Vibratory stimulation at 70 and 150 Hz on the neck overlying the larynx increased the frequency of spontaneous swallowing. Simultaneously vibration also enhanced hemodynamic responses in the motor cortex to swallows when recorded with functional near-infrared spectroscopy (fNIRS). As vibrotactile stimulation on the neck enhanced cortical activation for swallowing in healthy participants, it may be useful for enhancing swallowing in patients with dysphagia.
Keywords: functional near-infrared spectroscopy, sensory stimulation, motor cortex, sensory cortex, hemodynamic response
Abstract
Sensory input can alter swallowing control in both the cortex and brainstem. Electrical stimulation of superior laryngeal nerve afferents increases reflexive swallowing in animals, with different frequencies optimally effective across species. Here we determined 1) if neck vibration overlying the larynx affected the fundamental frequency of the voice demonstrating penetration of vibration into the laryngeal tissues, and 2) if vibration, in comparison with sham, increased spontaneous swallowing and enhanced cortical hemodynamic responses to swallows in the swallowing network. A device with two motors, one over each thyroid lamina, delivered intermittent 10-s epochs of vibration. We recorded swallows and event-related changes in blood oxygenation level to swallows over the motor and sensory swallowing cortexes bilaterally using functional near infrared spectroscopy. Ten healthy participants completed eight 20-min conditions in counterbalanced order with either epochs of continuous vibration at 30, 70, 110, 150, and 70 + 110 Hz combined, 4-Hz pulsed vibration at 70 + 110 Hz, or two sham conditions without stimulation. Stimulation epochs were separated by interstimulus intervals varying between 30 and 45 s in duration. Vibration significantly reduced the fundamental frequency of the voice compared with no stimulation demonstrating that vibration penetrated laryngeal tissues. Vibration at 70 and at 150 Hz increased spontaneous swallowing compared with sham. Hemodynamic responses to swallows in the motor cortex were enhanced during conditions containing stimulation compared with sham. As vibratory stimulation on the neck increased spontaneous swallowing and enhanced cortical activation for swallows in healthy participants, it may be useful for enhancing swallowing in patients with dysphagia.
NEW & NOTEWORTHY Vibratory stimulation at 70 and 150 Hz on the neck overlying the larynx increased the frequency of spontaneous swallowing. Simultaneously vibration also enhanced hemodynamic responses in the motor cortex to swallows when recorded with functional near-infrared spectroscopy (fNIRS). As vibrotactile stimulation on the neck enhanced cortical activation for swallowing in healthy participants, it may be useful for enhancing swallowing in patients with dysphagia.
swallowing is a highly intricate process that is both automatic and modifiable by volitional control. In animals, swallowing can be altered by manipulating bolus properties (Miller and Sherrington 1915) and can be induced by peripheral stimulation (Doty et al. 1967). One mechanism involves stimulation of laryngeal afferents in the internal superior laryngeal nerve, which can trigger the central pattern generators (CPGs) for swallowing in the brainstem (Sumi 1963). Sensory input to the brainstem CPGs is also relayed from the nucleus of the solitary tract (NTS) via the thalamus bilaterally to each cortical hemisphere (Car et al. 1975). Stimulation of superior laryngeal nerve afferents initiated reflexive swallowing in animals and maximally increased the rate of swallowing at certain frequencies specific to each animal species tested; the optimal frequency for dogs was 20 to 30 Hz, while it was 60 to 80 Hz in rabbits (Doty 1951).
Similarly, human studies have shown that swallowing physiology may be altered by changes in sensory input. Swallowing may change in response to bolus properties in healthy participants and in patients with dysphagia (Gatto et al. 2013; Hamdy et al. 2003; Mulheren et al. 2016). Furthermore, swallowing can be disrupted by a reduction in sensation following a bilateral chemical block of the internal superior laryngeal nerve (Jafari et al. 2003; Månsson and Sandberg 1975).
As in animals, the mechanism of sensory modulation of human swallowing likely involves afferent connections to the swallowing CPGs in the brain stem as well as projections to the cortical swallowing network. During spontaneous swallowing, functional (f)MRI studies demonstrated increased activation bilaterally in the sensory and motor cortexes (Teismann et al. 2007; Zald and Pardo 1999). Sour taste also enhanced cortical activation in the motor and sensory regions during swallowing (Mulheren et al. 2016).
Functional near-infrared spectroscopy (fNIRS) measures changes in oxygenated hemoglobin associated with cortical activation (Malonek and Grinvald 1996). With this neuroimaging system, light is emitted 1.5 cm into the cortex, attenuated by cortical tissue, and the output detected reflects changes in oxygenation during functional activity (Quaresima et al. 2012). Changes in the absorption of light represent changes in oxygenated and deoxygenated hemoglobin, referred to as the hemodynamic response (Boas et al. 2001; Quaresima et al. 2012; Villringer and Chance 1997). fNIRS is noninvasive and can be conducted in an upright sitting position. Additionally, fNIRS analysis allows for extraction of motion artifact and relatively high temporal resolution compared with fMRI. Hemodynamic responses to swallowing peak 2–7 s after the swallow, with motion artifact during swallowing occurring between 0 and 2 s (Birn et al. 1999).
Although electrical stimulation of laryngeal afferents at certain frequencies enhances swallowing in animals (Chi-Fishman et al. 1994; Doty 1951), laryngeal stimulation has not been examined in adult humans. Air puff stimulation to the faucial pillars in the mouth can upregulate reflexive swallowing in healthy and older adults (Theurer et al. 2005, 2009) presumably by stimulation of glossopharyngeal afferents, which can regulate the CPGs for swallowing in the brainstem. However, this approach requires an apparatus in the mouth for air puff stimulation, which can interfere with oral intake for feeding. A recent study indicated that surface vibration to the neck significantly increased the rate of swallowing in healthy infants without significant alterations in respiration (Hegyi Szynkiewicz et al. 2016). This method of noninvasive vibratory stimulation on the neck would not interfere with oral intake and thus may be suitable for dysphagia management. Additionally, a previous study reported that vibratory stimulation can improve voice production in patients with dysphonia (Kosztyła-Hojna et al. 2012).
We hypothesized first that vibration on the surface of the neck over the thyroid cartilage would penetrate to the laryngeal tissues, thus altering the fundamental frequency of the voice during stimulation in comparison with voicing without stimulation. By affecting the laryngeal tissues, vibration could stimulate afferents of the superior laryngeal nerve to upregulate brain stem control of swallowing. We then examined the second hypothesis that vibrotactile stimulation over the thyroid cartilage would increase swallowing frequency. For the third hypothesis, we examined whether vibration activated the cortical swallowing network on fNIRS recordings during stimulation epochs in healthy adults. Fourth, we hypothesized that vibratory stimulation would enhance cortical responses to swallows regardless of whether swallows occurred during stimulation or between stimulation periods during 20-min stimulation conditions in comparison with sham conditions. We also expected that increases in swallowing frequency and activation in the cortical swallowing network would vary with vibratory frequency and the continuity of stimulation (either continuous or pulsed).
MATERIALS AND METHODS
Participants.
