Currently, there is no method to accelerate emergence from anesthesia. Patients “wake” when they clear the anesthetic from their systems. Previously, we have shown that caffeine can accelerate emergence from anesthesia. In this study, we show that caffeine is effective even at high levels of anesthetic. We also show that caffeine operates by both elevating intracellular cAMP levels and by blocking adenosine receptors. This complicated pharmacology makes caffeine especially effective in accelerating emergence from anesthesia.
Keywords: emergence from anesthesia, isoflurane, caffeine, cAMP-elevating drugs, adenosine receptors
Abstract
Various studies have explored different ways to speed emergence from anesthesia. Previously, we have shown that three drugs that elevate intracellular cAMP (forskolin, theophylline, and caffeine) accelerate emergence from anesthesia in rats. However, our earlier studies left two main questions unanswered. First, were cAMP-elevating drugs effective at all anesthetic concentrations? Second, given that caffeine was the most effective of the drugs tested, why was caffeine more effective than forskolin since both drugs elevate cAMP? In our current study, emergence time from anesthesia was measured in adult rats exposed to 3% isoflurane for 60 min. Caffeine dramatically accelerated emergence from anesthesia, even at the high level of anesthetic employed. Caffeine has multiple actions including blockade of adenosine receptors. We show that the selective A2a adenosine receptor antagonist preladenant or the intracellular cAMP ([cAMP]i)-elevating drug forskolin, accelerated recovery from anesthesia. When preladenant and forskolin were tested together, the effect on anesthesia recovery time was additive indicating that these drugs operate via different pathways. Furthermore, the combination of preladenant and forskolin was about as effective as caffeine suggesting that both A2A receptor blockade and [cAMP]i elevation play a role in caffeine’s ability to accelerate emergence from anesthesia. Because anesthesia in rodents is thought to be similar to that in humans, these results suggest that caffeine might allow for rapid and uniform emergence from general anesthesia in humans at all anesthetic concentrations and that both the elevation of [cAMP]i and adenosine receptor blockade play a role in this response.
NEW & NOTEWORTHY Currently, there is no method to accelerate emergence from anesthesia. Patients “wake” when they clear the anesthetic from their systems. Previously, we have shown that caffeine can accelerate emergence from anesthesia. In this study, we show that caffeine is effective even at high levels of anesthetic. We also show that caffeine operates by both elevating intracellular cAMP levels and by blocking adenosine receptors. This complicated pharmacology makes caffeine especially effective in accelerating emergence from anesthesia.
there are currently no drugs available to the clinician or scientist to reverse the coma-like state induced by general anesthetics (Solt et al. 2011). Identification of such drugs would be of considerable utility in clinical and laboratory settings. Over 40 yr ago, Cohn et al. (1975) showed that direct intraventricular application of a membrane permeant cAMP analog could accelerate recovery from a variety of anesthetics and sedatives. Previously, we have shown that drugs that elevate intracellular cAMP dramatically accelerated emergence from anesthesia (Wang et al. 2014). Three intravenous drugs that raise cAMP levels were tested; forskolin, theophylline, and caffeine. Of the three drugs, caffeine was most effective in accelerating emergence from anesthesia. Other groups have reported approaches to accelerating emergence from anesthesia using different agents. In several recent papers, Solt and colleagues (Chemali et al. 2012; Solt et al. 2011; Taylor et al. 2031) demonstrated that intravenous administration of methylphenidate, which works through the inhibition of dopamine reuptake, could shorten the time it took adult rats to emerge from anesthesia. The authors suggest that the increased extracellular dopamine caused by methylphenidate, acting via D1 dopamine receptors (Taylor et al. 2013), mediates its observed effect (Kenny et al. 2015; Taylor et al. 2016). Of note, activation of D1 receptors typically results in the elevation of intracellular cAMP ([cAMP]i) (Snyder et al. 1998; Zhuang et al. 2000).
Forskolin and caffeine both elevate [cAMP]i but by different mechanisms. Forskolin stimulates adenylate cyclase (Laurenza et al. 1989; Simonds 1999), leading to increased production of cAMP while caffeine inhibits phosphodiesterase (Rang et al. 2007), thereby preventing the breakdown of cAMP. Forskolin and caffeine, when coadministered, were no more effective at accelerating emergence than caffeine by itself suggesting that the two drugs were operating via a common mechanism, namely increasing intracellular cAMP. It is known that in addition to elevating [cAMP]i caffeine has a variety of other effects. Most notably caffeine is an antagonist at all adenosine receptors. Blockade of the A2a adenosine receptor mediates caffeine’s arousal effects (El Yacoubi et al. 2000; Lazarus et al. 2011; Svenningsson et al. 1997). Caffeine is also an inositol triphosphate receptor antagonist and a voltage-independent activator of ryanodine receptors (Rang et al. 2007).
Our earlier studies revealed caffeine’s ability to accelerate emergence from anesthesia at a single anesthetic concentration of 2% isoflurane. In the current study, we sought to determine whether caffeine could exert its effect at higher anesthetic concentrations, which would suggest that it is likely that it will do so at all clinically relevant anesthetic concentrations. Furthermore, in this study we wanted to determine whether inhibiting A2a receptors plays a role in caffeine’s ability to accelerate emergence from anesthesia.
MATERIALS AND METHODS
Anesthetizing adult rats.
All studies on rats were approved by The University of Chicago Animal Use Committee.
Isoflurane.
