Superficial neurons in the rat granular retrosplenial cortex (GRS) show distinctive late-spiking (LS) firing property. However, little is known about spatiotemporal dynamics of signal transduction in the GRS. We demonstrated LS neuron network relaying thalamic inputs to deep layers and anisotropic distribution of inhibition between coronal and horizontal planes. Since deep layers of the GRS receive inputs from the subiculum, GRS circuits may work as an integrator of multiple sources such as sensory and memory information.
Keywords: retrosplenial cortex, cortical layers, synaptic transmission, voltage-sensitive dye imaging
Abstract
Rodent granular retrosplenial cortex (GRS) has dense connections between the anterior thalamic nuclei (ATN) and hippocampal formation. GRS superficial pyramidal neurons exhibit distinctive late spiking (LS) firing property and form patchy clusters with prominent apical dendritic bundles. The aim of this study was to investigate spatiotemporal dynamics of signal transduction in the GRS induced by ATN afferent stimulation by using fast voltage-sensitive dye imaging in rat brain slices. In coronal slices, layer 1a stimulation, which presumably activated thalamic fibers, evoked propagation of excitatory synaptic signals from layers 2–4 to layers 5–6 in a direction perpendicular to the layer axis, followed by transverse signal propagation within each layer. In the presence of ionotropic glutamate receptor antagonists, inhibitory responses were observed in superficial layers, induced by direct activation of inhibitory interneurons in layer 1. In horizontal slices, excitatory signals in deep layers propagated transversely mainly from posterior to anterior via superficial layers. Cortical inhibitory responses upon layer 1a stimulation in horizontal slices were weaker than those in the coronal slices. Observed differences between coronal and horizontal planes suggest anisotropy of the intracortical circuitry. In conclusion, ATN inputs are processed differently in coronal and horizontal planes of the GRS and then conveyed to other cortical areas. In both planes, GRS superficial layers play an important role in signal propagation, which suggests that superficial neuronal cascade is crucial in the integration of multiple information sources.
NEW & NOTEWORTHY Superficial neurons in the rat granular retrosplenial cortex (GRS) show distinctive late-spiking (LS) firing property. However, little is known about spatiotemporal dynamics of signal transduction in the GRS. We demonstrated LS neuron network relaying thalamic inputs to deep layers and anisotropic distribution of inhibition between coronal and horizontal planes. Since deep layers of the GRS receive inputs from the subiculum, GRS circuits may work as an integrator of multiple sources such as sensory and memory information.
the granular retrosplenial cortex (GRS) is one of the largest cortical regions in the rodent posterior cingulate cortex, which forms dense reciprocal connections with the anterior thalamic nuclei (ATN) and hippocampal formation (HCF). These three areas have been assumed to operate conjointly to support spatial learning and memory (Aggleton et al. 2010; Chen et al. 1994; Cooper and Mizumori 2001; Dumont et al. 2010; Garden et al. 2009; Pothuizen et al. 2008; Vann et al. 2009; Vogt and Miller 1983), default mode network (Buckner et al. 2008; Uddin et al. 2009; Vann et al. 2009), and emotional evaluation of behavioral contexts (Maddock 1999). Connections between the GRS and ATN strongly affect association learning (Gabriel et al. 1980a, 1980b). Furthermore, the hippocampal-retrosplenial network is responsible for spatial representation (Honda et al. 2011). These findings strongly suggest that the GRS serves as a bridge, which links the ATN and HCF and integrates these two input signals.
Superficial layers of the GRS consist mainly of closely packed, callosally projecting small pyramidal neurons (Ichinohe et al. 2008; Sripanidkulchai and Wyss 1987; Wyss et al. 1990). Their apical dendrites form prominent bundles, which spread out in layer 1a and colocalize with patchy thalamic terminations, mainly from the ATN (Ichinohe et al. 2003; Odagiri et al. 2011; Shibata 1993; Sripanidkulchai and Wyss 1986; van Groen and Wyss 1990, 2003; Wyss et al. 1990). The most remarkable feature of layer 2–3 neurons is their late spiking (LS) firing, which presumably facilitates comparison or integration of differently timed synaptic inputs, consistent with the proposed role of the GRS in memory-related processes (Kurotani et al. 2013a).
Despite a need to clarify an integrated view of GRS function, most of the previous studies were devoted to provide an anatomical identification of major inputs and outputs to the area (Aggleton et al. 2010; Fisk and Wyss 1999; Shibata et al. 2004; Sripanidkulchai and Wyss 1986; Van Groen and Wyss 1990, 1992, 2003; Wyss and Van Groen 1992) and we have only limited knowledge on GRS functional connectivity, which was revealed by current source-density (CSD) analysis of field potentials (Hedberg et al. 1993; Nixima et al. 2013). Thus, to understand the GRS function, it is necessary to elucidate how ATN inputs are processed in the GRS microcircuitry, especially by the distinctive superficial neurons. We conducted an optical recording of neuronal activity using fast voltage-sensitive dye in rat GRS brain slice preparation. Our results demonstrated that thalamic inputs first activate superficial and then deeper GRS layers. Given that HCF input directly projects to deeper GRS layers, ATN and HCF inputs temporally modulated by superficial neuronal cascades may trigger synaptic plasticity in deeper layers. We also revealed that the superficial layers were essential for the transverse propagation of excitation along the cortical layers to other cortical areas, suggesting a possible role of the GRS as an “information hub.”
MATERIALS AND METHODS
Slice preparation and staining procedure.
All experiments and procedures were approved by the Institutional Animal Care and Use Committee of the University of Tokyo. A total of 41 brain slices were prepared from 20 male Wistar rats (postnatal days 22–28; weight 31–62 g). Rats were deeply anesthetized by isoflurane (Forane, Abbott Japan, Tokyo, Japan) and decapitated. The whole rat brain was removed and immersed into the ice-cold artificial cerebrospinal fluid (ACSF; 126 NaCl, 3 KCl, 2.4 CaCl2, 1.3 MgSO4, 1.2 NaH2PO4, 26 NaHCO3, and 10 mM glucose; 297–305 mosmol/kg) saturated with 95% O2 and 5% CO2. Coronal (n = 25; bregma −5.30 to −3.30 mm) and horizontal (n = 16; dorsoventral (DV) −1.20 to −0.80 mm) slices, which included the GRS, were dissected using a vibrating microtome (Pro7, Dosaka, Kyoto, Japan; thickness 400 μm). The slices were transferred on membrane filters (Omni Pore membrane filter, JCWP01300, Millipore, Billerica, MA) to an interface-type incubation chamber perfused with warm, oxygenated ACSF (30–35°C) for recovery. After incubation for at least 1 h, we carried out the staining procedure. Dye preparation and staining procedure were as described by Tominaga et al. (2000). The fast voltage-sensitive dye (Di-4-ANEPPS, Invitrogen, Waltham, MA, dye concentration, 3.3 mg/ml) was dissolved in a 2:1 mixture of ethanol and 10% Cremophor EL (Sigma-Aldrich, St. Louis, MO, vol/vol in distilled water), and stored at −30°C as a stock solution. The stock solution was dissolved in a mixture of ACSF and fetal bovine serum (1:1 ratio), so that the final concentration of the dye in the slice staining solution was 0.2 mM. Each slice was transferred into a silicon well and stained with 100 µl of the staining solution for 30 min in the incubation chamber. After being well rinsed with the ACSF and left recovered for at least 1 h, each stained slice was transferred into an interface-type recording chamber (30–35°C) and perfused with the ACSF. Neural structures, such as layers 2–3 of the GRS or the corpus callosum, were clearly visible in each slice with a dissecting microscope.
Optical recording.
Light emitted by a LED (LEX2, LED light source unit 40087, Brainvision, Tokyo, Japan) was projected onto stained slices after being passed through an excitation filter (center wave length λ = 531 nm). Fluorescence signals were conveyed through an absorption filter (transition wavelength λ = 580 ± 5 nm) to a CMOS camera (resolution: 192 × 160 pixels) and data were collected through an Input/Output interface (MiCAM02, Brainvision). A 2.69 × 2.13 mm2 imaging area (188 × 160 active pixels) was covered with a ×5 objective lens (1044723, NA: 0.5, Leica Microsystems, Wetzlar, Germany). A total of 256 frames (307.20 ms) of ΔF/F transitions were recorded at 833.33 Hz (sampling interval 1.20 ms) during each trial. To avoid any disturbance caused by the LED system or rapid bleaching of the dye, we turned on the LED light source 100 ms before the start of the recording and turned it off following the completion of the data acquisition. The data were acquired by Brainvision Analysis Software (Brainvision).
At the beginning of a recording sequence (for each stimulus and nonstimulus trial), the initial baseline fluorescence was recorded for the first 9.60 ms, to normalize the optical signals (i.e., to calculate parameter ΔF/F) for each trial. Stimuli were then delivered after a 30-ms delay to stabilize the optical baseline. A monopolar tungsten stimulating electrode was placed in layer 1a (DV = 1.07 ± 0.03 mm, n = 20) to focally stimulate projection fibers from the ATN (e.g., Sripanidkulchai and Wyss 1986; see details in results) or layer 5 (DV = 1.13 ± 0.05 mm, anteroposterior = 0.50 ± 0.04 mm, n = 5) of the coronal slices. The electrode was placed in layer 1a (3.0 mm anterior to the posterior end of the slice, n = 16) of the horizontal slices (gray arrows in each figure). The stimulating electrode was manipulated and placed according to the rat brain atlas (Paxinos and Watson 1998). The ground electrode (an Ag-AgCl wire) was placed in the ASCF more than 1 mm away from the monopolar electrode. Constant-current pulses (duration 0.1 ms; frequency 0.033 Hz) were applied so as to be tip negative for the tungsten electrode, using the stimulus isolator (BSI-2, Bak Electronics, Umatilla, FL). To avoid unexpected artifacts or bleaching by LED light exposure, we employed subtraction of nonstimulus trial data from stimulus data for averaging and analysis. Electrical stimulations were given every 30 s and optical signals were recorded every 15 s for stimulus and nonstimulus trials alternately. A subtracted image (= stimulus trial − nonstimulus trial) was calculated using each trial pair. The data were averaged 16 times to increase the signal-to-noise ratio.
Data analysis.
In this study, we measured the change in the intensity of fluorescence (ΔF) in each pixel relative to the initial intensity of fluorescence (F). The fractional change in light intensity (ΔF/F) was used for analysis to normalize the difference in the amount of dye in the slice. The ΔF/F was superimposed on the initial fluorescent image of each frame and represented by pseudo-colors: red indicated membrane depolarization (i.e., a decrease in fluorescence defined as a positive signal) and blue indicated membrane hyperpolarization (a negative signal). Images were analyzed with Brainvision Analysis Software. The software spatial filter (boxcar, 7 × 7 pixels) was applied twice to the acquired image in each frame. The optical signal data in each layer were averaged across slices, so the data are expressed as the mean ± SE (standard error of the mean) of a total number (n) of tested slices. For statistical analysis of data obtained from optical experiments, paired t-test or one-way ANOVA was employed unless otherwise mentioned.
Stimulus intensity and component identification.
Five coronal slices were used to determine the appropriate stimulus intensity for GRS slices. One monopolar tungsten stimulating electrode was placed in layer 1a to stimulate projection fibers from the ATN. For each slice, the recording process consisted of four sessions. In each session, nine stimuli of stepwise-increasing intensity from the 0.00 to 0.40 mA (0.05-mA increment) were applied at a 15-s interval.
