In younger adults, more sensitive and more powerful long-interval intracortical inhibitory circuits are evident in the hemisphere controlling the more dexterous hand; this is not the case in older adults, for whom long-interval intracortical inhibitory circuits are symmetric and more variable than in younger adults. We speculate that the highly sensitive and powerful long-interval intracortical inhibition circuits in the dominant hemisphere play a role in manual dexterity.
Keywords: intracortical inhibition, motor control aging, transcranial magnetic stimulation, cortical excitability
Abstract
Aging is typically accompanied by a decline in manual dexterity and handedness; the dominant hand executes tasks of manual dexterity more quickly and accurately than the nondominant hand in younger adults, but this advantage typically declines with age. Age-related changes in intracortical inhibitory processes might play a role in the age-related decline in manual dexterity. Long-interval intracortical inhibition (LICI) is asymmetric in young adults, with more sensitive and more powerful LICI circuits in the dominant hemisphere than in the nondominant hemisphere. Here we investigated whether the hemispheric asymmetry in LICI in younger adults persists in healthy older adults. Paired-pulse transcranial magnetic stimulation was used to measure LICI in the dominant and nondominant hemispheres of younger and older adults; LICI stimulus-response curves were obtained by varying conditioning stimulus intensity at two different interstimulus intervals [100 ms (LICI100) and 150 ms]. We have replicated the finding that LICI100 circuits are more sensitive and more powerful in the dominant than the nondominant hemisphere of young adults and extend this finding to show that the hemispheric asymmetry in LICI100 is lost with age. In the context of behavioral observations showing that dominant hand movements in younger adults are more fluent than nondominant hand movements in younger adults and dominant hand movements in older adults, we speculate a role of LICI100 in the age-related decline in manual dexterity.
NEW & NOTEWORTHY In younger adults, more sensitive and more powerful long-interval intracortical inhibitory circuits are evident in the hemisphere controlling the more dexterous hand; this is not the case in older adults, for whom long-interval intracortical inhibitory circuits are symmetric and more variable than in younger adults. We speculate that the highly sensitive and powerful long-interval intracortical inhibition circuits in the dominant hemisphere play a role in manual dexterity.
aging is typically accompanied by a decline of manual dexterity. Growing evidence implicates age-related structural and functional changes across the cerebral cortex—such as decreases in cortical thickness, gray matter volume, and white matter infrastructure and diffuse task-related cortical activation—in age-related functional decline (for reviews see Grady 2012; Seidler et al. 2010). The contribution of age-related changes in the inhibitory control of voluntary movement, which is important for the execution of dexterous movement, has been the focus of recent research (Levin et al. 2014). Studies in young adults show that intracortical inhibition, acting within the primary motor cortex (M1), is reduced before and during muscle contraction (Buccolieri et al. 2004; Hammond and Vallence 2007; Reynolds and Ashby 1999; Ridding et al. 1995; Zoghi et al. 2003) and the reduction in intracortical inhibition during muscle contraction is greater during a precision grasp than an isolated tonic contraction (Kouchtir-Devanne et al. 2012). Intracortical inhibition is thought to be important for selectivity of motor activity during the execution of dexterous movement; in particular, surround inhibition—the suppression of excitability of muscles surrounding the active muscle—appears to be mediated by intracortical inhibition (Sohn and Hallett 2004; Thirugnanasambandam et al. 2015). A shift from intracortical inhibition to intracortical facilitation is thought to play an important role in dexterous movement with advancing age in older adults, with atypical intracortical facilitation associated with poor manual dexterity (Marneweck et al. 2011). These findings support the functional importance of intracortical inhibition in the preparation and execution of dexterous movement.
Paired-pulse transcranial magnetic stimulation (TMS) can be used to measure the excitability of several different inhibitory and excitatory processes within M1. One major inhibitory process that has been identified is long-interval intracortical inhibition (LICI) (Valls-Solé et al. 1992; Wassermann et al. 1996). When a suprathreshold conditioning stimulus (CS) precedes a suprathreshold test stimulus (TS) by ~50–200 ms, the amplitude of the motor evoked potential (MEP) elicited by the TS is suppressed because of the activation of long-interval intracortical inhibitory circuits (LICI) (Valls-Solé et al. 1992; Wassermann et al. 1996). Pharmacological studies provide strong evidence that LICI is mediated by GABAB receptor activity (McDonnell et al. 2006; Müller-Dahlhaus et al. 2008; Sanger et al. 2001; Werhahn et al. 1999). The recruitment of the inhibitory interneurons that mediate LICI can be measured by varying the intensity of the CS. The ratio of the amplitude of the conditioned MEP to that of the test MEP is a U-shaped function of CS intensity (Chen et al. 1998; Hammond and Garvey 2006; Ilić et al. 2002; Kujirai et al. 1993; Vallence et al. 2014). The initial descending limb of the LICI stimulus-response curve, where net inhibition increases with increasing CS intensity, represents the progressive recruitment of populations of inhibitory interneurons within M1 that mediate LICI; the subsequent ascending limb, where net inhibition decreases with increasing CS intensity, represents progressive recruitment of excitatory interneurons. Thus the descending limb of the LICI stimulus-response curve provides a measure of inhibitory influences mediated by GABAB receptor activity.
In young adults, LICI has been linked with handedness, the asymmetric pattern of hand use in which the dominant hand is typically used for exploration and manipulation of objects and the nondominant hand for stabilizing objects. LICI is asymmetric in young healthy right-handed adults: 1) LICI emerges at a lower CS intensity in the dominant than the nondominant hemisphere, showing that the inhibitory interneurons that mediate LICI are more sensitive in the dominant than the nondominant hemisphere; and 2) LICI is greater in magnitude in the dominant than the nondominant hemisphere (Hammond and Garvey 2006), showing that the inhibitory interneurons that mediate LICI exert a more powerful inhibition of M1 output in the dominant than the nondominant hemisphere. Therefore, in young adults LICI circuits are more sensitive and more powerful in the hemisphere controlling the more dexterous hand.
Handedness is affected by aging: while the dominant hand executes tasks of manual dexterity more quickly and accurately than the nondominant hand in younger adults, this asymmetry is typically lost in older adults (see, for example, Clark et al. 2011; Francis and Spirduso 2000; Marneweck et al. 2011; Przybyla et al. 2011). Given that LICI circuits are more sensitive and more powerful in the hemisphere controlling the dominant hand in younger adults, and an imbalance between intracortical inhibition and intracortical facilitation is associated with dexterity of the dominant hand in older adults, we hypothesized that the hemispheric asymmetry in LICI will decline with age.
To investigate this hypothesis, paired-pulse TMS was used to measure LICI in the dominant and nondominant hemispheres of younger and older adults; LICI stimulus-response curves were obtained by varying CS intensity at two different interstimulus intervals (ISIs) (specifically, 100 and 150 ms, as previous research suggests that different inhibitory processes might contribute to LICI measured at these two ISIs: Chu et al. 2008; Opie et al. 2015a, 2015b; Vallence et al. 2014). Dexterity was measured for each hand with subtests of the Purdue Pegboard task.