Adult Sprague-Dawley rats, weighing 300–700 g, were placed in a gas-tight anesthesia box where they were exposed to 3% anesthesia (in 4 l/min O2) for 10 min. The 3% isoflurane corresponds to the anesthesia machine chamber concentration and is not a measurement of the end-tidal concentration. In rodents, end-tidal values for anesthetic are not typically measured. Nonetheless, the end-tidal value will almost certainly be <3%. During this time, the rats became unconscious and were insensitive to tail pinch. After 10 min, the rats were removed from the gas-tight box and then weighed. Next, an anesthesia nose cone was put in place, which delivered 3% isoflurane (in 4 l/min O2). At this time, an intravenous line was inserted into a tail vein. The rats were then put back into the gas tight anesthesia box for an additional period such that, in total, they were exposed to 60 min of 3% isoflurane in 4 l/min O2. Sixty minutes was chosen to mimic a typical “brief” surgery. Ten minutes before the anesthesia was terminated, the rats were injected with either saline, DMSO, or a solution containing a drug (forskolin or caffeine or preladenant or preladenant and forskolin). After anesthesia was terminated, the rats were placed on their backs in the middle of a large table. Recovery time from anesthesia was the time from when the animals were removed from the anesthesia chamber to when they stood with four paws on the table. Because there was a small variation in responsiveness to anesthesia between groups of rats, all rats were used as their own controls. Pure caffeine (Sigma) was dissolved in saline before injection. Rats were injected with caffeine (test) in 1 wk, and then they were injected with saline (control) a week later, while all aspects of the anesthesia protocol were repeated. Sometimes control injections preceded test injections. Caffeine injection was adjusted for the weight of the rat. Forskolin or preladenant or preladenant and forskolin were all dissolved in DMSO. Rats injected with forskolin or preladenant or forskolin (Sigma) and preladenant (Ark Pharmaceuticals) were injected with DMSO as control. These drugs were not adjusted for the rat’s weight since rats do not tolerate large injections of DMSO, and threfore, the same small amounts of DMSO (either 0.1 or 0.2 ml) were injected in every rat. The small injection volume precluded adjustments for rat size.
A paired t-test was used to test for statistical significance.
The same rats were used for both control and test conditions. In a previous study, we found that there could be significant variability in emergence time from anesthesia for different groups of rats (Wang et al. 2014).
Due to the speed of drug availability, rats received intravenous injections exclusively.
RESULTS
Caffeine accelerates emergence from anesthesia even when using very high levels of isoflurane.
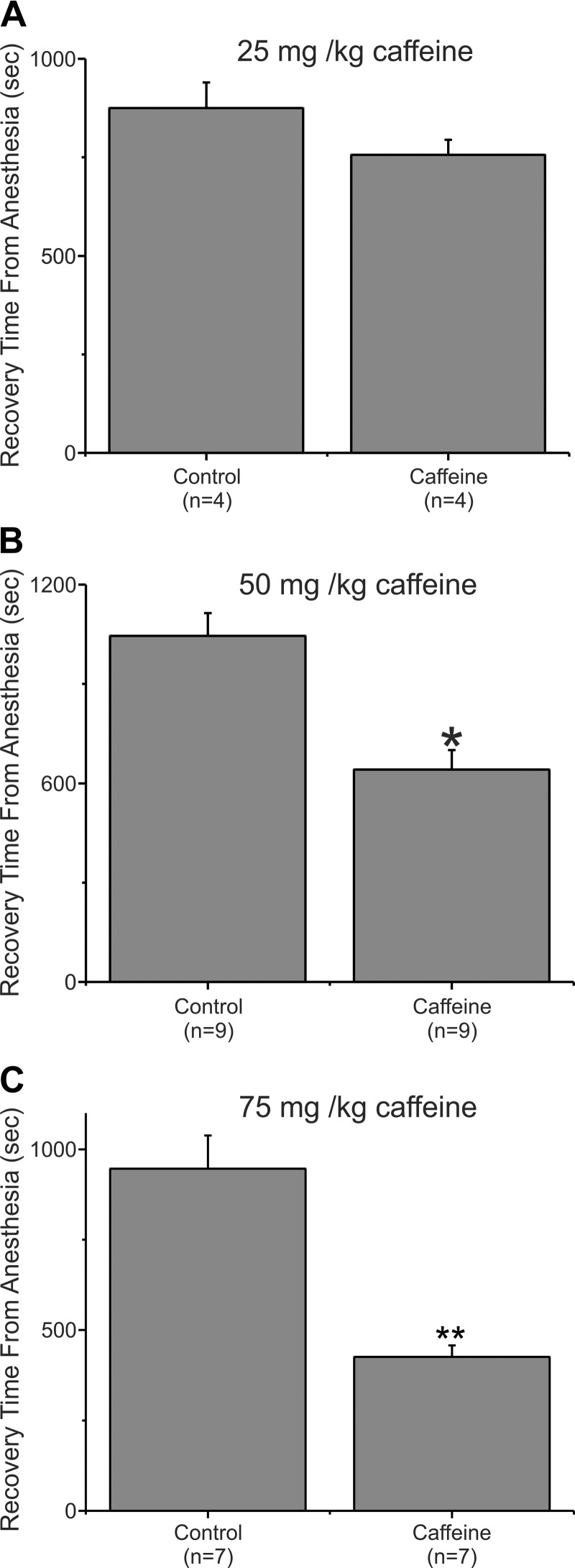
Our earlier studies showed that drugs that elevated intracellular cAMP, including caffeine, accelerated emergence from isoflurane. In that study, we maintained anesthesia with 2% isoflurane exclusively (Wang et al. 2014), leaving unanswered the question of whether caffeine alters recovery from higher concentrations of anesthesia. To address this question, we examined emergence from anesthesia in adult male rats exposed to 3% isoflurane (in 4 l/min O2) for a total of 60 min. During anesthesia, an intravenous line was inserted into a tail vein. Ten minutes before the anesthesia was terminated, the rats were injected with either saline or saline containing caffeine. After the isoflurane was terminated, the intravenous line was removed and the rats were placed on their backs on a table where they were allowed to wake in room air at ambient temperature (~68°F). Upon emerging from anesthesia, rats, when placed on their backs, will roll over and stand on four paws. Recovery time from anesthesia for rats is defined as the time from when the animals are removed from the anesthesia chamber to when they stand upright with four paws on the table. Figure 1A shows data from four animals that underwent to this protocol. In week 1, they received an injection of 25 mg/kg caffeine following 60 min of anesthesia with 3% isoflurane and a week later they were processed with an identical protocol except that they received an injection of saline. This concentration of caffeine, 25 mg/kg, was previously shown to produce a maximal acceleration of recovery (~60%) from anesthesia when rats were anesthetized with 2% isoflurane. Figure 1A shows that this concentration of caffeine had a limited effect on recovery from anesthesia when 3% isoflurane was employed to anesthetize the rats. In this experiment, animals injected with saline (Control) took on average 875.25 ± 64.46 s (SE; n = 4) to emerge from anesthesia while animals injected with caffeine averaged 755.75 ± 392.2 s (n = 4). This 13.65% reduction in waking time was not significant (P = 0.164).
Fig. 1.
