A novel implantable lumbar epidural catheter-subcutaneous port system enables targeted drug delivery and induction of temporary paraplegia in awake, behaving nonhuman primates. Three macaques displayed loss of voluntary leg movement for 60–90 min after injection of lidocaine, followed by full recovery. This technique for the first time will enable ethical live recording from the proximal central nervous system during the acute onset, maintenance, and resolution of paraplegia.
Keywords: paralysis, primate, spinal cord injury, monkey, interface
Abstract
Lower limb paralysis from spinal cord injury (SCI) or neurological disease carries a poor prognosis for recovery and remains a large societal burden. Neurophysiological and neuroprosthetic research have the potential to improve quality of life for these patients; however, the lack of an ethical and sustainable nonhuman primate model for paraplegia hinders their advancement. Therefore, our multidisciplinary team developed a way to induce temporary paralysis in awake behaving macaques by creating a fully implantable lumbar epidural catheter-subcutaneous port system that enables easy and reliable targeted drug delivery for sensorimotor blockade. During treadmill walking, aliquots of 1.5% lidocaine with 1:200,000 epinephrine were percutaneously injected into the ports of three rhesus macaques while surface electromyography (EMG) recorded muscle activity from their quadriceps and gastrocnemii. Diminution of EMG amplitude, loss of voluntary leg movement, and inability to bear weight were achieved for 60–90 min in each animal, followed by a complete recovery of function. The monkeys remained alert and cooperative during the paralysis trials and continued to take food rewards, and the ports remained functional after several months. This technique will enable recording from the cortex and/or spinal cord in awake behaving nonhuman primates during the onset, maintenance, and resolution of paraplegia for the first time, thus opening the door to answering basic neurophysiological questions about the acute neurological response to spinal cord injury and recovery. It will also negate the need to permanently injure otherwise high-value research animals for certain experimental paradigms aimed at developing and testing neural interface decoding algorithms for patients with lower extremity dysfunction.
NEW & NOTEWORTHY A novel implantable lumbar epidural catheter-subcutaneous port system enables targeted drug delivery and induction of temporary paraplegia in awake, behaving nonhuman primates. Three macaques displayed loss of voluntary leg movement for 60–90 min after injection of lidocaine with epinephrine, followed by a full recovery. This technique for the first time will enable ethical live recording from the proximal central nervous system during the acute onset, maintenance, and resolution of paraplegia.
paraplegia from spinal cord injury (SCI) or neurological disease carries a poor prognosis for recovery, even today (Waters et al. 1992). The prevalence of SCI in the United States alone is ~240,000–337,000 (National Spinal Cord Injury Statistical Center 2015) with an estimated total annual economic burden of $18.5 billion (Berkowitz 1998; Gooch et al. 2017). Neurophysiological research, neural interfaces, and novel rehabilitation techniques have the potential to drastically improve the quality of life for patients suffering from lower limb paralysis (Krucoff et al. 2016). Neurally controlled exoskeletons (Banala et al. 2006; Costa and Caldwell 2006; Dollar and Herr 2008; Donati et al. 2016; Eliseyev et al. 2014), wheelchairs (Rajangam et al. 2016), electrical stimulation devices (Bajd et al. 1999; Capogrosso et al. 2016; Minassian et al. 2012), and prostheses (Hargrove et al. 2009; Martinez-Villalpando et al. 2008; Windrich et al. 2016) have already displayed excellent potential. However, the advancement of these technologies and the ability to study the proximal neurophysiological response to acute paraplegia is hindered by the lack of an ethical and sustainable model for paralysis in nonhuman primates.
Monkeys provide essential advantages over lower animal models (e.g., rats) for developing neural interfaces and thus cannot be replaced (Courtine et al. 2005, 2007; Lemon 2008; Lemon and Griffiths 2005). Unfortunately, to study paralysis in monkeys currently means performing cord transections (Capogrosso et al. 2016; Courtine et al. 2005; Craggs 1975; Freund et al. 2006), lesioning nerve roots (rhizotomies) (Knapp et al. 1963), or inducing compression/ischemia (Doppman et al. 1973; Ramsey and Doppman 1973), all of which cause irreversible neurological deficits (Capogrosso et al. 2016; Courtine et al. 2005; Craggs 1975; Freund et al. 2006). These types of lesions often come with the undesired effects of disrupting autonomic functions including bowel and bladder control (Freund et al. 2006; Lawrence and Kuypers 1968) and may be followed by self-mutilation due to persistent lack of sensation in an extremity, thus raising both ethical and cost-related concerns about these procedures (Courtine et al. 2007). These issues are especially important in high-value (both in monetary cost and time invested) animals with chronic implants that otherwise might be able to generate years of data (Krüger et al. 2010; Nicolelis et al. 2003; Schwarz et al. 2014). Therefore, our group set out to develop an ethical, temporary, and sustainable model for inducing paraplegia in awake, behaving macaques such that the proximal nervous system can be studied and neural interfaces can be developed without inducing a permanent neurological deficit.
In common medical practice, local anesthetic agents are used as part of peripheral, epidural, and spinal nerve blocks to temporarily disrupt nerve conduction so that otherwise painful procedures can be completed on awake patients. However, these procedures require a cooperative patient and careful triangulation, therefore making it nearly impossible to perform in a laboratory setting on an awake animal. To address this issue, Pohlmeyer et al. developed fully implantable nerve cuffs to enable easy percutaneous delivery of drug directly to the nerves of the forearm through a subcutaneous port (Pohlmeyer et al. 2009). This group then used their method to demonstrate brain-controlled functional electrical stimulation (FES) of the arm to recapitulate grasp in the temporarily paralyzed hands of macaques (Ethier et al. 2012). While the nerve-cuff method might be translatable to the legs to simulate a peripheral nerve injury (e.g., sciatic, femoral, or obturator nerve), it would be very difficult to achieve complete paraplegia using this technique due to the complexity of the nervous innervation of the legs, especially considering the retroperitoneal location of the lumbar plexus and hip flexor muscles and nerves. With this in mind, our multidisciplinary group adapted the idea of a fully implantable port system to target the lumbar epidural space with the aim of anesthetizing enough exiting nerve roots to effectively produce bilateral lower extremity paraplegia and simulate the physiological effect of a lumbothoracic SCI: the blockade of nerve transmission. In doing so, we have developed the first temporary paraplegia model in nonhuman primates.
MATERIALS AND METHODS
Study design.