METHODS
Subjects
Thirty eight right-handed subjects participated in the study: 19 younger adults (3 men, 16 women; median 20 yr, range: 18–30 yr) and 19 older adults (2 men, 17 women; median 74 yr, range: 64–82 yr). The protocol was performed in accordance with the Declaration of Helsinki and was approved by the Murdoch University Human Research Ethics Committee. All subjects gave written informed consent before testing and were screened for conditions that would contraindicate TMS (Rossi et al. 2009, 2011). The Montreal Cognitive Assessment (MoCA; Nasreddine et al. 2005) was used as a screening tool for older adults: the mean score for the MoCA was 28.42 (SD 1.46).
Manual Dexterity
The Purdue Pegboard task was used to assess manual dexterity according to the standardized testing procedure (Lafayette Instrument). The peg-moving subtest required participants to pick up small pegs from a well with one hand and to insert them, one at a time, into a vertical array of holes on the pegboard (from top to bottom); the subtest was performed separately with the right and left hands. Participants were instructed to complete the tests as quickly as possible. The number of pegs moved and placed in a 30-s period was measured.
Transcranial Magnetic Stimulation
Subjects were seated with their head and neck supported throughout the session. Electromyographic (EMG) activity was recorded from the relaxed first dorsal interosseous (FDI) of the dominant (right) and nondominant (left) hands with surface electrodes placed in a belly-tendon montage. The EMG signal was amplified (1,000×; CED 1902 amplifier), band-pass filtered (20–1,000 Hz), and digitized at a sampling rate of 2 kHz (CED 1401 interface). A Magstim BiStim 2002 stimulator (Magstim, Whitland, UK) generated single- and paired-pulse stimuli (monophasic pulse waveform); stimuli were delivered through a figure-of-eight coil (90-mm diameter) placed tangentially to the scalp with the handle pointing backward and at a 45° angle away from the midline to induce a posterior-anterior current in the cortex.
The site for optimal stimulation and the resting motor threshold (RMT) were determined for the dominant (right) FDI with left M1 stimulation and for the nondominant (left) FDI with right M1 stimulation. To determine the optimal stimulation site, suprathreshold pulses were delivered over the target M1 at a number of sites in order to identify the site from which MEPs were evoked consistently in the target FDI. These sites were marked on the scalp with water-soluble ink to allow reliable placement of the coil throughout the experiment. RMT was defined as the minimum stimulus intensity [% of maximal stimulator output (MSO)] required to elicit MEPs of at least 50 µV in at least 5 of 10 consecutive trials in the relaxed target FDI.
Experimental Protocol
LICI was measured at rest so that MEP amplitude, and hence LICI measures, was not affected by other intracortical inhibitory and excitatory processes that are recruited during voluntary activation. LICI stimulus-response curves were obtained by delivering single-pulse TS-alone trials and paired-pulse trials with a range of CS intensities at two ISIs (100 and 150 ms); LICI stimulus-response curves were obtained separately for the dominant and nondominant hemispheres (order counterbalanced across subjects). Single-pulse TS-alone trials were delivered at an intensity of 120% RMT. Paired-pulse trials were delivered with CS intensities ranging from 100% RMT to 120% RMT (increments of 5% RMT), a TS intensity of 120% RMT, and ISIs of 100 and 150 ms. This resulted in 11 conditions: 5 different CS intensities for ISIs of 100 and 150 ms and the TS-alone condition.
Blocks of trials comprised TS-alone trials and paired-pulse trials of a single CS intensity. A single block of trials comprised 12 TS-alone trials, 12 paired-pulse trials (at a single CS intensity) with an ISI of 100 ms, and 12 paired-pulse trials (at a single CS intensity) with an ISI of 150 ms; trial conditions were pseudorandomized with an intertrial interval of 5 s (±20%) within each block. A total of 5 blocks (each with 36 trials; 1 block for each CS intensity) were delivered to each hemisphere, with a grand total of 10 blocks of trials (1 block for each of the 5 CS intensities targeting the dominant hemisphere and 1 block for each of the 5 CS intensities targeting the nondominant hemisphere). All five blocks of trials were delivered to the target hemisphere before switching to the other hemisphere and delivering the remaining five blocks of stimuli; the order in which the hemispheres were tested was counterbalanced across participants. Each block lasted ~3 min.
Data Analysis
Individual trials were excluded if prestimulus EMG activity exceeded 10 µV during the 100 ms before the CS or during the interval between the CS and the MEP evoked by the TS (5% of trials were excluded because of this background noise). The peak-to-peak MEP amplitude (in mV) was obtained from 40 ms of EMG activity beginning 15 ms after the TS.
To test for differences in RMT of the dominant and nondominant hands, paired-samples t-tests were performed on the RMT of the left and right hemispheres, with separate tests for younger and older adults. To test for differences in the mean TS-alone MEP amplitude between blocks of stimuli delivered at each CS intensity, repeated-measures ANOVAs (RM-ANOVAs) were performed on the mean single-pulse MEP amplitude from each of the stimulation blocks with within-subject factors Hemisphere (2 levels: dominant and nondominant) and CS Intensity Block (5 levels: CS 100%, 105%, 110%, 115%, 120% RMT). Separate ANOVAs were performed on the data for younger and older adults. For younger adults, there was no main effect of Hemisphere (F1,18 = 0.97, P = 0.338, = 0.05), no main effect of CS Intensity Block (F1,18 = 1.30, P = 0.279, = 0.07), and no Hemisphere × CS Intensity Block interaction (F1,18 = 1.36, P = 0.256, = 0.07). Similarly, for older adults, there was no main effect of Hemisphere (F1,18 = 0.09, P = 0.774, = 0.01), no main effect of CS Intensity Block (F1,18 = 0.85, P = 0.496, = 0.05), and no Hemisphere × CS Intensity Block interaction (F1,18 = 0.57, P = 0.683, = 0.03).
LICI was quantified by expressing the mean paired-pulse MEP amplitude for each CS intensity as a ratio of the mean TS-alone MEP amplitude. Normality was assessed by visual inspection of box plots, histograms, and Q-Q plots. To deal with nonnormally distributed LICI ratios at some CS intensities at some ISIs, all ratios were log-transformed. The results of the ANOVAs performed on the log-transformed ratios and the nontransformed ratios were the same. In light of this, and the knowledge that most parametric tests are relatively robust to moderate violations (Tabachnick and Fidell 2007), we report results from analyses performed on nontransformed ratios for ease of interpretation.
To test for differences in manual dexterity of the dominant and nondominant hands, a RM-ANOVA was performed on the Purdue Pegboard scores of the right and left hands with the within-subject factor Hand (2 levels: dominant and nondominant) and the between-subject factor Age (younger, older). In addition, paired-samples t-tests were performed on the Purdue Pegboard scores of the right and left hands, with separate tests for younger and older adults.
