Astrocytes in the retrotrapezoid nucleus (RTN) are known to function as respiratory chemoreceptors; however, it is not clear whether changes in voltage contribute to astrocyte chemoreception. We showed that depolarization of RTN astrocytes at constant CO2 levels is sufficient to modulate RTN chemoreception by a purinergic-dependent mechanism. These results support the possibility that astrocyte depolarization can facilitate purinergic enhancement of respiratory drive from the RTN.
Keywords: astrocytes, breathing, chemoreceptor, purinergic signaling, ventrolateral medulla
Abstract
Evidence indicates that CO2/H+-evoked ATP released from retrotrapezoid nucleus (RTN) astrocytes modulates the activity of CO2-sensitive neurons. RTN astrocytes also sense H+ by inhibition of Kir4.1 channels; however, the relevance of this pH-sensitive current remains unclear since ATP release appears to involve CO2-dependent gating of connexin 26 hemichannels. Considering that depolarization mediated by H+ inhibition of Kir4.1 channels is expected to increase sodium bicarbonate cotransporter (NBC) conductance and favor Ca2+ influx via the sodium calcium exchanger (NCX), we hypothesize that depolarization in the presence of CO2 is sufficient to facilitate ATP release and enhance respiratory output. Here, we confirmed that acute exposure to fluorocitrate (FCt) reversibly depolarizes RTN astrocytes and increased activity of RTN neurons by a purinergic-dependent mechanism. We then made unilateral injections of FCt into the RTN or two other putative chemoreceptor regions (NTS and medullary raphe) to depolarize astrocytes under control conditions and during P2-recepetor blockade while measuring cardiorespiratory activities in urethane-anesthetized, vagotomized, artificially ventilated male Wistar rats. Unilateral injection of FCt into the RTN increased phrenic (PNA) amplitude and frequency without changes in arterial pressure. Unilateral injection of pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS, a P2-receptor antagonist) into the RTN dampened both PNA amplitude and frequency responses to FCt. Injection of MRS2179 (P2Y1-receptor antagonist) into the RTN did not affect the FCt-induced respiratory responses. Fluorocitrate had no effect on breathing when injected into the NTS or raphe. These results suggest that depolarization can facilitate purinergic enhancement of respiratory drive from the RTN.
NEW & NOTEWORTHY Astrocytes in the retrotrapezoid nucleus (RTN) are known to function as respiratory chemoreceptors; however, it is not clear whether changes in voltage contribute to astrocyte chemoreception. We showed that depolarization of RTN astrocytes at constant CO2 levels is sufficient to modulate RTN chemoreception by a purinergic-dependent mechanism. These results support the possibility that astrocyte depolarization can facilitate purinergic enhancement of respiratory drive from the RTN.
neurons located in the medial portion of the retrotrapezoid nucleus (RTN) function as respiratory chemoreceptors (Guyenet and Bayliss 2015; Nattie and Li 2012). Chemosensitive RTN neurons have a well-defined molecular phenotype: they express VGLUT2 and the transcription factor Phox2b but not choline acetyl-transferase (ChAT) or tyrosine hydroxylase (TH) (Kang et al. 2007; Stornetta et al. 2006; Takakura et al. 2006). These excitatory neurons are activated by hypercapnia via their intrinsic proton sensitivity (Kumar et al. 2015; Mulkey et al. 2004) and by CO2/H+-evoked ATP release presumably from local astrocytes (Gourine et al. 2005, 2010; Huckstepp et al. 2010b; Turovsky et al. 2016; Wenker et al. 2012). Genetic or pharmacological depletion of RTN chemoreceptor neurons reduces the central respiratory chemoreflex by an average of 80% (Ramanantsoa et al. 2011; Takakura et al. 2014; Kumar et al. 2015).
Astrocytes are increasingly recognized as modulators of neural activity (Araque et al. 2014; Haydon and Carmignoto 2006; Khakh and Sofroniew 2015; Panatier et al. 2011) and have been shown to release a variety of signaling molecules such as ATP, glutamate, and norepinephrine (Halassa et al. 2009; Haydon and Carmignot 2006). At the level of the RTN, astrocytes have been shown to function as chemoreceptors by sensing CO2/H+ by inhibition of Kir4.1 channels (Wenker et al. 2010) and release of ATP most likely through connexin 26 (Cx26) hemichannels (Gourine et al. 2010; Huckstepp et al. 2010a, 2010b; Meigh et al. 2013; Wenker et al. 2012). Furthermore, it is becoming clear that CO2/H+-evoked ATP release is a component of RTN chemoreception (Huckstepp et al. 2010b; Meigh et al. 2013; Wenker et al. 2012). However, uncertainty remains regarding how astrocytes perform this function. Specifically, it is not clear whether H+-mediated depolarization is required for CO2-induced ATP release.
To test this possibility, we used fluorocitrate (FCt) to depolarize astrocytes while recording activity of neurons in the RTN or two other putative chemoreceptor regions [i.e., the commissural nucleus of the solitary tract (cNTS) and the raphe pallidus/parapyramidal region (RPa/PPy)] (Richerson 2004; Sobrinho et al. 2014). Fluorocitrate is considered a metabolic toxin that is selectively taken up by astrocytes (Erlichman and Leiter 2010), where it can initiate membrane depolarization presumably by disruption of the Na+/K+ ATPase. Previous evidence suggests that subtoxic doses of FCt can reversibly depolarize CO2/H+-sensitive RTN astrocytes (Wenker et al. 2010). Here, we confirm that exposure to FCt depolarized membrane potential of CO2/H+-sensitive RTN astrocytes. Furthermore, the FCt difference current reverses near the reversal potential for K+, suggesting that FCt directly or indirectly decreases a subthreshold K+ conductance. Considering that inward-rectifying K+ channels like Kir4.1 are activated by an increase in extracellular K+ (Nwaobi et al. 2016) and since the FCt-sensitive current is fairly voltage independent, we suspect that FC-mediated depolarization results from decreased K+ efflux via background K+ channels that are known to regulate resting membrane potential in astrocytes from other brain regions (Olsen et al. 2015). We have also shown previously (Wenker et al. 2010) and confirmed here that blocking P2 receptors decreased FCt-mediated activation of RTN neurons, thus suggesting that FCt-induced depolarization of astrocytes activates chemosensitive RTN neurons by a purinergic-dependent mechanism. Here, we go on to show that injection of FCt into the RTN, but not cNTS or raphe, increased respiratory drive by a P2 receptor-dependent mechanism. These results support the possibility that depolarization in the presence of CO2 contributes to the mechanism by which RTN astrocytes release ATP and activate downstream chemosensitive neurons.