Three adult rhesus macaques, two male (monkey M, 11.8 kg, age 14 yr; and monkey R, 11.4 kg, age 8 yr) and one female (monkey N, 6.6 kg, age 16 yr), were utilized for this study. All animal procedures were performed in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals and were approved by the Duke University Institutional Animal Care and Use Committee (IACUC). Each animal was trained to walk bipedally on a treadmill before implantation, and they were acclimated to protective vests to cover the future incision site.
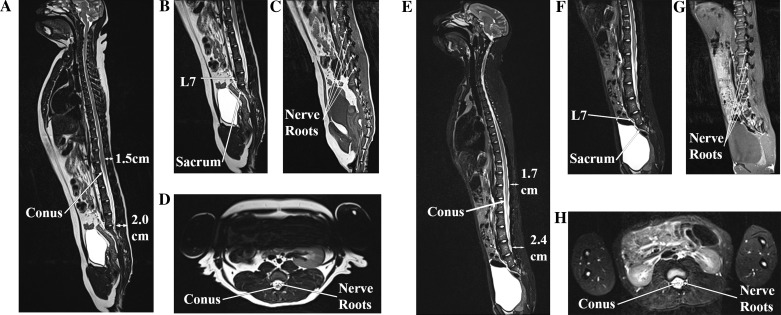
To plan the epidural catheter placement, magnetic resonance images (MRI) were obtained of one male (monkey R, age 6 yr at the time of imaging) and one female (monkey J, 8.8 kg, age 7 yr) rhesus macaque (Fig. 1). The epidural space was noted to be ~1.5–2.4 cm deep to the skin edge depending on the level chosen and the size of the monkey (the more cephalad the level, the shallower the depth) (Fig. 1, A and E). The conus medullaris was found to be located around L3–L4, given that rhesus macaques have seven lumbar vertebrae (Hartman and Straus 1933; Thomas and Combs 1965) (Fig. 1, A and E). The trajectory of the spinous processes was noted to be similar to human anatomy (pointing caudally and dorsally as they leave the laminae and approach the skin), and the nerve roots could be seen exiting the neural foraminae just caudal to their respective pedicles (also similar to human anatomy) (Fig. 1, C and G).
Fig. 1.
MRI of a female (A–D) and male (E–H) rhesus macaque. A and E: sagittal T2 sequences of the entire spinal column. The conus medullaris is seen at L3–L4, and the depth of the lumbar epidural space ranges from 1.5 to 2.4 cm. B and F: sagittal lumbar T2 sequences showing the transition from lumbar to sacral vertebrae. C and G: parasagittal (just off midline) lumbar T1 sequences showing the nerve roots exiting out their respective neural foraminae. D and H: axial lumbar T2 sequences showing the conus medullaris and surrounding nerve roots (which become the cauda equina inferiorly).
Surgical implantation of epidural catheter and port.
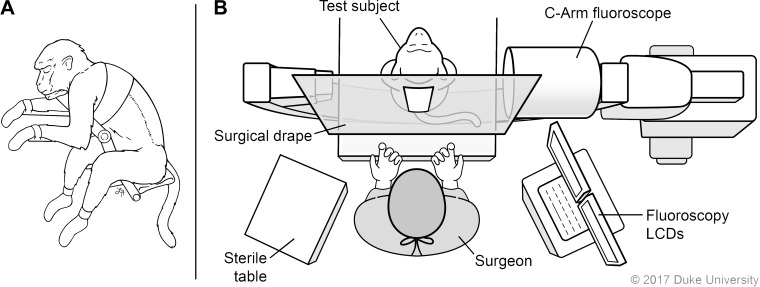
The day before surgery the monkey’s back was shaved with an electrical clipper. On the day of implantation, the animal underwent general anesthesia with endotracheal tube placement by our veterinary staff. Induction was performed using ketamine [3 mg/kg intramuscular (IM)] and Dexdomitor (0.15 mg/kg IM), and, once intubated, isoflurane inhalant was titrated between 0.5 and 1.5% to maintain sedation. An intravenous (IV) catheter was placed in the saphenous vein. The animal was positioned upright in a specially designed seat to place its back in flexion and maximize the intralaminar distance (the posterior target for access to the epidural space) (Fig. 2A).
Fig. 2.
Surgical setup for implantation of epidural catheter-port. A: the monkey is positioned upright and with back in flexion. B: the c-arm is placed under the sterile drape so images can be acquired during placement. LCD, liquid crystal display computer monitor. Illustrated by Lauren Halligan, MSMI; copyright Duke University; with permission under a CC BY-ND 4.0 license.
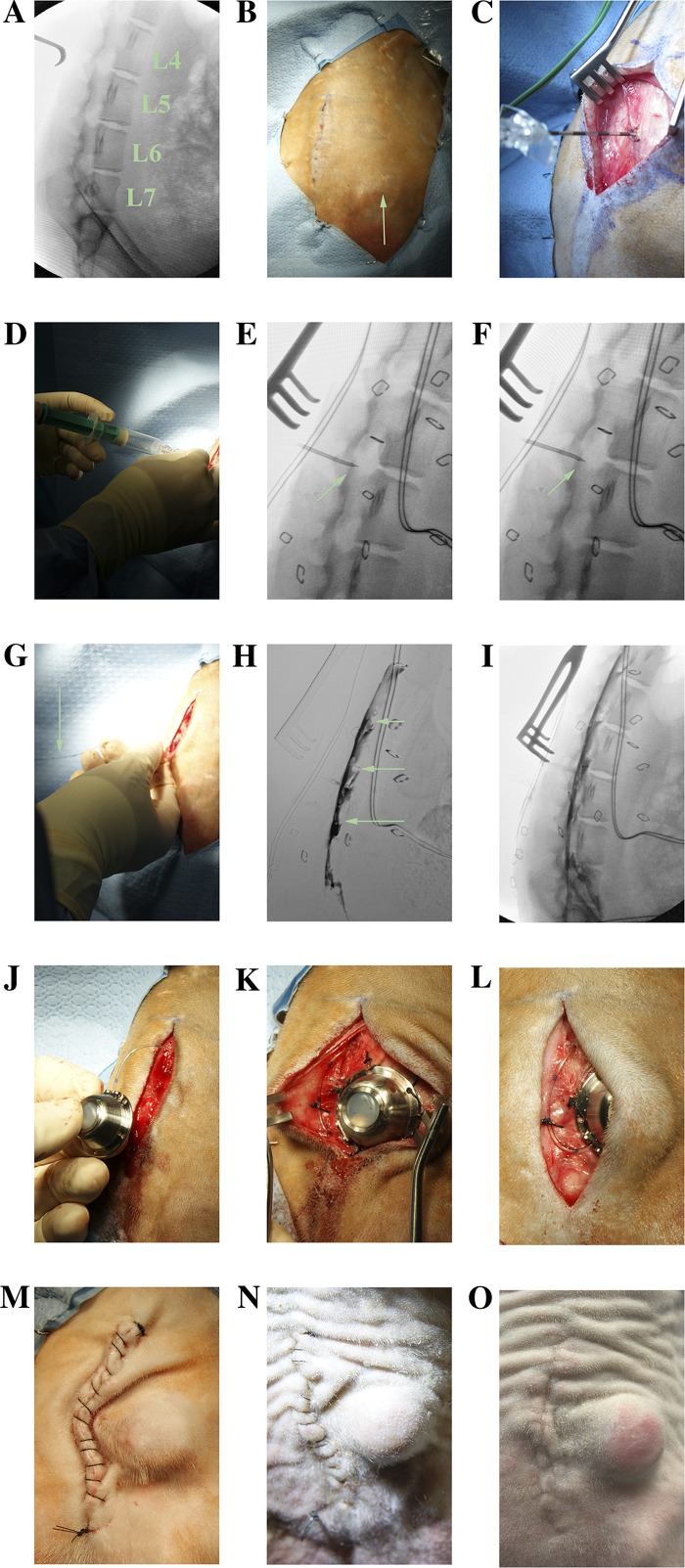
The surgical skin area was scrubbed with a chlorhexidine gluconate 4% solution (Scrub Care, CareFusion) and then prepped with a chlorhexidine gluconate 2% wt/vol and isopropyl alcohol 70% vol/vol stick (ChloraPrep, CareFusion). A surgical drape was applied over the fluoroscopy machine (c-arm), establishing a sterile field (Fig. 2B). A fluoroscopic X-ray was then obtained to identify the target level (Fig. 3A), and the planned incision was marked over the respective midline spinous processes (Fig. 3B). The location of the iliac crest was noted as a caudal border for the port placement (Fig. 3B).
