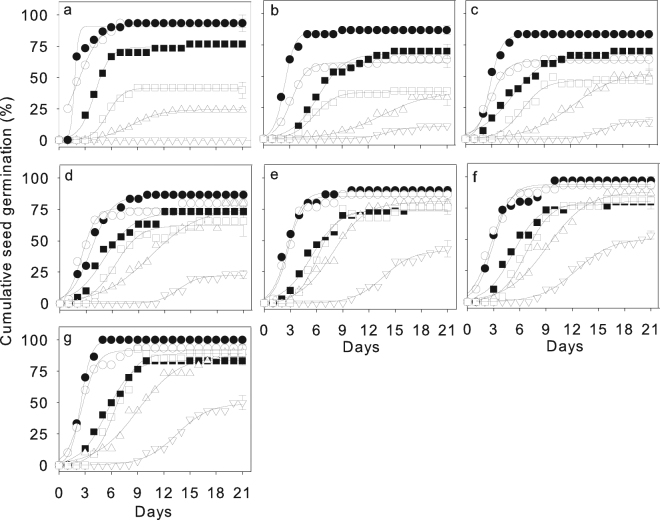
The FGP of the six Caragana species was significantly affected by species, GA3 and their interaction (Table 2). The GA3 solution increased the FGP of all the species, especially C. boisi and C. stipatata at high GA3 concentrations (Fig. 4). Compared with the 0 μg g−1 control, T50 increased when the seeds were treated with 100 μg g−1, but tended to decrease with increasing GA3 concentration (Figs 4 and S4, Table S3) in all species.
Figure 4.
Cumulative seed germination of six Caragana species under gibberellic acid treatments. Seeds of species from arid (C. korshinskii, ●), semiarid (C. intermedia, ⚬, and C. microphylla, ■), semihumid (Caragana arborescens, □, and C. boisi, △), and humid (C. stipitata, ▽) habitats incubated with gibberellic acid (GA3) concentrations of 0 μg g−1 (a), 100 μg g−1 (b), 250 μg g−1 (c), 500 μg g−1 (d), 1000 μg g−1 (e), 1500 μg g−1 (f), 2500 μg g−1 (g) at 20/10 °C. The seed coat of C. boisi and C. stipitata was cracked. Data are means ± one SE of the mean of the final germination percentage when larger than the symbol (n = 5); the lines are the fitted logistic curve (see Methods).
Seed coat structure and ABA content
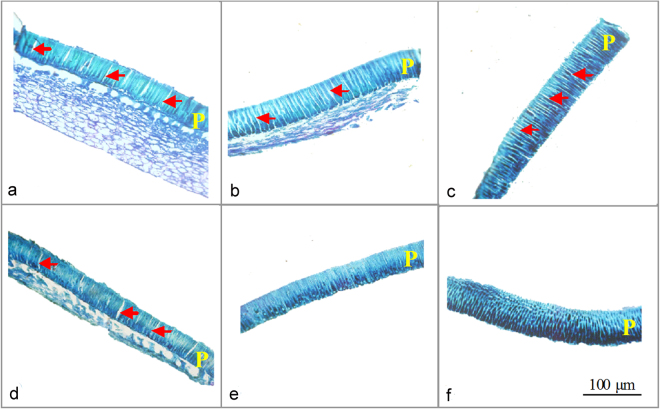
The seed coat consisted mainly of a palisade layer composed of macrosclereids, with gaps between the macrosclereids in C. korshinskii, C. intermedia, C. microphylla and C. arborescens, but with no gaps between the macrosclereids in C. boisi (Fig. 5). For C. stipatata, the palisade layer was composed of brachysclereids, with no gaps between the brachysclereids (Fig. 5).
Figure 5.
Seed coat structure. Micrographs using light microscopy of the seed coat structure of six Caragana species from arid (C. korshinskii, a), semiarid (C. intermedia, b, and C. microphylla, c), semihumid (C. arborescens, d, and C. boisi, e) and humid (C. stipitata, f) habitats. Arrows show the gaps in the palisade tissue in (a) to (d); P, palisade layer.
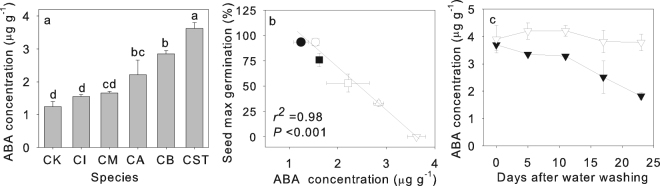
The ABA concentration in the seeds ranged from 1.2–1.6 μg g−1 in C. korshinskii, C. intermedia and C. microphylla, 2.2–2.8 μg g−1 in C. arborescens and C. boisi, and 3.7 μg g−1 in C. stipatata (Fig. 6a). Across the six species, there was a significant negative relationship between maximum FGP and ABA concentration (Fig. 6b). The ABA concentration in cracked seeds of C. stipatata decreased gradually with daily washing with water (Fig. 6c) and as the seeds germinated (Fig. 3g).