Measures of LICI were analyzed with RM-ANOVA with polynomial contrasts, and a Greenhouse-Geisser correction was used for analyses in which the assumption of sphericity was violated (Mauchly’s test of sphericity). To test for differences in LICI measured at different CS intensities between the two hemispheres, a two-way RM-ANOVA with the within-subject factors Hemisphere (2 levels: dominant and nondominant) and CS Intensity (5 levels: 100%, 105%, 110%, 115%, and 120% RMT). Separate ANOVAs were performed on the data for younger adults and older adults (to allow clear interpretation of interaction effects). Dependent on a significant main effect of Hemisphere, post hoc one-sample t-tests were performed on the LICI measure calculated for each CS intensity to determine the CS intensities at which conditioned MEP amplitudes were inhibited (i.e., LICI onset). Statistical significance was accepted at α < 0.05 unless there were multiple comparisons, in which case a Bonferroni correction was used (0.05/number of comparisons). Data are presented as means ± SD, except in the figures, where the SE is presented.
The threshold for inhibiting the conditioned MEP by 50% (i.e., LICI ratio of 0.5) was obtained by fitting a linear regression to the descending limb of each individual’s LICI stimulus-response curve (which represents the progressive recruitment of populations of inhibitory interneurons that mediate LICI) and using the regression formula to determine the CS intensity at which the linear regression was equal to 0.5. Participants were excluded from the analysis if the fit of the linear regression was ±2 SDs from the mean. Correlational analyses were performed to examine associations between each individual’s asymmetry in Purdue Pegboard performance and asymmetry in LICI (determined with the threshold for inhibiting the conditioned MEP by 50%). Asymmetry ratios were calculated for the Purdue Pegboard task (dominant and nondominant hands) and for LICI (dominant and nondominant hemispheres). For all asymmetry ratios, “nondominant” was expressed as a ratio of “dominant”; ratios >1 indicate dominant advantage (over nondominant), and ratios <1 indicate nondominant advantage (over dominant).
RESULTS
Behavioral Results: Manual Dexterity Is Asymmetric in Younger but Not Older Adults
The median Edinburgh Handedness Inventory score was 100 (range 75–100) for younger adults and 100 (range 75–100) for older adults. Figure 1 shows performance on the Purdue Pegboard subtests for younger and older adults. At the group level, younger adults moved more pegs with their dominant than their nondominant hand, whereas older adults moved the same number of pegs with each hand, showing a functional asymmetry in motor performance in younger adults but not older adults. A two-way ANOVA performed on the Purdue data showed main effects of Hand (F1,36 = 7.68, P = 0.009, = 0.18) and Age (F1,36 = 6.95, P = 0.012, = 0.16) and no Hand × Age interaction (F1,36 = 0.98, P = 0.329, = 0.03). Post hoc paired-samples t-tests showed that performance of the dominant hand was significantly better than performance of the nondominant hand in younger adults (t18 = 2.52, P = 0.022) but not older adults (t18 = 1.34, P = 0.197). [Note that with the young individual who placed the fewest number of pegs with the left hand removed from the analysis, the mean number of pegs placed with the dominant and nondominant hands for the younger adult sample is 14.89 and 14.17, respectively; a paired-samples t-test shows that this difference is statistically significant (t17 = 2.40, P = 0.028)].
Fig. 1.
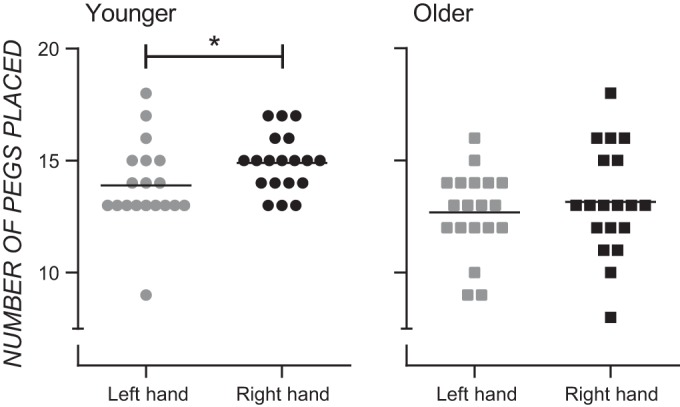
Column scatter graphs showing the number of pegs moved on the Purdue Pegboard with the left and right hands for younger (left) and older (right) adults. Each data point represents an individual participant, and the horizontal line in each graph represents the mean. *P < 0.025, Bonferroni correction for multiple comparisons.
Neurophysiological Results
Resting motor threshold and test MEP amplitude.
RMTs were similar in both hemispheres and both age groups. For younger adults, the mean RMT was 55% (6.9%) MSO for the dominant hemisphere and 53% (8.6%) MSO for the nondominant hemisphere (t18 = 1.41, P = 0.175). For older adults, the mean RMT was 56% (6.9%) MSO for the dominant hemisphere and 55% (9.1%) MSO for the nondominant hemisphere (t18 = 0.27, P = 0.794). MEP amplitude evoked by the TS-alone stimuli (TMS intensity of 120% RMT) was similar in the dominant and nondominant hemispheres for both younger [dominant: 1.53 mV, nondominant: 1.21 mV; t18 = 0.72, P = 0.482, 95% confidence interval (CI) [−0.50, 1.01]] and older (dominant: 0.74 mV, nondominant: 0.79 mV; t18 = 0.53, P = 0.606, 95% CI [−0.52, 0.31]) adults. In the dominant hemisphere, the test MEP amplitude was significantly larger in younger than older adults (t36 = 3.26, P = 0.002, 95% CI [0.30, 1.29]); there was no difference in test MEP amplitude in the nondominant hemisphere between younger and older adults (t36 = 0.96, P = 0.343, 95% CI [−0.48, 1.34]).
Younger adults: LICI asymmetry.