Fig. 3.
Implantation of the catheter-port system. A: X-ray is used to identified the target level (L4–L5) for planning of the incision. B: incision planned on the midline spinous processes. The right iliac crest is seen (green arrow) and show be noted as an inferior margin for port placement. C: subcutaneous, suprafascial dissection (location of retractor tongs) is carried out to make a pocket for the port, then the Tuohy needle is carefully advanced until it can stay perpendicular without being held. D: loss of resistance technique. E: Tuohy bevel up (green arrow). F: Tuohy bevel down (green arrow). G: epidural catheter (green arrow) is advanced through the Tuohy needle. H: digitally subtracted fluoroscopic dye injection showing epidural spread of contrast and wrapping of exiting nerve roots (green arrows). I: nonsubtracted fluoroscopic X-ray showing location of dye relative to vertebral bodies. J: attachment of port. K and L: port and catheter are secured to the underlying fascia. M: incision is closed. N: healing incision at 2 wk, just before suture removal. O: incision completely healed at 2 mo.
Prior to incision, a dose of IV antibiotics was administered (cefazolin 22 mg/kg) to minimize skin flora. An incision was made over the midline spinous processes, and blunt dissection was carried out lateral to midline in the plane below the skin but above the fascia, thus creating a pocket for the port. An 18-gauge × 5 cm epidural Tuohy needle (Perifix, Braun Medical) was then advanced just under the spinous process of the target level (L4) aiming perpendicular or slightly cephalad to the spinal column with the bevel facing cephalad and carefully noting the depth. The L4–L5 interspace was chosen because L4 is the middle lumbar vertebra, and the goal was to anesthetize all lumbosacral levels. Also, this was the level just below the conus, so accidental penetration into the thecal sac would be less likely to result in a neurological injury. Once the Tuohy needle approached the target depth and could hold itself perpendicular to the spine (a sign that it was in the ligamentum flavum) (Fig. 3C), the stylet was removed and a loss of resistance syringe (Perifix, Braun Medical) was attached to the Tuohy needle. Resistance was assessed after each millimeter of careful further advancement, as loss of resistance signaled entry of the bevel into the epidural space (Fig. 3D).
Once the bevel of the Tuohy needle was in the epidural space (Fig. 3E), it was turned 180° to face caudally (Fig. 3F) (cephalad advancement of the epidural catheter was found to preferentially cause cephalad flow of anesthetic in one of our preliminary trials, whereas a caudal trajectory allowed for both caudal and cephalad spread of dye). Next, a 20-gauge closed tip epidural catheter (Perifix, Braun Medical) was advanced through the Tuohy needle (Fig. 3G) into the epidural space just far enough so that all the holes at the distal end of the catheter were in the epidural space (~2.0 cm past the tip of the Tuohy needle). Next, the Tuohy needle was removed and the catheter was gently aspirated to confirm lack of blood or cerebrospinal fluid (CSF).
After ensuring the aspiration remained dry, 2 ml of contrast dye (iopamidol 61%, organically bound iodine 30%, Isovue-300, Bracco Diagnostics) was injected to assess for epidural location and extent of spread (Fig. 3, H and I). Ideally the dye should cover all lumbar and sacral levels. In the second and third monkeys (monkey M and monkey R), an anterior-posterior fluoroscopic X-ray was also obtained to ensure bilateral spread of contrast. After confirmation, the catheter was cut to length and inserted into a sterile titanium injection port (TuBo Port, Access Technologies) (Fig. 3J). The port and catheter were secured with 2-0 silk sutures (Fig. 3, K and L), and a final confirmatory fluoroscopic X-ray with dye injection was obtained through the port to ensure there was no leak. The system was then flushed with preservative-free saline (sodium chloride 0.9%) and the subcutaneous area was copiously irrigated with bacitracin-infused saline (50,000 units bacitracin per 1 liter sodium chloride 0.9%). Finally, the subcutaneous layer was closed with 3-0 vicryl sutures, bringing the skin opening lateral to the port (to avoid tension on the suture line), and the skin was closed with a running 3-0 nylon suture (Fig. 3M). The incision was covered with bacitracin ointment, and then the surgical site was covered with a nonadherent dressing (Telfa, Covidien) and stapled to the skin to prevent access by the monkey, and the protective vest was replaced. The reversal agent atipamezole 1.5 mg/kg IM was given at the conclusion of the procedure. Postoperative analgesia was achieved using acetaminophen 120 mg per rectum before awakening and then 5–10 mg/kg orally every 8 h for a total of 3 days, as well as buprenorphine 0.005-0.01 mg/kg IM every 8 h for a total of three doses. The dressing was removed after 5–10 days, and the sutures were removed 14 days postoperatively (Fig. 3N). The port was flushed with at least 1 ml of heparin flush solution (100 USP units per 1 ml, PosiFlush, Becton Dickinson) daily for the first 3 days postoperatively, then every 3–7 days after that to prevent clotting. A 22-gauge noncoring Huber needle (Access Technologies) was used to access the port percutaneously each time.
