Abstract
With the release of the 2016 edition of the World Health Organization (WHO) Classification of Central Nervous System Tumors, brain invasion in meningiomas has been added as a stand-alone criterion for atypia and can therefore impact grading and indirectly adjuvant therapy. Regarding this rising clinical importance, we have reviewed the current knowledge about brain invasion with emphasis on its implications on current and future clinical practice. We found various definitions of brain invasion and approaches for evaluation in surgically obtained specimens described over the past decades. This heterogeneity is reflected by weak correlation with prognosis and remains controversial. Similarly, associated clinical factors are largely unknown. Preoperative, imaging-guided detection of brain invasion is unspecific, and intraoperative assessment using standard and new high-magnification microscopic techniques remains imprecise. Despite the increasing knowledge about molecular alterations of the tumor/ brain surface, pharmacotherapeutic options targeting brain invasive meningiomas are lacking. Finally, we summarize the impact of brain invasion on histopathological grading in the WHO classifications of brain tumors since 1979.
In conclusion, standardized neurosurgical sampling and neuropathological analyses could improve diagnostic reliability and reproducibility of future studies. Further research is needed to improve pre- and intraoperative visualization of brain invasion and to develop adjuvant, targeted therapies.
Keywords: brain invasion, grading, meningioma, prognosis, resection
Importance of the study
In the 2016 edition of the WHO classification of brain tumors, brain invasion in meningiomas has gained the highest clinical relevance, as it now directly impacts grading. Hence, brain invasion can now influence considerations about patients’ prognosis and inclusion in clinical trials and can indirectly impact adjuvant therapy. However, varying approaches for histopathological analyses are described and the prognostic value of brain invasion remains controversial. Additionally, several studies investigated correlations of brain invasion with clinical variables and imaging findings. We reviewed the current knowledge and published studies about brain invasion with emphasis on its implications on current and future clinical neurooncological and neurosurgical practice, including preoperative imaging, surgery, and adjuvant therapy. We discuss the variability of neuropathological approaches to evaluate brain invasion, elucidate the prognostic value of brain-invasive growth among the studies published so far, and summarize its impact on histopathological grading in the WHO classifications of brain tumors published since 1979.
Based on the increased risk of tumor progression, the 2016 edition of the World Health Organization (WHO) Classification of Central Nervous System Tumors now lists brain invasion as a stand-alone grading criterion for the diagnosis of an atypical grade II meningioma. Although explicitly specified as tumor tissue within the adjacent brain without a separating connective tissue layer,1 a variety of other definitions and assessment methods of brain invasion have been described.2–4 Several studies have focused on brain invasion and its associated variables, mainly emphasizing the characterization of the surface between the brain and the tumor,5–10 associated changes in the extracellular matrix,7,11–17 genetic alterations,18–29 as well as correlations with clinical parameters and prognosis.2–5,10,16,24,29–40 Moreover, few authors have analyzed the pre- and intraoperative assessment of brain invasive growth, as well as targeted pharmacological options.2,4,30,41,42 Despite providing increasing insights into these topics, varying key aspects, methods, and approaches to evaluate brain invasion in these studies, as well as different WHO classifications1,28,43,44 used over a long period of time, have led to inconclusive results and different interpretations. Accordingly, a broad variety and a lack of correlations between brain invasion and patients’ prognosis have been shown in some studies.32,45 Thus, although not listed as a grading criterion for more than 2 decades, worse prognosis of brain invasive meningiomas has been described in the WHO classifications of brain tumors since 1993.43 With the release of the 2016 edition, this heterogeneity gained highest clinical relevance, as microscopic evidence of brain invasion, even in the absence of further histopathological criteria of atypia, is now sufficient to impact tumor grading and therefore indirectly adjuvant therapy as well as inclusion in clinical trials.1,46
Regarding this increasing importance in routine meningioma diagnostics and treatment, we here provide an overview of the clinical implications of brain invasion based on an extensive Medline research. In particular, we aimed to (i) elucidate the different pre-, intra-, and postoperative methods for assessing brain invasion as well as (ii) the impact of brain invasion on tumor progression and survival. Moreover, we aimed to (iii) describe the current knowledge about specific adjuvant treatment options for brain invasive meningiomas. Finally, we intended to (iv) summarize the impact of brain invasion on histopathological grading in the WHO classifications of brain tumors published since 1979 and aimed to explore the implication of the 2016 changes on both clinical practice and trials.
Materials and Methods
An extensive Medline search was performed for the MeSH terms “meningioma” in combination with “brain invasion,” “brain infiltration,” and “brain invasive.” Initial search was performed on November 5, 2016 and repeated on January 17, 2017. No additional relevant articles were found from the repeated searches. Retrieved records were published between 1950 and January 2017. Titles, abstracts, and full texts obtained, in addition to the cited studies, were systematically screened by 2 independent observers and included in this review, if they were relevant to the topics described above. Case reports, clinical studies, clinical trials, comparative studies, and comments were considered independent of the follow-up period, WHO classification used for grading, and date of publication. Abstracts were only included if providing detailed and explicit information about methods and results. Both full texts and abstracts not written in English were not further considered. Implications for brain invasion in the 1979, 1993, 2000, 2007, and 2016 WHO classifications of CNS tumors were also reviewed and summarized in a separate section. Hence, of 476 records assessed for eligibility, 58 sources were included in this review (Fig. 1). For a concise description in this manuscript, brain invasive grade II tumors with no further histopathological criteria of atypia are labeled “otherwise benign” meningiomas.
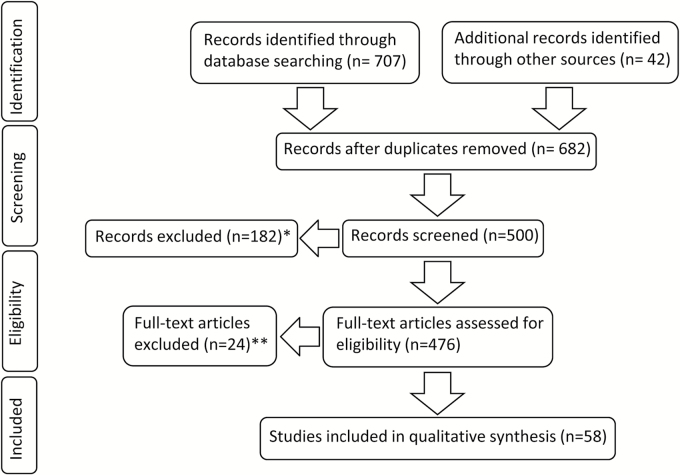
Fig. 1.
Flowchart showing the selection process of relevant studies. In addition to 707 PubMed results identified through database searching, 42 records including quotations, 5 editions of the WHO classification of brain tumors, and recent studies known to the authors were screened. In 182 excluded cases, the abstracts did not provide detailed information about methods and results and full texts were lacking (*). Moreover, 24 full texts could not be considered, since they were not written in English (**).
