Visual extrapolation represents a potential neural solution to afford motor interactions with the environment in the face of missing information. We investigated relative contributions by temporoparietal junction (TPJ), hMT/V5+, and intraparietal cortex (IPS), cortical areas potentially involved in these processes. Parallel organization visual extrapolation processes emerged with respect to the target’s motion causal nature: TPJ was primarily involved for visual motion congruent with gravity effects, IPS for arbitrary visual motion, whereas hMT/V5+ contributed at earlier processing stages.
Keywords: gravity, internal model, visual motion extrapolation, predictive processing, manual interception
Abstract
The ability to catch objects when transiently occluded from view suggests their motion can be extrapolated. Intraparietal cortex (IPS) plays a major role in this process along with other brain structures, depending on the task. For example, interception of objects under Earth’s gravity effects may depend on time-to-contact predictions derived from integration of visual signals processed by hMT/V5+ with a priori knowledge of gravity residing in the temporoparietal junction (TPJ). To investigate this issue further, we disrupted TPJ, hMT/V5+, and IPS activities with transcranial magnetic stimulation (TMS) while subjects intercepted computer-simulated projectile trajectories perturbed randomly with either hypo- or hypergravity effects. In experiment 1, trajectories were occluded either 750 or 1,250 ms before landing. Three subject groups underwent triple-pulse TMS (tpTMS, 3 pulses at 10 Hz) on one target area (TPJ | hMT/V5+ | IPS) and on the vertex (control site), timed at either trajectory perturbation or occlusion. In experiment 2, trajectories were entirely visible and participants received tpTMS on TPJ and hMT/V5+ with same timing as experiment 1. tpTMS of TPJ, hMT/V5+, and IPS affected differently the interceptive timing. TPJ stimulation affected preferentially responses to 1-g motion, hMT/V5+ all response types, and IPS stimulation induced opposite effects on 0-g and 2-g responses, being ineffective on 1-g responses. Only IPS stimulation was effective when applied after target disappearance, implying this area might elaborate memory representations of occluded target motion. Results are compatible with the idea that IPS, TPJ, and hMT/V5+ contribute to distinct aspects of visual motion extrapolation, perhaps through parallel processing.
NEW & NOTEWORTHY Visual extrapolation represents a potential neural solution to afford motor interactions with the environment in the face of missing information. We investigated relative contributions by temporoparietal junction (TPJ), hMT/V5+, and intraparietal cortex (IPS), cortical areas potentially involved in these processes. Parallel organization of visual extrapolation processes emerged with respect to the target’s motion causal nature: TPJ was primarily involved for visual motion congruent with gravity effects, IPS for arbitrary visual motion, whereas hMT/V5+ contributed at earlier processing stages.
motor interactions with the environment are often challenged by lacking visual information, as when trying to intercept objects that disappear momentarily. The brain may solve this problem by extrapolating the object motion, i.e., by elaborating predictions of visual events, which “fill in” missing information. This process relies on multiple sources of information. Visual signals about target position, velocity, and curvature are known to influence timing and spatial aspects of interceptive actions (Brouwer et al. 2002; Dessing et al. 2009; Dubrowski et al. 2000; Mrotek and Soechting 2007; Soechting and Flanders 2008; Soechting et al. 2009; Teixeira et al. 2006). Predictive estimates of interceptive actions can be based also on memory representations of target motion, derived from past history, cognitive cues, and internal models of the physical properties of the environment built over experience (De Lucia 2004; de Rugy et al. 2012; Lacquaniti et al. 2015; Makin et al. 2008, 2009a; Zago et al. 2008). For example, time-to-contact information for occluded targets affected by gravity can be obtained by combining visual motion with prior knowledge of gravity effects on targets’ motion (Bosco et al. 2012, 2015; de la Malla and López-Moliner 2015; Katsumata and Russell, 2012; La Scaleia et al. 2014, 2015; Vishton et al. 2010; Zago et al. 2010).
Potential neural correlates of an internal representation of gravity have been identified by neuroimaging studies in multimodal brain regions of the vestibular network (Brandt and Dieterich 1999; Indovina et al. 2005, 2013; Maffei et al. 2010; Miller et al. 2008). Following this neuroimaging evidence, transcranial magnetic stimulation (TMS) experiments have shown that the temporoparietal junction (TPJ), a core region of the vestibular network, can influence the timing of the interceptive action selectively for visual information congruent with the effects of gravity (Bosco et al. 2008). More recently, a role for the right TPJ also in the processing of relative timing has been implied by simultaneity judgment responses of patients with TPJ lesions and of healthy subjects following TMS of TPJ (Agosta et al. 2017).
Persistent activity after the target disappearance, reflecting extrapolation of the occluded motion, has been, in turn, observed in the intraparietal cortex (IPS). Electrophysiological recordings in monkeys trained to interact with constant-velocity targets have shown, in fact, that lateral intraparietal sulcus (LIP) neuronal activities recorded during periods of visual occlusion could be related to the target velocities before disappearance, perhaps reflecting internal memory representations of inferred motion (Assad and Maunsell 1995; Eskandar and Assad 1999). Further evidence obtained by neuroimaging studies in humans has pointed out that, during visual extrapolation tasks, intraparietal activations can be accompanied by analogous activation profiles in brain structures generally involved in sensorimotor and temporal estimation processing, such as visual motion areas hMT/V5+ and MST, premotor and prefrontal areas, cerebellum, and basal ganglia (Beudel et al. 2009; Lencer et al. 2004; Makin et al. 2009b; Ogawa and Inui 2007; Olson et al. 2004; O’Reilly et al. 2008; Shuwairi et al. 2007). The elevated fMRI signals after target motion disappearance found in area hMT/V5+ suggest that even cortical areas considered primarily for their sensory function might contribute to visual motion extrapolation processes (Olson et al. 2004). Compatible with this idea, it has been shown that temporal predictions underlying manual interceptions and apparent motion perception can be affected by TMS of area hMT/V5+ (Bosco et al. 2008; Dessing et al. 2013; Kaas et al. 2010; Vetter et al. 2015). Despite this evidence, however, direct involvement of area hMT/V5+ in visual motion extrapolation processes is still considered controversial (Williams et al. 2003).
In the present series of experiments, we sought to investigate further the cortical network controlling the interception of visually occluded targets, by examining the relative contributions of TPJ, hMT/V5+, and IPS, as three of the cortical areas potentially involved in the underlying predictive processes. To this end, we disrupted their neural activities with triple-pulse TMS (tpTMS, 3 pulses at 10 Hz) while subjects intercepted computer-simulated projectile trajectories. Target motion was either perturbed or not with altered gravity effects at variable intervals before landing and was occluded 500 ms thereafter. We aimed at separating the contribution of each cortical area to the visual motion extrapolation processes by applying event-related tpTMS at the time of either the target motion perturbation or disappearance. We presumed that tpTMS time-locked to the trajectory perturbation could provide insight on potential differences in the processing by each cortical area while target motion information was available, whereas with tpTMS time-locked to the target disappearance we could interfere with memory representations of the occluded target motion, thereby probing the contribution by each cortical area at this processing stage.
We hypothesized that this event-related TMS paradigm might reveal contributions by TPJ, hMT/V5+, and IPS at different stages of the visual motion extrapolation processing. These would emerge as differential effects of TMS of the three cortical sites on the interceptive responses with respect to the type of target motion and to whether the target is visible or not at the time of the magnetic stimulation. Thus we might expect that disruption of TPJ activity, being related to an internal representation of gravity, would affect mostly interceptive responses to visual motion congruent with a natural environment. Effects of TPJ stimulation timed at the target disappearance might indicate also contributions by this area to memory representations of occluded 1-g motion. Disruption of the processing of incoming visual motion signals by area hMT/V5+, instead, would affect interceptive responses irrespective of the target kinematics. Moreover, hMT/V5+ stimulation after the target disappearance would provide further insight on whether this area can play a direct role in visual extrapolation processes. Finally, given the role IPS is believed to play in maintaining internal representations of the occluded target motion, we might presume that interfering with its activity after the target disappearance would affect strongly the interceptive responses.
We found that magnetic stimulation of TPJ, hMT/V5+. and IPS did affect differentially the interceptive responses depending on the gravity acceleration imposed to the target motion after the trajectory perturbation and on the availability of visual information when cortical activity was disrupted. In particular, the specific contributions by each cortical area emerging from the effects of event-related tpTMS appeared compatible with parallel organization of visual motion extrapolation processes.
METHODS
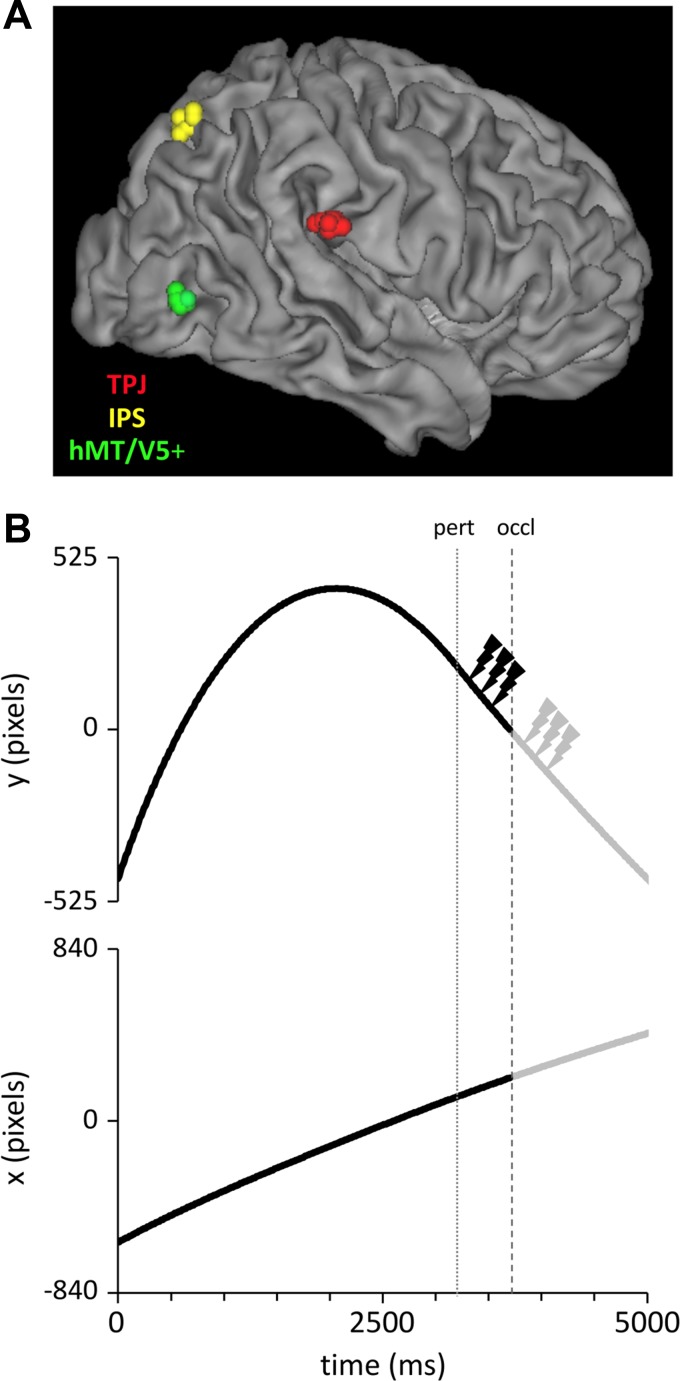
Thirty subjects [18 men and 12 women, mean age 26.37 yr ± 4.30 (SD)] participated in the experiment, receiving a monetary reward of 40 Euros. They were screened for history of neurological disorders and all met the safety criteria for enrollment to TMS studies (Rossini et al. 2015; Wassermann 1998). Subjects provided informed written consent to the experimental procedures, which were approved by the ethics committee at the University of Rome “Tor Vergata” and carried out in accordance with the Declaration of Helsinki. Participants had normal or corrected-to-normal vision and were either right-handed or ambidextrous (1 subject), as assessed by means of the abbreviated version of the Edinburgh Handedness Inventory (Oldfield 1971). Subjects sat 60 cm in front of a 22-in. LCD screen (Viewsonic, model VX2268WM, 1,680 × 1,050 pixels resolution) and kept their head fixed on a chin rest. They were instructed to maintain ocular fixation during the experimental trials on a designated point of the visual scene (the windmill blade in Fig. 1A), whereas they could move their eyes freely or blink between trials.