METHODS
Animals.
Animal use was in accordance with guidelines approved by the University of São Paulo Institutional Animal Care and Use Committee (CEUA: 118/109-2) and by National Institutes of Health and University of Connecticut Animal Care and Use Guidelines, both of which approved our experiments. All in vivo experiments were performed on male Wistar rats weighing 250–350 g (8–10 mo old). In vitro experiments were performed on neonatal rats (7–12 days postnatal). All efforts were made to minimize animal discomfort and the number of animals used.
Surgical procedures.
All surgical procedures were similar to those described previously (Takakura and Moreira 2011; Sobrinho et al. 2014). Briefly, animals were first anesthetized with 5% halothane in 100% O2. After anesthesia, rats were tracheostomized and connected to a ventilation pump, and the halothane was reduced to 1.4–1.5% until the end of surgery. The femoral artery and vein were cannulated (polyethylene tubing, 0.6 mm od, 0.3 mm id; Scientific Commodities, Lake Havasu City, AZ) for measurement of arterial pressure (AP) and administration of drugs and fluids. After an occipital trepanation, a micropipette was placed in the medulla oblongata via a dorsal transcerebellar approach for microinjection of drugs. The phrenic nerve was accessed by a dorsolateral approach after retraction of the right shoulder blade. To prevent any influence of artificial ventilation on phrenic nerve activity (PNA), the vagus nerve was cut bilaterally. Upon completion of the surgical procedures, halothane was withdrawn gradually while an isotonic solution of urethane (1.2 g/kg) was slowly administered intravenously (iv). Rats were also paralyzed with a muscle relaxant (1 mg/kg iv pancuronium, with additional doses of 0.2 mg/kg when needed). In the absence of pancuronium, adequacy of anesthesia was monitored by athe bsence of a withdrawal reflex and, under paralysis, by a limited increase in blood pressure (BP; <10 mmHg) and lack of activation of the phrenic nerve in response to a firm paw pinch. Approximately hourly supplements of one-third of the initial dose of urethane were needed to satisfy these criteria throughout the recording period (2 h). Rectal temperature was monitored and maintained at 37°C. End-tidal CO2 was monitored throughout each experiment with a capnometer (CWE, Ardmore, PA). This instrument provided a reading of <0.1% CO2 during inspiration in animals breathing 100% O2 and an asymptotic, nearly horizontal reading during expiration.
In vivo physiological recordings.
As previously described (Sobrinho et al. 2014; Wenker et al. 2012), recordings of mean arterial pressure (MAP), PNA, and end-expiratory CO2 (etCO2) were digitized with a micro1401 (Cambridge Electronic Design), stored on a computer, and processed offline with Spike 2 software version 6 (Cambridge Electronic Design). Integrated phrenic nerve activity (iPNA) was obtained after rectification and smoothing (τ = 0.015 s) of the original signal, which was acquired with a 30- to 300-Hz band-pass filter. PNA amplitude was defined as the area under the curve of the rectified and smoothed phrenic bursts, and these values were normalized by assigning a value of 100 to the maximum value recorded at a high etCO2 in the absence of any nerve stimulation and a value of 0 to the noise recorded between bursts. Then, these values were used to verify the changes of response in the presence of purinergic antagonists.
Brain stem slices.
Brain stem slices were prepared as described previously (Wenker et al. 2012). Briefly, neonatal rats (7–12 days postnatal) were decapitated under ketamine-xylazine anesthesia, and transverse brain stem slices (300 μm) were cut using a microslicer (DSK 1500E; Dosaka, Kyoto, Japan) in ice-cold substituted Ringer solution containing (in mM) 260 sucrose, 3 KCl, 5 MgCl2, 1 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, and 1 kynurenic acid. Slices were incubated for ∼30 min at 37°C and subsequently at room temperature in normal Ringer solution containing (in mM) 130 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose. Both substituted and normal Ringer solutions were bubbled with 95% O2–5% CO2.
Slices containing the RTN and raphe were harvested between −11.7 and −11.4 mm relative to bregma (Paxinos and Watson 1998). Within these slices, we targeted CO2/H+-sensitive RTN neurons located ventral to the facial motor nucleus within ∼50 μm of the ventral surface, and raphe neurons that exhibited slow regular discharge activity located between the pyramidal tract in the region of the raphe pallidus. Slices containing the NTS were taken 12.5 mm caudal and 0.8 mm lateral from bregma (Paxinos and Watson 1998).
Cellular electrophysiology.
Individual slices were transferred to a recording chamber mounted on a fixed-stage microscope (Zeiss Axioskop) and perfused continuously (∼2 ml/min) with normal Ringer solution bubbled with 95% O2-5% CO2 (bath pH = 7.30). The pH of the bicarbonate-based bath solution was decreased to a pH of 6.9 by bubbling with 15% CO2. All cellular recordings were made with an Axopatch 200B patch clamp amplifier, digitized with a Digidata 1322A A/D converter, and recorded using pCLAMP 10.0 software (Molecular Devices). Recordings were obtained at room temperature, with patch electrodes pulled from borosilicate glass capillaries (Warner Instruments) on a two-stage puller (P89; Sutter Instrument) to a DC resistance of 4–7 MΩ when filled with an internal solution containing the following (in mM): 120 KCH3SO3, 4 NaCl, 1 MgCl2, 0.5 CaCl2, 10 HEPES, 10 EGTA, 3 Mg-ATP, and 0.3 GTP-Tris (pH 7.2); electrode tips were coated with Sylgard 184 (Dow Corning). Voltage-clamp recordings from astrocytes were made at a holding potential of −80 mV and in the presence of tetrodotoxin (TTX, 0.1 μM) to block neuronal activity. Holding current, conductance, and current-voltage (I-V) relationships were determined using voltage steps between −150 and 40 mV. It is important to recognize that in a whole cell recording circuit, recording cells with a low membrane resistance (e.g., passive astrocytes) in series with a large access resistance (Ra) can result in voltage-clamping error. In our experiments, we limited Ra to <10 MΩ by preparing tissue from young animals; capacitance and Ra compensation (70%) were used to minimize voltage errors, as described previously (Matthias et al. 2003). Recordings were discarded if Ra varied >10% during an experiment. A liquid junction potential of 10 mV was corrected offline.