The study was approved by the Institutional Review Boards of James Madison University and Sentara Rockingham Memorial Hospital. Before admission to the study, participants completed a medical history form to ensure no reports of history of dysphagia or psychiatric or neurological abnormalities. A Handedness Inventory (Oldfield 1971) was used to include only participants who were right-hand dominant. Written informed consent was obtained. As each participant received an anatomical MRI scan for neuronavigation for optode placement, participants were screened with magnetic resonance (MR) safety questionnaires; the protocol recommended for MR safety standards established by the American College of Radiology (Kanal et al. 2013).
Study 1: the effects of vibration on voice fundamental frequency.
To examine the first hypothesis that vibration on the surface of the neck over the thyroid cartilage would penetrate to the laryngeal tissues, we determined the fundamental frequency of the voice during stimulation in comparison with voicing without stimulation in six participants (Table 1, study 1). This was important to establish if the upregulation of reflexive swallowing with vibration could be due to vibration penetrating to the laryngeal tissues with mechanoreceptors innervated by the internal branch of the superior laryngeal nerve.
Table 1.
Conditions and measures of studies
| Conditions | Measures |
|---|---|
| Study 1: Effects of vibration on laryngeal tissues (n = 6 HV) | |
| Phonation | Spectrum to identify fundamental frequency |
| Phonation with vibration | Spectrum to identify fundamental frequency |
| Vibration without phonation | Spectrum to identify fundamental frequency |
| Study 2: Vibration effects on swallowing and cortical hemodynamic response (n = 10 HV) | |
| Conditions | Measure: Swallow Frequency | Measure: Cortical Activation Level | Measures: Event-Related Peaks |
|---|---|---|---|
| 20 min Containing 30 epochs of 10-s vibration or sham with interstimulus intervals between 30 and 45 s | Numbers of swallows in 20 min | Mean Z score for HbO2 level over 10-s stimulation block relative to baseline means and SD HbO2 level | Mean peak HbO2 level Z score between 4 and 7 s and between 14 and 17 s relative to baseline means and SD HbO2 level for swallows during stimulation and between stimulations |
| Two motors at 30 Hz continuous | Number of swallows | Mean HbO2 Z for 10 s | Peak Z between 4 and 7 s and 14–17 s |
| Two motors at 70 Hz continuous | Number of swallows | Mean HbO2 Z for 10 s | Peak Z between 4 and 7 s and 14–17 s |
| Two motors at 110 Hz continuous | Number of swallows | Mean HbO2 Z for 10 s | Peak Z between 4 and 7 s and 14–17 s |
| Two motors at 150 Hz continuous | Number of swallows | Mean HbO2 Z for 10 s | Peak Z between 4 and 7 s and 14–17 s |
| One motor at 70 and one at 110 Hz continuous | Number of swallows | Mean HbO2 Z for 10 s | Peak Z between 4 and 7 s and 14–17 s |
| One motor at 70 and one at 110-Hz pulsed at 4 Hz |
Number of swallows | Mean HbO2 Z for 10 s | Peak Z between 4 and 7 s and 14–17 s |
| Sham 1 (wearing device with no vibration) | Number of swallows | Mean HbO2 Z for 10 s | Peak Z between 4 and 7 s and 14–17 s |
| Sham 2 (wearing device with no vibration | Number of swallows | Mean HbO2 Z for 10 s | Peak Z between 4 and 7 s and 14–17 s |
HV, healthy volunteers.
A device with two motors, each placed on the skin covering a lamina of the thyroid cartilage, was used to deliver vibratory stimulation to the laryngeal tissues. The device was developed for the study by Passy Muir, Inc. (Irvine, CA). A molded plastic butterfly shaped neck piece contained the two motors, one on each side. Each motor was attached to a plunger that was placed on the skin overlying a thyroid lamina on each side (Fig. 1). Different pairs of motors were used in the six participants; two participants had 70-Hz motors on each side, two had 150-Hz motors on each side, and two others had a 70-Hz motor on one side and a 110-Hz motor on the other side.
Fig. 1.
A vibratory device with 2 motors, each placed over the lamina on one side of the thyroid cartilage. The neck motors are connected to contacts and contained in a molded plastic neck piece. The neck piece is attached with soft adjustable ties and is connected to a controller unit. The device was supplied by Passy Muir.
Procedures to determine the effects of vibration on voice.
To determine if vibration on the skin overlying the thyroid cartilage affected tissues inside the larynx, acoustic voice recordings were analyzed to determine the fundamental frequency during voicing with stimulation on and with stimulation off. Vibratory motion induced by the motors on the skin was also recorded and analyzed when the subject was not voicing using a piezoelectric accelerometer (model 8778A500; Kistler, Amherst, NY) attached to the skin over the thyroid notch. For the voice recordings, a microphone was attached to the participants’ clothing at 10 in. from the mouth. Participants sustained the vowel /i/ for 10 s. This was performed six times, with 20 s or more of rest between each trial. Online digitized recordings were made on an AD Instruments Powerlab 16-bit digital converter at 4 kHz (model ML880; AD Instruments, Colorado Springs, CO). The motors were turned on during half of the trials without warning the participant.
Analysis of fundamental frequency with and without vibration.
Voice analysis was conducted using LabChart (AD Instruments). The phonatory signal was selected from microphone recordings to include up to 8 s when the phonation was stable. The Spectrum function in LabChart V 7 was used to compute a fast Fourier transform (FFT) using a Hamming window at 1 k (1,024). The FFT was displayed using a 2-kHz horizontal scaling. The resulting display was an average of between 300 and 400 FFTs from ~8 s of phonation (Fig. 2). The frequency of the first FFT peak was measured in Hertz in LabChart and recorded as the lowest frequency peak, which for a phonatory signal is considered the fundamental frequency (Fo) of the voice. This procedure was performed for each phonation when the motor was turned on and when it was not. Furthermore, to determine the frequency of the motor, we recorded when the motor was turned on and the participant was not phonating and analyzed the accelerometer signal using the Spectrum window to determine the lowest vibratory frequency of the motor when the participant was not phonating.
Fig. 2.

A: results of the fast Fourier transform (FFT) used in LabChart 7 to analyze the spectrum and identify the lowest frequency component from the microphone signal recorded during phonation of the vowel /i/ for ~8 s with the motor turned off. The lowest peak frequency was identified at the arrow which was 195 Hz in this female subject. B: FFT of the accelerometer signal when recorded with the motor turned on while the participant was not phonating. The lowest motor frequency at the arrow was 66 Hz. C: FFT was computed from signal from the microphone recording of the same female subject when the motor was turned on and the peak frequency of the lowest frequency component at the arrow was 97 Hz. The y-axis is the dB down of the power spectrum and the x-axis is the frequency from 0 to 2 kHz.
To determine if the lowest frequency peak of the voice changed when the motor was turned on, a paired t-test compared the lowest frequency of the voice with the motor on and off across the six participants.
Study 2: the effects of vibration on swallowing, cortical activation during stimulation, and cortical activation during swallowing.