Caffeine accelerated recovery from isoflurane anesthesia. A: adult rats were anesthetized with 3% isoflurane (4 l/min O2) for 10 min in an anesthesia machine. An intravenous line was inserted into a tail vein, while anesthesia was maintained with a nose cone (3% isoflurane, 4 l/min O2). The rats were then put back into the anesthesia machine such that they received 60 min of anesthesia in total. Ten minutes before discontinuing the anesthetic was discontinued, the animals received an intravenous injection of either saline (control) or saline with 25 mg/kg caffeine. The animals were removed from the chamber and allowed to recover on a table top breathing room air. They were placed on their backs, and the time to recover from isoflurane anesthesia was the time from terminating the 3% anesthetic exposure until the animals had all 4 paws on the table. A: the bar chart represents the average time to emerge from anesthesia. The same animals were used for control and caffeine with anesthetic exposures 1 wk apart. The 13.7% difference in time to emerge from anesthesia was not significant. P = 0.16425, significant difference; n = 4). B: data from a 2nd set of rats that were injected with either saline (Control) or with 50 mg/kg caffeine. This amount of caffeine corresponds to a doubling of the dosage of that shown in A. *P = 0.004, significant difference; n = 9. In this experiment, rats injected with saline (Control) emerged from anesthesia significantly more slowly than did rats injected with caffeine. Caffeine reduced the emergence time by 38.6%. C: data from rats that were injected with either saline (Control) or with 75 mg/kg caffeine. In this experiment, caffeine reduced the time it took to emerge from the isoflurane anesthesia by ~55% as compared with rats injected with saline. **P < 0.0002, significant difference; n = 7.
Figure 1B shows data from a second set of rats where they were injected with either saline (Control) or with 50 mg/kg caffeine, a doubling of the dosage from the experiment shown in Fig. 1A. Figure 1B shows data from nine animals that underwent this protocol. The same cohort of animals was used for both the saline and caffeine injection. In this experiment, animals injected with saline (Control) averaged 1044.89 ± 69.2 s (n = 9) to emerge from anesthesia while animals injected with caffeine (50 mg/kg) averaged 641.22 ± 58.7 s (n = 9). This 38.6% reduction in waking time was significant (P = 0.004). Nonetheless, doubling the caffeine concentration from 25 to 50 mg/kg, while producing a dramatic acceleration in emergence from anesthesia, did not recapitulate the ~60% reduction in recovery time observed in our earlier studies with 25 mg/kg caffeine at 2% isoflurane (Wang et al. 2014).
Figure 1C shows data from a cohort of animals that were injected with either saline (Control) or caffeine (75 mg/kg). Saline-injected rats emerged from anesthesia at 946.57 ± 91.67 s (n = 7) on average, while caffeine (75 mg/kg)-injected rats averaged 426 s ± 31.31 (n = 7). This difference was very significant, P < 0.0002 . The magnitude of the acceleration in emergence time produced by 75 mg/kg caffeine in animals anesthetized with 3% isoflurane was similar to that previously observed in animals injected with 25 mg/kg caffeine at 2% isoflurane. This result, a 55% reduction in emergence times, is very similar to the ~60% reduction observed for 25 mg/kg caffeine when rats were anesthetized with 2% isoflurane (Wang et al. 2014). Caffeine accelerates emergence from anesthesia even at very high anesthetic levels.
Inhibition of A2a receptors plays a role in accelerating emergence from anesthesia.
Our previous studies showed that caffeine was more effective at accelerating emergence from anesthesia than forskolin, a drug that elevates intracellular cAMP but does not share the other cellular targets of caffeine (adenosine receptors, inositol triphosphate, and ryanodine receptors). Studies involving A2A receptor knockouts or pharmacological blockade of these receptors have implicated them in the mechanism of caffeine-mediated arousal (El Yacoubi et al. 2000; Lazarus et al. 2011; Svenningsson et al. 1997). We sought to assess whether blockade of A2a receptors underlie caffeine’s ability to accelerate emergence from anesthesia.
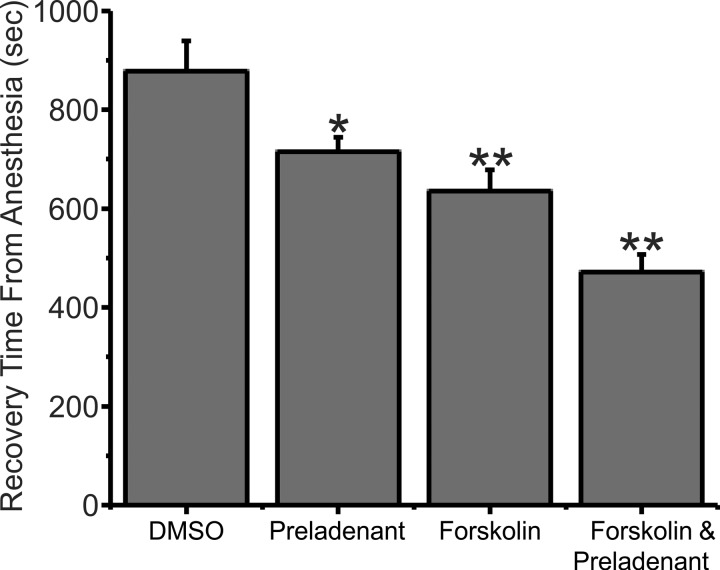
Preladenant is a selective A2a adenosine receptor antagonist. It was tested in a cohort of animals that were either injected with preladenant (0.5 mg, dissolved in 0.1 ml DMSO) or injected with DMSO (0.1 ml) by itself (Control). Rats injected with DMSO emerged from anesthesia on average at 877.75 ± 61.9 s (n = 8) while preladenant-injected rats averaged 715.25 ± 28.52 s (n = 8), as shown in Fig. 2. This 18.5% reduction in time to emerge from anesthesia was significant, P < 0.05. Because the rats could only tolerate a limited volume of DMSO, preladenant was administered in a 0.1-ml injection volume. At these small injection volumes, we could not adjust the preladenant dosage for the weight of the rats. In addition, due to the limited solubility of preladenant, the 0.5-mg dose of preladenant used in this experiment represented the maximum we could administer. These results suggest that A2a receptor blockade contributes significantly to caffeine’s ability to accelerate emergence from anesthesia.
Fig. 2.