Results
Different Approaches of Brain Invasion Detection
For this section, all 58 eligible records were reviewed. According to the broad variety of studies published over the past decades, several definitions of brain invasion have been described, and a number of different approaches for detection in both research and clinical context have been proposed.
Among all related publications which were analyzed for this review, brain invasion was further specified in 16 manuscripts2–4,6,8–12,18,30,32,38,45,47,48 and aside from one exception was2 mostly delineated in reference to Perry et al,32 who defined brain invasive growth in 1997 as the presence of meningioma tissue within the adjacent brain without a separating connective tissue layer. Prior to that publication, the definition of brain invasion was rather inconsistent and included2,3,8 or excluded18,38 the spreading of tumor cells within the Virchow–Robin spaces. While mostly not further specified, only 7 studies have described the partially distinct infiltrative growth patterns.6,9,10,12,18,45,47 Among those studies, tongue- or finger-like protrusion of the tumor into the adjacent brain tissue is the most frequently reported invasion subtype.6,9,10,12,45,47 Additionally, several studies found diffuse (single cells spreading into the brain parenchyma18,45) and cluster-like (clustered “nests”/islands of tumor cells6,18,45,47) invasion of the tumor into the adjacent brain parenchyma (Fig. 2). Moreover, gender-specific patterns of infiltration were found in one study (see below).45
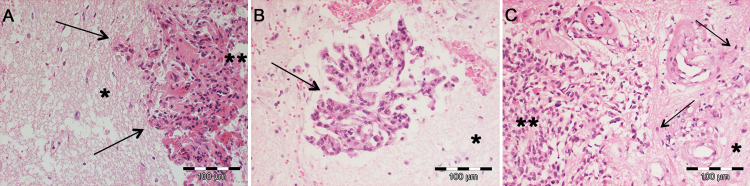
Fig. 2.
Examples of different microscopic patterns of brain invasion. Although finger-like invasion is mostly described (A, arrows), several studies also showed meningioma cell clusters/islands in the adjacent CNS parenchyma distant from the main tumor (B, arrow) or single tumor cells diffusely infiltrating the brain (arrows). (A–C) Hematoxylin and eosin staining; *brain tissue; **main meningioma tissue.
Microscopically detectable cerebral tissue was considered mandatory to determine brain invasive growth in only 7 of the 47 (15%)4,10,23,30,32,33,37 reviewed studies with available data, while this was not further specified in the vast majority of papers and one group also requested macroscopically detectable brain on the microscopic slides.6 In 2014, Pizem et al showed that “extensive sampling” of resected tumors, in terms of increasing the number of tissue blocks analyzed, raised the frequency of detection of brain invasion.4 Accordingly, although with some limitations, since WHO grades of the tumors in the different studies were not generally considered, we found a correspondingly higher average frequency of brain invasion in studies in which the presence of neural tissue on microscopic slides was mandatory to evaluate brain invasion (50%, range 26%–77% vs 16%, range 3%–46%).
Among 31 studies with available data, brain invasion was uniformly light-microscopically diagnosed without using immunohistochemical staining. However, when brain invasion was suspected, but not clearly determinable on standard hematoxylin and eosin slides, staining for glial fibrillary acid protein, CD44, and epithelial membrane antigen2,4,9,10,12 was shown to increase sensitivity for detecting brain invasion in 5 studies.
According to the different approaches to assess brain invasion, reported incidences varied among all studies and further depended on the WHO grades of the tumors included in each analysis. Thus, studies investigating meningiomas of all WHO grades revealed brain invasion in 5%–78%, while rates were increased as predicted, when only investigating atypical (13%–70%) and anaplastic meningiomas (70%–100%).4,17,19,23,33,39,45
Correlation of Brain Invasion with Clinical Variables
Fifteen studies were found reporting information about the correlation between clinical variables and brain invasion.2,4,16,29,30,32,36–38,45,49–53 Among those studies, the incidence of brain invasion was generally not correlated to patients’ age and has been observed in pediatrics harboring both sporadic and neurofibromatosis 2–associated tumors.38,52,53 Among the latter, brain invasive growth was found to be less common than in individuals with sporadic tumors.52
Two studies demonstrated a slight trend of a higher frequency of invasive growth in males than in females29,30 which we could confirm in a statistically significant manner.45 However, in the same series, brain invasion was strongly correlated with the presence of further histopathological grading criteria. Moreover, invasion was shown to have a mostly finger-like pattern in males, while in females the pattern showed a predominantly cluster-like infiltrative growth.
Although the degree of resection according to the Simpson classification54 was independent of brain invasive tumor growth in 2 series,16,45 Pizem et al4 reported a correlation of microscopically detectable brain invasion and intraoperative cleavability of the tumor with the brain surface and therefore contradicted previous findings from Vranic et al.30 However, despite a lack of correlation between the extent of resection and brain invasive tumor growth,16,45 several studies32,36,37,45 demonstrated that the prognostic impact of brain invasion might vary depending on the grade of resection (see below).
Generally, studies investigating potential associations between brain invasion and patients symptoms are sparse. In a series of 86 meningiomas, Vranic et al reported a higher frequency of behavior changes in brain invasive tumors in univariate analyses.51 Additionally, an increased risk of seizures appears supposable regarding both cortical infiltration after disruption of the pial layer and potentially distinctive peritumoral edema of brain invasive meningiomas.2,7,50,55–57 In fact, an increased incidence of seizures in patients with macroscopically brain invasive grade II and III meningiomas, compared with non-invasive meningiomas, has been described.50 However, brain infiltrative growth was not confirmed by microscopic analyses in this study.
Tumor location was found to be independent of brain invasion in all studies with available data,16,30,45,49 and to date no other radiologically detectable parameter was shown to be sufficient to predict brain invasion preoperatively by either MRI or CT. Although Mantle et al reported a strong correlation of invasive growth and peritumoral edema and demonstrated a 20% increase of brain invasion incidence for each centimeter of edema (as illustrated in Fig. 3),2 this finding is nonspecific and was later contradicted by Pizem et al.4 Moreover, expression of vascular endothelial growth factor, a driving factor for developing peritumoral edema in meningiomas,58,59 was not correlated with brain invasion.12,22
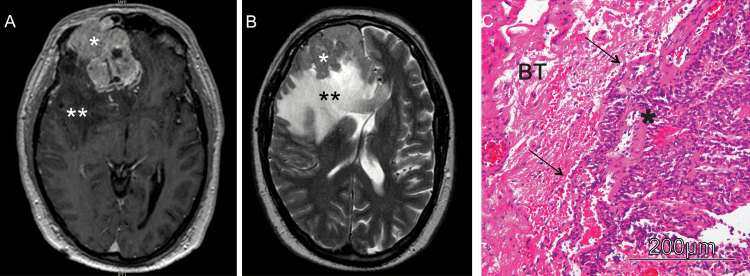
Fig. 3.
