Considerable recent evidence has suggested important links between inflammation and the pathological mechanisms underlying hypertension. The present study describes cellular mechanisms through which acute and long-term exposure of tumor necrosis factor-α (TNF-α) influences the activity of subfornical organ neurons by modulating the voltage-gated transient Na+ current. This provides critical new information regarding the specific pathological mechanisms through which inflammation and TNF-α in particular may result in the development of hypertension.
Keywords: tumor necrosis factor-α, subfornical organ, patch-clamp electrophysiology, inflammation, angiotensin II
Abstract
Tumor necrosis factor-α (TNF-α) is a proinflammatory cytokine implicated in cardiovascular and autonomic regulation via actions in the central nervous system. TNF-α−/− mice do not develop angiotensin II (ANG II)-induced hypertension, and administration of TNF-α into the bloodstream of rats increases blood pressure and sympathetic tone. Recent studies have shown that lesion of the subfornical organ (SFO) attenuates the hypertensive and autonomic effects of TNF-α, while direct administration of TNF-α into the SFO increases blood pressure, suggesting the SFO to be a key site for the actions of TNF-α. Therefore, we used patch-clamp techniques to examine both acute and long-term effects of TNF-α on the excitability of Sprague-Dawley rat SFO neurons. It was observed that acute bath application of TNF-α depolarized SFO neurons and subsequently increased action potential firing rate. Furthermore, the magnitude of depolarization and the proportion of depolarized SFO neurons were concentration dependent. Interestingly, following 24-h incubation with TNF-α, the basal firing rate of the SFO neurons was increased and the rheobase was decreased, suggesting that TNF-α elevates SFO neuron excitability. This effect was likely mediated by the transient sodium current, as TNF-α increased the magnitude of the current and lowered its threshold of activation. In contrast, TNF-α did not appear to modulate either the delayed rectifier potassium current or the transient potassium current. These data suggest that acute and long-term TNF-α exposure elevates SFO neuron activity, providing a basis for TNF-α hypertensive and sympathetic effects.
NEW & NOTEWORTHY Considerable recent evidence has suggested important links between inflammation and the pathological mechanisms underlying hypertension. The present study describes cellular mechanisms through which acute and long-term exposure of tumor necrosis factor-α (TNF-α) influences the activity of subfornical organ neurons by modulating the voltage-gated transient Na+ current. This provides critical new information regarding the specific pathological mechanisms through which inflammation and TNF-α in particular may result in the development of hypertension.
hypertension is the leading risk factor for the development of cardiovascular disease (Lim et al. 2012). While the consequences of hypertension are well understood, its etiology remains elusive. One line of thought suggests that autonomic nervous system dysfunction may contribute to the development of hypertension by increasing sympathetic output and inducing the release of vasoconstricting agents such as catecholamines and angiotensin II (ANG II; Seravalle et al. 2014). Interestingly, elevated sympathetic nerve activity has been observed in patients with borderline hypertension, suggesting that autonomic dysfunction may play an important role in the development of hypertension (Anderson et al. 1989; Julius et al. 1991).
Tumor necrosis factor-α (TNF-α), a proinflammatory cytokine, is elevated in the plasma of patients with hypertension and cardiovascular disease (Aukrust et al. 1998; Glezeva and Baugh 2014, Tedgui and Mallat 2006). Recent evidence indicates that TNF-α acts within the central nervous system (CNS) to increase blood pressure and activate key autonomic centers, such as the paraventricular nucleus (PVN) of the hypothalamus (Zhang et al. 2003). Strikingly, TNF-α−/− mice do not develop hypertension or cardiac hypertrophy (Sriramula et al. 2008a) in an ANG II-salt model of hypertension. These data implicate the involvement of TNF-α in blood pressure regulation and sympathetic output. However, CNS sites at which TNF-α may exert its sympathetic and pressor effects have only been recently explored.
The subfornical organ (SFO) is a circumventricular forebrain structure that lacks a complete blood-brain barrier (BBB), making it an ideal site for sensing circulating signals in the periphery that do not diffuse across the normal BBB (Ganong 2000; Smith and Ferguson 2010). It has been well established that the SFO serves as a fundamental site for fluid homeostasis and cardiovascular regulation due to its ability to mediate ANG II dipsogenic (Moreau et al. 2012; Simpson and Routtenberg 1973; Tanaka et al. 2003) and pressor effects (Mangiapane and Simpson 1980). Strikingly, the SFO is largely responsible for the induction of hypertension by ANG II-salt loading (Osborn et al. 2012), which signifies that it may be a site at which pathological mechanisms of hypertension may converge. Electrical stimulation of the SFO increases blood pressure (Mangiapane and Brody 1983) and induces vasopressin release by the PVN (Ferguson and Kasting 1986). Moreover, efferent SFO projections innervate PVN neurons that project to the dorsal medulla, a critical site for sympathetic output (Ferguson et al. 1984). Neurons within the SFO express TNF-α receptor 1 (TNF-R1; Hindmarch and Ferguson 2016), and intriguingly, it is colocalized with the type 1 ANG II receptor (AT1R; Wei et al. 2015). It has been determined that injection of TNF-α into the carotid artery of rats increases PVN action potential firing rate, sympathetic nerve activity, and arterial pressure (Zhang et al. 2003), and the effects on sympathetic nerve activity and arterial pressure are attenuated by lesioning the SFO (Wei et al. 2013). This suggests that TNF-α may have direct actions on the SFO. In agreement with this hypothesis, it was found that direct microinjection of TNF-α into the SFO dramatically elevates blood pressure and expression of AT1R in both the SFO and PVN (Wei et al. 2015). Together, these findings suggest that the SFO plays an important role in the pressor and sympathetic effects of TNF-α. However, the underlying mechanisms by which TNF-α influences the SFO to mediate these effects are currently unknown.
The goal of the present study was to investigate the effects of TNF-α on the activity of SFO neurons. To test this, we performed whole cell and perforated patch-clamp experiments on dissociated SFO neurons in the presence of TNF-α and observed changes in activity and membrane excitability. Collectively, our data suggest that acute and long-term application of TNF-α excites SFO neurons, and the long-term effects are mediated by the voltage-gated transient sodium current (INa).
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Charles River, Quebec, Canada) that were 28–35 days old and weighed between 100 and 150 g were used for all experiments. Rats were housed at 22°C under a 12-h light-dark cycle and were provided food and water ad libitum. All animal procedures were approved and in compliance with the Queen’s University Animal Care Committee, as well as with the Canadian Council on Animal Care guidelines.
Chemicals and Drugs
All chemicals and drugs were obtained from Sigma-Aldrich (Oakville, ON) unless otherwise stated. Tetrodotoxin was obtained from Alomone Laboratories (Jerusalem, Israel) and stored at −20°C. TNF-α was obtained from Phoenix Pharmaceuticals (Burlingame, CA) and stored at −20°C. All drugs were prepared in artificial cerebrospinal fluid (aCSF) or intracellular solution the day of experimentation.