Another five coronal slices were used for simultaneous extracellular recordings of field potentials (FPs) to identify neural activity-based optical signals. Glass microelectrodes (GD-1.5, Narishige Scientific Instrument Laboratory. Tokyo, Japan) filled with 0.9% NaCl containing 2% Direct Blue 1 (Tokyo Chemical Industry, Tokyo, Japan) (average impedance 10.9 MΩ at 100 Hz) were inserted into layer 5 of the GRS, 0.50 ± 0.02 mm lateral to the stimulating electrode. The electrical signals were band-pass filtered (low and high cutoff frequencies 1 Hz and 3 kHz, respectively), 1,000 × amplified, and digitized by an analog-to-digital converter (resolution 12 bits and sampling rate 10 kHz).
Pharmacological blockade of synaptic transmission.
To confirm that optical signals evoked by the stimulation of layer 1a were synaptic, we examined their sensitivity to antagonists of ionotropic glutamate receptors in 10 coronal and 6 horizontal slices. After recording in normal ACSF, we perfused the ACSF containing 40 µM 6,7-dinitroquinoxaline-2,3-dione (DNQX, Tocris Bioscience, Bristol, UK) and 50 µM dl-2-amino-5-phosphonopentanoic acid (dl-AP5, Tocris Bioscience) for over 30 min to block excitatory synaptic transmission. Optical recordings were then repeated following the same set of procedures. In all coronal slices tested (n = 5), negative signals were detected even in the presence of DNQX and dl-AP5, while positive signals were almost abolished (see results). To establish whether those negative signals represented inhibitory synaptic transmission, we next perfused the ACSF containing a high (20 µM) concentration of bicuculline methiodide (BMI, Sigma-Aldrich), in addition to 40 µM DNQX and 50 µM dl-AP5, for over 30 min to block both inhibitory and excitatory synaptic transmission. Similarly, we used additional five coronal slices to examine the effect of layer 5 stimulation using the same pharmacological procedure.
Fiber-cutting experiments.
We observed transverse signal propagation in deep layers evoked by layer 1a stimulation in both coronal and horizontal slices (see details in results). This propagation might be attributed to transverse connections confined within deep layers. Another possibility is that mutual vertical connections between deep and superficial layers were necessary to attain transverse propagation. To examine these possibilities, we used another 10 slices (5 coronal and 5 horizontal), which had a vertical cut made in deep layers (perpendicular to the layer axis; horizontal cut for coronal slices; coronal cut for horizontal slices) to check whether the cut disturbed transverse propagation within deep layers of the GRS. If propagation in deep layers was blocked by the cut, we would conclude that excitatory signals are mainly transmitted within the deep layer connections and do not depend on the activation of superficial neurons. After allowing each stained slice to recover for 30 min in the incubation chamber, we performed a cortical cut in GRS deep layers (layers 5 and 6; 0.27 ± 0.03 mm dorsal to a stimulation electrode for coronal slices; 0.36 ± 0.02 mm anterior for horizontal slices) using a razor blade under a dissecting microscope. Then the slices were allowed to recover for another 30 min. The optical recordings (layer 1a stimulation) were conducted using the same procedure as described above for intact slices.
In horizontal slices, transverse excitations in deep layers were not completely blocked by vertical (coronal) cuts (see details in results). To examine whether transverse propagation of excitatory signals could also be mediated by deep layer transverse connections, we tested another five horizontal slices with a cut parallel to the layer axis (parasagittal cut) just under superficial layers (layers 2–3). Cutting and recording (layer 1a stimulation) procedures were the same as described above.
Histology.
After optical and electrophysiological studies, the slices were fixed with a phosphate-buffered 4% paraformaldehyde solution for more than 3 days at 4°C. The slices were rinsed with 0.01 M phosphate buffered-saline (PBS) and infused with 30% sucrose/PBS for more than 1 h. Histological sections (50 µm in thickness) were then cut by a freezing microtome and Nissl-stained with cresyl violet. The borders of GRS layers and other brain regions were determined by the size and density of neurons using light microscopy.
Patch-clamp recordings and morphological analysis.
To examine the spatial distribution and time course of inhibitory responses observed in coronal slices in voltage-sensitive dye (VSD) experiments, we performed patch-clamp recordings from interneurons in layer 1 or LS neurons in layers 2–3 of the GRS. These neurons were visualized by infrared differential interference contrast video microscopy and whole-cell recordings were conducted with patch pipettes filled with an internal solution containing (in mM), 130 K-gluconate, 10 NaCl, 5 MgSO4, 10 HEPES, 2 Na-ATP, 0.6 Na-GTP, 10 tris-creatine phosphate, 0.6 EGTA (pH 7.3 with KOH, 305 mosmol/kg resistance 5–7 MΩ). For the recordings from layer 1 interneurons, biocytin (Nε-biotinyl-l-lysine, 3 mg/ml, Nacalai Tesque, Kyoto, Japan) was dissolved in the internal solution to visualize morphology of the cells. After the whole-cell configuration was established, the firing pattern of the cell was recorded by injecting DC step currents in current-clamp mode and the cell were kept for 10–15 min to allow biocytin to diffuse. After the recording session, slices were fixed with 4% paraformaldehyde (in 0.1 M phosphate buffer) overnight. The fixed slices were reacted with avidin-biotin complex system and stained with 3,3′-diaminobenzidine (VECTASTAIN Elite ABC Standard Kit, PK-6100 and DAB Peroxidase Substrate Kit, SK-4100, Vector Laboratories, Burlingame, CA) according to the protocol recommended by the manufacturer. The morphology of each interneuron was photographed by BZ-X700 microscope (KEYENCE, Osaka, Japan) and spatial distribution of dendrites and axons were measured to estimate the extent of area that could receive inhibitory input from that single interneuron. For the recordings from layer 2 neurons, a monopolar tungsten stimulating electrode was placed in layer 1a, as described above, and an electrical pulse (intensity, 0.20 mA and duration 100 μs, tip negative) was applied with 10-s interval. To isolate monosynaptic inhibitory postsynaptic potentials (IPSPs) to measure the time course, glutamate antagonists (DNQX and dl-AP5) were administered in ACSF. LS neurons in layer 2 were identified by their small pyramidal shapes and further confirmed by their LS firing pattern evoked by depolarizing DC current pulse. To show the waveform clearly and to measure the parameters of the time course consistently, IPSPs in LS neurons were recorded at −60 mV in current-clamp mode with DC current injection. The time course of IPSPs was compared with that of the inhibitory responses observed in VSD recordings.
RESULTS
Identification of the recording sites.
According to previous anatomical studies (van Groen and Wyss 1990, 1992, 2003; Wyss and Sripanidkulchai 1984), the retrosplenial cortex is subdivided into three parts, dysgranular (DRS), granular a (GRSa), and granular b (GRSb) cortices. The GRSb can be clearly identified in Nissl-stained sections. In the GRSb, layers 2–3 are wider, layer 2 cells are smaller and darkly stained, and neuronal cell bodies in layer 5 tend to be larger than cell bodies in other subregions of the retrosplenial cortex. Using these criteria and the rat brain atlas (Paxinos and Watson 1998), we confirmed that the recording sites mainly included the GRSb, DRS, and corpus callosum in coronal slices (Fig. 1A), and predominantly GRSb in horizontal slices (Fig. 1B). We also confirmed layer organization (from layer 1 to 6; layer 3 was usually adjoined to layer 2) by the neuron size and density in Nissl-stained sections (Fig. 1, C and D).
Fig. 1.
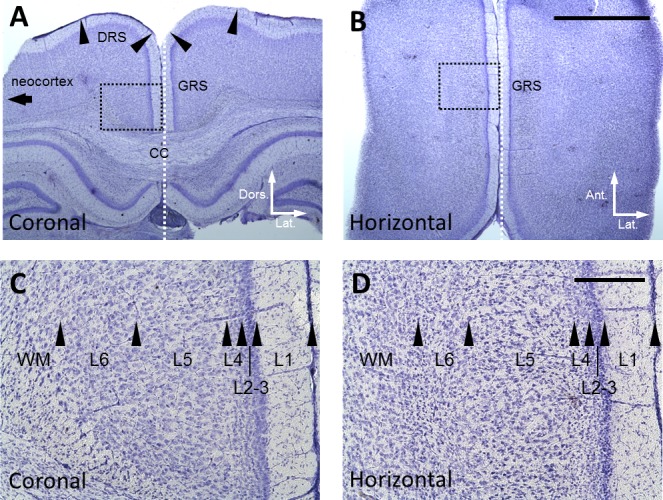
Location of recording sites. A: Nissl-stained coronal section of the GRS. DRS, dysgranular retrosplenial cortex; and CC, corpus callosum. Arrowheads indicate the borders of cortical areas. B: Nissl-stained horizontal section of the GRS. Scale bar, 2 mm, is also applied to A. White dotted line in A and B shows the midline of the brain. C and D: Magnified view of the area indicated by black dotted rectangle in A and B: respectively. Arrowheads indicate the borders of cortical layers. Scale bar, 200 µm, in D is also applied to C.
Stimulus intensity and component identification.
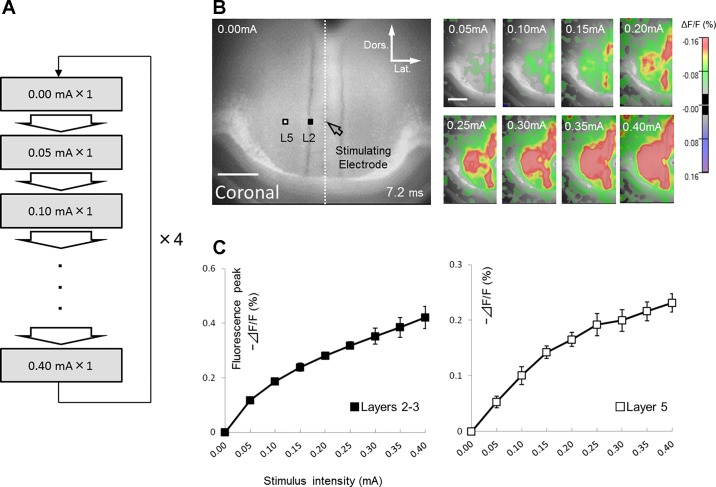
In the initial series of experiments, we systematically explored the appropriate stimulus intensity for GRS slices. Image data recorded with zero strength stimulus was subtracted respectively from images recorded in eight other sessions to control the optical baseline. This procedure was repeated four times and the four data points corresponding to respective stimulus intensities were averaged to obtain the final movie data. This acquisition procedure helped to avoid any temporal effects caused by long-term bleaching of the dye during the recording (Fig. 2A). Figure 2, B and C illustrates the relationship between the stimulus intensity (0.05 mA steps from 0.00 to 0.40 mA) and optical signals (−ΔF/F) in superficial layers (layers 2–3) and the deep layer 5 (0.20 and 0.46 mm lateral to the stimulating electrode, black and white squares in Fig. 2B, respectively). Based on the observed relationship, we eventually employed 0.20 mA stimulus strength for GRS stimulation, which evoked ~60% of the maximal responses observed at 0.40 mA.
Fig. 2.