Illustrative cranial MRI of a patient with a brain invasive meningioma. (A) Preoperative, axial post-gadolinium (GD) T1-weighted MRI revealing a large right frontal, contrast-enhancing meningioma. (B) Axial T2-weighted image shows the distinct peritumoral edema. In neuropathological analyses, the tumor showed finger-like invasion into the adjacent brain tissue (C; hematoxylin and eosin staining); *meningioma; **peritumoral brain edema; BT, brain tissue.
Impact of Brain Invasion on Prognosis
Twenty-one studies2–5,10,16,30–37,40,45,48,49,60 were found investigating brain invasion in correlation with patients’ prognosis with a varying median follow-up period between 32 months to over 10 years (Table 1). Of those, 14 (70%) reported an association of brain invasion with tumor recurrence after uni- or multivariate analyses with a broad variety of hazard ratios (Fig. 4),2,4,5,30,32–35,37,39,40,45,48,60 or showed increased incidence of invasive growth in recurrent meningiomas.4 However, several studies consistently reported invasive growth to be only prognostic after gross but not after subtotal tumor resection.32,36,37,45 Although a correlation of brain invasion with prognosis is therefore generally postulated, several studies reported significant associations of brain invasion with further histopathological criteria of atypia,4,10,45,60 probably additionally contributing to a worse prognosis in some of these cases.
Table 1.
Studies investigating brain invasion in correlation with prognosis
| Year | Author | No. of Patients and WHO Grades Included | WHO Classification | Microscopic Brain Tissue | Mortality | Progression | Median Follow-up |
|---|---|---|---|---|---|---|---|
| 1970 | Crompton et al5 | 70, grades N/A | N/A | No | N/A | Trend, no statistical analyses | N/A |
| 1993 | McLean et al3 | 28 °II–III | 1993 | No | N/A | n.s. | N/A |
| 1997 | Perry et al32 | 89, grades N/A | 1993 | Yes | N/A | Correlation in UVA and MVAa after GTR | N/A |
| 1999 | Perry et al34 | 116 °I–III | N/A | N/a | N/A | Correlation in UVA and MVAa after GTR | N/A |
| 1999 | Mantle et al2 | 135 °I–III | N/A | No | N/A | Correlation in UVA and MVA | 9 y |
| 2002 | Ho et al35 | 58 °I, 25 °II | N/A | No | N/A | Correlation in UVA | 159 mo |
| 2008 | Yang et al33 | 33 °II, 41° III | 2000 | Yes | Correlation in °III tumors in UVA | Correlation in irradiated °II in MVA | 43 mo |
| 2008 | Moradi et al40 | 329 °I, 41 °II, 8 °III | 2000 | No | N/A | Correlation in UVA | N/A |
| 2009 | Lee et al39 | 43 °I, 13° II, 3 °III | 2007 | No | N/A | Correlation in UVA | 34 mo |
| 2010 | Vranic et al30 | 76 °II, 10 °III | 2000 | Yes | Correlation in UVA | Correlation in MVAa in °III tumors | 96 mo |
| 2010 | Ruiz et al16 | 208 °I, 39 °II | 2007 | No | n.s. | n.s. | 8 y |
| 2014 | Backer- Grondal et al10 | 34 °I, 33°II | 2007 | Yes | n.s. | n.s. | >8 y |
| 2014 | Pizem et al4 | 233 °I, 51 °II, 10 °III | 2000 | Yes | N/A | Correlation in UVA | 51 mo |
| 2014 | Sun et al37 | 151 °II after GTR | 2007 | No | N/A | Correlation in UVA and MVAa | 45 mo |
| 2014 | Sun et al36 | 210 °II after STR | 2007 | No | N/A | n.s. | 67 mo |
| 2015 | Klinger et al31 | 45 °II | 2000 + 2007 | No | N/A | n.s. | 65 mo |
| 2015 | Yoon et al60 | 158 °II | 2000 | No | n.s. | Correlation in UV | 32 mo |
| 2015 | Sun et al48 | 50 °II | 2007 | Yes | N/A | Correlation in UVA and MVAa | 86 mo |
| 2016 | Spille et al45 | 401 °I, 60 °II, 6 °III | 2007 | No | n.s. | Correlation in UVA and only after GTR | 91 mo |
| 2016 | Telegu et al49 | 194 °I, 24 °II, 6 °III | 2007 | No | N/A | n.s. | N/A |
| 2016 | Baumgarten et al79 | 229 °II | 2016 | No | n.s. | n.s | 22 mo |
Abbreviations: N/A = not applicable; n.s. = not significant; GTR = gross total resection; UVA = univariate analyses; MVA = multivariate analyses.
aIncluding one or more further histopathological features of atypia. Varying WHO classifications, grades of included tumors, and follow-up periods were used within the included studies. However, most studies report correlations with tumor recurrence, while increased mortality in brain invasive meningiomas is rarely reported.
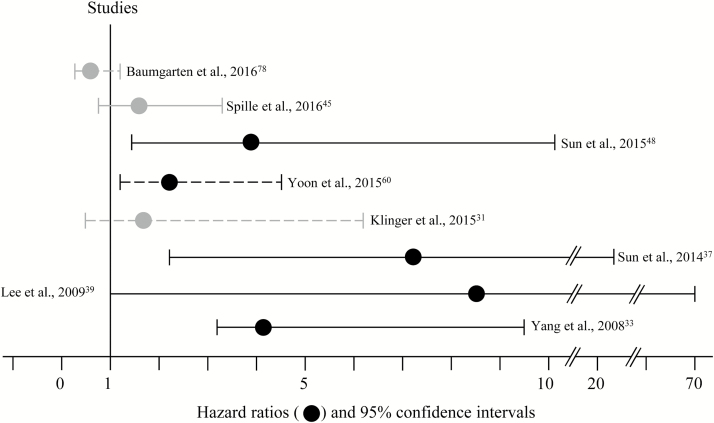
Fig. 4.
Risk of tumor recurrence comparing brain invasive and non-invasive meningiomas. In most studies, brain invasion was significantly (black graphs) correlated with an increased hazard ratio of tumor recurrence in uni- (dashed lines) or multivariate (solid lines) analyses. However, no statistically significant correlation between tumor recurrence and brain invasion was found in some studies (gray graphs). For this figure, studies without information about hazard ratios and confidence intervals were excluded.
