Abstract
Background
Although poliomyelitis has almost been eradicated worldwide, cases of a polio-like disease with asymmetrical flaccid paralysis of variable severity have been seen repeatedly in recent years.
Methods
Data were collected on children treated in hospitals in the German federal states of Bavaria and Lower Saxony in 2016. The frequency of disease across Germany was estimated on the basis of voluntary reporting to the Robert Koch Institute. 16 cases were registered there for the entire year 2016.
Results
7 children with flaccid paralysis of acute onset were treated in the participating hospitals in the summer and fall of 2016. We describe two illustrative cases, one with a mild course and one with a severe course. Rapid diagnosis requires not only clinical neurological assessment but also neurophysiological studies, magnetic resonance imaging (MRI), and targeted microbiological testing. The characteristic features include damage to the anterior horn of the spinal cord that can be seen on MRI and/or electrophysiologically demonstrable abnormalities indicating motor neuron damage. A pathogen can hardly ever be identified in the cerebrospinal fluid, but the epidemiological context and the detection of viruses in the stool or respiratory secretions indicate that enteroviruses may be responsible.
Conclusion
The prognosis of this disease cannot be reliably assessed at first, and no specific treatment is currently available.
In summer and autumn 2016, an increased number of cases with sudden onset of flaccid paralysis were observed in Germany. Both with regard to disease manifestation and numbers, these cases were unusual and of concern. Four of these cases occurred in August 2016 among children in Bavaria, another three were treated in Lower Saxony (table 1). In the National Reference Center for Poliomyelitis and Enteroviruses at the Robert Koch Institute (RKI), altogether 16 pediatric cases from six German Federal States were examined (6 from Bavaria, 3 from North Rhine–Westphalia, 4 from Lower Saxony, and 1 each from Bremen, Rhineland–Palatinate, and Baden–Wuerttemberg; [data from the National Reference Center for Poliomyelitis and Enteroviruses]), (table 2). The enterovirus detection rate was high in this patient population (7 out of 16 patients); however, it was not possible to clearly distinguish between a pathogen causing the disease and a commensal bystander. National enterovirus surveillance (EVSurv) in Germany collects data on patients with aseptic meningitis and acute flaccid paralysis (AFP). These data do not show a significant overall increase in the number of AFP cases in comparison with previous years. In August 2016, however, a marked rise in cases was observed compared with previous years (figure 1), but it should be taken into consideration that few or no clinical data are available for some of these cases registered at RKI and consequently paralysis of other origin cannot be ruled out with certainty.
Table 1. 7 children with AFP; these cases occurred in summer 2016 and were treated in Bavaria and Lower Saxony.
| Patient | Year of birth | Zip code | Onset of symptoms | Fever | CRP | Initial neurological symptoms | Later neurological symptoms | Magnetic resonance imaging | CSF cells | Liquor PCR | Enterovirus testing (stool) | Vaccinations |
| 1 | 2001 | 82xxx | 07/ 2016 | Initially up to 39 °C | CRP + | Headache, nuchal rigidity, fever | Urinary retention, vomiting, paraparesis predominantly in legs | Questionable discrete leptomeningeal enhancement along spinal cord | ++ | HSV1/2 & enteroviruses PCR negative | neg | STIKO +TBE |
| 2 | 2007 | 83xxx | 07/ 2016 | Initially, yes | CRP + | Headache, fever, fatigue After 3 days: proximal paralysis of right arm, facial nerve paralysis | Tetraplegia requiring mechanical ventilation | Hyperintense changes in cervical spinal cord, especially anterior horns | 131/µL | HSV1/2 & enteroviruses PCR negative | neg | 4 × pentavalent, 1 × MMR |