Figure 6.
ABA concentration, the correlations between ABA concentration and seed maximum germination percentage of six Caragana species and ABA concentration after daily washing in C. stipitata. (a) ABA concentration of six Caragana species from arid (C. korshinskii, CK), semiarid (C. intermedia, CI, and C. microphylla, CM), semihumid (C. arborescens, CA, and C. boisi, CB) and humid (C. stipitata, CST) habitats, (b) relationship between ABA concentration and maximum final seed germination of each species (C. korshinskii, ●, C. intermedia, ○, C. microphylla, ■, C. arborescens, □, and cracked seeds of C. boisi, △ and C. stipitata, ▽), and (c) change with time in the ABA concentration of cracked seed after daily washing with (▼) and without (▽) water in C. stipitata. Data are means + one SE (a) or ± one SE (b,c) of the mean of the final germination percentage when larger than the symbol (n = 5). In (b), the line is the fitted linear regression with the correlation coefficient (r 2) and probability values (P) given.
Discussion
The uplift of the Qinghai-Tibetan Plateau, especially the onset of the Himalayan uplift, is considered to have induced the formation of an arid climate in northwest China31, and the latter, in turn, induced the origin of Caragana in the steppe areas. Subsequently the ancestor of Caragana radiated from the arid areas of northwest China to the humid and forested areas of East Asia29, 30, where they differentiated into species with different ecological niches30, 32. The general consensus from a number of studies is that a phylogenetic signal in both functional traits and niche preference is fairly common in plants3, 9, 33–35, and seed germination is largely influenced by such a phylogenetic signal. That is, germination strategies can be stable evolutionary traits that do not vary with environment and remain similar within a genus or family3, 6, 10–14. However, other studies have reported that species within a family or a genus develop different seed germination behaviors that depend on the local environment1, 15. In this study, we used a comparative analysis of the germination behavior of the Caragana species in their natural habitat as a test for phylogenetic constraints. The results showed that FGP decreased from more than 95% in the species from an arid habitats to 0% in the species from a humid habitat and, based on Pagel’s λ, seed germination does not represent a strongly-conserved phylogenetic niche in the genus, but has adapted to the environments in which the different species evolved.
In arid and semiarid regions, it is presumed that seed germination in summer bears a high risk of seedling loss due to long periods of drought and high temperature, and that most species in these regions appear to avoid germination in summer and autumn17. However, 70–95% of the mature seeds in Caragana species from arid and semiarid areas germinated within 2–4 d after the beginning of incubation (high FGP, low T50). We suggest that the reason for the rapid germination is that the regions where Caragana species occur are influenced by the east-Asian monsoon, which results in more than 70% of the annual precipitation falling during the May–September growing season31, while only 40–60 mm of the annual precipitation falls in winter and spring. To ensure population regeneration, the timing of seed germination needs to coincide with a period of rainfall and the seedlings need to have a reasonable growth period after germination to grow large and acquire resources for subsequent survival and growth5. Therefore, Caragana species from arid and semiarid habitats are early flowering, have early seed maturation, and early and rapid seed germination after maturity in summer. The present results suggest these species use ‘opportunistic’ strategies to ensure rapid emergence and establishment after only one rainfall event36. In addition, high temperatures in summer in these areas result in high rates of transpiration and drought stress. Therefore, seed germination has adapted to rainfall-induced low temperature (increasing FGP in response to low temperature), and the ability to tolerate drought stress (germination even at osmotic potentials of −1.2 MPa). Further, as almost all seeds are eaten by seed predators within one week of falling to the ground, there is no advantage for seeds of plants from arid and semiarid habitats delaying germination until the beginning of in the next growing season37.