Determination of the optimal stimulus intensity. A: recording procedure for stimulus intensity experiment. B: representative examples of optical responses recorded from a GRS coronal slice stained with Di-4-ANNEPS. Left: reference image of slice. The location of stimulus site is indicated by open arrow. In this and following figures, scale bar, 500 µm, is applied. White and black squares show the sites in layer 2 and 5, respectively where the optical responses were taken to draw input-output relationship graphs shown in C. In this case, the location of each site was at around 0.20 mm and 0.46 mm lateral to the stimulating electrode, respectively. Right: optical responses evoked in left hemisphere (region of interest, ROI) by 0.05 to 0.4 mA stimulation (indicated in each panel). Optical responses are presented as pseudo-color images. Red color illustrates a decrease in fluorescence, reflecting membrane depolarization. These optical responses were taken 7.2 ms after stimulation. In this and following figures, value indicated at the bottom of images indicates the time when the images were taken from the stimulation. C: the relationship between the stimulus intensity (0.05 mA steps from 0.00 to 0.40 mA) and optical signals (−ΔF/F). Data are presented as mean ± SE (n = 5).
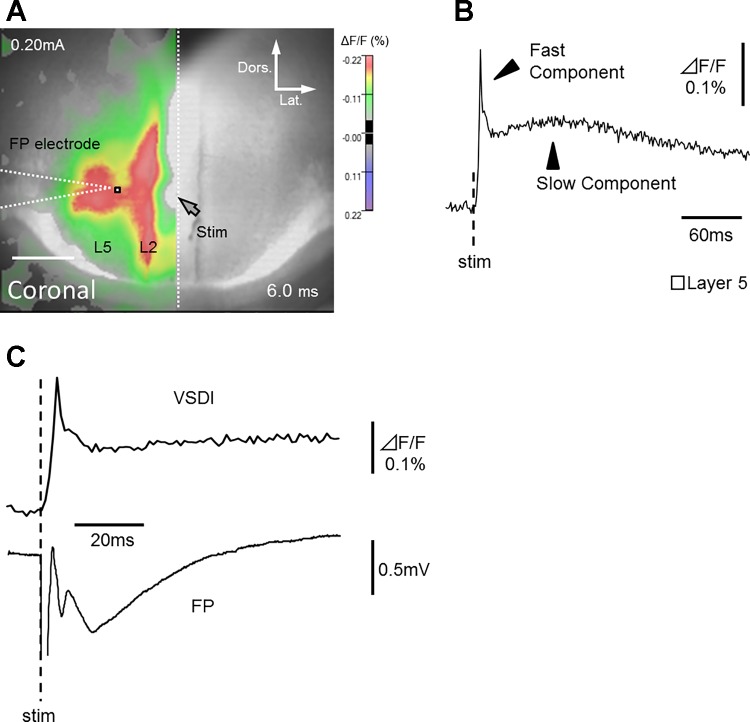
Next, to identify optical signals generated by neural activity, we conducted simultaneous FP recordings using extracellular glass microelectrodes in layer 5 (0.50 ± 0.02 mm lateral of the stimulating electrode, white square in Fig. 3A). As shown in Fig. 3B, we observed two major types of optical responses: fast and slow fluorescence signals. The time course of the fast optical response was similar to that of prominent FPs in all slices tested (n = 5; Table 1; Fig. 3C). Slow signals, however, were not detected in FP waveforms. The slow fluorescence signals generated by electrical stimulation have been reported in some previous studies, which utilized Di-4-ANEPPS (Iijima et al. 1996; Kajiwara et al. 2007; Koganezawa et al. 2008; Wang et al. 2011; Yuste et al. 1997). Yuste et al. (1997) indicated that these slow signals do not reflect true voltage changes but occur due to altered scattering properties of the slice. They reported that slow signals are independent of the wavelength and can be isolated at the emission zero crossing point, unlike the fast signals.
Fig. 3.
Comparison of optical responses with simultaneously recorded field potentials. A: optical signal superimposed on reference image. Location of glass micro-electrode used for FP recording is also indicated as white dashed line. White dotted line indicates cortical midline. Only the optical signals of responsible area (left hemisphere) is shown. B: trace of fast and slow optical components in layer 5 (white square in A). C: fast optical component and simultaneously recorded FP.
Table 1.
Properties of signals obtained during voltage-sensitive dye imaging and electrophysiological recordings
| Recording Site | Onset Latency, ms | Peak Latency, ms | Peak Amplitude | |
|---|---|---|---|---|
| VSDI | Layer 5 (n = 5) | 1.0 ± 0.24 | 4.8* | −0.23 ± 0.011% |
| FP | Layer 5 (n = 5) | 4.8 ± 0.60 | 11.8 ± 0.60 | −1.56 ± 0.094 mV |
Properties of signals obtained during voltage-sensitive dye imaging (VSDI) and electrophysiological recordings. Note that the time course of the fast optical signal (onset and peak latency from electrical stimulation) is similar to that of a prominent FP. Data are presented as the mean ± SE.
SE was much less than the sampling time.
Based on these and our own observations, only the fast optical components were regarded as true reflection of the neural activity in this study. Therefore, in the following sections, we analyze fast signals (data at the peak latency) induced by 0.20 mA electrical stimulation.
Signal propagation in coronal slices.
Layer 1a of the GRS exclusively receive thalamic inputs from the ATN (Ichinohe et al. 2003; Odagiri et al. 2011; Shibata 1993; Sripanidkulchai and Wyss 1986; Van Groen and Wyss 1990, 2003; Wyss et al. 1990). Therefore, we assumed that a series of synaptic events in the GRS activated by afferent inputs from the ATN could be replicated by a selective stimulation of layer 1a in the slice preparation (open arrow in each figure). Indeed, layer 1a stimulation produced prominent synaptic FPs in the GRS, without any antidromic activations (Nixima et al. 2013).
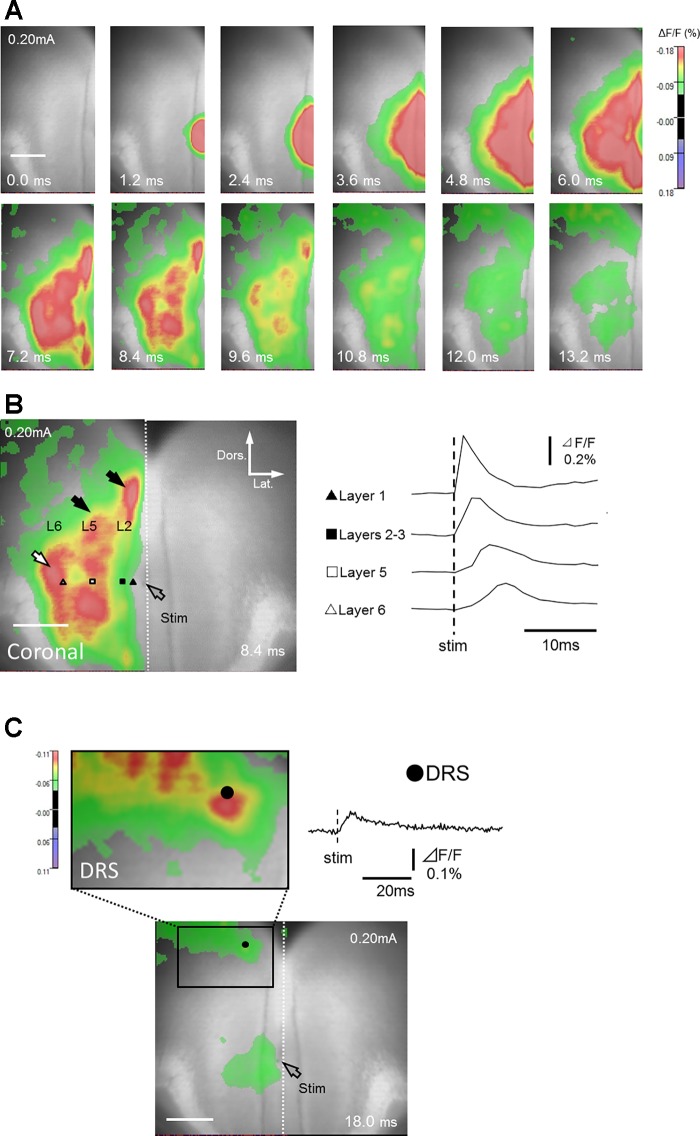
In coronal slices, layer 1a stimulation induced excitatory responses first in layer 1 (Table 2; data around 0.086 mm lateral to the stimulating electrode, black triangle in Fig. 4B) and in layers 2–3 (Table 2; data around 0.20 mm lateral to the electrode, black square in Fig. 4B), followed by the propagation of excitatory signals to layer 5 (Table 2; data around 0.46 mm lateral to the electrode, white square in Fig. 4B) in all five slices tested (Fig. 4 and Supplemental Movie S1; Supplemental Material for this article is available online at the Journal website). These responses were accompanied by transversely propagating signals within superficial and deep layers (Fig. 4B; black arrows). In deep layers, the excitation was first transmitted to layer 5 and then to layer 6 (Table 2; Fig. 4B; white arrow; n = 5/5; data around 0.76 mm lateral to the electrode, white triangle in Fig. 4B). In all five slices tested, the excitation was not confined only within the GRS but also spread to the DRS (especially to its superficial layers) (Table 2; Fig. 4C; black circle).
Table 2.
Properties of fEPSPs and fIPSPs in coronal slices
| Recording Site | Onset Latency | Peak Latency | Peak Fluorescence ΔF/F, % | |
|---|---|---|---|---|
| fEPSPs | Layer 1 | 0.0* | 1.2* | −0.44 ± 0.021 |
| (Normal ACSF) | Layers 2–3 | 0.0* | 2.6 ± 0.24 | −0.25 ± 0.010 |
| (layer 1a stimulation, n = 5) | Layer 5 | 0.7 ± 0.29 | 5.0 ± 0.24 | −0.17 ± 0.015 |
| Layer 6 | 1.7 ± 0.29 | 7.0 ± 0.45 | −0.14 ± 0.032 | |
| DRS | 4.1 ± 0.48 | 13.9 ± 1.54 | −0.10 ± 0.014 | |
| fIPSPs | Layer 1 (n = 5) | 3.1 ± 0.22 | 7.0 ± 0.45 | +0.093 ± 0.016 |
| (DNQX,dl-AP5) | Layers 2–3 (n = 5) | 4.3 ± 0.29 | 7.7 ± 0.48 | +0.031 ± 0.0049 |
| (layer 1a stimulation) | ||||
| fIPSPs | Layer 1 (n = 5) | 5.5 ± 0.29 | 8.9 ± 0.61 | +0.035 ± 0.0040 |
| (DNQX,dl-AP5) | Layers 2–3 (n = 3) | 5.6 ± 0.40 | 8.4 ± 0.69 | +0.027 ± 0.0092 |
| (layer 5 stimulation) |
Onset latency, peak latency, and peak fluorescence amplitude of fluorescent excitatory postsynaptic potentials (fEPSPs) and fluorescent inhibitory postsynaptic potentials (fIPSPs) in coronal slices. Onset and peak latencies were longer in deep layers than in superficial layers. Note that onset and peak latencies of fIPSPs were relatively longer than those of fEPSPs.
SE was much less than the sampling time.
Fig. 4.