Fig. 1.
Visual scene and ball trajectories. A: the visual scene simulated the baseball’s flyball. The yellow trace represents one of the unperturbed 1-g parabolic trajectories used in the experiments (the ball is indicated by the white circle). Subjects intercepted the ball by displacing the running outfielder either rightward or leftward (see orange arrows) with a computer mouse and by pressing the mouse button to time the interceptive event. The semitransparent circle on the outfielder’s hand delimited the valid interception zone. In experiment 1, ball trajectories were occluded by making the ball disappear either 1,250 or 750 ms before landing (the occluded portion of the trajectory is displayed as transparent). In experiment 2, instead, trajectories were entirely visible until landing. Subjects held ocular fixation throughout the trial on the white windmill blade. B: 0-g (blue), 1-g (red), and 2-g (green) trajectories occluded 1,250 ms before landing. Crosses and open circles mark trajectory perturbation and occlusion onsets, respectively. As in A, the occluded portion of ball trajectories is displayed as transparent. Note that, in 0-g and 2-g trials, trajectories were occluded invariably 500 ms after the perturbation onset. The black dot indicates the location of the ocular fixation point. C: same as in B for ball trajectories occluded 750 ms before landing.
Visual Scene and Ball Trajectories
Visual stimuli consisted of a reduced subset of the computer-simulated baseball’s flyball trajectories presented in two earlier studies (Bosco et al. 2012; Delle Monache et al. 2015). Simulations were designed by means of the graphics software Presentation (version 14.9, Neurobehavioral Systems) and displayed at 100-Hz refresh rate (Fig. 1A). Ball motion (ball diameter: 7 pixels, 0.18° visual angle) along the parabolic path was confined to the frontoparallel plane (yellow trace in Fig. 1A), even though static pictorial elements added depth cues to the scene through perspective view and relative size. The longest trajectory, for example, reproduced a flyball spanning the scene horizontally and vertically for 26.70 and 23.86 m in real-world coordinates (corresponding to 26.85° and 24.00° of visual angle, respectively).
The ascending motion of the ball was modeled by taking into account the effects of gravity and air drag (see Bosco et al. 2012 and Brancazio 1985 for more details). In two-thirds of the trials the descending motion of the ball was perturbed by altered gravity effects either by imposing constant-velocity motion (0 g) or by doubling the value of its acceleration (2 g). In the remaining third of the trials the ball retained the natural gravitational law of motion (unperturbed 1- trials). Trajectory perturbations occurred either 1,750 or 1,250 ms before ball landing (see Fig. 1, B and C). For each perturbation interval, we designed two ball trajectories, with initial velocities (V0) of either 24.95 or 25.92°/s of visual angle, and fixed launch angle of 76.5° relative to the horizontal. Unperturbed trajectories varied in both initial direction (either 71.4° or 76.6°) and ball V0 (either 25.43°/s or 25.92°/s of visual angle), so that half of them landed close to the perturbed 0-g and the other half close to the perturbed 2-g trajectories (see Fig. 1, B and C). In experiment 1, vision of the terminal portion of the ball trajectories was prevented by making the ball disappear 500 ms after the trajectory perturbation, that is, either 1,250 or 750 ms before ball landing (see Fig. 1, B and C, and Fig. 2B). In experiment 2, instead, ball motion was visible throughout the trajectory. Each experiment was, therefore, based on a set of 12 ball trajectories, obtained by factoring 2 V0 × 2 perturbation durations × 3 ball laws of motion.
Fig. 2.
tpTMS paradigm and stimulation site locations. A: the location of individual target cortical sites stimulated on the 3 groups of participants to experiment 1 are represented on a 3D fiducial rendering of the MNI brain template. Red, yellow, and green symbols identify TPJ, IPS, and hMT/V5+ stimulation sites, respectively. B: the time course of one exemplificative perturbed 0-g trajectory is illustrated with respect to its X (bottom) and Y (top) coordinates. Vertical dotted and dashed gray lines mark the onset of the 0-g perturbation and of the visual occlusion, respectively. In experiment 1, 3 TMS pulses, indicated by thunderbolts, were delivered at 10 Hz, 100 ms after the onset of either the trajectory perturbation (black thunderbolts; tpTMS-pert) or the occlusion (filled gray thunderbolts; tpTMS-occl). In experiment 2 trajectories were entirely visible and tpTMS was delivered at corresponding time frames as tpTMS-pert (tpTMS-100) and tpTMS-occl (tpTMS-600).
Interception Task
Using a gaming mouse (Razer copperhead, Razer), participants displaced the image of an outfielder, starting from a “home” position at the center of the screen, horizontally toward the presumed landing location of the ball trajectory and pressed the mouse button at the expected time of ball arrival to indicate interception. A white semitransparent circle, centered on the outfielder's hand, delimited the valid interception area (diameter: 0.56° visual angle). Since the ocular fixation point (the windmill blade in Fig. 1A) was placed above the interception height, the image of outfielder’s hand fell always on peripheral regions of the retina.
Before the experiment, participants familiarized with the behavioral task by intercepting visual targets moving across a uniform gray background with equal average luminance to that of the baseball scene. A white cross, located at the same screen coordinates as the windmill blade of the baseball scene, indicated the ocular fixation point. A red rectangular object (0.61° visual angle), symbolizing a ball launcher, appeared at the beginning of the trial on the upper edge of the visual scene. The position of the ball launcher and its orientation relative to vertical were varied from trial to trial. The ball, identical to that of the baseball scene, was launched toward the lower edge of the screen and moved along the rectilinear path imposed by the orientation of the ball launcher. This training session consisted of 90 trials, i.e., five randomized repetitions of 18 different ball trajectories obtained by factoring three launcher positions, three motion directions relative to the vertical (values between −22.5° and 38.6°), and three ball accelerations along the ball direction of motion (0, 0.6, 1.2 m/s2).
Transcranial Magnetic Stimulation
TMS site locations and coil positioning.
Target site locations in the right hemisphere were determined a priori in normalized stereotactic space (Montreal Neurological Institute, MNI), based on previous fMRI and TMS findings. In experiment 1, participants were assigned randomly to three separate groups of 10 subjects each. Each group received TMS on one of the target sites (TPJ | hMT/V5+ | IPS) and on the vertex. Vertex stimulation was used as control condition for the somatosensory and acoustic artifacts associated with the magnetic coil discharge, which may induce intersensory facilitation of motor responses (Marinovic et al. 2014, 2015; Pascual-Leone et al. 1992; Terao et al. 1997; Tresilian and Plooy 2006).
Group 1 (3 women, 7 men, mean age 26.80 ± 3.97 yr) underwent stimulation of TPJ. To this end, we targeted the MNI coordinates (X = 67, Y = −26, Z = 23) of one of the TPJ sites that a previous TMS study found causally related to the timing of interceptive responses to visual motion congruent with gravity (Bosco et al. 2008; see Donaldson et al. 2015 for an extensive review of TPJ stimulation sites). In group 2 (6 women, 4 men, mean age 24.80 ± 4.21 yr), TMS was applied to a scalp site corresponding to hMT/V5+. The site was centered on the MNI coordinates (X = 52, Y = −71, Z = 0) of the fMRI activation peak reported by Orban et al. (2003), which was also targeted by our earlier TMS study (Bosco et al. 2008). This site location was also congruent with the hMT/V5+ location targeted by other recent TMS studies (Dessing et al. 2013; Haarmeier and Kammer 2010; Tadin et al. 2011). Group 3 (3 women, 7 men, mean age 27.50 ± 4.67 yr) underwent stimulation of IPS. The IPS site was based on topographic and functional mapping studies, showing a good degree of functional homology between the monkey LIP and the posterior/medial bank of the human IPS (Sereno et al. 2001; Sereno and Tootell 2005; Swisher et al. 2007). In particular, we targeted the MNI coordinates (X = 35, Y = −67; Z = 52) of the fMRI activation focus responding to remembered visual locations reported by Sereno et al. (2001).
Vertex location was determined directly on the subjects’ scalp by intersecting the midsagittal plane (defined by the nasion-inion line) with the midcoronal plane (delimited by the line between the intertrachial notches of the ears).
Another group of 10 subjects, recruited among cohorts of the first experiment, participated in experiment 2 (3, 4, and 3 subjects from groups 1, 2, and 3 of experiment 1, respectively). In separate blocks of this experiment, each subject received tpTMS on either TPJ or hMT/V5+. These experimental sessions were carried out, on average, 15.8 mo (± 6.1 SD) after those of experiment 1.
The TMS coil was attached to a multiple degrees of freedom mechanical arm (Magic Arm, Manfrotto, Italy) and positioned on the scalp sites corresponding to the target areas by using neuronavigation procedures based on individual subjects’ MRI anatomical brain images (Brainsight, Rogue Research). MRI anatomical brain images for each participant were acquired on a Siemens Magnetom Allegra 3-tesla head-only scanning system (Siemens Medical Systems, Erlangen, Germany), equipped with a quadrature volume RF head coil. Scan parameters were T1-weighted Magnetization Prepared Rapid Gradient Echo, MPRAGE, sequence, TR = 2.00 s, TE = 4.38 ms, flip angle = 8°, 161 mm in-plane resolution, matrix 256 × 256, 176 contiguous 1-mm thick sagittal slices, bandwidth = 130 Hz/pixel.
To locate the target stimulation sites on the brain images of individual subjects using standard MNI coordinates, the original MRI volumes were normalized to the T1–256 MNI template by applying FLIRT’s affine transformation (FSL, UK). Once the MNI coordinates of the target sites were located on the normalized brain images, we identified on the original brain volumes, with the aid of anatomical landmarks, the corresponding voxel coordinates used for neuronavigation. For the vertex site, the scalp position was determined directly on the subject’s head, based on the anatomical landmarks mentioned above. At each scalp site, fine adjustments of the coil orientation were performed to minimize muscle twitches and eye blinks elicited by TMS. Once in place, the TMS coil position was monitored at a sampling rate of 20 Hz. Displacements of the coil hot spot exceeding 5 mm halted the experiment to allow repositioning of the TMS coil.