In semihumid and humid environments the requirements for seed germination are different. C. arborescens, a species from a semihumid habitat, had a permeable seed coat and absorbed water, but the FGP was about 50%, while seeds of C. boisis, the other species from a semihumid habitat, and seeds of C. stipitata, a species from a humid habitat, had an impermeable seed coat and did not germinate. The results from this study suggest that germination decreased (increasing degree of dormancy) with increasing precipitation36. C. arborescens is found at altitude ~200 m, where the air temperature is relatively high when the seeds mature, and the length of the growing season for seedlings is relatively long (from early August to mid-October). Therefore, some mature seeds germinate when the conditions are suitable. On the other hand, C. boisi is found on the south-eastern Tibetan Plateau, and C. stipitata grows at the top of the Hua mountains, where the altitude is more than 1800 m. The time for peak flowering of the two species is in early June and seeds mature in mid-August, more than two months later than those of the species in arid habitats. Thus, if germination were to occur, the period for seedling growth would be very short and low temperatures and/or early frost would kill the seedlings14, 17, 38, 39. Therefore, the seeds of C. boisi and C. stipitata have strong seed dormancy when mature, but germination in the following growing season would be advantageous as water is not a limiting factor (700–1000 mm precipitation per year, 70% in the growing season). Accordingly, seed germination was sensitive to drought stress (decreasing water/osmotic potential) and tended to increase with increasing temperature. Besides temperature and water status, other factors, such as light, may influence seed germination, and the investigation of their influence on seed germination would be beneficial.
The seed coat acts as a barrier to water permeability as a result of the arrangement (how tightly packed) and chemical coating/impregnation of cells in the palisade layer23–25. For seeds to germinate, the water-impermeable layers of the seed coat must become permeable to allow passage of water to the embryo. In this study, we found there are a number of gaps between the macrosclereids in the seed coat of species from arid, semiarid and one semihumid habitat, enabling the seeds to absorb water and germinate, while there were no gaps between the macrosclereids/brachysclereids in the seed coat of the species from the other semihumid habitat and the humid habitat, the seeds did not absorb water and were dormant. Therefore, the findings showed that divergence in seed coat structure is important in determining whether the seed germination behavior in Caragana species is ‘opportunistic’ or dormancy-induced. To enable germination of seeds without gaps in the palisade layer, we suggest that after dispersal they form a seed bank in the soil and mechanical damage and/or abrasion of the seed coat by soil disturbance and microbial action result in the development of cracks in the seed coat that results in germination24, 40, 41.
Many studies on a wide variety of plant species have demonstrated abscisic acid (ABA) is a positive regulator of the induction of dormancy during seed maturation, and a negative regulator of seed germination4, 28 as ABA suppresses differential and mitotic cell division27. For example, Arabidopsis mutants that over-accumulate ABA have enhanced dormancy levels or delays in germination42, but mutants that lack the capacity to produce ABA showed the absence of primary dormancy in mature seeds, while some ABA-insensitive mutants also lack, or have decreased, primary dormancy in mature seeds43. Further, in tomato, higher ABA levels enhanced dormancy levels, and ABA deficiency resulted in non-dormancy44. A direct correlation between dormancy and high endogenous ABA content has also been reported in tree seeds45. In this study, the maximum FGP (after eliminating the seed coat effect) decreased linearly with an increase in seed ABA concentration, suggesting that ABA concentration might determine physiological dormancy in Caragana species, and consequently result in different FGP in different species. In order to test this, a range of GA3 treatments was applied, as gibberellins (GA) have the opposite effect to ABA and promote seed germination28, 46. The results showed that GA3 promoted seed germination, especially in species from semihumid and humid habitats, supporting the conclusion that ABA is regulating seed dormancy and seed germination in Caragana. The findings are consistent with previous studies showing that exogenous application of gibberellic acid (GA3) to intact, unstratified seeds is effective in breaking the dormancy of seeds of Myrica esculenta and Phellodendron amurense 45, 47. Furthermore, the findings also showed that, besides seed coat structure, seed ABA concentration is a key factor determining dormancy-induced seed germination in Caragana species. Seeds of C. stipitata have strong dormancy and usually form a seed bank in the sand and shallow soil on the top of the Hua mountains. Due to frequent rainfall, they are regularly washed by water, which may be an effective means of breaking dormancy, as cracked seed in combination with washing with water decreased the ABA concentration in the seed and increased germination.
In conclusion, the results presented in this study demonstrate that there was no significant phylogenetic signal in the germination of Caragana species. Seeds of species from arid and semiarid habitats lacked dormancy, seeds of species from semihumid habitats had physiological dormancy or physical (seed coat) and physiological dormancy, while seeds of species from humid habitats had physical and physiological dormancy. Furthermore, variation in seed germination of Caragana species was related to seed coat structure and ABA concentration. The results provide a better understanding of the existing patterns of species’ distribution and adaptation. However, as global climate change is altering the environmental conditions48, the strong environmental cues in seed germination behavior shown in this study suggest that climate change will undoubtedly affect seed germination, and subsequently recruitment of plants and population dynamics.