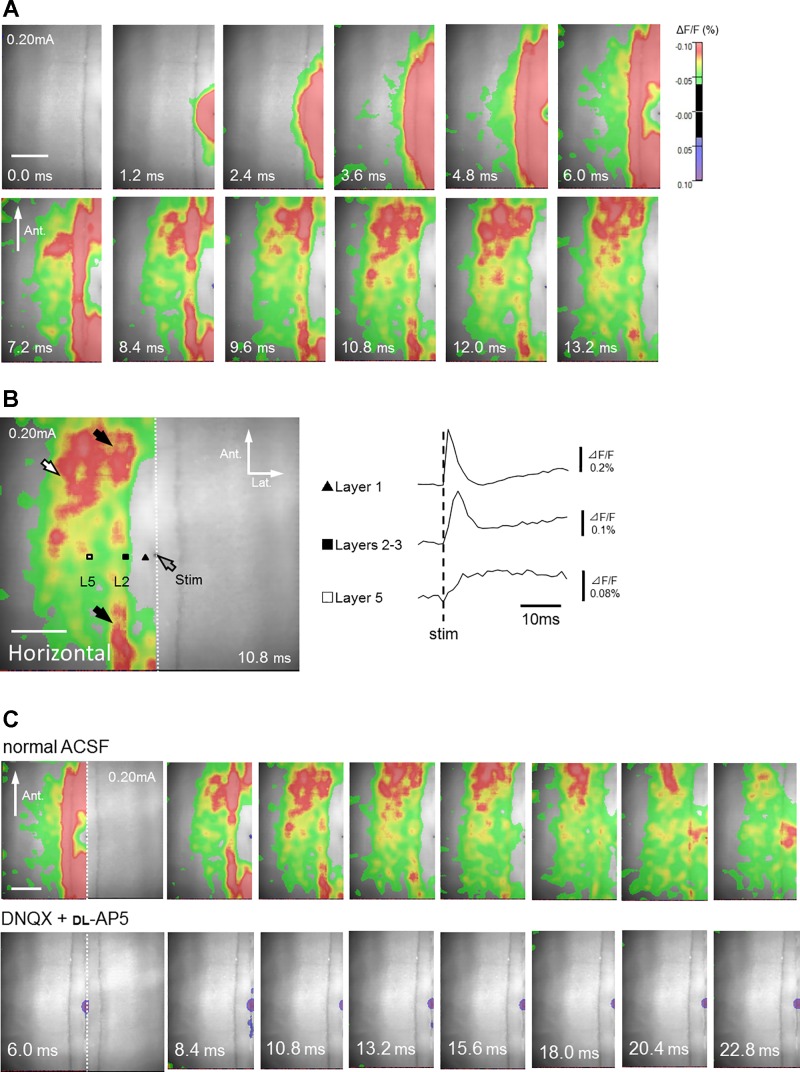
Optical responses recorded from coronal slices. A: time course of changes in fluorescence in response to a single electrical stimulation of layer 1a (see also Supplemental Movie S1). Only left hemisphere (ROI) is shown. B: traces of optical signals acquired from layer 1 (black triangle), layers 2–3 (black square), layer 5 (white square), and layer 6 (white triangle). Layer 1a stimulation induced excitatory signals in layers 2–3, and then in layer 5, which later propagated transversely within the same layers (black arrows). The excitation wave also propagated to layer 6 (white arrow). C: excitatory signals reached the DRS (black circle).
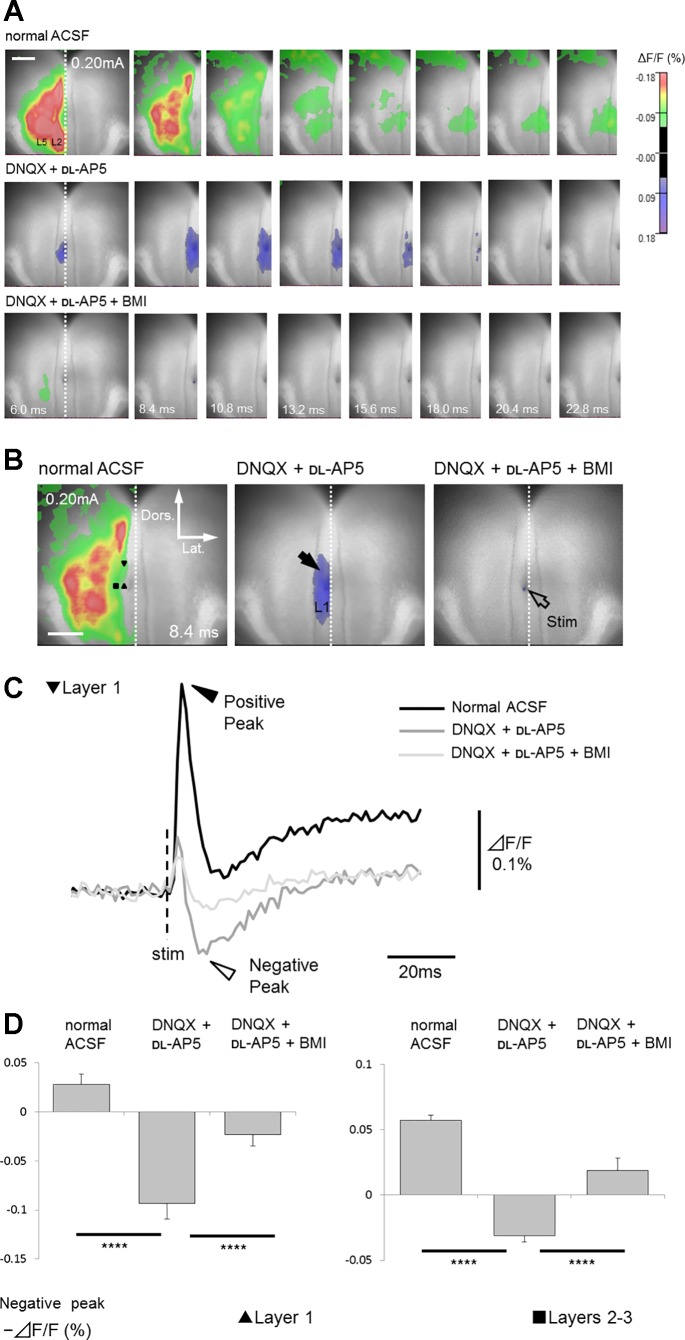
To confirm that the observed excitation was synaptically evoked (and they are not antidromic responses), DNQX and dl-AP5 were perfused. The depolarizing responses in superficial and deep layers were almost abolished by the glutamate antagonists (cf. upper and middle panels in Fig. 5A; n = 5 slices of 5), indicating that they were evoked by orthodromic excitatory synaptic transmission.
Fig. 5.
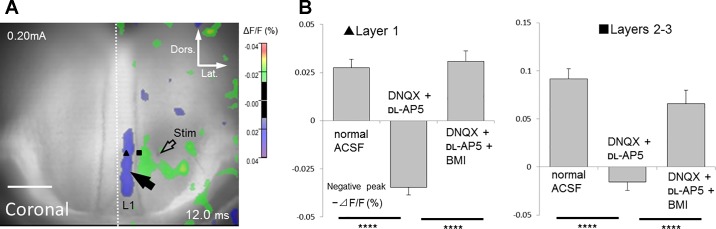
Effects of glutamate and GABA antagonists application on optical responses recorded from coronal slice. A: top panels show the time course of signal transmission in normal ACSF. As shown in middle panels, excitatory events were mostly blocked by antagonists of ionotropic glutamate receptors (40 µM DNQX and 50 µM dl-AP5) both in superficial (layers 2–3, ΔF/F = 0.085 ± 0.013%; P < 0.001) and deep layers (layer 5, ΔF/F = 0.038 ± 0.011%; P < 0.005) (n = 5 slices of 5; data at the peak latency in normal ACSF). In the presence of these antagonists, negative signals (blue color indicates an increase in fluorescence reflecting membrane hyperpolarization) were occasionally detected mainly in superficial layers (see also Supplemental Movie S2). These negative signals were almost completely inhibited by the GABAA receptor antagonist bicuculline (20 µM, bottom panels), indicating that they were monosynaptic inhibitory responses (fIPSPs). Only left hemisphere (ROI) is shown. B: comparison of optical response recorded in normal ACSF with those in the presence of DNQX and dl-AP5, or DNQX, dl-AP5 and BMI. All responses were recorded 8.4 ms after stimulation, corresponding to the time when the maximum inhibitory response in layer 1 was observed in the presence of DNQX and dl-AP5. Black arrow shows fIPSPs in superficial layers. Open arrow indicates stimulation site. C: comparison of the time course of optical responses from layer 1. Black and white arrowheads show positive and negative peaks in fluorescence change traces, respectively. D: comparisons of the negative-peak amplitude (minimum amplitude after positive-peak latency) between each drug conditions in superficial layers (layer 1, black triangle; layers 2–3, black square). Effects of drug conditions were verified by one-way ANOVA [layer 1, F(2, 8) = 91.7, P < 0.001; layers 2–3, F(2, 8) = 48.5, P < 0.001)]. Significant differences are indicated by asterisks (****P < 0.001).
Interestingly, after the disappearance of those excitatory responses by application of DNQX and dl-AP5, we observed negative signals, which represented membrane hyperpolarization, in all five slices tested (middle panels in Fig. 5A, black arrow in Fig. 5B and white arrow head in Fig. 5C; Supplemental Movie S2). The negative signals were transversely spread within superficial layers (layer 1, layers 2–3; Table 2 and Fig. 5D) (one slice included additional negative signals in layer 5; onset latency 6.0 ms; peak latency 10.8 ms; peak amplitude +0.040%). To verify whether these negative signals reflected inhibitory synaptic components, a high concentration of BMI, in addition to DNQX and dl-AP5, was perfused to block both inhibitory and excitatory synaptic responses. Application of BMI abolished negative signals, indicating that they represented GABAA receptor-mediated inhibitory monosynaptic responses in superficial layers (fIPSPs; fluorescent inhibitory postsynaptic potentials, bottom panels in Fig. 5A; n = 5/5). To investigate whether those fIPSPs could be induced exclusively by layer 1a stimulation, we performed deep layer 5 stimulation in five additional coronal slices. As shown in Fig. 6, in the presence of DNQX and dl-AP5, layer 5 stimulation (open arrow) induced negative signals limited to superficial layers (Table 2) (layer 1; n = 5/5; black arrow; layers 2–3; n = 3/5). One exceptional slice displayed additional negative signals even in deep layers. Similar to events elicited by layer 1a stimulation, these negative responses were abolished by a combination of BMI, DNQX, and dl-AP5. This observation indicated that superficial layers of the GRS could be selectively suppressed by both superficial and deep layer stimulation.
Fig. 6.
A: fIPSPs in superficial layers induced by layer 5 stimulation in coronal slice. The black arrow indicates a fIPSP in superficial layers. B: comparisons of the negative-peak amplitude (minimum amplitude after positive-peak latency) between drug conditions in superficial layers (layer 1, black triangle in A; layers 2–3, black square in A). Effects of drug conditions were examined by one-way ANOVA [layer 1, F(2, 8) = 70.7, P < 0.001; layers 2–3, F(2, 8) = 51.1, P < 0.001)]. Significant differences are indicated by asterisks (****P < 0.001).