Preladenant, an A2a adenosine receptor antagonist, reduced the time it took rats to emerge from isoflurane anesthesia. Forskolin, a drug that elevates intracellular cAMP levels, reduced the time it took rats to emerge from isoflurane anesthesia. The effect of forskolin was additive with that of preladenant. Plotted are data from rats that were injected with either 0.1 ml of DMSO (Control) or with 0.5 mg/preladenant, dissolved in 0.1 ml of DMSO or with forskolin (0.1 mg dissolved in 0.1 ml of DMSO) or with both forskolin and preladenant. In this experiment, preladenant reduced the time it took to emerge from isoflurane anesthesia by 18.5% compared with control. *P = 0.042, significant difference; n = 8. Forskolin by itself reduced the time it took to emerge from isoflurane anesthesia by ~27.6% compared with control. **P < 0.0007, significant reduction. The combination of forskolin and preladenant reduced the time it took to emerge from isoflurane anesthesia by 46.3% compared with control. **P < 0.0000022, significant reduction. This reduction was also significantly different from that observed with forskolin alone (P < 0.007). The same cohort of 8 animals was employed for all 3 conditions.
Combining forskolin and preladenant recapitulates the actions of caffeine.
Forskolin is a drug that activates adenylate cyclase, thereby elevating intracellular cAMP levels. In a previous study, we found that 0.1 mg/kg of forskolin produced a maximal acceleration in emergence time from isoflurane anesthesia. In the current study, we injected DMSO alone or 0.1 mg forskolin dissolved in 0.1 ml of DMSO. Although not a precise estimate, most of the rats used in the study were ~500 g in weight. This would correspond to an injection of ~0.2 mg/kg forskolin. Figure 2 shows that this amount of forskolin reduced the time it took to emerge from anesthesia by ~27.6%. DMSO-injected animals took on average 877.75 ± 61.9 s (n = 8) to emerge from anesthesia while forskolin-injected animals averaged 635.63 ± 42.44 s (n = 8). This reduction was significant, P < 0.0007. This acceleration of emergence from anesthesia was not significantly different than was previously observed for 0.1 mg forskolin and 2% isoflurane.
In our earlier study combining maximal doses of caffeine and forskolin produced no faster emergence from anesthesia than did caffeine alone. This complete lack of additivity, which we reproduced in the current study (data not shown), suggests that forskolin and caffeine operate via a common pathway, the elevation of intracellular cAMP. Figure 2 shows that forskolin and preladenant together were more effective than either drug alone. Rats injected with DMSO alone (Control) emerged from anesthesia at an average time of 877.75 ± 61.9 s (n = 8) while rats injected with forskolin and preladenant emerged on average at 471.5 ± 35.7 s (n = 8). This 46.3% reduction was significant, P < 0.0000022. Administered alone, forskolin reduced waking times by 27.6%, and preladenant reduced waking times by 18.5%. The reduction observed when both drugs were used together was 46.3%, suggesting that the effects of the drugs were additive. The reduction in waking time in experiments where forskolin alone was injected was significantly different than those experiments where forskolin and preladenant were injected together (P < 0.007).
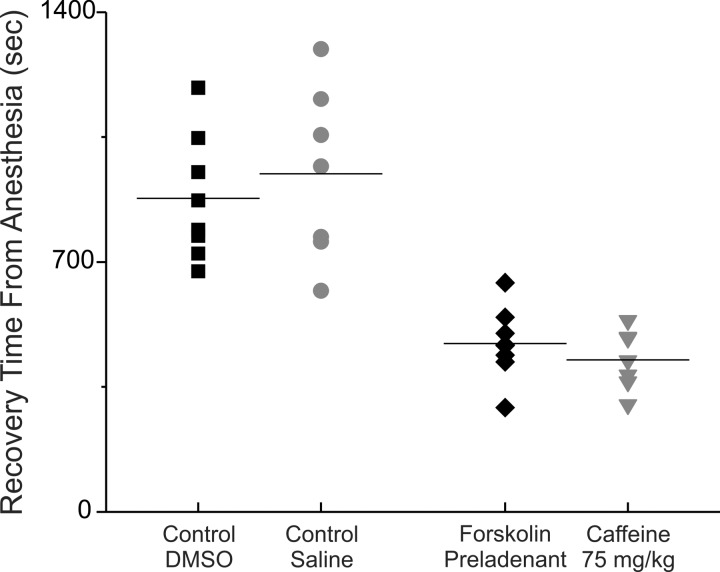
Figure 3 compares emergence times from anesthesia for rats injected with caffeine to those of rats injected with preladenant and forskolin. Emergence times from anesthesia for both control and drug-injected rats are plotted. These data are from two different cohorts of rats. The data shows that rats injected with saline (Control) emerged from anesthesia at about the same time as did rats injected with DMSO by itself (Control). Both the average values and the distribution of waking times were similar. Similarly, the data show that rats injected with caffeine (75 mg/kg) emerged from anesthesia at about the same time as did rats injected with forskolin and preladenant. The forskolin and preladenant recapitulated caffeine’s ability to accelerate mergence from anesthesia. Comparing the two data sets suggests that there was no difference P > 0.36.
Fig. 3.
The distribution of emergence times from anesthesia for the combination of forskolin and preladenant was very similar to that of caffeine at 75 mg/kg. Plotted is the distribution of emergence from isoflurane anesthesia times. Left: 2 control distributions. Right: the 2 test distributions. Note that the control distribution for caffeine (■) employed a saline injection, while that for forskolin and preladenant (●) employed a DMSO injection; waking times were similar for rats injected with either saline or DMSO. Similarly, waking times were similar for rats injected with either a high dose of caffeine (♦, 75 mg/kg) or with both preladenant and forskolin (▼).
[Control rats were usually injected with saline or DMSO. In some experiments, they were injected with nothing. We observed no difference in the time to emerge from anesthesia in rats injected with DMSO compared with rats injected with saline or to rats that were not injected with anything (data not shown, but see Fig. 3 for a comparison of saline and DMSO)].
DISCUSSION
Anesthetics have been used successfully for over a century and a half, but there are no methods for clinicians or scientists to accelerate emergence from anesthesia. The ability to reverse anesthesia or at least accelerate emergence from anesthesia would be useful in clinical and laboratory settings. Over 40 yr ago, Cohn et al. (1975) showed that intraventricular application of membrane-permeant cAMP could accelerate recovery from eight different anesthetic or sedative agents. While emergence was assessed in a manner similar to our current study, that work employed rats that were likely only a few weeks old based on their small size. Since then, other groups have attempted to devise strategies and identify agents capable of accelerating recovery from anesthesia. These studies have employed a variety of differing methodologies and anesthetic concentrations, suggesting caution in comparing results among them or to our current work, at least in some cases.