Methods
Plant materials
Caragana species are all early-flowering, small trees or woody perennial shrubs. They occur in distinct habitats from desert to deciduous woodland environments along an annual precipitation gradient from 100 mm to 1000 mm29, 30, 49–52 (Table S4, Fig. S5, for further details see Supplementary Information Appendix S1). The period of seed maturation gradually becomes later in more humid environments, from mid-June in species from arid habitats to mid-August in the species from humid habitats (Fig. S6). The seed mass of the species was similar (between 12 and 38 mg seed−1) with the exception of C. korshinskii which has a larger seed of over 80 mg seed−1 (Table S4).
From June to August 2012, seeds of the 12 Caragana species were collected from more than 20 unique individual plants each at physiological maturity when the pods change colour from green to brown. Ripe pods were spread out to allow them to open and the seed removed. Some seeds were germinated at the site where they were collected under natural conditions and the remaining seeds were transported to the laboratory where they were used to assess their viability following the tetrazolium test procedure described by Santos et al.53, and germinated under control conditions.
Experiment 1- natural site germination and phytogenetic tree
Seeds of the 12 species were germinated under the natural environmental temperatures at the site of collection in three-fold filter paper in 90-mm diameter by 15-mm deep Petri dishes covered with soil to keep the seeds in dark. Distilled water was added to each Petri dish until the seeds floated, but were not inundated, and fresh distilled water was added each day. Each species had five replications of 30 seeds. The experiment continued until FGP became constant at which time the FGP was recorded. The mean daytime temperature during the seed incubation period was recorded at a weather station near to each site.
A phylogenetic tree of the 12 species was constructed based on three commonly sequenced chloroplast gene regions: ITS, rbcL and trnS-trnG DNA sequences were retrieved from the GenBank of NCBI for 11 of the Caragana species, or were isolated from fresh leaves for C. intermedia; Hedysarum alpinum, a member of a sister species to Caragana was used to establish the relationship between the Caragana species (Table S5). The three markers were amplified by polymerase chain reaction (PCR) with primers published in literature29. The sequences of the three genes were aligned using ClustalW (http://www.ebi.ac.uk/clustalw/), followed by manual adjustments in BioEdit v7.0.9 (Carlsbad, CA). The phylogenetic tree was built through the MEGA software package (version 5.0, www.megasoftware.net).
Experiment 2- Seed germination under controlled conditions
From the 12 species used in Experiment 1, one typical wide-spread species from an arid habitat, C. korshinskii, two typical wide-spread species from semiarid habitats, C. intermedia and C. microphylla, two species from semihumid habitats, C. arborescens and C. boisi, and one species from a humid habitat, C. stipitata, were chosen for seed germination under controlled conditions in the laboratory. To minimize the effect of after-ripening, the experiments were started shortly after the seeds had been transported to the laboratory from their sites of collection. Intact plump seeds were surface sterilized for 600 s with 75% ethanol. They were then placed on three-fold filter paper in 90-mm diameter by 15-mm deep Petri dishes and distilled water, or a solution of polyethylene glycol (PEG) 6000, or gibberellic acid (GA3), was added to each dish until the seeds floated, but were not inundated. Each treatment had five replications of 30 seeds.
To examine the effects of temperature on seed germination, the seeds were incubated in distilled water, which was added as required to maintain the depth as on the first day, in separate incubators with alternating (12/12h) temperature regimes of 20/10 °C, 25/15 °C, 30/20 °C and kept in constant darkness. The number of germinating seeds was recorded daily and the experiment continued until FGP became constant.
To examine the effects of water status on seed germination, seeds were incubated with solutions of PEG-6000 of known osmotic potential, 0, −0.2, −0.4, −0.6, −0.8, −1.0, −1.2, −1.6, −1.8, −2.0 MPa at 20 °C54, completely randomized within each incubator, and maintained at an alternating (12/12 h) temperature of 20/10 °C. Each day the solution in each Petri dish was removed, a further 10 ml test solution added and removed again as completely as possible, before the test solution was added as required to maintain the depth as on the first day55. The experiment continued until FGP became constant.
As seeds of C. boisi and C. stipatata did not absorb water and failed to germinate in the above treatments, the structure of the seed coat of the six species was analyzed by microscopic observation to compare differences between species in which the seeds absorbed water with species in which the seeds did not absorb water. Then, in order to eliminate the effect of seed coat impermeability on FGP, the seed coat of C. boisi and C. stipatata was cracked (cracked seeds) before germination under the three temperature and ten water status regimes above.