Drugs.
All drugs were purchased from Sigma and prepared and administered as described in previous studies (Costa et al. 2013; Sobrinho et al. 2014; Wenker et al. 2012). Fluorocitrate (obtained from DL-fluorocitric acid barium salt) was dissolved in 0.1 M HCl. The barium salt was precipitated by the addition of 0.1 M Na2SO4. This solution was buffered with 0.1 M NaPO4 and centrifuged at 800 rpm for 10 min. The supernatant containing the barium salt was removed, and a saline solution was added to a final concentration of 1 mM. For electrophysiological experiments, fluorocitrate was used at a final concentration of 100 µM (pH = 7.3).
The nonspecific P2 receptor antagonist pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) and the P2Y1-receptor antagonist MRS2179 were diluted to 3 mM and 100 μM, respectively, in sterile saline (pH 7.4) and injected into the RTN using single-barrel glass pipettes (tip diameter of 20 μm) connected to a pressure injector (Picospritzer III; Parker Hannifin, Cleveland, OH). For each injection, we delivered a volume of 50 nl over a period of 5 ms. RTN injections were made using the lambda as reference: AP = −2.3 mm; ML 1.8 and −8.3 mm ventral to the dura mater. Raphe pallidus injections were made using the lambda as reference: AP = −2.3 mm; ML 1.0 and −8.3 mm ventral to the dura mater. Injections in the caudal aspect of the NTS (cNTS) were centered ∼400 μm caudal to the calamus scriptorius in the midline and 300–500 μm below the dorsal surface of the brainstem. Fluorescent latex microbeads (5%; Lumafluor, New City, NY) were included with all drug applications to mark the injection sites and verify the spread of the injected material (Sobrinho et al. 2014; Takakura and Moreira 2011).
Histology.
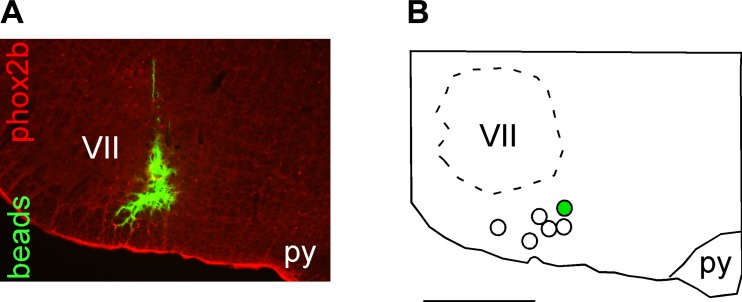
Upon completion of the experiments, the rats received an additional dose of urethane and were transcardially perfused with PBS (pH 7.4), followed by paraformaldehyde (4% in 0.1 M phosphate buffer, pH 7.4). The brains were removed and maintained in a 20% sucrose solution for 24 h. The brains were frozen, and coronal slices of 40 µm thickness were obtained using a microtome. Sections of different brains were mounted and the injection sites confirmed with an Axioskop2 microscope (Zeiss, Oberkochen, Germany) equipped with epifluorescence light. We considered positive injections to be those in which the brains contained a deposit of fluorescent latex microbeads close to the ventral surface. The positive injections in this region were also confirmed by the presence of microbeads between positive Phox2b-expressing neurons identified by immunohistochemistry (Fig. 4). Phox2b was detected by using a free-floating method according to previously described protocols (Takakura et al. 2014), which included a primary rabbit antibody (1:800 dilution; gift of J. F. Brunet, Ecole Normale Superieure, Paris, France) and a secondary antibody, donkey anti-rabbit, conjugated to Cy3 (Jackson Immunoresearch, West Grove, PA).
Fig. 4.
Histological confirmation of injection sites in the RTN. A: photomicrograph of a typical injection of fluorocitrate (FCt) into the RTN. Note that the FCt injection (demonstrated by presence of green fluorescent microbeads) targets the region that contains the highest density of phox2B immunoreactive neurons (in red). B: computer-assisted plots of the center of the FCt injection sites targeted to the RTN revealed by the presence of fluorescent microbeads. All sites are projected on a single section located at Bregma −11.6 mm according to Paxinos and Watson (1998). py, Pyramidal tract; VII, facial motor nucleus. Scale, 1 mm.
Statistics.
Data are reported as means ± SE. Statistical analysis was performed using Sigma Stat software version 3.0 (Jandel, Point Richmond, CA). A t-test, paired t-test, or one-way ANOVA followed by the Newman-Keuls multiple-comparisons test was performed as appropriate (P < 0.05).
RESULTS
FCt can be used to depolarize astrocytes.