To examine the second hypothesis, we determined whether vibrotactile stimulation over the thyroid cartilage would increase swallowing frequency. To compare the effects of different frequencies of vibration with sham stimulation (wearing the device but not turned on) on swallowing frequency and cortical activation, 10 participants were studied (Table 1, study 2). All participants completed eight conditions of 20 min each in counterbalanced order: two motors continuously vibrating at 30 Hz each, 70 Hz each, 110 Hz each, 150 Hz each, or one vibrating at 70 Hz and the other at 110 Hz, or motors pulsed at 4 Hz with one at 70 Hz and the other at 110 Hz (150 ms on and 100 ms off), and two sham conditions with no vibration for 20 min while wearing the device. Each participant completed two sessions each consisting of one sham condition and three experimental conditions in counterbalanced order. In each condition, 30 epochs of 10 s of stimulation or sham were separated by interstimulus intervals randomized between 30 to 45 s to reduce expectation of the time of stimulation.
The five sets of two motors used to deliver vibratory stimulation were developed for the study by Passy Muir. These were the same as some of the motor sets used in the first study to examine the effects of vibration on the fundamental frequency of the voice (Fig. 1). All devices were uniform in size, shape, and appearance. The devices were adjusted around the neck using tracheostomy ties with Velcro. To have a constant pressure of the motors on the skin across subjects and sessions, the air-filled bulb of the IOPI pressure transducer (model 2.3; IOPI Medical, Redmond, WA) was placed between the neck piece and the skin and the ties were adjusted to provide a pressure reading of 2 kPa.
e-Prime v2.0 (Psychology Software Tools, Sharpsburg, PA) was used to drive the motors by turning them on for 10 s and off. e-Prime was also used to randomize the duration of the interstimulus intervals between 30 and 45 s during a condition. Stimulation epochs were continuous vibration for 10 s for all motors except during the 4-Hz pulsed stimulation mode, “on” periods of 150 ms were alternated with “off “periods of 100 ms for 10 s. During the sham conditions, the vibrator from the previous condition was left in place on the neck to control for the tactile and pressure effect of the neck piece on the throat with the motors turned off. During the sham conditions, pulses were recorded from e-Prime on PowerLab to mark sham periods of no stimulation and interstimulus intervals of randomized durations between 30 and 45 s for analysis of sham conditions.
Throughout the study, participants were not instructed about swallowing, and all swallows were uncued. They were not provided with information about which conditions would or would not include stimulation.
Analysis of numbers of swallows in each condition.
Simultaneous online digital recordings during each 20-min condition were digitized and displayed on a PowerLab 16/30 SP unit (16-bit analog-to-digital converter, sampling rate 1 kHz; model ML880; AD Instruments). Each session was simultaneously recorded by video camera. The PowerLab recordings included piezoelectric accelerometry placed over the thyroid notch to detect laryngeal elevation at the onset of the pharyngeal phase of swallowing (model 8778A500; Kistler); Inductotrace signals from the ribcage and abdomen used to detect periods of swallowing respiratory apnea (model 10.9000; Ambulatory Monitoring, Ardsley, NY); and pulses produced when an observer pushed a button to indicate they visually confirmed a swallow (Fig. 3).
Fig. 3.
An example of marking a swallow in LabChart. A large initial spike in the piezoelectric accelerometer signal (A) indicates hyolaryngeal elevation at swallowing onset. The first derivative of the sum Inductotrace channel (B), which flattens around zero on the y-axis at ~217.4 s, with arrows indicating the onset and offset of the apneic period. A square wave in the pulse generator (C) indicates a button press when a swallow was observed by a trained assistant.
The number of swallows in each 20-min condition was determined by reviewing the PowerLab recordings including the piezoelectric accelerometer, Inductotrace, and push button signals in LabChart 7 (AD Instruments). Three events were required to identify the occurrence of a swallow: 1) a rapid change in position in the accelerometer signal representing hyolaryngeal elevation at the onset of swallowing; 2) a respiratory apnea on the inductotrace signal, indicated by a flat line showing an absence of ribcage/abdomen movement; and 3) a pulse from a button press by the trained observer when they saw thyroid cartilage elevation associated with a swallow. To confirm a respiratory apnea, the first derivative of the sum of the ribcage and abdominal signals was calculated using a sliding window to select the apneic interval for each swallow (Fig. 3). A flat signal lasting more than 350 ms indicated a respiratory apnea on exhalation that was required for a swallow to be identified (Klahn and Perlman 1999; Martin-Harris et al. 2005).
The percent change in the number of swallows over 20 min during a stimulation condition in comparison with a sham condition during the same recording session was computed for each participant in each condition. Directional Z tests were used to determine if the rate of swallowing increased or not for each stimulation frequency.
fNIRS recording procedures.
Simultaneous with the Powerlab recordings, continuous fNIRS cortical recordings were made over regions in the primary motor (M1) and sensory (S1) areas in both hemispheres previously shown to be active during swallowing (Lowell et al. 2008; Söros et al. 2008) (Table 2). Before the study each participant underwent a T-1 weighted anatomical MRI 1.5 Tesla scan with a field of view that included the tip of the nose, both ears, and the maxilla. Brainsight 2.0 (Rogue Research, Montreal, QC, Canada) neuronavigation software with a two-dimensional Polaris camera with eyeglasses with two locators and a pointer with two locators that identified the nasion and each tragus were used to coregister each participant’s MRI brain scan inside his or her head. After fitting a participant’s brain to three-dimensional Montreal Neurological Institute coordinate space, optode pairs with 3 cm between an emitter-detector pair were placed over the regions of interest for lateral motor and sensorimotor cortices in the right and left hemispheres using (Fig. 4). Templates were created from rubber sheeting and placed on the head with self-adhesive bandage to hold the optodes in place. Power levels for wavelengths of 690 and 830 nm were set to 3 and 6 mW, respectively, on the fNIRS system (model CW6; Techen, Milford, MA). Continuous data were recorded at a sampling rate of 50 Hz. The e-Prime signal for onset of vibration was connected to an auxiliary CW6 channel to synchronize fNIRS recordings with stimulation epochs. fNIRS signals were monitored, and the gain levels were set before the data were recorded and were maintained at the same levels throughout the study for an individual.
Table 2.
Coordinates for hemodynamic regions of interest (Montreal Neurological Institute coordinates)
| X | Y | Z | |
|---|---|---|---|
| Right | |||
| S1 | 59 | −17 | 35 |
| M1 | 57 | 8 | 23 |
| Left | |||
| S1 | −56 | −16 | 35 |
| M1 | −55 | 8 | 23 |
Based on Sörös et al. (2008) and Lowell et al. (2008).
Fig. 4.
A 3-dimensional MRI in Brainsight 2.0 software showing the location of the regions of interest in Montreal Neurological Institute standardized space in the right and left hemispheres, including motor (anterior) and sensory (posterior) areas.
Measures of hemodynamic activity during 10-s blocks of vibratory stimulation.
To examine the third hypothesis that vibration activated the cortical swallowing network on fNIRS recordings during stimulation epochs in healthy adults, we analyzed fNIRS data with HomER 2 software (Boas et al. 2012) in MATLAB 2013 (The MathWorks, Natick, MA). The recordings were high- and low-pass filtered at 0.016 and 0.8, respectively, to remove physiological signals (e.g., Mayer’s waves, respiratory, and cardiac).