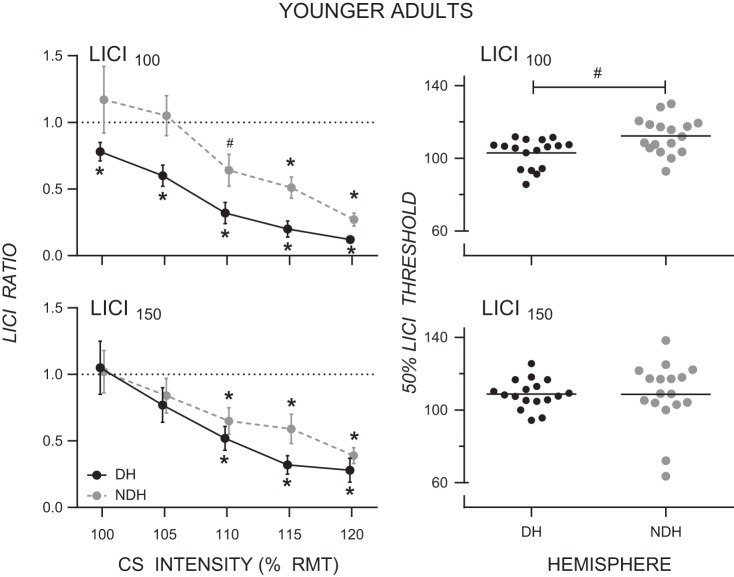
Figure 2 shows LICI as a function of CS intensity for the dominant and nondominant hemispheres in younger adults at the 100-ms ISI (Fig. 2, top left) and the 150-ms ISI (Fig. 2, bottom left). As expected, LICI100 and LICI150 both increased with CS intensity, reflecting the progressive recruitment of inhibitory interneurons with increasing CS intensity. LICI100 was greater in the dominant than the nondominant hemisphere in younger adults. A two-way ANOVA performed on the LICI100 data showed main effects of Hemisphere (F1,18 = 15.49, P = 0.001, = 0.46) and CS Intensity (F1.8,31.5 = 21.20, P < 0.001, = 0.54) and no Hemisphere × CS Intensity interaction (F2.0,36.0 = 0.64, P = 0.533, = 0.03). There was no difference in LICI150 between the two hemispheres. The LICI150 ANOVA showed a main effect of CS Intensity (F1.8,32.8 = 10.04, P = 0.001, = 0.36) but no main effect of Hemisphere (F1,18 = 1.96, P = 0.178, = 0.10) and no Hemisphere × CS Intensity interaction (F2.4,43.3 = 0.74, P = 0.506, = 0.04).
Fig. 2.
Left: mean LICI ratios for the dominant (DH) and nondominant (NDH) hemispheres as a function of conditioning stimulus (CS) intensity for LICI100 (top) and LICI150 (bottom) in younger adults (note: data points are offset for clarity). LICI100 is greater in the dominant than the nondominant hemisphere, and the onset of LICI100 occurs at a lower CS intensity in the dominant than the nondominant hemisphere. Right: threshold for inhibiting the conditioned MEP by 50% (i.e., LICI ratio of 0.5) for the dominant (DH) and nondominant (NDH) hemispheres for LICI100 (top) and LICI150 (bottom) in younger adults. The threshold (as % of RMT) for inhibiting the conditioned MEP by 50% at the ISI of 100 ms was lower in the dominant than the nondominant hemisphere. *LICI ratio significantly different from 1.0 at P ≤ 0.005, Bonferroni correction for multiple comparisons; #LICI ratio significantly different from 1.0 at P < 0.05.
As shown in Fig. 2, top left, LICI100 in younger adults emerged at a lower CS intensity in the dominant than the nondominant hemisphere. One-sample t-tests were performed to determine the CS intensity at which the conditioned MEP amplitude was significantly inhibited (Bonferroni correction for multiple comparisons, statistical significance for 1-sample t-tests accepted at P ≤ 0.005). Figure 2, top left, shows that LICI100 was significant at the lowest two CS intensities (CS100 and CS105) in the dominant hemisphere (both t18 > 3.16, P ≤ 0.005) but not the nondominant hemisphere (both t18 < 0.67, P > 0.514); at the intensity CS110, LICI100 was significant in the dominant hemisphere (t = 8.74, P < 0.001) but not the nondominant hemisphere when corrected for multiple comparisons (t = 3.05, P = 0.007). At the CS intensities CS115 and CS120, LICI100 was significant in both hemispheres (both t > 6.25, both P < 0.001). Figure 2, right, shows the CS intensity threshold (as % of RMT) required to inhibit the conditioned MEP by 50% (i.e., a LICI ratio of 0.5). (Note that linear regression did not give satisfactory fits for LICI100 and LICI150 for 2 younger adults; therefore, they were excluded from analyses.) The threshold for inhibiting the conditioned MEP by 50% at an ISI of 100 ms was lower in the dominant hemisphere (mean: 103% RMT, SD: 8.3% RMT) than the nondominant hemisphere (mean: 113% RMT, SD: 10.1% RMT; t16 = 2.58, P = 0.020). Moreover, unlike LICI100, LICI150 was similar in both hemispheres. One-sample t-tests showed that LICI150 was significant at CS110, CS115, and CS120 for both the dominant and nondominant hemispheres (all t > 3.58, all P < 0.002). There was no statistically significant difference between the threshold for inhibiting the conditioned MEP by 50% in the dominant (mean: 110% RMT, SD: 8.2% RMT) and the nondominant (mean: 103% RMT, SD: 31% RMT) hemisphere at an ISI of 150 ms (Fig. 2, bottom right). Finally, in the dominant hemisphere, LICI100 was significant at a lower CS intensity than LICI150; at the intensities CS100 and CS105 in the dominant hemisphere, LICI100 but not LICI150 was significant.
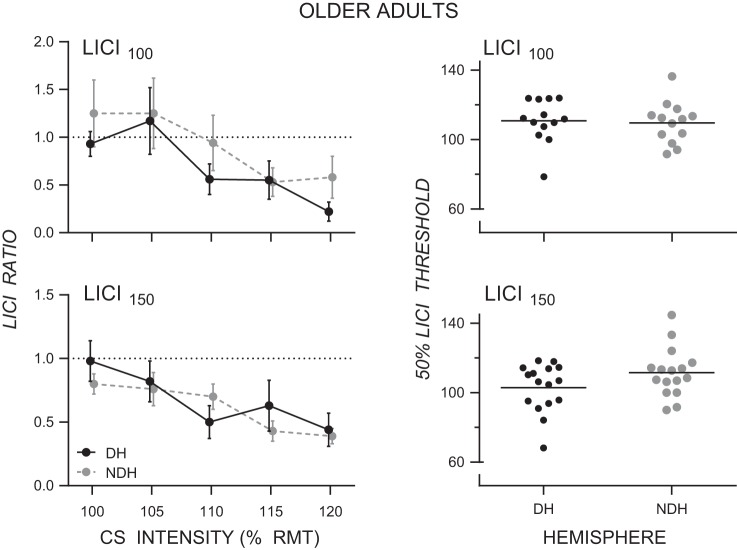
Older adults: no LICI asymmetry.
Figure 3 shows LICI as a function of CS intensity for both the dominant and nondominant hemispheres in older adults at the 100-ms ISI (Fig. 3, top left) and the 150-ms ISI (Fig. 3, bottom left). LICI100 and LICI150 both increased with CS intensity. There was no difference in either LICI100 or LICI150 between the dominant and nondominant hemispheres in older adults. Separate two-way RM-ANOVAs were performed for LICI100 and LICI150. For both LICI100 and LICI150 there was a main effect of CS Intensity (both F4,72 > 4.22, both P < 0.017, > 0.19) but no main effect of Hemisphere (both F1,18 < 3.55, both P > 0.076, < 0.17) and no Hemisphere × CS Intensity interaction (both F4,72 < 1.02, P > 0.387, < 0.05). The threshold for inhibiting the conditioned MEP by 50% at an ISI of 100 ms was similar in the dominant (mean: 111% RMT, SD: 12.6% RMT) and nondominant (mean: 110% RMT, SD: 12.0% RMT; see Fig. 3, right) hemispheres. The threshold for inhibiting the conditioned MEP by 50% at an ISI of 150 ms was numerically lower in the dominant (mean: 103% RMT, SD: 13.9% RMT) than the nondominant (mean: 112% RMT, SD 14.1% RMT) hemisphere, although this difference was not statistically significant (t15 = 1.91, P = 0.075). (Note that linear regression did not give satisfactory fits for 6 older adults for LICI100 and for 3 older adults for LICI150; therefore, they were excluded from analyses.)