| 3 | 2012 | 94xxx | 07/ 2016 | Initially up to 38.5 °C | CRP neg | 1 week fever, then headache, nuchal rigidity, neck pain, paresis of left arm | Flaccid paresis of left arm | Long hyperintensity C1 to T1 | 107/µL | HSV1/2 & enteroviruses PCR negative | Coxsackievirus A2 | STIKO +TBE |
| 4 | 2014 | 84xxx | 08/ 2016 | Initially >39 °C | CRP neg | Fever, 2 days after fever cervical dystonia, after another day flaccid paresis of arm and neck extensor muscles, paradoxical breathing | Flaccid paresis of right upper arm, paresis of neck extensor muscles | Long diffuse inhomogeneous hyperintensity C2 to C7 | 3 days after onset: 3/µL | HSV1/2 & enteroviruses PCR negative | neg | STIKO |
| 5 | 2015 | 30xxx | 10/ 2016 | Initially, febrile upper airway infection | CRP + | Febrile airway infection, fatigue, diarrhea | Unsteady gait, flaccid paralysis of left arm | Left parasagittal T2 signal enhancement C3/4 to C6 | Status normal, intrathecal IgA synthesis 24% | HSV, picorna negative | Picornavirus RNA incl. enteroviruses positive | STIKO |
| 6 | 2014 | 30xxx | 08/ 2016 | Subfebrile upper airway infection | CRP neg | Airway infection, then right leg weakness and pain | Flaccid paresis of legs, R>L, constipation | Elongated T2 signal enhancement right lateral in lower thoracic spinal cord | 5/µL | HSV1/2 & enteroviruses PCR negative | neg | STIKO |
| 7 | 2014 | 30xxx | 10/ 2016 | Initially, febrile upper airway infection | CRP + | Mild airway infection, transient limping gait followed by brief full recovery, then pain in left hip | Paresis of left leg, inability to walk | At level of T10/11 weak punctiform intramedullary T2 signal enhancement, left side | 1/µL | HSV1/2 & enteroviruses PCR negative | neg | STIKO |
AFP, acute flaccid paralysis; T, thoracic vertebra; CRP, C-reactive protein; TBE, tick-borne encephalitis; HSV, herpes simplex virus; C, cervical vertebra; L, left; MMR, measles, mumps, rubella vaccine; neg, negative, PCR, polymerase chain reaction; R, right; RNA, ribonucleic acid; STIKO, vaccinations according to the recommendations of the German Standing Committee on Vaccination; ++, many
Table 2. Compilation of the pediatric patients with severe acute flaccid paralysis examined in the Robert Koch Institute in 2016 (including patients listed in Table 1).
| Case | Age(years) | Sex | Place of residence | Clinical findings | Onset of symptoms | Enterovirus detection in stool |
| 1 | 1.8 | male | Hamburg | Pred. proximal pareses; requiring mechanical ventilation | March 2016 | n.d. |
| 2 | 2.0 | female | East Wuerttemberg | Right-sided hemiparesis, no head control, myelitis | July 2016 | ECHO virus 30 |
| 3 | 4.8 | male | Lower Bavaria | AFP, meningitis | July 2016 | n.d. |
| 4 | 8.6 | female | n.a. | AFP (transverse myelitis) | August 2016 | n.d. |
| 5 | 2.7 | female | Hannover region | n.a. *2 | August 2016 | n.d. |
| 6 | 10.0 | male | Upper Bavaria | Polio-like, tetraplegia | August 2016 | n.d. |
| 7 | 3.7 | female | Rhineland | AFP (left arm), oral antibiotic therapy for pneumonia | August 2016 | n.d. |
| 8 | 2.8 | male | Lower Bavaria | AFP (arm), upper airway infection, paradoxical breathing, no head control |
August 2016 | Coxsackievirus A2 |
| 9 | 14.3 | male | Upper Bavaria | AFP-proximal and distal muscle weakness L>R | August 2016 | n.d. |
| 10 | 2.3 | female | Rhenish Hesse | AFP (lower extremities), bronchopneumonia (10 days earlier) |
August 2016 | Enterovirus D68 |
| 11 | 6.3 | male | Lower Saxony | AFP (lower extremities), respir. disease, myelitis | August 2016 | n.d. |
| 12 | 2.2 | female | Rhineland | AFP, meningitis | September 2016 | Enterovirus A71 |
| 13 | 1.7 | female | Hannover region | AFP | October 2016 | Coxsackievirus A2 |
| 14 | 4.5 | female | n.a. | AFP, requiring mechanical ventilation | n.a. | pos* |
| 15 | 3.1 | male | Upper Swabia | Cervical myelitis, facial nerve paralysis | n.a. | n.d. |
| 16 | 2.4 | male | n.a. | AFP, myelitis | n.a. | pos* |
*, without typing; AFP, acute flaccid myelitis; L, left; n.a, not available; n.d., not detected; pos, positive; pred., predominantly; R, right
Figure 1.