Compared with tumor progression, mortality was investigated only in the minority of studies (n = 8).3,10,16,24,30,33,45,60 Among those, brain invasion was of minor prognostic value and correlated with mortality in uni- but not in multivariate analyses in 3 series only.24,30,33
Surgery for Brain Invasive Meningiomas
Among all reviewed records, 8 studies were found investigating the role of brain invasion during microsurgery for meningioma.4,16,30,32,36,37,41,45 Since sensitivity of in vivo detection of brain invasion using the standard intraoperative microscope is considered inferior to histopathological analyses and correlation with cleavability remains controversial,4,30 Peyre et al investigated the feasibility of in vivo and ex vivo confocal microscopy to detect brain invasion in a rodent model. After both the brain–meningioma interface as well as the tumor spread along the Virchow–Robin spaces were clearly identifiable in both native rodent meningioma samples and after the administration of fluorescein, the authors subsequently proposed to conduct further studies to investigate the feasibility of confocal (fluorescence) microscopy to detect brain invasion in humans.41 While intraoperative 5-aminolevulinic acid–induced protoporphyrin IX fluorescence was shown to enable visualization of bone invasion in meningiomas,61 increased sensitivity for detection of brain invasive growth has not been reported yet. Interestingly, although an increased intraoperative cleavability of the tumor does not necessarily indicate brain invasion4,30 and the extent of resection is considered to be independent of invasive growth,16,45 several studies showed that brain invasion is only prognostic after gross but not after subtotal tumor resection (see discussion).32,36,37,45
Irradiation in Brain Invasive Meningiomas
The prognostic influence of adjuvant irradiation in high-grade meningiomas is widely investigated and remains controversial.62–64 However, although shown to harbor distinct genetic alterations,18–20 brain invasive meningiomas were found to be investigated by only 2 separate studies in this context.37,48 In 2014, Sun et al reported no prognostic impact of adjuvant external beam radiation therapy (EBRT) in a small series of gross totally resected brain invasive atypical meningiomas.37 In a larger series of 50 radiation-naive patients who received either adjuvant EBRT or stereotactic radiosurgery for residual or recurrent atypical meningioma, brain invasion was correlated with a 3.5-fold increased risk of tumor recurrence compared with non-invasive atypical meningiomas.48
Pharmacological Approaches Directly Targeting Brain Invasion
One study was found investigating a drug directly targeting brain invasive meningiomas.42 In gliomas, alterations of integrin-mediated signaling pathways have been shown to promote brain invasion,65 and the effects of cilengitide, a specific integrin inhibitor, have been investigated in phase I, II, and III trials.66–68 After integrins have also been shown to play an important role for invasive growth in meningiomas,69,70 Wilisch-Neumann et al investigated the effects of cilengitide in meningioma cell lines and mouse models.42 Although not affecting survival or tumor volume, the authors report an inhibition of cell migration and invasive growth in vitro and correspondingly reduced brain invasion in mice after administration of cilengitide. Since performing adjuvant irradiation after cilengitide revealed some evidence of impacting tumor volume in subgroup analyses, the authors propose further studies to clarify the utility of a combined treatment.
Brain Invasion and WHO Grading
Regarding the above-mentioned variety of descriptions and definitions, the consideration of brain invasion and its impact on patients’ prognosis and histopathological grading underwent substantial evolution within the past 4 decades and 5 editions of WHO classifications of brain tumors. With the release of the 1979 edition, several meningioma subtypes as well as proposals for histopathological grading were described,71 while brain invasion as a grading criterion was initially mentioned in the subsequent series in 1993.43 Referring to a consensus conference, the authors state that some neuropathologists “maintained that gross brain invasion (. . .) qualifies a tumor for the designation malignant,” therefore rather vaguely proposing invasive growth as a criterion for anaplasia. Later on, the 2000 WHO classification initially specified brain invasion as “characterized by irregular groups of tumor cells infiltrating the adjacent cerebral parenchyma, without an intervening layer of leptomeninges.”28 Similar to 1993, the authors note that brain invasion can be found in otherwise histopathologically benign, atypical, or anaplastic meningiomas. Referring to the findings by Perry et al in 1997,32 the 2000 WHO classification further reports a similar prognosis of brain invasive but histopathologically benign and atypical meningiomas, while brain invasion itself was not listed as a criterion for either atypia or anaplasia. In fact, although the authors state that brain invasive but otherwise benign meningiomas “should prognostically be considered WHO grade II,” they do not conclude that these tumors should also histopathologically be diagnosed as grade II. Interestingly, although this appears to be critical with regard to a uniform histopathological grading, the following 2007 edition did not provide any further details to clarify the role of brain invasion in meningioma grading.44 Accordingly, while regarded as a criterion for atypia by most authors, brain invasion was not included in several studies published within the 20004,6 and 200729,49 WHO classification eras as a criterion for histopathological grading. About 23 years after the first description, the revised and currently published 2016 WHO classification now further specifies brain invasion and its impact on histopathological grading by indexing it as a stand-alone criterion for atypia.1
Discussion
This systematic literature review revealed various definitions of brain invasion and different methods of assessment. While its definition was rather vague and partially included tumor spreading along the Virchow–Robin spaces in studies published in the 80s and early 90s,2,3,8,18,38 most reports released after 1997 defined invasive growth more precisely and uniformly according to the pioneering work by Perry et al.32 However, the mandatory presence of neural tissue on the microscopically analyzed slides to provide reliable evaluation of brain invasion as described in this study still remains controversial. In 2014, Pizem et al further contributed to this discussion when showing that extensive neuropathological sampling increases the frequency of detection of brain invasion.4 In fact, evaluating brain invasive growth in the absence of neural tissue and the brain/tumor surface appears to be critical, and the average frequency of brain invasion in meningiomas in this review was found to be higher in studies in which the neural tissue on the analyzed microscopic slides was mandatory. While therefore a more uniform neuropathological assessment was proposed,72 standardization of surgical sampling might additionally help to improve accuracy of neuropathological diagnostics.73
Assessment of brain invasion using the standard operative microscope is critical,4,30 and although promising, the feasibility of intraoperative fluorescence confocal endomicroscopy for the detection of brain invasion needs to be further investigated.41 In neurosurgical practice, meningiomas are usually resected piecemeal by intraoperative suction and Cavitron Ultrasonic Surgical Aspirator, and en-bloc excision is rarely performed. Although we did not find any evidence that brain invasion impacts extent of resection,16,45 a considerable tissue portion might therefore get lost prior to neuropathological analysis also after gross total removal, and the risk of leaving behind (diagnostic, eg, brain invasive) tumor tissue is even higher after subtotal resection.73 In fact, several studies consistently reported invasive growth to be prognostic only after gross but not after subtotal tumor resection.32,36,37,45 One possible explanation for this might be the extent of resection, which could impact prognosis much more74 than brain invasive growth after subtotal resections or biopsies. However, this observation might also reflect a fundamental shortcoming when evaluating brain invasion neuropathologically in surgically obtained tumor specimens of subtotally resected meningiomas.