Fig. 3.
Left: mean LICI ratios for the dominant (DH) and nondominant (NDH) hemispheres as a function of conditioning stimulus intensity for LICI100 (top) and LICI150 (bottom) in older adults (note: data points are offset for clarity). Right: threshold for inhibiting the conditioned MEP by 50% (i.e., LICI ratio of 0.5) for the dominant (DH) and nondominant (NDH) hemispheres for both LICI100 (top) and LICI150 (bottom) in older adults.
The threshold for inhibiting the conditioned MEP by 50% at an ISI of 100 ms in the dominant hemisphere was numerically lower in younger (mean: 103% RMT, SD: 8.3% RMT) than older (mean: 111% RMT, SD: 12.6% RMT) adults, and this difference approached statistical significance (t29 = 2.04, P = 0.050). In contrast, LICI150 showed a similar pattern in younger and older adults in both the dominant and nondominant hemispheres. The between-subject variability in LICI was examined by calculating the coefficient of variation for LICI ratios at each of the CS intensities in younger and older adults (Table 1). For LICI100, the coefficient of variation was numerically smaller in younger adults than older adults in both the dominant and nondominant hemispheres at all CS intensities. For LICI150, the coefficient of variation was similar in younger and older adults in both the dominant and nondominant hemispheres.
Table 1.
Coefficient of variation for LICI ratios at each of the CS intensities in younger and older adults
| CS Intensity, %RMT |
|||||
|---|---|---|---|---|---|
| 100 | 105 | 110 | 115 | 120 | |
| Left hemisphere: LICI100 | |||||
| Younger | 0.40 | 0.57 | 1.05 | 1.37 | 1.20 |
| Older | 0.63 | 1.29 | 1.26 | 1.58 | 2.01 |
| Right hemisphere: LICI100 | |||||
| Younger | 0.92 | 0.62 | 0.81 | 0.67 | 0.86 |
| Older | 1.23 | 1.29 | 1.37 | 1.25 | 1.63 |
| Left hemisphere: LICI150 | |||||
| Younger | 0.85 | 0.72 | 0.71 | 0.92 | 1.42 |
| Older | 0.71 | 0.84 | 1.10 | 1.36 | 1.27 |
| Right hemisphere: LICI150 | |||||
| Younger | 0.66 | 0.65 | 0.66 | 0.78 | 0.68 |
| Older | 0.43 | 0.75 | 0.60 | 0.82 | 0.69 |
Asymmetry in Motor Function and Asymmetry in LICI
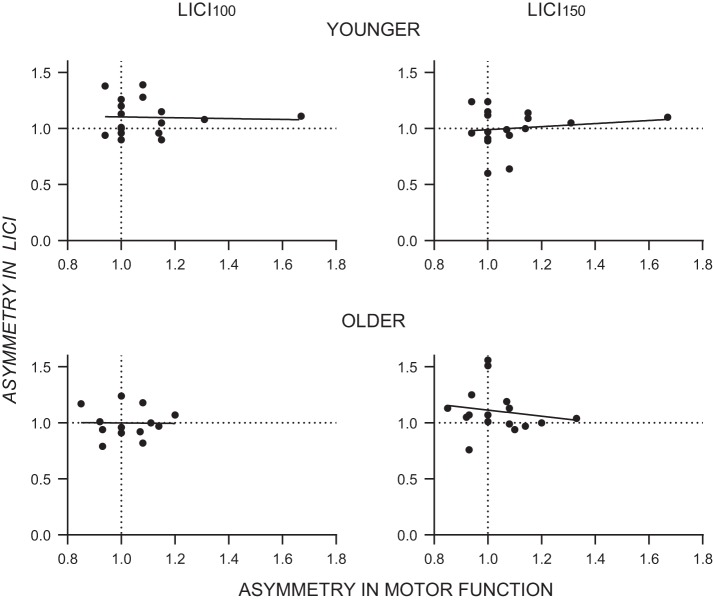
For each individual, asymmetry ratios were calculated to quantify the asymmetry in motor function (performance on the Purdue Pegboard with the dominant and nondominant hands). The average ratio of the dominant to nondominant hand performance on the Purdue Pegboard task was 1.09 for younger adults and 1.04 for older adults. Asymmetry ratios were also calculated to quantify the asymmetry in LICI (threshold for inhibiting the conditioned MEP by 50% in the dominant and nondominant hemispheres; analyses were performed on the subset of participants for whom the linear regressions gave satisfactory fits). Asymmetry in motor function was unrelated to asymmetry in LICI100 or LICI150 in either younger or older adults (Fig. 4).
Fig. 4.
Relationships between asymmetry in motor function and asymmetry in LICI100 (left) and LICI150 (right) for younger (top) and older (bottom) adults. Ratios >1 indicate dominant advantage (over nondominant), and ratios <1 indicate nondominant advantage (over dominant).
DISCUSSION
Three key findings emerge from the present study. First, in younger adults manual dexterity is greater in the dominant than the nondominant hand and LICI100 circuits are more sensitive and more powerful in the dominant than the nondominant hemisphere. Second, in older adults both manual dexterity and LICI show no asymmetry at the group level. Third, although both LICI100 and dexterity are asymmetric in younger adults and symmetric in older adults at the group level, these variables were not associated within individuals in either age group.
Manual Dexterity Asymmetry in Younger but Not Older Adults
The results of the Purdue Pegboard showed an asymmetry in manual dexterity, favoring dominant hand performance, in younger but not older adults. Consistent with previous research, younger adults placed a greater number of pegs with the dominant hand than with the nondominant hand (Francis and Spirduso 2000; Marneweck et al. 2011). In contrast to younger adults, and consistent with previous research, older adults placed the same number of pegs with the dominant and nondominant hands (Clark et al. 2011; Francis and Spirduso 2000; Marneweck et al. 2011). Although performance on the Purdue Pegboard reflects the use of the hands in daily activities, and was sufficient here to show the asymmetry in manual dexterity in younger adults and no asymmetry in manual dexterity in older adults, it is not a sensitive measure as shown by the overlapping distributions of left- and right-hand performance in right-handed young adults. The outcome measure is the number of pegs placed in a 30-s period: the statistically significant difference in performance of the dominant and nondominant hands in younger adults was, on average, only one peg. Tasks such as the grip-and-lift and motor tracking tasks using a digitizing tablet provide multiple, more sensitive measures of motor control (Aoki et al. 2016) and could better quantify asymmetry in manual dexterity across the life span.