SFO Neuron Preparation
Dissociated SFO neurons were prepared as previously described (Ferguson et al. 1997; Kuksis and Ferguson 2015). Rats were euthanized by decapitation and their brains were dissected out and placed in ice-cold, carbogenated (95% O2-5% CO2) aCSF containing the following (in mM): 124 NaCl, 20 NaHCO3, 10 glucose, 2.27 CaCl2, 2.5 KCl, 1.3 MgSO4, and 1.24 KH2PO4. The SFOs from three euthanized rats were dissociated and cultured together each week for experimentation. The SFOs were then microdissected and placed in a drop of Hibernate media (ThermoFisher Scientific, Burlington, ON, Canada) containing B27 supplement (ThermoFisher Scientific). After dissection, the SFOs were incubated in 5 ml of Hibernate media containing 10 mg papain (Worthington Biochemical, Lakewood, NJ) for 30 min at 30°C in a warm water bath to allow for digestion of the extracellular matrix proteins. The SFOs were then rinsed and triturated three times each with Hibernate containing B27 to dissociate the SFOs into individual cells. The resulting cell suspension was centrifuged at 200 g for 8 min. The supernatant was removed and the pellet was resuspended in 30 μl Neurobasal-A media (ThermoFisher Scientific) containing B27, 100 U/ml penicillin-streptomycin (ThermoFisher Scientific), 0.4 mM l-glutamine (ThermoFisher Scientific), and 5 mM glucose. Ten-microliter drops were plated individually on 35 × 10 mm polystyrene dishes (Corning Life Sciences, Corning, NY) and were incubated at 37°C in 5% CO2 for 1.5 h to allow the cells to adhere to the dish. Following the incubation period, 2 ml of Neurobasal-A media were added to the dishes and the cells were incubated for at least 24 h before experiments were performed. Twenty-four hours before experiments on neurons incubated in TNF-α, the Neurobasal-A media were replaced with a Neurobasal-A media containing 10 ng/ml (575 pM) TNF-α, a concentration that was chosen based on a previous study showing the effects of TNF-α on mouse cortical neurons (Chen et al. 2015).
Electrophysiology
Whole cell and perforated patch-clamp recordings were performed on SFO neurons using the Multiclamp 700B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA) and were filtered at 2 kHz and digitized to 5 kHz. Spike2 (v7) and Signal (v6) software programs (Cambridge Electronics Design, Cambridge, UK) were used to record the data and analyze it post hoc. Micropipettes of 3–5 MΩ were prepared using borosilicate glass (World Precision Instruments, Sarasota, FL) using a P47 Flaming-Brown Micropipette Puller (Sutter Instrument, Novato, CA). The dishes containing SFO neurons were perfused at a rate of 2 ml/min with 37°C aCSF that contained the following (in mM): 140 NaCl, 10 HEPES, 5 KCl, 5 glucose, 5 mannitol, 2 CaCl2, and 1 MgCl2 (280–300 mosM) and had a pH adjusted to 7.3 using NaOH. Perfusion was performed using a gravity perfusion system, and the flow of aCSF was established with a vacuum pump. A MP-225 micromanipulator (Sutter Instrument) was used to guide the micropipette containing the electrode and intracellular solution toward a SFO cell membrane. Once a membrane was reached, negative pressure was applied to allow the formation of a >1-GΩ seal. For whole cell patch-clamp experiments, further negative pressure was applied until whole cell access was achieved. For perforated patch-clamp experiments, time was given for the Amphotericin B (400 μg/ml, 1% DMSO) within the micropipette intracellular solution to form pores in the membrane until adequate whole cell access was achieved (Linley 2013). It was determined whether the cell was a neuron based on the presence of a fast-activating voltage-gated inward conductance during a depolarizing voltage-step protocol. For voltage-clamp experiments, only recordings with a series resistance (Rs) of 5–20 MΩ were kept for analysis.
Whole cell and perforated current-clamp experiments.
For whole cell and perforated current-clamp experiments, the aCSF described above was used unless otherwise specified. An intracellular solution containing the following (in mM): 125 K-gluconate, 10 KCl, 10 HEPES, 2 MgCl2, 2 Na2ATP, 1.1 EGTA, and 0.3 CaCl2 (270–290 mosM) was used for current-clamp experiments. This intracellular solution pH was adjusted to 7.3 using KOH and had a calculated liquid junction potential of −15.0 mV. To measure acute changes in membrane potential, 4 ml TNF-α (5 fM to 5 nM) were bath perfused into the dish. Before bath application of TNF-α, a >100-s baseline was established and neurons were only analyzed whether action potentials were >60 mV. Each dish was tested with only a single bath application of TNF-α. To measure firing rate, the neurons were held at −65 mV with a holding current and the firing rate was obtained from a 100-s bin. The rheobase for each neuron was obtained by resting them at a membrane potential of −80 mV with a holding current to prevent spontaneous activity, and current pulses (500 ms) of increasing magnitude were applied until one action potential was observed.
Isolation and measurement of the transient sodium current.
To isolate the INa during whole cell voltage-clamp experiments, modified aCSF and intracellular solution were used to remove the contribution of K+ and Ca2+ currents. The modified aCSF contained the following (in mM): 110 NaCl, 20 TEA-Cl, 10 HEPES, 5 glucose, 5 CsCl, 1 MgCl2, and 0.3 CdCl2 (280–300 mosM), and its pH was adjusted to 7.3 using NaOH. In addition, the modified intracellular solution contained the following (in mM): 125 CsMeSO4, 10 CsCl, 10 HEPES, 2 MgCl2, 2 Na2ATP, 1.1 EGTA, and 0.3 CaCl2 (270–290 mosM), and its pH was adjusted to 7.3 using CsOH. This intracellular solution had a liquid junction potential of −18.1 mV. To measure the activation kinetics and the peak magnitude of the INa, a voltage-step protocol was used. The neurons were held at −75 mV, and then a prestep was performed to −90 mV for 100 ms, followed by 250-ms voltage steps from −95 to −35 mV in 5-mV increments. Peak inward currents for each voltage step were measured and conductance values (GNa) were calculated using the equation below:
where INa is the peak inward current, ENa is the equilibrium potential for INa (+107.1 mV), and Vm is the voltage step that induced INa. Conductance values were normalized to the maximum conductance of that neuron, and the normalized conductance values were then plotted against the pertaining voltage step. To measure the INa inactivation kinetics, the neurons were clamped to −75 mV, followed by a series of presteps from −125 to −35 mV for 500 ms in 10-mV increments and succeeded by a voltage step to −25 mV for 100 ms. Just as was done for the activation kinetics of the INa, the peak inward currents corresponding to the −25-mV voltage step and its preceding prestep were measured and conductance values were calculated using the formula above. These conductance values were normalized to the maximum conductance of that neuron and were plotted against its corresponding voltage step.
Isolation and measurement of the delayed rectifier potassium current.
SFO neurons have two primary potassium currents that contribute to the majority of the outward current: the IK and IA (Anderson et al. 2001). These currents can be separated from one another using modified intracellular and extracellular solutions in combination with manipulation of cell membrane potential. For whole cell voltage-clamp experiments to isolate the IK, modified aCSF and intracellular solution were used to remove the contribution of Na+ and Ca2+ currents. The modified aCSF contained the following (in mM): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, 5 glucose, 5 mannitol, 0.3 CdCl2, and 0.001 tetrodotoxin (280–300 mosM) and was adjusted to a pH of 7.3 using NaOH. The intracellular solution contained the following (in mM): 125 K-gluconate, 10 KCl, 10 HEPES, 5.5 EGTA, 2 MgCl2, 2 Na2ATP, and 0.3 CaCl2 (270–290 mosM) and was adjusted to a pH of 7.3 using KOH. To measure the peak magnitude of the IK, a voltage-step protocol was used that clamped the neurons at −75 mV, followed by a prestep to −40 mV for 100 ms, and then voltage steps from −80 to 20 mV for 250 ms in 10-mV increments. The prestep to −40 mV inactivates the IA component of the total outward current. The peak outward magnitudes in the last 50 ms of the voltage step were taken and plotted against its corresponding voltage step.
Isolation and measurement of the transient potassium current.
