Abstract
The fields of tumor metabolism and immune oncology have both independently received considerable attention over the last several years. The majority of research in tumor metabolism has largely focused on the Warburg effect and its resulting biologic consequences, including energy and macromolecule production. However, recent investigations have identified elegant, multifaceted strategies by which alterations in tumor metabolism can also contribute to a potent tolerogenic immune environment. One of the most notable is increased tryptophan metabolism through activation of indoleamine 2,3-dioxygenase 1 (IDO1) and tryptophan 2,3-dioxygenase (TDO). However, this pathway represents one of numerous metabolic pathways that may modulate the immune system. For example, metabolites associated with aerobic glycolysis, adenosine, arginine, and prostaglandin metabolism have all been implicated in cancer-mediated immune tolerance and represent attractive therapeutic targets. In this review, we will provide an overview of the emerging interface between these 2 timely areas of cancer research and provide an overview of strategies currently being tested to target these next-generation metabolic immune checkpoints.
Keywords: glioblastoma, immune checkpoint, tumor metabolism
The role the immune system plays in modulating tumor progression has been recognized since the late 1800s. William Coley initially demonstrated that injecting microbes directly into inoperable tumors provoked an immune response that resulted in tumor regression.1 Despite encouraging results obtained in his case series, his work received criticism and was only accepted after his offspring continued with this line of work over the next several decades.2 In parallel to these early scientific explorations that would eventually develop into the field of immuno-oncology, seminal studies were being conducted in the early 1920s by Otto Warburg, who postulated that changes in metabolism were the fundamental cause of cancer.3,4 Although these emerging fields of cancer research appeared to lose favor over the years, including a focus on treating cancer with radiation therapy and chemotherapy and research emphasizing genetic mutations as primary drivers of tumorigenesis, both fields have independently received considerable attention and renewed interest. Overcoming tumor-mediated immune suppression using immune checkpoint inhibitors has led to striking improvements in clinical outcomes in multiple cancers, revolutionizing our approach to cancer therapy,5 and technological advancements have provided researchers an unprecedented opportunity to uncover an intricate set of metabolic programs driving the aggressive phenotype of malignancies far beyond alterations in glycolysis that underlie the Warburg effect.6,7 With continued advancement in both of these research disciplines, the intimate relationship between multifaceted alterations in tumor metabolism and their subsequent influence on immune regulation has become increasingly recognized as an important factor contributing to tumor growth and progression. Recent studies using global metabolomic profiling designed to identify metabolic programs that may be driving the aggressive phenotype of glioblastoma have identified numerous alterations in cellular metabolism that may play a contributory role in immune regulation.8 In this review, using these metabolomic findings as a framework, we will provide an overview of the emerging interface between these 2 timely areas of cancer research—bringing attention to how metabolic remodeling can actively and passively contribute to an immune suppressive state— as well as an overview of strategies currently being tested to target these next-generation, metabolic immune checkpoints.
Aerobic Glycolysis: A Shared Metabolic Adaptation of Both Tumor and Immune Cells
Cancer cells need to fulfill unique bioenergetic and biosynthetic demands to support their rapid growth and continued unregulated proliferation. One strategy they employ involves altering their energy metabolism to favor glycolysis, even under aerobic conditions, to support rapid energy generation and efficient macromolecule biosynthesis. This aerobic form of glycolysis is also known as the Warburg effect. Thus, tumor cells get most of their energy through high consumption of glucose and its conversion into lactic acid by glycolysis, as opposed to mitochondrial oxidative phosphorylation in normal cells. In addition to providing efficient energy, this shift fosters biosynthesis by providing the requisite macromolecules for DNA and lipid synthesis and maintenance of redox balance required for continued growth, termed anabolic metabolism,6,7 which has recently been identified to be particularly relevant in glioblastoma.8 Further, this glycolytic switch is also a useful adaptation for cancer cells to survive in regions of hypoxia, which is typical in the tumor microenvironment.