Hemispheric Asymmetry in LICI100 in Younger but Not Older Adults
In younger adults, LICI100 was more sensitive and more powerful in the dominant hemisphere than the nondominant hemisphere. Specifically, LICI100 emerged at a lower CS intensity and was larger in magnitude in the dominant hemisphere than the nondominant hemisphere, consistent with previous research (Hammond and Garvey 2006). This finding suggests that the intracortical inhibitory circuits mediated by GABAB receptor activity, which are predominantly active at 100 ms, are more excitable and have a stronger inhibitory influence in the dominant than the nondominant hemisphere in younger adults. Taken together with behavioral findings, this shows that LICI circuits are more sensitive and more powerful in the hemisphere that controls the more dexterous hand. Movements of the dominant hand are more fluent, are timed more consistently, and are accompanied by less muscle cocontraction than those of the nondominant hand (see, for example, Aoki et al. 2016; Goble and Brown 2007; Hammond et al. 1988; Heuer 2007). The more sensitive and powerful LICI100 circuits in the dominant than the nondominant hemisphere in younger adults may contribute to the more fluent movements of the dominant than the nondominant hand. This hypothesis, however, remains to be tested.
A novel finding here is that, at the group level, there is no hemispheric asymmetry in LICI100 in older adults. Taken together with the results from younger adults, this suggests that the asymmetry in the sensitivity and power of LICI circuits acting predominantly at 100 ms is lost with age. Our neurophysiological results showing an asymmetry in LICI100 in younger but not older adults complement our behavioral results showing an asymmetry in manual dexterity in younger but not older adults. This suggests that LICI100 circuits could be part of a neural substrate for handedness and help determine the direction of asymmetry in manual dexterity. Therefore, the loss of a hemispheric asymmetry in the sensitivity and power of inhibitory interneurons that mediate LICI100 with age might play a role, in part, in the loss of asymmetry in manual dexterity in older adults.
In the present study, we characterize the excitability of LICI circuits within the M1 of each hemisphere in younger and older participants by probing the excitability of these circuits with varying CS intensities. It was important to make the measurements with the muscle at rest for two reasons. First, taking the measurements at rest avoided contamination of the MEP by other intracortical inhibitory and excitatory processes that are recruited during voluntary activation. Second, the similar time courses of LICI determined with the paired-pulse protocol and the cortical silent period (cSP) elicited by a single pulse make measurements of LICI during voluntary contraction problematic. During contraction, the CS will elicit a cSP and, depending on its duration, the TS will be delivered either during or after the cSP. As a result, the amplitude of the MEP elicited by the TS, and hence the LICI measure, will vary depending on the timing of the TS relative to the cSP. Because cSP duration varies both between individuals and within individuals from trial to trial, measures of LICI taken during voluntary contraction would be subject to uncontrolled variation.
Previous research shows that the cSP in intrinsic hand muscles is evoked by lower TMS intensities and is longer in duration in the dominant than the nondominant hemisphere in younger adults and this asymmetry in the cSP is lost with age (Lo and Fook-Chong 2005; Matsunaga et al. 1998). Given the pharmacological studies showing that the cSP and LICI are both mediated by GABAB receptor activity (Siebner et al. 1998; Werhahn et al. 1999), the previous cSP findings, together with our LICI findings, show that long-acting inhibitory processes are asymmetric both at rest and during voluntary contraction in younger adults but not older adults. Short-interval intracortical inhibition (SICI) is also asymmetric in younger adults: SICI emerges at a lower CS intensity in the dominant than the nondominant hemisphere (Hammond et al. 2004). It is likely that several intracortical inhibitory and excitatory processes contribute to the greater dexterity of the dominant than the nondominant hand in younger adults and play a role in the age-related decline in manual dexterity.
Relationships Between Hemispheric Asymmetries in LICI and Manual Dexterity
Despite younger adults showing a dominant hand advantage for motor performance and a dominant hemisphere advantage for LICI100, there was no relationship between individuals’ asymmetry in motor function and asymmetry in LICI in this age group. This finding shows that the hemispheric asymmetry in LICI100 is not associated with the degree of asymmetry in manual dexterity. The more sensitive and powerful LICI100 circuits in the hemisphere controlling the more dexterous hand could be part of a neural substrate for handedness and help determine the direction of asymmetry in manual dexterity. However, it is important to note that the present results cannot conclusively rule out an association between the degree of hemispheric asymmetry in LICI100 and the degree of asymmetry in manual dexterity. While the Purdue Pegboard is sufficiently sensitive to show the asymmetry in manual dexterity in younger adults, it might not be sufficiently sensitive to show a relationship between asymmetry in manual dexterity and asymmetry in LICI100 in this group. Such an association between the degree of asymmetry in manual dexterity and cortical lateralization is based on the view that dexterity is unidimensional, with the dominant hand superior to the nondominant hand. In contrast, there is good evidence for qualitatively different control processes for upper limb movements of the dominant and nondominant sides (Sainburg 2005). Specifically, movements of the dominant upper limb are controlled by a predictive mechanism, whereas movements of the nondominant upper limb are controlled by a feedback-based mechanism (Sainburg and Schaefer 2004). From this perspective, LICI100 may play a role, among other neurophysiological processes, in the predictive control processes that control the dominant hand.
Given the hemispheric asymmetry in LICI100 and the asymmetry in manual dexterity at the group level in younger adults, we might expect that, with age, maintaining a “younglike” asymmetry in LICI100 would be associated with a “younglike” asymmetry in motor performance. However, there was no association between asymmetry in LICI100 and asymmetry in Purdue Pegboard performance in older adults. The symmetry in LICI100 in older but not younger adults fits with the growing literature that shows more symmetric and diffuse cortical activation in older than younger adults (see, for example, Calautti et al. 2001; Heuninckx et al. 2005; Mattay et al. 2002; Ward 2006). Whether this symmetric and diffuse activation in older adults is compensatory in nature or indicates dedifferentiation (that is, less distinctive) in cortical representations remains debated (for review see Seidler et al. 2010).
The loss of asymmetry with age is not global: although many studies report an attenuation or loss of manual dexterity asymmetry with age (e.g., Przybyla et al. 2011; Raw et al. 2012; Teixeira 2008; Wang et al. 2011), others report no change in asymmetry with age (see for review Francis and Spirduso 2000; Raw et al. 2015; Teixeira 2008) or even an increase in asymmetry with age (Weller and Latimer-Sayer 1985). These findings suggest that age-related changes in manual dexterity are task specific (Teixeira 2008), which might be due, in part, to the sensorimotor requirements of the tasks (Raw et al. 2015; Teixeira 2008). However, the task characteristics that show different age-related effects on manual asymmetry are unknown. Furthermore, age-related peripheral changes (e.g., sarcopenia, cutaneous sensitivity) and cortical changes that are not directly related to manual dexterity (e.g., decision making) likely influence age-related changes in manual asymmetry.