Cracked seeds of C. boisi germinated, but cracked seeds of C. stipatata still failed to germinate. To stimulate the break of dormancy of the C. stipatata seeds, a heat shock treatment, a cold stratification treatment, a smoke treatment and an ethanol treatment were applied (details in Supplementary Information Appendix S2). In addition, cracked seeds of C. stipatata were either washed for 3 h each day in running water at 20 °C (washing treatment) or not washed during the incubation period. After each of these treatments, seeds were germinated in an incubator at 20/10 °C as described above. Cracked seeds (about 0.5 to 1.0 g dry weight) with and without washing for 3 h each day were randomly removed at 0, 5, 11, 17, and 21 days during incubation to measure the abscisic acid (ABA) concentration of the seeds.
To examine the effect of GA3 on seed germination, seeds of C. korshinkii, C. intermedia, C. microphylla and C. arborescens, and cracked seeds of C. boisi and C. stipitata were incubated with solutions of GA3 of 0, 100, 250, 500, 1000, 1500, 2000, 2500 μg g−1 at an alternating (12/12 h) temperature of 20/10 °C. The solution was added in the same way as the PEG solutions. The number of germinating seeds was recorded daily and the experiment continued until FGP became constant.
The cumulative seed germination data were fitted by a logistic curve56:
in which GP is the germination percentage at t time (days), A estimates the FGP, t0 is the time required for each replicate set of seeds to reach 50% of final FGP and b is related to the rate of seed germination.
Microscopic observations
To determine the structure of the seed coat, seeds were embedded in epoxy resin after fixing in 4% FAA solution (formaldehyde, 70% ethanol and acetic acid), and transverse and longitudinal sections approximately 8–10 µm thick were cut with a microtome. These sections, stained by an aqueous solution of 1% toluidine blue, were observed using a light microscope (Axioskop II plus; Zeiss, Oberkochen, Germany) and photographed with a digital camera (Nikon Digital Sight, Nikon, Tokyo, Japan).
ABA determination
Seed ABA was extracted following the methods described by Tombesi et al.57. Analyses were performed on an Agilent 1260 HPLC (Agilent Ltd., California, USA) equipped with an Agilent C18 ZORBAX (5 mm × 150 mm × 4.6 mm) column at a flow rate of 0.013 ml s−1. The injection volume was 10 μL and the detection was made at 265 nm. The mobile phase of acetonitrile/methanol/water (8:40:52 v/v/v, 0.6% acetic acid) was previously filtered and degassed. ABA was identified by comparing the retention times with those of standard ABA, and the peak area quantified by an external standard method. Stock solutions of ABA standards was prepared by diluting a solution (0.1 mg mL−1 in acetonitrile) to obtain a range of concentrations from 0.1 to 10 μg mL−1.
Statistical analysis
The phylogenetic signal in FGP of species was tested by estimating Pagel’s λ with the ‘fitContinuous’ function in the R package ‘geiger’ v1.99-358, which uses a maximum likelihood framework to estimate the parameter λ. Pagel’s λ measures correlations relative to the correlation expected under Brownian movement59 and can vary from 0 (no phylogenetic signal) to 1 (strong phylogenetic signal)6. We tested for the significance of phylogenetic signal against the assumption of no signal (λ = 0) and strong signal (λ = 1) using a likelihood-ratio test58.
The germination data were analyzed by analysis of variance (ANOVA) using SPSS 15.0 (SPSS, Chicago, IL). Data were log-transformed to meet the requirement of normal distribution. Mean values for treatments were compared using least significant differences (LSD) at P = 0.05. A logistic curve was fitted for the relationship between cumulative FGP with germination days using Sigmaplot 10.0 (Systat Software, Inc., Chicago, IL, USA). Maximum final seed germination of six species (using cracked seeds in C. boisi and C. stipitata) among temperature- and water-regime treatments were used to fit linear regressions with seed ABA content.
Data availability statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Electronic supplementary material
Acknowledgements
We thank Ming-Xi Yu, Xiu Jia and Qin-Shan Niu for their assistance with measurements. We also thank the reviewers for their helpful comments and feedback. The research was partially supported by the National Natural Science Foundation of China (Nos 31422011, 31370423, 31670404, 31460162, 31160118), and the Fundamental Research Funds for the Central Universities (Lzujbky-2016-K12, Lzujbky-2017-ot07) and Feitian Project (860059).