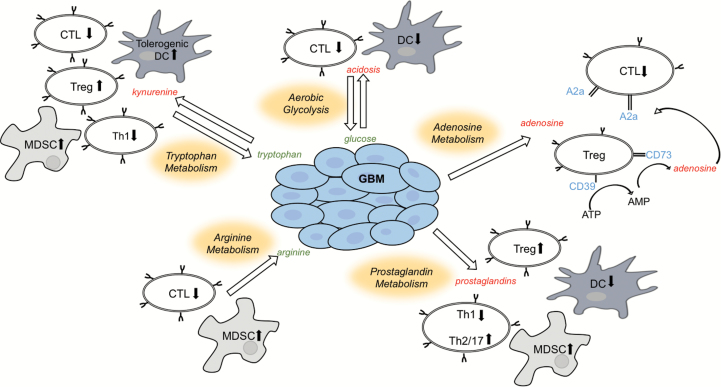
Alterations in metabolic programming that drive enhanced glycolysis to support proliferation are not unique to cancer cells but represent an important metabolic shift that is able to support the bioenergetic needs of a variety of normal cells, including immune cells.6,9 The immune system encompasses a heterogeneous population of cells that are naïve in the steady state but can rapidly respond to infection and inflammation. Enhanced glycolysis helps support the rapid and dynamic transitions between naïve and activated states in a variety of immune cells.9 For example, dendritic cells (DCs), whose main function is priming T-cell response by processing and presenting antigen on the T-cell surface, typically oxidize glucose in the mitochondria and produce relatively little lactate. However, after stimulation, they undergo dramatic metabolic reprogramming consistent with the Warburg effect, including increased glucose uptake, decreased carbon flux into the mitochondria, and increased lactate production.10,11 This transition from a quiescent to an activated state is perhaps most notable in T cells, which demonstrate extensive and rapid proliferation upon activation.9 When naïve T cells recognize antigen, they undergo energetically demanding developmental programs characterized by rapid growth, proliferation, and acquisition of specialized functions. To allow for this level of growth and proliferation, a switch from catabolic to anabolic metabolism in effector T cells is required, which is largely supported through enhanced glycolysis. Further, this shift to glycolysis has also been described in a variety of other immune cells, including macrophages, neutrophils, B cells, and natural killer (NK) cells.9,12,13 Therefore, the availability of glucose is of clear importance in initiating an effective immune response. However, enhanced glycolysis observed in tumors that appear to be driving its aggressive phenotype leads to a microenvironment depleted of glucose. Therefore, an immune suppressive microenvironment results as a passive consequence to the metabolic shift to aerobic glycolysis in tumors. In addition, rapid utilization of glucose and growing oxygen demands in tumors result in hypoxia, which can further shape the immune system. Tumor hypoxia results in the induction of the transcription factor hypoxia-inducible factor 1α, which increases production of transforming growth factor (TGF)-β that can further inhibit NK cells and stimulate immune suppressive CD4+ T cells.14 Further, the accumulation of lactic acid resulting from aerobic glycolysis in tumor cells also has direct immune suppressive effects at many levels,13 including inhibition of the differentiation of monocytes to DCs, increasing transcription and secretion of tumor promoting cytokines, including interleukin (IL)-23, and suppression of T-cell response (Figure 1).12,13,15,16 Therefore, the shift to aerobic glycolysis in tumors has both active and passive consequences on the immune microenvironment.
Fig. 1.
Schematic illustrating the interplay between metabolic remodeling and immune tolerance in cancer. CTL: cytotoxic T lymphocyte. Green and red represent decreased and increased concentrations, respectively, of the represented metabolites in the tumor microenvironment.
Tryptophan Catabolism
Tryptophan is an essential amino acid, is required for protein synthesis, and serves as a precursor to the neurotransmitters serotonin and melatonin. In addition, tryptophan can be further metabolized to kynurenine, which is driven by the rate-limiting enzymes indoleamine 2,3-dioxygenase 1 (IDO1) and tryptophan 2,3-dioxygenase (TDO). Over a decade ago, Munn et al demonstrated that modulating this pathway resulted in the rejection of allogenic fetuses, suggesting that this pathway is necessary for maintaining immune tolerance and fetal protection from maternal immune rejection in the placenta.17 Numerous studies have since identified many potential mechanisms of immune suppression offered by this metabolic pathway.18–20 This includes tryptophan depletion within the microenvironment, which represents a passive consequence of IDO1/TDO pathway activation in both tumor and immune cells. Tryptophan depletion results in cell cycle arrest and/or anergy in effector T cells while simultaneously fostering maturation and activation of regulatory T cells (Tregs).21 In addition, the kynurenine produced through pathway activation can be exported to the microenvironment, where it has the capacity to balance regulatory and effector responses of the immune system. This includes binding and activation of aryl hydrocarbon receptors (AHRs), a cytoplasmic transcription factor, resulting in reduced proliferation and infiltration of effector T cells.22 Kynurenine-activated AHRs are also responsible for providing a tolerogenic phenotype in DCs, resulting in increased production of Tregs and reduced type 1 T helper (Th1) cells (Figure 1).23,24 Activation of AHRs also results in increased production of TGF-β1, reduced production of interferon (IFN)-γ and IL-17,25 and regulation of IL-1β production. This suggests that AHRs work by reducing inflammatory cytokines and upregulating cytokines that drive conversion of naïve CD4+ cells toward a suppressive Treg phenotype, promoting tumorigenesis.22 Further, IDO pathway activation and upregulation of Tregs have been demonstrated to recruit and activate myeloid derived suppressor cells (MDSCs), furthering immune suppression.26 In addition to kynurenine, downstream metabolic intermediaries of tryptophan metabolism, including kynurenic acid, have been shown to activate AHRs, resulting in upregulation of IL-6 in the presence of IL-1β,27–29 which is important for maintaining the suppressive activity of MDSCs.