Figure 2A shows on a 3D fiducial rendering of the MNI brain template, the location of the individual target cortical sites stimulated in the three subject groups of experiment 1 [TPJ, red symbols, mean MNI coordinates (± SD): X = 68.2 (± 1.7), Y = −25.6 (± 2.2), Z = 22.9 (± 0.9); hMT/V5+, green symbols, mean MNI coordinates (± SD): X = 53.8 (± 0.8), Y = −70.8 (± 1.1), Z = −0.5 (± 0.8); IPS, yellow symbols, mean MNI coordinates (± SD): X = 34.8 (±1.4), Y = −69.0 (±1.6), Z = 52.9 (±2.1)]. The mean MNI coordinates (± SD) of TPJ and hMT/V5+ sites stimulated in the group of participants to experiment 2 were, respectively: X = 67.1 (± 1.1), Y = −25.7 (± 0.6), Z = 22.8 (± 1.2), and X = 52.2 (± 1.1), Y = −70.4 (± 1.1), Z = 0.0 (± 1.1).
TMS paradigm.
TMS pulses were delivered through a figure-of-eight coil (Double 70-mm coil) connected to a SuperRapid2 stimulation unit (Magstim, Whitland, Wales, UK). We adopted an event-related triple-pulse TMS paradigm (tpTMS), consisting of three pulses delivered at 10 Hz (Sack et al. 2005; Striemer et al. 2011). The stimulation intensity was fixed for all subjects to 70% of the stimulator output, in line with the stimulation intensities reported by other studies adopting the same TMS paradigm (Sack et al. 2005; Striemer et al. 2011; Wang et al. 2016). In determining the stimulation intensity, we also considered that the vestibular area we targeted in the temporoparietal junction may extend deep into the Sylvian sulcus, and a similar situation may apply also to the intraparietal site.
By assuming that each TMS pulse could disrupt the cortical function for ~50–100 ms, tpTMS interfered with cortical activity for a temporal window of ~300 ms after the onset of the TMS train (Grotheer et al. 2016; Pascual-Leone et al. 2000, Siebner and Rothwell 2003; Striemer et al. 2011; Wang et al. 2016). In experiment 1, tpTMS was applied 100 ms after either the trajectory perturbation (for unperturbed 1-g trajectories at corresponding time markers) or the target disappearance. Throughout the paper, we will be referring to these two stimulation events as tpTMS-pert and tpTMS-occl, respectively (see also Fig. 2B). In experiment 2, tpTMS was applied either 100 or 600 ms after the onset of the trajectory perturbation and at corresponding time markers of unperturbed 1-g trajectories. Thus, for this experiment, the two stimulation events will be referred to as tpTMS-100 and tpTMS-600. tpTMS-100 and tpTMS-600 matched, respectively, the timing of tpTMS-pert and tpTMS-occl applied during experiment 1. In this way, by combining the results of experiment 1 (groups 1 and 2) and experiment 2, we could compare the effects of tpTMS of TPJ and hMT/V5+ on the interceptive responses while the same portion of the ball trajectory was either visible or occluded.
Participants did not report altered visual perception following TMS, even though potential effects of TMS on visual perception might have been concealed by the demanding requirements of the interceptive action. Moreover, to reduce facilitating effects of TMS auditory artifacts on motor responses (Pascual-Leone et al. 1992; Terao et al. 1997), white noise was played continuously to subjects through in-ear earphones (Bose). Before the experiment, we delivered single-test TMS pulses, and subjects adjusted manually the player volume until masking the clicking noise associated to the magnetic coil discharge, which is estimated to be ~90 dB at 70% of the output of the stimulator and at a distance of 5 cm from the coil (Dhamne et al. 2014).
Experimental Sessions
Figure 3 illustrates schematically the layout of the two experiments. Each subject performed two blocks of 216 trials. Each block consisted of a pseudorandom sequence of 6 repetitions of 36 experimental conditions, obtained by factoring 3 gravity levels, 2 trajectory perturbation intervals, 2 ball V0s, and 3 tpTMS conditions (no-tpTMS, tpTMS-pert, tpTMS-occl in experiment 1; no-tpTMS, tpTMS-100, tpTMS-600 in experiment 2). Thus unperturbed and perturbed trajectories as well as trials with and without tpTMS were randomly interleaved within each block. In each block of experiment 1, tpTMS was applied either to a target site (TPJ | hMT/V5+ | IPS, depending on the experimental group) or to the vertex. In each of the two experimental blocks of experiment 2, tpTMS was applied either to TPJ or to hMT/V5+. The order of blocks with different stimulation sites was balanced among subjects of each experimental group.
Fig. 3.
Synoptic scheme of the experimental conditions. The left panel summarizes the ball trajectory conditions, which were identical in the 2 experiments. Individual trajectories were obtained by factoring 3 gravity levels (0 g; 1 g; 2 g), 2 perturbation durations (1,250; 1,750 ms), and 2 target initial velocities (V01 = 25.5 and 26.0 m/s for perturbed and unperturbed trajectories, respectively; V02 = 26.5 m/s for all trajectories). In experiment 1 (top right panel), trajectories were occluded 500 ms after the perturbation (or at corresponding times of unperturbed trajectories) and tpTMS was applied 100 ms after either the perturbation or the occlusion event (tpTMS-pert and tpTMS-occl). In separate blocks of trials, tpTMS was applied to either 1 target cortical site (TPJ, hMT/V5+, IPS, depending on the subject group) or to the vertex. In experiment 2 (bottom right panel), ball motion was always visible and tpTMS was applied either 100 ms or 600 ms after the perturbation event (tpTMS-100 and tpTMS-600). In separate blocks of trials, subjects underwent tpTMS on either TPJ or hMT/V5+.
Data Acquisition
Interceptive responses.
Button press responses were acquired through a PC data-acquisition interface with 1-ms temporal resolution (CED Power 1401), while x-y positions of the mouse cursor were sampled at 200 Hz through the USB port (Neurobehavioral Systems). Mouse cursor horizontal positions were used for offline analyses of the interceptive responses as well as for real-time rendering of the outfielder’s displacements. In this respect, since the mouse cursor samples were taken every 5 ms and rendered at a refresh rate of 100 Hz, we could estimate maximal rendering delays of ~5 ms.
Eye position.
Subjects’ eye positions were recorded by means of an EyeLink II tracker (SR Research; sampling frequency: 500 Hz). Eye-tracker signals were calibrated every 54 trials with a nine-point calibration grid, and drift corrections were applied every 27 trials. To determine whether subjects broke ocular fixation at any moment during the trial, eye traces were processed with a custom-made algorithm written in MATLAB language (MATLAB 14, Mathworks). Cyclopean eye-position time series, computed by averaging bin-by-bin signals from both eyes, were numerically differentiated and filtered with zero-lag, second-order, low-pass Butterworth filter (cut-off frequency = 40 Hz). Saccadic movements were detected by using combined eye velocity (>30 degrees/s) and acceleration (>2,000 degrees/s2) thresholds. Slower ocular drifts from the fixation point were assessed by computing bin-by-bin Euclidean distances between cyclopean eye positions and the fixation point (Bennett and Barnes 2003; Cesqui et al. 2013, 2015). We considered fixation broken if either ocular drift exceeded 2° of visual angle from the fixation point for more than 200 ms or saccadic movements were detected and discarded the corresponding trials. Overall, 471 of 12,960 (3.63%) trials and 40 of 4,320 (0.93%) trials were discarded in experiment 1 and experiment 2, respectively.
Analysis of Interceptive Responses
Interceptive responses were analyzed by extracting from button press and mouse cursor position time series 1) the timing error (TE), i.e., the difference between the time of the button press and the time at which the ball trajectory actually intersected the nominal interception point (negative values of TE denoted early responses); and 2) the position error (PE), defined as the difference in millimeters between the horizontal position of the mouse cursor at the time of the button press and the nominal interception point (negative values corresponded to horizontal underestimations of the ball’s landing position). Signed TEs and PEs, by denoting under- and overestimates of the target trajectories, could be informative of the predictive processes underlying control of the interceptive action (see Bosco et al. 2012 for further details).
To quantify the effects of tpTMS on the interceptive behavior, we computed, for each subject, mean values of TE and PE across trials of each experimental condition. Based on previous evidence that ball V0 had marginal effects on the interception of these ballistic trajectories, we also merged data from conditions with identical laws of motion and perturbation intervals but different V0s, thereby factoring out this latter experimental variable from further statistical analyses (Bosco et al. 2012). For each experimental block, we then subtracted the mean TE and PE values of experimental conditions during which we applied tpTMS from those of corresponding conditions without tpTMS (ΔTE and ΔPE). Statistical analyses on datasets of ΔTE and ΔPE values were carried out with SPSS ver. 22.0 (IBM).
First, we determined whether the effects of tpTMS applied to the target sites on the interceptive responses differed significantly from the effects of vertex tpTMS and to which extent they depended on the ball acceleration after the perturbation, the visual occlusion duration, and the timing of the magnetic stimulation. For this purpose, ΔTE and ΔPE values drawn from each group of subjects of experiment 1 were submitted to Generalized Linear Mixed Models with gravity level (0 g | 1 g | 2 g), visual occlusion duration (750 ms | 1,250 ms), tpTMS timing (tpTMS-pert | tpTMS-occl), block type (target site tpTMS | vertex tpTMS) as “within-subjects” factors, and the presentation order of the experimental blocks as “between-subjects” factor. Generalized Linear Mixed Models included also two- and three-way interactions between block type and all other “within-subjects” factors. These interaction terms accounted for potential changes in the interceptive responses evoked by stimulation of each target cortical site (TPJ, hMT/V5+, or IPS) compared with the vertex, depending on the gravity level, the timing of tpTMS, and the duration of the visual occlusion. We examined further overall effects of vertex stimulation on the interceptive responses by applying also two-tailed, one-sample t-tests on ΔTE and ΔPE datasets pooled across the three group of participants, against the null hypothesis that differences between the interceptive responses to stimulated and nonstimulated trials were nil. Moreover, to make differences between the effects of magnetic stimulation of the target areas and the vertex more readily noticeable, results of experiment 1 will be presented graphically in Figs. 4–6 as mean differences between the ΔTE and ΔPE values evoked by stimulation of each target site and those evoked by vertex stimulation.
Fig. 4.
Effects of TPJ compared with vertex magnetic stimulation on the interceptive responses of experiment 1. A: average differences among subjects between ΔTEs resulting from TPJ and vertex tpTMS in corresponding ball trajectory conditions are plotted against gravity levels. Error bars represent standard errors of the mean (SE). The effects of tpTMS applied at the time of either the trajectory perturbation (tpTMS-pert) or the visual occlusion (tpTMS-occl) are indicated by filled and open black circles, respectively. B: same as A for tpTMS effects on mouse cursor positioning (ΔPE).
Fig. 6.
Effects of IPS compared with vertex magnetic stimulation on the interceptive responses of experiment 1. Same layout as Fig. 4.
Second, we tested the possibility that tpTMS of the three target areas produced differential effects on the interceptive responses. For this purpose, we pooled across the three groups of participants in experiment 1, ΔTE and ΔPE values separately for blocks with either target areas or vertex tpTMS. These datasets were submitted to Generalized Linear Mixed Models with TMS timing (tpTMS-pert | tpTMS-occl), gravity level (0 g | 1 g | 2 g) as “within-subjects” factors, and group (group 1 | group 2 | group 3) and order of the experimental blocks (target site tpTMS first | vertex tpTMS first) as “between-subjects” factors. Models included also two- and three-way interaction terms of group with tpTMS timing and ball law of motion, as they could account for differential effects of tpTMS among experimental groups, depending on the gravity level applied to the target motion after the perturbation and on whether tpTMS was applied at the onset of the trajectory perturbation or at the ball disappearance. Thus, for the dataset drawn from blocks with TPJ, hMT/V5+, and IPS stimulation, main and interaction effects of the factor “group” could denote differential effects of magnetic stimulation among the three cortical areas. Conversely, for the dataset drawn from blocks with vertex tpTMS, the control condition applied to all subject groups, main and interaction effects of the factor “group” could indicate significant variability among subject groups.