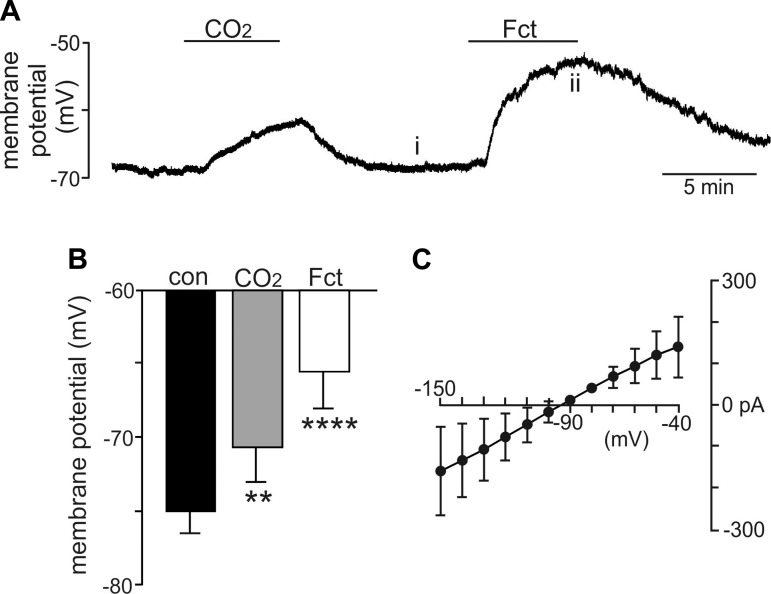
In the first series of experiments, we confirmed that 3–5 min of exposure to FCt (100 µM) depolarized membrane potential of CO2/H+-sensitive RTN astrocytes by 9.9 ± 1.5 mV (n = 6, P < 0.001; Fig. 1, A and B). Furthermore, the FCt difference current reverses near the reversal potential for K+ (Fig. 1C), suggesting that FCt directly or indirectly decreased a subthreshold K+ conductance. Considering that inward-rectifying K+ channels like Kir4.1 are activated by an increase in extracellular K+ (Nwaobi et al. 2016), and since the FCt-sensitive current is fairly voltage independent (Fig. 1C), we suspect FC-mediated depolarization results from decreased K+ efflux via background K+ channels that are known to regulate resting membrane potential in astrocytes from other brain regions (Olsen et al. 2015). These results are consistent with previous work showing that FCt can depolarize astrocytes (identified based on a very negative resting membrane potential and passive electrical signature) in part by inhibition of a K+ conductance (Hülsmann et al. 2000).
Fig. 1.
Bath application of fluorocitrate (FCt) results in a strong and reversible depolarization of chemosensitive astrocytes on the retrotrapezoid nucleus in vitro. A: trace of membrane potential from a chemosensitive retrotrapezoid nucleus (RTN) astrocyte shows that exposure to FCt (100 µM) caused a large depolarization. B: summary data (n = 6) show the membrane potential response to 15% CO2 and FCt. C: current-voltage (I-V) relationship of the FCt-sensitive difference current was determined at voltages between −150 and −40 mV by subtracting I-V relationships obtained under control conditions from those recorded during exposure to FCt. The FCt-sensitive current is fairly linear over this range of voltages and reverses at −95 mV, suggesting that FCt decreased conductance of 1 or more subthreshold K+ channels. Different from control (repeated-measures/1-way ANOVA; F2,5 = 28.004). **P = 0.02; ****P < 0.0001.
Consistent with our previous evidence (Wenker et al. 2010), we found that bath application of FCt increased activity of RTN neurons by 1.51 ± 0.49 Hz (n = 4), and this response was blunted by P2 receptor blockade (1.0 ± 0.73 Hz; n = 4, P = 0.012) (data not shown). These results suggest that FCt activates chemosensitive RTN neurons by a purinergic-dependent mechanism presumably facilitated by astrocyte depolarization. We also tested the effects of FCt on neural activity in other chemosensitive regions, including the cNTS and medullary raphe. We found that exposure to FCt (100 µM) increased activity of cNTS (0.89 ± 0.27; n = 5) and raphe neurons (0.57 ± 0.15; n = 4); however, unlike in the RTN, neurons in the cNTS and raphe did not respond to repeated FCt exposures and showed suppressed basal activity after the initial FCt exposure (data not shown). It is not clear whether FCt directly affected neural activity in these regions by release of neuromodulators or by disruption of homeostatic processes like K+ buffering, transmitter uptake, or pH regulation. In any case, since our main focus is not related to study neural activity in the absence of functional astrocytes, we did not continue these experiments.
RTN injection of FCt increased respiratory output by a purinergic-dependent mechanism.
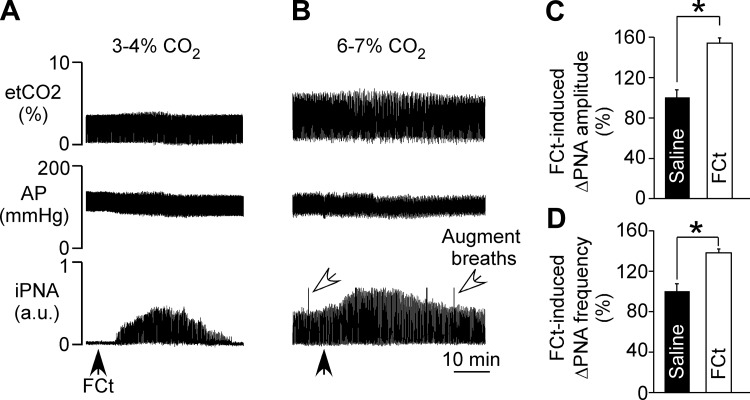
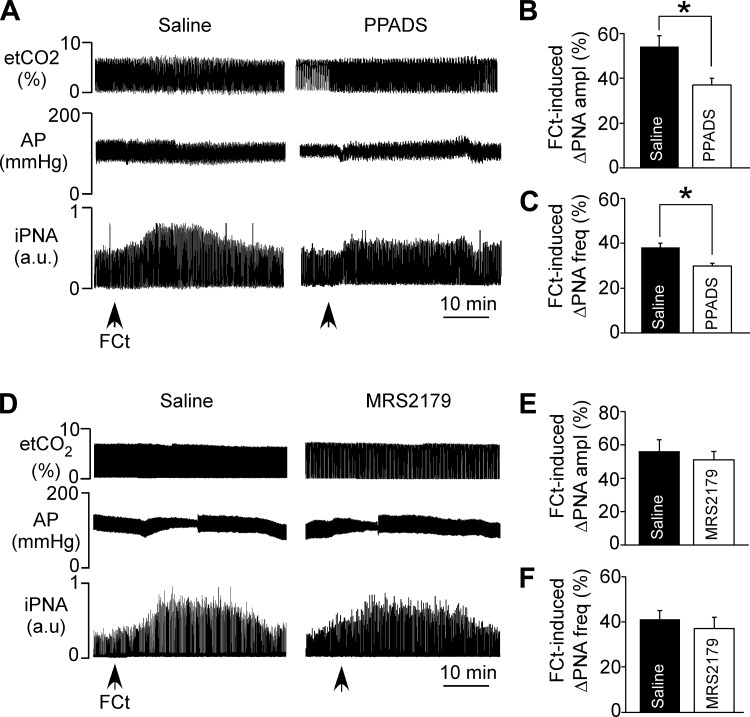
The respiratory effects produced by injection of FCt (1 mM, 50 nl) into the RTN were evaluated in anesthetized, vagotomized, artificially ventilated rats at low (3–4% etCO2) and high (6–7% etCO2) levels of end-expiratory CO2 under control conditions and during P2 receptor blockade. Below the PNA threshold (etCO2: 3–4%), unilateral injection of FCt increased PNA ∼5 min after FCt injection (range: 3–7 min) (Fig. 2A). When the respiratory central pattern generator was active (etCO2: 6–7%), unilateral injection of FCt produced an increase in PNA amplitude (54 ± 5% of control, P = 0.02) and frequency (38 ± 2% of control, P = 0.031) (Figs. 2B–D). We did not detect a significant