Interestingly, a variety of tumors, including glioblastoma, have evolved mechanisms to co-opt this potent mode of immune tolerance to evade the host immune system.20 This relationship between cancer and elevated tryptophan catabolism was first recognized in the 1950s with the identification of an accumulation of its metabolic intermediaries in the urine of cancer patients.30,31 A more direct role the IDO1 pathway plays in tumor immune evasion was later proposed, demonstrating a more robust T-cell response and delayed growth in vivo following pathway inhibition.32 Several recent studies have demonstrated that tryptophan catabolism, through both IDO1 and TDO, is particularly relevant in glioblastoma. Global metabolomic profiling identified an accumulation of several metabolic intermediaries of tryptophan metabolism in glioblastoma.8 Opitz et al demonstrated robust TDO-mediated pathway activation in a panel of glioma cell lines that resulted in inhibition of T-cell proliferation, and modulating this pathway influenced growth in vivo.22 Wainwright et al demonstrated significant IDO1 expression in glioblastoma that promoted an immunosuppressive environment through recruitment of Tregs33 and went on to show that the therapeutic efficacy of IDO1 inhibition can be significantly enhanced when combined with other immune checkpoint inhibitors.34 Preclinical studies also suggest that IDO1 inhibition can enhance radiation response in glioblastoma,35 and downstream metabolic intermediaries of tryptophan metabolism, including NAD+ and quinolinic acid, may play additional immune and non-immune roles in gliomagenesis.36
Arginine Metabolism
L-arginine is a semi-essential amino acid with several critical functions and metabolic fates. It is metabolically interconvertible with the amino acids proline and glutamate and serves as a precursor for the synthesis of protein, nitric oxide (NO), polyamines, and urea.37,38 These diverse processes are differentially regulated according to cell type, which includes several important roles in the regulation of immune response. Perhaps the most notable is in macrophages, which are categorized as M1 or M2, each displaying profoundly different phenotypes based on how arginine is metabolized.38 Macrophages classified as M1, which have been described to engage an immune response targeted to tumor cells, metabolize arginine to NO required for its cytotoxic activity via NO synthase. Conversely, M2 macrophages, which have been described as tumor promoting, use arginase to metabolize arginine, leading to the production of ornithine and urea, resulting in both anti-inflammatory properties and suppression of CD4+ T cell–mediated antitumoral immunity.38 The fate of arginine to NO and arginase production is regulated by Th1 and Th2 cytokines, respectively. It is unclear if these similar modes of polarization translate to microglia, which represent resident macrophages of the CNS. For example, both M1 and M2 microglia have been described39–41; however, their existence and biologic relevance and the role arginine plays in their polarization are still under investigation. In addition, the importance of arginine metabolism in myeloid cells has been well established. Specifically, both arginase and NO synthase activity in MDSCs contribute to immunosuppression by inhibiting T-cell proliferation through arginine depletion and peroxynitrate production (Figure 1).37,38 Recent literature also suggests that downstream metabolites of ornithine, when produced by DCs or MDSCs positive for arginase 1, result in activation of IDO1 and induction of TGF-β1. Consequently coexpression of arginase 1 and IDO1 in DCs results in formation of tolerogenic DCs.42 Therefore, both IDO1 and arginase pathway upregulation may work coordinately in promoting an immune suppressive microenvironment in glioblastoma.
Arginine depletion is actively being investigated as a treatment strategy in a variety of human malignancies. This approach is largely based on successes achieved in leukemia involving L-asparaginase. It was identified that leukemia cells often lack the biosynthetic enzyme asparagine synthase and are subsequently reliant on exogenous sources of asparagine. Therefore, treatment with L-asparaginase would limit this conditionally essential amino acid in these tumors.43,44 In a similar context, arginosuccinate synthase 1 (ASS1) is absent in many tumors, resulting in a reliance on exogenous arginine.44 Although the biologic advantage gained from this deletion in cancer is unclear, it does appear to contribute to a more aggressive phenotype with increased proliferation and invasion by limiting aspartate availability for nucleotide biosynthesis.45 It was initially demonstrated that arginine depletion in the tumor microenvironment was a result of MDSCs, which subsequently downregulated T-cell receptor zeta chains, reducing antitumor T-cell response.46,47 Arginine auxotrophy was reported in a panel of glioblastoma cell lines sensitive to arginine deprivation, which was determined by ASS1 expression,48 and involved in cell adhesion, invasion, and cytoskeletal organization.49 Conversely, additional studies identified increased expression of arginase in a variety of tumors and implicated pathway activation to tumor progression through polyamine synthesis or downregulation of NO-mediated tumor cytotoxicity.37 In glioblastoma, increased expression of arginine transporters has been reported,50 and our work involving global metabolomic profiling identified accumulation of several metabolites associated with arginine metabolism, including citrulline, arginosuccinate, urea, and ornithine.8 These findings suggest that this metabolic pathway is intact, although further study evaluating its intermediary metabolism is needed to provide insight into the biologic relevance of these findings. Although arginine metabolism has been implicated in tumorigenesis, the interplay of these metabolic changes with immune response is not as intuitive. Similar to the above described scenario of glucose and tryptophan depletion in the microenvironment, increased utilization of arginine in tumors could result in an arginine-depleted microenvironment. Further, acidosis of the microenvironment from enhanced glycolysis has been shown to increase arginase 1 expression in immune suppressive tumor associated macrophages (TAMs), further limiting antitumor immune response. However, others have shown that arginine depletion therapy may also induce MDSCs and subsequently blunt antitumor T-cell response.51 Therefore, further work needs to be performed to better understand both the immune and tumoral consequences of modulating arginine metabolism in glioblastoma.