Summary of the cases with acute flaccid paralysis registered as part of the Enterovirus Surveillance (EVSurv) at the Robert Koch Institute for the years 2010 to 2017. EV, enterovirus
The children who came to the attention of RKI were between 1.7 and 10 years old and half of them were male (8/16). Common initial signs and symptoms were headache and fever as well as nuchal rigidity, progressing in one case to tetraplegia (case 2). The cases varied widely with regard to severity and clinical course. In all of these children, lumbar punctures were performed for suspected encephalitis, but cerebrospinal fluid (CSF) analysis did not detect a pathogen in any of these cases. In the following, two case reports will be presented: one with a benign course and good recovery, the other with a severe course and as yet only very limited recovery from paralysis.
Case 1: Two weeks prior to the onset of the neurological symptoms, the 14-month-old female patient had a common cold with rhinitis and cough, resolving spontaneously within a short time. Until then, her statomotor development had been normal. She had received all scheduled vaccinations as recommended by the German Standing Committee on Vaccination (STIKO, Ständige Impfkommission) and had a last MMR vaccination (MMR, measles, mumps, rubella vaccine) one week before she developed acute symptoms of mild fever and fatigue followed by otitis. The patient became increasingly apathetic and sleepy and on day 4 she lost the ability to raise her left arm above the horizontal level and developed a complete flaccid paralysis of her left arm within hours. She could move her fingers at all times.
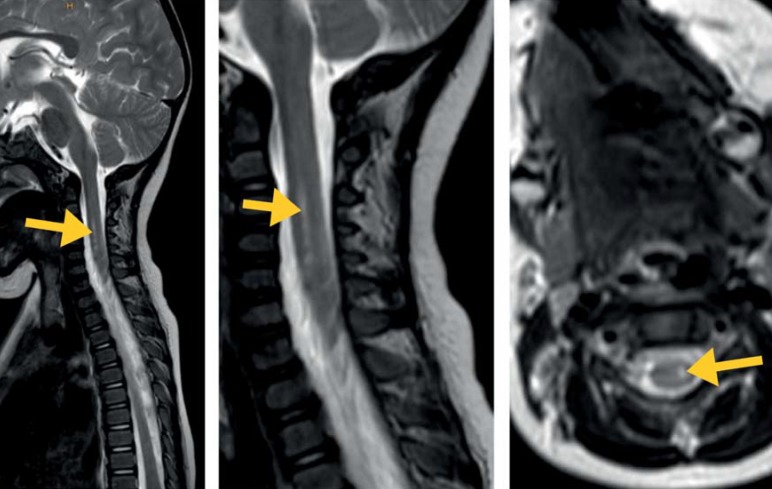
She was started on clindamycin and cefuroxime for initially suspected osteomyelitis. However, since further investigations found no evidence of an inflammatory process in the bone and could not rule out borreliosis, antibiotic treatment was switched to cefotaxime. This treatment was continued until repeated Borrelia serology had yielded negative results. Laboratory testing found no increase in serum inflammatory markers. Further serological testing found IgM antibodies to mumps, indicating that the child had been vaccinated. While microbiological examination of CSF did not identify any pathogen, picornavirus RNA was detected in throat swaps and fecal samples. Coxsackievirus A2 was detected in stool. Besides normal cell count and protein level, CSF analysis demonstrated an intrathecal IgA synthesis of 24% (normal <10%) along with intact blood–brain barrier (BBB) function, without any other signs of CNS inflammation. Magnetic resonance imaging (MRI) showed a focal myelopathy (figure 2) in the cervical cord at about the level of the upper endplate of cervical vertebrae 4 to 6. Neurography found no sign of peripheral nerve damage.
Figure 2.