MAP response to RTN injection of FCt (115 ± 6 vs. resting: 118 ± 7 mmHg, P > 0.05; Fig. 2, A and B). Consistent with our previous in vitro data (Wenker et al. 2010), we found in vivo that unilateral injection of the P2 receptor antagonist PPADS (100 μM, 50 nl) into the RTN attenuated the ventilatory response to subsequent application of FCt in terms of both PNA amplitude (37 ± 3%, vs. saline + FCt: 54 ± 5%, P = 0.036) and PNA frequency (30 ± 1%, vs. saline + FCt: 38 ± 2%, P = 0.041) (Fig. 3, A–C). However, application of PPADS into the RTN had no measureable effect on baseline MAP (113 ± 7 mmHg, vs. saline: 115 ± 4 mmHg, P > 0.05) or PNA amplitude (55 ± 3%. vs. saline: 58 ± 6%, P > 0.05) or frequency (37 ± 4%, vs. saline: 42 ± 4%, P > 0.05) (Fig. 3A).
Fig. 2.
RTN injection of fluorocitrate (FCt) increases respiratory activity. A and B: recordings of end-expiratory CO2 (etCO2), arterial pressure (AP), and integrated phrenic nerve activity (iPNA) in urethane-anesthetized and artificially ventilated rats show that injection of FCt (1 mM, 50 nl) into the RTN increased breathing in 2 experimental conditions, i.e., below (etCO2: 3–4%; A) and above the PNA threshold (etCO2: 6–7%; B). C and D: summary data show changes in PNA amplitude (ΔPNA amplitude; C) and PNA frequency (ΔPNA frequency; D) produced by unilateral injections of saline or FCt into the RTN in a condition of 6–7% etCO2. *Different from saline (n = 6/group).
Fig. 3.
P2 receptor blockade at the level of the RTN blunted the ventilatory response to fluorocitrate (FCt). A: recordings of end-expiratory CO2 (etCO2), arterial pressure (AP), and integrated phrenic nerve activity (iPNA) in urethane-anesthetized and artificially ventilated rats show that injection of pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS; P2 receptors antagonist, 100 μM) into the RTN reduced the respiratory response to FCt. B and C: summary data show that PPADS injection into the RTN reduced the increase in PNA amplitude (ΔPNA amplitude; B) and PNA frequency (ΔPNA frequency; C) elicited by FCt. D: recordings of etCO2, AP and iPNA in urethane-anesthetized and artificially ventilated rats show that injection of MRS2179 (P2Y1a receptor antagonist, 100 μM) into the RTN did not change the respiratory response to FCt. E and F: summary data show that MRS2179 injection into the RTN had no effect on FCt-induced changes in PNA amplitude (ΔPNA amplitude; B) or PNA frequency (ΔPNA frequency; C). *Different from saline (n = 6/group).
The above evidence suggests that FCt triggers the release of ATP, and considering that P2Y1 receptors regulate the activity of presympathetic C1 neurons located near the RTN (Sobrinho et al. 2014), we also tested effects of the P2Y1 receptor antagonist MRS2179 on breathing and blood pressure responses to FCt. We found that unilateral injection of MRS2179 (100 µM, 50 nl) into the RTN did not change baseline PNA amplitude (58 ± 3% vs. saline: 54 ± 6%, P > 0.05) or PNA frequency (42 ± 4% vs. saline: 43 ± 5%, P > 0.05), nor did it affect resting MAP (118 ± 4 mmHg, vs. saline: 117 ± 5 mmHg, P > 0.05). Furthermore, MRS2179 failed to block the respiratory effects elicited by FCt in the RTN region. For example, P2Y1 receptor blockade did not abrogate the increase in PNA amplitude (58 ± 6%, vs. saline + FCt: 55 ± 5%, P > 0.05) or PNA frequency (41 ± 3%, vs. saline + FCt: 39 ± 4%, P > 0.05) (Fig. 3, D–F). In addition, RTN injection of MRS2179 did not reveal an effect of FCt on MAP.
Our injections (FCt, PPADS, or MRS2179) were placed unilaterally in the RTN region (Figs. 4, A and B). The injection center was 200–230 μm below the facial motor nucleus, 200 μm rostral to the caudal end of this nucleus, and 1.8 mm lateral to the midline, as previously demonstrated (Barna et al. 2016; Sobrinho et al. 2014; Takakura and Moreira 2011). Note that the representative injection site targets the region of the RTN that contains the highest density of Phox2b-expressing neurons (Fig. 4A).
Injections of FCt into the cNTS or in the medullary raphe had a negligible effect on respiratory output.