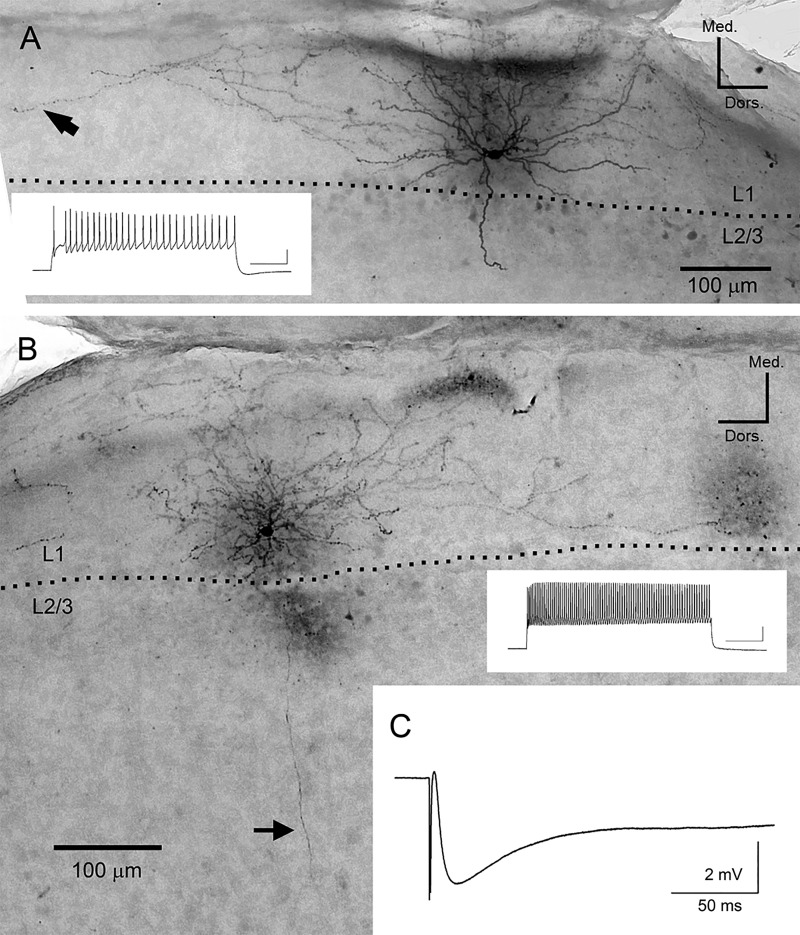
To further investigate the spatiotemporal distribution of inhibitory responses observed in superficial layers, we performed whole-cell patch-clamp recordings and DAB staining in 14 interneurons (5 coronal slices prepared from 2 rats) in layer 1. These interneurons showed extensive axon branching in layer 1 (e.g., black arrow in Fig. 7A). The average size of axon branching along dorsoventral axis was 990 ± 185 μm, well corresponding to the spatial distribution of inhibitory responses observed in VSD experiment (cf. Fig. 5B). Some of them (n = 6 of 14) demonstrated a descending axon toward the deep layers (average length, 382 ± 93 μm), often reaching to deep layers (black arrow in Fig. 7B). We also recorded IPSPs from LS neurons in layers 2–3, with electrical stimulation in layer 1a (0.2 mA). The average time course of IPSPs recorded from 10 LS neurons is shown in Fig. 7C. The onset delay, time to peak from electrical stimulation and half width of average IPSPs were 2.4 ± 0.1, 18.1 ± 1.9, and 121 ± 32 ms, respectively, which approximately corresponded to those of the inhibitory responses in VSD experiment. The average amplitude of IPSPs held at −60 mV was 5.0 ± 0.5 mV. IPSPs could be recorded at the site 1 mm away from the stimulus site, which also well corresponded to the morphological analysis of layer 1 interneurons and to the spatial distribution of inhibitory responses observed in VSD experiment.
Fig. 7.
Photomicrographs of representative examples of layer 1 interneurons and average trace of IPSPs recorded from LS neurons. A: elongated neurogliaform cell. Inset trace indicates the firing pattern of the cell. Scale bar, 20 mV and 200 ms. Dotted line represents the border between layer 1 and layers 2–3. B: similar to A, but demonstrating single bouquet cell. Arrow indicates a descending axon toward deep layers. Morphology of neurons was determined according to the description in the previous studies (Cadwell et al. 2016; Muralidhar et al. 2014). C: average trace of IPSPs (average of 50 traces recorded from 10 LS neurons) evoked by layer 1 stimulation (0.20 mA).
In summary, in coronal slices, layer 1a stimulation first induced excitatory synaptic transmission to superficial neurons, followed by transverse propagation of excitation among these neurons. Simultaneously, the stimulation also induced excitation of inhibitory interneurons in layer 1, which caused IPSPs spreading mainly in superficial layers. Thereafter, superficial neurons transmitted the signals to deep layer cells (first in layer 5 and then in layer 6), accompanied by successive transverse propagation of excitation in these layers.
Signal propagation in horizontal slices.
In all five horizontal slices tested, layer 1a stimulation first induced excitatory signals in layer 1 (Table 3; data around 0.13 mm lateral to the stimulating electrode, black triangle in Fig. 8B) and layers 2–3 (Table 3; data around 0.27 mm lateral to the stimulating electrode, black square in Fig. 8B), followed by excitatory signal transmission in layers 5–6 (Table 3; data around 0.61 mm lateral to the stimulating electrode, white square in Fig. 8B, Supplemental Movie S3). This propagation pattern in horizontal slices was quite similar to that in coronal slices. Likewise, vertical signal transmission was accompanied by transverse signal transmission within superficial (Fig. 8B; black arrow) and deep layers (Fig. 8B; white arrow). In most cases (n = 4/5), the direction of transversely propagated signals was predominantly from posterior to anterior (Fig. 8).
Table 3.
Properties of fEPSPs induced by layer 1a stimulation in horizontal slices
| Recording Site | Onset Latency | Peak Latency | Peak Fluorescence ΔF/F, % | |
|---|---|---|---|---|
| Normal ACSF | Layer 1 (n = 6) | 0.0* | 1.2* | −0.41 ± 0.028 |
| Layers 2–3 (n = 6) | 0.0* | 3.4 ± 0.22 | −0.19 ± 0.0085 | |
| Layers 5–6 (n = 6) | 1.0 ± 0.22 | 8.0 ± 1.00 | −0.12 ± 0.018 |
Onset latency, peak latency, and peak fluorescence amplitude of fEPSPs in horizontal slices. Onset and peak latencies were longer in deep layers than in superficial layers.
SE was much less than the sampling time.
Fig. 8.
Optical responses recorded from horizontal slices. A: time course of fluorescence changes in response to a single electrical stimulation of layer 1a in a horizontal slice (see also Supplemental Movie S3). Only left hemisphere (ROI) is shown. B: time course of optical signals acquired from layer 1 (black triangle), layers 2–3 (black square), and layer 5 (white square). Layer 1a stimulation induced excitatory signals in layers 2–3, and then in layer 5, which later propagated transversely within the same layers (black and white arrows, respectively). The direction of excitation was predominantly from posterior to anterior. C, top: signal transmission time course in normal ACSF. Bottom: excitatory responses were almost abolished by antagonists of ionotropic glutamate receptors (40 µM DNQX and 50 µM dl-AP5) both in superficial (layers 2–3, ΔF/F = 0.039 ± 0.016%; P < 0.001) and deep layers (layer 5, ΔF/F = 0.0067 ± 0.0043%; P < 0.001) (n = 6 slices of 6; data at the peak latency in normal ACSF). Note that inhibitory responses were rare in horizontal slices. Only left hemisphere (ROI) is shown.
Signal propagation in superficial and deep layers was largely abolished by the application of glutamate antagonists DNQX and dl-AP5, indicating that these signals were mediated by excitatory synapses (n = 6 of 6 slices; bottom panels in Fig. 8C). In the majority of horizontal slices (n = 5/6), negative signals (i.e., inhibitory synaptic transmission), which were clearly observed in the coronal slices, were not detected after incubation with glutamate antagonists.
In summary, layer 1a stimulation in horizontal slices evoked a similar signal propagation dynamics to that observed in coronal slices, although fewer or no inhibitory responses were induced. The direction of transversely propagated excitation within each layer was predominantly from posterior to anterior.
Transverse signal propagation.
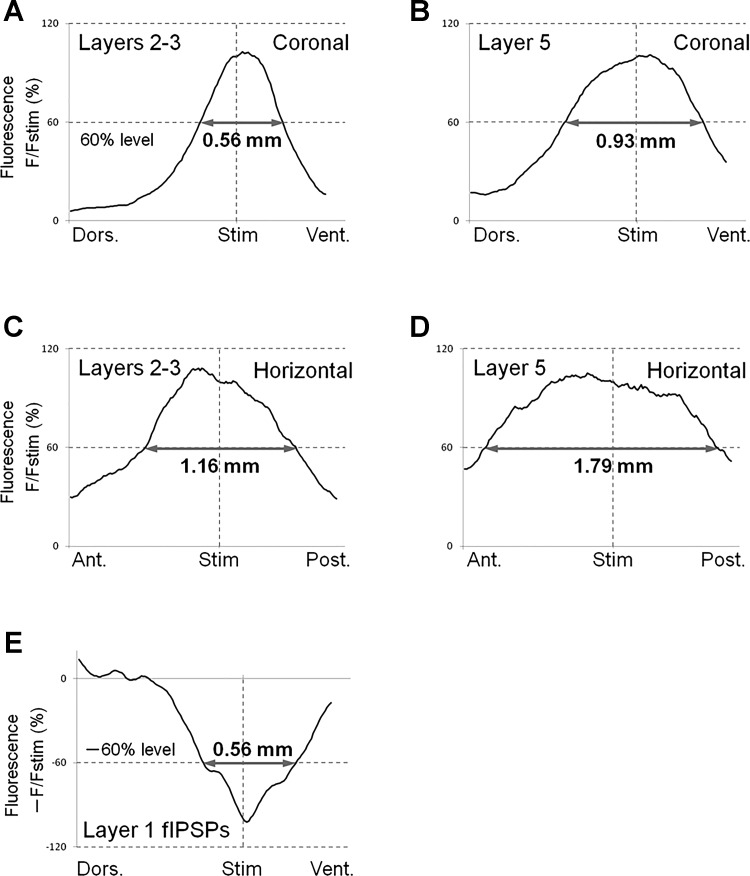
To compare functional connectivity of the microcircuitry of coronal and horizontal planes, we quantified spatial profile of transverse excitation within each layer at the time of the response peak (Fig. 9). The width of spatial profiles at 60% of the peak height in layers 2–3 and 5 were compared in coronal and horizontal planes (Fig. 9, A and D). We used the value of 60% of the peak height in our analysis because half-width values could not be measured in some horizontal slices (see Fig. 9, C and D).
Fig. 9.
Spatial profiles of transverse signal propagation in coronal and horizontal slices. A–D: we used 5 coronal and 6 horizontal slices to obtain averaged spatial profiles of excitation propagation in each plane. For this purpose, spatial distribution of optical responses, in each slice tested, along lines parallel to layers 2–3 or 5 were measured at peak latency. Then, grand average (average of averages) was calculated and plotted as a function of distance from the stimulus site. The lines for analysis were drawn 0.20 mm (layers 2–3) and 0.46 mm (layer 5) lateral to the stimulation electrode in coronal slices, and 0.27 mm (layers 2–3) and 0.61 mm (layer 5) in horizontal slices, respectively. To estimate how spatially widespread the responses in those layers were, we measured 60%–width of the responses (gray arrows in A to D). Note that, in both superficial and deep layers, transverse signal propagation was much broader in horizontal slices, than in coronal slices. E: spatial profile of transverse propagation of inhibitory signals at peak latency in coronal slices. The procedure to draw this graph was similar to that employed in A–D. Data were taken from coronal slices in the presence of DNQX and dl-AP5. The gray arrow shows the 60%–width of the peak response. The track for analysis was 0.086 mm (layer 1) lateral to the stimulating electrode.