Author Contributions
X.W.F., N.C.T., F.M.L. and J.P. designed the experiments and wrote the manuscript; T.P.G. and C.H.Z. performed data analysis and prepared the figures; J.J.Z. and D.H.X. conducted most of the experiments. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-11294-x
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arana MV, et al. Seed dormancy responses to temperature relate to Nothofagus species distribution and determine temporal patterns of germination across altitudes in Patagonia. New Phytol. 2016;209:507–520. doi: 10.1111/nph.13606. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, et al. Nitric oxide-induced rapid decrease of abscisic acid concentration is required in breaking seed dormancy in Arabidopsis. New Phyt. 2009;183:1030–1042. doi: 10.1111/j.1469-8137.2009.02899.x. [DOI] [PubMed] [Google Scholar]
- 3.Norden N, et al. The relationship between seed mass and mean time to germination for 1037 tree species across five tropical forests. Funct. Ecol. 2009;23:203–210. doi: 10.1111/j.1365-2435.2008.01477.x. [DOI] [Google Scholar]
- 4.El-Maarouf-Bouteau H, et al. Reactive oxygen species, abscisic acid and ethylene interact to regulate sunflower seed germination. Plant Cell Environ. 2015;38:364–374. doi: 10.1111/pce.12371. [DOI] [PubMed] [Google Scholar]
- 5.Donohue K, Rubio de Casas. R, Burghardt L, Kovach K, Willis CG. Germination, postgermination adaptation, and species ecological ranges. Ann. Rev. Ecol. Evol. S. 2010;41:293–319. doi: 10.1146/annurev-ecolsys-102209-144715. [DOI] [Google Scholar]
- 6.Zhang C, et al. The community-level effect of light on germination timing in relation to seed mass: a source of regeneration niche differentiation. New Phytol. 2014;204:496–506. doi: 10.1111/nph.12955. [DOI] [PubMed] [Google Scholar]
- 7.Vranckx G, Vandelook F. A season- and gap-detection mechanism regulates seed germination of two temperate forest pioneers. Plant Biol. 2012;14:481–490. doi: 10.1111/j.1438-8677.2011.00515.x. [DOI] [PubMed] [Google Scholar]
- 8.Carta A, Probert R, Moretti M, Peruzzi L, Bedini G. Seed dormancy and germination in three Crocus ser. Verni species (Iridaceae): implications for evolution of dormancy within the genus. Plant Biol. 2014;16:1065–1074. doi: 10.1111/plb.12168. [DOI] [PubMed] [Google Scholar]
- 9.Losos JB. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett. 2008;11:995–1003. doi: 10.1111/j.1461-0248.2008.01229.x. [DOI] [PubMed] [Google Scholar]
- 10.Figueroa JA, Armesto JJ. Community-wide germination strategies in a temperate rainforest of Southern Chile: ecological and evolutionary correlates. Aust. J. Bot. 2001;49:411–425. doi: 10.1071/BT00013. [DOI] [Google Scholar]
- 11.Moles AT, et al. Factors that shape seed mass evolution. Proc. Nat. Acad. Sci. USA. 2005;102:3010540–10544. doi: 10.1073/pnas.0501473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carta C, Hanson S, Müller JV. Plant regeneration from seeds responds to phylogenetic relatedness and local adaptation in Mediterranean Romula (Iridaceae) species. Ecol. Evol. 2016;6:4166–4178. doi: 10.1002/ece3.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoltz LP, Snyder JC. Embryo growth and germination of American ginseng seed in response to stratification temperature. Hortscience. 1985;20:261–262. [Google Scholar]
- 14.Baskin JM, Baskin CC. Seed germination ecophysiology of Jeffersonia diphylla, a perennial herb of mesic deciduous forests. Am. J. Bot. 1989;76:1073–1080. doi: 10.2307/2444529. [DOI] [Google Scholar]
- 15.Vandelook F, Van de Moer D, Van Assche JA. Environmental signals for seed germination reflect habitat adaptations in four temperate Caryophyllaceae. Funct. Ecol. 2008;22:470–478. doi: 10.1111/j.1365-2435.2008.01385.x. [DOI] [Google Scholar]
- 16.Walck J, Hidayati S, Dixon KW, Thompson K, Poschlod P. Climate change and plant regeneration from seed. Global Change Biol. 2011;17:2145–2161. doi: 10.1111/j.1365-2486.2010.02368.x. [DOI] [Google Scholar]
- 17.Schütz W. Ecology of seed dormancy and germination in sedges (Carex) Perspect Plant Ecol. 2000;3:67–89. doi: 10.1078/1433-8319-00005. [DOI] [Google Scholar]