Anesthetic injections and EMG recordings.
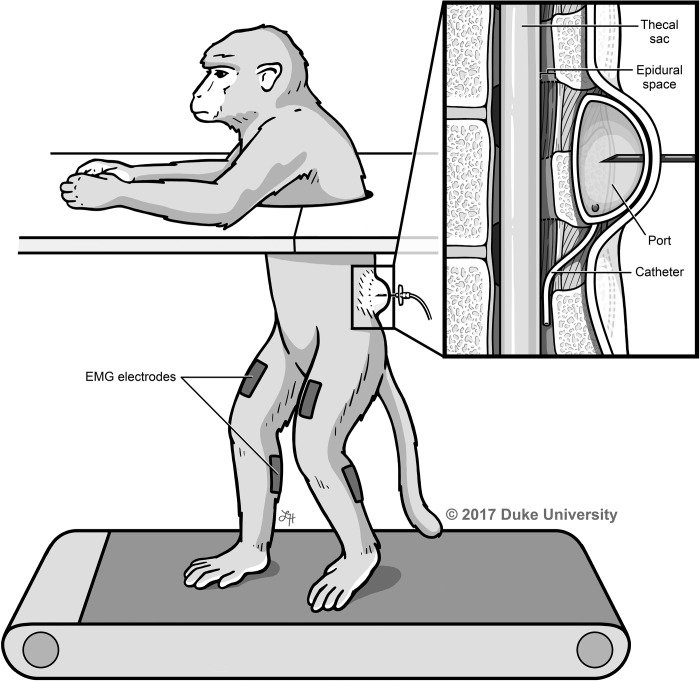
To quantify the paralytic effect of the anesthetic, the animals were placed in the treadmill setup and surface electromyography (EMG) electrodes (Bagnoli-8, Delsys) were secured to the quadriceps and calf muscles using Vet-Flex, Dura-Tech (Fig. 4).
Fig. 4.
Treadmill and EMG experimental setup. The monkey is placed on a treadmill with upper extremities separated from lower extremities, and surface EMGs are recorded from the quadriceps and gastrocnemius muscles while the port is injected with varying anesthetic doses. Illustrated by Lauren Halligan, MSMI; copyright Duke University; with permission under a CC BY-ND 4.0 license.
Prior to injection of anesthetic, EMGs were recorded for 5 min of normal walking to establish baseline levels of activation. Various doses (1–3 ml) of lidocaine 1–2% with and without epinephrine 1:200,000 was injected percutaneously into the port using a 22-gauge noncoring Huber needle (Access Technologies) to achieve motor blockade. The skin was cleaned with two to three antiseptic alcohol prep pads (isopropyl alcohol 70%, Allegiance, CardinalHealth) before each injection, and hair over the port site had to be periodically shaved with an electric clipper. The animal was observed in the treadmill setup until weight bearing ability had returned, and the port was flushed with heparinized saline at the conclusion of each experiment. These experiments were repeated no more frequently than every 72 h.
EMG analysis.
The purpose of these recordings was to establish objective evidence of motor blockade, so only a simple analysis was required. EMGs were sampled at 2,000 Hz, filtered from 20 to 500 Hz, and analyzed in MATLAB R2016a. Mean EMG amplitudes during 3-min intervals after injection were compared with their respective means before injection, and the mean amplitude of the pure noise signal was subtracted from the result such that a value of 1 represented the average EMG amplitude during baseline walking and 0 represented no activity. Standard error of the mean was used to calculate a 95% confidence interval for true mean estimation during each time interval after injection.
RESULTS
All three animals healed well from the catheter-port implantation and were able to have their sutures removed in 10–14 days (Fig. 3O). Each port continued to be patent, the longest of which was 4 mo. Successful bilateral lower extremity paraplegia was achieved in both monkeys with minimal movement in either leg and inability to bear weight for 60–90 min after injection of a total of 3 ml of 1–2% lidocaine with or without 1:200,000 epinephrine (Fig. 5, Supplemental Video; Supplemental Material for this article is available online at the Journal website.). The animals tolerated the experiments with minimal distress after the initial trials and eagerly took fruit rewards.
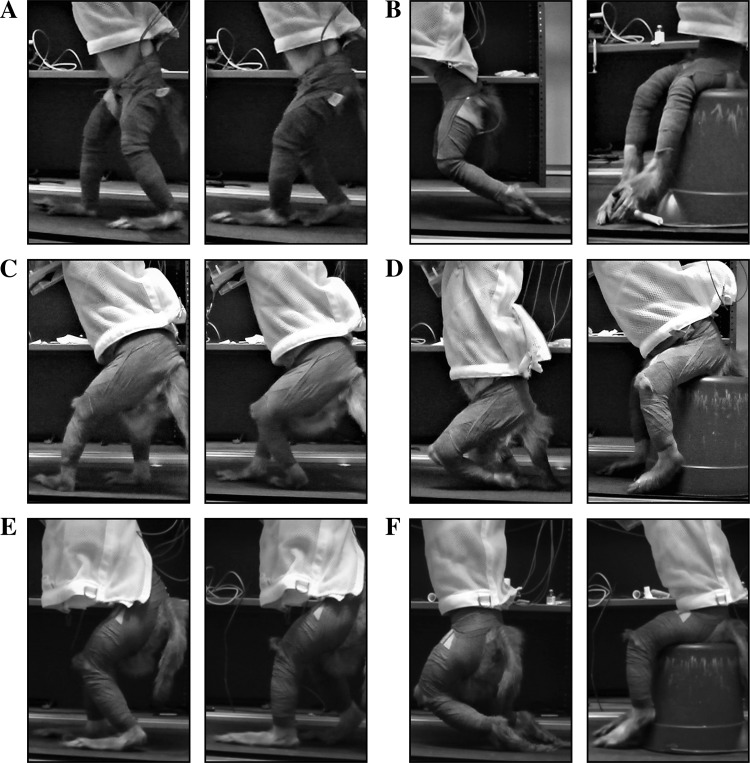
Fig. 5.
Treadmill walking pre- and postinjection, monkey N (A and B), monkey M (C and D), and monkey R (E and F). A, C, and E: walking before anesthetic injection. B, D, and F: no leg movement after injection. The animals required weight-bearing support to prevent exhaustion pending return of leg function.