Values of the width of averaged signals at 60% of the peak height in coronal slices were 0.57 ± 0.38 mm in layers 2–3 and 0.92 ± 0.07 mm in layer 5 (Fig. 9, A and B; the graph shows grand-averaged data, n = 5) (grand average: average of averages of subsamples). On the other hand, in horizontal slices, 60%-width values comprised 1.18 ± 0.17 mm in layers 2–3 and 1.60 ± 0.17 mm in layer 5 (Fig. 9, C and D, n = 6). These results indicated that transverse spread of excitation in each layer was much broader in horizontal plane than in coronal plane (Fig. 9).
We also quantified the inhibitory signal propagation in superficial layers of coronal slices. Figure 9E shows the spatial profile of transverse fIPSP, which propagated in layer 1, demonstrating that its 60%-width value was 0.56 ± 0.13 mm (n = 5). Such a prominent propagation of inhibitory signals in the coronal plane might be one of the reasons why the width of transverse excitation propagation in coronal plane was narrower than that in horizontal plane (see discussion).
Fiber-cutting experiments.
Transverse signal propagation along the layer axis shown in the present study has been previously observed in slices of rat visual cortex in conditions of partially suppressed cortical inhibition (Tanifuji et al. 1994). In our study, we took the same strategy to investigate how transverse connections, parallel to cortical laminae, contribute to the information processing in the GRS by examining the effects of partial cuts through cortical layers of GRS slices.
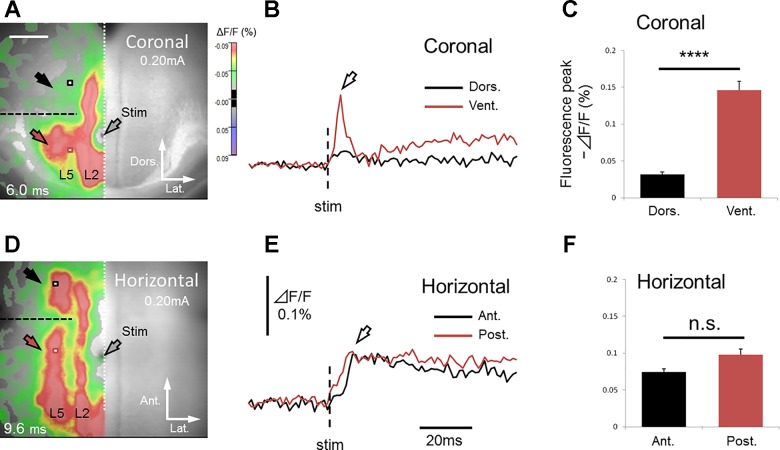
We used 10 (5 coronal and 5 horizontal) slices and made a vertical cut selectively in deep layers of the GRS (horizontal cut for coronal slices; coronal cut for horizontal slices) (black dashed line in Fig. 10, A and D). In all coronal slices tested (n = 5; Fig. 10, A–C), the cut blocked transverse signal propagation in deep layers (black and red arrows in Fig. 10A), whereas transverse signal propagation in superficial layers remained intact. This result suggests that transverse excitation in deep layers is conveyed mainly by the connections within those layers and occurs rather independently from excitatory events in superficial layers (Fig. 11A).
Fig. 10.
Effect of vertical cut on transverse excitation propagation in coronal and horizontal slices (horizontal cut for coronal slices; coronal cut for horizontal slices). The data was normalized (see the text for more detail). A–C: coronal slice. Red and black squares in A show the sites 0.40 mm ventral and dorsal to the cut (dashed line), respectively (0.46 mm lateral to the stimulating electrode). Correspondingly, red and black traces in B indicate the time course of fluorescence changes at these sites. The effect of the cut in 5 coronal slices is summarized in C. Dorsal peak fluorescence ΔF/F (−0.032 ± 0.0075%, black bar in C) was significantly [t(4) = 10.2, P < 0.01, paired t-test] smaller than ventral peak fluorescence ΔF/F (−0.15 ± 0.027%, red bar in C). The cut in deep layers (dorsal to the stimulating electrode) interrupted transverse signal transmission (white arrow in B), suggesting that excitatory events in deep layers were transmitted just within the layers. D–F: horizontal slice. Red and black squares in D show the sites 0.40 mm anterior and posterior to the cut (dashed line), respectively (0.61 mm lateral to the stimulating electrode). Anterior peak fluorescence ΔF/F (−0.075 ± 0.0095%, black bar in F) was not significantly different [t(4) = 2.35, P > 0.05, paired t-test] from posterior peak fluorescence ΔF/F (−0.098 ± 0.017%, red bar in F). In contrast to coronal slices, transverse signal propagation was not completely blocked (white arrow in E) by the cut (anterior to the stimulating electrode) in horizontal slices. This finding indicates that some excitatory signals in deep layers were propagated via superficial layers.
Fig. 11.
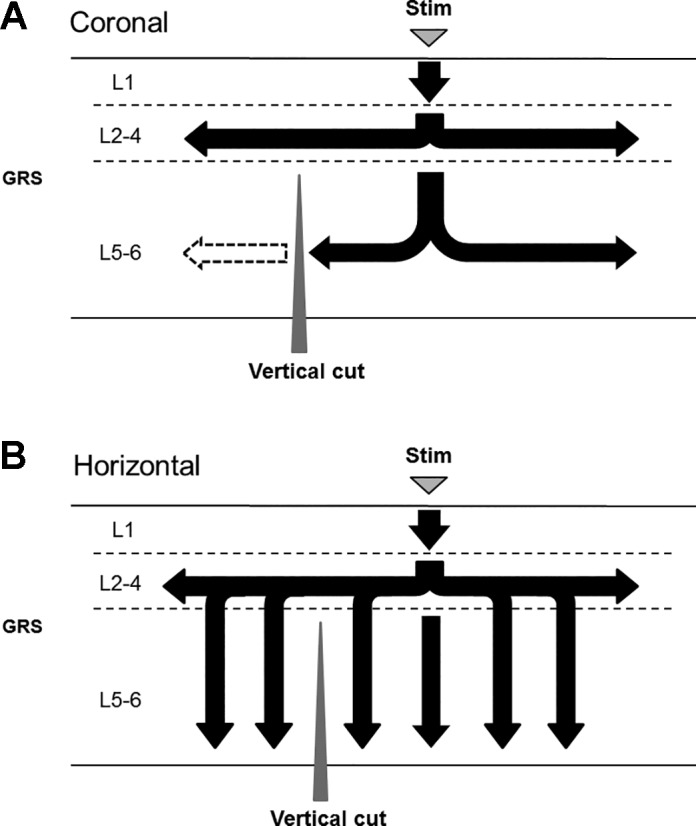
Schematic drawing of transverse signal propagation in the GRS, which can be suggested by the results of vertical cut experiment (Fig. 10). Black arrows show excitatory synaptic transmission. A: coronal plane. The vertical (horizontal) cut blocked transverse signal propagation in deep layers, suggesting that transverse excitation in deep layers is conveyed mainly by the intrinsic connections within those layers. B: Horizontal plane. Transversely propagating signals in deep layers proceeded beyond the vertical (coronal) cut and continued to travel further, suggesting that they are attributed to transverse neural connections within superficial layers.
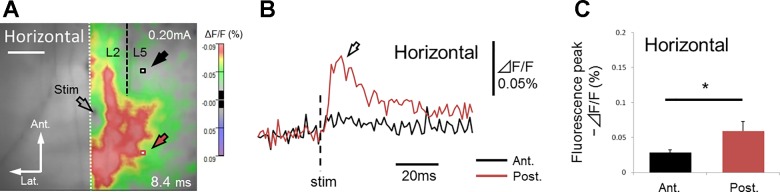
In contrast, in horizontal slices (n = 5; Fig. 10, D–F), transverse signal propagation in deep layers proceeded beyond the cut and continued to advance further (black and red arrows in Fig. 10D). This suggests that excitation of superficial layers is necessary to invoke signals in the lower deep layers. The apparent transverse signal propagation in deep layers can be mediated by the excitation of superficial layers (Fig. 11B). To test whether the connections within deep layers alone have enough capability to keep the transverse excitation propagation going without the excitation of the superficial layers, we used five additional horizontal slices in which we made a parasagittal cut parallel to the layer axis just beneath superficial layers (layers 2–3, black dashed line in Fig. 12A). In these slices, we observed that excitation of deep layers under the cut was weaker than that on the intact side (Fig. 12, B and C; anterior peak fluorescence ΔF/F = −0.028 ± 0.0041%, black arrow; posterior peak fluorescence ΔF/F = −0.059 ± 0.014%, red arrow; data around 0.53 mm anterior or posterior, and 0.61 mm lateral to the stimulating electrode) (such propagation pattern has been also observed in the horizontal slices with the parasagittal cut only beneath the stimulation locus, in which we observed signal propagation in deep layers further from the stimulation locus, while the excitation just under the cut has been prevented; n = 9/13; data not shown). The result confirmed that the connections within deep layers alone were not enough to generate and maintain the observed transverse signal propagation in that area. Thus, in horizontal slices, transverse signal propagation in deep layers can be attributed to transverse neural connections within superficial layers, rather than to intrinsic connections within deep layers (Fig. 11B).
Fig. 12.
Only the transverse signal propagation within deep layer was abolished by parasagittal cut made beneath superficial layer in horizontal slice. A: black dashed line represents a cut made parallel to the layer axis beneath superficial layer. Red and black squares show the sites 0.53 mm anterior and posterior (0.61 mm lateral) to the stimulation electrode, respectively. B: time course of fluorescence changes at red and black square sites in A. C: averaged values of optical signals at positive peaks of fast components at red and black square sites in A, respectively. Note that amplitude of excitatory signals in deep layers under the cut (anterior side, black arrow in A) was significantly smaller [t(4) = 3.08, P < 0.05, asterisk, paired t-test] than that of the intact (posterior side, red arrow in A). In horizontal slices, transverse excitation in deep layers was primarily mediated by neural connections in superficial layers.
DISCUSSION
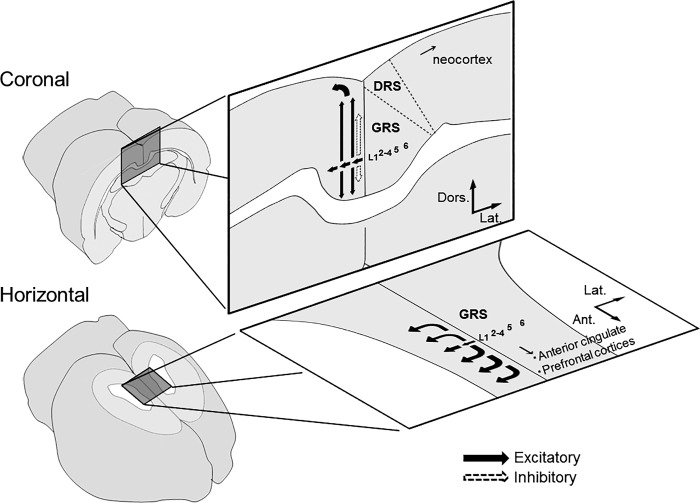
The results of the present study are schematically summarized in Fig. 13. Electrical stimulation of layer 1a of the GRS mimicking the thalamic input initially induced excitation of superficial layers. Then, excitatory signals propagated vertically to deep layers of the GRS. This stimulation also induced transverse excitation within superficial and deep layers, which could reach the DRS and the anterior cingulate area in coronal and horizontal slices, respectively. Such patterns are unique to the GRS, compared with previous VSD researches in other brain regions. Tanifuji et al. (1994) reported a similar transverse propagation of excitation along cortical layers using VSD imaging in slice preparation of the rat primary visual cortex. One major difference between their and our results is that induction of the long-range transverse propagation of excitation required partial suppression of the inhibition by perfusing BMI (>1 μM in ACSF) in the visual cortex, while such propagation could be induced without BMI in the GRS. This suggests that the inhibitory circuits may be differently organized in each cortical region. The present study, in fact, also revealed that inhibitory responses in the superficial layers were prominent in coronal slices while they were not in horizontal slices, suggesting an anisotropic distribution of inhibitory circuits between coronal and horizontal planes of the GRS. In both planes, superficial layers play an important role in processing information from thalamic inputs and, thus, in integrating thalamic signals with other inputs, such as those from the HCF, which may underlie various types of learning and memory.