We did not consider visual occlusion duration among the model factors, based on preliminary analyses with regression models that either included or not visual occlusion duration and its related interaction effects. The Akaike-corrected and Bayesian information indexes, which measure the trade-off between the goodness of fit and the model complexity, indicated, in fact, that predictors associated with the visual occlusion duration did not contribute significant additional information.
ΔTE and ΔPE datasets drawn from experiment 2 were analyzed with similar Generalized Linear Mixed Models applied to the datasets drawn from each group of participants to experiment 1. Thus Generalized Linear Mixed Models included the following predictors—ball law of motion (3 levels: 0 g, 1 g, 2 g), perturbation duration (2 levels: 1,250 ms, 1,750 ms), tpTMS timing (2 levels: tpTMS-100, tpTMS-600), block type (2 levels: TPJ tpTMS, hMT/V5+ tpTMS)—as “within-subjects” factors, the presentation order of the experimental blocks as “between-subjects” factor, and the two- and three-way interactions between block type and all other “within-subjects” factors. Note that, since ball trajectories were entirely visible, the factor “occlusion duration” was replaced by “perturbation duration.”
For each Generalized Linear Mixed Model, we tested pairwise comparisons between all factor levels. Confidence intervals and significance values of pairwise tests were Bonferroni-adjusted to account for multiple comparisons. Statistical significance cut-off was set for all analyses to P = 0.05.
RESULTS
Experiment 1
The analysis of ΔTE and ΔPE datasets drawn from this experiment indicated that tpTMS of TPJ, hMT/V5+, and IPS affected mainly the timing of the interceptive responses. Response timing was affected differentially by stimulation of the three cortical areas, depending on the acceleration imposed to the target motion after the perturbation and on whether magnetic stimulation was applied before or after the target disappearance. These effects were statistically discernible from those evoked by stimulation of the vertex, which was used as control site for potential artefactual effects of the magnetic stimulation. Overall, vertex tpTMS produced rather weak effects on the interceptive responses. We observed, on average, a slight shortening of the response timing compared with trials without stimulation. This effect was more pronounced with tpTMS delivered at the trajectory perturbation, without reaching, however, statistical significance [tpTMS-pert: −12.6 ms ± 30.1 (SE), P = 0.071, 2-tailed, 1-sample t-test; tpTMS-occl: −0.6 ms ± 32.2 (SE), P = 0.937, 2-tailed, 1-sample t-test]. The effects of vertex tpTMS on mouse cursor placements were, also, more consistent with tpTMS-pert, reaching, in this case, statistical significance [tpTMS-pert: −1.3 mm ± 1.5 (SE), P < 0.001, 2-tailed, 1-sample t-test; tpTMS-occl: −0.3 mm ± 1.5 (SE), P = 0.28, 2-tailed, 1-sample t-test]. Finally, we noted that vertex stimulation effects on the interceptive responses did not differ among the three groups of participants from experiment 1 and did not depend on the gravity level imposed to the targets after the perturbation (see GLMM results in Table 4), suggesting they might simply reflect intersensory facilitation of the motor responses by TMS somatosensory artifacts (Pascual-Leone et al. 1992).
Table 4.
Results of Generalized Linear Mixed Model analyses on ΔTE and ΔPE data sets pooled across experiments, separately for blocks with target sites and vertex magnetic stimulation
| Target Sites |
Vertex |
||||
|---|---|---|---|---|---|
| Factors | dof | F value | P value | F value | P value |
| ΔTE | |||||
| Gravity level | 2, 341 | 4.419 | 0.013 | 1.176 | 0.31 |
| tpTMS timing | 1, 341 | 28.352 | 0 | 6.016 | 0.015 |
| Block order | 1, 341 | 0 | 0.997 | 0.314 | 0.576 |
| Group | 2, 341 | 0.858 | 0.425 | 0.638 | 0.529 |
| Group × gravity level | 4, 341 | 3.411 | 0.009 | 0.229 | 0.922 |
| Group × tpTMS timing | 2, 341 | 4.394 | 0.013 | 1.377 | 0.254 |
| Group × tpTMS timing × gravity level | 6, 341 | 2.552 | 0.02 | 0.572 | 0.752 |
| ΔPE | |||||
| Gravity level | 2, 341 | 1.807 | 0.166 | 2.247 | 0.107 |
| tpTMS timing | 1, 341 | 10.014 | 0.002 | 10.477 | 0.001 |
| Block order | 1, 341 | 0.093 | 0.76 | 0 | 0.99 |
| Group | 2, 341 | 1.51 | 0.222 | 0.924 | 0.398 |
| Group × gravity level | 4, 341 | 1.953 | 0.101 | 0.503 | 0.734 |
| Group × tpTMS timing | 2, 341 | 1.111 | 0.331 | 0.335 | 0.716 |
| Group × tpTMS timing × gravity level | 6, 341 | 0.6 | 0.73 | 1.326 | 0.245 |
Statistically significant effects (P < 0.05) are in bold.
Effects of TPJ magnetic stimulation.
Table 1 and Fig. 4 illustrate the effects of TPJ and vertex tpTMS on the interceptive responses of group 1 subjects. We found that the effects on the interceptive timing were significantly stronger when magnetic stimulation was timed at the trajectory perturbation (tpTMS-pert) than at the occlusion (tpTMS-occl). These differences between the effects of tpTMS-pert and tpTMS-occl depended further on the stimulation site (2-way interaction: block type × tpTMS timing). The differences between the effects of tpTMS-pert and tpTMS-occl applied to TPJ were, in fact, large and highly significant (t219 = 5.43; P < 0.001), whereas the differences observed with vertex tpTMS were much smaller, albeit significant (t219 = 2.16; P = 0.032). Moreover, whereas with tpTMS-pert interceptive responses following TPJ stimulation were significantly more anticipated than those following vertex stimulation (t219 = 2.25; P = 0.025), with tpTMS-occl we did not observe significant differences between the two stimulation sites.
Table 1.
Results of Generalized Linear Mixed Model analyses applied to ΔTE and ΔPE data sets drawn from group 1 participants to experiment 1 (target site: TPJ)
| ΔTE |
ΔPE |
||||
|---|---|---|---|---|---|
| Factors | dof | F value | P value | F value | P value |
| Gravity level | 2, 219 | 0.975 | 0.379 | 1.996 | 0.138 |
| Occlusion duration | 1, 219 | 0.002 | 0.965 | 0.227 | 0.634 |
| tpTMS timing | 1, 219 | 25.831 | <0.001 | 16.741 | <0.001 |
| Block type | 1, 219 | 1.570 | 0.212 | 0.056 | 0.814 |
| Block order | 1, 219 | 0.499 | 0.481 | 0.430 | 0.513 |
| Block type × gravity level | 2, 219 | 1.285 | 0.279 | 0.438 | 0.646 |
| Block type × occlusion duration | 1, 219 | 1.803 | 0.181 | 0.043 | 0.836 |
| Block type × tpTMS timing | 1, 219 | 9.464 | 0.002 | 0.341 | 0.560 |
| Block type × tpTMS timing × gravity level | 4, 219 | 4.206 | 0.003 | 1.946 | 0.104 |
| Block type × gravity level × occlusion duration | 4, 219 | 6.807 | <0.001 | 0.393 | 0.813 |
| Block type × tpTMS timing × occlusion duration | 2, 219 | 8.047 | <0.001 | 0.008 | 0.992 |
Statistically significant effects (P < 0.05) are in bold. dof, Degrees of freedom.
Remarkably, the differential effects between TPJ and vertex tpTMS-pert depended significantly on the level of gravity applied to the target motion after the trajectory perturbation (3-way interaction: block type × tpTMS timing × gravity level). Figure 4A illustrates clearly this finding, by plotting the mean differences between ΔTEs evoked by TPJ and vertex stimulation against the gravity level. The largest differences in response timing were evident with 1-g motion and consisted in significantly greater anticipation of the interceptive responses following TPJ compared with vertex stimulation (t219 = 5.78; P < 0.001). Pairwise tests detected also significantly stronger effects of TPJ tpTMS-pert on the interceptive responses to 1-g compared with 2- trajectories (t219 = 3.03; P = 0.008), whereas differences between responses to 1-g and 0-g trials were borderline significant (t219 = 2.09; P = 0.076).
Differential effects of tpTMS among gravity levels and stimulation sites were related also to the visual occlusion duration (3-way interaction: gravity level × block type × occlusion duration). In fact, TPJ and vertex stimulation effects were significantly different only for interceptive responses to 1-g trials occluded for 1,250 ms (t219 = 2.82; P = 0.005), and the differences between the effects of TPJ tpTMS on 1-g and 0-g responses reached statistical significance only for trials occluded for 1,250 ms (t219 = 2.81; P = 0.016). Finally, additional variance in the distribution of ΔTEs was predicted by the three-way interaction, block type × tpTMS timing × occlusion duration.
The effects of TPJ magnetic stimulation on the mouse cursor positioning were rather small and less consistent than those on the interceptive timing (see Fig. 4B). Magnetic stimulation effects on PEs depended on whether stimulation was timed at the trajectory perturbation or at the occlusion (main effect of tpTMS timing). However, the effects of TPJ and vertex stimulation were not significantly different, suggesting that changes in mouse cursor placements following TMS could not be entirely attributable to TPJ cortical disruption.
Effects of magnetic stimulation of visual area hMT/V5+.
Table 2 summarizes the results obtained with magnetic stimulation of area hMT/V5+ and vertex in group 2 subjects. The effects of tpTMS on the interceptive timing depended strongly on the stimulation site (main effect of block type) and on whether it was timed at the trajectory perturbation or at the visual occlusion (main effect tpTMS timing). In particular, tpTMS-pert induced significantly earlier interceptive responses when applied to area hMT/V5+ compared with the vertex (t219 = 2.71; P = 0.007), whereas with tpTMS-occl no significant differences were evident between stimulation sites. Therefore, the effects of tpTMS-pert were significantly stronger than those induced by tpTMS-occl only following hMT/V5+ stimulation (t219 = 3.27; P = 0.001). Unlike what was observed in group 1 subjects with TPJ stimulation, the distribution of ΔTEs was not influenced significantly by the ball acceleration (see Table 2). In fact, hMT/V5+, compared with vertex stimulation, shortened the timing of the interceptive responses similarly across types of ball trajectory. This result is evident in Fig. 5A where the differences between the ΔTEs evoked by hMT/V5+ and vertex stimulation are plotted against the level of gravity imposed to the ball after the perturbation.
Table 2.