For whole cell voltage-clamp experiments to isolate the IA, the modified aCSF and intracellular solution that were used for the isolation of the IK above were used here. A similar voltage-step protocol was used, but instead a prestep to −90 mV was performed to prevent the inactivation of the IA. This voltage-step protocol was performed on the same neuron and in direct succession to the voltage-step protocol that was performed to isolate the IK. The peak outward current magnitudes within the first 50 ms of the voltage steps were subtracted from the peak outward current magnitudes observed during the IK voltage-step protocol, which generated a set of values pertaining to the IA component of the total outward current. The IA at each voltage step was normalized to the maximum IA of that neuron and then plotted against its corresponding voltage step.
Statistical Analysis
All data are reported as means ± SE. Changes in membrane potential following acute bath application of TNF-α were deemed significant if the magnitude of the change was at least two times the SD of the resting membrane potential before application. All comparisons of the mean values between two groups were analyzed using unpaired Student’s t-tests. A Boltzmann function was used to calculate the V50 and slope of the INa activation and inactivation profiles, as well as the IA activation profile. The liquid junction potential was corrected for in all the data analysis and figures. All data analysis was performed using GraphPad Prism 6.07 (La Jolla, CA). The level of significance for all statistical tests was set at P < 0.05.
RESULTS
Acute Bath Application of TNF-α Depolarizes SFO Neurons
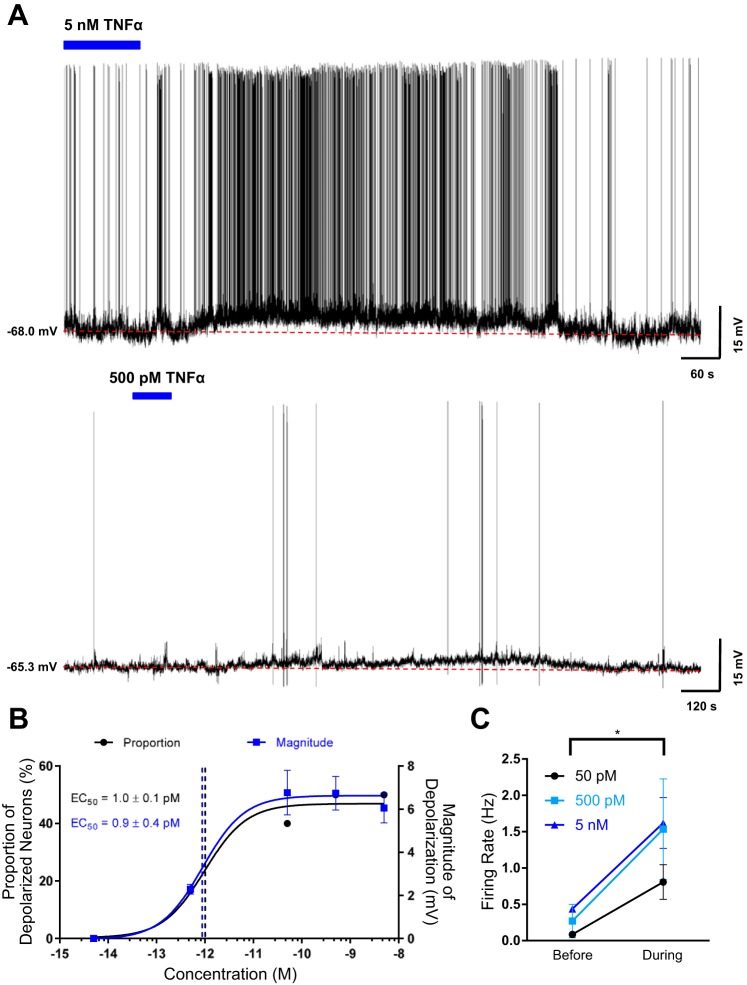
Based on previous reports that microinjection of TNF-α into the SFO elevates blood pressure and PVN firing rate (Wei et al. 2015), we hypothesized that TNF-α increases the excitability of SFO neurons to cause these effects. To determine the effects of TNF-α on the excitability SFO neurons, we conducted perforated current-clamp experiments on 62 dissociated SFO neurons obtained from 39 animals and observed changes in membrane potential and firing rate following bath application of TNF-α. We found that bath application of 5 nM TNF-α depolarized 50% (n = 5/10) of SFO neurons with a mean magnitude of depolarization of 6.4 ± 0.6 mV (n = 5; Fig. 1, A and B). As illustrated in Fig. 1, A and B, additional groups of SFO neurons were tested with TNF-α at concentrations between 5 fM and 5 nM, and collectively a total of 35% depolarized (n = 22/62). The depolarizations were typically accompanied by an increase in firing rate (Fig. 1C) and lasted around 10–30 min. It should be noted that some neurons, such as in Fig. 1A, bottom, exhibited a minimal or nonspontaneous firing rate before and during bath application of TNF-α. There was no significant difference in the resting membrane potential between nonresponsive and depolarized SFO neurons (nonresponsive = −56.5 ± 1.1 mV, n = 40; depolarized = −56.9 ± 1.5 mV, n = 22; P = 0.842), suggesting that TNF-α effects, or lack thereof, were not due to differences in the resting membrane potential. Interestingly, both the proportion of depolarized SFO neurons and the magnitude of depolarization induced by TNF-α were concentration dependent and had nearly identical EC50 values (EC50 = 1.0 ± 0.1 pM, and EC50 = 0.9 ± 0.4 pM, respectively; Fig. 1B). These data suggest that TNF-α is able to acutely influence the activity of SFO neurons in a concentration-dependent manner.
Fig. 1.
Acute bath application of TNF-α depolarizes subfornical organ (SFO) neurons. A: representative current-clamp recordings from 2 dissociated SFO neurons highlighting that bath application of 5 nM (top) and 500 pM (bottom) TNF-α depolarizes SFO neurons. The blue bar denotes the time and duration of bath application of TNF-α, and the dashed red line denotes the baseline membrane potential before TNF-α bath application. B: proportion of depolarizing SFO neurons (black) and the magnitude of depolarization (blue) at each concentration are shown (n = 62). The data sets were superimposed with a sigmoid concentration-response function, and the EC50 values are highlighted by the dashed vertical lines and as numerical values. C: action potential firing rate before and during bath application of 5 nM (blue), 500 pM (light blue), and 50 pM (black) TNF-α. The firing rates during bath application of TNF-α relative to the firing rates before for each concentration were significantly different (5 nM: Δfiring rate = 0.9 ± 0.3 Hz, *P = 0.04; 500 pM: Δfiring rate = 1.6 ± 0.7 Hz, *P = 0.05; 50 pM: Δfiring rate = 0.8 ± 0.2 Hz, *P = 0.02).