Luo and Leung (2009) demonstrated that injection of histaminergic agonists into the nucleus basalis magnocellularis was able to accelerate emergence from 1 h of 2.1% isoflurane anesthesia by over 50%. Alkire et al. (2009) injected an antibody to the Kv1.2 potassium channel into the thalamus of anesthetized rats and observed that 17% of the rats woke during the injection. Unfortunately about one-third of the animals so treated exhibited seizures. In a second study from the same group (Alkire et al. 2007), nicotine was injected into thalamus in an effort to reverse the effects of anesthesia. The therapeutic window was small, as too little nicotine had no effect and too much induced seizures. Even the optimal dose produced either no effect or seizures at higher rates than it did arousal. Neither of these two studies assessed time to emergence after termination of anesthesia.
Cholinergic activation has been implicated in anesthesia reversal (Leung et al. 2011; Tai et al. 2014). Infusion of either a cholinesterase inhibitor or a muscarinic agonist intracerebroventricularly (Hudetz et al. 2003) decreased anesthesia. In this study, Hudetz et al. (2003) did not assess emergence from anesthesia, monitoring brain wave activity instead. Although compelling, these studies are of limited clinical utility as they involve intracranial injections, which are difficult even in a laboratory setting.
Surprisingly, subanesthetic doses of ketamine dramatically accelerated emergence from isoflurane anesthesia in rats (Hambrecht-Wiedbusch et al. 2017). In this study, it was observed that ketamine produced a large elevation in acetylcholine release, consistent with the hypothesis that cholinergic activation can play a role in reversing anesthesia.
Cholinergic activation was studied in a human trial to determine whether it reversed anesthesia. The cholinesterase inhibitor physostigmine reversed postoperative somnolence (Hill et al. 1977) and reversed propofol anesthesia (Meuret et al. 2000). Unfortunately, physostigmine was less reliable when used with the volatile anesthetic sevoflurane (Plourde et al. 2003). Physostigmine usefulness may also be limited by peripheral autonomic side-effects.
Methylphenidate, a dopamine and norepinephrine reuptake inhibitor (Pascoli et al. 2005) used to treat attention deficit hyperactivity disorder and other disorders, has been shown in rats to dramatically emergence from anesthesia (Chemali et al. 2012; Solt et al. 2011). Of note, the rats in these studies were much more lightly anesthetized than those in the current study. Solt and colleagues (Chemali et al. 2012; Solt et al. 2011; Taylor et al. 2013) proposed that inhibition of dopamine transport and the subsequent elevation of extracellular dopamine and activation of D1 dopamine receptors was key to the ability of methylphenidate to accelerate the emergence from anesthesia. D1 dopamine receptors are typically coupled to the G proteins Gs or Golf, which stimulate adenylate cyclase thereby elevating [cAMP]i (Snyder et al. 1998; Zhuang et al. 2000). Thus methylphenidate/dopamine/D1 receptors may be operating via the same mechanism as caffeine or forskolin.
Earlier work from our group suggested that anesthetics inhibit neurotransmitter release (Herring et al. 2009, 2011). Data from a number of groups have provided evidence for a presynaptic site of action for anesthetics (Hemmings 2009; Hemmings et al. 2005; Westphalen et al. 2009, 2013). Other studies suggest that inhibition of neurotransmitter release might play an important role in anesthesia (Herring et al. 2011; Herring et al. 2009; Metz et al. 2007; Saifee et al. 2011; van Swinderen et al. 1999; Winegar and MacIver 2006). This opened the possibility that drugs that reversed the inhibitory effects of anesthetics on neurotransmitter release could affect recovery from anesthesia. It is well known that cAMP signaling modulates neurotransmitter release; elevating intracellular cAMP levels augments neurotransmitter release in neurons and in secretory cells (Burgoyne and Morgan 2003; Byrne and Kandel 1996; Fujita-Yoshigaki 1998; Fujita-Yoshigaki et al. 1998; Hilfiker et al. 2001; Kasai et al. 2012; Kuromi and Kidokoro 2000, 2003; Lonart et al. 1998, 2003; Machado et al. 2001; Sakaba and Neher 2001, 2003; Seino and Shibasaki 2005; Takahashi et al. 1999;). Consistent with this hypothesis, a Caenorhabditis elegans mutant that results in elevated levels of intracellular cAMP levels is resistant to isoflurane anesthesia (Saifee et al. 2011). In addition, in a previous study from our laboratory (Wang et al. 2014), we reported that methylphenidate was able to reverse the inhibition of neurotransmitter release engendered by isoflurane, perhaps by elevating [cAMP]i (Pascoli et al. 2005). This ability of methylphenidate to reverse the anesthesia-induced inhibition of neurotransmitter release may play a role in accelerating recovery from anesthesia. Further studies are required to link the inhibition of neurotransmitter release to the anesthetic state and to determine whether overcoming anesthetic-induced blockade of neurotransmitter release by elevation of [cAMP]i is causally linked to accelerated recovery from anesthesia. Because elevated [cAMP]i has important postsynaptic effects (Lee and Messing 2008) as well, it is also possible that these may play a role in accelerating emergence from anesthesia.
Forskolin, used since ancient times as a treatment for heart disorders, is known to potently stimulate adenylyl cyclase (Laurenza et al. 1989; Simonds 1999), thereby elevating intracellular cAMP. More recently it has been used to treat congestive heart failure, asthma and glaucoma. When tested in vitro, forskolin completely reversed isoflurane-mediated inhibition of the neurotransmitter release machinery (Wang et al. 2014), and in vivo accelerated recovery from anesthesia in rats.
The drug with the highest efficacy in our previous studies was caffeine (Wang et al. 2014). Caffeine is a psychoactive stimulant drug that is legal and unregulated. Caffeine is the most commonly used psychoactive drug (Nehlig et al. 1992), and in the United States more than 90% of adults use it daily. Caffeine has a variety of effects but the two most noteworthy effects are its ability to block adenosine receptors (Lazarus et al. 2011) and to elevate cytosolic cAMP levels (Rang et al. 2007) by inhibiting phosphodiesterase. Clinically, caffeine is primarily used to treat neonatal apnea and certain types of headache. Caffeine binds with similar affinity to A1 and A2a receptors and antagonizes both (Lazarus et al. 2011) at concentrations much lower than shown in Fig. 1 (Chen et al. 2013). Adenosine regulates sleep and waking (Huang et al. 2011; Lazarus et al. 2011). Caffeine blocks adenosine receptors nonselectively, but it appears that A2a adenosine receptors mediate caffeine’s arousal effects since knocking out this receptor or blocking it pharmacologically suppresses caffeine-mediated arousal (El Yacoubi et al. 2000; Huang et al. 2005; Lazarus et al. 2011; Svenningsson et al. 1997). The results in this study show that some of the accelerated recovery from anesthesia produced by caffeine is most likely mediated by block of adenosine receptors but that elevation of [cAMP]i accounts for the majority of the effect.