In fact, the risk of “undergrading” of meningiomas in these cases can directly impact clinical decisions (eg, in terms of withholding adjuvant EBRT) and have become even more critical with the release of the new WHO classification of brain tumors.1 Based on the results of the reviewed studies, we therefore recommend the following considerations to improve the accuracy of detection of brain invasion in meningiomas:
Neurosurgical Sampling
Inform the neuropathologist if brain invasion is suspected based on the extent of peritumor edema on preoperative MRI (illustrated in Fig. 3).
Try to obtain specimens from the tumor surface at different sites.
Explicitly flag these specimens as tumor surface.
If applicable, record sites of resection of superficial tumor specimen using intraoperative neuronavigation.
Inform the neuropathologist about the extent of resection to allow conclusion about tumor remnants left in situ.
Neuropathological Analyses
Define brain invasion strictly according to the WHO classification of brain tumors.
Analyze specimens marked as “superficial” separately from further tumor samples.
Explicitly label if brain tissue is detectable on the analyzed slides.
If no brain tissue is detectable, delineate that evaluation of brain invasion is not possible.
If detectable, brain invasion and the impact on grading should be explicitly mentioned.
Immunohistochemical staining should be considered in cases where brain invasion is suspected but not clearly visible on hematoxylin and eosin staining.
Among all analyzed clinical variables, brain invasion was found to be independent of patients’ age despite the higher proportion of atypical and anaplastic meningiomas in older patients,75,76 but more frequent in males compared with females.30,45,60 Although brain invasive growth was not correlated with estrogen or progesterone receptor expression in a recent series,77 this finding might at least partially contribute to a higher frequency of atypical and anaplastic meningiomas in males.44,76 However, a higher rate of grades II and III meningiomas at the convexity compared with the skull base has also been described,78 while brain invasion was independent of tumor location in all series with available data.16,30,45,49 Although no radiological imaging modality was found to reliably predict brain invasion, peritumoral edema was found to be distinctly higher in brain invasive than in non-invasive meningiomas2 and should therefore be indicated in communication between the neurosurgeon and the neuropathologist. Both increased edema and disruption of the pial surface in brain invasive tumors might lead to cortical dysfunction and subsequent development of neurological symptoms. Hence, an increased risk of seizures in patients with macroscopic brain invasion was reported.50 However, brain infiltrative growth was not confirmed by microscopic analyses in this study and intraoperative assessment of brain invasive growth using the standard operative microscope remains controversial.4,30 Similarly, an increased risk of behavior changes was found in microscopically brain invasive meningiomas in another series in univariate analyses.51 Remarkably, behavior changes were also significantly associated with brain edema, which is commonly observed in brain invasive tumors,2 and multivariate analyses including both brain invasion and edema were not provided.
Several series investigating brain invasion in correlation with tumor progression were found. Among those, brain invasion was correlated with increased risk of tumor recurrence in the majority of studies. However, 4 of 8 more recent studies (50%) referring to the 2007 WHO classification44 with a median follow-up period of 34 months to over 8 years reported no correlation between brain invasion and tumor recurrence.10,16,36,49,79 In contrast, as a part of multivariate analyses in papers studying predictors for tumor relapse after gross total resection37 and radiotherapy of residual or recurrent atypical meningiomas,48 Sun et al calculated hazard ratios for recurrence in tumors with brain invasion of 3.8 and 7.1, respectively. The incidence of tumor relapse in brain invasive tumors was 2-fold increased only in univariate analyses in another recent series.45 As the follow-up period in the studies which did not report any correlation with brain invasive tumor growth3,10,16,31,36,49 was generally long enough to detect recurrences, the question arises whether different approaches to evaluate brain invasion, such as the mandatory evidence of neural tissue on the analyzed microscopic slides, partially contribute to the lack of correlation in some cases. However, one of 2 studies which evaluated brain invasion where there was the presence of microscopically detectable neural tissue reported a lack of correlation between invasive growth and tumor progression.10 As correlation of brain invasion with further histopathological criteria of atypia, such as increased mitotic activity, geographic necrosis, etc, was reported in several studies and possibly additionally contributes to a worse prognosis,4,10,45,60 inclusion of these features in multivariate analyses30,32,34,37,48 (marked with * in Table 1) is necessary to evaluate brain invasive growth more precisely as a stand-alone prognostic variable and criterion of atypia.
Only a few retrospective studies investigating adjuvant therapy options specifically in brain invasive meningiomas were identified. Among those, adjuvant EBRT was not shown to reduce tumor recurrence in gross totally resected tumors, and brain invasion was even correlated with increased risk of tumor recurrence after EBRT for residual or recurrent atypical meningioma.37,48 Regarding pharmacological options targeting brain invasion in meningiomas, cilengitide, a specific integrin inhibitor, was shown to reduce brain invasive growth in rodents.42 Although preliminary, this finding encourages future studies to further elucidate the effects of cilengitide in invasive meningiomas. However, a literature search revealed that the role of both chemotherapeutic drugs and irradiation in brain invasive meningiomas is largely unexplored and still needs to be further established.
Since its initial consideration in 1993, the role of brain invasion for meningioma grading underwent substantial changes in the 4 released editions of the WHO classification of CNS tumors to date.1,28,43,44 Most significantly, describing a similar prognosis in brain invasive but otherwise histopathologically benign compared with atypical meningiomas in general without explicitly listing brain invasion as a grading criterion in the 2000 and 2007 editions28,44 has probably led to a partially inconsistent grading.4,6,29,49 However, the explicit inclusion of brain invasion as a stand-alone grading criterion for atypia in the newly released 2016 edition1 might lead to a more unique grading in future analyses.
In conclusion, despite a variety of described methods to detect and define brain invasion over decades, the approaches used in the majority of the current studies were similar. However, more precise grading will require standardized neuropathological evaluation regarding both the extent of sampling and the mandatory presence of cerebral tissue on analyzed slides. Additionally, subtotal resection as well as loss of tissue prior to neuropathological analyses can impair detection of brain invasive growth.
Male gender is the only clinical or demographic risk factor found to be associated with brain invasion. Brain invasive growth can be poorly predicted on preoperative MRI or assessed intraoperatively and hence doesn’t impact resectability. However, confocal microscopy might offer a future method for intraoperative detection. Although brain invasion was correlated with increased risk of tumor progression in most studies, frequently reported correlation with further histopathological criteria of atypia and anaplasia is noteworthy. The effect of irradiation in brain invasive meningiomas is presumably minor, and adjuvant chemotherapeutic options directly targeting brain invasive growth are largely unexplored. Hence, we recommend considering the following aspects when regarding brain invasion in meningiomas in clinical research and daily neurosurgical practice:
Neurosurgical techniques and incomplete resection can impair neuropathological analyses and therefore grading.
Neuropathological evaluation of brain invasion is not standardized and methods vary among published studies.