TNF-α Incubation Increases SFO Neuron Excitability
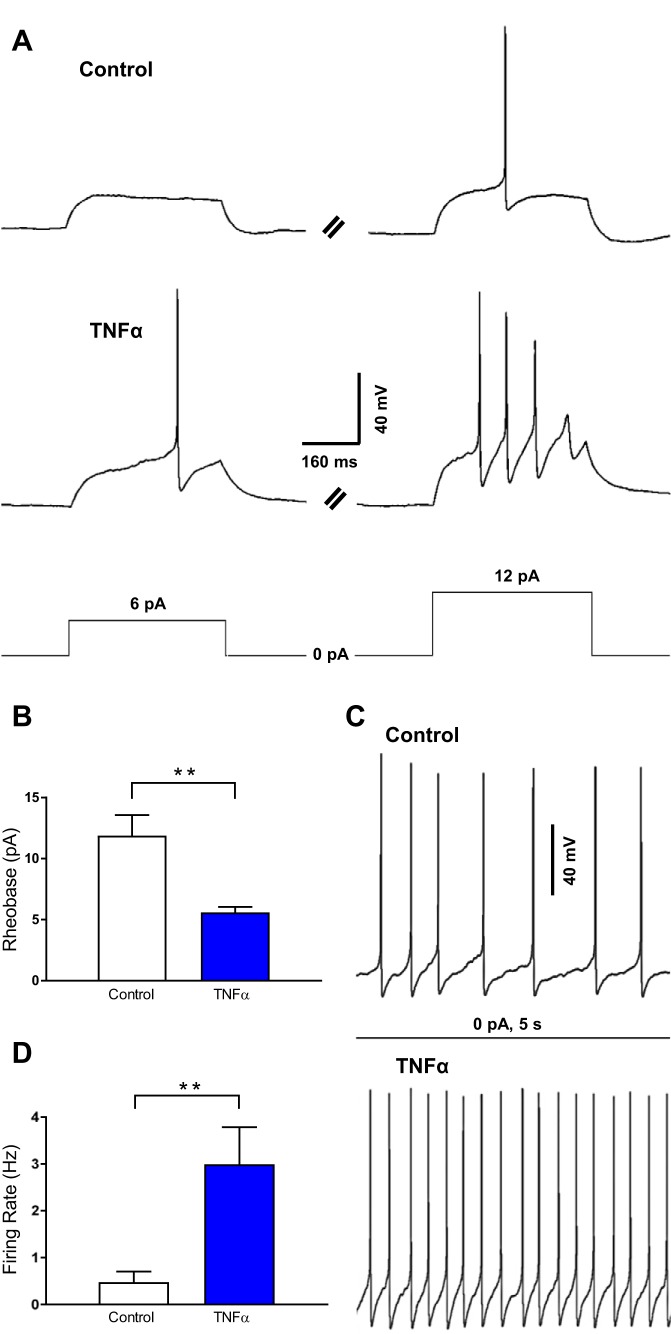
To determine the long-term effects of TNF-α on SFO neuron excitability, we incubated SFO neurons in 10 ng/ml (575 pM) TNF-α for 24 h and then performed perforated current-clamp experiments to examine their properties. We determined the magnitude of the minimum current required to induce a single action potential (rheobase) in neurons incubated in either TNF-α or control media (control; Fig. 2A). To do this, the SFO neuron membrane potential was held at −80 mV to allow for the measurement of membrane excitability without the presence of spontaneous activity. It was observed that neurons incubated in TNF-α exhibited a rheobase of smaller magnitude relative to control (control = 11.9 ± 1.7 pA, n = 8; TNF-α = 5.6 ± 0.5 pA, n = 10; P = 0.007; Fig. 2, A and B). Both the resting membrane potential and the input resistance were not significantly different between the control group and the TNF-α-treated group (resting membrane potential: control = −79.7 ± 0.4 mV, n = 8; TNF-α = −79.3 ± 0.4 mV, n = 10; P = 0.541; input resistance: control = 1.6 ± 0.2 GΩ, n = 8; TNF-α = 1.8 ± 0.3 GΩ, n = 10; P = 0.684). These data suggest that TNF-α increases SFO neuron membrane excitability and this effect was due to changes in the intrinsic properties of these neurons.
Fig. 2.
TNF-α incubation increases SFO neuron excitability. A: representative current-clamp traces of a control neuron (−80 mV baseline; top) and a neuron incubated in 10 ng/ml (575 pM) TNF-α (−80 mV baseline; middle) during current pulses (bottom) of 6 pA (left) and 12 pA (right). B: rheobase of control neurons (white) compared with neurons incubated in TNF-α (blue), highlighting that neurons incubated in TNF-α have a significantly smaller rheobase (**P = 0.007). C: representative current-clamp traces of the firing rate of control neurons (−65 mV; top) compared with neurons incubated in TNF-α (−65 mV; bottom) following no stimulus. D: basal firing rate of control neurons (white) and of neurons incubated in TNF-α (blue), highlighting that neurons incubated in TNF-α have a significantly higher firing rate (**P = 0.019).
Based on the observation that TNF-α incubation lowers the rheobase magnitude, we then hypothesized that incubation in TNF-α would increase the firing rate of SFO neurons. To test this we measured the firing rate of neurons incubated in TNF-α and compared with control. It was found that 70% (n = 7/10) of neurons in each group exhibited spontaneous action potentials (Fig. 2C). Of the neurons that were spontaneously active, the neurons incubated in TNF-α had a significantly elevated firing rate relative to that of control (control = 0.5 ± 0.2 Hz, n = 7; TNF-α = 3.0 ± 0.8 Hz, n = 7; P = 0.019; Fig. 2, C and D). The mean resting membrane potentials between the groups were not significantly different (control = −64.8 ± 0.5 mV, n = 7; TNF-α = −65.5 ± 0.8 mV, n = 7; P = 0.428), indicating that the difference in firing rate was not due to a difference in the membrane potential. Overall, these data suggest that TNF-α incubation increases SFO neuron membrane excitability and basal activity.
TNF-α Incubation Enhances the Transient Sodium Current
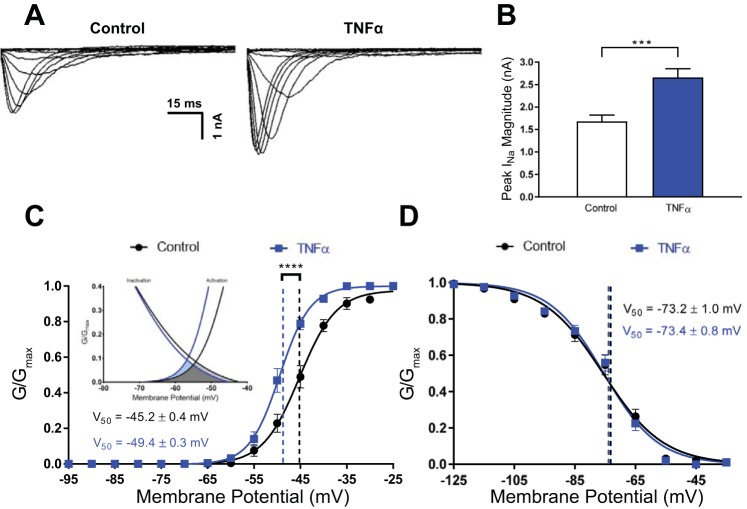
Based on the effects of TNF-α incubation on rheobase and firing rate, we hypothesized that TNF-α was potentiating the INa. To investigate this, we incubated neurons in 575 pM TNF-α and then performed whole cell voltage-clamp experiments on these neurons using a voltage-step protocol (see materials and methods). We explored the effects of TNF-α on the peak INa magnitude and found that neurons incubated in TNF-α had a significantly larger peak INa magnitude relative to control (control = 1.7 ± 0.1 nA, n = 15; TNF-α = 2.7 ± 0.2 nA, n = 12; P < 0.001; Fig. 3, A and B). Intriguingly, we also observed that neurons incubated in TNF-α exhibited a hyperpolarized V50 compared with control (control = −45.2 ± 0.4 mV, n = 15; TNF-α = −49.4 ± 0.3 mV, n = 12; P < 0.001; Fig. 3C), but TNF-α had no effect on the slope of the activation curve (control = 4.0 ± 0.4, n = 15, TNF-α = 3.3 ± 0.2, n = 12; P = 0.147). Thus TNF-α lowered the membrane potential at which 50% of the INa activates, which may explain why TNF-α incubation elevated the firing rate and decreased the rheobase. Next, we investigated the effects of TNF-α on the inactivation kinetics of INa and found that TNF-α incubation did not influence INa inactivation (V50: control = −73.2 ± 1.0 mV, n = 11; TNF-α = −73.4 ± 0.8 mV, n = 16; P = 0.889; slope: control = −10 ± 1.0, n = 11; TNF-α = −8.8 ± 0.7, n = 16; P = 0.323; Fig. 3D). Taken together, these data suggest that TNF-α incubation increases the excitability of SFO neurons by enhancing the INa.