Adenosine Metabolism
Numerous studies have identified adenosine as a potent anti-inflammatory molecule involved in restoration of tissue homeostasis through modulation of innate and adaptive immune response.52,53 One mechanism contributing to adenosine-mediated immune suppression involves its interplay between regulatory and effector T cells. Tregs have been demonstrated to express the ectoenzymes CD39 and CD73, allowing them to generate pericellular adenosine from extracellular nucleotides (Figure 1).54 The generated adenosine then binds to adenosine receptors, which are upregulated in activated effector T cells, generating an immunosuppressive signaling loop.55 In addition, immune suppression associated with adenosine metabolism has been demonstrated through regulation of neutrophil and macrophage activation.53,56,57 Although these findings may not extend to microglia, a recent study demonstrated that microglial cells produce high amounts of quinolinate, which can be utilized by glioblastoma to perform de novo synthesis of NAD+.36,58 NAD+ could consequently contribute to immune suppression by Treg-mediated conversion of NAD+ to adenosine using the adenosinergic pathway. Therefore, it may be possible that microglial cells also play an important role in immune suppression in glioblastoma. While extracellular adenosine levels are typically very low, tissue breakdown and hypoxia, which are typical in the tumor microenvironment, generate high levels of extracellular adenosine, thereby contributing to an immune suppressive state.57 Further, increased expression of adenosine receptors has been identified in a variety of tumors, including glioblastoma, and implicated in numerous oncogenic processes.56 The most notable is their role in stimulating the proliferation and migration of endothelial cells and vascular endothelial growth factor–mediated angiogenesis. In addition to adenosine receptors, tumors have been shown to express the ectoenzyme CD73, thereby in a fashion similar to Tregs increasing levels of this immune suppressive metabolite into the microenvironment.59,60 CD39 and CD73 activity in ovarian cancer was shown to contribute to the recruitment of TAMs that further amplified the adenosine-dependent immunosuppressive microenvironment.61 Further, studies evaluating glioblastoma stem cells have identified a unique capacity of these intrinsically aggressive cells to generate and export adenosine, which, in addition to contributing to therapeutic resistance in an immune compromised mouse model,62 may play a role in fostering an immune suppressive microenvironment.
Prostaglandins
Prostaglandins are derived from the metabolism of arachidonic acid, which is driven by cyclooxygenase (COX) and prostaglandin synthases. Prostaglandin E2 (PGE2) represents one of the most well studied downstream metabolic intermediaries of this pathway, contributing to many diverse biologic processes, from neuronal signaling to regulation of blood flow and vascular permeability. In addition, this metabolite, whose synthesis is controlled by local expression and activity of cyclooxygenase, is a key mediator in immunopathology, regulating inflammation at many levels.63,64 PGE2 has a paradoxical role of both promoting active inflammation but also shifting from an antitumor to an immunosuppressive response within the tumor microenvironment. For example, although PGE2 can recruit neutrophils, macrophages, and mast cells, it appears to modify their phenotype toward a tolerogenic state. In addition, PGE2 plays several important roles in antigen-specific immune responses. PGE2 along with COX2 form a positive-feedback loop that redirects the development of DCs to MDSCs that suppress the cytotoxic activity of effector T cells.65 PGE2 can also directly suppress T-cell activation through suppression of IL-2 production, shifting CD4+ T-cell responses from the aggressive antitumor Th1 subtype to the anti-inflammatory Th2/Th17 subtype. It can also promote the development of Tregs and shift macrophages from an M1 to an M2 phenotype (Figure 1).63 Further, this immune suppressive pathway appears to work coordinately with other immune checkpoints, including programmed cell death protein 166 and adenosine metabolism,67 and has been demonstrated to induce TDO expression,68 stimulating tryptophan metabolism.
Several studies have identified potential implications of prostaglandins in glioblastoma biology. For example, COX2 expression has been demonstrated to be aberrantly expressed in glioblastoma and to have prognostic significance.69,70 Nakano et al demonstrated that glioma cells could stimulate PGE2 production in macrophages, resulting in an immune suppressive state,71 and later studies went on to demonstrate pathway activation following therapy.72 Our metabolomic data identified several intermediaries of prostaglandin metabolism to be elevated in glioblastoma,8 suggesting relevance of this immune regulatory pathway in this tumor. Although more work is required to better understand the immunologic consequences of these findings, collectively they suggest that this metabolic switch may contribute to glioblastoma immune suppression.