MRI of the cervical cord shows a comparatively well demarcated myelopathy signal in the area of the left anterior horn cells of the gray matter (sagittal T2-weighted TSE; Institute for Diagnostic Imaging and Interventional Radiology, HELIOS-Klinikum Hildesheim, Germany)
Constant clinical improvement started about 6 days after the onset of neurological symptoms while the patient was still in hospital. On clinical follow-up 6 weeks after the onset of disease, spontaneous motor function of the arm and hand appeared normal; only occasionally a slight simultaneous movement of the shoulder along with upward reaching movements, preferential use of the right hand (patient had been left-handed before), and a minor loss of strength during prolonged elevation of the left arm were noted.
Case 2: A 9-year-old boy, without significant medical history, suffered from headache and fever for five days; three days prior to hospitalization, he developed progressive, more proximal paralysis of his right arm and right-sided peripheral facial nerve paralysis. Cranial CT and MRI were unremarkable; a lumbar puncture was performed, showing 353/3 cells/µL with predominantly mononuclear pattern. CSF testing was negative for herpes simplex 1 and 2, human herpesvirus 6, cytomegalovirus, varicella-zoster virus, Cryptococcus, E. coli, Haemophilus influenzae, Listeria monocytogenes, as well as meningococcus and pneumococcus. Testing for neuronal antibodies, including anti-aquaporin-4 antibodies, was negative, with no findings indicative of autoimmunity.
After initial treatment with antibiotics and acyclovir, the patient developed progressive proximal flaccid paresis, now also of the left arm, hypoglossal nerve paresis, and right-sided accessory nerve paresis. MRI of head and cervical spine showed swelling of the cervical cord and mild leptomeningeal contrast enhancement. Because no pathogen had been detected, antibiotic and antiviral treatment was discontinued. With progressive neurological signs and symptoms and progressive respiratory failure, the patient was intubated and mechanically ventilated; because symptoms persisted, a tracheostomy was performed. A treatment attempt with intravenous immunoglobulins had no effect. After altogether 35 days, the boy was transferred to a neurological rehabilitation facility. At that time, his neurological symptoms had only slightly improved and he was still on mechanical ventilation. In the meantime, he has experienced partial remission with improvement of his respiratory function and some minor signs of recovery in arms and legs; however, the patient is still in need of care.
The condition of acute flaccid paralysis
The Global Polio Eradication Initiative of the World Health Organization (WHO) and its partners is well on its way to becoming a success story: Of the 3 serotypes, the wild-type poliovirus type 2 (WPV2) has not been detected anywhere in the world since 1999 and was declared eradicated in 2015. The last detected WPV3 dates to November 2012; consequently, at the moment only WPV1 is naturally circulating. In 2016, altogether only 37 cases were reported globally: 13 in Afghanistan, 20 in Pakistan, and 4 in Nigeria. Thus, the world is at the verge of eradicating polio—a disease that has caused tremendous suffering and permanent disability (1). However, in case of insufficient immunity in the population, live-vaccine–derived polioviruses may circulate and cause outbreaks (5 cases in 2016).
Against this background, it is even more of concern to note that since 2012 increasing numbers of severe cases with flaccid paralysis have been observed in various countries. In these cases, clinical presentation was very similar to that observed in poliomyelitis, but the condition was caused by other pathogens which frequently remained unidentified (2). For example, a case of flaccid paralysis related to enterovirus D68 (EV-D68) has recently been reported from the Netherlands (3). In Sweden, a series of severe cases with EV-D68 infection was observed of which 3 patients had flaccid paralysis (4); in Norway, two cases were reported, which occurred in 2014 (5). In France, 59 cases of flaccid paralysis were reported in children showing severe neurological damage, including 15 children with rhombencephalitis, 10 with encephalitis, 4 with myelitis, and 2 with cranial nerve radiculitis. In these cases, enterovirus A71 (EV-A71) and EV-D68 were detected (6). The European Centre for Disease Prevention and Control (ECDC) published a rapid risk assessment on August 8, 2016 in which cases from Denmark, France, the Netherlands, Spain, Sweden, the United Kingdom, and Ireland were documented (2).
Initially, the condition was referred to as “polio-like disease.“ In the meantime, the term “acute flaccid paralysis with anterior myelitis“ or “acute flaccid myelitis“ (AFM) has been adopted so that this condition can be distinguished from classical poliomyelitis. Characteristic features of AFM include damage to the anterior horn of the spinal cord detectable in MRI (7) or electrophysiologically detected lesions as signs of motor neuron damage (8). In contrast to Guillain Barré syndrome (GBS), there are no signs of demyelination. The sensory nerve is not affected. Unlike transverse myelitis, AFM results from damage to the 2nd motor neuron without involvement of the 1st motor neuron or sensory afferent nerves; thus only motor function is affected.