Fig. 3.
TNF-α enhances the transient sodium current. A: representative overlaid voltage-clamp traces of the sodium current (INa) during each voltage step in control neurons (left) compared with neurons incubated in TNF-α (right), both of which were performed in the presence of extracellular and intracellular solutions that blocked K+ and Ca2+ currents. B: peak INa magnitude of control neurons (white) compared with neurons incubated in TNF-α (blue). C: normalized conductance at each voltage step of the activation protocol was plotted for both control neurons (black) and neurons incubated in TNF-α (blue). The data sets were fitted with a Boltzmann sigmoidal function and the representative curves were overlaid. The voltage (V50) for the data sets is highlighted by the dashed vertical lines and the numerical values (****P < 0.001). Inset: overlapping window currents for the activation and inactivation curves of TNF-α (light blue and dark grey) and control (light grey and dark grey). D: normalized conductance at each voltage step of the inactivation protocol was plotted for both control neurons (black) and neurons incubated in TNF-α (blue). The data sets were fitted with a Boltzmann sigmoidal function and the representative curves were overlaid. The V50 for the data sets is highlighted by the dashed vertical lines and the numerical values. GNa, conductance values.
TNF-α Has No Effect on the Delayed Rectifier Potassium Current or the Transient Potassium Current
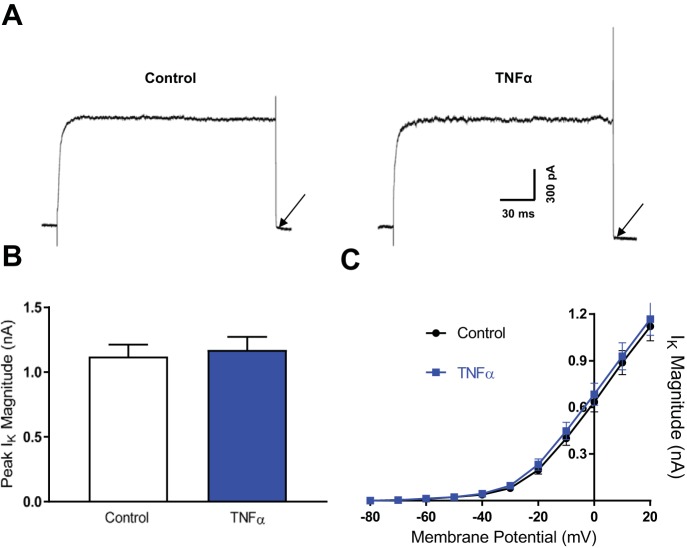
We next investigated whether voltage-gated potassium currents in SFO neurons were also modulated by incubation in TNF-α. We observed no significant difference between peak magnitude of the IK in neurons incubated in 575 pM TNF-α compared with control (control = 1.1 ± 0.1 nA, n = 11; TNF-α = 1.2 ± 0.1 nA, n = 11; P = 0.713; Fig. 4, A–C). Although the V50 or the slope could not be reported (as the IK magnitude did not reach a plateau at the larger voltage steps), we compared the current magnitudes at each voltage step between the two groups and found no significant differences (Fig. 4C).
Fig. 4.
TNF-α has no effect on the delayed rectifier potassium current. A: representative voltage-clamp traces of the IK in control neurons (left) and neurons incubated in TNF-α (right) following a voltage step to 20 mV. These recordings were performed in the presence of extracellular and intracellular solutions that blocked Na+ and Ca2+ currents. The black arrows highlight the very small tail currents observed when voltage is stepped back to −75 mV (close to reversal potential of −82 mV). B: peak IK magnitude at 20 mV for control neurons (white) and neurons incubated in TNF-α (blue). C: magnitude of the IK at each voltage step for control neurons (black) and neurons incubated in TNF-α (blue). Individual data points within each data set were connected with a straight line between each data point.
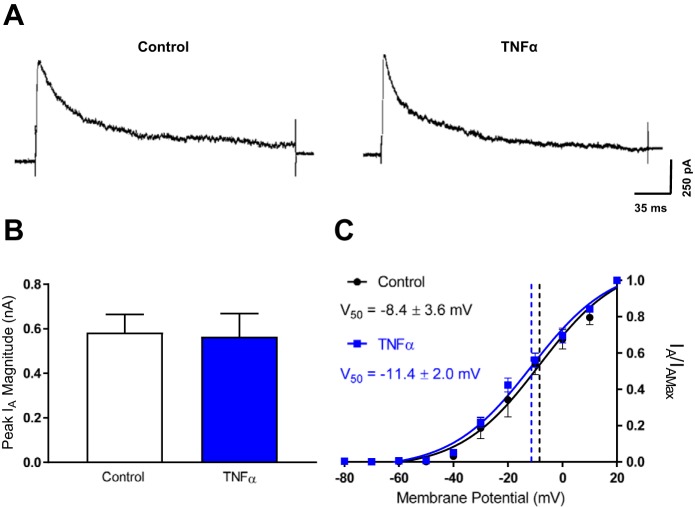
Similarly, we tested whether TNF-α incubation had an effect on the IA (Fig. 5A). It was determined that TNF-α incubation had no significant effect on the peak magnitude of the IA (control = 0.6 ± 0.1 nA, n = 11; TNF-α = 0.6 ± 0.1 nA, n = 8; P = 0.893; Fig. 5B). Furthermore, TNF-α incubation had no significant effect on the activation kinetics of the IA (V50: control = −8.4 ± 3.6 mV, n = 11; TNF-α = −11.4 ± 2.0 mV, n = 7; P = 0.546; slope: control = 14.6 ± 2.8, n = 11; TNF-α = 14.7 ± 1.7, n = 7; P = 0.977; Fig. 5C). These data suggest that TNF-α incubation has no effect on the IK or the IA, and therefore, these currents do not contribute to TNF-α effects on SFO neuron excitability.
Fig. 5.
TNF-α has no effect on the transient potassium current. A: representative voltage-clamp traces of the IA in control neurons (left) and neurons incubated in TNF-α (right) following a voltage step to 20 mV. These recordings were performed in the presence of extracellular and intracellular solutions that blocked Na+ and Ca2+ currents. B: peak IA magnitude at 20 mV for control neurons (white) and neurons incubated in TNF-α (blue). C: normalized current values at each voltage step of the activation protocol for the IA was plotted for both control neurons (black) and neurons incubated in TNF-α (blue). The data sets were fitted with a Boltzmann sigmoidal function and the representative curves were overlaid. The V50 for the data sets is highlighted by the dashed vertical lines and the numerical values.