To analyze the mean level of oxygenated hemoglobin during each 10-s stimulation epoch during each vibratory condition (continuous stimulation at 30, 70, 110, 150, 70 + 110, pulsed stimulation at 70 + 110 Hz, and sham), all stimulation epochs during a condition were labeled for each optode recording in a participant. The onset and offset of each 10-s stimulation epoch were defined by the auxiliary signal from e-Prime. An average of mean block level of oxygenated hemoglobin was computed from the 30 epochs contained in each frequency or sham condition for each participant at each cortical location in each hemisphere.
Measures of event-related hemodynamic responses to swallows during stimulation, between stimulations, and during sham conditions.
Our fourth hypothesis was that vibratory stimulation would enhance cortical responses to swallows regardless of whether swallows occurred during 10-s stimulation epochs or between stimulation periods during 20-min stimulation conditions in comparison with sham conditions. We analyzed the peak of event-related averages of hemodynamic responses to three groups of swallows in each participant: all spontaneous swallows that occurred during 10-s epochs of vibratory stimulation; all spontaneous swallows that occurred during interstimulus intervals between stimulations that were without stimulation for 30- to 45-s duration; and all spontaneous swallows that occurred during the sham conditions epochs (that did not contain any stimulation for 20 min). We used the ePrime auxiliary channel to determine whether swallows occurred during vibration, between vibratory periods, or during sham conditions.
The onsets of swallows were identified using the onset of hyolaryngeal elevation from the accelerometer signal for averaging events from 5 s before the onset of swallows to 35 s after swallow onsets.
The event-related averages of hemodynamic signals during swallows were reviewed to identify the period of artifact between 0 and 2 s associated with the swallowing motion (Birn et al. 1999), which was indicated by simultaneous increases in both the oxygenated and deoxygenated hemoglobin signals (Cui et al. 2010; Devor et al. 2003). The identification of the peak hemodynamic response to a swallow was between 4 and 7 s after onset (Birn et al. 1999). To confirm a valid hemodynamic response and not a motion artifact, the oxygenated hemoglobin and the deoxygenated hemoglobin signals had to be negatively correlated in the event-related averages between 4 and 7 s with the oxygenated hemoglobin (increasing) and deoxygenated hemoglobin (decreasing) (Cui et al. 2010; Devor et al. 2003) (Fig. 5). A second, later hemodynamic response in the event-related averages, also with the same negative correlation between the oxygenated and deoxygenated hemoglobin signals, was found between 14 and 17 s and designated a late response in the event-related average for each location in each participant for each condition (Fig. 5).
Fig. 5.

An example of the plot of the event-related average of Z score changes in one location in one hemisphere in one participant showing oxygenated hemoglobin (solid line) and deoxygenated hemoglobin (dotted line) changes over time from 5 s before the onset of the swallows (−5s) to 22 s after the onset of swallows. All events are time-locked averages based on the time of onset of laryngeal elevation (at 0 s) for the onset of the pharyngeal phase of swallowing. This average is for swallows occurring between stimulations. The period of potential motion artifact for swallowing is from 0 to 2 s, the time of the early peak is between 4 and 7 s from the swallow onset and for the late peak is from 14 to 17 s from the swallow onset.
Baseline activity was measured between −5 and 0 s before stimulation onset for stimulation blocks and before swallow onsets in the event-related average signals. The baseline means ± SD were used to convert measures of amplitude within each participant into Z score plots normalized by baseline activity for that condition and that location in a participant. Z scores were calculated relative to the means and SD between −5 and 0 s of oxygenated hemoglobin at baseline .
Mean Z scores were then computed for each block of vibration or sham. For event-related averages of each of the three types of swallows, the peak Z score between 4 and 7 s after swallow onset was identified in the event-related average for each location in a participant. Similarly, the peak Z score between 14 and 17 s after swallow onset was measured from the event-related average for each location in each participant for each of the three types of swallows (during vibration, between vibrations, and during sham).
Repeated-measures ANOVAs were conducted to examine each hypothesis separately. First, the effects of stimulation mode (continuous vs. pulsed) when using the hybrid motors were compared using a repeated-measures ANOVA. Second, a repeated-measures ANOVA examined the effects of stimulation vibratory frequency, and presence of stimulation vs. sham, on the mean Z score in each hemisphere and cortical location. Third, a repeated-measures ANOVA examined session (1 or 2) effects, swallowing type effects (during stimulation, between vibrations, or during sham condition), and timing of response (early or late) effects on peak Z scores in each hemisphere and cortical location. The significance level was set at 0.05 for each repeated-measures ANOVA with Bonferroni adjustments for post hoc comparisons.
RESULTS
Effects of vibration on fundamental frequency of voice.
To examine our first hypothesis that vibration on the surface of the neck over the thyroid cartilage would penetrate to the laryngeal tissues, we measured the fundamental frequency of the voice during stimulation in comparison with voicing without stimulation. Six participants were recorded, three males and three females ranging in age from 30 to 57 yr. All phonated the vowel /i/ for 10 s in a speaking voice on each trial. Between two and three trials in each condition were recorded with the motor turned on and off and analyzed using the FFT function in LabChart Pro. Within each participant in the same condition, each of the FFT analyses had the same Fo yielding one measure for each condition for each participant. Fo was significantly reduced during vibration [t(5) = 2.885, P = 0.034; Fig. 6]. As vibration altered vocal fold vibration during phonation, vibration was shown to penetrate to the tissues inside the larynx and likely affected mechanoreceptors innervated by the internal branch of the superior laryngeal nerve (Davis and Nail 1987).
Fig. 6.

A line graph showing the peak frequency in Hz of the lowest frequency component during 8 s of phonation with the motor turned off (Phonation) and the peak frequency in Hz of the lowest frequency component during 8 s of phonation with the motor turned on (Phonation + Vibration). The solid lines are the results of the 3 male participants; the dashed lines are the results of the 3 female participants.
Effects of vibration on the numbers of swallows.
To examine our second hypothesis that vibratory stimulation over the thyroid cartilage would increase swallowing frequency, 10 participants were included in the analysis of swallowing frequency over 20 min in each condition that contained epochs of stimulation for comparisons with the sham condition (6 female; mean age: 45.4 yr). The numbers of swallows increased significantly from sham for the 150-Hz (Z = 1.721, P = 0.043) and 70-Hz (Z = 1.642, P = 0.05) motor conditions, with a nonsignificant trend for the combination of the 70- and 110-Hz motors (Z = 1.614, P = 0.053; Fig. 7). No statistically significant increase in swallowing occurred during stimulation with the 30- or 110-Hz motors.
Fig. 7.
Line graphs depict the percent change from the sham condition in the rate of swallowing for 70-Hz stimulation (A), 150-Hz stimulation (B), and 70/110-Hz hybrid (C) stimulation. Each line is a single participant.
Mean hemodynamic levels during vibratory stimulation.
To examine our third hypothesis that vibration activated the cortical swallowing network on fNIRS recordings over blocks during stimulation epochs in healthy adults, we tested 10 participants. However, as two participants did not have valid fNIRS recording due to artifact or optode displacement, eight participants were included in the analysis of hemodynamic levels during 10-s blocks of vibration (5 female; mean age: 45.5 yr). A repeated-measures ANOVA compared block Z score means during continuous and pulsed stimulation when using the 70- and 110-Hz motors and found no significant differences in block means between the two conditions [F(1,7) = 1.317, P = 0.289]. Thus the pulsed condition was not included in the following analyses.