Results of Generalized Linear Mixed Model Analyses applied to ΔTE and ΔPE data sets drawn from group 2 participants to experiment 1 (target site: hMT/V5+)
| ΔTE |
ΔPE |
||||
|---|---|---|---|---|---|
| Factors | dof | F value | P value | F value | P value |
| Gravity level | 2, 219 | 0.428 | 0.652 | 2.183 | 0.115 |
| Occlusion duration | 1, 219 | 0.027 | 0.869 | 0.553 | 0.458 |
| tpTMS timing | 1, 219 | 9.055 | 0.003 | 4.367 | 0.038 |
| Block type | 1, 219 | 4.740 | 0.031 | 0.561 | 0.455 |
| Block order | 1, 219 | 0.085 | 0.771 | 3.895 | 0.050 |
| Block type × gravity level | 2, 219 | 0.088 | 0.916 | 1.435 | 0.240 |
| Block type × occlusion duration | 1, 219 | 1.881 | 0.172 | 0.009 | 0.924 |
| Block type × tpTMS timing | 1, 219 | 5.137 | 0.024 | 0.048 | 0.826 |
| Block type × tpTMS timing × gravity level | 4, 219 | 0.409 | 0.802 | 1.447 | 0.220 |
| Block type × gravity level × occlusion duration | 4, 219 | 1.758 | 0.138 | 2.322 | 0.058 |
| Block type × tpTMS timing × occlusion duration | 2, 219 | 0.453 | 0.636 | 0.955 | 0.386 |
Statistically significant effects (P < 0.05) are in bold.
Fig. 5.
Effects of hMT/V5+ compared with vertex magnetic stimulation on interceptive responses of experiment 1. Same layout as Fig. 4.
Changes in mouse cursor positioning evoked by tpTMS were rather small and inconsistent also for group 2 subjects (see Fig. 5B). We did find statistically significant differences between the effects of tpTMS-pert and tpTMS-occl (main effect of tpTMS timing). However, these effects could not be attributed reliably to disruption of hMT/V5+ activity because they did not depend significantly on the experimental block with either hMT/V5+ or vertex stimulation.
Effects of IPS magnetic stimulation.
Table 3 summarizes the results of the Generalized Linear Mixed Models analyses applied to the interceptive responses of group 3 subjects. A large fraction of the variance in the ΔTE distribution was explained by the gravity level and by the two-way interaction between gravity level and block type involving either IPS or vertex stimulation. Figure 6A shows, indeed, that IPS stimulation, compared with that of the vertex, delayed responses to 0-g trials, shortened responses to 2-g trials, while leaving unaltered responses to 1-g motion. Accordingly, pairwise tests indicated that IPS stimulation effects were significantly different between responses to 0-g and either 2-g or 1-g motion (t219 = 11.06; P < 0.001; t219 = 2.4; P = 0.034), whereas those of vertex stimulation were not significantly different among gravity levels (P > 0.1).
Table 3.
Results of Generalized Linear Mixed Model analyses applied to ΔTE and ΔPE data sets drawn from group 3 participants to experiment 1 (target site: IPS)
| ΔTE |
ΔPE |
||||
|---|---|---|---|---|---|
| Factors | dof | F value | P value | F value | P value |
| Gravity level | 2, 219 | 5.499 | 0.005 | 4.860 | 0.009 |
| Occlusion duration | 1, 219 | 1.696 | 0.194 | 0.846 | 0.359 |
| tpTMS timing | 1, 219 | 0.369 | 0.544 | 5.754 | 0.017 |
| Block type | 1, 219 | 0.051 | 0.822 | 2.766 | 0.098 |
| Block order | 1, 219 | 1.618 | 0.205 | 6.021 | 0.015 |
| Block type × gravity level | 2, 219 | 3.522 | 0.031 | 1.776 | 0.172 |
| Block type × occlusion duration | 1, 219 | 4.340 | 0.038 | 0.102 | 0.749 |
| Block type × tpTMS timing | 1, 219 | 0.239 | 0.626 | 0.162 | 0.688 |
| Block type × tpTMS timing × gravity level | 4, 219 | 0.568 | 0.686 | 0.627 | 0.644 |
| Block type × gravity level × occlusion duration | 4, 219 | 3.507 | 0.008 | 2.912 | 0.022 |
| Block type × tpTMS timing × occlusion duration | 2, 219 | 1.297 | 0.275 | 0.028 | 0.972 |
Statistically significant effects (P < 0.05) are in bold.
The different effects of IPS magnetic stimulation observed among the different gravity levels depended also on the visual occlusion duration (3-way interaction: block type × gravity level × occlusion duration). In fact, differences between the effects of IPS and vertex stimulation were significantly larger for interceptive responses to 0-g motion occluded for 1,250 ms compared with motion occluded for 750 ms (t219 = 2.04; P = 0.043). Finally, we found a significant two-way interaction of block type × occlusion duration, explained by different vertex stimulation effects on responses to motion occluded for 750 and 1,250 ms (t219 = 2.78; P = 0.006).
Unlike TPJ and area hMT/V5+, magnetic stimulation of IPS induced small but consistent changes of the mouse cursor positioning. These changes showed a similar dependency on the ball acceleration after the perturbation as that observed for the interceptive timing (see Fig. 6B). Accordingly, we found statistically significant effects of gravity level and of the three-way interaction gravity level × block type × occlusion duration. Pairwise tests indicated that IPS stimulation affected differently responses to 0-g and 2-g motion occluded for 1,250 ms (t219 = 2.86; P = 0.014), whereas vertex stimulation failed to induce such changes. Moreover, ΔPEs depended on whether tpTMS was timed at the trajectory perturbation or at the target disappearance (main effect of tpTMS timing), as well as on the order of experimental blocks.
Differential contributions of TPJ, hMT/V5+ and IPS on the interceptive responses to occluded targets.
The results of the analyses of ΔTE and ΔPE data sets drawn separately from the three groups of participants to experiment 1 suggested that tpTMS of each target cortical area induced distinctive effects on the interceptive responses. To examine this issue further, we pooled ΔTE and ΔPE datasets across subject groups, separately for blocks with either target areas’ or vertex tpTMS. Generalized Linear Mixed Models, in this case, tested for different tpTMS effects among subject groups, depending on whether magnetic stimulation was applied to either the target cortical areas or the vertex. With vertex stimulation used as control condition in all subject groups, testing for differences among subject groups following vertex compared with target areas tpTMS could, therefore, disambiguate genuine differential effects of tpTMS among TPJ, hMT/V5+, and IPS sites from mere effects of intergroup variability.
Results compatible with bona fide differential effects of TPJ, hMT/V5+, and IPS on the interceptive responses did emerge from analysis of the ΔTEs. A significant fraction of the variance of ΔTEs pooled from experimental blocks with tpTMS of the target cortical areas was explained by interaction effects of the factor “group” with “tpTMS timing” and “gravity level” (see Table 4, top). That is, tpTMS of TPJ, hMT/V5+, and IPS produced different effects, depending on the ball acceleration after the perturbation and on whether the stimulation was applied before or after the target disappearance. On the contrary, we did not find statistically significant effects of the factor “group” following vertex stimulation, making it unlikely that differences observed with tpTMS of the target cortical areas were merely due to intergroup variability.
Finally, GLMM analysis applied to the ΔPEs failed to show significantly different tpTMS effects among experimental groups for both datasets drawn from blocks with either stimulation of the target cortical areas or of the vertex.
Experiment 2
Experiment 1 indicated that magnetic stimulation of TPJ and hMT/V5+ affected significantly the interceptive timing while the ball motion was visible (tpTMS-pert), but not after its disappearance (tpTMS-occl). In this experiment, however, the different timing of tpTMS-pert and tpTMS-occl relative to the ball landing (see Fig. 2A) represented a potential confound with respect to the presumed influence of the different availability of visual motion information on the efficacy of tpTMS to alter the interceptive timing. We sought to address this confound with experiment 2 by using entirely visible trajectories and by applying tpTMS on TPJ and hMT/V5+ at time intervals, relative to ball landing, identical to experiment 1 (see methods and Fig. 3). Thus, by comparing results between the two experiments, we could determine whether the effects of TPJ and hMT/V5+ magnetic stimulation were genuinely related to the availability of visual motion information or were also influenced by the stimulation timing. With experiment 2, we could get also direct evidence about differential effects of tpTMS among target areas, since TPJ and hMT/V5+ sites were stimulated in the same group of subjects, rather than in separate groups as in experiment 1.
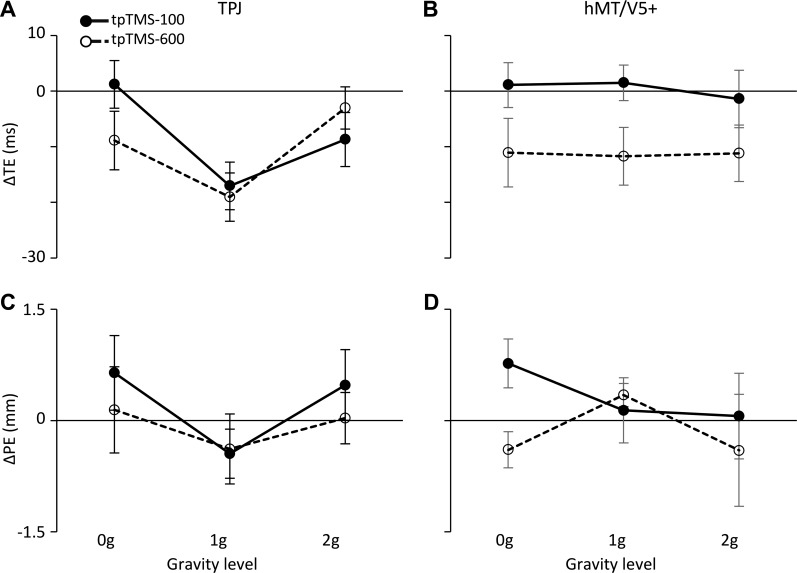
Table 5 summarizes the results of this experiment. The effects of tpTMS on the interceptive timing were accounted for mainly by the factor gravity level and by the two-way interaction between gravity level and the experimental block with either TPJ or hMT/V5+ stimulation. TPJ stimulation produced significantly larger anticipation of the interceptive responses to 1-g motion compared with 0-g and 2-g motion (t219 = 4.49; P < 0.001, and t219 = 4.62; P < 0.001, respectively), whereas tpTMS of hMT/V5+ induced uniform anticipation of the interceptive timing across ball accelerations. Moreover, the effects of TPJ stimulation on responses to 1-g trials were significantly stronger than those evoked by hMT/V5+ stimulation (t219 = 2.16; P = 0.032). Remarkably, these distributions of mean ΔTEs following TPJ and hMT/V5+ stimulation, illustrated in Fig. 7, A and B, resembled those observed in experiment 1 with group 1 and group 2 subjects, respectively. Additional variance in the distribution of ΔTEs was explained by the two-way interaction tpTMS timing × block type. This interaction effect could be appreciated by comparing Fig. 7A with Fig. 7B. In fact, whereas with TPJ stimulation (Fig. 7A), tpTMS-100 and tpTMS-600 affected equally the interceptive responses, with hMT/V5+ stimulation (Fig. 7B), tpTMS-100 induced significantly stronger effects than tpTMS-600 (t219 = 2.61; P = 0.01). Moreover, pairwise tests indicated that the effects induced by tpTMS-100 of TPJ and hMT/V5+ were significantly different (t219 = 2.28; P = 0.024).
Table 5.