DISCUSSION
In the present study, we investigated the effects of acute and long-term TNF-α exposure on the excitability of SFO neurons and the potential mechanisms underlying these long-term effects. We found that TNF-α depolarizes SFO neurons in a concentration-dependent manner and these depolarizations were accompanied by an increase in firing rate. Following an incubation period with TNF-α, we identified an elevated basal firing rate and a decrease in rheobase in SFO neurons, suggesting an increase in overall membrane excitability. Furthermore, TNF-α incubation increased the magnitude of the INa and shifted the activation curve of the INa to a more hyperpolarized state. However, TNF-α incubation had no effect on magnitude and activation kinetics of the IK or the IA. The concentrations of TNF-α used in the present in vitro study are higher than normal physiological ranges and at best reflect high pathophysiological concentrations previously reported in humans and animals (Drímal et al. 2008; Maury and Teppo 1989). Therefore, caution should be taken in the interpretation and application of these findings. Future studies will be necessary to determine whether these observations translate when physiological concentrations of TNF-α are induced in intact animal models of inflammation. Despite this limitation, our data show that acute and long-term application of TNF-α excites SFO neurons and the long-term effects are mediated by modulation of the INa but not the IK or the IA.
The Role of TNF-α in Central Cardiovascular and Sympathetic Regulation
The current study provides evidence for excitatory actions of TNF-α in the SFO, providing a basis for its central pressor and sympathetic effects. The SFO acts in a circuit with critical autonomic centers of the brain, such as the PVN, to control blood pressure and sympathetic output (Smith and Ferguson 2010). The consequences of activating the SFO include elevating blood pressure (Mangiapane and Brody, 1983), inducing vasopressin release (Ferguson and Kasting 1986), and increasing renal sympathetic nerve activity (Llewellyn et al. 2012). The SFO is known to project to the PVN, and efferent SFO fibers innervate distinct PVN neuron subtypes that regulate neuroendocrine and autonomic output (Kawano and Masuko 2010). Furthermore, ANG II, which has excitatory effects on SFO (Anderson et al. 2001; Cancelliere and Ferguson 2017) and PVN neurons (Li and Ferguson 1993a), has been shown to be released as a neurotransmitter from SFO efferents onto PVN neurons (Bains and Ferguson 1994; Li and Ferguson 1993b). Therefore, activation of the SFO may lead to synaptic release of ANG II (and potentially other neurotransmitters) within the PVN, and this may lead to an increase in blood pressure and sympathetic output. Direct administration of TNF-α into the SFO elevates the PVN firing rate, sympathetic nerve activity, and blood pressure (Wei et al. 2013). In combination with our data, these results implicate TNF-α as a modulator of this circuit and thus a modulator of neuroendocrine and autonomic activity.
Despite this, the time courses at which these effects take place remain to be elucidated. In the present study, the acute effects of TNF-α on SFO neurons typically lasted 10–30 min, whereas the increase in blood pressure caused by direct administration of TNF-α into the SFO persisted beyond 4 h (Wei et al. 2015). A possible explanation for these seemingly inconsistent observations may be that acute activation of the SFO by TNF-α may potentiate the activity of the downstream pathways that regulate blood pressure and sympathetic activity in the long term to allow for a constant and prolonged pressor response. Electrical stimulation of the SFO causes immediate increases in blood pressure that terminate immediately after cessation of stimulation (Mangiapane and Brody 1983), while administration of TNF-α into the SFO increases expression of TNF-R1 as well as AT1R in the SFO and PVN (Wei et al. 2015). Overall, these data suggest that TNF-α is not just able to increase SFO neuron activity acutely but also potentiates the circuit to allow for a prolonged pressor response. It should also be considered that the routes of administration (i.e., bath application in the present study and microinjection in Wei et al. 2015) and the concentration of TNF-α (5 fM to 5 nM for bath application and 7 µM for microinjection) were different between the studies, which may potentially explain the discrepancy in the latency of sympathetic activation seen following microinjection of TNF-α. Lastly, it is known that TNF-α signaling induces reactive oxygen species generation (Barth et al. 2009), a process that is important for the pressor effects of ANG II (Zimmerman et al. 2004) and also has effects on other cell types, such as microglia and astroglia (Olmos and Lladó 2014). Perhaps these mechanisms allow for further neuronal activation in vivo on a longer time scale, which may contribute to the increased sympathetic activity seen following TNF-α administration into the SFO. Therefore, future studies should explore the contributions of these other potential actions of TNF-α within the SFO to sympathetic activation and blood pressure regulation. It remains unknown how the pressor and sympathetic effects of TNF-α translate into a pathological state such as hypertension. Future studies should investigate the potential pathological manifestations of TNF-α effects within the SFO.
A further consideration to our studies relates to the fact that all of our recordings were obtained from SFO neurons derived from male rats. Similarly, all of the work to date examining TNF-α action in SFO of intact animals has been carried out in males. There is now an extensive literature describing sex differences in many animal models of hypertension (Hay et al. 2014), including ANG II-induced hypertension (Xue et al. 2005, 2013), where estrogen receptors (Xue et al. 2015) and endoplasmic reticulum stress (Dai et al. 2016; Young et al. 2012) in the SFO have been implicated. While it is beyond the scope of the present study, in the future it will be intriguing to determine whether there are sex-related differences in the responsiveness of SFO neurons to TNF-α.
TNF-α and ANG II Cross Talk
ANG II is a peptide that plays a critical role in cardiovascular and autonomic regulation and is implicated in cardiovascular disease (Kishi 2012; Lastra and Sowers 2013). Centrally, ANG II is known to act at the SFO to increase blood pressure (Mangiapane and Simpson 1980). There appears to be extensive cross talk between TNF-α and ANG II in regards to cardiovascular regulation, primarily through an interaction within the PVN (Sriramula et al. 2013; Kang et al. 2008) and the SFO (Wei et al. 2015). It has been shown that TNF-α−/− mice do not develop ANG II-induced hypertension or cardiac hypertrophy (Sriramula et al. 2008a). Intriguingly, ANG II-induced hypertension is mediated in large part by the SFO, as lesioning of this structure attenuates the pressor effect of ANG II (Osborn et al. 2012). Following administration of TNF-α into the SFO, AT1R and other components of the renin-angiotensin system, such as angiotensin converting enzyme, are upregulated in the SFO and PVN (Wei et al. 2015). Furthermore, TNF-R1 and AT1R are colocalized in the same SFO neurons (Wei et al. 2015). In the present study, TNF-α incubation of SFO neurons for 24 h (which attempts to mimic elevated circulating TNF-α during an immune challenge and in pathophysiological states like hypertension and heart failure in which the immune system is chronically activated) increases their membrane excitability and basal activity. In conjunction, these data suggest that 1) TNF-α is a vital component to central pressor effects of ANG II, and 2) increased TNF-α activity within the SFO modulates ANG II activity. One could therefore speculate that a pathological consequence of long-term increases in the circulating proinflammatory cytokine TNF-α may be priming the SFO to be more responsive to ANG II, thus allowing ANG II to induce a potentiated pressor response and subsequent hypertension. It has been previously shown that proinflammatory cytokines potentiate the pressor response to central ANG II (Ufnal et al. 2006; Zera et al. 2008; Sriramula et al. 2008b), but the mechanisms underlying these effects remain unknown. Future studies should examine whether previous TNF-α exposure potentiates the responsiveness of SFO neurons to ANG II and the signaling pathways by which this may occur.