We compared the block mean Z score for the vibration epochs of 10 s from the five frequency continuous stimulation conditions with the 10-s block mean Z scores from the sham condition (when no stimulation was presented for 20 min). The repeated-measures ANOVAs also examined the main effects of vibratory frequency, hemisphere, and cortical location and their interactions with stimulation effects. One Z score of participant 106 was identified as an outlier, was examined, and was found to be a recording artifact rather than a calculation error. It was removed before analysis of the data.
The group mean Z scores for blocks with vibratory stimulation (means = −4.90, SD = 2.55) were significantly lower than for blocks without stimulation during sham [means = −3.01, SD = 1.90; F(1,7) = 11.964, P = 0.011, ƞ2 = 0.631]. No statistically significant main effects of vibratory frequency, side, or location were found on block mean Z scores. A significant interaction occurred between stimulation condition (30, 70, 110, 150, and 70/110 Hz and sham) and hemisphere [F(5,35) = 2.796, P = 0.032, ƞ2 = 0.285]. On post hoc testing, no statistically significant differences were found between the same conditions in the right or left hemisphere. On examination of the data (Fig. 8), block means tended to be higher in the left hemisphere than in the right during stimulation at 70 and 150 Hz and higher in the right hemisphere than in the left hemisphere during 110-Hz stimulation. In all other conditions, similar block means occurred in both hemispheres.
Fig. 8.

Mean Z scores with 95% confidence intervals (CI) of difference in oxygenated hemoglobin during vibratory stimulation and sham (no stimulation) from baseline mean and SD between −5 and 0 s before stimulation onset for different vibratory conditions 30, 70, 110, 150, 70 + 110 Hz (Hybrid), and sham (no vibration) in the left hemisphere (squares) and the right hemisphere (circles).
Event-related hemodynamic responses to swallows during and between stimulations within a condition and during the sham condition.
Our fourth hypothesis was that vibratory stimulation would enhance cortical responses to swallows regardless of whether swallows occurred during stimulation or between stimulation periods during 20-min stimulation conditions in comparison with during sham conditions without stimulation. As described earlier, the event-related averages of oxygenated hemoglobin Z scores showed early response peaks between 4 and 7 s following swallow onset and a later response peaks between 14 and 17 s after swallow onset (Fig. 5). The peak Z scores were compared among three types of swallows: swallows during and between stimulation periods in the stimulation conditions and swallows during sham conditions on repeated-measures ANOVAs. The analyses examined main effects for session (1 or 2), swallowing type (during stimulations, between stimulations, or during sham conditions), and timing of responses (early or late) on peak Z scores in each hemisphere. There were significant main effects of condition (during stimulation, between stimulations, or sham) [F(2,14) = 8.46, P = 0.004, ƞ2 = 0.547]. No significant main effects were found for location, timing (early or late), or side. On post hoc comparisons, the differences between swallows during stimulation (means = 0.597, SD = 2.337) and swallows during sham (means = −2.593, SD = 1.585 were statistically significant (P = 0.05). Furthermore, the difference between swallows between stimulations (means = 1.826, SD = 3.458) and the swallows during sham condition approached significance (P = 0.057). As no significant differences were found between swallow peak Z scores during stimulations compared with swallow peak Z scores between stimulations, it suggests that the occurrence of stimulation during the 20-min enhanced responses to swallows whether the swallows were during stimulation or between periods of stimulation. These results indicated that the hemodynamic responses to swallows were lower during the sham condition (when no stimulation did not occur for 20 min) than during conditions when stimulation occurred intermittently over 20 min (Fig. 9).
Fig. 9.
Mean peak Z scores with 95% confidence intervals (CI) of early and late responses combined showing difference in oxygenated hemoglobin to swallowing in the sham condition (Sham), swallowing between stimulation periods (Swallow stim off), and swallowing during stimulation periods (Swallow stim on) from baseline mean and SD between −5 and 0 s before swallow onset. The data is divided by motor (M1; diamonds) and sensory (S1; circles) regions and by right (RIGHT) and left (LEFT) hemispheres.
From the same repeated-measures ANOVA analysis, the interaction between the location (motor vs. sensory) and timing (early or late) on peak Z score responses to swallows was significant [F(1,7) = 12.292, P = 0.01, ƞ2 = 0.637]. On post hoc testing, there were significant differences between the early and late responses in the motor region [F(1,7) = 7.482, P = 0.029, ƞ2 = 0.517] but not in the sensory region (Fig. 10). Responses to swallows in the motor cortex were higher 4–7 s after swallowing onset (means = 0.4755, SD = 1.96) than 14–17 s after swallowing onset (means = −1.86, SD = 2.267) (Fig. 10).
Fig. 10.
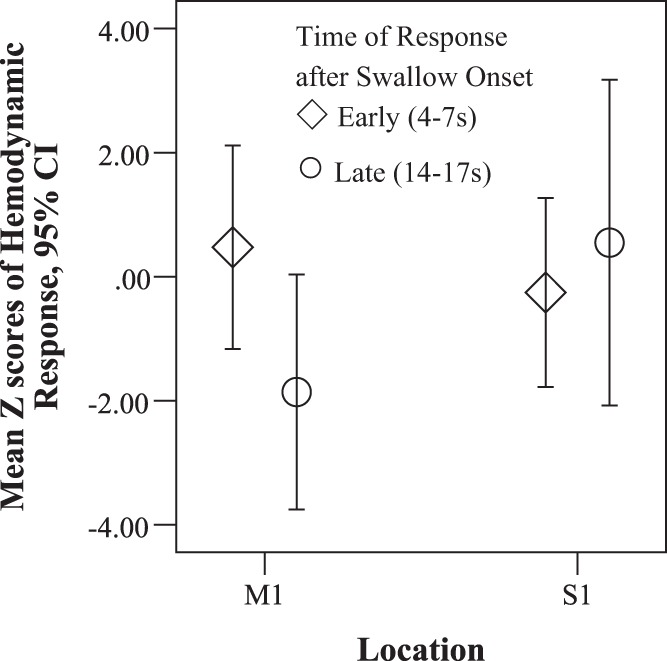
Mean peak Z scores with 95% confidence intervals (CI) of responses in oxygenated hemoglobin to swallowing in the motor (M1) and sensory (S1) regions for early responses between 4 and 7 s (diamonds) and late responses between 14 and 17 s (circles) after swallowing onset.
A three-way interaction from the same repeated-measures ANOVA showed a significant interaction between condition (swallows during stimulations, between stimulations, or during sham), hemisphere, and location [F(2,14) = 4.655, P = 0.028, ƞ2 = 0.399]. A simple two-way interaction between condition and location was statistically significant in the right hemisphere [F(2,14) = 5.598, P = 0.016, ƞ2 = 0.444] but not in the left hemisphere. The main effect of condition in the right hemisphere was significant in the motor region [F(2,14) = 8.633, P = 0.004, ƞ2 = 0.552] but not in the sensory area. In post hoc comparisons between conditions in the right motor region, the differences between the peak Z scores to swallows in the sham condition (means = −5.25, SD = 4.41) and swallows during stimulation (means = 1.39, SD = 3.64) approached statistical significance (P = 0.068), as did the difference between peak Z scores for swallows between stimulations (means = 2.12, SD = 3.49) and those occurring in the sham condition (P = 0.057). Based on these results, lower Z scores occurred in swallows in the sham condition than to swallows during and between stimulations specific to the right motor area (Fig. 9), whereas in other regions there was no appreciable differences between conditions.