- 18.Brink D, Hendriksma HP, Bruun HH. Habitat specialization through germination cueing: a comparative study of herbs from forests and open habitats. Ann. Bot. 2013;111:283–292. doi: 10.1093/aob/mcs253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosbakh S, Poschlod P. Initial temperature of seed germination as related to species occurrence along a temperature gradient. Funct. Ecol. 2015;29:5–14. doi: 10.1111/1365-2435.12304. [DOI] [Google Scholar]
- 20.Walder T, Erschbamer B. Temperature and drought drive differences in germination responses between congeneric species along altitudinal gradients. Plant Ecol. 2015;216:1297–1309. doi: 10.1007/s11258-015-0509-1. [DOI] [Google Scholar]
- 21.Thompson PA. Germination of species of Caryophyllaceae in relation to their geographical distribution in Europe. Ann. Bot. 1970;34:427–449. doi: 10.1093/oxfordjournals.aob.a084381. [DOI] [Google Scholar]
- 22.Santiago A, Herranz JM, Copete E, Ferrandis P. Species-specific environmental requirements to break seed dormancy: implications for selection of regeneration niches in three Lonicera (Caprifoliaceae) species. Botany. 2013;91:1–9. doi: 10.1139/cjb-2012-0169. [DOI] [Google Scholar]
- 23.Egerton-Warburton LM. A smoke-induced alteration of the sub-testa cuticle in seeds of the post-fire recruiter, Emmenanthe penduliflora Benth.(Hydrophyllaceae) J. Exp. Bot. 1998;49:1317–1327. doi: 10.1093/jxb/49.325.1317. [DOI] [Google Scholar]
- 24.Baskin JM, Baskin CC, Li X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Spec. Biol. 2000;15:139–152. doi: 10.1046/j.1442-1984.2000.00034.x. [DOI] [Google Scholar]
- 25.Willis CG, et al. The evolution of seed dormancy: environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytol. 2014;203:300–309. doi: 10.1111/nph.12782. [DOI] [PubMed] [Google Scholar]
- 26.Nambara E, Marion-Poll A. ABA action and interasctions in seeds. Trends Plant Sci. 2003;8:1360–1385. doi: 10.1016/S1360-1385(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y, Chung MC, Yeung EC, Lee N. Dynamic distribution and the role of abscisic acid during seed development of a lady’s slipper orchid, Cypripedium formosanum. Ann. Bot. 2015;116:403–411. doi: 10.1093/aob/mcv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang J, et al. Abscisic acid transporters cooperate to control seed germination. Nat. Commun. 2015;6:8113. doi: 10.1038/ncomms9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang ML, Fritsch PW, Cruz BC. Phylogeny of Caragana (Fabaceae) based on DNA sequence data from rbcL, trnS–trnG, and ITS. Mol. Phylogenet Evol. 2009;50:547–559. doi: 10.1016/j.ympev.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Zhang ML, Xiang XG, Xue JJ, Sanderson SC, Fritsch PW. Himalayan uplift shaped biomes in Miocene temperate Asia: evidence from leguminous Caragana. Sci. Rep. 2016;6:36528. doi: 10.1038/srep36528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An ZS, Kutzbach JE, Prell WL, Porter SC. Evolution of Asian monsoons and phased uplift of the Himalaya—Tibetan plateau since Late Miocene times. Nature. 2001;411:62–66. doi: 10.1038/35075035. [DOI] [PubMed] [Google Scholar]
- 32.Fang XW, et al. Limits to the height growth of Caragana korshinskii resprouts. Tree Physiol. 2013;33:275–284. doi: 10.1093/treephys/tpt006. [DOI] [PubMed] [Google Scholar]
- 33.Crisp MD, et al. Phylogenetic biome conservatism on a global scale. Nature. 2009;458:754–756. doi: 10.1038/nature07764. [DOI] [PubMed] [Google Scholar]
- 34.Davies TJ, et al. Phylogenetic conservatism in plant phenology. J. Ecol. 2013;101:1520–1530. doi: 10.1111/1365-2745.12154. [DOI] [Google Scholar]
- 35.Cornwell WK, et al. Functional distinctiveness of major plant lineages. J. Ecol. 2014;102:345–356. doi: 10.1111/1365-2745.12208. [DOI] [Google Scholar]
- 36.Gutterman Y. (2000) Environmental factors and survival strategies of annual plant species in the Negev desert, Israel. Plant Spec. Biol. 2001;15:113–125. doi: 10.1046/j.1442-1984.2000.00032.x. [DOI] [Google Scholar]
- 37.Zhao X, Ren J. Influence of seed predation on regeneration of three Caragana species. Biodivers. Sci. 2005;13:514–519. doi: 10.1360/biodiv.050160. [DOI] [Google Scholar]
- 38.Shimono Y, Kudo G. Comparisons of germination traits of alpine plants between fellfield and snowbed habitats. Ecol. Res. 2005;20:189–197. doi: 10.1007/s11284-004-0031-8. [DOI] [Google Scholar]
- 39.Inouye DW. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecol. 2008;89:353–362. doi: 10.1890/06-2128.1. [DOI] [PubMed] [Google Scholar]