Results of Generalized Linear Mixed Model analyses on ΔTE and ΔPE data sets drawn from experiment 2 (target sites: TPJ, hMT/V5+)
| ΔTE |
ΔPE |
||||
|---|---|---|---|---|---|
| Factors | dof | F value | P value | F value | P value |
| Gravity level | 2, 219 | 5.016 | 0.007 | 1.473 | 0.232 |
| Perturbation duration | 1, 219 | 1.619 | 0.205 | 1.013 | 0.315 |
| tpTMS timing | 1, 219 | 3.727 | 0.055 | 6.817 | 0.010 |
| Block type | 1, 219 | 0.861 | 0.354 | 0.008 | 0.927 |
| Block order | 1, 219 | 2.792 | 0.096 | 4.494 | 0.035 |
| Block type × gravity level | 2, 219 | 5.421 | 0.005 | 1.278 | 0.281 |
| Block type × perturbation duration | 1, 219 | 1.336 | 0.249 | 1.018 | 0.314 |
| Block type × tpTMS timing | 1, 219 | 5.967 | 0.015 | 0.225 | 0.636 |
| Block type × tpTMS timing × gravity level | 4, 219 | 1.001 | 0.408 | 2.467 | 0.046 |
| Block type × gravity level × perturbation duration | 4, 219 | 1.051 | 0.382 | 1.336 | 0.258 |
| Block type × tpTMS timing × perturbation duration | 2, 219 | 0.433 | 0.649 | 2.608 | 0.076 |
Statistically significant effects (P < 0.05) are in bold.
Fig. 7.
Effects of tpTMS of TPJ (left column graphs) and hMT/V5+ (right column graphs) on the interceptive responses of experiment 2. TE and PE differences between conditions with and without tpTMS (ΔTE and ΔPE) are averaged among subjects (±SE) and plotted as a function of the gravity level in A and B, and in C and D, respectively. Black filled circles joined by solid lines refer to tpTMS-100 conditions, whereas open circles joined by dotted lines indicate tpTMS-600 conditions.
The effects of magnetic stimulation on mouse cursor positioning were rather small. Nevertheless, they could be related significantly to the tpTMS timing, the block order, and the three-way interaction tpTMS timing × gravity level × block type (see Fig. 7, C and D). This interaction was mostly explained by different effects induced by tpTMS-100 of TPJ and hMT/V5+ on the interceptive responses to 1-g motion (t219 = 2.16; P = 0.032). TPJ tpTMS-100 produced significantly different effects on responses to 1-g motion compared with either 0-g (t219 = 2.95; P = 0.011) or 2-g responses (t219 = 2.35; P = 0.039). Moreover, interceptive responses to 0-g motion were affected differently by tpTMS-100 and tpTMS-600 applied to hMT/V5+ (t219 = 4.36; P < 0.001).
DISCUSSION
In this study, we investigated the relative contributions by TPJ, hMT/V5+, and IPS to interception of occluded ballistic trajectories, by disrupting their neural activities with tpTMS timed to either perturbations of the trajectory kinematics or visual occlusions. tpTMS of the three cortical areas affected mostly the interceptive timing by inducing, generally, earlier responses. tpTMS effects varied among cortical areas with respect to the acceleration imposed to the ball after the trajectory perturbations and to the availability of visual information at the time of stimulation, implying they could relate to different aspects of predictive processes underlying occluded targets interception.
TPJ and A Priori Information about Gravity
TPJ stimulation affected preferentially the responses to the unperturbed 1-g trajectories, which simulated natural conditions on Earth. This result, therefore, extended to projectile motion evidence that TPJ activity could influence the interceptive timing to vertical motion compatible with gravity effects (Bosco et al. 2008; Indovina et al. 2005, 2013, 2015; Maffei et al. 2010; Miller et al. 2008). A novel perspective on TPJ contribution to interception of 1-g motion emerged from a comparison between the effects of tpTMS in the two experiments, indicating that effects of TPJ disruption depended on availability of visual motion information. During experiment 1, tpTMS affected preferentially the interceptive responses to unperturbed 1-g motion when applied with the targets visible (tpTMS-pert) but not after their disappearance (tpTMS-occl). Conversely, during experiment 2, targets were visible throughout the trajectories and preferential effects on responses to 1-g trajectories were observed following both tpTMS-100 and tpTMS-600. The ineffectiveness of TPJ stimulation applied after the target disappearance might suggest that TPJ is not involved directly in maintaining memory representations of the occluded target motion. Instead, predictive information about occluded target motion could be maintained in memory by other regions of the multisensory vestibular network that are engaged preferentially by interception of visual motion congruent with gravity (Indovina et al. 2005; Lacquaniti et al. 2013; Miller et al. 2008). Vestibular areas related more closely to the motor output, such as SMA and premotor cortex, could represent plausible candidates. Indeed, SMA shows increased BOLD signal during motor timing tasks requiring visual motion extrapolation (O’Reilly et al. 2008). Moreover, ramp-to-threshold activities, related to elapsed time, as well as predictive of the interceptive timing, can be recorded from monkey medial premotor and motor cortexes (Merchant and Averbeck 2017; Merchant and Bartolo 2017; Merchant and Georgopoulos 2006; Merchant et al. 2004a, 2004b, 2009, 2011). Finally, the idea that different areas of the vestibular network may contribute to distinct processes related to the internal representation of gravity is also in line with recent evidence that lesions of parietal operculum and posterior insula are associated with abnormal interceptive timing and visual vertical estimates, respectively (Maffei et al. 2016)
Contributions by hMT/V5+ to the Predictive Processing of Interceptive Timing
Magnetic stimulation of area hMT/V5+ shortened the timing of the interceptive responses to all types of target motion, congruent with earlier results suggesting that hMT/V5+ might contribute to temporal predictions for the interceptive action regardless of the causality of visual motion (Bosco et al. 2008). Interestingly, a similar role in the processing of timing information has been implied by TMS and neuroimaging studies indicating that hMT/V5+ may encode temporal intervals and contribute to a dedicated pathway for time perception (Battelli et al. 2007; Bueti et al. 2010; Salvioni et al. 2013). Our experiments showed further that the effects of hMT/V5+ disruption on the interceptive timing depended critically on whether visual information was available at the time of the magnetic stimulation. In fact, whereas tpTMS applied during experiment 1 at the target disappearance (tpTMS-occl) did not alter consistently the interceptive timing, stimulation applied during experiment 2 at corresponding time frames with the target visible (tpTMS-600) induced significantly earlier responses across trajectory types. Remarkably, also tpTMS-pert applied before the target occlusion during experiment 1, induced earlier interceptive responses to all trajectory types. However, we also observed an incongruence between the result of the two experiments in that tpTMS-100, applied during experiment 2 with identical timing as tpTMS-pert in experiment 1, did not alter significantly the interceptive responses. We might relate this apparent incongruence to the different availability of visual information about the terminal segment of ball trajectories in the two experiments, since targets disappeared 200 ms after tpTMS-pert in experiment 1, whereas they remained visible until landing in experiment 2 (Fig. 2B). We could presume, in fact, that in experiment 2 the transient disruptive effect of tpTMS-100 on hMT/V5+ activity was overridden by the visual motion signals available thereafter for at least 950 ms before ball landing. Conversely, transient hMT/V5+ disruption just before the ball disappearance in experiment 1 might have altered the processing of short-term visual memory signals by cortical areas downstream hMT/V5+, thereby affecting indirectly the interceptive responses (Huk and Shadlen 2005).
The lack of significant effects of hMT/V5+ stimulation applied after the target visual occlusion appeared also compatible with electrophysiological results, which failed to reveal consistent relationships between hMT/V5+ neuronal activities and occluded target motion (Williams et al. 2003). Overall, we suggest that hMT/V5+ may not be involved directly in maintaining memory representations of inferred target motion, albeit it may contribute indirectly by integrating visual information with top-down modulatory signals from parietal areas (Kaas et al. 2010).
IPS Contributions to Memory Representations of Inferred Motion
We found that IPS stimulation affected the interceptive responses, whether applied before or after ball disappearance. This result could be expected based on previous neurophysiological and neuroimaging evidence, indicating that the intraparietal cortex can play a crucial role in maintaining internal representations of inferred target motion (Assad and Maunsell 1995; Beudel et al. 2009; Eskandar and Assad 1999; Ogawa and Inui 2007; Olson et al. 2004; O’ Reilly et al. 2008; Williams et al. 2003). Disruption of IPS activity delayed responses to 0-g motion, anticipated responses to 2-g motion, and had only marginal effects on 1-g trials. In principle, this pattern may reflect altered memory representations of the target motion based on the velocity just before its disappearance (Assad and Maunsell 1995; Eskandar and Assad 1999). However, the changes in interceptive timing induced by IPS magnetic stimulation were only weakly related to the ball velocities before disappearance (on average, R = 0.047). This result suggests that IPS target motion representations may not be based strictly on the target speed before the occlusion, but also on higher-order time derivatives not tested by the aforementioned studies. Moreover, IPS activity is known to reflect attentional processes, as well as motor intentions. Along these lines, we might interpret the anticipated responses to 2-g motion as denoting increased urgency to intercept balls falling at higher acceleration than usual. By the same token, delayed responses to 0-g trajectories could reflect decreased urgency at intercepting even more unusual nonaccelerating targets. From a neurophysiological standpoint, changes in urgency could be related to disrupted ramp-to-threshold activities of intraparietal neurons, which have been shown to be associated to motor timing and perceptual estimates (Janssen and Shadlen 2005; Jazayeri and Shadlen 2015; Leon and Shadlen 2003; Maimon and Assad 2006). Finally, dependence of IPS stimulation effects on the likelihood of time-varying properties of the visual stimuli may seem compatible with experimental evidence that internal timing mechanisms adapt to the temporal statistics of the environment (Jazayeri and Shadlen 2010).
TPJ, hMT/V5+, and IPS Contribution to Spatial Aspects of Interception
tpTMS effects on mouse positioning were less consistent compared with the interceptive timing, perhaps because of the motor strategy generally adopted by subjects of displacing the mouse cursor close to the presumed interception location before the ball disappearance, performing only small movements afterwards (Bosco et al. 2012). With the occluded trajectories presented during experiment 1, only magnetic stimulation of the IPS site affected consistently the mouse positioning. The effects resembled those observed with the interceptive timing, suggesting a common basis. Thus increased urgency to intercept 2-g motion, besides inducing earlier response timing, might have shortened also mouse displacements toward the presumed interception location. In a similar vein, decreased urgency to intercept 0-g motion, by postponing the interceptive responses, might have determined wider mouse displacements.
With the fully visible trajectories of experiment 2, also magnetic stimulation of the TPJ site affected mouse positioning with a seemingly consistent pattern, which resembled that observed with the interceptive timing. This correspondence between the effects on response timing and on mouse placements might suggest contributions by TPJ also to the spatial control of interception. However, in the light of the inconsistent effects of TPJ magnetic stimulation on mouse placements observed in experiment 1, this interpretation must be taken cautiously. Finally, the finding that TMS of area hMT/V5+ did not alter systematically the positioning of the mouse cursor appeared in line with the lack of significant effects on horizontal interception errors reported by a previous TMS study (Dessing et al. 2013).