Preoperative, imaging-based as well as intraoperative macroscopic assessment of brain invasion is not reliable.
Microscopic evidence of brain invasion is correlated with tumor progression in most series.
Although frequently associated with other histopathological criteria of atypia, clinical risk factors correlated with brain invasion are sparse.
Funding
This work was supported by Deutsche Forschungsgemeinschaft (MA2530/6-1 and MA2530/8-1 to C.M.); Wilhelm Sander-Stiftung (2014.092.1 to C.M.); Deutsche Krebshilfe (111853 to C.M.); Stiftung Neurochirurgische Forschung der Deutschen Gesellschaft für Neurochirurgie (to B.B.); Wilhelm Tönnis Stiftung der Deutschen Gesellschaft für Neurochirurgie (to B.B.); Maria Möller Stiftung (to B.B.).
Conflict of interest statement
All authors declare no conflict of interest.
Acknowledgments
Cordial thanks to Petra and Phil Aston for providing support and critically reviewing the manuscript.
References
- 1. Perry A, Louis DN, von Deimling A, et al. Meningiomas. In: Louis DN, Ohgaki H, Wiestler ODet al. , eds. WHO Classification of Tumors of the Central Nervous System. Lyon: International Agency on Cancer Research; 2016:232–245. [Google Scholar]
- 2. Mantle RE, Lach B, Delgado MR, Baeesa S, Bélanger G. Predicting the probability of meningioma recurrence based on the quantity of peritumoral brain edema on computerized tomography scanning. J Neurosurg. 1999;91(3):375–383. [DOI] [PubMed] [Google Scholar]
- 3. McLean CA, Jolley D, Cukier E, Giles G, Gonzales MF. Atypical and malignant meningiomas: importance of micronecrosis as a prognostic indicator. Histopathology. 1993;23(4):349–353. [DOI] [PubMed] [Google Scholar]
- 4. Pizem J, Velnar T, Prestor B, Mlakar J, Popovic M. Brain invasion assessability in meningiomas is related to meningioma size and grade, and can be improved by extensive sampling of the surgically removed meningioma specimen. Clin Neuropathol. 2014;33(5):354–363. [DOI] [PubMed] [Google Scholar]
- 5. Crompton MR, Gautier-Smith PC. The prediction of recurrence in meningiomas. J Neurol Neurosurg Psychiatry. 1970;33(1):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fritz J, Roser F, Tatagiba M, Bornemann A. The basement membrane at the tumour-brain interface of brain-invasive grade I meningiomas. Neuropathol Appl Neurobiol. 2005;31(3):339–342. [DOI] [PubMed] [Google Scholar]
- 7. Nakasu S, Fukami T, Jito J, Matsuda M. Microscopic anatomy of the brain-meningioma interface. Brain Tumor Pathol. 2005;22(2):53–57. [DOI] [PubMed] [Google Scholar]
- 8. Nakasu S, Hirano A, Llena JF, Shimura T, Handa J. Interface between the meningioma and the brain. Surg Neurol. 1989;32(3):206–212. [DOI] [PubMed] [Google Scholar]
- 9. Zeltner L, Schittenhelm J, Mittelbronn M, et al. The astrocytic response towards invasive meningiomas. Neuropathol Appl Neurobiol. 2007;33(2):163–168. [DOI] [PubMed] [Google Scholar]
- 10. Backer-Grøndahl T, Moen BH, Arnli MB, Torseth K, Torp SH. Immunohistochemical characterization of brain-invasive meningiomas. Int J Clin Exp Pathol. 2014;7(10):7206–7219. [PMC free article] [PubMed] [Google Scholar]
- 11. Coven İ, Ozer O, Ozen O, Şahin Fİ, Altinors N. Presence of matrix metalloproteinase-2 and tissue inhibitor matrix metalloproteinase-2 gene polymorphisms and immunohistochemical expressions in intracranial meningiomas. J Neurosurg. 2014;121(6):1478–1482. [DOI] [PubMed] [Google Scholar]
- 12. Schittenhelm J, Mittelbronn M, Roser F, Tatagiba M, Mawrin C, Bornemann A. Patterns of SPARC expression and basement membrane intactness at the tumour-brain border of invasive meningiomas. Neuropathol Appl Neurobiol. 2006;32(5):525–531. [DOI] [PubMed] [Google Scholar]
- 13. von Randow AJ, Schindler S, Tews DS. Expression of extracellular matrix-degrading proteins in classic, atypical, and anaplastic meningiomas. Pathol Res Pract. 2006;202(5):365–372. [DOI] [PubMed] [Google Scholar]
- 14. Kliese N, Gobrecht P, Pachow D, et al. miRNA-145 is downregulated in atypical and anaplastic meningiomas and negatively regulates motility and proliferation of meningioma cells. Oncogene. 2013;32(39):4712–4720. [DOI] [PubMed] [Google Scholar]
- 15. Kilic T, Bayri Y, Ozduman K, et al. Tenascin in meningioma: expression is correlated with anaplasia, vascular endothelial growth factor expression, and peritumoral edema but not with tumor border shape. Neurosurgery. 2002;51(1):183–192; discussion 192–183. [DOI] [PubMed] [Google Scholar]
- 16. Ruiz J, Martínez A, Hernández S, et al. Clinicopathological variables, immunophenotype, chromosome 1p36 loss and tumour recurrence of 247 meningiomas grade I and II. Histol Histopathol. 2010;25(3):341–349. [DOI] [PubMed] [Google Scholar]
- 17. Mawrin C, Wolke C, Haase D, et al. Reduced activity of CD13/aminopeptidase N (APN) in aggressive meningiomas is associated with increased levels of SPARC. Brain Pathol. 2010;20(1):200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rempel SA, Schwechheimer K, Davis RL, Cavenee WK, Rosenblum ML. Loss of heterozygosity for loci on chromosome 10 is associated with morphologically malignant meningioma progression. Cancer Res. 1993;53(10 Suppl):2386–2392. [PubMed] [Google Scholar]
- 19. Simon M, von Deimling A, Larson JJ, et al. Allelic losses on chromosomes 14, 10, and 1 in atypical and malignant meningiomas: a genetic model of meningioma progression. Cancer Res. 1995;55(20):4696–4701. [PubMed] [Google Scholar]
- 20. Weber RG, Boström J, Wolter M, et al. Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: toward a genetic model of meningioma progression. Proc Natl Acad Sci U S A. 1997;94(26):14719–14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kandenwein JA, Park-Simon TW, Schramm J, Simon M. uPA/PAI-1 expression and uPA promoter methylation in meningiomas. J Neurooncol. 2011;103(3):533–539. [DOI] [PubMed] [Google Scholar]
- 22. Denizot Y, De Armas R, Caire F, et al. The quantitative analysis of bFGF and VEGF by ELISA in human meningiomas. Mediators Inflamm. 2006;2006(6):36376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van de Nes JA, Griewank KG, Schmid KW, Grabellus F. Immunocytochemical analysis of glucose transporter protein-1 (GLUT-1) in typical, brain invasive, atypical and anaplastic meningioma. Neuropathology. 2015;35(1):24–36. [DOI] [PubMed] [Google Scholar]