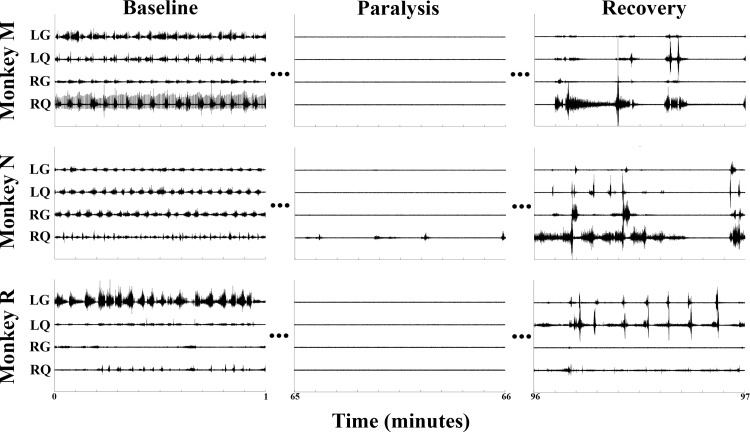
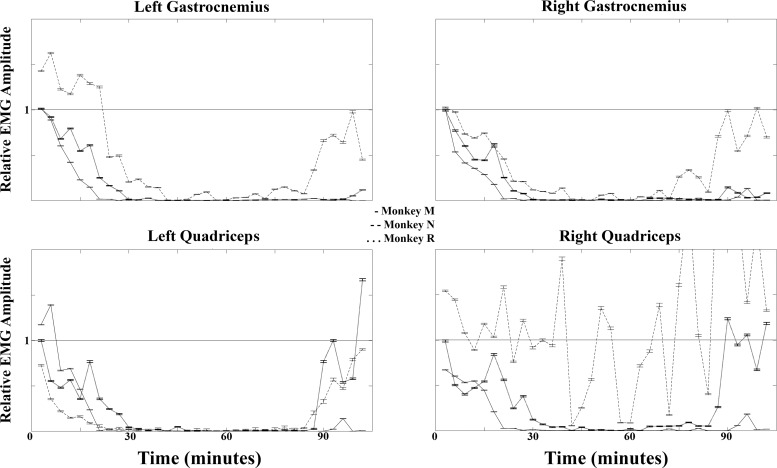
Figure 6 shows 1 min excerpts of EMG signals recorded during baseline walking, paralysis, and recovery periods from each animal. The recovery EMGs shown here represent the transition period back to a normal state of function as the lidocaine continued to wear off. After each experiment, normal cage behavior was observed without signs of distress or deficit (usually around 2–3 h after the initial injection). For clarity of presentation, a typical data set is presented in Figs. 6 and 7 that correlates with the photographs in Fig. 5 and the Supplemental Video. Repeated experiments consistently demonstrated similar results. While monkey N attained only partial motor block of the right quadriceps (Figs. 6 and 7), this did not affect the functional outcome of paraplegia, as that leg was still nonfunctional during the trial epoch (Fig. 5, Supplemental Video). The lack of complete block here was likely because the catheter tip was biases to the left at the level of the L5 root. Therefore, anesthetic was delivered to this nerve root and bilaterally as it spread both cephalad and caudally, but the right L5 nerve root had the least exposure to the anesthetic. This was avoided in the next animals (monkey M and monkey R) by obtaining an anterior-posterior fluoroscopic X-ray (in addition to lateral views) before closure to ensure bilateral spread of anesthetic at all levels. The maintenance of EMGs in this muscle group served as an internal control and proof of maintained effort by the animal. Therefore, the lack of any observed EMG signal in all muscle groups except the right quadriceps of monkey N is proof of complete motor blockade during the paralysis period. At no point was any upper extremity weakness observed.
Fig. 6.
EMGs. One-minute excerpts of raw EMG data from all 3 animals showing preinjection, postinjection paralysis, and postinjection recovery periods. LG, left gastrocnemius; LQ, left quadriceps; RG, right gastrocnemius; RQ, right quadriceps.
Fig. 7.
Average EMG amplitudes during 3-min intervals postinjection from each monkey relative to baseline walking ( = 1). Error bars are 95% confidence intervals.
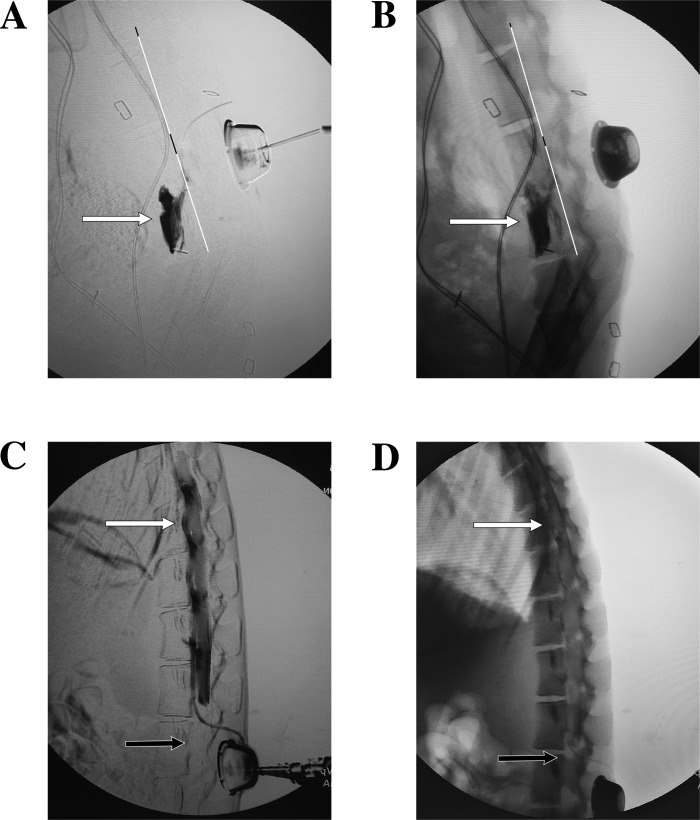
Radiopaque dye injection was found to be a reliable way to confirm the placement of the catheter and to anticipate the spread of anesthetic. Figure 8 shows two examples where radiopaque dye injection revealed nonideal spread of anesthetic, and the catheter had to be revised. The extent of cephalad and caudal spread of anesthetic was found to be volume dependent, and injections of 2 ml at a time were enough to achieve near complete or complete lumbar coverage with the catheters entering at the L4–L5 interlaminar space and aiming caudally. A repeat injection ~15 min later was found to be necessary to deliver a sufficient dose to achieve a complete motor blockade, likely because the repeat injection redelivered the dose to the same levels as opposed further spreading. In the experiments shown in Figs. 5–7 and the Supplemental Video, a total of 3 ml of 1.5% lidocaine with 1:200,000 epinephrine divided into two doses separated by 15 min achieved excellent results in monkey M and monkey N, whereas it took 4 ml split into three doses (given at 0, 15, and 25 min) to achieve complete paraplegia in monkey R. Generally, lidocaine 1–2% with or without epinephrine 1:200,000 was used and was successful in achieving similar levels of blockade. Increasing lidocaine concentration and addition of epinephrine appeared to both strengthen and prolong the motor blockade, but this was not rigorously examined as all concentrations within this range performed well, and laboratories that utilize this technique can vary and individualize anesthetic and dosing regimens to fit their needs.