Targeting the Immunometabolic Interface in Glioblastoma
As described in this review, tumors have co-opted several metabolic strategies that, in addition to playing a contributory role in the intrinsic growth of these tumors, promote an immune suppressive microenvironment. Therefore, directly targeting these metabolic nodes has promise in serving as next-generation immune checkpoint inhibitors. As discussed above, availability of glucose in the tumor microenvironment is a passive consequence of enhanced glycolysis typical in glioblastoma. Therefore, reverting this metabolic program may provide the requisite glucose for immune cells to stimulate an antitumor response. Hexokinase 2 (HK2) is an enzyme that converts glucose to glucose-6-phosphate. In addition to serving as an essential rate-limiting step for glycolysis, it traps glucose intracellularly, committing this important metabolite to cellular functions. It has been previously shown that upregulation and overexpression of HK2 play a key role in regulating aerobic glycolysis in glioblastoma, and targeting HK2 has therapeutic potential.73,74 Therefore, further studies defining the immune consequence of glycolysis inhibition in immune competent preclinical models would be of interest. Another strategy for targeting aerobic glycolysis is by modulating its downstream metabolism. Pyruvate dehydrogenase (PDH), which metabolizes pyruvate to acetyl CoA, represents an important gatekeeping enzyme regulating glycolytic flux into the mitochondria. Its activity is regulated by reversible phosphorylation. A family of PDH kinases (PDK) inhibit enzyme activity through phosphorylation, thereby shifting glucose metabolism toward fermentation, while dephosphorylation by PDH phosphatases stimulate enzyme activity, resulting in increased oxidative phosphorylation.75 The metabolic modulator dichloroacetate (DCA) inhibits PDK, thereby activating PDH and allowing for increased glycolytic flux into the mitochondria and oxidative phosphorylation, a strategy that has demonstrated activity in glioblastoma.76 It is unclear how modulating downstream glycolysis may influence glucose utilization and its subsequent availability in the microenvironment; however, both DCA along with HK2 inhibition would likely decrease lactic acid levels, thereby mitigating the potential immune suppressive effects of acidosis.77
One of the most clinically advanced strategies for targeting the immunometabolic interface in cancer involves tryptophan metabolism, with several IDO inhibitors currently being tested. These include PF-06840003, indoximod, 1-methyl-D-tryptophan, epacadostat (INCB024360), and GDC-0919, along with peptide vaccines, several of which are being studied in glioblastoma, as reviewed by Vacchelli et al.78 Further, as alterations in metabolic programming in tumors can potentially be studied using advanced imaging, incorporating correlative studies into clinical trials, including kynurenine/tryptophan ratios79 and C11 L-tryptophan-based PET tracers,80 represents a promising strategy to enrich for patients most likely to benefit from therapy and/or evaluate target engagement of an individual agent.
Arginine deprivation therapy is an active area of investigation based on the hypothesis that certain tumors are reliant on exogenous sources of this conditionally essential amino acid. Therefore, arginine degrading enzymes, including ADI-PEG 2044 and recombinant human arginase (PEG-BCT-100),48 represent strategies that are actively being investigated and, in addition to antitumor activity, may have broad immunological consequences. As current data supporting arginine auxotrophy as a metabolic phenotype in glioblastoma are mixed, more rigorous studies designed to determine the impact of arginine restriction on both tumor growth and immune response will better establish the clinical potential of this approach, as arginine depletion may also have an unintended consequence of promoting further immune suppression.51
Strategies designed to target adenosine metabolism have largely focused on its most well studied receptor, A2a. PBF-509 and CPI-444 represent 2 such antagonists currently being investigated in clinical trials; however, it is important to note that several similar compounds have already gone through phase III testing in Parkinson’s disease.57 Another strategy for modulating adenosine metabolism is by targeting the aberrantly expressed ectoenzyme CD73, which generates adenosine from extracellular nucleotides.81 As adenosine has multiple receptors, intra- and extracellular targets, and a range of biologic consequences dependent on the developmental stage of a given target, careful consideration of the timing of blockade and rationale combinatorial strategies will be important to extend promising laboratory findings clinically. Promising combinatorial strategies include evaluating adenosine modulation in combination with vaccines, chemotherapy, other immune checkpoint agents, and adoptive T-cell therapy, as reviewed by Leone et al.57
The initial interest in targeting prostaglandin metabolism was based on its anti-inflammatory roles, largely focused on COX2 inhibition in the management of arthritis. Its clinical development in arthritis was tempered by cardiovascular side effects (although recent data have questioned the severity of these events82), but COX2 inhibition continues to be actively investigated in oncology research, as both a chemopreventive and a therapeutic agent, as reviewed by Stasinopoulos et al.83 In addition to independent activity, these agents also have the potential for enhancing the activity of cancer vaccines and immune checkpoint inhibitors.66 Moving forward, next-generation agents are currently being developed to more specifically target the downstream intermediary metabolism of prostaglandin, including agonists and antagonists of individual PGE2 receptors and modulation of 15-hydroxyprostaglandin dehydrogenase, which controls PGE2 degradation.63