- 24. Ke RH, Wang Y, Mao Y, Zhang J, Xiong J. Decreased expression of LASS2 is associated with worse prognosis in meningiomas. J Neurooncol. 2014;118(2):369–376. [DOI] [PubMed] [Google Scholar]
- 25. Küster O, Simon P, Mittelbronn M, et al. Erythropoietin receptor is expressed in meningiomas and lower levels are associated with tumour recurrence. Neuropathol Appl Neurobiol. 2009;35(6):555–565. [DOI] [PubMed] [Google Scholar]
- 26. Mawrin C, Sasse T, Kirches E, et al. Different activation of mitogen-activated protein kinase and Akt signaling is associated with aggressive phenotype of human meningiomas. Clin Cancer Res. 2005;11(11):4074–4082. [DOI] [PubMed] [Google Scholar]
- 27. Simon M, Park TW, Leuenroth S, Hans VH, Löning T, Schramm J. Telomerase activity and expression of the telomerase catalytic subunit, hTERT, in meningioma progression. J Neurosurg. 2000;92(5):832–840. [DOI] [PubMed] [Google Scholar]
- 28. Louis DN, Scheithauer BW, Budka H, von Deimling A, Kepes JJ. Meningiomas. In: Kleihues P, Cavenee WK, eds. Pathology and Genetics: Tumours of the Nervous System. Lyon: IARC Press; 2000:176–184. [Google Scholar]
- 29. Yun S, Koh JM, Lee KS, Seo AN, Nam KH, Choe G. Expression of c-MET in invasive meningioma. J Pathol Transl Med. 2015;49(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vranic A, Popovic M, Cör A, Prestor B, Pizem J. Mitotic count, brain invasion, and location are independent predictors of recurrence-free survival in primary atypical and malignant meningiomas: a study of 86 patients. Neurosurgery. 2010;67(4):1124–1132. [DOI] [PubMed] [Google Scholar]
- 31. Klinger DR, Flores BC, Lewis JJ, et al. Atypical meningiomas: recurrence, reoperation, and radiotherapy. World Neurosurg. 2015;84(3):839–845. [DOI] [PubMed] [Google Scholar]
- 32. Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. 1997;21(12):1455–1465. [DOI] [PubMed] [Google Scholar]
- 33. Yang SY, Park CK, Park SH, Kim DG, Chung YS, Jung HW. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry. 2008;79(5):574–580. [DOI] [PubMed] [Google Scholar]
- 34. Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85(9):2046–2056. [DOI] [PubMed] [Google Scholar]
- 35. Ho DM, Hsu CY, Ting LT, Chiang H. Histopathology and MIB-1 labeling index predicted recurrence of meningiomas: a proposal of diagnostic criteria for patients with atypical meningioma. Cancer. 2002;94(5):1538–1547. [DOI] [PubMed] [Google Scholar]
- 36. Sun SQ, Cai C, Murphy RK, et al. Management of atypical cranial meningiomas, part 2: predictors of progression and the role of adjuvant radiation after subtotal resection. Neurosurgery. 2014;75(4):356–363; discussion 363. [DOI] [PubMed] [Google Scholar]
- 37. Sun SQ, Kim AH, Cai C, et al. Management of atypical cranial meningiomas, part 1: predictors of recurrence and the role of adjuvant radiation after gross total resection. Neurosurgery. 2014;75(4):347–354; discussion 354–355; quiz 355. [DOI] [PubMed] [Google Scholar]
- 38. Deen HG, Jr, Scheithauer BW, Ebersold MJ. Clinical and pathological study of meningiomas of the first two decades of life. J Neurosurg. 1982;56(3):317–322. [DOI] [PubMed] [Google Scholar]
- 39. Lee JW, Kang KW, Park SH, et al. 18F-FDG PET in the assessment of tumor grade and prediction of tumor recurrence in intracranial meningioma. Eur J Nucl Med Mol Imaging. 2009;36(10):1574–1582. [DOI] [PubMed] [Google Scholar]
- 40. Moradi A, Semnani V, Djam H, et al. Pathodiagnostic parameters for meningioma grading. J Clin Neurosci. 2008;15(12):1370–1375. [DOI] [PubMed] [Google Scholar]
- 41. Peyre M, Clermont-Taranchon E, Stemmer-Rachamimov A, Kalamarides M. Miniaturized handheld confocal microscopy identifies focal brain invasion in a mouse model of aggressive meningioma. Brain Pathol. 2013;23(4):371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilisch-Neumann A, Kliese N, Pachow D, et al. The integrin inhibitor cilengitide affects meningioma cell motility and invasion. Clin Cancer Res. 2013;19(19):5402–5412. [DOI] [PubMed] [Google Scholar]
- 43. Kleihues P, Burger PC, Scheithauer BW. Histological typing of tumours of the central nervous system. In: Sobin LH, ed. World Health Organization, International Classification of Tumours. Berlin: Springer; 1993:33–37. [Google Scholar]
- 44. Perry A, Louis DN, Scheithauer BW, Budka H, von Deimling A. Meningiomas. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. WHO Classification of Tumors of the Central Nervous System. Lyon: International Agency on Cancer Research; 2007:164–172. [Google Scholar]
- 45. Spille DC, Heß K, Sauerland C, et al. Brain invasion in meningiomas: incidence and correlations with clinical variables and prognosis. World Neurosurg. 2016;93:346–354. [DOI] [PubMed] [Google Scholar]
- 46. Jenkinson MD, Javadpour M, Haylock BJ, et al. The ROAM/EORTC-1308 trial: radiation versus observation following surgical resection of atypical meningioma: study protocol for a randomised controlled trial. Trials. 2015;16:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99(3):379–391. [DOI] [PubMed] [Google Scholar]
- 48. Sun SQ, Cai C, Murphy RK, et al. Radiation therapy for residual or recurrent atypical meningioma: the effects of modality, timing, and tumor pathology on long-term outcomes. Neurosurgery. 2015;79(1):23–32. [DOI] [PubMed] [Google Scholar]
- 49. Telugu RB, Chowhan AK, Rukmangadha N, et al. Histopathological and immunohistochemical evaluation of meningiomas with reference to proliferative markers p53 and Ki-67. J Clin Diagn Res. 2016;10(1):EC15–EC19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chernov M. Letter to the editor: Seizures and invasive meningiomas. J Neurosurg. 2016;125(6):1615–1616. [DOI] [PubMed] [Google Scholar]