Fig. 8.
Aberrant dye distributions. A and B: subtracted (A) and nonsubtracted (B) fluoroscopic X-rays showing dye leaving the L5–L6 neural foramen. Note the dye is collecting anterior to the dorsal aspect of the vertebral body (white line) and not in the epidural space, suggesting the catheter has followed the nerve root out that foramen. C and D: subtracted (C) and nonsubtracted (D) fluoroscopic X-rays showing the catheter in the ventral epidural space at L3; however, dye is noted to be spreading far more cephalad (white arrows) than caudally (black arrows). Compare these results with Fig. 3, H and I.
DISCUSSION
Here we describe the first known method for achieving temporary paraplegia in awake, behaving rhesus macaques via a fully implanted lumbar epidural catheter-port anesthetic delivery system. While epidural catheters have been used in rhesus macaques to provide peripartum analgesia (DeWeert et al. 1995), to study the pharmacokinetics of drug delivery (Katz et al. 1993; MacAllister et al. 2016; Thompson et al. 1986), and to model SCI in anesthetized animals (Nesathurai et al. 2006), they have never been used to provide a motor blockade in awake behaving macaques for neurophysiological analysis and neural interface development, to our knowledge. We foresee that this model will lead to important advancements in the study of how the proximal central nervous system (CNS) responds to acute injury, maintenance of paralysis, and recovery of function. Additionally, it should aid in the development of many lower extremity neural interfacing paradigms, as now the neurological function of chronically implanted macaques that have extensively trained for this type of neural interface work can be preserved while developing these techniques. Furthermore, the temporary paraplegia achieved here is done in an ethical, sustainable, and nonpainful manner.
While this paraplegia model is applicable to many neurophysiological study modalities, it is not applicable to all, and it does have limitations. Epidural injection of anesthetic simulates the flaccid paralysis induced by, for example, an acute and relatively large lumbosacral lesion. Therefore, assistive interfaces that decode signals from either the brain or proximal spinal cord to activate simulations on a computer, exoskeletons, leg musculature via FES, or distal peripheral nerves will be the most likely to benefit from this type of model. Also, sensory feedback modalities, such as spinal cord or somatosensory cortex (S1) stimulation, can now be tested in awake macaque paraplegia models. However, paradigms that seek to record from or stimulate the lumbosacral spinal cord will be unlikely to benefit from this model, as the anesthetic inhibits the lumbosacral spinal cord and its exiting nerve roots. Notably, patients with lumbosacral lesions would also be unlikely to benefit from those types of interventions anyway. Also, this technique is not meant to model an intrinsic SCI, but instead recapitulate its macroscopic physiological effects (i.e., blockage of nerve transmission resulting in paralysis). Therefore, while interfaces aimed at rehabilitating CNS lesions via induction of plasticity or regrowth of long tracts could use this model to develop their decoding and actuating algorithms, the rehabilitative potential of such paradigms would still ultimately need to be tested on a permanent CNS lesion. Also, spasticity, loss of autonomic function, and reduction of cortical leg representation (e.g., from a chronic injury) are not recapitulated here.
The main focus of this work was to establish the technique for catheter placement and demonstrate the induction of paralysis. An in-depth analysis of anesthetic types, dosing, and pharmacokinetics is outside the scope of this initial study, as laboratories that adopt this technique will be able to tailor their anesthetic and dosing to their specific application. As learned from video fluoroscopy during catheter placement, increasing the volume of the anesthetic during a single injection increased its spread to adjacent levels. Therefore, if the desire is to block more neighboring nerve roots, an increased volume of injection can be used. Increasing the concentration of the anesthetic, and/or dividing up the doses over time, can be used to help deliver more drug to an already targeted segment. Additionally, proper placement of the catheter is crucial, as slight misplacement of the catheter to the left was the likely reason that the right quadriceps in monkey N was only partially blocked. We improved our result in the second and third animals, monkey M and monkey R, by verifying midline placement intraoperatively with anterior-posterior fluoroscopy and radiopaque dye injection.
The longevity of these catheters remains to be seen. However, if they were to become nonfunctional due to clotting, scarring, kinking, movement, or disconnection, revision of the catheters is a relatively minimal procedure. especially when considered against complications resulting from permanent lesions.
It should be noted that there are several nontrivial risks (numbered items) to using this technique that can be minimized using the following prevention techniques (lettered items):
-
Misplacement of the catheter intrathecally (into the CSF space) or intravascularly
-
Aspirate from the catheter to assess for return of clear fluid (CSF) or blood.
If CSF is noted, the catheter should be removed and replaced at an adjacent level, or the procedure should be aborted and tried again later.
If blood is noted, the catheter should be withdrawn ~0.5 cm and reaspirated. This can be repeated until the holes of the catheter are no longer in the epidural space, at which time the catheter should be withdrawn and replaced at an adjacent level.
Use radiopaque dye injection with fluoroscopy to confirm epidural placement and spread of anesthetic.
Minimize the amount of catheter placed into the epidural space (~2 cm, or just enough to pass all holes into the space), as excess catheter length can lead to unpredictable final location of the catheter (e.g., out a neural foramen) (Fig. 8, A and B).
-
-
Overdose or overly cephalad spread of anesthetic
Confirm epidural (as opposed to intrathecal) placement and appropriate distribution of anesthetic (see step 1).
Minimize bolus volumes, as the spread is volume dependent, and boluses can be repeated.
Keep the animal upright to minimize cephalad spread with gravity.
Have respiratory support and resuscitative measures available in case of emergency during initial trials, or when increasing the volume of anesthetic to be injected.
-
Infection
-
Superficial infections
Use careful sterile technique during implantation of the catheter-port (as described in methods).
Irrigate subcutaneous space with bacitracin-infused saline before closure.
Provide careful skin closure and wound coverage, as well as maintenance of a protective vest until well healed.
Use IV antibiotics before incision to minimize skin flora.
-
Epidural infections
Cleanse the skin carefully with alcohol prep pad(s) before each injection.