Time-Dependent LICI Processes
In younger adults, LICI is asymmetric at an ISI of 100 ms but not at an ISI of 150 ms. Furthermore, within the dominant hemisphere in younger adults, LICI100 is evident at a lower CS intensity than LICI150, replicating previous research (Vallence et al. 2014). These findings contribute to a somewhat dispersed, but growing, body of literature suggesting the contribution of different processes to LICI100 and LICI150. Most recently, Opie et al. (2015a, 2015b) showed an increase in LICI100 but a decrease in LICI150 during voluntary muscle contraction. Earlier work from Chu et al. (2008) showed that LICI-SICI interactions in the resting muscles are differentially affected by ISI, with SICI inhibited in the presence of LICI100 but not LICI150. In addition, experimental induction of motor cortical plasticity differentially affects LICI measured at different ISIs: theta burst stimulation applied to the cerebellum changed LICI100 but not LICI150 (Koch et al. 2008). It is difficult to interpret this somewhat dispersed body of literature, but it does suggest the contribution of partially independent neural processes to LICI measured at different times after activation.
Limitations
The MEP amplitude evoked by TS-alone stimuli over the dominant hemisphere was significantly larger in younger than older adults. It is known that as the intensity of the TS increases, the magnitude of LICI100 and LICI150 decreases (Opie and Semmler 2014; Sanger et al. 2001), likely because the effect of the inhibitory interneurons is overridden by excitatory processes activated by the TS. Thus the apparently higher effective TS intensity to the dominant hemisphere of the younger than the older adults would have decreased the measure of LICI100 in the dominant hemisphere in younger adults, and so slightly underestimated the magnitude of the difference in LICI100 in the dominant hemisphere between younger and older adults. Furthermore, any such effect would have reduced the magnitude of the asymmetry of LICI100 in the younger adults, and so the greater effective TS intensity over the dominant hemisphere of younger than older adults does not affect the main conclusion that LICI100 is asymmetric in young but not old adults. It is also important to note the sex imbalance in the present sample; only 16% of younger adults and 11% of older adults were male. Previous work suggests an interaction of sex and age on excitability, whereby older women but not older men show reduced corticospinal excitability measured with single-pulse TMS input/output curves (Pitcher et al. 2003; Smith et al. 2011), as well as influences of the menstrual cycle on cortical excitability (Smith et al. 1999). Therefore, replication of the present results with a sex-balanced sample is warranted. Finally, the sample of older adults was not random: although study advertisements were placed at a variety of community centers, the majority of the older adults who participated were from physical activity groups. Therefore, the conclusions from the present study are limited to healthy older adults. Future work quantifying physical activity levels and additional lifestyle factors is warranted.
Conclusions
We have replicated the finding that LICI100 circuits are more sensitive and more potent in the dominant than the nondominant hemisphere of young adults and extend this finding to show that the hemispheric asymmetry in LICI100 is lost with age. We also show that the hemispheric asymmetry in LICI in young adults is evident at an ISI of 100 ms but not 150 ms, adding to the growing literature that suggests time-dependent LICI processes. Gaining a better understanding of the functional importance of hemispheric asymmetry in LICI might further elucidate the functional roles of LICI processes.
GRANTS
A.-M. Vallence is supported by a National Health and Medical Research Council Early Career Fellowship (GNT1088295).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.-M.V. and E.S. analyzed data; A.-M.V., E.S., P.D., and G.H. interpreted results of experiments; A.-M.V. prepared figures; A.-M.V. drafted manuscript; A.-M.V., E.S., P.D., and G.H. edited and revised manuscript; A.-M.V., E.S., P.D., and G.H. approved final version of manuscript; E.S. performed experiments.
REFERENCES
- Aoki T, Rivlis G, Schieber MH. Handedness and index finger movements performed on a small touchscreen. J Neurophysiol 115: 858–867, 2016. doi: 10.1152/jn.00256.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccolieri A, Abbruzzese G, Rothwell JC. Relaxation from a voluntary contraction is preceded by increased excitability of motor cortical inhibitory circuits. J Physiol 558: 685–695, 2004. doi: 10.1113/jphysiol.2004.064774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti C, Serrati C, Baron JC. Effects of age on brain activation during auditory-cued thumb-to-index opposition: a positron emission tomography study. Stroke 32: 139–146, 2001. doi: 10.1161/01.STR.32.1.139. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Bütefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol 80: 2870–2881, 1998. [DOI] [PubMed] [Google Scholar]
- Chu J, Gunraj C, Chen R. Possible differences between the time courses of presynaptic and postsynaptic GABAB mediated inhibition in the human motor cortex. Exp Brain Res 184: 571–577, 2008. doi: 10.1007/s00221-007-1125-7. [DOI] [PubMed] [Google Scholar]
- Clark J, Loftus A, Hammond G. Age-related changes in short-interval intracortical facilitation and dexterity. Neuroreport 22: 499–503, 2011. doi: 10.1097/WNR.0b013e3283487480. [DOI] [PubMed] [Google Scholar]
- Francis KL, Spirduso WW. Age differences in the expression of manual asymmetry. Exp Aging Res 26: 169–180, 2000. doi: 10.1080/036107300243632. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Brown SH. Task-dependent asymmetries in the utilization of proprioceptive feedback for goal-directed movement. Exp Brain Res 180: 693–704, 2007. doi: 10.1007/s00221-007-0890-7. [DOI] [PubMed] [Google Scholar]
- Grady C. The cognitive neuroscience of ageing. Nat Rev Neurosci 13: 491–505, 2012. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G, Bolton Y, Plant Y, Manning J. Hand asymmetries in interresponse intervals during rapid repetitive finger tapping. J Mot Behav 20: 67–71, 1988. doi: 10.1080/00222895.1988.10735433. [DOI] [PubMed] [Google Scholar]
- Hammond G, Faulkner D, Byrnes M, Mastaglia F, Thickbroom G. Transcranial magnetic stimulation reveals asymmetrical efficacy of intracortical circuits in primary motor cortex. Exp Brain Res 155: 19–23, 2004. doi: 10.1007/s00221-003-1696-x. [DOI] [PubMed] [Google Scholar]
- Hammond G, Vallence AM. Modulation of long-interval intracortical inhibition and the silent period by voluntary contraction. Brain Res 1158: 63–70, 2007. doi: 10.1016/j.brainres.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Hammond GR, Garvey CA. Asymmetries of long-latency intracortical inhibition in motor cortex and handedness. Exp Brain Res 172: 449–453, 2006. doi: 10.1007/s00221-006-0349-2. [DOI] [PubMed] [Google Scholar]