Best documented and analyzed are the cases from the United States which occurred between 2012 and 2015, primarily in California, Colorado, and Utah. In 2016, 136 cases in 37 states were reported to the Center for Disease Control and Prevention (CDC) (9). The CDC analyzed 120 cases which occurred in 2014 (10). These cases were children and young people under the age of 21 and occurred in 34 states. The median age was 7.1 years; 59% of patients were male. Altogether 56% of patients had fever in combination with respiratory tract infection, 25% only respiratory tract infection (without fever), and 9% only fever. Neurological symptoms appeared between 0 and 18 days after the respiratory tract infection or fever, with a median interval of 5 days. Except for 1 patient, all patients were admitted to hospital and in 20% mechanical ventilation was required. The upper extremities were affected in 34%, the lower extremities in 23%. In 43% of patients, both the upper and lower extremities were affected and in 47% of patients a clearly one-sided asymmetrical pattern was noted. Cranial nerve involvement was observed in 28% of patients.
According to WHO data, flaccid paralysis occurs in 1/100 000 children younger than age 15 years. For Germany that would amount to about 120 cases annually; however, this number was never reached between 1997 and 2010, i.e. in the time when the AFP surveillance was established in Germany to monitor the polio-free status of the country. The national enterovirus surveillance in Germany, collecting data on aseptic meningitis/encephalitis and AFP, has recorded 47 to 78 cases/year since 2010 (according to RKI data).
Spinal MRI scans showed in 87% involvement of the cervical cord, while in 80% thoracic and in 47% medullary involvement was noted. In 96% of patients, more than 1 spinal segment was affected. CSF studies revealed pleocytosis (median cell count of 44 cells/µL): 91% of patients had CSF cell counts above 5/µL, typically with lymphocytic predominance. In only 1 patient, EV-D68 was detected in CSF; however, the authors questioned the validity of this finding. All other CSF studies did not detect any pathogen, despite comprehensive testing, including metagenomics analysis in some cases, by the CDC. By contrast, EV-D68 was detected in nasopharyngeal secretions of 20% of patients; likewise, other enteroviruses and rhinoviruses were found in 20% of patients. In respiratory specimens collected within less than 1 week from the onset of the disease, the detection rate was higher.
The differential diagnosis of AFP includes immunological disorder, such as Guillain–Barré syndrome (GBS) and transverse myelitis, toxic effects, severe electrolyte imbalances, and, most importantly, acute injury-related damages.
In 87% of the patients with data available, either immunoglobulins (73%), corticosteroids (54%), plasmapheresis (15%) and/or other immunosuppressants (2%) had been used. Information about the clinical course was only available for 47% of patients, with follow-up periods ranging between 1 and 7.5 months. Of these patients, 14% required full care, while 68% had functional impairments. Only 18% had recovered to such an extent that they did not need further support and in only 5% the initial strength was fully restored. Results for longer follow-up periods have not yet been reported (10).
It is known that enterovirus infections are associated with acute flaccid paralysis (2, 8, 10). The enteroviruses include coxsackieviruses and echoviruses, each containing numerous serotypes (11). Some serotypes, for example EV-D68 (12) and EV-A71 (13), have occurred more frequently in recent years and have been associated with severe clinical courses (14). Non-polio enterovirus infections of the central nervous system have been known for a long time and associations between numerous serotypes and aseptic meningitis/encephalitis have been described (15). The Western Nile virus, the Japanese encephalitis virus, and the Zika virus have been detected in patients with AFP.
Conclusion
Polio-like courses of acute encephalitis with flaccid paralysis in children have been described in many European countries and in the US in recent years. Epidemiological associations indicate that these conditions are caused by enteroviruses, even though CSF testing for pathogens tends to return negative results in almost all cases. More frequently, it is possible to detect pathogens in stool or respiratory secretions, but this does not prove a pathogenetic relationship. Other causes (bacterial or viral infections) and Guillain–Barré syndrome must be ruled out. The characteristic MRI pattern plays an important role in the differential diagnosis of the condition.