Fig. 13.
Schematic drawing of functional connectivity in the intracortical circuit of the rat GRS in coronal and horizontal planes. Black and dotted arrows indicate excitatory and inhibitory synaptic transmission, respectively.
Functional connectivity in the rat retrosplenial region.
Layer 1a stimulation first evoked synaptic excitation of superficial layers (layers 1–3), which then propagated to deeper layers 5–6, corroborating results of our previously reported CSD analysis (Nixima et al. 2013).
The excitation of superficial layers was always followed by transverse signal propagation, suggesting that superficial neurons form transverse connections within these layers, comprising special units, which we termed “superficial neuronal cascades.” Correspondingly, we found that layer 2 stimulation evoked transversely propagating signals, so that FPs of considerable amplitudes could be recorded more than 1 mm away from the stimulation electrode (Nixima et al. 2013). We also observed that layer 2 stimulation evoked quite similar excitation propagation pattern to that evoked by layer 1a stimulation, both in coronal (n = 3) and horizontal (n = 3) slices of the GRS (K. Nixima, data not shown). Such transverse excitation can be attributed to flat basal dendrites or axon collaterals of superficial neurons extending within layers 2 and 3 (Ichinohe et al. 2008; Kurotani et al. 2013a).
The signals are then transmitted to deep layers of the GRS or to the DRS. GRS deep layer neurons can receive signal inputs from the HCF (e.g., postsubiculum and subiculum) and thus integrate signals from the ATN with those from the HCF (see the following sections). Deep layer neurons in the GRS have been also reported to project into anterior brain regions, including the anterior cingulate cortex and prefrontal cortices (Morris et al. 1999; Shibata et al. 2004; Vann et al. 2009). In agreement with these studies, we observed that the direction of transverse signal propagation in deep layers was predominantly from posterior to anterior. DRS neurons have been reported to project to the neocortex, in particular to visual areas 18b and 17 (van Groen and Wyss 1992), which is necessary for visual information processing and navigation (Taube 2007; Vann and Aggleton 2005; Vann et al. 2009). Signal propagation from the GRS to the DRS observed in the present study may constitute a part of such information processing pathways between visual areas and the GRS. Overall, computed signals in the rat GRS circuit are possibly transmitted to frontal lobes or visual areas, indicating that the GRS can be a “hub” among cortical and subcortical regions as suggested by Vann et al. (2009).
Signal transmission in superficial and deep layer neuronal cascades.
In coronal slices, layer 1a or 5 stimulation directly activated inhibitory neurons, evoking inhibitory synaptic responses localized to superficial layers. Previous studies have reported the existence of GABAergic interneurons in superficial layers of the GRS, which colocalized with apical dendritic bundles. It has been suggested that these structures mediate feed-forward inhibition to layer 2 pyramidal neurons or lateral inhibition between their modules such as dendritic bundles or patchy clusters of LS neurons (Ichinohe 2012; Ichinohe and Rockland 2002). Garden et al. (2009) also reported GABAA receptor-mediated inhibitory signals in superficial neurons of the GRS, which could undergo a long-term depression. In our whole-cell patch-clamp recordings, we observed monosynaptic IPSPs in LS neurons induced by layer 1a stimulation in the presence of glutamate antagonists (Fig. 7C), suggesting that layer 1 interneurons make synaptic contact with LS neurons. We also analyzed the spatial distribution of axon branching in labeled interneurons in layer 1 and found that the axons extended largely within the superficial layers or further to deep layers (layers 5–6). Such morphological patterns of interneurons well corresponded to the spatial distribution of inhibitory responses observed in VSD experiment (Figs. 5B and 6A). We concluded that the transverse propagation of fIPSPs in coronal slices is mediated by GABAergic interneurons, which possibly suppress or modulate superficial neuronal cascade activities (see the following section). On the other hand, in horizontal slices, inhibitory signals were not prominent. This anisotropy may be one of the causes of differences in transverse signal propagation patterns between coronal and horizontal planes. Inhibitory responses in coronal slices can produce lateral inhibition between superficial neuron modules (Figs. 5 and 9), presumably interrupting wider transverse excitation in deep layers transmitted via superficial neuronal cascades. Indeed, fiber-cutting experiments demonstrated that transverse excitatory signals in deep layers were mediated by intrinsic connections of those layers themselves and were not mainly relayed by superficial neuronal cascades (Fig. 10, A–C). Meanwhile, in horizontal slices, transverse excitatory signals in deep layers were much broader (Fig. 9D). This excitation can be transmitted by way of further transverse connections between superficial neurons because inhibitory responses in horizontal slices were much less prominent compared with those observed in coronal slices (Fig. 8C). Such a propagation pattern was verified by our fiber-cutting experiments, where we demonstrated that transverse excitation in deep layers was mainly due to transverse connections within superficial layers (Figs. 10, D–F, and 11B). Overall, anisotropic signal propagation can play a crucial role in signal processing by the GRS microcircuitry, suggesting its module-like activity.
The functional implications of the GRS intracortical circuit.
Previous lesion studies reported that the GRS contributes to spatial learning, memory (Van Groen et al. 2004), and simple classical conditioning, taking part in the formation of associations between multiple stimuli (Radwanska et al. 2010; Robinson et al. 2011). To accomplish such associative learning, signal inputs to the GRS need to be explicitly paired at the actual neural level (Hebbian learning; Bliss and Collingridge 1993; McGann and Brown 2000).
Our results demonstrated that ATN signals can be selectively “filtered” by superficial neuronal cascades and then transmitted to deep layers. In superficial layers of the rat GRS, more than 90% of pyramidal neurons demonstrate late-spiking firing property (Kurotani et al. 2013a). Moreover, these superficial neurons form apical dendritic bundles (Sripanidkulchai and Wyss 1986), which are thought to facilitate the synchronization of neuronal activity (Fleischhauer 1974). If this is the case, thalamic signals are filtered (delayed, suppressed, or synchronized) by superficial neuronal cascades (see the previous sections). Indeed, thalamic inputs evoked polysynaptic-like field potentials with multiple peaks in layer 5, which means that ATN signals are polysynaptically filtered by chains of superficial neuron modules (K. Nixima, data not shown). Oscillation-like activity in the GRS has also been observed in previous electrophysiological studies (Hedberg et al. 1993; Hedberg and Stanton 1995, 1996).
In contrast, signal inputs from the HCF are thought to be directly transmitted to deep layers of the GRS. Anatomical studies reported that apical dendrites of deep layer neurons in the GRS extend into layer 1, but they arborize mainly in layer 2 (Wyss et al. 1990). Vogt (1985) also demonstrated that layer 5 neurons have a higher spine density in layer 1b and 1c than in layer 1a. Furthermore, layers 1b to 3 of the GRS receive inputs from the postsubiculum and the subiculum (Van Groen and Wyss 2003; Wyss and Van Groen 1992). These studies suggest that layer 5 pyramids receive direct (“nonfiltered”) inputs from the HCF. In accordance with these reports, stimulation of the subiculum mainly activated deep layer neurons rather than superficial neurons of the GRS in our VSD imaging experiment (n = 2; Supplemental Movie S4).
Using intracellular recordings, Finch et al. (1984) found that the integration of ATN signals with those from the HCF occurs in layer 5 neurons of the GRS. Together with our results, layer 5 neurons can receive and integrate signals from the ATN relayed by superficial neuronal cascades with the direct signal input from the HCF, suggesting that the superficial neuronal cascades (including LS neurons) play a crucial role for the signal integration. This hypothesis is corroborated by a previous computational modeling study, which implicated that neural circuits including LS neurons can encode various time-delay chains (Beggs et al. 2000) and play a stimulus time-buffering role among multiple signal inputs during aversive conditioning (McGann and Brown 2000; Tieu et al. 1999). Correspondingly, lesions of the rat GRS impaired trace fear conditioning behavior (Kurotani et al. 2013b) and temporal discrimination learning (Todd et al. 2015).
In conclusion, superficial neuronal cascades can play an important role in processing information from thalamic inputs and, thus, in integrating thalamic signals with other inputs, such as those from the HCF, which may underlie various types of learning and memory.
GRANTS
This work was supported by the Okanoya Emotional Information Project of the Japan Science and Technology Agency (JST-ERATO), the Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) initiative supported by the Japan Agency for Medical Research and Development (AMED), RIKEN Brain Science Institute (BSI), KAKENHI no. 26119509 to K. Okanoya, KAKENHI no. 22500370 to T. Kurotani, and KAKENHI-AREA no. 4903-LH06380 to K. Okanoya.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.N., K.O., N.I., and T.K. conceived and designed research; K.N. and T.K. performed experiments; K.N. and T.K. analyzed data; K.N., K.O., and T.K. interpreted results of experiments; K.N. and T.K. prepared figures; K.N., K.O., and T.K. drafted manuscript; K.N., K.O., N.I., and T.K. edited and revised manuscript; K.N., K.O., N.I., and T.K. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Takashi Tominaga for helpful comments on the VSD imaging technique.