TNF-α Effects on Ion Currents
Only recently have the central effects of TNF-α been explored. TNF-α is known to play a role in many neuronal processes including synaptic plasticity (Liu et al. 2017), neurodegeneration (Montgomery and Bowers 2012), and modulation of ion currents (Vezzani and Viviani 2015). In the present study, we identified the INa as a potential current underlying the long-term excitatory effects of TNF-α. A previous study in mouse cortical neurons showed that following TNF-α incubation nearly identical effects were seen on rheobase, firing rate, and INa kinetics when compared with the present study (Chen et al. 2015). On the other hand, TNF-α appeared to have no effect on voltage-gated K+ currents in other neuron types (Czeschik et al. 2008). These data are consistent with the data presented in the current study. What remains unknown is how TNF-α acutely depolarizes SFO neurons. Intriguingly, interleukin-1β (IL-1β), another proinflammatory cytokine, has similar depolarizing effects on SFO neurons that were shown to be mediated by a nonselective cationic conductance (Desson and Ferguson 2003). TNF-R1 and the IL-1β receptor share similar downstream signaling pathways in neurons (see Vezzani and Viviani 2015 for review), and thus, it is possible that TNF-α may also influence a nonselective cationic conductance. Future studies examining the mechanism(s) behind the depolarization induced by TNF-α may bring insight into how TNF-α elevates blood pressure and sympathetic activity.
In conclusion, we demonstrated that TNF-α increases the excitability of SFO neurons acutely and in the long term and that the long-term effects are mediated by the INa, thus implicating the SFO as a putative site for TNF-α to exert its central effects. In conjunction with previous findings, the actions of TNF-α at the SFO may promote activation of downstream autonomic and cardiovascular centers of the brain, such as the PVN, to elevate blood pressure and sympathetic tone. Our data, in combination with other studies, set a basis for future research to identify whether and how the effects of TNF-α in this circuit can pathologically manifest into hypertension.
GRANTS
This research was supported by the Canadian Institutes of Health Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.J.S. and A.V.F. conceived and designed research; N.J.S. performed experiments; N.J.S. and A.V.F. analyzed data; N.J.S. and A.V.F. interpreted results of experiments; N.J.S. prepared figures; N.J.S. and A.V.F. drafted manuscript; N.J.S. and A.V.F. edited and revised manuscript; N.J.S. and A.V.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Pauline M. Smith for technical assistance, as well as comments and suggestions during the creation of the manuscript.
REFERENCES
- Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension 14: 177–183, 1989. doi: 10.1161/01.HYP.14.2.177. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Smith PM, Ferguson AV. Subfornical organ neurons projecting to paraventricular nucleus: whole cell properties. Brain Res 921: 78–85, 2001. doi: 10.1016/S0006-8993(01)03093-1. [DOI] [PubMed] [Google Scholar]
- Aukrust P, Ueland T, Müller F, Andreassen AK, Nordøy I, Aas H, Kjekshus J, Simonsen S, Frøland SS, Gullestad L. Elevated circulating levels of C-C chemokines in patients with congestive heart failure. Circulation 97: 1136–1143, 1998. doi: 10.1161/01.CIR.97.12.1136. [DOI] [PubMed] [Google Scholar]
- Bains JS, Ferguson AV. Angiotensin II neurotransmitter actions in paraventricular nucleus are potentiated by a nitric oxide synthase inhibitor. Regul Pept 50: 53–59, 1994. doi: 10.1016/0167-0115(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Barth BM, Stewart-Smeets S, Kuhn TB. Proinflammatory cytokines provoke oxidative damage to actin in neuronal cells mediated by Rac1 and NADPH oxidase. Mol Cell Neurosci 41: 274–285, 2009. doi: 10.1016/j.mcn.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancelliere NM, Ferguson AV. Subfornical organ neurons integrate cardiovascular and metabolic signals. Am J Physiol Regul Integr Comp Physiol 312: R253–R262, 2017. doi: 10.1152/ajpregu.00423.2016. [DOI] [PubMed] [Google Scholar]
- Chen W, Sheng J, Guo J, Gao F, Zhao X, Dai J, Wang G, Li K. Tumor necrosis factor-α enhances voltage-gated Na+ currents in primary culture of mouse cortical neurons. J Neuroinflammation 12: 126, 2015. doi: 10.1186/s12974-015-0349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeschik JC, Hagenacker T, Schäfers M, Büsselberg D. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neurosci Lett 434: 293–298, 2008. doi: 10.1016/j.neulet.2008.01.070. [DOI] [PubMed] [Google Scholar]
- Dai SY, Fan J, Shen Y, He JJ, Peng W. Endoplasmic reticulum stress in the brain subfornical organ contributes to sex differences in angiotensin-dependent hypertension in rats. Acta Physiol (Oxf) 217: 33–44, 2016. doi: 10.1111/apha.12635. [DOI] [PubMed] [Google Scholar]
- Desson SE, Ferguson AV. Interleukin 1beta modulates rat subfornical organ neurons as a result of activation of a non-selective cationic conductance. J Physiol 550: 113–122, 2003. doi: 10.1113/jphysiol.2003.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drímal J, Knezl V, Paulovicová E, Drímal D. Enhanced early after-myocardial infarction concentration of TNF-alpha subsequently increased circulating and myocardial adrenomedullin in spontaneously hypertensive rats. Gen Physiol Biophys 27: 12–18, 2008. [PubMed] [Google Scholar]
- Ferguson AV, Bicknell RJ, Carew MA, Mason WT. Dissociated adult rat subfornical organ neurons maintain membrane properties and angiotensin responsiveness for up to 6 days. Neuroendocrinology 66: 409–415, 1997. doi: 10.1159/000127266. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Day TA, Renaud LP. Subfornical organ stimulation excites paraventricular neurons projecting to dorsal medulla. Am J Physiol Regul Integr Comp Physiol 247: R1088–R1092, 1984. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Kasting NW. Electrical stimulation in subfornical organ increases plasma vasopressin concentrations in the conscious rat. Am J Physiol Regul Integr Comp Physiol 251: R425–R428, 1986. [DOI] [PubMed] [Google Scholar]
- Ganong WF. Circumventricular organs: definition and role in the regulation of endocrine and autonomic function. Clin Exp Pharmacol Physiol 27: 422–427, 2000. doi: 10.1046/j.1440-1681.2000.03259.x. [DOI] [PubMed] [Google Scholar]
- Glezeva N, Baugh JA. Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart Fail Rev 19: 681–694, 2014. doi: 10.1007/s10741-013-9405-8. [DOI] [PubMed] [Google Scholar]
- Hay M, Xue B, Johnson AK. Yes! Sex matters: sex, the brain and blood pressure. Curr Hypertens Rep 16: 458, 2014. doi: 10.1007/s11906-014-0458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarch CC, Ferguson AV. Physiological roles for the subfornical organ: a dynamic transcriptome shaped by autonomic state. J Physiol 594: 1581–1589, 2016. doi: 10.1113/JP270726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius S, Krause L, Schork NJ, Mejia AD, Jones KA, van de Ven C, Johnson EH, Sekkarie MA, Kjeldsen SE, Petrin J, Schmouder R, Gupta R, Ferraro J, Nazzaro P, Weissfeld J. Hyperkinetic borderline hypertension in Tecumseh, Michigan. J Hypertens 9: 77–84, 1991. doi: 10.1097/00004872-199101000-00012. [DOI] [PubMed] [Google Scholar]
- Kang YM, Ma Y, Elks C, Zheng JP, Yang ZM, Francis J. Cross-talk between cytokines and renin-angiotensin in hypothalamic paraventricular nucleus in heart failure: role of nuclear factor-kappaB. Cardiovasc Res 79: 671–678, 2008. doi: 10.1093/cvr/cvn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H, Masuko S. Region-specific projections from the subfornical organ to the paraventricular hypothalamic nucleus in the rat. Neuroscience 169: 1227–1234, 2010. doi: 10.1016/j.neuroscience.2010.05.065. [DOI] [PubMed] [Google Scholar]