The currently available evidence is not sufficient to prove conclusively that corticosteroids, immunoglobulins, plasmapheresis, or antiviral medications are effective in the treatment of AFP (16).
The national enterovirus surveillance (EVSurv) in Germany is part of the efforts to monitor the polio-free status of the country. Any suspected cases can be reported to EVSurv (www.rki.de/DE/Content/Infekt/NRZ/Polio/Polio_node.html). Furthermore, all pediatric and neurological departments of hospitals in Germany are offered free enterovirus testing of fecal and CSF samples to assist with the differential diagnostic workup of viral meningitis/encephalitis and acute flaccid paralysis.
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Lopalco PL. Wild and vaccine-derived poliovirus circulation, and implications for polio eradication. Epidemiol Infect. 2017;145:413–441. doi: 10.1017/S0950268816002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ECDC. Enterovirus detections associated with severe neurological symptoms in children and adults in European countries. ECDC Rapid Risk Assessment. 2016:1–9. [Google Scholar]
- 3.Knoester M, Schölvinck EH, Poelman R, et al. Upsurge of Enterovirus D68, the Netherlands, 2016. Emerg Infect Dis. 2017;23:140–143. doi: 10.3201/eid2301.161313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dyrdak R, Grabbe M, Hammas B, et al. Outbreak of enterovirus D68 of the new B3 lineage in Stockholm, Sweden, August to September 2016. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.46.30403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeiffer HC, Bragstad K, Skram MK, et al. Two cases of acute severe flaccid myelitis associated with enterovirus D68 infection in children, Norway, autumn 2014. Euro Surveill. 2015;10 doi: 10.2807/1560-7917.es2015.20.10.21062. [DOI] [PubMed] [Google Scholar]
- 6.Antona D, Kossorotoff M, Schuffenecker I, et al. Severe paediatric conditions linked with EV-A71 and EV-D68, France, May to October 2016. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.46.30402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maloney JA, Mirsky DM, Messacar K, Dominguez SR, Schreiner T, Stence N V. MRI findings in children with acute flaccid paralysis and cranial nerve dysfunction occurring during the 2014 enterovirus D68 outbreak. Am J Neuroradiol. 2015;36:245–250. doi: 10.3174/ajnr.A4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crone M, Tellier R, Wei XC, et al. Polio-like illness associated with outbreak of upper respiratory tract infection in children. J Child Neurol. 2016;31:409–414. doi: 10.1177/0883073815596613. [DOI] [PubMed] [Google Scholar]
- 9.CDC. Accute Flaccid Paralysis. www.cdc.gov/acute-flaccid-myelitis/afm-surveillance.html (last accessed on 8 June 2017) [Google Scholar]
- 10.Sejvar JJ, Lopez AS, Cortese MM, et al. Acute flaccid myelitis in the United States, August-December 2014: Results of nationwide surveillance. Clin Infect Dis. 2016;63:737–745. doi: 10.1093/cid/ciw372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Crom SCM, Rossen JWA, van Furth AM, Obihara CC. Enterovirus and parechovirus infection in children: a brief overview. Eur J Pediatr. 2016;175:1023–1029. doi: 10.1007/s00431-016-2725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holm-Hansen CC, Midgley SE, Fischer TK. Global emergence of enterovirus D68: a systematic review. Lancet Infect Dis. 2016;16:e64–e75. doi: 10.1016/S1473-3099(15)00543-5. [DOI] [PubMed] [Google Scholar]
- 13.Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus. Lancet Infect Dis. 2010;71:778–790. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- 14.Pons-Salort M, Parker EPK, Grassly NC. The epidemiology of non-polio enteroviruses. Curr Opin Infect Dis. 2015;28:479–487. doi: 10.1097/QCO.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudolph H, Schroten H, Tenenbaum T. Enterovirus infections of the central nervous system in children - an update. Pediatr Infect Dis J. 2016;35:567–569. doi: 10.1097/INF.0000000000001090. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Acute flaccid myelitis interim considerations for clinical management. www.cdc.gov/acute- flaccid-myelitis/downloads/interim-considerations-afm.pdf (last accessed on 8 June 2017) [Google Scholar]