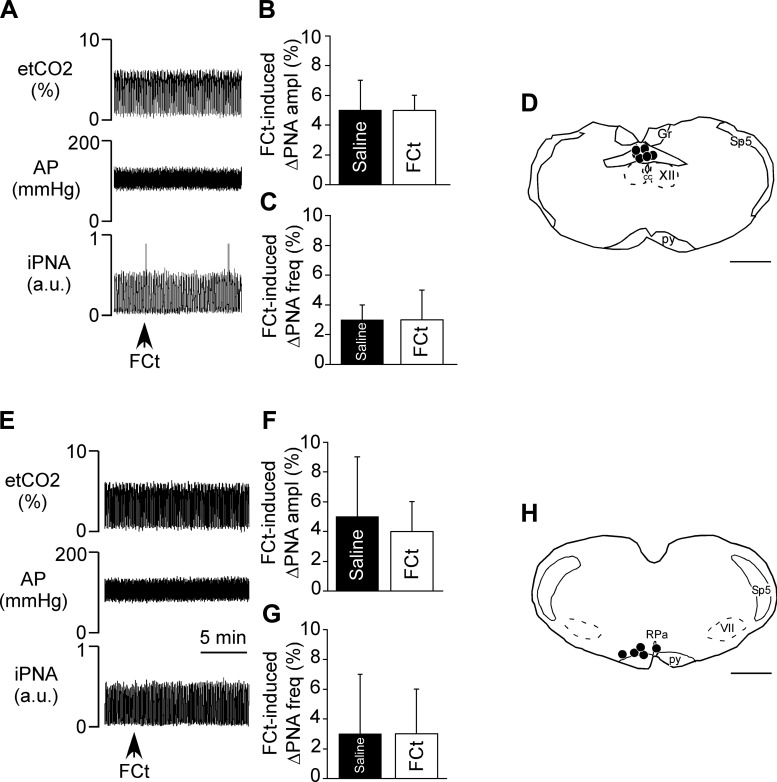
To determine whether pharmacological activation of astrocytes in other chemosensitive regions also stimulates cardiorespiratory output, we made FCt injections into the cNTS or RPa while measuring breathing and blood pressure in urethane-anesthetized rats. For these experiments, we targeted the raphe pallidus/parapyramidal region (RPa/PPy; n = 5) and the commissural NTS (n = 6) because cells in these regions reportedly function as chemoreceptors (Nattie and Li 2002; Richerson 2004; Sobrinho et al. 2014). The injections into the NTS were centered ∼400 μm caudal to the calamus scriptorius (Fig. 5D) (Sobrinho et al. 2014). According to the distribution of the fluorescent microbeads, injected material spread bilaterally ∼450–500 μm from the injection center and ∼250–300 μm from the injection center in the rostro-caudal direction, as previously demonstrated (Favero et al. 2011). Medullary raphe injections targeted the RPa/PPy 200–230 μm below the facial motor nucleus, 200 μm rostral to the caudal end of this nucleus, and 1 mm lateral to the midline, as previously demonstrated (Sobrinho et al. 2014; Mulkey et al. 2004) (Fig. 5H). Contrary to our cellular data, we found that injections of FCt into either the cNTS (Fig. 5, A–D) or RPa (Fig. 5, E–H) had a negligible effect on baseline PNA amplitude or frequency and resting MAP. These results are consistent with previous evidence that FCt injection into the cNTS minimally affected respiratory activity (Erlichman et al. 2008; Holleran et al. 2001) and suggest that astrocytes in these other putative chemoreceptor regions do not contribute directly to breathing.
Fig. 5.
Injections of fluorocitrate (FCt) into the commissural nucleus of the solitary tract (cNTS) or raphe pallidus (RPa) failed to stimulate breathing. A: recordings of end-expiratory CO2 (etCO2), arterial pressure (AP), and integrated phrenic nerve activity (iPNA) in urethane-anesthetized and artificially ventilated rats show that injection of FCt (1 mM, 50 nl) into the nucleus of the solitary tract (NTS) did not affect AP or respiratory motor output. B and C: summary data show that NTS injection of FCt had no effect on PNA amplitude (ΔPNA ampl; B) or PNA frequency (ΔPNA freq; C). D: computer-assisted plots showing the center of fluorescent microbeads within the NTS. E: recordings of etCO2, AP, and iPNA in urethane-anesthetized and artificially ventilated rats show that injection of FCt (1 mM, 50 nl) into the RPa did not affect AP or respiratory motor output. F and G: summary data show that RPa injection of FCt had no effect on ΔPNA ampl (F) or ΔPNA freq (G). H: computer-assisted plots showing the center of fluorescent microbeads within the RPa. Scale bars in D and H, 1 mm.
DISCUSSION
Evidence suggests that CO2/H+-evoked ATP release from ventral surface astrocytes contributes to RTN chemoreception (Gourine et al. 2010; Huckstepp et al. 2010b; Wenker et al. 2012). The mechanisms by which RTN astrocytes sense and respond to CO2/H+ are thought to involve CO2-dependent gating of Cx26 (Huckstepp et al. 2010a; Wenker et al. 012) and depolarization mediated by H+-inhibition of Kir4.1 channels (Wenker et al. 2010). It is also possible that RTN astrocytes release ATP by Ca2+-dependent exocytosis (Gourine et al. 2010; Kasymov et al. 2013). However, the potential contribution of changes in voltage to astrocyte chemosensitivity has yet to be demonstrated. Here, we confirm that FCt reversibly depolarizes RTN astrocytes, and we show that application of FCt into the RTN, but not cNTS or raphe, stimulates breathing by a purinergic-dependent mechanism. These results suggest that depolarization in the presence of CO2 is sufficient to modulate RTN chemoreception by a mechanism involving purinergic signaling rather than extracellular acidification.
It is not clear how depolarization contributes to ATP release by RTN astrocytes. Recent work shows that acid activation of the sodium bicarbonate cotransporter (NBC) and reverse mode operation of the NCX leads to an increase in intracellular Ca2+ in RTN astrocytes (Turovsky et al. 2016). Depolarization will increase NBC activity and favor Ca2+ influx via the sodium calcium exchanger (NCX), and an increase in intracellular Ca2+ will conceivably facilitate ATP release by exocytosis or influencing connexin hemichannel gating. However, it is also possible that depolarization-mediated activation of the NBC results in an extracellular acidification that potentiates chemoreceptor activity. Although this possibility is consistent with evidence that FCt application to the RTN resulted in an extracellular acidification in conjunction with an increase in breathing (Erlichman et al. 2010; Holleran et al. 2001), it is not consistent with previous cellular data (Wenker et al. 2010) or present in vivo results showing that excitatory effects of FCt on RTN chemoreceptor function could be blocked by P2 receptor blockade (Fig. 3, A–C).