-
-
Detachment, movement, or clotting of the catheter
Secure the catheter carefully into the port at the time of surgery.
Verify there is no leak with fluoroscopic dye injection into the port before closure.
Secure the port carefully to the fascia with permanent sutures (e.g., silk).
Secure the catheter at the fascial entry site with a purse-string suture, and tack the remaining catheter length down to the fascia with silk sutures.
Flush the catheter at regular intervals with heparinized saline.
In conclusion, our group has developed a simple and well-tolerated mechanism for inducing temporary paraplegia in awake behaving macaques so that recordings from the proximal CNS can be obtained during induction, maintenance, and resolution of paralysis in an ethical manner. This information can be used to study the underlying proximal neurophysiological response to acute lower extremity paralysis, as well as to develop and test decoding paradigms for lower extremity neuroprostheses and neural interfaces without needing to permanently injure experimental animals. Laboratories that use this technique will be able to adjust anesthetics and dosing paradigms to fit their needs; our group was able to reliably produce paraplegia for over an hour, which is an ideal time period for our applications (Vouga et al. 2017). Hopefully, this model will prove to be both cost and time effective, as rhesus macaques have the potential to produce many years of important scientific data (Roth et al. 2004).
GRANTS
This work was supported by a R25 grant from the National Institute of Neurological Disorders and Stroke (NINDS 5R25NS065731-08) awarded to M. O. Krucoff and a grant from the National Institute of Mental Health (NIMH DP1MH099903) awarded to Miguel A. L. Nicolelis.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.O.K., K.Z., D.B.M., L.O., and M.A.L. conceived and designed research; M.O.K., R.J.M., L.O., and M.A.L. performed experiments; M.O.K., A.Y., Y.W.B., R.J.M., and M.A.L. analyzed data; M.O.K., K.Z., D.B.M., A.Y., Y.W.B., R.J.M., D.A.T., L.O., and M.A.L. interpreted results of experiments; M.O.K. prepared figures; M.O.K. drafted manuscript; M.O.K., K.Z., D.B.M., A.Y., Y.W.B., D.A.T., L.O., and M.A.L. edited and revised manuscript; M.O.K., K.Z., D.B.M., A.Y., Y.W.B., R.J.M., D.A.T., L.O., and M.A.L. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
All illustrations were done by Lauren Halligan at Duke University. Thanks to laboratory manager Tamara Phillips and engineer Gary Lehew for all their help. Thanks to our Division of Laboratory Animal Resources (DLAR) veterinary staff for taking extraordinary care of our animals. Thanks to Miguel Nicolelis, MD, PhD, for his continued support. We thank Lee Miller, PhD, and the members of his laboratory for their collaboration. We also thank the Department of Neurosurgery at Duke University for its continued support.
REFERENCES
- Bajd T, Kralj A, Stefancic M, Lavrac N. Use of functional electrical stimulation in the lower extremities of incomplete spinal cord injured patients Artif Organs 23: 403–409, 1999. doi: 10.1046/j.1525-1594.1999.06360.x. [DOI] [PubMed] [Google Scholar]
- Banala SK, Agrawal SK, Fattah A, Krishnamoorthy V, Hsu WL, Scholz J, Rudolph K. Gravity-balancing leg orthosis and its performance evaluation. IEEE Trans Robot 22: 1228–1239, 2006. doi: 10.1109/TRO.2006.882928. [DOI] [Google Scholar]
- Berkowitz M. Spinal Cord Injury: An Analysis of Medical and Social Costs. New York: Demos Medical Publishing, 1998. [Google Scholar]
- Capogrosso M, Milekovic T, Borton D, Wagner F, Moraud EM, Mignardot J-B, Buse N, Gandar J, Barraud Q, Xing D, Rey E, Duis S, Jianzhong Y, Ko WK, Li Q, Detemple P, Denison T, Micera S, Bezard E, Bloch J, Courtine G. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature 539: 284–288, 2016. doi: 10.1038/nature20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa N, Caldwell DG. Control of a biomimetic “soft-actuated” 10DoF lower body exoskeleton. Proc First IEEE/RAS-EMBS Int Conf Biomed Robot Biomechatronics 2006: 495–501, 2006. [Google Scholar]
- Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, Maier I, Martin J, Nudo RJ, Ramon-Cueto A, Rouiller EM, Schnell L, Wannier T, Schwab ME, Edgerton VR. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med 13: 561–566, 2007. doi: 10.1038/nm1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Roy RR, Raven J, Hodgson J, McKay H, Yang H, Zhong H, Tuszynski MH, Edgerton VR. Performance of locomotion and foot grasping following a unilateral thoracic corticospinal tract lesion in monkeys (Macaca mulatta). Brain 128: 2338–2358, 2005. doi: 10.1093/brain/awh604. [DOI] [PubMed] [Google Scholar]
- Craggs MD. Cortical control of motor prostheses: using the cord-transected baboon as the primate model for human paraplegia Adv Neurol 10: 91–101, 1975. [PubMed] [Google Scholar]
- DeWeert TM, Golub MS, Kaaekuahiwi MA. Long-term epidural catheterization of rhesus macaques: loss of resistance technique Lab Anim Sci 45: 94–97, 1995. [PubMed] [Google Scholar]
- Dollar AM, Herr H. Lower extremity exoskeletons and active orthoses: challenges and state-of-the-art. IEEE Trans Robot 24: 144–158, 2008. doi: 10.1109/TRO.2008.915453. [DOI] [Google Scholar]
- Donati ARC, Shokur S, Morya E, Campos DSF, Moioli RC, Gitti CM, Augusto PB, Tripodi S, Pires CG, Pereira GA, Brasil FL, Gallo S, Lin AA, Takigami AK, Aratanha MA, Joshi S, Bleuler H, Cheng G, Rudolph A, Nicolelis MAL. Long-term training with a brain-machine interface-based gait protocol induces partial neurological recovery in paraplegic patients. Sci Rep 6: 30383, 2016. doi: 10.1038/srep30383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doppman JL, Ramsey R, Thies RJ II. A percutaneous technique for producing intraspinal mass lesions in experimental animals. J Neurosurg 38: 438–447, 1973. doi: 10.3171/jns.1973.38.4.0438. [DOI] [PubMed] [Google Scholar]