Although the data are clear in that preladenant has a significant effect in accelerating emergence from anesthesia, a comprehensive explanation given the underlying pharmacology is not so straightforward. Preladenant is a selective A2a adenosine receptor blocker. A2a receptors are normally linked to the activation of the G-proteins Gs or Golf, thereby activating adenylate cyclase (Brown et al. 2008; Schiffmann et al. 2007). Blockers of the A2a receptor, like caffeine, inhibit this pathway. However, because caffeine is also a phosphodiesterase inhibitor, it will raise intracellular cAMP inside all cells, including those that express A2a receptors. In this case, blocking one path that elevates [cAMP]i while enabling a second pathway that elevates cAMP will still result in elevated cAMP. The consequence of this unusual signaling is to accelerate emergence from anesthesia. The simplest explanation for this somewhat complicated result is that A2a receptors have other signaling properties independent of cAMP. Alternatively, A2a receptors appear to exist as heteroreceptors with D2 dopamine receptors, and this association may lead to complicated downstream signaling (Fuxe et al. 2015; Navarro et al. 2016).
An important question raised by our studies is whether caffeine can accelerate recovery from anesthesia in humans. Because caffeine is so widely used in the general population, it might provide a relatively innocuous and inexpensive way to accelerate recovery from anesthesia in patients who are slow to emerge from anesthesia. It is tempting to speculate about other possible benefits of caffeine. Even after emerging from anesthesia, patients can exhibit cognitive impairment that lasts for hours (Larsen et al. 2000). The cognitive abilities of elderly patients can be impaired for even longer periods after anesthesia was terminated (Chen et al. 2001). Even subanesthetic doses of drugs like sevoflurane cause significant cognitive impairment (Galinkin et al. 1997; Janiszewski et al. 1999). If caffeine accelerates cognitive recovery, similar to what it does for emergence, that would be extremely useful. It might allow patients to be released more rapidly leading to better outcomes and lower costs. If caffeine does operate in this manner, it will be interesting to determine from a mechanistic perspective, whether blockade of A2a adenosine receptors, elevation of [cAMP]i, or both play significant roles in reversing post-anesthesia cognitive deficits.
GRANTS
This study was funded by a National Institute of General Medical Sciences Grant GM-116119 (to A. P. Fox and Z. Xie).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.F., A.P.F., and Z.X. conceived and designed research; R.F., S.K., A.N.C., K.G.X., J.H.-Y.W., A.P.F., and Z.X. performed experiments; R.F., S.K., A.N.C., K.G.X., J.H.-Y.W., A.P.F., and Z.X. interpreted results of experiments; R.F., A.P.F., and Z.X. edited and revised manuscript; R.F., S.K., A.N.C., K.G.X., J.H.-Y.W., A.P.F., and Z.X. approved final version of manuscript; A.P.F. analyzed data; A.P.F. prepared figures; A.P.F. drafted manuscript.
REFERENCES
- Alkire MT, Asher CD, Franciscus AM, Hahn EL. Thalamic microinfusion of antibody to a voltage-gated potassium channel restores consciousness during anesthesia. Anesthesiology 110: 766–773, 2009. doi: 10.1097/ALN.0b013e31819c461c. [DOI] [PubMed] [Google Scholar]
- Alkire MT, McReynolds JR, Hahn EL, Trivedi AN. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology 107: 264–272, 2007. doi: 10.1097/01.anes.0000270741.33766.24. [DOI] [PubMed] [Google Scholar]
- Brown RA, Spina D, Page CP. Adenosine receptors and asthma. Br J Pharmacol 153, Suppl 1: S446–S456, 2008. doi: 10.1038/bjp.2008.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev 83: 581–632, 2003. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. J Neurosci 16: 425–435, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemali JJ, Van Dort CJ, Brown EN, Solt K. Active emergence from propofol general anesthesia is induced by methylphenidate. Anesthesiology 116: 998–1005, 2012. doi: 10.1097/ALN.0b013e3182518bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets—what are the challenges? Nat Rev Drug Discov 12: 265–286, 2013. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhao M, White PF, Li S, Tang J, Wender RH, Sloninsky A, Naruse R, Kariger R, Webb T, Norel E. The recovery of cognitive function after general anesthesia in elderly patients: a comparison of desflurane and sevoflurane. Anesth Analg 93: 1489–1494, 2001. [DOI] [PubMed] [Google Scholar]
- Cohn ML, Cohn M, Taylor FH, Scattaregia F. A direct effect of dibutyryl cyclic AMP on the duration of narcosis induced by sedative, hypnotic, tranquiliser and anaesthetic drugs in the rat. Neuropharmacology 14: 483–487, 1975. doi: 10.1016/0028-3908(75)90051-9. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Ménard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. Br J Pharmacol 129: 1465–1473, 2000. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita-Yoshigaki J. Divergence and convergence in regulated exocytosis: the characteristics of cAMP-dependent enzyme secretion of parotid salivary acinar cells. Cell Signal 10: 371–375, 1998. doi: 10.1016/S0898-6568(97)00178-2. [DOI] [PubMed] [Google Scholar]
- Fujita-Yoshigaki J, Dohke Y, Hara-Yokoyama M, Furuyama S, Sugiya H. Snare proteins essential for cyclic AMP-regulated exocytosis in salivary glands. Eur J Morphol 36, Suppl: 46–49, 1998. [PubMed] [Google Scholar]
- Fuxe K, Guidolin D, Agnati LF, Borroto-Escuela DO. Dopamine heteroreceptor complexes as therapeutic targets in Parkinson’s disease. Expert Opin Ther Targets 19: 377–398, 2015. doi: 10.1517/14728222.2014.981529. [DOI] [PubMed] [Google Scholar]
- Galinkin JL, Janiszewski D, Young CJ, Klafta JM, Klock PA, Coalson DW, Apfelbaum JL, Zacny JP. Subjective, psychomotor, cognitive, and analgesic effects of subanesthetic concentrations of sevoflurane and nitrous oxide. Anesthesiology 87: 1082–1088, 1997. doi: 10.1097/00000542-199711000-00012. [DOI] [PubMed] [Google Scholar]