It is important to point out that disruption of metabolic activity in astrocytes may affect neural activity by several mechanisms, including disruption of extracellular pH, K+ buffering, or neurotransmitter uptake. At the RTN level, a significant portion of the FCt ventilatory response could be blocked by prior injection of a P2 receptor antagonist. However, RTN injections of PPADS did not totally eliminate the ventilatory response to FCt. The residual FCt response in PPADS may involve astrocyte-dependent release of other transmitters or dysregulation of extracellular pH or K+. For example, Holleran et al. 2001 and Erlichman et al. 2010 showed that RTN injections of FCt resulted in an extracellular acidification in conjunction with an increase in breathing. Furthermore, a non-purinergic-dependent mechanism likely contributes to FCt activation of neurons in the cNTS and raphe. In support of this possibility, we have shown that CO2/H+-sensitive raphe neurons do not respond to exogenous ATP, and although NTS chemoreceptors are activated by exogenous ATP, applications of purinergic blockers failed to promote changes in chemosensitive response (Sobrinho et al. 2014). Perhaps more importantly, these cellular results are inconsistent with our in vivo data showing that injections of FCt into the NTS (Fig. 5, A–D) or raphe (Fig. 5, E–H) had a negligible effect on respiratory output. We speculate that differences in experimental preparations may account for these divergent results. For example, under in vivo conditions, blood flow helps buffer pH and ionic composition (namely K+) of the extracellular fluid. Therefore, it is possible that under in vitro conditions where there is no blood flow, FCt may increase extracellular H+ and K+ to the extent that neural activity increases, whereas in vivo blood flow is sufficient to limit the accumulation of these factors, and so neural activity remains stable. Despite these inconsistencies, our cellular evidence that purinergic receptor blockade strongly suppressed FCt-mediated activation of neurons in the RTN is consistent with the possibility that depolarization of RTN astrocytes facilitates purinergic activation of RTN chemoreceptors.
The results presented here are limited to the use of imperfect pharmacological tools that in addition to depolarizing astrocytes may also disrupt other astrocyte functions that also affect neural activity. Furthermore, commonly used molecular markers of astrocytes like glial fibrillary acidic protein can also be expressed by neurons (Liu et al. 2010), and considering that astrocytes can exhibit diverse cellular, molecular, and functional properties (John Lin et al. 2017), there is a need to identify genetic markers that can distinguish between functional subtypes of astrocytes, including chemosensitive and homeostatic astrocytes in the RTN. Recent technical advances in single-cell gene profiling make this need attainable; however, these experiments are beyond the scope of this study.
In summary, our findings suggest that depolarization is an important component of astrocyte chemoreception. Our results also support the possibility that astrocytes in the RTN, but not NTS or raphe, are specialized for purinergic modulation of chemoreceptor function. However, our results do not exclude the possibility that astrocytes in the NTS or RPa influence neural activity (Gordon et al. 2009; Costa et al. 2013). Additional experiments will also be needed to elucidate the role of NTS and RPa astrocytes in long-term homeostatic control.
GRANTS
This research was supported by public funding from the São Paulo Research Foundation (FAPESP; grants 2014/22406-1 to ACT and 2015/23376-1 and 2016/22069-0 to T. S. Moreira) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; grant 471263/2013-3 to A. C. Takakura), FAPESP fellowship (2011/13462-7 and 2015/12827-2 to CRS), and CNPq fellowship (301219/2016-8 to ACT and 301904/2015-4 to T. S. Moreira). This work was also supported by the National Institutes of Health Grant HL-104101 (D. K. Mulkey) and Connecticut Department of Public Health Grant 150263.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.R.S., C.M.G., and T.S.M. performed experiments; C.R.S., C.M.G., A.C.T., and T.S.M. analyzed data; C.R.S., C.M.G., A.C.T., D.K.M., and T.S.M. interpreted results of experiments; C.R.S., C.M.G., A.C.T., D.K.M., and T.S.M. prepared figures; C.R.S., A.C.T., D.K.M., and T.S.M. drafted manuscript; C.R.S., C.M.G., A.C.T., D.K.M., and T.S.M. edited and revised manuscript; C.R.S., C.M.G., A.C.T., D.K.M., and T.S.M. approved final version of manuscript; C.M.G., A.C.T., and T.S.M. conceived and designed research.
REFERENCES
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron 81: 728–739, 2014. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barna BF, Takakura AC, Mulkey DK, Moreira TS. Purinergic receptor blockade in the retrotrapezoid nucleus attenuates the respiratory chemoreflexes in awake rats. Acta Physiol (Oxf) 217: 80–93, 2016. doi: 10.1111/apha.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa KM, Moraes DJ, Machado BH. Acute inhibition of glial cells in the NTS does not affect respiratory and sympathetic activities in rats exposed to chronic intermittent hypoxia. Brain Res 1496: 36–48, 2013. doi: 10.1016/j.brainres.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Leiter JC. Glia modulation of the extracellular milieu as a factor in central CO2 chemosensitivity and respiratory control. J Appl Physiol (1985) 108: 1803–1811, 2010. doi: 10.1152/japplphysiol.01321.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlichman JS, Leiter JC, Gourine AV. ATP, glia and central respiratory control. Respir Physiol Neurobiol 173: 305–311, 2010. doi: 10.1016/j.resp.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlichman JS, Putnam RW, Leiter JC. Glial modulation of CO2 chemosensory excitability in the retrotrapezoid nucleus of rodents. Adv Exp Med Biol 605: 317–321, 2008. [DOI] [PubMed] [Google Scholar]