Limitations and Further Investigations
It is important to note that in this review, we did not discriminate between isocitrate dehydrogenase 1 (IDH1) mutant and wild-type glioblastoma. Although mutation in IDH1 results in the formation of a neomorphic enzyme with the capacity of generating the oncometabolite 2-hydroxyglutarate,84 its role in tumorigenesis appears to be epigenetic in nature.85 Further work is required to determine if IDH1 mutation in glioblastoma also results in global changes in metabolic programs that may interface with immune regulation in unique ways compared with IDH1 wild-type tumors. In addition, glioblastoma represents an archetypal example of a heterogeneous malignancy, harboring regions of invasion, necrosis, and vascularization. Although the influence of regional changes associated with tumor hypoxia on immune suppression was discussed in this review, further research evaluating if unique sets of metabolic programs driving growth in these distinct intratumoral biomes differentially modulate host immune response is warranted, potentially providing further insight into the multifaceted interface between these 2 oncogenic processes. For example, although glioblastoma has been typically characterized as a glycolytic tumor, several recent studies have shown that these tumors also rely heavily on oxidative phosphorylation,86,87 which may be driven by regional differences in the tumor microenvironment. Further, therapies may have unintended consequences on metabolism that can potentiate the immune tolerant microenvironment. For example, anti-angiogenic therapy has been reported to induce hypoxia in glioblastoma, resulting in a more glycolytic tumor,88 which as described above, may potentially contribute to further immune suppression. Therefore, further studies evaluating these complex interactions are warranted.
Conclusion
The fields of tumor metabolism and immuno-oncology have both independently received considerable attention over the last several years and recent investigations have identified elegant, multifaceted strategies by which alterations in tumor metabolism can also contribute to a potent tolerogenic immune environment. Continued investigations are required to better understand the individual contributions of these metabolic programs on immune tolerance in glioblastoma and how they interface with other known anti-immune strategies currently being tested clinically. Such studies may provide further insight into the complex modes of immune suppression co-opted by these tumors and identify novel combinatorial strategies that may build upon the recent successes of immunotherapy in cancer.
Funding
This work was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (R21NS090087) and the American Cancer Society (RSG-11-029-01) (P.C.).
Conflict of interest statement. None.
References
- 1. Coley WB. II. Contribution to the knowledge of sarcoma. Ann Surg. 1891;14(3):199–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154–158. [PMC free article] [PubMed] [Google Scholar]
- 3. Warburg O, Posener K, Negelein E. Uber den Stoffwechsel der Carcinomzelle. Biochem Zeitschr. 1924;152:309– 344. [Google Scholar]
- 4. Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21(3):297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chinnaiyan P, Kensicki E, Bloom G, et al. The metabolomic signature of malignant glioma reflects accelerated anabolic metabolism. Cancer Res. 2012;72(22):5878–5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38(4):633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Everts B, Amiel E, van der Windt GJ, et al. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120(7):1422–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krawczyk CM, Holowka T, Sun J, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115(23):4742–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Biswas SK. Metabolic reprogramming of immune cells in cancer progression. Immunity. 2015;43(3):435–449. [DOI] [PubMed] [Google Scholar]
- 13. Ghesquière B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature. 2014;511(7508):167–176. [DOI] [PubMed] [Google Scholar]
- 14. Hasmim M, Noman MZ, Messai Y, et al. Cutting edge: hypoxia-induced Nanog favors the intratumoral infiltration of regulatory T cells and macrophages via direct regulation of TGF-β1. J Immunol. 2013;191(12):5802–5806. [DOI] [PubMed] [Google Scholar]
- 15. Becker JC, Andersen MH, Schrama D, Thor Straten P. Immune-suppressive properties of the tumor microenvironment. Cancer Immunol Immunother. 2013;62(7):1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singer K, Gottfried E, Kreutz M, Mackensen A. Suppression of T-cell responses by tumor metabolites. Cancer Immunol Immunother. 2011;60(3):425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–1193. [DOI] [PubMed] [Google Scholar]
- 18. Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34(3):137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 2012;72(21):5435–5440. [DOI] [PubMed] [Google Scholar]
- 20. van Baren N, Van den Eynde BJ. Tryptophan-degrading enzymes in tumoral immune resistance. Front Immunol. 2015;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Munn DH, Sharma MD, Hou D, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114(2):280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. [DOI] [PubMed] [Google Scholar]