- Hambrecht-Wiedbusch VS, Li D, Mashour GA. Paradoxical emergence: administration of subanesthetic ketamine during isoflurane anesthesia induces burst suppression but accelerates recovery. Anesthesiology 126: 482–494, 2017. doi: 10.1097/ALN.0000000000001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings HC., Jr Sodium channels and the synaptic mechanisms of inhaled anaesthetics. Br J Anaesth 103: 61–69, 2009. doi: 10.1093/bja/aep144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings HC Jr, Yan W, Westphalen RI, Ryan TA. The general anesthetic isoflurane depresses synaptic vesicle exocytosis. Mol Pharmacol 67: 1591–1599, 2005. doi: 10.1124/mol.104.003210. [DOI] [PubMed] [Google Scholar]
- Herring BE, McMillan K, Pike CM, Marks J, Fox AP, Xie Z. Etomidate and propofol inhibit the neurotransmitter release machinery at different sites. J Physiol 589: 1103–1115, 2011. doi: 10.1113/jphysiol.2010.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring BE, Xie Z, Marks J, Fox AP. Isoflurane inhibits the neurotransmitter release machinery. J Neurophysiol 102: 1265–1273, 2009. doi: 10.1152/jn.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker S, Czernik AJ, Greengard P, Augustine GJ. Tonically active protein kinase A regulates neurotransmitter release at the squid giant synapse. J Physiol 531: 141–146, 2001. doi: 10.1111/j.1469-7793.2001.0141j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill GE, Stanley TH, Sentker CR. Physostigmine reversal of postoperative somnolence. Can Anaesth Soc J 24: 707–711, 1977. doi: 10.1007/BF03006714. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci 8: 858–859, 2005. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Urade Y, Hayaishi O. The role of adenosine in the regulation of sleep. Curr Top Med Chem 11: 1047–1057, 2011. doi: 10.2174/156802611795347654. [DOI] [PubMed] [Google Scholar]
- Hudetz AG, Wood JD, Kampine JP. Cholinergic reversal of isoflurane anesthesia in rats as measured by cross-approximate entropy of the electroencephalogram. Anesthesiology 99: 1125–1131, 2003. doi: 10.1097/00000542-200311000-00019. [DOI] [PubMed] [Google Scholar]
- Janiszewski DJ, Galinkin JL, Klock PA, Coalson DW, Pardo H, Zacny JP. The effects of subanesthetic concentrations of sevoflurane and nitrous oxide, alone and in combination, on analgesia, mood, and psychomotor performance in healthy volunteers. Anesth Analg 88: 1149–1154, 1999. doi: 10.1213/00000539-199905000-00034. [DOI] [PubMed] [Google Scholar]
- Kasai H, Takahashi N, Tokumaru H. Distinct initial SNARE configurations underlying the diversity of exocytosis. Physiol Rev 92: 1915–1964, 2012. doi: 10.1152/physrev.00007.2012. [DOI] [PubMed] [Google Scholar]
- Kenny JD, Taylor NE, Brown EN, Solt K. Dextroamphetamine (but not atomoxetine) induces reanimation from general anesthesia: implications for the roles of dopamine and norepinephrine in active emergence. PLoS One 10: e0131914, 2015. doi: 10.1371/journal.pone.0131914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Tetanic stimulation recruits vesicles from reserve pool via a cAMP-mediated process in Drosophila synapses. Neuron 27: 133–143, 2000. doi: 10.1016/S0896-6273(00)00015-5. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Two synaptic vesicle pools, vesicle recruitment and replenishment of pools at the Drosophila neuromuscular junction. J Neurocytol 32: 551–565, 2003. doi: 10.1023/B:NEUR.0000020610.13554.3c. [DOI] [PubMed] [Google Scholar]
- Larsen B, Seitz A, Larsen R. Recovery of cognitive function after remifentanil-propofol anesthesia: a comparison with desflurane and sevoflurane anesthesia. Anesth Analg 90: 168–174, 2000. doi: 10.1097/00000539-200001000-00035. [DOI] [PubMed] [Google Scholar]
- Laurenza A, Sutkowski EM, Seamon KB. Forskolin: a specific stimulator of adenylyl cyclase or a diterpene with multiple sites of action? Trends Pharmacol Sci 10: 442–447, 1989. doi: 10.1016/S0165-6147(89)80008-2. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Shen HY, Cherasse Y, Qu WM, Huang ZL, Bass CE, Winsky-Sommerer R, Semba K, Fredholm BB, Boison D, Hayaishi O, Urade Y, Chen JF. Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci 31: 10067–10075, 2011. doi: 10.1523/JNEUROSCI.6730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Messing RO. Protein kinases and addiction. Ann N Y Acad Sci 1141: 22–57, 2008. doi: 10.1196/annals.1441.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LS, Petropoulos S, Shen B, Luo T, Herrick I, Rajakumar N, Ma J. Lesion of cholinergic neurons in nucleus basalis enhances response to general anesthetics. Exp Neurol 228: 259–269, 2011. doi: 10.1016/j.expneurol.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Lonart G, Janz R, Johnson KM, Südhof TC. Mechanism of action of rab3A in mossy fiber LTP. Neuron 21: 1141–1150, 1998. doi: 10.1016/S0896-6273(00)80631-5. [DOI] [PubMed] [Google Scholar]
- Lonart G, Schoch S, Kaeser PS, Larkin CJ, Südhof TC, Linden DJ. Phosphorylation of RIM1alpha by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell 115: 49–60, 2003. doi: 10.1016/S0092-8674(03)00727-X. [DOI] [PubMed] [Google Scholar]
- Luo T, Leung LS. Basal forebrain histaminergic transmission modulates electroencephalographic activity and emergence from isoflurane anesthesia. Anesthesiology 111: 725–733, 2009. doi: 10.1097/ALN.0b013e3181b061a0. [DOI] [PubMed] [Google Scholar]
- Machado JD, Morales A, Gomez JF, Borges R. cAmp modulates exocytotic kinetics and increases quantal size in chromaffin cells. Mol Pharmacol 60: 514–520, 2001. [PubMed] [Google Scholar]
- Metz LB, Dasgupta N, Liu C, Hunt SJ, Crowder CM. An evolutionarily conserved presynaptic protein is required for isoflurane sensitivity in Caenorhabditis elegans. Anesthesiology 107: 971–982, 2007. doi: 10.1097/01.anes.0000291451.49034.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret P, Backman SB, Bonhomme V, Plourde G, Fiset P. Physostigmine reverses propofol-induced unconsciousness and attenuation of the auditory steady state response and bispectral index in human volunteers. Anesthesiology 93: 708–717, 2000. doi: 10.1097/00000542-200009000-00020. [DOI] [PubMed] [Google Scholar]