Conclusions
Altogether, the findings reported here may suggest parallel processing within the network of brain areas potentially involved in the interception of visually occluded projectile motion. Figure 8 illustrates schematically the parallel organization scheme that seems to emerge from our results. According to this hypothetical framework, area hMT/V5+ would represent an early processing stage upstream of IPS and the vestibular network, responsible for elaborating visual motion signals and feeding downstream areas also predictive timing information, irrespective of the causal nature of the target motion (Battelli et al. 2007; Bosco et al. 2008; Bueti et al. 2010; Salvioni et al. 2013). Parallel processing with respect to the natural attributes of the target motion would rather take place between the multimodal areas of the vestibular network and IPS. In this regard, our current results supported the idea that predictions of motion congruent with gravity effects engage specifically the vestibular network (Bosco et al. 2015; Lacquaniti et al. 2013; Zago et al. 2008, 2009). In particular, the effects of TPJ magnetic stimulation applied either before or after the target disappearance indicated that this area contributes with interceptive timing information based on a priori knowledge of gravity while visual information is available, without maintaining memory representations of temporarily occluded motion. Furthermore, the lack of effects of IPS magnetic stimulation on interceptive responses to 1-g motion implied marginal contribution by this cortical region to the processing of memory representations of occluded 1-g motion. We hypothesize that representation of occluded 1-g motion may occur in other vestibular network areas, which drive the build-up activity of cortical motor areas related to motor timing (Merchant and Averbeck 2017; Merchant and Bartolo 2017; Merchant and Georgopoulos 2006; Merchant et al. 2004a, 2004b, 2009, 2011). A parallel pathway through IPS, instead, would be critical for the extrapolation of arbitrary visual motion, like the 0-g and 2-g stimuli employed by our experiments. Our findings support strongly the idea that IPS activity maintains internal representations of the inferred motion, suggesting further that these representations may not be based strictly on target velocity before the occlusion, by including also acceleration information as well as urgency cues (Assad and Maunsell 1995; O’ Reilly et al. 2008).
Fig. 8.
Schematic illustration of the hypothetical parallel organization within the cortical network underlying visual extrapolation of occluded motion. Gray thunderbolts indicate the cortical areas targeted by TMS in the present study. Arrows linking elements of the cortical network indicate the hypothetical flow of information, without representing necessarily direct anatomical connections.
GRANTS
This work was supported by the Italian University Ministry (PRIN Grant 2015HFWRYY_002), the Italian Space Agency (COREA Grant 2013-084-R.0), the Horizon 2020 Robotics Program (ICT-23-2014 under Grant Agreement 644727-CogIMon), and the University of Tor Vergata (Consolidating the Foundation Grant 2015). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.D.M., F.L., and G.B. conceived and designed research; S.D.M. and G.B. performed experiments; S.D.M. analyzed data; S.D.M., F.L., and G.B. interpreted results of experiments; S.D.M. and G.B. prepared figures; S.D.M. and F.L. edited and revised manuscript; S.D.M., F.L., and G.B. approved final version of manuscript; G.B. drafted manuscript.
REFERENCES
- Agosta S, Magnago D, Tyler S, Grossman E, Galante E, Ferraro F, Mazzini N, Miceli G, Battelli L. The pivotal role of the right parietal lobe in temporal attention. J Cogn Neurosci 29: 805–815, 2017. doi: 10.1162/jocn_a_01086. [DOI] [PubMed] [Google Scholar]
- Assad JA, Maunsell JH. Neuronal correlates of inferred motion in primate posterior parietal cortex. Nature 373: 518–521, 1995. doi: 10.1038/373518a0. [DOI] [PubMed] [Google Scholar]
- Battelli L, Pascual-Leone A, Cavanagh P. The “when” pathway of the right parietal lobe. Trends Cogn Sci 11: 204–210, 2007. doi: 10.1016/j.tics.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett SJ, Barnes GR. Human ocular pursuit during the transient disappearance of a visual target. J Neurophysiol 90: 2504–2520, 2003. doi: 10.1152/jn.01145.2002. [DOI] [PubMed] [Google Scholar]
- Beudel M, Renken R, Leenders KL, de Jong BM. Cerebral representations of space and time. Neuroimage 44: 1032–1040, 2009. doi: 10.1016/j.neuroimage.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Bosco G, Carrozzo M, Lacquaniti F. Contributions of the human temporoparietal junction and MT/V5+ to the timing of interception revealed by transcranial magnetic stimulation. J Neurosci 28: 12071–12084, 2008. doi: 10.1523/JNEUROSCI.2869-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco G, Delle Monache S, Lacquaniti F. Catching what we can’t see: manual interception of occluded fly-ball trajectories. PLoS One 7: e49381, 2012. doi: 10.1371/journal.pone.0049381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco G, Delle Monache S, Gravano S, Indovina I, La Scaleia B, Maffei V, Zago M, Lacquaniti F. Filling gaps in visual motion for target capture. Front Integr Nuerosci 9: 13, 2015. doi: 10.3389/fnint.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancazio PJ. Looking into Chapman’s homer: the physics of judging a fly ball. Am J Phys 53: 849–855, 1985. doi: 10.1119/1.14350. [DOI] [Google Scholar]
- Brandt T, Dieterich M. The vestibular cortex. Its locations, functions, and disorders. Ann N Y Acad Sci 871: 293–312, 1999. doi: 10.1111/j.1749-6632.1999.tb09193.x. [DOI] [PubMed] [Google Scholar]
- Brouwer AM, Brenner E, Smeets JBJ. Hitting moving objects: is target speed used in guiding the hand? Exp Brain Res 143: 198–211, 2002. doi: 10.1007/s00221-001-0980-x. [DOI] [PubMed] [Google Scholar]
- Bueti D, Bahrami B, Walsh V, Rees G. Encoding of temporal probabilities in the human brain. J Neurosci 30: 4343–4352, 2010. doi: 10.1523/JNEUROSCI.2254-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesqui B, de Langenberg R, Lacquaniti F, d’Avella A. A novel method for measuring gaze orientation in space in unrestrained head conditions. J Vis 13: 28, 2013. doi: 10.1167/13.8.28. [DOI] [PubMed] [Google Scholar]
- Cesqui B, Mezzetti M, Lacquaniti F, d’Avella A. Gaze behavior in one-handed catching and its relation with interceptive performance: what the eyes can’t tell. PLoS One 10: e0119445, 2015. doi: 10.1371/journal.pone.0119445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Malla C, López-Moliner J. Predictive plus online visual information optimizes temporal precision in interception. J Exp Psychol Hum Percept Perform 41: 1271–1280, 2015. doi: 10.1037/xhp0000075. [DOI] [PubMed] [Google Scholar]
- de Rugy A, Marinovic W, Wallis G. Neural prediction of complex accelerations for object interception. J Neurophysiol 107: 766–771, 2012. doi: 10.1152/jn.00854.2011. [DOI] [PubMed] [Google Scholar]
- Delle Monache S, Lacquaniti F, Bosco G. Eye movements and manual interception of ballistic trajectories: effects of law of motion perturbations and occlusions. Exp Brain Res 233: 359–374, 2015. doi: 10.1007/s00221-014-4120-9. [DOI] [PubMed] [Google Scholar]
- DeLucia PR. Time-to-contact judgments of an approaching object that is partially concealed by an occluder. J Exp Psychol Hum Percept Perform 30: 287–304, 2004. doi: 10.1037/0096-1523.30.2.287. [DOI] [PubMed] [Google Scholar]
- Dessing JC, Oostwoud Wijdenes L, Peper CLE, Beek PJ. Adaptations of lateral hand movements to early and late visual occlusion in catching. Exp Brain Res 192: 669–682, 2009. doi: 10.1007/s00221-008-1588-1. [DOI] [PubMed] [Google Scholar]
- Dessing JC, Vesia M, Crawford JD. The role of areas MT+/V5 and SPOC in spatial and temporal control of manual interception: an rTMS study. Front Behav Neurosci 7: 15, 2013. doi: 10.3389/fnbeh.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamne SC, Kothare RS, Yu C, Hsieh TH, Anastasio EM, Oberman L, Pascual-Leone A, Rotenberg A. A measure of acoustic noise generated from transcranial magnetic stimulation coils. Brain Stimul 7: 432–434, 2014. doi: 10.1016/j.brs.2014.01.056. [DOI] [PubMed] [Google Scholar]
- Donaldson PH, Rinehart NJ, Enticott PG. Noninvasive stimulation of the temporoparietal junction: A systematic review. Neurosci Biobehav Rev 55: 547–572, 2015. doi: 10.1016/j.neubiorev.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Dubrowski A, Lam J, Carnahan H. Target velocity effects on manual interception kinematics. Acta Psychol (Amst) 104: 103–118, 2000. doi: 10.1016/S0001-6918(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Eskandar EN, Assad JA. Dissociation of visual, motor and predictive signals in parietal cortex during visual guidance. Nat Neurosci 2: 88–93, 1999. doi: 10.1038/4594. [DOI] [PubMed] [Google Scholar]
- Grotheer M, Ambrus GG, Kovács G. Causal evidence of the involvement of the number form area in the visual detection of numbers and letters. Neuroimage 132: 314–319, 2016. doi: 10.1016/j.neuroimage.2016.02.069. [DOI] [PubMed] [Google Scholar]
- Haarmeier T, Kammer T. Effect of TMS on oculomotor behavior but not perceptual stability during smooth pursuit eye movements. Cereb Cortex 20: 2234–2243, 2010. doi: 10.1093/cercor/bhp285. [DOI] [PubMed] [Google Scholar]
- Huk AC, Shadlen MN. Neural activity in macaque parietal cortex reflects temporal integration of visual motion signals during perceptual decision making. J Neurosci 25: 10420–10436, 2005. doi: 10.1523/JNEUROSCI.4684-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indovina I, Maffei V, Bosco G, Zago M, Macaluso E, Lacquaniti F. Representation of visual gravitational motion in the human vestibular cortex. Science 308: 416–419, 2005. doi: 10.1126/science.1107961. [DOI] [PubMed] [Google Scholar]
- Indovina I, Maffei V, Pauwels K, Macaluso E, Orban GA, Lacquaniti F. Simulated self-motion in a visual gravity field: sensitivity to vertical and horizontal heading in the human brain. Neuroimage 71: 114–124, 2013. doi: 10.1016/j.neuroimage.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Indovina I, Mazzarella E, Maffei V, Cesqui B, Passamonti L, Lacquaniti F. Sound-evoked vestibular stimulation affects the anticipation of gravity effects during visual self-motion. Exp Brain Res 233: 2365–2371, 2015. doi: 10.1007/s00221-015-4306-9. [DOI] [PubMed] [Google Scholar]
- Janssen P, Shadlen MN. A representation of the hazard rate of elapsed time in macaque area LIP. Nat Neurosci 8: 234–241, 2005. doi: 10.1038/nn1386. [DOI] [PubMed] [Google Scholar]
- Jazayeri M, Shadlen MN. Temporal context calibrates interval timing. Nat Neurosci 13: 1020–1026, 2010. doi: 10.1038/nn.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri M, Shadlen MN. A Neural mechanism for sensing and reproducing a time interval. Curr Biol 25: 2599–2609, 2015. doi: 10.1016/j.cub.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas A, Weigelt S, Roebroeck A, Kohler A, Muckli L. Imagery of a moving object: the role of occipital cortex and human MT/V5+. Neuroimage 49: 794–804, 2010. doi: 10.1016/j.neuroimage.2009.07.055. [DOI] [PubMed] [Google Scholar]