Another statistically significant three-way interaction was found on the same repeated-measures ANOVA, comparing peak Z scores for swallows between stimulation condition, time of responses, and hemisphere [F(2,14) = 8.079, P = 0.005, ƞ2 = 0.536]. The simple two-way interaction between swallows during or between stimulation and during sham conditions and time of responses (early or late) approached statistical significance in the left hemisphere [F(2,14) = 3.46, P = 0.06, partial ƞ2 = 0.331] and was not significant in the right hemisphere. Simple one-way ANOVAs were significant for stimulation condition in left hemisphere for the early response [F(2,14) = 6.455, P = 0.01, ƞ2 = 0.48] and late response [F(2,14) = 10.626, P = 0.002, ƞ2 = 0.603; Fig. 11]. Post hoc comparisons between peak Z scores for swallows in the sham condition and swallows between stimulations approached statistical significance for the early left response (P = 0.06). Other post hoc comparisons between Z scores on swallows in the different conditions showed that late responses in the left region differed (P = 0.004), with higher responses to swallows during stimulation (means = 0.479, SD = 1.966) and between stimulations (means = 2.47, SD = 5.87) than during the sham condition (means = −4.81, SD = 2.78). However, no differences in Z scores for swallows during and between stimulation were found. Overall, Z scores for early and late responses during swallows were consistently lower in the sham condition than when swallows occurred during and between stimulations in the left hemisphere with a similar trend in the right hemisphere (Fig. 11). This suggests that during stimulation conditions, responses to swallows were potentiated by the occurrence of stimulation over 20 min regardless of whether stimulation occurred during or between swallows during that time.
Fig. 11.
Mean peak Z scores with 95% confidence intervals (CI) of responses in oxygenated hemoglobin to swallowing are shown on the y-axis, with the following conditions on the x-axis: Sham, swallowing between stimulation periods (Swallow stim off), and swallowing during stimulation periods (Swallow stim on). The timing of responses occurred 4–7 s (diamonds) and 14–17 s (circles) after swallowing onset. The data were divided into separate graphs for the right (RIGHT) and left (LEFT) hemispheres.
DISCUSSION
This study demonstrated in study 1 that vibration on the neck over the thyroid cartilage affected tissues inside the larynx by changing the fundamental frequency of the voice. This supported our first hypothesis that vibration over the thyroid lamina could activate mechanoreceptors inside the larynx that are innervated by the internal branch of the superior laryngeal nerve (Davis and Nail 1987). Our second hypothesis was that intermittent periods of vibration would increase spontaneous swallowing. This was supported by the results of study 2 and suggests that the mechanoreceptors excited the central pattern generator for swallowing in the brain stem in the healthy volunteers. We determined that vibration at 70 and 150 Hz both increased spontaneous swallowing over sham conditions that did not contain any stimulation for 20 min in these participants. Thus vibration likely potentiated activation of the central pattern generator in the brain stem for swallowing. Electrical stimulation of the internal branch of the superior laryngeal nerve in cats, rabbits, and dogs have been shown to induce fictive swallowing (Chi-Fishman et al. 1994; Doty 1951). The optimal rates of vibration we found for increasing spontaneous swallowing in humans (between 70 and 150 Hz) were higher than the rates of electrical superior laryngeal nerve stimulation in rats, which were between 5 and 50 Hz with a shorter latency to swallowing induction at higher rates of electrical stimulation (Kitagawa et al. 2009). Perhaps because of the need for vibration to penetrate the thyroid cartilage and the tissues attached to it, higher rates of vibration than electrical nerve stimulation in animals were needed to induce swallowing increases in humans. Likely, some damping of tissue vibration occurred during transmission from the neck surface to the tissues inside the larynx, reducing both the frequency and amplitude of vibration to the mechanoreceptors inside the larynx.
Our third hypothesis was that during epochs of vibratory stimulation cortical activation would increase in the swallowing network on fNIRS recordings in healthy adults. Therefore, it was unexpected that the mean level of oxygenated hemoglobin decreased in cortical pre- and postcentral regions during periods of vibratory stimulation in comparison with comparable epochs during the sham condition. This decrease in cortical activation occurred during stimulation epochs despite an increase in the frequency of spontaneous swallowing that occurred throughout the 20-min stimulation conditions in comparison with the 20-min sham conditions.
When we examined our fourth hypothesis that vibratory stimulation would enhance cortical responses to swallows regardless of whether swallows occurred during stimulation or between stimulation periods during 20-min stimulation conditions in comparison with sham conditions, the results were as expected. The peak Z scores of event-related levels of oxygenated hemoglobin for spontaneous swallows were increased both during vibratory stimulation and between periods of vibration in comparison with the swallows that occurred during sham conditions without any stimulation for 20 min.
The differences in the effects of vibration on the frequency of spontaneous swallowing and the decreases in overall cortical levels of activation suggest that different components of the swallowing system may be affected differently by vibration. Vibration increased spontaneous swallowing during conditions when vibration occurred in comparison with the sham condition without any stimulation, indicating that vibration of the laryngeal afferents may have increased swallowing at the level of the brainstem similar to the effects of electrical stimulation of afferents in the superior laryngeal nerve in animals. The suppression of cortical activation during 10-s stimulation epochs did not vary by stimulation frequency or cortical location suggesting that it was a general depression of the cortex due to vibration. However, this decrease in cortical activation during stimulation epochs did not alter the effects of cortical activation for spontaneous swallows as the peaks of event-related activity for swallowing were increased equally both during stimulation epochs and between stimulation epochs. Thus the cortical suppression during vibration was not associated with a reduced cortical activation for swallowing, rather the opposite. Peak event-related activity was increased for swallow events when the swallows occurred during or between stimulation epochs as compared with the sham condition with no stimulation for 20 min.
Our results might suggest that vibration of the laryngeal tissues has different effects on different cortical and brain stem components of the swallowing system and their interaction during spontaneous swallowing. Although vibration had an overall suppression effect on cortical activity, it did not reduce event-related hemodynamic responses to swallowing and was associated with increased frequency of spontaneous swallowing. In fact, swallows were accompanied by increased hemodynamic responses in the cortex when they occurred during 20-min periods with intermittent stimulation in comparison with sham periods with no stimulation. Even though cortical activity was reduced during vibration, the cortical activation during spontaneous swallows was greater during 20-min periods containing epochs of vibration. Thus not only did the generation of spontaneous swallowing increase in frequency, but the hemodynamic responses during swallowing were enhanced in the cortex by periods of intermittent vibration.