- 23. Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185(6):3190–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nguyen NT, Kimura A, Nakahama T, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A. 2010;107(46):19961–19966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quintana FJ, Basso AS, Iglesias AH, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. [DOI] [PubMed] [Google Scholar]
- 26. Holmgaard RB, Zamarin D, Li Y, et al. Tumor-expressed IDO recruits and activates MDSCs in a treg-dependent manner. Cell Rep. 2015;13(2):412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DiNatale BC, Murray IA, Schroeder JC, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115(1):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hollingshead BD, Beischlag TV, Dinatale BC, Ramadoss P, Perdew GH. Inflammatory signaling and aryl hydrocarbon receptor mediate synergistic induction of interleukin 6 in MCF-7 cells. Cancer Res. 2008;68(10):3609–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67(20):10019–10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boyland E, Williams DC. The metabolism of tryptophan. 2. The metabolism of tryptophan in patients suffering from cancer of the bladder. Biochem J. 1956;64(3):578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhai L, Lauing KL, Chang AL, et al. The role of IDO in brain tumor immunotherapy. J Neurooncol. 2015;123(3):395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friberg M, Jennings R, Alsarraj M, et al. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int J Cancer. 2002;101(2):151–155. [DOI] [PubMed] [Google Scholar]
- 33. Wainwright DA, Balyasnikova IV, Chang AL, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012;18(22):6110–6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20(20):5290–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li M, Bolduc AR, Hoda MN, et al. The indoleamine 2,3-dioxygenase pathway controls complement-dependent enhancement of chemo-radiation therapy against murine glioblastoma. J Immunother Cancer. 2014;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sahm F, Oezen I, Opitz CA, et al. The endogenous tryptophan metabolite and NAD+ precursor quinolinic acid confers resistance of gliomas to oxidative stress. Cancer Res. 2013;73(11):3225–3234. [DOI] [PubMed] [Google Scholar]
- 37. Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158(3):638–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rath M, Müller I, Kropf P, Closs EI, Munder M. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol. 2014;5:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miron VE, Boyd A, Zhao JW, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16(9):1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tang Y, Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol. 2016;53(2):1181–1194. [DOI] [PubMed] [Google Scholar]
- 41. Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19(8):987–991. [DOI] [PubMed] [Google Scholar]
- 42. Mondanelli G, Bianchi R, Pallotta MT, et al. A relay pathway between arginine and tryptophan metabolism confers immunosuppressive properties on dendritic cells. Immunity. 2017;46(2):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jaffe N, Traggis D, Das L, et al. Favorable remission induction rate with twice weekly doses of L-asparaginase. Cancer Res. 1973;33(1):1–4. [PubMed] [Google Scholar]
- 44. Phillips MM, Sheaff MT, Szlosarek PW. Targeting arginine-dependent cancers with arginine-degrading enzymes: opportunities and challenges. Cancer Res Treat. 2013;45(4):251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rabinovich S, Adler L, Yizhak K, et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature. 2015;527(7578):379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rodriguez PC, Zea AH, DeSalvo J, et al. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol. 2003;171(3):1232–1239. [DOI] [PubMed] [Google Scholar]
- 47. Rodriguez PC, Quiceno DG, Zabaleta J, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64(16):5839–5849. [DOI] [PubMed] [Google Scholar]
- 48. Khoury O, Ghazale N, Stone E, El-Sibai M, Frankel AE, Abi-Habib RJ. Human recombinant arginase I (Co)-PEG5000 [HuArgI (Co)-PEG5000]-induced arginine depletion is selectively cytotoxic to human glioblastoma cells. J Neurooncol. 2015;122(1):75–85. [DOI] [PubMed] [Google Scholar]
- 49. Pavlyk I, Rzhepetskyy Y, Jagielski AK, et al. Arginine deprivation affects glioblastoma cell adhesion, invasiveness and actin cytoskeleton organization by impairment of β-actin arginylation. Amino Acids. 2015;47(1):199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kobayashi K, Ohnishi A, Promsuk J, et al. Enhanced tumor growth elicited by L-type amino acid transporter 1 in human malignant glioma cells. Neurosurgery. 2008;62(2):493–503; discussion 503. [DOI] [PubMed] [Google Scholar]
- 51. Fletcher M, Ramirez ME, Sierra RA, et al. l-Arginine depletion blunts antitumor T-cell responses by inducing myeloid-derived suppressor cells. Cancer Res. 2015;75(2):275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414(6866):916–920. [DOI] [PubMed] [Google Scholar]
- 53. Thiel M, Caldwell CC, Sitkovsky MV. The critical role of adenosine A2A receptors in downregulation of inflammation and immunity in the pathogenesis of infectious diseases. Microbes Infect. 2003;5(6):515–526. [DOI] [PubMed] [Google Scholar]
- 54. Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mandapathil M, Hilldorfer B, Szczepanski MJ, et al. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem. 2010;285(10):7176–7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gessi S, Merighi S, Sacchetto V, Simioni C, Borea PA. Adenosine receptors and cancer. Biochim Biophys Acta. 2011;1808(5):1400–1412. [DOI] [PubMed] [Google Scholar]