- 40.Van Assche JA, Debucquoy KLA, Rommens WAF. Seasonal cycles in the germination capacity of buried seeds of some Leguminosae (Fabaceae) New Phytol. 2003;158:315–323. doi: 10.1046/j.1469-8137.2003.00744.x. [DOI] [Google Scholar]
- 41.Karaki T, Watanabe Y, Konda T, Koike T. Strophiole of seeds of the black locust acts as a water gap. Plant Spec. Biol. 2012;27:226–232. doi: 10.1111/j.1442-1984.2011.00343.x. [DOI] [Google Scholar]
- 42.Nonogaki M, Sall K, Nambara E, Nonogaki H. Amplification of ABA biosynthesis and signaling through a positive feedback mechanism in seeds. Plant J. 2015;78:527–539. doi: 10.1111/tpj.12472. [DOI] [PubMed] [Google Scholar]
- 43.Lefebvre V, et al. Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 2006;45:309–319. doi: 10.1111/j.1365-313X.2005.02622.x. [DOI] [PubMed] [Google Scholar]
- 44.Thompson AJ, et al. Ectopic expression of a tomato 9-cis-epoxycarotenoid dioxygenase gene causes over-production of abscisic acid. Plant J. 2000;23:163–174. doi: 10.1046/j.1365-313x.2000.00789.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen SY, Chien CT, Baskin JM, Baskin CC. Storage behavior and changes in concentrations of abscisic acid and gibberellins during dormancy break and germination in seeds of Phellodendron amurense var. wilsonii (Rutaceae) Tree Physiol. 2009;30:275–284. doi: 10.1093/treephys/tpp111. [DOI] [PubMed] [Google Scholar]
- 46.Holdsworth MJ, Bentsink L, Soppe WJ. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008;179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 47.Bhatt ID, Rawal RS, Dhar U. Improvement in seed germination of Myrica esculenta Buch.-Ham. ex D. Don: a high value tree species of Kumaun Himalaya, India. Seed Sci. Technol. 2000;28:597–605. [Google Scholar]
- 48.Ishida A, et al. Effective use of high CO2 efflux at the soil surface in a tropical understory plant. Sci. Rep. 2015;5:8991. doi: 10.1038/srep08991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma CC, Gao YB, Guo HY, Wang JL. Interspecific transition among Caragana microphylla, C. davazamcii and C. korshinskii along geographic gradient. I. Characteristics of photosynthesis and water metabolism. Acta Bot. Sin. 2003;45:1228–1237. [Google Scholar]
- 50.Fang XW, et al. The distribution of four Caragana species is related to their differential responses to drought stress. Plant Ecol. 2014;215:133–142. doi: 10.1007/s11258-013-0285-8. [DOI] [Google Scholar]
- 51.Xie LN, Ma CC, Guo HY, Li QF, Gao YB. Distribution pattern of Caragana species under the influence of climate gradient in the Inner Mongolia region, China. J. Arid Land. 2014;6:311–323. doi: 10.1007/s40333-013-0227-2. [DOI] [Google Scholar]
- 52.Xie LN, Gou HY, Ma CC. Alterations in flowering strategies and sexual allocation of Caragana stenophylla along a climatic aridity gradient. Sci. Rep. 2016;6:33602. doi: 10.1038/srep33602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santos MAO, Novembre ADLC, Marcos-Filho J. Tetrazolium test to assess viability and vigour of tomato seeds. Seed Sci. Technol. 2007;35:213–223. doi: 10.15258/sst.2007.35.1.19. [DOI] [Google Scholar]
- 54.Michel BE, Kaufmann MR. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973;51:914–916. doi: 10.1104/pp.51.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song J, Feng G, Tian C, Zhang F. Strategies for adaptation of Suaeda physophora, Haloxylon ammodendron and Haloxylon persicum to a saline environment during seed-germination stage. Ann. Bot. 2005;96:399–405. doi: 10.1093/aob/mci196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fang X, Turner NC, Yan GJ, Li FM, Siddique KHM. Flower numbers, pod production, pollen viability, and pistil function are reduced and flower and pod abortion increased in chickpea (Cicer arietinum L.) under terminal drought. J. Exp. Bot. 2010;61:335–345. doi: 10.1093/jxb/erp307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tombesi S, et al. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015;5:12449. doi: 10.1038/srep12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harmon L, et al. GEIGER: analysis of evolutionary diversification. R package version. 2013;1:99–3. [Google Scholar]
- 59.Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).