- Eliseyev A, Mestais C, Charvet G, Sauter F, Abroug N, Arizumi N, Cokgungor S, Costecalde T, Foerster M, Korczowski L, Moriniere B, Porcherot J, Pradal J, Ratel D, Tarrin N, Torres-Martinez N, Verney A, Aksenova T, Benabid A-L. CLINATEC® BCI platform based on the ECoG-recording implant WIMAGINE® and the innovative signal-processing: preclinical results. 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, 26–30 Aug. 2014. Conf Proc IEEE Eng Med Biol Soc 2014: 1222–1225, 2014. doi: 10.1109/EMBC.2014.6943817. [DOI] [PubMed] [Google Scholar]
- Ethier C, Oby ER, Bauman MJ, Miller LE. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature 485: 368–371, 2012. doi: 10.1038/nature10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med 12: 790–792, 2006. doi: 10.1038/nm1436. [DOI] [PubMed] [Google Scholar]
- Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol 81: 479–484, 2017. doi: 10.1002/ana.24897. [DOI] [PubMed] [Google Scholar]
- Hargrove LJ, Huang H, Schultz AE, Lock BA, Lipschutz R, Kuiken TA. Toward the development of a neural interface for lower limb prosthesis control. Proc 31st Annu Int Conf IEEE Eng Med Biol Soc Eng Futur Biomed EMBC 2009: 2111–2114, 2009. doi: 10.1109/IEMBS.2009.5334303. [DOI] [PubMed] [Google Scholar]
- Hartman CG, Straus WL Jr, editors. The Anatomy of the Rhesus Monkey. Baltimore, MD: Williams & Wilkins, 1933. [Google Scholar]
- Katz JA, Sehlhorst CS, Thompson GA, Denson DD, Coyle D, Bridenbaugh PO. Pharmacokinetics of intravenous and epidural ropivacaine in the rhesus monkey. Biopharm Drug Dispos 14: 579–588, 1993. doi: 10.1002/bdd.2510140704. [DOI] [PubMed] [Google Scholar]
- Knapp HD, Taub E, Berman AJ. Movements in monkeys with deafferented forelimbs. Exp Neurol 7: 305–315, 1963. doi: 10.1016/0014-4886(63)90077-3. [DOI] [PubMed] [Google Scholar]
- Krucoff MO, Rahimpour S, Slutzky MW, Edgerton VR, Turner DA. Enhancing nervous system recovery through neurobiologics, neural interface training, and neurorehabilitation. Front Neurosci 10: 584, 2016. doi: 10.3389/fnins.2016.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger J, Caruana F, Volta RD, Rizzolatti G. Seven years of recording from monkey cortex with a chronically implanted multiple microelectrode. Front Neuroeng 3: 6, 2010. doi: 10.3389/fneng.2010.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HGJM. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain 91: 1–14, 1968. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 31: 195–218, 2008. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve 32: 261–279, 2005. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- MacAllister RP, McCully CML, Bacher J, Thomas ML, Cruz R, Wangari S, Warren KE. Minimally invasive lumbar port system for the collection of cerebrospinal fluid from rhesus macaques (Macaca mulatta). Comp Med 66: 349–352, 2016. [PMC free article] [PubMed] [Google Scholar]
- Martinez-Villalpando EC, Weber J, Elliott G, Herr H. . Design of an agonist-antagonist active knee prosthesis. Proc 2nd Benn IEEE/RAS-EMBS Int Conf Biomed Robot Biomechatronics 2008: 529–534, 2008. doi: 10.1109/BIOROB.2008.4762919. [DOI] [Google Scholar]
- Minassian K, Hofstoetter U, Tansey K, Mayr W. Neuromodulation of lower limb motor control in restorative neurology. Clin Neurol Neurosurg 114: 489–497, 2012. doi: 10.1016/j.clineuro.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center. Spinal cord injury facts and figures at a glance [Online]. https://www.nscisc.uab.edu/Public/Facts%202015.pdf, 2015.
- Nesathurai S, Graham WA, Mansfield K, Magill D, Sehgal P, Westmoreland SV, Prusty S, Rosene DL, Sledge JB. Model of traumatic spinal cord injury in Macaca fascicularis: similarity of experimental lesions created by epidural catheter to human spinal cord injury. J Med Primatol 35: 401–404, 2006. [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL, Dimitrov D, Carmena JM, Crist R, Lehew G, Kralik JD, Wise SP. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc Natl Acad Sci USA 100: 11041–11046, 2003. doi: 10.1073/pnas.1934665100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmeyer EA, Jordon LR, Kim P, Miller LE. A fully implanted drug delivery system for peripheral nerve blocks in behaving animals. J Neurosci Methods 182: 165–171, 2009. doi: 10.1016/j.jneumeth.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajangam S, Tseng P-H, Yin A, Lehew G, Schwarz D, Lebedev MA, Nicolelis MAL. Wireless cortical brain-machine interface for whole-body navigation in primates. Sci Rep 6: 22170, 2016. doi: 10.1038/srep22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey R, Doppman JL. The effects of epidural masses on spinal cord blood flow. An experimental study in monkeys. Radiology 107: 99–103, 1973. doi: 10.1148/107.1.99. [DOI] [PubMed] [Google Scholar]
- Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science 305: 1423–1426, 2004. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- Schwarz DA, Lebedev MA, Hanson TL, Dimitrov DF, Lehew G, Meloy J, Rajangam S, Subramanian V, Ifft PJ, Li Z, Ramakrishnan A, Tate A, Zhuang KZ, Nicolelis MA. Chronic, wireless recordings of large-scale brain activity in freely moving rhesus monkeys. Nat Methods 11: 670–676, 2014. doi: 10.1038/nmeth.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Combs CM. Spinal cord segments. B. Gross structure in the adult monkey. Am J Anat 116: 205–216, 1965. doi: 10.1002/aja.1001160110. [DOI] [PubMed] [Google Scholar]
- Thompson GA, Turner PA, Bridenbaugh PO, Stuebing RC, Denson DD. The influence of diazepam on the pharmacokinetics of intravenous and epidural bupivacaine in the rhesus monkey Anesth Analg 65: 151–155, 1986. [PubMed] [Google Scholar]
- Vouga T, Zhuang KZ, Olivier J, Lebedev MA, Nicolelis MA, Bouri M, Bleuler H. EXiO—a brain-controlled lower limb exoskeleton for rhesus macaques. IEEE Trans Neural Syst Rehabil Eng 25: 131–141, 2017. doi: 10.1109/TNSRE.2017.2659654. [DOI] [PubMed] [Google Scholar]
- Waters RL, Yakura JS, Adkins RH, Sie I. Recovery following complete paraplegia Arch Phys Med Rehabil 73: 784–789, 1992. [PubMed] [Google Scholar]
- Windrich M, Grimmer M, Christ O, Rinderknecht S, Beckerle P. Active lower limb prosthetics: a systematic review of design issues and solutions. Biomed Eng Online 15, Suppl 3: 140, 2016. doi: 10.1186/s12938-016-0284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.