- 57. Leone RD, Lo YC, Powell JD. A2aR antagonists: next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J. 2015;13:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morandi F, Morandi B, Horenstein AL, et al. A non-canonical adenosinergic pathway led by CD38 in human melanoma cells induces suppression of T cell proliferation. Oncotarget. 2015;6(28):25602–25618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. de Lourdes Mora-García M, García-Rocha R, Morales-Ramírez O, et al. Mesenchymal stromal cells derived from cervical cancer produce high amounts of adenosine to suppress cytotoxic T lymphocyte functions. J Transl Med. 2016;14(1):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Saldanha-Araujo F, Ferreira FI, Palma PV, et al. Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res. 2011;7(1):66–74. [DOI] [PubMed] [Google Scholar]
- 61. Montalbán Del Barrio I, Penski C, Schlahsa L, et al. Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages—a self-amplifying, CD39- and CD73-dependent mechanism for tumor immune escape. J Immunother Cancer. 2016;4:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Torres A, Vargas Y, Uribe D, et al. Adenosine A3 receptor elicits chemoresistance mediated by multiple resistance-associated protein-1 in human glioblastoma stem-like cells. Oncotarget. 2016;7(41):67373–67386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang D, DuBois RN. The role of prostaglandin E(2) in tumor-associated immunosuppression. Trends Mol Med. 2016;22(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118(20):5498–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zelenay S, van der Veen AG, Böttcher JP, et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. 2015;162(6):1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mandapathil M, Szczepanski MJ, Szajnik M, et al. Adenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. J Biol Chem. 2010;285(36):27571–27580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ochs K, Ott M, Rauschenbach KJ, et al. Tryptophan-2,3-dioxygenase is regulated by prostaglandin E2 in malignant glioma via a positive signaling loop involving prostaglandin E receptor-4. J Neurochem. 2015;doi: 10.1111/jnc.13503. [DOI] [PubMed] [Google Scholar]
- 69. Joki T, Heese O, Nikas DC, et al. Expression of cyclooxygenase 2 (COX-2) in human glioma and in vitro inhibition by a specific COX-2 inhibitor, NS-398. Cancer Res. 2000;60(17):4926–4931. [PubMed] [Google Scholar]
- 70. Shono T, Tofilon PJ, Bruner JM, Owolabi O, Lang FF. Cyclooxygenase-2 expression in human gliomas: prognostic significance and molecular correlations. Cancer Res. 2001;61(11):4375–4381. [PubMed] [Google Scholar]
- 71. Nakano Y, Kuroda E, Kito T, et al. Induction of prostaglandin E2 synthesis and microsomal prostaglandin E synthase-1 expression in murine microglia by glioma-derived soluble factors. Laboratory investigation. J Neurosurg. 2008;108(2):311–319. [DOI] [PubMed] [Google Scholar]
- 72. Authier A, Farrand KJ, Broadley KW, et al. Enhanced immunosuppression by therapy-exposed glioblastoma multiforme tumor cells. Int J Cancer. 2015;136(11):2566–2578. [DOI] [PubMed] [Google Scholar]
- 73. Rempel A, Mathupala SP, Griffin CA, Hawkins AL, Pedersen PL. Glucose catabolism in cancer cells: amplification of the gene encoding type II hexokinase. Cancer Res. 1996;56(11):2468–2471. [PubMed] [Google Scholar]
- 74. Vartanian A, Agnihotri S, Wilson MR, et al. Targeting hexokinase 2 enhances response to radio-chemotherapy in glioblastoma. Oncotarget. 2016;7(43):69518–69535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Prabhu A, Sarcar B, Miller CR, et al. Ras-mediated modulation of pyruvate dehydrogenase activity regulates mitochondrial reserve capacity and contributes to glioblastoma tumorigenesis. Neuro Oncol. 2015;17(9):1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Michelakis ED, Sutendra G, Dromparis P, et al. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2(31):31ra34. [DOI] [PubMed] [Google Scholar]
- 77. Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vacchelli E, Aranda F, Eggermont A, et al. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2014;3(10):e957994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhai L, Dey M, Lauing KL, et al. The kynurenine to tryptophan ratio as a prognostic tool for glioblastoma patients enrolling in immunotherapy. J Clin Neurosci. 2015;22(12):1964–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kamson DO, Mittal S, Robinette NL, et al. Increased tryptophan uptake on PET has strong independent prognostic value in patients with a previously treated high-grade glioma. Neuro Oncol. 2014;16(10):1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Loi S, Pommey S, Haibe-Kains B, et al. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A. 2013;110(27):11091–11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nissen SE, Yeomans ND, Solomon DH, et al. ; PRECISION Trial Investigators. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375(26):2519–2529. [DOI] [PubMed] [Google Scholar]
- 83. Stasinopoulos I, Shah T, Penet MF, Krishnamachary B, Bhujwalla ZM. COX-2 in cancer: Gordian knot or Achilles heel? Front Pharmacol. 2013;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lu C, Ward PS, Kapoor GS, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483(7390):474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Maher EA, Marin-Valencia I, Bachoo RM, et al. Metabolism of [U-13 C]glucose in human brain tumors in vivo. NMR Biomed. 2012;25(11):1234–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Marin-Valencia I, Yang C, Mashimo T, et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15(6):827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fack F, Espedal H, Keunen O, et al. Bevacizumab treatment induces metabolic adaptation toward anaerobic metabolism in glioblastomas. Acta Neuropathol. 2015;129(1):115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]