- Heuer H. Control of the dominant and nondominant hand: exploitation and taming of nonmuscular forces. Exp Brain Res 178: 363–373, 2007. doi: 10.1007/s00221-006-0747-5. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP. Neural basis of aging: the penetration of cognition into action control. J Neurosci 25: 6787–6796, 2005. doi: 10.1523/JNEUROSCI.1263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilić TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol 545: 153–167, 2002. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Mori F, Marconi B, Codecà C, Pecchioli C, Salerno S, Torriero S, Lo Gerfo E, Mir P, Oliveri M, Caltagirone C. Changes in intracortical circuits of the human motor cortex following theta burst stimulation of the lateral cerebellum. Clin Neurophysiol 119: 2559–2569, 2008. doi: 10.1016/j.clinph.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Kouchtir-Devanne N, Capaday C, Cassim F, Derambure P, Devanne H. Task-dependent changes of motor cortical network excitability during precision grip compared to isolated finger contraction. J Neurophysiol 107: 1522–1529, 2012. doi: 10.1152/jn.00786.2011. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol 471: 501–519, 1993. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin O, Fujiyama H, Boisgontier MP, Swinnen SP, Summers JJ. Aging and motor inhibition: a converging perspective provided by brain stimulation and imaging approaches. Neurosci Biobehav Rev 43: 100–117, 2014. doi: 10.1016/j.neubiorev.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Lo YL, Fook-Chong S. The silent period threshold as a measure of corticospinal inhibition. J Clin Neurophysiol 22: 176–179, 2005. [PubMed] [Google Scholar]
- Marneweck M, Loftus A, Hammond G. Short-interval intracortical inhibition and manual dexterity in healthy aging. Neurosci Res 70: 408–414, 2011. doi: 10.1016/j.neures.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Matsunaga K, Uozumi T, Tsuji S, Murai Y. Age-dependent changes in physiological threshold asymmetries for the motor evoked potential and silent period following transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 109: 502–507, 1998. doi: 10.1016/S1388-2457(98)00020-0. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in human motor function. Neurology 58: 630–635, 2002. doi: 10.1212/WNL.58.4.630. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABAB receptors in intracortical inhibition in the human motor cortex. Exp Brain Res 173: 86–93, 2006. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- Müller-Dahlhaus JF, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J Physiol 586: 495–514, 2008. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: 695–699, 2005. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Opie GM, Ridding MC, Semmler JG. Age-related differences in pre- and post-synaptic motor cortex inhibition are task dependent. Brain Stimul 8: 926–936, 2015a. doi: 10.1016/j.brs.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Opie GM, Ridding MC, Semmler JG. Task-related changes in intracortical inhibition assessed with paired- and triple-pulse transcranial magnetic stimulation. J Neurophysiol 113: 1470–1479, 2015b. doi: 10.1152/jn.00651.2014. [DOI] [PubMed] [Google Scholar]
- Opie GM, Semmler JG. Age-related differences in short- and long-interval intracortical inhibition in a human hand muscle. Brain Stimul 7: 665–672, 2014. doi: 10.1016/j.brs.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Pitcher JB, Ogston KM, Miles TS. Age and sex differences in human motor cortex input-output characteristics. J Physiol 546: 605–613, 2003. doi: 10.1113/jphysiol.2002.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla A, Haaland KY, Bagesteiro LB, Sainburg RL. Motor asymmetry reduction in older adults. Neurosci Lett 489: 99–104, 2011. doi: 10.1016/j.neulet.2010.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raw RK, Kountouriotis GK, Mon-Williams M, Wilkie RM. Movement control in older adults: does old age mean middle of the road? J Exp Psychol Hum Percept Perform 38: 735–745, 2012. doi: 10.1037/a0026568. [DOI] [PubMed] [Google Scholar]
- Raw RK, Wilkie RM, White A, Williams JH, Mon-Williams M. The “Goldilocks Zone”: getting the measure of manual asymmetries. PLoS One 10: e0128322, 2015. doi: 10.1371/journal.pone.0128322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C, Ashby P. Inhibition in the human motor cortex is reduced just before a voluntary contraction. Neurology 53: 730–735, 1999. doi: 10.1212/WNL.53.4.730. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol 487: 541–548, 1995. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Screening questionnaire before TMS: an update. Clin Neurophysiol 122: 1686, 2011. doi: 10.1016/j.clinph.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A; Safety of TMS Consensus Group . Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120: 2008–2039, 2009. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL. Handedness: differential specializations for control of trajectory and position. Exerc Sport Sci Rev 33: 206–213, 2005. doi: 10.1097/00003677-200510000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Schaefer SY. Interlimb differences in control of movement extent. J Neurophysiol 92: 1374–1383, 2004. doi: 10.1152/jn.00181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol 530: 307–317, 2001. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34: 721–733, 2010. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve 21: 1209–1212, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- Smith AE, Sale MV, Higgins RD, Wittert GA, Pitcher JB. Male human motor cortex stimulus-response characteristics are not altered by aging. J Appl Physiol (1985) 110: 206–212, 2011. doi: 10.1152/japplphysiol.00403.2010. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Keel JC, Greenberg BD, Adams LF, Schmidt PJ, Rubinow DA, Wassermann EM. Menstrual cycle effects on cortical excitability. Neurology 53: 2069–2072, 1999. doi: 10.1212/WNL.53.9.2069. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Hallett M. Surround inhibition in human motor system. Exp Brain Res 158: 397–404, 2004. doi: 10.1007/s00221-004-1909-y. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Experimental Designs Using ANOVA. Belmont, CA: Thomson/Brooks/Cole, 2007. [Google Scholar]
- Teixeira LA. Categories of manual asymmetry and their variation with advancing age. Cortex 44: 707–716, 2008. doi: 10.1016/j.cortex.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Thirugnanasambandam N, Khera R, Wang H, Kukke SN, Hallett M. Distinct interneuronal networks influence excitability of the surround during movement initiation. J Neurophysiol 114: 1102–1108, 2015. doi: 10.1152/jn.00791.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallence AM, Schneider LA, Pitcher JB, Ridding MC. Long-interval facilitation and inhibition are differentially affected by conditioning stimulus intensity over different time courses. Neurosci Lett 570: 114–118, 2014. doi: 10.1016/j.neulet.2014.03.060. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol 85: 355–364, 1992. doi: 10.1016/0168-5597(92)90048-G. [DOI] [PubMed] [Google Scholar]
- Wang J, Przybyla A, Wuebbenhorst K, Haaland KY, Sainburg RL. Aging reduces asymmetries in interlimb transfer of visuomotor adaptation. Exp Brain Res 210: 283–290, 2011. doi: 10.1007/s00221-011-2631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS. Compensatory mechanisms in the aging motor system. Ageing Res Rev 5: 239–254, 2006. doi: 10.1016/j.arr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res 109: 158–163, 1996. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Weller MP, Latimer-Sayer DT. Increasing right hand dominance with age on a motor skill task. Psychol Med 15: 867–872, 1985. doi: 10.1017/S0033291700005109. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol 517: 591–597, 1999. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghi M, Pearce SL, Nordstrom MA. Differential modulation of intracortical inhibition in human motor cortex during selective activation of an intrinsic hand muscle. J Physiol 550: 933–946, 2003. doi: 10.1113/jphysiol.2003.042606. [DOI] [PMC free article] [PubMed] [Google Scholar]