- Katsumata H, Russell DM. Prospective versus predictive control in timing of hitting a falling ball. Exp Brain Res 216: 499–514, 2012. doi: 10.1007/s00221-011-2954-y. [DOI] [PubMed] [Google Scholar]
- La Scaleia B, Lacquaniti F, Zago M. Neural extrapolation of motion for a ball rolling down an inclined plane. PLoS One 9: e99837, 2014. doi: 10.1371/journal.pone.0099837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scaleia B, Zago M, Lacquaniti F. Hand interception of occluded motion in humans: a test of model-based vs. on-line control. J Neurophysiol 114: 1577–1592, 2015. doi: 10.1152/jn.00475.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacquaniti F, Bosco G, Gravano S, Indovina I, La Scaleia B, Maffei V, Zago M. Gravity in the brain as a reference for space and time perception. Multisens Res 28: 397–426, 2015. doi: 10.1163/22134808-00002471. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Bosco G, Indovina I, La Scaleia B, Maffei V, Moscatelli A, Zago M. Visual gravitational motion and the vestibular system in humans. Front Integr Nuerosci 7: 101, 2013. doi: 10.3389/fnint.2013.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer R, Nagel M, Sprenger A, Zapf S, Erdmann C, Heide W, Binkofski F. Cortical mechanisms of smooth pursuit eye movements with target blanking. An fMRI study. Eur J Neurosci 19: 1430–1436, 2004. doi: 10.1111/j.1460-9568.2004.03229.x. [DOI] [PubMed] [Google Scholar]
- Leon MI, Shadlen MN. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron 38: 317–327, 2003. doi: 10.1016/S0896-6273(03)00185-5. [DOI] [PubMed] [Google Scholar]
- Maffei V, Macaluso E, Indovina I, Orban G, Lacquaniti F. Processing of targets in smooth or apparent motion along the vertical in the human brain: an fMRI study. J Neurophysiol 103: 360–370, 2010. doi: 10.1152/jn.00892.2009. [DOI] [PubMed] [Google Scholar]
- Maffei V, Mazzarella E, Piras F, Spalletta G, Caltagirone C, Lacquaniti F, Daprati E. Processing of visual gravitational motion in the peri-sylvian cortex: evidence from brain-damaged patients. Cortex 78: 55–69, 2016. doi: 10.1016/j.cortex.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Maimon G, Assad JA. A cognitive signal for the proactive timing of action in macaque LIP. Nat Neurosci 9: 948–955, 2006. doi: 10.1038/nn1716. [DOI] [PubMed] [Google Scholar]
- Makin AD, Poliakoff E, El-Deredy W. Tracking visible and occluded targets: changes in event related potentials during motion extrapolation. Neuropsychologia 47: 1128–1137, 2009b. doi: 10.1016/j.neuropsychologia.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Makin AD, Stewart AJ, Poliakoff E. Typical object velocity influences motion extrapolation. Exp Brain Res 193: 137–142, 2009a. doi: 10.1007/s00221-008-1678-0. [DOI] [PubMed] [Google Scholar]
- Makin ADJ, Poliakoff E, Chen J, Stewart AJ. The effect of previously viewed velocities on motion extrapolation. Vision Res 48: 1884–1893, 2008. doi: 10.1016/j.visres.2008.05.023. [DOI] [PubMed] [Google Scholar]
- Marinovic W, Flannery V, Riek S. The effects of preparation and acoustic stimulation on contralateral and ipsilateral corticospinal excitability. Hum Mov Sci 42: 81–88, 2015. doi: 10.1016/j.humov.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Marinovic W, Tresilian JR, de Rugy A, Sidhu S, Riek S. Corticospinal modulation induced by sounds depends on action preparedness. J Physiol 592: 153–169, 2014. doi: 10.1113/jphysiol.2013.254581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant H, Averbeck BB. The computational and neural basis of rhythmic timing in medial premotor cortex. J Neurosci 37: 4552–4564, 2017. doi: 10.1523/JNEUROSCI.0367-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant H, Bartolo R. Primate beta oscillations and rhythmic behaviors. J Neural Transm (Vienna) 16: 390, 2017. doi: 10.1007/s00702-017-1716-9. [DOI] [PubMed] [Google Scholar]
- Merchant H, Battaglia-Mayer A, Georgopoulos AP. Neural responses during interception of real and apparent circularly moving stimuli in motor cortex and area 7a. Cereb Cortex 14: 314–331, 2004b. doi: 10.1093/cercor/bhg130. [DOI] [PubMed] [Google Scholar]
- Merchant H, Battaglia-Mayer A, Georgopoulos AP. Neural responses in motor cortex and area 7a to real and apparent motion. Exp Brain Res 154: 291–307, 2004a. doi: 10.1007/s00221-003-1664-5. [DOI] [PubMed] [Google Scholar]
- Merchant H, Georgopoulos AP. Neurophysiology of perceptual and motor aspects of interception. J Neurophysiol 95: 1–13, 2006. doi: 10.1152/jn.00422.2005. [DOI] [PubMed] [Google Scholar]
- Merchant H, Zarco W, Pérez O, Prado L, Bartolo R. Measuring time with different neural chronometers during a synchronization-continuation task. Proc Natl Acad Sci USA 108: 19784–19789, 2011. doi: 10.1073/pnas.1112933108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant H, Zarco W, Prado L, Pérez O. Behavioral and neurophysiological aspects of target interception. Adv Exp Med Biol 629: 201–220, 2009. doi: 10.1007/978-0-387-77064-2_10. [DOI] [PubMed] [Google Scholar]
- Miller WL, Maffei V, Bosco G, Iosa M, Zago M, Macaluso E, Lacquaniti F. Vestibular nuclei and cerebellum put visual gravitational motion in context. J Neurophysiol 99: 1969–1982, 2008. doi: 10.1152/jn.00889.2007. [DOI] [PubMed] [Google Scholar]
- Mrotek LA, Soechting JF. Predicting curvilinear target motion through an occlusion. Exp Brain Res 178: 99–114, 2007. doi: 10.1007/s00221-006-0717-y. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Mesulam MM, Nobre AC. The cerebellum predicts the timing of perceptual events. J Neurosci 28: 2252–2260, 2008. doi: 10.1523/JNEUROSCI.2742-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Inui T. Lateralization of the posterior parietal cortex for internal monitoring of self- versus externally generated movements. J Cogn Neurosci 19: 1827–1835, 2007. doi: 10.1162/jocn.2007.19.11.1827. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olson IR, Gatenby JC, Leung HC, Skudlarski P, Gore JC. Neuronal representation of occluded objects in the human brain. Neuropsychologia 42: 95–104, 2004. doi: 10.1016/S0028-3932(03)00151-9. [DOI] [PubMed] [Google Scholar]
- Orban GA, Fize D, Peuskens H, Denys K, Nelissen K, Sunaert S, Todd J, Vanduffel W. Similarities and differences in motion processing between the human and macaque brain: evidence from fMRI. Neuropsychologia 41: 1757–1768, 2003. doi: 10.1016/S0028-3932(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Solé J, Wassermann EM, Brasil-Neto J, Cohen LG, Hallett M. Effects of focal transcranial magnetic stimulation on simple reaction time to acoustic, visual and somatosensory stimuli. Brain 115: 1045–1059, 1992. doi: 10.1093/brain/115.4.1045. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience--virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol 10: 232–237, 2000. doi: 10.1016/S0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 126: 1071–1107, 2015. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack AT, Camprodon JA, Pascual-Leone A, Goebel R. The dynamics of interhemispheric compensatory processes in mental imagery. Science 308: 702–704, 2005. doi: 10.1126/science.1107784. [DOI] [PubMed] [Google Scholar]
- Salvioni P, Murray MM, Kalmbach L, Bueti D. How the visual brain encodes and keeps track of time. J Neurosci 33: 12423–12429, 2013. doi: 10.1523/JNEUROSCI.5146-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science 294: 1350–1354, 2001. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Tootell RBH. From monkeys to humans: what do we now know about brain homologies? Curr Opin Neurobiol 15: 135–144, 2005. doi: 10.1016/j.conb.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Shuwairi SM, Curtis CE, Johnson SP. Neural substrates of dynamic object occlusion. J Cogn Neurosci 19: 1275–1285, 2007. doi: 10.1162/jocn.2007.19.8.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res 148: 1–16, 2003. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- Soechting JF, Flanders M. Extrapolation of visual motion for manual interception. J Neurophysiol 99: 2956–2967, 2008. doi: 10.1152/jn.90308.2008. [DOI] [PubMed] [Google Scholar]
- Soechting JF, Juveli JZ, Rao HM. Models for the extrapolation of target motion for manual interception. J Neurophysiol 102: 1491–1502, 2009. doi: 10.1152/jn.00398.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striemer CL, Chouinard PA, Goodale MA. Programs for action in superior parietal cortex: a triple-pulse TMS investigation. Neuropsychologia 49: 2391–2399, 2011. doi: 10.1016/j.neuropsychologia.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Swisher JD, Halko MA, Merabet LB, McMains SA, Somers DC. Visual topography of human intraparietal sulcus. J Neurosci 27: 5326–5337, 2007. doi: 10.1523/JNEUROSCI.0991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadin D, Silvanto J, Pascual-Leone A, Battelli L. Improved motion perception and impaired spatial suppression following disruption of cortical area MT/V5. J Neurosci 31: 1279–1283, 2011. doi: 10.1523/JNEUROSCI.4121-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira LA, Chua R, Nagelkerke P, Franks IM. Use of visual information in the correction of interceptive actions. Exp Brain Res 175: 758–763, 2006. doi: 10.1007/s00221-006-0740-z. [DOI] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, Suzuki M, Sakai K, Hanajima R, Gemba-Shimizu K, Kanazawa I. Shortening of simple reaction time by peripheral electrical and submotor-threshold magnetic cortical stimulation. Exp Brain Res 115: 541–545, 1997. doi: 10.1007/PL00005724. [DOI] [PubMed] [Google Scholar]
- Tresilian JR, Plooy AM. Effects of acoustic startle stimuli on interceptive action. Neuroscience 142: 579–594, 2006. doi: 10.1016/j.neuroscience.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Vetter P, Grosbras M-H, Muckli L. TMS over V5 disrupts motion prediction. Cereb Cortex 25: 1052–1059, 2015. doi: 10.1093/cercor/bht297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishton PM, Reardon KM, Stevens JA. Timing of anticipatory muscle tensing control: responses before and after expected impact. Exp Brain Res 202: 661–667, 2010. doi: 10.1007/s00221-010-2172-z. [DOI] [PubMed] [Google Scholar]
- Wang H, Callaghan E, Gooding-Williams G, McAllister C, Kessler K. Rhythm makes the world go round: An MEG-TMS study on the role of right TPJ theta oscillations in embodied perspective taking. Cortex 75: 68–81, 2016. doi: 10.1016/j.cortex.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol 108: 1–16, 1998. doi: 10.1016/S0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Elfar JC, Eskandar EN, Toth LJ, Assad JA. Parietal activity and the perceived direction of ambiguous apparent motion. Nat Neurosci 6: 616–623, 2003. doi: 10.1038/nn1055. [DOI] [PubMed] [Google Scholar]
- Zago M, Iosa M, Maffei V, Lacquaniti F. Extrapolation of vertical target motion through a brief visual occlusion. Exp Brain Res 201: 365–384, 2010. doi: 10.1007/s00221-009-2041-9. [DOI] [PubMed] [Google Scholar]
- Zago M, McIntyre J, Senot P, Lacquaniti F. Internal models and prediction of visual gravitational motion. Vision Res 48: 1532–1538, 2008. doi: 10.1016/j.visres.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Zago M, McIntyre J, Senot P, Lacquaniti F. Visuo-motor coordination and internal models for object interception. Exp Brain Res 192: 571–604, 2009. doi: 10.1007/s00221-008-1691-3. [DOI] [PubMed] [Google Scholar]