- Kishi T. Heart failure as an autonomic nervous system dysfunction. J Cardiol 59: 117–122, 2012. doi: 10.1016/j.jjcc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Kuksis M, Ferguson AV. Actions of a hydrogen sulfide donor (NaHS) on transient sodium, persistent sodium, and voltage-gated calcium currents in neurons of the subfornical organ. J Neurophysiol 114: 1641–1651, 2015. doi: 10.1152/jn.00252.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastra G, Sowers JR. Obesity and cardiovascular disease: role of adipose tissue, inflammation, and the renin-angiotensin-aldosterone system. Horm Mol Biol Clin Investig 15: 49–57, 2013. doi: 10.1515/hmbci-2013-0025. [DOI] [PubMed] [Google Scholar]
- Li Z, Ferguson AV. Angiotensin II responsiveness of rat paraventricular and subfornical organ neurons in vitro. Neuroscience 55: 197–207, 1993a. doi: 10.1016/0306-4522(93)90466-S. [DOI] [PubMed] [Google Scholar]
- Li Z, Ferguson AV. Subfornical organ efferents to paraventricular nucleus utilize angiotensin as a neurotransmitter. Am J Physiol Regul Integr Comp Physiol 265: R302–R309, 1993b. [DOI] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2260, 2012. [Erratum. Lancet 381: 628, 2013]. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley JE. Perforated whole cell patch-clamp recording. Methods Mol Biol 998: 149–157, 2013. doi: 10.1007/978-1-62703-351-0_11. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhou LJ, Wang J, Li D, Ren WJ, Peng J, Wei X, Xu T, Xin WJ, Pang RP, Li YY, Qin ZH, Murugan M, Mattson MP, Wu LJ, Liu XG. TNF-α differentially regulates synaptic plasticity in the hippocampus and spinal cord by microglia-dependent mechanisms after peripheral nerve injury. J Neurosci 37: 871–881, 2017. doi: 10.1523/JNEUROSCI.2235-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn T, Zheng H, Liu X, Xu B, Patel KP. Median preoptic nucleus and subfornical organ drive renal sympathetic nerve activity via a glutamatergic mechanism within the paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol 302: R424–R432, 2012. doi: 10.1152/ajpregu.00403.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiapane ML, Brody MJ. Electrical stimulation of subfornical organ (SFO) increases arterial pressure and regional vascular resistance (Abstract). Fed Proc 42: 584, 1983. [Google Scholar]
- Mangiapane ML, Simpson JB. Subfornical organ: forebrain site of pressor and dipsogenic action of angiotensin II. Am J Physiol Regul Integr Comp Physiol 239: R382–R389, 1980. [DOI] [PubMed] [Google Scholar]
- Maury CP, Teppo AM. Tumor necrosis factor in the serum of patients with systemic lupus erythematosus. Arthritis Rheum 32: 146–150, 1989. doi: 10.1002/anr.1780320206. [DOI] [PubMed] [Google Scholar]
- Montgomery SL, Bowers WJ. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J Neuroimmune Pharmacol 7: 42–59, 2012. doi: 10.1007/s11481-011-9287-2. [DOI] [PubMed] [Google Scholar]
- Moreau JM, Iqbal W, Turner JK, Wagner GF, Ciriello J. Stanniocalcin-1 in the subfornical organ inhibits the dipsogenic response to angiotensin II. Am J Physiol Regul Integr Comp Physiol 303: R921–R928, 2012. doi: 10.1152/ajpregu.00057.2012. [DOI] [PubMed] [Google Scholar]
- Olmos G, Lladó J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm 2014: 861231, 2014. doi: 10.1155/2014/861231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn JW, Hendel MD, Collister JP, Ariza-Guzman PA, Fink GD. The role of the subfornical organ in angiotensin II-salt hypertension in the rat. Exp Physiol 97: 80–88, 2012. doi: 10.1113/expphysiol.2011.060491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seravalle G, Mancia G, Grassi G. Role of the sympathetic nervous system in hypertension and hypertension-related cardiovascular disease. High Blood Press Cardiovasc Prev 21: 89–105, 2014. doi: 10.1007/s40292-014-0056-1. [DOI] [PubMed] [Google Scholar]
- Simpson JB, Routtenberg A. Subfornical organ: site of drinking elicitation by angiotensin II. Science 181: 1172–1175, 1973. doi: 10.1126/science.181.4105.1172. [DOI] [PubMed] [Google Scholar]
- Smith PM, Ferguson AV. Circulating signals as critical regulators of autonomic state–central roles for the subfornical organ. Am J Physiol Regul Integr Comp Physiol 299: R405–R415, 2010. doi: 10.1152/ajpregu.00103.2010. [DOI] [PubMed] [Google Scholar]
- Sriramula S, Cardinale J, Pariaut R, Francis J. Central nervous system blockade of tumor necrosis factor attenuates angiotensin II induced hypertension (Abstract). Circulation 118: S383, 2008b. [Google Scholar]
- Sriramula S, Cardinale JP, Francis J. Inhibition of TNF in the brain reverses alterations in RAS components and attenuates angiotensin II-induced hypertension. PLoS One 8: e63847, 2013. doi: 10.1371/journal.pone.0063847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension 51: 1345–1351, 2008a. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Miyakubo H, Fujisawa S, Nomura M. Reduced dipsogenic response induced by angiotensin II activation of subfornical organ projections to the median preoptic nucleus in estrogen-treated rats. Exp Neurol 179: 83–89, 2003. doi: 10.1006/exnr.2002.8054. [DOI] [PubMed] [Google Scholar]
- Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev 86: 515–581, 2006. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- Ufnal M, Dudek M, Zera T, Szczepańska-Sadowska E. Centrally administered interleukin-1 beta sensitizes to the central pressor action of angiotensin II. Brain Res 1100: 64–72, 2006. doi: 10.1016/j.brainres.2006.04.122. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 96: 70–82, 2015. doi: 10.1016/j.neuropharm.2014.10.027. [DOI] [PubMed] [Google Scholar]
- Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension 65: 1126–1133, 2015. doi: 10.1161/HYPERTENSIONAHA.114.05112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SG, Zhang ZH, Beltz TG, Yu Y, Johnson AK, Felder RB. Subfornical organ mediates sympathetic and hemodynamic responses to blood-borne proinflammatory cytokines. Hypertension 62: 118–125, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Johnson AK, Hay M. Sex differences in angiotensin II- and aldosterone-induced hypertension: the central protective effects of estrogen. Am J Physiol Regul Integr Comp Physiol 305: R459–R463, 2013. doi: 10.1152/ajpregu.00222.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol 288: H2177–H2184, 2005. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- Xue B, Zhang Z, Beltz TG, Guo F, Hay M, Johnson AK. Genetic knockdown of estrogen receptor-alpha in the subfornical organ augments ANG II-induced hypertension in female mice. Am J Physiol Regul Integr Comp Physiol 308: R507–R516, 2015. doi: 10.1152/ajpregu.00406.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, Davisson RL. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest 122: 3960–3964, 2012. doi: 10.1172/JCI64583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zera T, Ufnal M, Szczepańska-Sadowska E. Central TNF-alpha elevates blood pressure and sensitizes to central pressor action of angiotensin II in the infarcted rats. J Physiol Pharmacol 59, Suppl 8: 117–121, 2008. [PubMed] [Google Scholar]
- Zhang ZH, Wei SG, Francis J, Felder RB. Cardiovascular and renal sympathetic activation by blood-borne TNF-alpha in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol 284: R916–R927, 2003. doi: 10.1152/ajpregu.00406.2002. [DOI] [PubMed] [Google Scholar]
- Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res 95: 532–539, 2004. doi: 10.1161/01.RES.0000139957.22530.b9. [DOI] [PubMed] [Google Scholar]