Two cortical responses were found in association with spontaneous swallows, one peaking between 4 and 7 s and a second response between 14 and 17 s. Responses were higher from 4 to 7 s than from 14 to 17 s after swallow onsets in the motor regions (Fig. 10). The heightened early motor response suggests that swallows combined with stimulation upregulated cortical activation during the execution of swallowing. The early response was in the time period that a cortical response to a swallow would be expected based on the study of Birn et al. (1999). The second response was much later peaking between 14 to 17 and likely onset around 10 s after a swallow onset. This may not be related to swallowing execution but to consequences that follow a swallow. Furthermore, both the early and late cortical responses to swallows were significantly higher when swallows occurred either during vibration or between vibrations than during sham conditions (Figs. 9 and 11). This suggests that stimulation potentiated activation in the cortical swallowing network for both types of responses to spontaneous swallows throughout the 20 min conditions and vibration was equally potentiating whether vibration occurred during or between swallows (Figs. 9 and 11). Thus intermittent vibration can have a potentiating effect on the swallowing cortical network over 20 min whether or not vibration is present during a swallow. Furthermore, the potentiation was greater in the motor regions than the in the postcentral regions in comparison with the sham condition (Fig. 9) particularly for the early responses between 4 and 7 s (Fig. 10). The heightened early motor responses to swallows occurring both during and between 10-s stimulation periods suggests that stimulation upregulated cortical activation for the motor execution of swallows throughout the 20-min intermittent stimulation condition.
The origin of the early and late responses cannot be distinguished based on the results of this study as both were similarly enhanced by intermittent vibration over 20 min in comparison with 20 min without stimulation. The time of the late response in this study is similar to the interval when the level of oxygenated hemoglobin increased in response to a sour taste in a previous study (Mulheren et al., 2016). There we posited that the late response might be a sensory response; however, here the early and late responses showed similar increases during spontaneous swallowing during intermittent vibration. This may indicate that the early and late responses are controlled by similar components in the swallowing system.
Previous studies have indicated that both hemispheres are active during spontaneous swallowing. Similar amplitudes of hemodynamic response in the left and right hemispheres have been reported (Dziewas et al. 2003; Martin et al. 2001), and overall similar patterns were seen on the left and right sides when stimulation accompanied swallowing in this study (Figs. 9 and 11). Here vibration combined with swallowing seemed to equally affect both hemispheres to a similar degree. Perhaps in patients with lesions in one hemisphere, vibration might upregulate the swallowing network in both the injured and the noninjured hemispheres.
Limitations.
This study focused on swallowing in healthy participants to determine the mechanisms that may underlie the upregulation of swallowing by vibration. Although the results indicated that vibration effectively increased swallowing frequency and cortical activation during swallows in these healthy volunteers, the findings may not be generalizable to a larger sample or to patients with disorders of swallowing. Due to evidence of individual variability in patterns of cortical activation during swallowing (Hamdy et al. 1999), further studies are needed to determine patterns of hemodynamic responses to vibratory stimulation in patients with dysphagia. Additionally, it needs to be determined if stimulation that increases the frequency of swallowing could facilitate the return of volitional swallowing or prevent deconditioning of spontaneous swallowing in patients unable to swallow. Studies are needed to determine if vibration may be applicable to patients with different anatomical or physiological swallowing impairments.
Instrumentation with fNIRS is limited to measurement of changes in cortical hemoglobin oxygenation at a depth of ~3 cm below the scalp. Therefore, we could not measure changes in subcortical portions of the swallowing network. fMRI studies are needed to identify which components of the cortical and subcortical systems are modulated by vibration during spontaneous swallowing.
Conclusion.
The results of this study indicated that vibratory stimulation on the surface of the neck overlying the larynx can penetrate to the laryngeal tissues that contain mechanoreceptors innervated by the internal branch of the superior laryngeal nerve to upregulate swallowing. The rate of swallowing was selectively increased from sham during 20-min conditions containing intermittent periods of 70- and 150-Hz vibratory stimulation. Although cortical activity was suppressed during the blocks of vibratory stimulation, the swallowing system was not suppressed. Rather spontaneous swallows were increased during 20-min intervals containing intermittent 10-s epochs of vibration, and the event-related averages of cortical activity during swallows increased throughout the 20 min that intermittent epochs of vibration occurred in comparison with sham conditions without any vibration for 20 min. Two hemodynamic responses were associated with swallows in the motor cortex in each hemisphere, an early peak between 4 and 7 s likely due to cortical responses associated with swallows as well as a late response between 14 and 17 s. Both were increased in amplitude throughout 20 min containing intermittent epochs of vibration in comparison with sham condition when no vibratory stimulation occurred for 20 min. Thus cortical activations for swallowing were enhanced by intermittent vibration despite a reduction in mean cortical activity during vibration. This suggests that vibratory stimulation of laryngeal tissues, which are innervated by the superior laryngeal nerve, has a facilitatory effect on both the elicitation of swallows as well as cortical responses to swallows in the motor area. As the swallowing system was upregulated throughout 20-min periods containing short epochs of vibration, possibly vibratory stimulation may have the potential to assist with treatment of patients with dysphagia by both increasing spontaneous swallowing and enhancing cortical activation in motor areas involved in the cortical control of swallowing.
GRANTS
The study was supported by James Madison University Grant JMU#550443 (to C. L. Ludlow) and Passy Muir.
DISCLOSURES
Partial support for this study and the vibrotactile devices were provided by Passy Muir, Inc. under an agreement between Passy Muir and James Madison University. C. L. Ludlow is an inventor on patents owned by National Institutes of Health (NIH) that pertain to the use of vibrotactile stimulation for treatment of dysphagia. Passy Muir has licensed these patents from NIH. C. L. Ludlow has previously received consultation payments from Passy Muir and Rymo Medical, Inc. for the development of vibrotactile stimulation for retraining swallowing postbrain injury. Neither company has had any input on the conduct of the research or this manuscript.
AUTHOR CONTRIBUTIONS
C.L.L. conceived and designed research; R.W.M. and C.L.L. performed experiments; R.W.M. and C.L.L. analyzed data; R.W.M. and C.L.L. interpreted results of experiments; R.W.M. and C.L.L. prepared figures; R.W.M. and C.L.L. drafted manuscript; R.W.M. and C.L.L. edited and revised manuscript; R.W.M. and C.L.L. approved final version of manuscript.