- 51. Vranic A, Gilbert F. Prognostic implication of preoperative behavior changes in patients with primary high-grade meningiomas. ScientificWorldJournal. 2014;2014:398295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perry A, Giannini C, Raghavan R, et al. Aggressive phenotypic and genotypic features in pediatric and NF2-associated meningiomas: a clinicopathologic study of 53 cases. J Neuropathol Exp Neurol. 2001;60(10):994–1003. [DOI] [PubMed] [Google Scholar]
- 53. Symons P, Tobias V, Pereira J, Vonau M. Brain-invasive meningioma in a 16-month-old boy. Pathology. 2001;33(2):252–256. [PubMed] [Google Scholar]
- 54. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(1):22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chernov MF, Kasuya H, Nakaya K, et al. ¹H-MRS of intracranial meningiomas: what it can add to known clinical and MRI predictors of the histopathological and biological characteristics of the tumor? Clin Neurol Neurosurg. 2011;113(3):202–212. [DOI] [PubMed] [Google Scholar]
- 56. Tamiya T, Ono Y, Matsumoto K, Ohmoto T. Peritumoral brain edema in intracranial meningiomas: effects of radiological and histological factors. Neurosurgery. 2001;49(5):1046–1051; discussion 1051–1042. [DOI] [PubMed] [Google Scholar]
- 57. Shepard MJ, Elias WJ. Editorial: seizures with meningioma. J Neurosurg. 2016;124(6):1549–1551. [DOI] [PubMed] [Google Scholar]
- 58. Hou J, Kshettry VR, Selman WR, Bambakidis NC. Peritumoral brain edema in intracranial meningiomas: the emergence of vascular endothelial growth factor-directed therapy. Neurosurg Focus. 2013;35(6):E2. [DOI] [PubMed] [Google Scholar]
- 59. Baumgarten P, Brokinkel B, Zinke J, et al. Expression of vascular endothelial growth factor (VEGF) and its receptors VEGFR1 and VEGFR2 in primary and recurrent WHO grade III meningiomas. Histol Histopathol. 2013;28(9):1157–1166. [DOI] [PubMed] [Google Scholar]
- 60. Yoon H, Mehta MP, Perumal K, et al. Atypical meningioma: randomized trials are required to resolve contradictory retrospective results regarding the role of adjuvant radiotherapy. J Cancer Res Ther. 2015;11(1):59–66. [DOI] [PubMed] [Google Scholar]
- 61. Millesi M, Kiesel B, Mischkulnig M, et al. Analysis of the surgical benefits of 5-ALA-induced fluorescence in intracranial meningiomas: experience in 204 meningiomas. J Neurosurg. 2016;125(6):1408–1419. [DOI] [PubMed] [Google Scholar]
- 62. Hasan S, Young M, Albert T, et al. The role of adjuvant radiotherapy after gross total resection of atypical meningiomas. World Neurosurg. 2015;83(5):808–815. [DOI] [PubMed] [Google Scholar]
- 63. Kaur G, Sayegh ET, Larson A, et al. Adjuvant radiotherapy for atypical and malignant meningiomas: a systematic review. Neuro Oncol. 2014;16(5):628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Paldor I, Awad M, Sufaro YZ, Kaye AH, Shoshan Y. Review of controversies in management of non-benign meningioma. J Clin Neurosci. 2016;31:37–46. [DOI] [PubMed] [Google Scholar]
- 65. VanMeter TE, Rooprai HK, Kibble MM, Fillmore HL, Broaddus WC, Pilkington GJ. The role of matrix metalloproteinase genes in glioma invasion: co-dependent and interactive proteolysis. J Neurooncol. 2001;53(2):213–235. [DOI] [PubMed] [Google Scholar]
- 66. Carter A. Integrins as target: first phase III trial launches, but questions remain. J Natl Cancer Inst. 2010;102(10):675–677. [DOI] [PubMed] [Google Scholar]
- 67. Nabors LB, Mikkelsen T, Rosenfeld SS, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25(13):1651–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stupp R, Hegi ME, Neyns B, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(16):2712–2718. [DOI] [PubMed] [Google Scholar]
- 69. Chen J, Xu X, Wang H. Expression of integrin-alpha(3) mRNA in meningiomas and its correlation with proliferation and invasion. J Huazhong Univ Sci Technolog Med Sci. 2009;29(1):94–96. [DOI] [PubMed] [Google Scholar]
- 70. Salehi F, Jalali S, Alkins R, et al. Proteins involved in regulating bone invasion in skull base meningiomas. Acta Neurochir (Wien). 2013;155(3):421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zülch KJ. Histological typing of tumours of the central nervous system. In: Organization TWH, ed. International Histological Classification of Tumors. Vol 21 Genf: Who’s Who; 1979:66. [Google Scholar]
- 72. Brokinkel B, Stummer W. Brain invasion in meningiomas: the rising importance of a uniform neuropathologic assessment after the release of the 2016 World Health Organization Classification of Central Nervous System Tumors. World Neurosurg. 2016;95:614–615. [DOI] [PubMed] [Google Scholar]
- 73. Jenkinson MD, Santarius T, Zadeh G, Aldape KD. Atypical meningioma—is it time to standardize surgical sampling techniques? Neuro Oncol. 2017;19(3):453–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gousias K, Schramm J, Simon M. The Simpson grading revisited: aggressive surgery and its place in modern meningioma management. J Neurosurg. 2016;125(3):551–560. [DOI] [PubMed] [Google Scholar]
- 75. Park JS, Sade B, Oya S, Kim CG, Lee JH. The influence of age on the histological grading of meningiomas. Neurosurg Rev. 2014;37(3):425–429; discussion 429. [DOI] [PubMed] [Google Scholar]
- 76. Brokinkel B, Holling M, Spille DC, et al. Surgery for meningioma in the elderly and long-term survival: comparison with an age- and sex-matched general population and with younger patients. J Neurosurg. 2017;126(4):1201–1211. [DOI] [PubMed] [Google Scholar]
- 77. Heß K, Spille DC, Wagner A, Stummer W, Paulus W, Brokinkel B. Letter to the editor: Brain invasion in meningiomas-sex-associated differences are not related to estrogen- and progesterone receptor expression. Neurosurgery. 2017 April 5. doi:10.1093/neuros/nyx147. [DOI] [PubMed] [Google Scholar]
- 78. Cornelius JF, Slotty PJ, Steiger HJ, Hänggi D, Polivka M, George B. Malignant potential of skull base versus non-skull base meningiomas: clinical series of 1663 cases. Acta Neurochir (Wien). 2013;155(3): 407–413. [DOI] [PubMed] [Google Scholar]
- 79. Baumgarten P, Gessler F, Schittenhelm J, et al. Brain invasion in otherwise benign meningiomas does not predict tumor recurrence. Acta Neuropathol. 2016;132(3):479–481. [DOI] [PubMed] [Google Scholar]