REFERENCES
- Aggleton JP, O’Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci 31: 2292–2307, 2010. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs JM, Moyer JR Jr, McGann JP, Brown TH. Prolonged synaptic integration in perirhinal cortical neurons. J Neurophysiol 83: 3294–3298, 2000. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39, 1993. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124: 1–38, 2008. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cadwell CR, Palasantza A, Jiang X, Berens P, Deng Q, Yilmaz M, Reimer J, Shen S, Bethge M, Tolias KF, Sandberg R, Tolias AS. Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat Biotechnol 34: 199–203, 2016. doi: 10.1038/nbt.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Lin LH, Green EJ, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex. I. Anatomical distribution and behavioral modulation. Exp Brain Res 101: 8–23, 1994. doi: 10.1007/BF00243212. [DOI] [PubMed] [Google Scholar]
- Cooper BG, Mizumori SJ. Temporary inactivation of the retrosplenial cortex causes a transient reorganization of spatial coding in the hippocampus. J Neurosci 21: 3986–4001, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont JR, Petrides M, Sziklas V. Fornix and retrosplenial contribution to a hippocampo-thalamic circuit underlying conditional learning. Behav Brain Res 209: 13–20, 2010. doi: 10.1016/j.bbr.2009.12.040. [DOI] [PubMed] [Google Scholar]
- Finch DM, Derian EL, Babb TL. Excitatory projection of the rat subicular complex to the cingulate cortex and synaptic integration with thalamic afferents. Brain Res 301: 25–37, 1984. doi: 10.1016/0006-8993(84)90399-8. [DOI] [PubMed] [Google Scholar]
- Fisk GD, Wyss JM. Associational projections of the anterior midline cortex in the rat: intracingulate and retrosplenial connections. Brain Res 825: 1–13, 1999. doi: 10.1016/S0006-8993(99)01182-8. [DOI] [PubMed] [Google Scholar]
- Fleischhauer K. On different patterns of dendritic bundling in the cerebral cortex of the cat. Kidney Int 5: 115–126, 1974. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Foster K, Orona E. Interaction of laminae of the cingulate cortex with the anteroventral thalamus during behavioral learning. Science 208: 1050–1052, 1980a. doi: 10.1126/science.7375917. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Foster K, Orona E, Saltwick SE, Stanton M. Neuronal activity of cingulate cortex, anteroventral thalamus and HCF in discriminative conditioning: encoding and extraction of the significance of conditional stimuli. Prog Psychobiol Physiol Psychol 9: 125–231, 1980b. [Google Scholar]
- Garden DLF, Massey PV, Caruana DA, Johnson B, Warburton EC, Aggleton JP, Bashir ZI. Anterior thalamic lesions stop synaptic plasticity in retrosplenial cortex slices: expanding the pathology of diencephalic amnesia. Brain 132: 1847–1857, 2009. doi: 10.1093/brain/awp090. [DOI] [PubMed] [Google Scholar]
- Hedberg TG, Simpson GV, Stanton PK. Microcircuitry of posterior cingulate cortex in vitro: electrophysiology and laminar analysis using the current source density method. Brain Res 632: 239–248, 1993. doi: 10.1016/0006-8993(93)91159-P. [DOI] [PubMed] [Google Scholar]
- Hedberg TG, Stanton PK. Long-term potentiation and depression of synaptic transmission in rat posterior cingulate cortex. Brain Res 670: 181–196, 1995. doi: 10.1016/0006-8993(94)01254-F. [DOI] [PubMed] [Google Scholar]
- Hedberg TG, Stanton PK. Long-term plasticity in cingulate cortex requires both NMDA and metabotropic glutamate receptor activation. Eur J Pharmacol 310: 19–27, 1996. doi: 10.1016/0014-2999(96)00371-8. [DOI] [PubMed] [Google Scholar]
- Honda Y, Furuta T, Kaneko T, Shibata H, Sasaki H. Patterns of axonal collateralization of single layer V cortical projection neurons in the rat presubiculum. J Comp Neurol 519: 1395–1412, 2011. doi: 10.1002/cne.22578. [DOI] [PubMed] [Google Scholar]
- Ichinohe N. Small-scale module of the rat granular retrosplenial cortex: an example of the minicolumn-like structure of the cerebral cortex. Front Neuroanat 5: 69, 2012. doi: 10.3389/fnana.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe N, Knight A, Ogawa M, Ohshima T, Mikoshiba K, Yoshihara Y, Terashima T, Rockland KS. Unusual patch-matrix organization in the retrosplenial cortex of the reeler mouse and Shaking rat Kawasaki. Cereb Cortex 18: 1125–1138, 2008. doi: 10.1093/cercor/bhm148. [DOI] [PubMed] [Google Scholar]
- Ichinohe N, Rockland KS. Parvalbumin positive dendrites co-localize with apical dendritic bundles in rat retrosplenial cortex. Neuroreport 13: 757–761, 2002. doi: 10.1097/00001756-200205070-00005. [DOI] [PubMed] [Google Scholar]
- Ichinohe N, Yoshihara Y, Hashikawa T, Rockland KS. Developmental study of dendritic bundles in layer 1 of the rat granular retrosplenial cortex with special reference to a cell adhesion molecule, OCAM. Eur J Neurosci 18: 1764–1774, 2003. doi: 10.1046/j.1460-9568.2003.02900.x. [DOI] [PubMed] [Google Scholar]
- Iijima T, Witter MP, Ichikawa M, Tominaga T, Kajiwara R, Matsumoto G. Entorhinal-hippocampal interactions revealed by real-time imaging. Science 272: 1176–1179, 1996. doi: 10.1126/science.272.5265.1176. [DOI] [PubMed] [Google Scholar]
- Kajiwara R, Tominaga T, Takashima I. Olfactory information converges in the amygdaloid cortex via the piriform and entorhinal cortices: observations in the guinea pig isolated whole-brain preparation. Eur J Neurosci 25: 3648–3658, 2007. doi: 10.1111/j.1460-9568.2007.05610.x. [DOI] [PubMed] [Google Scholar]
- Koganezawa N, Taguchi A, Tominaga T, Ohara S, Tsutsui K, Witter MP, Iijima T. Significance of the deep layers of entorhinal cortex for transfer of both perirhinal and amygdala inputs to the hippocampus. Neurosci Res 61: 172–181, 2008. doi: 10.1016/j.neures.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Kurotani T, Miyashita T, Wintzer M, Konishi T, Sakai K, Ichinohe N, Rockland KS. Pyramidal neurons in the superficial layers of rat retrosplenial cortex exhibit a late-spiking firing property. Brain Struct Funct 218: 239–254, 2013a. doi: 10.1007/s00429-012-0398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurotani T, Nixima K, Okanoya K. Distinct neuronal activities in the rat granular retrosplenial cortex and their possible involvement in the time interval encoding. Society for Neuroscience 43rd Annual Meeting; San Diego, Nov., 2013b. [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci 22: 310–316, 1999. doi: 10.1016/S0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- McGann JP, Brown TH. Fear conditioning model predicts key temporal aspects of conditioned response production. Psychobiology (Austin Tex) 28: 303–313, 2000. doi: 10.3758/BF03331989. [DOI] [Google Scholar]
- Morris R, Pandya DN, Petrides M. Fiber system linking the mid-dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. J Comp Neurol 407: 183–192, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- Muralidhar S, Wang Y, Markram H. Synaptic and cellular organization of layer 1 of the developing rat somatosensory cortex. Front Neuroanat 7: 52, 2014. doi: 10.3389/fnana.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixima K, Okanoya K, Kurotani T. Current source-density analysis of intracortical circuit in the granular retrosplenial cortex of rats: a possible role in stimulus time buffering. Neurosci Res 76: 52–57, 2013. doi: 10.1016/j.neures.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Odagiri S, Meguro R, Asano Y, Tani T, Ichinohe N. Single axon branching analysis in rat thalamocortical projection from the anteroventral thalamus to the granular retrosplenial cortex. Front Neuroanat 5: 63, 2011. doi: 10.3389/fnana.2011.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (4th ed.). San Diego: Academic, 1998. [Google Scholar]
- Pothuizen HHJ, Aggleton JP, Vann SD. Do rats with retrosplenial cortex lesions lack direction? Eur J Neurosci 28: 2486–2498, 2008. doi: 10.1111/j.1460-9568.2008.06550.x. [DOI] [PubMed] [Google Scholar]
- Radwanska A, Debowska W, Liguz-Lecznar M, Brzezicka A, Kossut M, Cybulska-Klosowicz A. Involvement of retrosplenial cortex in classical conditioning. Behav Brain Res 214: 231–239, 2010. doi: 10.1016/j.bbr.2010.05.042. [DOI] [PubMed] [Google Scholar]
- Robinson S, Keene CS, Iaccarino HF, Duan D, Bucci DJ. Involvement of retrosplenial cortex in forming associations between multiple sensory stimuli. Behav Neurosci 125: 578–587, 2011. doi: 10.1037/a0024262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H. Efferent projections from the anterior thalamic nuclei to the cingulate cortex in the rat. J Comp Neurol 330: 533–542, 1993. doi: 10.1002/cne.903300409. [DOI] [PubMed] [Google Scholar]
- Shibata H, Kondo S, Naito J. Organization of retrosplenial cortical projections to the anterior cingulate, motor, and prefrontal cortices in the rat. Neurosci Res 49: 1–11, 2004. doi: 10.1016/j.neures.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Sripanidkulchai K, Wyss JM. Thalamic projections to retrosplenial cortex in the rat. J Comp Neurol 254: 143–165, 1986. doi: 10.1002/cne.902540202. [DOI] [PubMed] [Google Scholar]
- Sripanidkulchai K, Wyss JM. The laminar organization of efferent neuronal cell bodies in the retrosplenial granular cortex. Brain Res 406: 255–269, 1987. doi: 10.1016/0006-8993(87)90790-6. [DOI] [PubMed] [Google Scholar]
- Tanifuji M, Sugiyama T, Murase K. Horizontal propagation of excitation in rat visual cortical slices revealed by optical imaging. Science 266: 1057–1059, 1994. doi: 10.1126/science.7973662. [DOI] [PubMed] [Google Scholar]
- Taube JS. The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci 30: 181–207, 2007. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Tieu KH, Keidel AL, McGann JP, Faulkner B, Brown TH. Perirhinal-amygdala circuit-level computational model of temporal encoding in fear conditioning. Psychobiology (Austin Tex) 27: 1–25, 1999. doi: 10.3758/BF03332095. [DOI] [Google Scholar]
- Todd TP, Meyer HC, Bucci DJ. Contribution of the retrosplenial cortex to temporal discrimination learning. Hippocampus 25: 137–141, 2015. doi: 10.1002/hipo.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T, Tominaga Y, Yamada H, Matsumoto G, Ichikawa M. Quantification of optical signals with electrophysiological signals in neural activities of Di-4-ANEPPS stained rat hippocampal slices. J Neurosci Methods 102: 11–23, 2000. doi: 10.1016/S0165-0270(00)00270-3. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp 30: 625–637, 2009. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Wyss JM. Retrosplenial cortex lesions of area Rgb (but not of area Rga) impair spatial learning and memory in the rat. Behav Brain Res 154: 483–491, 2004. doi: 10.1016/j.bbr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular a cortex in the rat. J Comp Neurol 300: 593–606, 1990. doi: 10.1002/cne.903000412. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol 315: 200–216, 1992. doi: 10.1002/cne.903150207. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol 463: 249–263, 2003. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. Selective dysgranular retrosplenial cortex lesions in rats disrupt allocentric performance of the radial-arm maze task. Behav Neurosci 119: 1682–1686, 2005. doi: 10.1037/0735-7044.119.6.1682. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci 10: 792–802, 2009. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Cingulate cortex. In: Cerebral Cortex, edited by Peters A, Jones EG. New York: Plenum, 1985, p. 88–149. [Google Scholar]
- Vogt BA, Miller MW. Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. J Comp Neurol 216: 192–210, 1983. doi: 10.1002/cne.902160207. [DOI] [PubMed] [Google Scholar]
- Wang L, Fontanini A, Maffei A. Visual experience modulates spatio-temporal dynamics of circuit activation. Front Cell Neurosci 5: 12, 2011. doi: 10.3389/fncel.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss JM, Sripanidkulchai K. The topography of the mesencephalic and pontine projections from the cingulate cortex of the rat. Brain Res 293: 1–15, 1984. doi: 10.1016/0006-8993(84)91448-3. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Van Groen T. Connections between the retrosplenial cortex and the hippocampal formation in the rat: a review. Hippocampus 2: 1–12, 1992. doi: 10.1002/hipo.450020102. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Van Groen T, Sripanidkulchai K. Dendritic bundling in layer I of granular retrosplenial cortex: intracellular labeling and selectivity of innervation. J Comp Neurol 295: 33–42, 1990. doi: 10.1002/cne.902950104. [DOI] [PubMed] [Google Scholar]
- Yuste R, Tank DW, Kleinfeld D. Functional study of the rat cortical microcircuitry with voltage-sensitive dye imaging of neocortical slices. Cereb Cortex 7: 546–558, 1997. doi: 10.1093/cercor/7.6.546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.