- Favero MT, Takakura AC, de Paula PM, Colombari E, Menani JV, Moreira TS. Chemosensory control by commissural nucleus of the solitary tract in rats. Respir Physiol Neurobiol 179: 227–234, 2011. doi: 10.1016/j.resp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Gordon GRJ, Iremonger KJ, Kantevari S, Ellis-Davies GCR, MacVicar BA, Bains JS. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron 64: 391–403, 2009. doi: 10.1016/j.neuron.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science 329: 571–575, 2010. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature 436: 108–111, 2005. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Bayliss DA. Neural control of breathing and CO2 homeostasis. Neuron 87: 946–961, 2015. doi: 10.1016/j.neuron.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61: 213–219, 2009. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86: 1009–1031, 2006. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Holleran J, Babbie M, Erlichman JS. Ventilatory effects of impaired glial function in a brain stem chemoreceptor region in the conscious rat. J Appl Physiol (1985) 90: 1539–1547, 2001. [DOI] [PubMed] [Google Scholar]
- Huckstepp RT, Eason R, Sachdev A, Dale N. CO2-dependent opening of connexin 26 and related β connexins. J Physiol 588: 3921–3931, 2010a. doi: 10.1113/jphysiol.2010.192096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, Id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol 588: 3901–3920, 2010b. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülsmann S, Oku Y, Zhang W, Richter DW. Metabotropic glutamate receptors and blockade of glial Krebs cycle depress glycinergic synaptic currents of mouse hypoglossal motoneurons. Eur J Neurosci 12: 239–246, 2000. doi: 10.1046/j.1460-9568.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- John Lin CC, Yu K, Hatcher A, Huang TW, Lee HK, Carlson J, Weston MC, Chen F, Zhang Y, Zhu W, Mohila CA, Ahmed N, Patel AJ, Arenkiel BR, Noebels JL, Creighton CJ, Deneen B. Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci 20: 396–405, 2017. doi: 10.1038/nn.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BJ, Chang DA, Mackay DD, West GH, Moreira TS, Takakura AC, Gwilt JM, Guyenet PG, Stornetta RL. Central nervous system distribution of the transcription factor Phox2b in the adult rat. J Comp Neurol 503: 627–641, 2007. doi: 10.1002/cne.21409. [DOI] [PubMed] [Google Scholar]
- Kasymov V, Larina O, Castaldo C, Marina N, Patrushev M, Kasparov S, Gourine AV. Differential sensitivity of brainstem versus cortical astrocytes to changes in pH reveals functional regional specialization of astroglia. J Neurosci 33: 435–441, 2013. doi: 10.1523/JNEUROSCI.2813-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18: 942–952, 2015. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SB, Lazarenko RM, Ludwig MG, Perez-Reyes E, Mohebbi N, Bettoni C, Gassmann M, Suply T, Seuwen K, Guyenet PG, Wagner CA, Bayliss DA. Regulation of breathing by CO2 requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science 348: 1255–1260, 2015. doi: 10.1126/science.aaa0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Namba T, Liu J, Suzuki R, Shioda S, Seki T. Glial fibrillary acidic protein-expressing neural progenitors give rise to immature neurons via early intermediate progenitors expressing both glial fibrillary acidic protein and neuronal markers in the adult hippocampus. Neuroscience 166: 241–251, 2010. doi: 10.1016/j.neuroscience.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, Hüttmann K, Matyash M, Kettenmann H, Steinhäuser C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci 23: 1750–1758, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigh L, Greenhalgh SA, Rodgers TL, Cann MJ, Roper DI, Dale N. CO2 directly modulates connexin 26 by formation of carbamate bridges between subunits. eLife 2: e01213, 2013. doi: 10.7554/eLife.01213 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreceptors: locations and functions. Compr Physiol 2: 221–254, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol (1985) 92: 2119–2130, 2002. doi: 10.1152/japplphysiol.01128.2001. [DOI] [PubMed] [Google Scholar]
- Nwaobi SE, Cuddapah VA, Patterson KC, Randolph AC, Olsen ML. The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol 132: 1–21, 2016. doi: 10.1007/s00401-016-1553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Khakh BS, Skatchkov SN, Zhou M, Lee CJ, Rouach N. New Insights on Astrocyte Ion Channels: Critical for Homeostasis and Neuron-Glia Signaling. J Neurosci 35: 13827–13835, 2015. doi: 10.1523/JNEUROSCI.2603-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Vallée J, Haber M, Murai KK, Lacaille J-C, Robitaille R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146: 785–798, 2011. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1998. [Google Scholar]
- Ramanantsoa N, Hirsch M-R, Thoby-Brisson M, Dubreuil V, Bouvier J, Ruffault P-L, Matrot B, Fortin G, Brunet J-F, Gallego J, Goridis C. Breathing without CO2 chemosensitivity in conditional Phox2b mutants. J Neurosci 31: 12880–12888, 2011. doi: 10.1523/JNEUROSCI.1721-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5: 449–461, 2004. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Sobrinho CR, Wenker IC, Poss EM, Takakura AC, Moreira TS, Mulkey DK. Purinergic signalling contributes to chemoreception in the retrotrapezoid nucleus but not the nucleus of the solitary tract or medullary raphe. J Physiol 592: 1309–1323, 2014. doi: 10.1113/jphysiol.2013.268490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26: 10305–10314, 2006. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Barna BF, Cruz JC, Colombari E, Moreira TS. Phox2b-expressing retrotrapezoid neurons and the integration of central and peripheral chemosensory control of breathing in conscious rats. Exp Physiol 99: 571–585, 2014. doi: 10.1113/expphysiol.2013.076752. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS. Contribution of excitatory amino acid receptors of the retrotrapezoid nucleus to the sympathetic chemoreflex in rats. Exp Physiol 96: 989–999, 2011. doi: 10.1113/expphysiol.2011.058842. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572: 503–523, 2006. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turovsky E, Theparambil SM, Kasymov V, Deitmer JW, Del Arroyo AG, Ackland GL, Corneveaux JJ, Allen AN, Huentelman MJ, Kasparov S, Marina N, Gourine AV. Mechanisms of CO2/H+ Sensitivity of Astrocytes. J Neurosci 36: 10750–10758, 2016. doi: 10.1523/JNEUROSCI.1281-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Kréneisz O, Nishiyama A, Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol 104: 3042–3052, 2010. doi: 10.1152/jn.00544.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Sobrinho CR, Takakura AC, Moreira TS, Mulkey DK. Regulation of ventral surface CO2/H+-sensitive neurons by purinergic signalling. J Physiol 590: 2137–2150, 2012. doi: 10.1113/jphysiol.2012.229666. [DOI] [PMC free article] [PubMed] [Google Scholar]