REFERENCES
- Birn RM, Bandettini PA, Cox RW, Shaker R. Event-related fMRI of tasks involving brief motion. Hum Brain Mapp 7: 106–114, 1999. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas D, Dubb J, Huppert T. Hemodynamic Evoked Response (HomER 2) (Software). http://homer-fnirs.org/. Boston, MA: Massachusetts General Hospital. [2012]. [Google Scholar]
- Boas DA, Gaudette T, Strangman G, Cheng X, Marota JJ, Mandeville JB. The accuracy of near infrared spectroscopy and imaging during focal changes in cerebral hemodynamics. Neuroimage 13: 76–90, 2001. doi: 10.1006/nimg.2000.0674. [DOI] [PubMed] [Google Scholar]
- Car A, Jean A, Roman C. A pontine primary relay for ascending projections of the superior laryngeal nerve. Exp Brain Res 22: 197–210, 1975. doi: 10.1007/BF00237689. [DOI] [PubMed] [Google Scholar]
- Chi-Fishman G, Capra NF, McCall GN. Thermomechanical facilitation of swallowing evoked by electrical nerve stimulation in cats. Dysphagia 9: 149–155, 1994. doi: 10.1007/BF00341258. [DOI] [PubMed] [Google Scholar]
- Cui X, Bray S, Reiss AL. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. Neuroimage 49: 3039–3046, 2010. doi: 10.1016/j.neuroimage.2009.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PJ, Nail BS. Quantitative analysis of laryngeal mechanosensitivity in the cat and rabbit. J Physiol 388: 467–485, 1987. doi: 10.1113/jphysiol.1987.sp016625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Dunn AK, Andermann ML, Ulbert I, Boas DA, Dale AM. Coupling of total hemoglobin concentration, oxygenation, and neural activity in rat somatosensory cortex. Neuron 39: 353–359, 2003. doi: 10.1016/S0896-6273(03)00403-3. [DOI] [PubMed] [Google Scholar]
- Doty RW. Influence of stimulus pattern on reflex deglutition. Am J Physiol 166: 142–158, 1951. [DOI] [PubMed] [Google Scholar]
- Doty RW, Richmond WH, Storey AT. Effect of medullary lesions on coordination of deglutition. Exp Neurol 17: 91–106, 1967. doi: 10.1016/0014-4886(67)90125-2. [DOI] [PubMed] [Google Scholar]
- Dziewas R, Sörös P, Ishii R, Chau W, Henningsen H, Ringelstein EB, Knecht S, Pantev C. Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. Neuroimage 20: 135–144, 2003. doi: 10.1016/S1053-8119(03)00285-4. [DOI] [PubMed] [Google Scholar]
- Gatto AR, Cola PC, Silva RG, Spadotto AA, Ribeiro PW, Schelp AO, Carvalho LR, Henry MA. Sour taste and cold temperature in the oral phase of swallowing in patients after stroke. CoDAS 25: 164–168, 2013. doi: 10.1590/S2317-17822013000200012. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Jilani S, Price V, Parker C, Hall N, Power M. Modulation of human swallowing behaviour by thermal and chemical stimulation in health and after brain injury. Neurogastroenterol Motil 15: 69–77, 2003. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol Gastrointestinal Physiol 277: G219–G225, 1999. [DOI] [PubMed] [Google Scholar]
- Hegyi Szynkiewicz S, Mulheren RW, Palmore KW, O’Donoghue CR, Ludlow CL. Using devices to upregulate nonnutritive swallowing in typically developing infants. J Appl Physiol (1985) 121: 831–837, 2016. doi: 10.1152/japplphysiol.00797.2015. [DOI] [PubMed] [Google Scholar]
- Jafari S, Prince RA, Kim DY, Paydarfar D. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol 550: 287–304, 2003. doi: 10.1113/jphysiol.2003.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanal E, Barkovich AJ, Bell C, Borgstede JP, Bradley WG, Froelich JW, Gimbel JR, Gosbee JW, Kuhni-Kaminski E, Larson PA, Lester JW, Nyenhuis J, Schaefer DJ, Sebek EA, Weinreb J, Wilkoff BL, Woods TO, Lucey L, Hernandez D; Expert Panel on MR Safety . ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging 37: 501–530, 2013. [DOI] [PubMed] [Google Scholar]
- Kitagawa J, Nakagawa K, Hasegawa M, Iwakami T, Shingai T, Yamada Y, Iwata K. Facilitation of reflex swallowing from the pharynx and larynx. J Oral Sci 51: 167–171, 2009. doi: 10.2334/josnusd.51.167. [DOI] [PubMed] [Google Scholar]
- Klahn MS, Perlman AL. Temporal and durational patterns associating respiration and swallowing. Dysphagia 14: 131–138, 1999. doi: 10.1007/PL00009594. [DOI] [PubMed] [Google Scholar]
- Kosztyła-Hojna B, Moskal D, Rogowski M, Falkowski D, Kasperuk J, Othman J. [The usage of modern physioterapeutic methods of rehabilitation in treatment of chosen kinds of dysphonia]. Otolaryngol Pol 66: 328–336, 2012. doi: 10.1016/j.otpol.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Lowell SY, Poletto CJ, Knorr-Chung BR, Reynolds RC, Simonyan K, Ludlow CL. Sensory stimulation activates both motor and sensory components of the swallowing system. Neuroimage 42: 285–295, 2008. doi: 10.1016/j.neuroimage.2008.04.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science 272: 551–554, 1996. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- Månsson I, Sandberg N. Oro-pharyngeal sensitivity and elicitation of swallowing in man. Acta Otolaryngol 79: 140–145, 1975. doi: 10.3109/00016487509124666. [DOI] [PubMed] [Google Scholar]
- Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol 85: 938–950, 2001. [DOI] [PubMed] [Google Scholar]
- Martin-Harris B, Brodsky MB, Michel Y, Ford CL, Walters B, Heffner J. Breathing and swallowing dynamics across the adult lifespan. Arch Otolaryngol Head Neck Surg 131: 762–770, 2005. doi: 10.1001/archotol.131.9.762. [DOI] [PubMed] [Google Scholar]
- Miller FR, Sherrington CS. Some observations on the bucco-pharyngeal state of reflex deglutition in the cat. Exp Physiol 9: 147–186, 1915. doi: 10.1113/expphysiol.1915.sp000201. [DOI] [Google Scholar]
- Mulheren RW, Kamarunas E, Ludlow CL. Sour taste increases swallowing and prolongs hemodymamic responses in the cortical swallowing network. J Neurophysiol 116: 2033–2042, 2016. doi: 10.1152/jn.00130.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Quaresima V, Bisconti S, Ferrari M. A brief review on the use of functional near-infrared spectroscopy (fNIRS) for language imaging studies in human newborns and adults. Brain Lang 121: 79–89, 2012. doi: 10.1016/j.bandl.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Sörös P, Lalone E, Smith R, Stevens T, Theurer J, Menon RS, Martin RE. Functional MRI of oropharyngeal air-pulse stimulation. Neuroscience 153: 1300–1308, 2008. doi: 10.1016/j.neuroscience.2008.02.079. [DOI] [PubMed] [Google Scholar]
- Sumi T. The activity of brain-stem respiratory neurons and spinal respiratory motoneurons during swallowing. J Neurophysiol 26: 466–477, 1963. [DOI] [PubMed] [Google Scholar]
- Teismann IK, Steinstraeter O, Stoeckigt K, Suntrup S, Wollbrink A, Pantev C, Dziewas R. Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci 8: 62, 2007. doi: 10.1186/1471-2202-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurer JA, Bihari F, Barr AM, Martin RE. Oropharyngeal stimulation with air-pulse trains increases swallowing frequency in healthy adults. Dysphagia 20: 254–260, 2005. doi: 10.1007/s00455-005-0021-1. [DOI] [PubMed] [Google Scholar]
- Theurer JA, Czachorowski KA, Martin LP, Martin RE. Effects of oropharyngeal air-pulse stimulation on swallowing in healthy older adults. Dysphagia 24: 302–313, 2009. doi: 10.1007/s00455-009-9207-2. [DOI] [PubMed] [Google Scholar]
- Villringer A, Chance B. Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci 20: 435–442, 1997. doi: 10.1016/S0166-2236(97)01132-6. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. The functional neuroanatomy of voluntary swallowing. Ann Neurol 46: 281–286, 1999. doi:. [DOI] [PubMed] [Google Scholar]