- Navarro G, Borroto-Escuela DO, Fuxe K, Franco R. Purinergic signaling in Parkinson’s disease. Relevance for treatment. Neuropharmacology 104: 161–168, 2016. doi: 10.1016/j.neuropharm.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev 17: 139–170, 1992. doi: 10.1016/0165-0173(92)90012-B. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Valjent E, Corbillé AG, Corvol JC, Tassin JP, Girault JA, Hervé D. cAMP and extracellular signal-regulated kinase signaling in response to d-amphetamine and methylphenidate in the prefrontal cortex in vivo: role of beta 1-adrenoceptors. Mol Pharmacol 68: 421–429, 2005. [DOI] [PubMed] [Google Scholar]
- Plourde G, Chartrand D, Fiset P, Font S, Backman SB. Antagonism of sevoflurane anaesthesia by physostigmine: effects on the auditory steady-state response and bispectral index. Br J Anaesth 91: 583–586, 2003. doi: 10.1093/bja/aeg209. [DOI] [PubMed] [Google Scholar]
- Rang HP, Dale MM, Ritter JM, Flower RJ. Rang and Dale’s Pharmacology (6th ed.). Amsterdam, The Netherlands: Elsevier, 2007. [Google Scholar]
- Saifee O, Metz LB, Nonet ML, Crowder CM. A gain-of-function mutation in adenylate cyclase confers isoflurane resistance in Caenorhabditis elegans. Anesthesiology 115: 1162–1171, 2011. doi: 10.1097/ALN.0b013e318239355d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Preferential potentiation of fast-releasing synaptic vesicles by cAMP at the calyx of Held. Proc Natl Acad Sci USA 98: 331–336, 2001. doi: 10.1073/pnas.98.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABA(B) receptor activation at a glutamatergic synapse. Nature 424: 775–778, 2003. doi: 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferré S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol 83: 277–292, 2007. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev 85: 1303–1342, 2005. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- Simonds WF. G protein regulation of adenylate cyclase. Trends Pharmacol Sci 20: 66–73, 1999. doi: 10.1016/S0165-6147(99)01307-3. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J Neurosci 18: 10297–10303, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt K, Cotten JF, Cimenser A, Wong KF, Chemali JJ, Brown EN. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology 115: 791–803, 2011. doi: 10.1097/ALN.0b013e31822e92e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nomikos GG, Ongini E, Fredholm BB. Antagonism of adenosine A2A receptors underlies the behavioural activating effect of caffeine and is associated with reduced expression of messenger RNA for NGFI-A and NGFI-B in caudate-putamen and nucleus accumbens. Neuroscience 79: 753–764, 1997. doi: 10.1016/S0306-4522(97)00046-8. [DOI] [PubMed] [Google Scholar]
- Tai SK, Ma J, Leung LS. Medial septal cholinergic neurons modulate isoflurane anesthesia. Anesthesiology 120: 392–402, 2014. doi: 10.1097/ALN.0b013e3182a7cab6. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kadowaki T, Yazaki Y, Ellis-Davies GC, Miyashita Y, Kasai H. Post-priming actions of ATP on Ca2+-dependent exocytosis in pancreatic beta cells. Proc Natl Acad Sci USA 96: 760–765, 1999. doi: 10.1073/pnas.96.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NE, Chemali JJ, Brown EN, Solt K. Activation of D1 dopamine receptors induces emergence from isoflurane general anesthesia. Anesthesiology 118: 30–39, 2013. doi: 10.1097/ALN.0b013e318278c896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NE, Van Dort CJ, Kenny JD, Pei J, Guidera JA, Vlasov KY, Lee JT, Boyden ES, Brown EN, Solt K. Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc Natl Acad Sci USA 113: 12826–12831, 2016. doi: 10.1073/pnas.1614340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE, Emery DG, Haydon PG. Direct modulation of the secretory machinery underlies PKA-dependent synaptic facilitation in hippocampal neurons. Neuron 17: 789–797, 1996. doi: 10.1016/S0896-6273(00)80210-X. [DOI] [PubMed] [Google Scholar]
- Trudeau LE, Fang Y, Haydon PG. Modulation of an early step in the secretory machinery in hippocampal nerve terminals. Proc Natl Acad Sci USA 95: 7163–7168, 1998. doi: 10.1073/pnas.95.12.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE, Parpura V, Haydon PG. Activation of neurotransmitter release in hippocampal nerve terminals during recovery from intracellular acidification. J Neurophysiol 81: 2627–2635, 1999. [DOI] [PubMed] [Google Scholar]
- van Swinderen B, Saifee O, Shebester L, Roberson R, Nonet ML, Crowder CM. A neomorphic syntaxin mutation blocks volatile-anesthetic action in Caenorhabditis elegans. Proc Natl Acad Sci USA 96: 2479–2484, 1999. doi: 10.1073/pnas.96.5.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Fong R, Mason P, Fox AP, Xie Z. Caffeine accelerates recovery from general anesthesia. J Neurophysiol 111: 1331–1340, 2014. doi: 10.1152/jn.00792.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen RI, Desai KM, Hemmings HC Jr. Presynaptic inhibition of the release of multiple major central nervous system neurotransmitter types by the inhaled anaesthetic isoflurane. Br J Anaesth 110: 592–599, 2013. doi: 10.1093/bja/aes448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen RI, Gomez RS, Hemmings HC Jr. Nicotinic receptor-evoked hippocampal norepinephrine release is highly sensitive to inhibition by isoflurane. Br J Anaesth 102: 355–360, 2009. doi: 10.1093/bja/aen387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegar BD, MacIver MB. Isoflurane depresses hippocampal CA1 glutamate nerve terminals without inhibiting fiber volleys. BMC Neurosci 7: 5, 2006. doi: 10.1186/1471-2202-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Belluscio L, Hen R. G(olf)alpha mediates dopamine D1 receptor signaling. J Neurosci 20: RC91, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]